Abstract
Environmental enrichment (EE) with a complex combination of physical, cognitive and social stimulations enhances synaptic plasticity and behavioral function. However, the mechanism remains to be elucidated in detail. We aimed to investigate dopamine-related synaptic plasticity underlying functional improvement after EE. For this, six-week-old CD-1 mice were randomly allocated to EE or standard conditions for two months. EE significantly enhanced behavioral functions such as rotarod and ladder walking tests. In a [18F]FPCIT positron emission tomography scan, binding values of striatal DAT were significantly decreased approximately 18% in the EE mice relative to the control mice. DAT inhibitor administrated to establish the relationship of the DAT down-regulation to the treatment effects also improved rotarod performances, suggesting that DAT inhibition recapitulated EE-mediated treatment benefits. Next, EE-induced internalization of DAT was confirmed using a surface biotinylation assay. In situ proximity ligation assay and immunoprecipitation demonstrated that EE significantly increased the phosphorylation of striatal DAT as well as the levels of DAT bound with protein kinase C (PKC). In conclusion, we suggest that EE enables phosphorylation of striatal DAT via a PKC-mediated pathway and causes DAT internalization. This is the first report to suggest an EE-mediated mechanism of synaptic plasticity by internalization of striatal DAT.
Keywords: Dopamine transporter, environmental enrichment, internalization, phosphorylation
Introduction
Environmental enrichment (EE) is a method of raising animals in a huge cage containing novel objects, running wheels and social interaction with a complex combination of physical, cognitive, and social stimulations.1 EE gives rise to biochemical changes and histological improvements such as neurogenesis, axonal sprouting, and dendritic branching even in the adult brain, consequently promoting behavioral functions.2,3 EE can affect neural plasticity via overexpression of neurothrophic factor and neurotransmitter receptors.4,5 A study also showed that EE enhanced endogenous angiogenesis and neurobehavioral functions.6 Likewise, many reports have shown that EE improves various behavioral performances, suggesting that the positive effects of EE may introduce potential therapeutic strategies for subjects recovering from brain damage.7–9
We recently reported the underlying mechanisms associated with long-term exposure to EE in the mouse brain by evaluating gene expression patterns.10 Among the gene expression changes, EE decreased the Na+/Cl−-dependent neurotransmitter transporters including dopamine transporter (DAT) in the brain.10 DAT is a pump for dopamine neurotransmitters, which takes the neurotransmitter out of the synapse back into the presynaptic neural cytosol, and dopamine reuptake via DAT controls the concentration of dopamine between presynaptic and postsynaptic neurons.11,12 Proper dopaminergic function is dependent on the reuptake activity by DAT, which is the primary mechanism responsible for the control of the dopamine levels in the neural synapse.13–15
Previous studies on the relationship between EE and DAT have been conducted in the prefrontal cortex. It has been reported that EE enables a more efficient use of neurotransmitters including dopamine, and modulates DAT dynamics in the prefrontal cortex.13,15 However, the underlying mechanism of DAT regulation, including phosphorylation, remains unclear.16 In particular, the regulation mechanism of DAT after exposure to EE, mainly distributed in the striatum, has yet to be elucidated.
Therefore, we investigated the regulation mechanism of striatal DAT in EE-induced improvement. In this study, we aimed to investigate dopamine-related synaptic plasticity underlying functional improvements after EE. According to a previous report which showed that presynaptic DAT was down-regulated,10 we hypothesized that EE may play a role in the internalization of striatal DAT. We suggest that EE enables the phosphorylation of DAT via a protein kinase C (PKC)-mediated pathway in the striatum, which causes internalization and may therefore control dopamine concentrations in the striatum.
Materials and methods
Enriched environment
The EE mice were housed in a huge cage (86 × 76 × 31 cm3) containing novel objects, such as tunnels, shelters, toys, and running wheels for voluntary exercise, and allowing for social interaction (10 mice/cage) for two months (Figure 1a), whereas the control mice were housed for the same duration in standard cages (27 × 22.5 × 14 cm3) (Figure 1b).10 For all experiments, CD-1 (ICR) mice were housed in a facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) and given food and water ad libitum under alternating 12-h light/dark cycles, according to animal protection regulations. The experimental procedure was approved by the Institutional Animal Care and Use Committee (IACUC) of Yonsei University Health System (permit number: 2014-0125). Experiments were blinded to both experimenters and analysts and were in compliance with the ARRIVE guidelines (Animal Research: Reporting in Vivo Experiments) for how to report animal experiments.
Figure 1.
Experimental design. (a) CD-1 (ICR) mice were randomly assigned to either an enriched environment (EE) or standard conditions starting at six weeks of age for two months. The EE was provided in a huge cage (86 × 76 × 31 cm3) containing various objects, such as tunnels, shelters, toys, and running wheels for voluntary exercise, and allowed for enriched social interaction (10 mice/cage). (b) Control mice were housed for the same duration in standard cages (27 × 22.5 × 14 cm3). (c) A schematic timeline of the experiments. A total of 20 CD-1 (ICR) mice were screened at 6 weeks of age, and randomly assigned to either control condition (n = 10) or the EE condition (n = 10) for behavioral assessment to investigate functional outcomes. Among the subjects, six mice were recruited for the PET imaging at eight weeks post-treatment (n = 3 per group). A total of six mice were also recruited to evaluate surface DAT levels using a biotinylation assay (n = 3 per group). For the PLA assay, eight mice were recruited to evaluate phosphorylation of DAT at eight weeks post-treatment (n = 4 per group). Mice were also recruited to confirm phosphorylation of DAT using immunoprecipitation (IP) (n = 10 per group). In an additional set, a total of 20 mice were used for the DAT inhibitor experiment. Mice from the control group (n = 5) and from the EE group (n = 5) were injected with DAT inhibitor, JHW0007, and mice from the control group (n = 5) and from the EE group (n = 5) were injected with saline to confirm that a DAT inhibitor-mediated inhibition could reverse functional outcomes.
Experimental grouping
In this study, a total of 20 CD-1 (ICR) mice were recruited at six weeks of age. For behavioral assessment, mice were randomly assigned to either an EE (n = 10) or standard condition as a control group (n = 10) to investigate functional outcomes. Among the subjects, six mice were recruited for the PET imaging at eight weeks post-treatment (n = 3 per group). A total of six mice were also recruited to evaluate surface DAT levels using a biotinylation assay (n = 3 per group). For the PLA assay, eight mice were recruited to evaluate phosphorylation of DAT at eight weeks post-treatment (n = 4 per group). A total of eight mice were also recruited to confirm phosphorylation of DAT using immunoprecipitation (IP) (n = 10 per group). In order to investigate whether a DAT inhibitor, JHW0007, mediated inhibition could reverse functional outcomes, a total of 20 mice were randomly assigned to either the JHW007-treated EE group, JHW007-treated control group, saline-treated EE group or the saline-treated control group (n = 5 per group). A schematic timeline of this experiment during the eight weeks is provided in Figure 1c.
Behavioral assessment
Rotarod behavioral test
A rotarod test was used to assess motor coordination and locomotor function. All animals received a pretreatment performance evaluation at 5–6 weeks of age. For this assessment, mice were placed on a rotarod treadmill (Ugo Basile), and the latency to fall, which is the length of time that the animals remained on the rolling rod, was measured. Rotarod tests were then performed at two-week intervals until eight weeks after the commencement of the treatment at an accelerating speed (4–80 rpm) and a constant speed (64 rpm).10 The latency period was measured twice for each test, and individual tests were terminated at a maximum latency of 300 s. To avoid any stress related to the tests, we conducted the tests gently.10
Ladder walking test
The ladder rung walking task allows discrimination between subtle disturbances of motor function by combining qualitative and quantitative analysis of skilled walking.17 The ladder walking test was performed eight weeks after treatment. In the ladder walking test, the mice were required to walk a distance of 1 m three times on a horizontal ladder with metal rungs (Jeung Do B&P) located at differing distances apart. The number of slips from the transverse rungs with each forelimb was measured by videotape analysis.6 A comparison between the control and EE groups was calculated as the difference in the percentage of slips on the transverse rungs of the ladder relative to the total number of steps taken by each forelimb, compared to the control groups.
PET imaging study
[18F]FPCIT was synthesized according to the previously described procedure.18 PET scanning was performed with the Siemens Inveon small animal PET scanner (Siemens Medical Solutions). The scanner has a peak absolute system sensitivity of <10% for the 250–750 eV energy window, an axial field of view of 12 cm and a transaxial field of view of 10 cm.19 Anesthesia was induced with 2.5% isoflurane and was maintained with 1.5% isoflurane for a 120-min long PET experiment. After administration of [18F]FPCIT (4.71 ± 0.94 MBq), mice were positioned in the center of gantry. Tracer accumulation in the brain was investigated by dynamic PET scans over 120 min after injection of [18F]FPCIT.
PET data were reconstructed in user-defined time frames and lengths (10 s × 6 frames, 30 s × 8 frames, 300 s × 5 frames, 1800 s × 4 frames) with a voxel size of 0.861386 × 0.861386 × 0.796000 mm by a two-dimentional order-subset expectation maximization (OSEM) algorithm (4 iterations and 16 subsets). Image files were evaluated by region-of-interest (ROI) analysis using the software AsiProVM software (Acquisition Sinogram Image Processing software, CTI Concorde Microsystems). ROIs associated with the striatum and cerebellum were drawn on all coronal brain images, guided by stereotactic coordinates.20 The decay-corrected time activity curves were presented in units of the standard uptake value (SUV), calculated as (% injected dose/cm3) × body weight (g) to normalize for the differences in rat weight and administered doses and to compare inter-rat and inter-organ data. All SUV values in the text are the means of the measurements on three mice. Non-displaceable binding potential (BPND), commonly used as an indication of receptor binding density, is the ratio of the peak values of the specific binding (SUVstriatum − SUVcerebellum) divided by the non-specific binding (SUVcerebellum) at the time of the peak. The cerebellum was used as the reference region because it contains very few dopamine transporters and receptors in adult mice.21,22 If the computed specific binding was negative, it was given a null value.
DAT inhibitor treatment
The BZT analogue, JHW007 [(N-(n-butyl)-3α-[bis(4′-fluorophenyl) methoxy]-tropane); Tocris], was synthesized as described previously.23,24 The purity of the compound was analyzed by magnetic resonance, which exceeded 98%. JHW007 was dissolved in 0.9% saline and sonicated until complete solubilization was achieved. It was then injected at doses of 15 mg/kg i.p. for two months.
Surface biotinylation assay
In the present study, Sulfo-NHS-biotin (Thermo) was used to isolate membrane-associated DAT protein prepared from the striatum region of the EE or control conditioned mice. DAT protein was subsequently identified by immunoreactivity using a DAT antibody. Larger amounts of striatal protein (400 µg) were used to determine NHS-biotin-labeled cell surface DAT.13 Chopped striatal tissues were washed with ice-cold PBS containing 1 mM MgCl2 and 0.1 mM CaCl2 (PBS++) and incubated with 1 mg/ml EZ-link Sulfo-NHS-SS-Biotin (Thermo) in PBS++ for 1 h at 4℃ with gentle shaking. The reaction was quenched by washing with 0.1% BSA in 4℃ PBS++. The cells were lysed with 100 μl cold RIPA buffer (50 mM Tris-HCl, pH 7.5), 1% Triton X-100, 150 mM NaCl, 0.1% sodium dodecyl sulfate (SDS), and 1% sodium deoxycholate with a protease inhibitor cocktail (Sigma). Protein lysates were then centrifuged at 13,000 rpm at 4℃ for 20 min. The supernatant was extracted, and incubated with NeutrAvidin agarose resin (Pierce) for 2 h at 4℃. The resin was washed and the bound proteins were eluted by mixing and incubating with 2× SDS sample buffer at 70℃ for 10 min. The eluted sample was analyzed by Western blotting.
In situ proximity ligation assay
The Duolink® kit (Olink Bioscience) is based on the use of two unique and bi-functional probes called PLA™. Each probe consists of a secondary antibody attached to a unique synthetic oligonucleotide that acts as a reporter. After exposure for eight weeks to the EE or control condition, the striatum region was stained with DAT and phosphoserine primary antibodies overnight at 4℃ to detect interacting DAT and phosphoserine proteins. After washing, the sections were incubated with the secondary oligonucleotide-linked antibodies (PLA probes) provided in the kit. The oligonucleotides bound to the antibodies were hybridized, ligated, amplified, and detected using a fluorescent probe (Detection Kit 563).25 Dots were detected by confocal imaging (LSM700, Zeiss).
Immunoprecipitation
To identify DAT and its phosphorylation regulated by EE, the striatum were lysed in 500 μl of cold RIPA buffer with a protease inhibitor cocktail (Sigma). Tissue lysates were then centrifuged at 13,000 rpm at 4℃ for 20 min, the supernatant was extracted and protein concentrations were measured using the Bradford method. For co-immunoprecipitation experiments, solubilized specific brain regions extracts (1 mg of protein) were incubated in the presence of primary anti-DAT antibodies (1:500; Chemicon) for 24 h at 4℃, followed by the addition of 20 μl of protein A/G agarose (Santa Cruz Biotechnology) for 3 h at 4℃. Then the solubilized specific brain regions extracts with the primary anti-DAT antibodies and protein A/G agarose were centrifuged at 3000 rpm at 4℃ for 1 min. Beads were washed four times in the buffer described above, boiled for 5 min in SDS sample buffer, and subjected to Western blot for anti-DAT and anti-phosphoserine. The proteins were visualized with enhanced chemiluminescence reagents as described (Amersham Biosciences).
To confirm the expression of PKC by control or EE group (n = 3 each), the striatum were lysed in 500 μl of cold RIPA buffer with a protease inhibitor cocktail (Sigma). All steps were performed as mentioned above. After IP and transfer onto a membrane, Western blot analysis was used to detect expression of anti-PKCβI (1:1,000, Santa Cruz Biotechnology).26 In addition, to validate that the DAT inhibition occurs via phosphorylation mechanism, SH-SY5Y neuroblastoma cells were treated with the DAT inhibitor (JHW007 15 µg/ml) for 2 min at 37℃.27 Larger amounts of cell protein (200 µg) were used to identify DAT and its phosphorylation regulated by DAT inhibitor using immunoprecipitation.
Statistical analysis
All data were expressed as means ± SEM. Statistical analyses were conducted using SPSS version 18.0. The results of behavioral tests, PET image assay, surface biotinylation assay, and immunoprecipitation were analyzed by the independent t-test which was used for the comparison of continuous variables between the EE and control groups. In addition, rotarod performance after the treatment of either a DAT inhibitor or saline was analyzed by the one-way ANOVA was used for the statistical analysis of variables among the groups. A P < 0.05 was considered statistically significant.
Results
The rotarod test showed that EE improved locomotor function
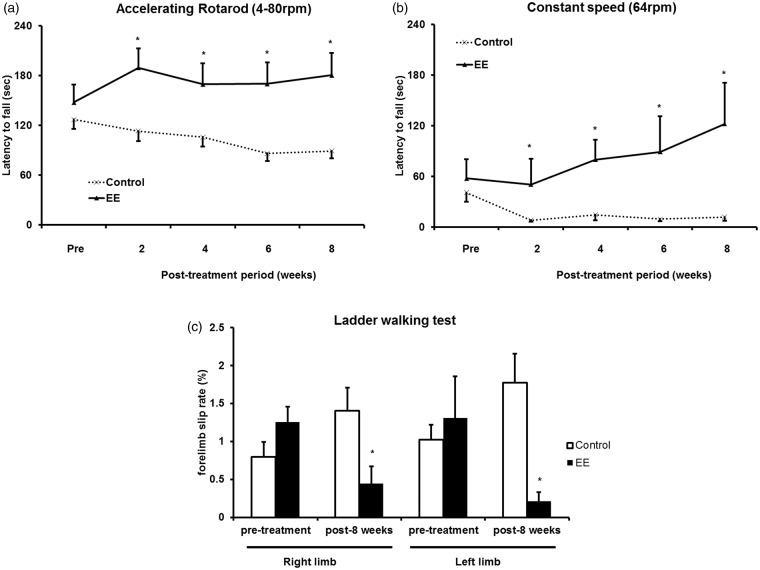
Six-week old CD-1 (ICR) mice were randomly allocated to either EE or standard conditions for two months (n = 10 per group) (Figure 1). To determine whether EE exposure improves motor function, rotarod tests were performed with accelerating (4–80 rpm) and constant (64 rpm) paradigms every two weeks. In the pretreatment evaluation, no statistical differences were seen between the groups. EE-induced improvement of rotarod performance relative to controls was significantly evident at two weeks post-treatment. A significant improvement was observed starting at two weeks post-treatment for the accelerating speed condition (192.4 ± 15.1 in EE and 109.9 ± 13.3 s in control, t = 4.104, P < 0.001; Figure 2a) and for the constant speed condition (50.6 ± 20.1 in EE and 7.8 ± 1.9 s in control, t = 2.115, P = 0.048; Figure 2b). This improved motor function was maintained throughout the study period. Finally, eight weeks after the treatment, the mean rotarod latencies of the EE mice increased significantly to 182.7 ± 17.9 s for the accelerating speed condition (t = 3.949, P < 0.001; Figure 2a) and to 122.4 ± 32.0 s for the constant-speed condition (t = 3.441, P = 0.001; Figure 2b), compared with the rotarod latencies of the control mice (102.6 ± 9.6 and 11.7 ± 3.3 s, respectively).
Figure 2.
Improved motor functions after exposure to EE. (a) For the accelerating speed (4–80 rpm) rotarod test, a significant improvement was observed starting two weeks after the exposure to an EE. Until eight weeks post-treatment, the mean rotarod latency of the EE mice was significantly higher than the latency of control mice. (b) For the constant speed (64 rpm) rotarod test, a significant improvement was also observed starting two weeks after exposure to an EE. Until eight weeks post-treatment, the mean rotarod latency of the EE mice was significantly higher than the latency of control mice. (c) In the ladder walking test, EE mice also showed a significant reduction in the percentage of the total slips among total steps with both forelimbs at eight weeks post-treatment.
Ladder walking test showed that EE improved fine motor function
To assess fine motor function more thoroughly between the EE and control groups (n = 10 each), ladder walking tests were performed at pre-treatment and eight weeks after EE (Figure 2c). In the ladder walking test, the percentage of slips on the transverse rungs of the ladder relative to the total number of steps by the right and left forelimbs were significantly decreased only in the EE mice (0.5 ± 0.2% and 0.2 ± 0.1%, respectively) compared with the forelimb slip rate (%) of the control mice (1.4 ± 0.3% and 1.8 ± 0.4%, respectively) at eight weeks post-treatment (t = 2.528, P = 0.016 in the right forelimb and t = 3.995, P = 0.001 in the left forelimb). Taken together, results in both the rotarod test and the ladder walking test suggest that EE can improve motor function.
PET image assay showed that EE down-regulated striatal DAT
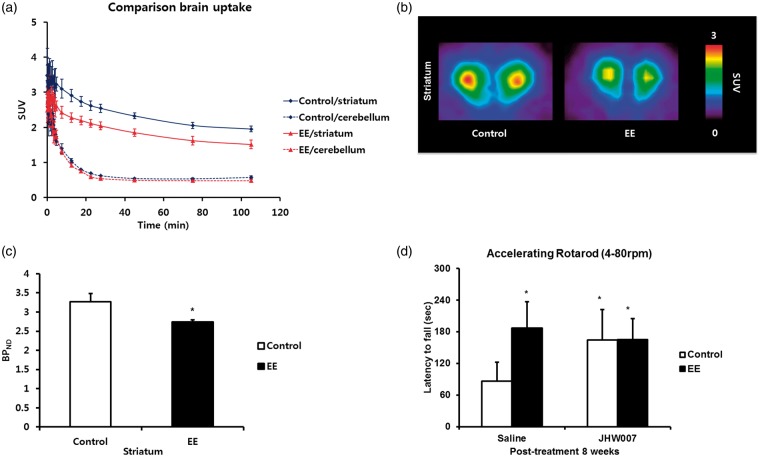
In this study, we performed a [18F]FPCIT PET scan in the mouse brain after EE to investigate changes of DAT expression. DAT was mainly expressed in the striatal region, but the cerebellum has little DAT expression. Therefore, the expression of the cerebellum was used as a negative control. In the time activity curves, cerebellar uptakes between EE and control group were similar. However, the striatal uptake of EE was lower than that of the control group (Figure 3a). Binding values of striatal DAT were significantly decreased approximately 18% in the EE mice (2.7 ± 0.05 BPND) relative to the control mice (3.3 ± 0.2 BPND) at eight weeks post-treatment (t = 2.28, P = 0.048; Figure 3b and c).
Figure 3.
EE-mediated down-regulation of DAT in the striatum. (a) Time activity curves in the specific brain regions for EE and control groups. While radioactivities in the cerebellum were similar each other, striatal uptake in EE mice was lower than that in control group. (b) Representative brain PET image in EE indicated decreased uptake of the DAT in the striatal region. (c) Binding values of striatal DAT were significantly decreased approximately 18% in the EE mice relative to the control mice at eight weeks post-treatment. (d) The effect of a DAT inhibitor, JHW007. Rotarod tests were performed with accelerating (4–80 rpm) paradigms eight weeks after exposure on either mice from the JHW007-treated EE and control groups or the saline-treated EE and control groups (n = 5 per group). In the pretreatment evaluation, no statistical differences were seen between the groups. At eight weeks post-treatment, the saline-treated EE and JHW007-treated mice induced improvement of rotarod performance relative to the saline-treated control mice.
DAT inhibitor recapitulated EE-mediated treatment benefits
To determine whether DAT inhibitor, JHW007, treated groups showed improvement on motor function, rotarod tests were performed with accelerating paradigms (4–80 rpm) eight weeks after treatment with either JHW007 or saline in the EE and control mice (n = 5 per group). In the pretreatment evaluation, no statistical differences were seen between the groups. EE and JHW007 treatment (196.8 ± 30.0 s in the saline-treated EE mice, 176.9 ± 25.5 s in the JHW007-treated EE mice, and 176.5 ± 33.8 s in the JHW007-treated control mice) resulted in significantly improved rotarod performance relative to the saline-treated controls (88.1 ± 21.0 s) at eight weeks post-treatment (F = 3.073, P = 0.038; Figure 3d).
Surface biotinylation assay showed that EE induced DAT internalization
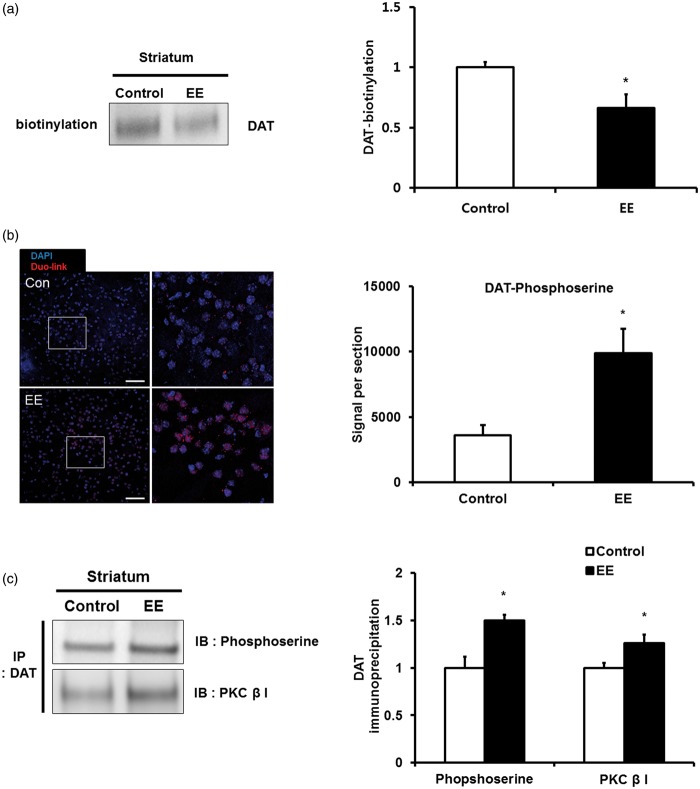
We considered that these patterns of decreased DAT in PET occurred due to internalization after EE. In order to confirm DAT internalization, we performed a biotinylation assay for striatal membrane DAT. EE significantly decreased the membrane DAT (0.7 ± 0.1 folds), which was approximately 30% lower than that of the control groups (1.0 ± 0.04 folds) at eight weeks post-treatment (t = 2.930, P = 0.015; Figure 4a), suggesting that membrane DAT is internalized to the intercellular region of the striatum after exposure to EE.
Figure 4.
Internalization and phosphorylation of striatal DAT induced by EE. (a) Internalization of striatal membrane DAT by a surface biotinylation assasy. After exposure to EE, membrane DAT significantly decreased compared with the control group at eight weeks post-treatment. (b) In situ proximity ligation assay for phosphorylation of DAT. The red color indicates the phosphorylation states showing increased fluorescent signal area of the DAT phosphorylation in the striatum after exposure to EE. The scale bar indicates 50 µm. (c) Immunoprecipitation (IP) for phosphorylation of DAT and for interaction between DAT and PKC. The levels of the DAT phosphorylation and the PKCβ bound with DAT significantly increased in the striatum after exposure to EE.
DAT phosphorylation increased after exposure to EE
To investigate DAT internalization by post-translational modification, we evaluated the phosphorylation level of DAT since DAT phosphorylation plays a role in DAT recycling and transporter internalization.28 In order to confirm phosphorylation of DAT in the striatal region, the interaction between endogenous DATs and phosphoserine was examined by an in situ proximity ligation assay, which enables visualization of the protein–protein interaction in close proximity (<40 nm) through ligation-mediated amplification of a specific oligonucleotide probe. After exposure to EE, fluorescent signal area of DAT phosphorylation significantly increased in the striatum compared with the control group (t = 3,180, P = 0.004; Figure 4b).
We then performed an immunoprecipitation (IP) assay using anti-DAT and anti-phosphoserine antibodies to confirm DAT phosphorylation by EE. After exposure to EE, DAT phosphorylation significantly increased in the striatum (1.5 ± 0.1 folds) relative to the control group (1.0 ± 0.1 folds) (t = 4.073, P = 0.002; Figure 4c), suggesting that DAT internalization occurred by phosphorylation. When we also validated the DAT inhibition occurred via phosphorylation mechanism with in vitro experiment, DAT inhibitor treatment showed DAT phosphorylation significantly increased in SH-SY5Y cells (1.6 ± 0.1 folds) relative to the control (t = 3.357, P = 0.028; Supplemental Figure S1). In addition, we investigated the interaction between DAT and PKC using IP to study whether DAT phosphorylation is controlled by PKC. We confirmed that DAT is bound with PKC, and the PKC levels increased in the striatum after exposure to EE (1.3 ± 0.1 folds) relative to the control group (1.0 ± 0.1 folds) (t = 2.464, P = 0.017; Figure 4c). Taken together, we suggest that EE enables phosphorylation of DAT via the PKC-mediated pathway in the striatum.
Discussion
Both enhanced physical activity and social interaction provide benefits to the brain; physical activity on its own improves cognitive performance due to a range of neural changes including synaptic plasticity and neurotransmitter subunit expression.29 However, the underlying mechanism remains to be elucidated in detail. Among the neurotransmitters, the role of dopamine as an important neurotransmitter has come to light.30–33 Based on the first clinical and preclinical studies showing that levodopa treatment could improve functional motor recovery in stroke patients and an experimental stroke model,34,35 dopamine system is responsible for many sensorimotor functions and motor learning involved in functional recovery. The dopamine levels in the neural synapse are controlled by DAT. DAT pumps dopamine neurotransmitters from the synapse back into the presynaptic neural cytosol. In this way, DAT plays a primary role in terminating dopaminergic signaling.33 In particular, dopamine reuptake via DAT is known to be integral in controlling the concentration of dopamine in the synaptic area between presynaptic and postsynaptic neurons.11
In a previous study,10 we performed quantitative real-time PCR to determine DAT expression levels in the mouse brain under EE. DAT significantly decreased after long-term exposure to EE, and DAT RNA levels show similar down-regulated expression patterns with microarray. In this study, we further elucidated the EE-mediated mechanism of synaptic plasticity by internalization of striatal DAT. The EE-induced functional improvement (Figure 2) was related to DAT down-regulation in striatum (Figure 3a–c) which was confirmed by [18F]FPCIT PET image assays and administration of a DAT inhibitor (Figure 3d). DAT-PET using [18F]FPCIT that is a specific radiotracer for dopamine transporter which is predominantly located in the striatum. Other brain regions had very low radioactivity concentration.36,37 Therefore, there were very small differences of radioactivity between extra-striatal brain regions and cerebellum (Supplemental Figure S2). In the present study, we focused on the changes of available dopamine transporter in the plasma membrane rather than cerebral perfusion. In [18F]FPCIT PET, cerebral perfusion effect could be negligible, because reference tissue has little dopamine transporters and a time activity curve from reference tissue has replaced that from the arterial blood. This methodology is previously validated and currently used in the clinical PET study for Parkinson’s disease.38
We then confirmed the internalization of striatal DAT using a surface biotinylation assay (Figure 4a), and focused on DAT regulation mechanisms such as internalization and phosphorylation.16,32 We also validated the DAT inhibition occurred via phosphorylation mechanism with in vitro experiment (Supplemental Figure S1). However, present study did not show that decreased DAT is associated with the increment of dopamine D2 receptor in EE (Supplemental Figure S3). Previous researches demonstrated that there was no evidence that EE influenced D2 receptor density, or the ratio of D2 receptor density to other receptor types,39 and destruction of DAT in Parkinsonian animal model did not show statistically significant increment of D2 receptors.40
DAT phosphorylation by multiple signaling systems causes DAT internalization.41,42 It has been reported that changes in DAT activity and cell surface expression occur in response to diverse mechanisms including several kinases and phosphatases.42–47 In addition, recent studies have shown that DAT and other neurotransmitter transporters are phosphoproteins which are regulated by protein kinases as a mechanism for maintaining synaptic neurotransmitter levels and neural signaling.41–44,48 Particularly, PKC reduces the rate of DAT plasma membrane recycling, further enhancing the level of transporter internalization.16,43 PKC-dependent phosphorylation of DAT has been demonstrated at sites that appear to be the most distal N-terminal serine.28 We evaluated DAT bound with PKCβ because it has been reported that PKCβ, but not PKCα or PKCγ, was co-immunoprecipitated with the DAT from striatal membranes.26
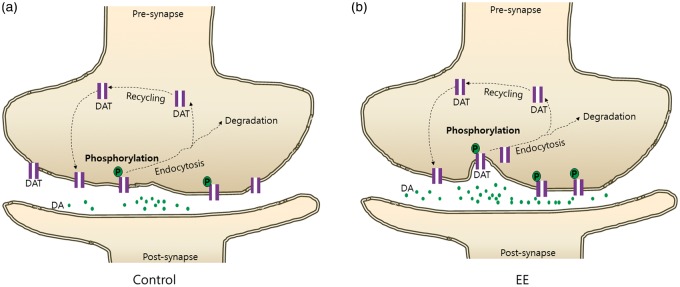
In this study, IP and in situ proximity assay demonstrated that EE increased phosphorylation of DAT via a PKC-mediated pathway as a DAT internalization mechanism, suggesting that EE caused DAT internalization by phosphorylation (Figure 4b and c). As a result of DAT internalization and dopamine reuptake inhibition in the presynaptic region, increased levels of dopamine in the neural synapse might lead to functional improvement. After this internalization, DAT undergoes several biological processes including degradation and recycling. The internalization process may play a key role in the intercellular regulation of DAT. Therefore, we focused on the role of EE in the early steps of DAT trafficking, and showed that EE substantially increased DAT phosphorylation (Figure 5). Taken together, we suggest that EE enables phosphorylation of DAT via a PKC-mediated pathway in the striatum. This is the first report to suggest the EE-mediated mechanism of synaptic plasticity by internalization of striatal DAT.
Figure 5.
EE-mediated internalization of DAT by phosphorylation in the presynaptic zone. As a result of DAT internalization and dopamine reuptake inhibition in the presynaptic region, dopamine levels in the neural synapse increased in the EE group (b) relative to the control group (a), which might cause functional improvement. After this internalization, DAT may undergo several biological processes including degradation and recycling. This internalization process plays a key role in the intercellular regulation of DAT. Therefore, we focused on the role of EE in the early steps of DAT trafficking, and showed that EE substantially increased DAT phosphorylation. Taken together, EE enables phosphorylation of DAT via a PKC-mediated pathway in the striatum, suggesting the EE-mediated mechanism of synaptic plasticity by internalization of striatal DAT.
Supplementary Material
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by grants from the National Research Foundation (NRF-2014R1A2A1A11052042), the Ministry of Science and Technology, Republic of Korea.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
M-SK and JHY performed whole experiments and wrote the manuscript. CHK, JYC, CHY,THC and YHR performed the PET image assays. JHS performed the animal experiments. M-YL performed the immunoprecipitation. JEL, BHL and HK contributed to the study concept and design. S-RC analyzed data, wrote the manuscript, developed the study concept, and supervised the project. All authors read and approved the manuscript.
Supplementary material
Supplementary material for this paper can be found at http://jcbfm.sagepub.com/content/by/supplemental-data
References
- 1.Rosenzweig MR, Bennett EL, Hebert M, et al. Social grouping cannot account for cerebral effects of enriched environments. Brain Res 1978; 153: 563–576. [DOI] [PubMed] [Google Scholar]
- 2.Will B, Galani R, Kelche C, et al. Recovery from brain injury in animals: relative efficacy of environmental enrichment, physical exercise or formal training (1990–2002). Prog Neurobiol 2004; 72: 167–182. [DOI] [PubMed] [Google Scholar]
- 3.Cummins RA, Walsh RN, Budtz-Olsen OE, et al. Environmentally-induced changes in the brains of elderly rats. Nature 1973; 243: 516–518. [DOI] [PubMed] [Google Scholar]
- 4.Zhu SW, Yee BK, Nyffeler M, et al. Influence of differential housing on emotional behaviour and neurotrophin levels in mice. Behav Brain Res 2006; 169: 10–20. [DOI] [PubMed] [Google Scholar]
- 5.Mlynarik M, Johansson BB, Jezova D. Enriched environment influences adrenocortical response to immune challenge and glutamate receptor gene expression in rat hippocampus. Ann N Y Acad Sci 2004; 1018: 273–280. [DOI] [PubMed] [Google Scholar]
- 6.Seo JH, Yu JH, Suh H, et al. Fibroblast growth factor-2 induced by enriched environment enhances angiogenesis and motor function in chronic hypoxic-ischemic brain injury. PLoS ONE 2013; 8: e74405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nithianantharajah J, Hannan AJ. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat Rev Neurosci 2006; 7: 697–709. [DOI] [PubMed] [Google Scholar]
- 8.Rosenzweig MR, Bennett EL. Psychobiology of plasticity: effects of training and experience on brain and behavior. Behav Brain Res 1996; 78: 57–65. [DOI] [PubMed] [Google Scholar]
- 9.van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci 2000; 1: 191–198. [DOI] [PubMed] [Google Scholar]
- 10.Lee MY, Yu JH, Kim JY, et al. Alteration of synaptic activity-regulating genes underlying functional improvement by long-term exposure to an enriched environment in the adult brain. Neurorehabil Neural Repair 2013; 27: 561–574. [DOI] [PubMed] [Google Scholar]
- 11.Manepalli S, Surratt CK, Madura JD, et al. Monoamine transporter structure, function, dynamics, and drug discovery: a computational perspective. Aaps J 2012; 14: 820–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pramod AB, Foster J, Carvelli L, et al. SLC6 transporters: structure, function, regulation, disease association and therapeutics. Mol Aspects Med 2013; 34: 197–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu J, Apparsundaram S, Bardo MT, et al. Environmental enrichment decreases cell surface expression of the dopamine transporter in rat medial prefrontal cortex. J Neurochem 2005; 93: 1434–1443. [DOI] [PubMed] [Google Scholar]
- 14.Shan J, Javitch JA, Shi L, et al. The substrate-driven transition to an inward-facing conformation in the functional mechanism of the dopamine transporter. PLoS ONE 2011; 6: e16350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wooters TE, Bardo MT, Dwoskin LP, et al. Effect of environmental enrichment on methylphenidate-induced locomotion and dopamine transporter dynamics. Behav Brain Res 2011; 219: 98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foster JD, Yang JW, Moritz AE, et al. Dopamine transporter phosphorylation site threonine 53 regulates substrate reuptake and amphetamine-stimulated efflux. J Biol Chem 2012; 287: 29702–29712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Metz GA, Whishaw IQ. The ladder rung walking task: a scoring system and its practical application. J Vis Exp 2009; 28: 1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee SJ, Oh SJ, Chi DY, et al. One-step high-radiochemical-yield synthesis of [18F]FP-CIT using a protic solvent system. Nucl Med Biol 2007; 34: 345–351. [DOI] [PubMed] [Google Scholar]
- 19.Constantinescu CC, Mukherjee J. Performance evaluation of an Inveon PET preclinical scanner. Phys Med Biol 2009; 54: 2885–2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paxinos G (6 ed.). The rat brain in stereotaxic coordinates. San Diego, CA, 2007.
- 21.Boja JW, Mitchell WM, Patel A, et al. High-affinity binding of [125I]RTI-55 to dopamine and serotonin transporters in rat brain. Synapse 1992; 12: 27–36. [DOI] [PubMed] [Google Scholar]
- 22.Cline EJ, Scheffel U, Boja JW, et al. In vivo binding of [125I]RTI-55 to dopamine transporters: pharmacology and regional distribution with autoradiography. Synapse 1992; 12: 37–46. [DOI] [PubMed] [Google Scholar]
- 23.Agoston GE, Wu JH, Izenwasser S, et al. Novel N-substituted 3 alpha-[bis(4′-fluorophenyl)methoxy]tropane analogues: selective ligands for the dopamine transporter. J Med Chem 1997; 40: 4329–4339. [DOI] [PubMed] [Google Scholar]
- 24.Velazquez-Sanchez C, Ferragud A, Murga J, et al. The high affinity dopamine uptake inhibitor, JHW 007, blocks cocaine-induced reward, locomotor stimulation and sensitization. Eur Neuropsychopharmacol 2010; 20: 501–508. [DOI] [PubMed] [Google Scholar]
- 25.Soderberg O, Gullberg M, Jarvius M, et al. Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat Methods 2006; 3: 995–1000. [DOI] [PubMed] [Google Scholar]
- 26.Johnson LA, Guptaroy B, Lund D, et al. Regulation of amphetamine-stimulated dopamine efflux by protein kinase C beta. J Biol Chem 2005; 280: 10914–10919. [DOI] [PubMed] [Google Scholar]
- 27.Furman CA, Chen R, Guptaroy B, et al. Dopamine and amphetamine rapidly increase dopamine transporter trafficking to the surface: live-cell imaging using total internal reflection fluorescence microscopy. J Neurosci 2009; 29: 3328–3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foster JD, Pananusorn B, Vaughan RA. Dopamine transporters are phosphorylated on N-terminal serines in rat striatum. J Biol Chem 2002; 277: 25178–25186. [DOI] [PubMed] [Google Scholar]
- 29.Farmer J, Zhao X, van Praag H, et al. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience 2004; 124: 71–79. [DOI] [PubMed] [Google Scholar]
- 30.Carlsson A, Lindqvist M, Magnusson T. 3,4-Dihydroxyphenylalanine and 5-hydroxytryptophan as reserpine antagonists. Nature 1957; 180: 1200. [DOI] [PubMed] [Google Scholar]
- 31.Carlsson A, Lindqvist M, Magnusson T, et al. On the presence of 3-hydroxytyramine in brain. Science 1958; 127: 471. [DOI] [PubMed] [Google Scholar]
- 32.Montagu KA. Catechol compounds in rat tissues and in brains of different animals. Nature 1957; 180: 244–245. [DOI] [PubMed] [Google Scholar]
- 33.Eriksen J, Jorgensen TN, Gether U. Regulation of dopamine transporter function by protein-protein interactions: new discoveries and methodological challenges. J Neurochem 2010; 113: 27–41. [DOI] [PubMed] [Google Scholar]
- 34.Scheidtmann K, Fries W, Muller F, et al. Effect of levodopa in combination with physiotherapy on functional motor recovery after stroke: a prospective, randomised, double-blind study. Lancet 2001; 358: 787–790. [DOI] [PubMed] [Google Scholar]
- 35.Ruscher K, Kuric E, Wieloch T. Levodopa treatment improves functional recovery after experimental stroke. Stroke 2012; 43: 507–513. [DOI] [PubMed] [Google Scholar]
- 36.Malison RT, Vessotskie JM, Kung MP, et al. Striatal dopamine transporter imaging in nonhuman primates with iodine-123-IPT SPECT. J Nucl Med 1995; 36: 2290–2297. [PubMed] [Google Scholar]
- 37.Lundkvist C, Halldin C, Ginovart N, et al. [18F] beta-CIT-FP is superior to [11C] beta-CIT-FP for quantitation of the dopamine transporter. Nucl Med Biol 1997; 24: 621–627. [DOI] [PubMed] [Google Scholar]
- 38.Yaqub M, Boellaard R, van Berckel BN, et al. Quantification of dopamine transporter binding using [18F]FP-beta-CIT and positron emission tomography. J Cereb Blood Flow Metab 2007; 27: 1397–1406. [DOI] [PubMed] [Google Scholar]
- 39.Anderson BJ, Gatley SJ, Rapp DN, et al. The ratio of striatal D1 to muscarinic receptors changes in aging rats housed in an enriched environment. Brain Res 2000; 872: 262–265. [DOI] [PubMed] [Google Scholar]
- 40.Choi JY, Kim CH, Jeon TJ, et al. Evaluation of dopamine transporters and D2 receptors in hemiparkinsonian rat brains in vivo using consecutive PET scans of [18F]FPCIT and [18F]fallypride. Appl Radiat Isot 2012; 70: 2689–2694. [DOI] [PubMed] [Google Scholar]
- 41.Ramamoorthy S, Shippenberg TS, Jayanthi LD. Regulation of monoamine transporters: Role of transporter phosphorylation. Pharmacol Ther 2011; 129: 220–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramamoorthy S, Blakely RD. Phosphorylation and sequestration of serotonin transporters differentially modulated by psychostimulants. Science 1999; 285: 763–766. [DOI] [PubMed] [Google Scholar]
- 43.Vaughan RA, Huff RA, Uhl GR, et al. Protein kinase C-mediated phosphorylation and functional regulation of dopamine transporters in striatal synaptosomes. J Biol Chem 1997; 272: 15541–15546. [DOI] [PubMed] [Google Scholar]
- 44.Vaughan RA, Foster JD. Mechanisms of dopamine transporter regulation in normal and disease states. Trends Pharmacol Sci 2013; 34: 489–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pristupa ZB, McConkey F, Liu F, et al. Protein kinase-mediated bidirectional trafficking and functional regulation of the human dopamine transporter. Synapse 1998; 30: 79–87. [DOI] [PubMed] [Google Scholar]
- 46.Chen R, Furman CA, Gnegy ME. Dopamine transporter trafficking: Rapid response on demand. Future Neurol 2010; 5: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moron JA, Zakharova I, Ferrer JV, et al. Mitogen-activated protein kinase regulates dopamine transporter surface expression and dopamine transport capacity. J Neurosci 2003; 23: 8480–8488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Torres GE. The dopamine transporter proteome. J Neurochem 2006; 97: 3–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.