Abstract
Previous studies have shown that intraparenchymal transplantation of neural stem cells ameliorates neurological deficits in animals with intracerebral hemorrhage. However, hemoglobin in the host brain environment causes massive grafted cell death and reduces the effectiveness of this approach. Several studies have shown that preconditioning induced by sublethal hypoxia can markedly improve the tolerance of treated subjects to more severe insults. Therefore, we investigated whether hypoxic preconditioning enhances neural stem cell resilience to the hemorrhagic stroke environment and improves therapeutic effects in mice. To assess whether hypoxic preconditioning enhances neural stem cell survival when exposed to hemoglobin, neural stem cells were exposed to 5% hypoxia for 24 hours before exposure to hemoglobin. To study the effectiveness of hypoxic preconditioning on grafted-neural stem cell recovery, neural stem cells subjected to hypoxic preconditioning were grafted into the parenchyma 3 days after intracerebral hemorrhage. Hypoxic preconditioning significantly enhanced viability of the neural stem cells exposed to hemoglobin and increased grafted-cell survival in the intracerebral hemorrhage brain. Hypoxic preconditioning also increased neural stem cell secretion of vascular endothelial growth factor. Finally, transplanted neural stem cells with hypoxic preconditioning exhibited enhanced tissue-protective capability that accelerated behavioral recovery. Our results suggest that hypoxic preconditioning in neural stem cells improves efficacy of stem cell therapy for intracerebral hemorrhage.
Keywords: Cell transplantation therapy, hypoxic preconditioning, intracerebral hemorrhage, neural stem cells, vascular endothelial growth factor
Introduction
Stroke is a leading cause of death and disability worldwide. In particular, intracerebral hemorrhage (ICH) constitutes 15% of all stroke cases, with 40% of patients dying within 30 days and survivors showing persistent, severe neurological deficits.1,2 Current methods for treating ICH have limited efficacy in reducing damage and improving recovery.3 Therefore, it is important to explore new approaches to treat ICH, such as stem cell transplants, blood pressure modulation, and intrahematoma tissue plasminogen activator injection.
ICH results from the rupture of blood vessels in the brain. The acute stage after ICH has several factors that contribute to cell death, including edema, hemoglobin (Hb) cytotoxicity, and inflammation. In particular, Hb causes secondary damage via oxidative stress and has been implicated in further perpetuating cell death.4
Recently, stem cell transplants have emerged as an exciting avenue for further research with potential clinical applications. Studies have shown the neuroprotection of intraparenchymal cell transplants that can ameliorate primary and secondary injury in animals with ICH, as well as enhance functional recovery.5–12 At the site of neuroprotection, the transplantation should be performed at the acute stage of ICH, because the irreversible brain damage extends as time goes by. However, the hostile host environment during the acute stage of ICH limits the efficacy of this strategy as more than 85% of transplanted cells are no longer present 8 weeks after transplant. A potential solution to this problem is to modify the cells prior to transplant to enhance their ability to survive and facilitate functional recovery.10–12
Several reports have shown that preconditioning induced by sublethal hypoxia or ischemia can markedly improve the tolerance of treated subjects to more severe insults.13–15 However, the effect of hypoxic preconditioning on cell transplantation therapy for ICH remains unclear. Therefore, we aimed to study the protective effects of hypoxic preconditioning on neural stem cells (NSCs) against Hb cytotoxicity and whether hypoxic preconditioned NSCs enhance the efficacy of cell transplantation in the ICH mouse model.
Materials and methods
Animals
All animals were treated in accordance with ARRIVE and Stanford University guidelines and the animal protocols were approved by Stanford University’s Administrative Panel on Laboratory Animal Care. NSCs were isolated from homozygous green fluorescent protein transgenic (GFP Tg) mice (C57BL/6-Tg [UBC-GFP] 30Scha/J; The Jackson Laboratory, Bar Harbor, ME, USA). We also used wild-type C57BL/6 mice (The Jackson Laboratory) for ICH models. Animals were randomized into groups by behavioral assessment 1 day after ICH. All methods and assessments described below were carried out by individuals blinded to the groups.
Isolation and culture of fetal neural stem cells
Neural stem cells were isolated from the subventricular zones of postnatal day 1 GFP Tg mice, as described previously.10 In brief, bilateral subventricular zones were dissected and mechanically dissociated. The cells were collected and suspended in Neurobasal-A medium (Invitrogen, Carlsbad, CA, USA) containing B-27 supplement (Invitrogen), L-glutamine (Invitrogen), 20 ng/ml mouse fibroblast growth factor-basic (PeproTech, Rocky Hill, NJ, USA), and 10 ng/ml mouse epidermal growth factor (PeproTech). Cells were grown as adherent monolayers. The medium was changed every 2 days and cells were passaged weekly. Cells that had been passaged 5 to 10 times were used for the experiments.
Hypoxic preconditioning
For in vitro experiments, the NSCs were incubated at 37℃ under 5% O2-5% CO2-90%N2 for 24 hours in a gas-tight humidified chamber (modular incubator chamber; Billups-Rothenberg, Del Mar, CA, USA).16
Cytotoxicity experiments in vitro
The NSCs were treated with Hb and H2O2 (216763; Sigma-Aldrich, St Louis, MO, USA). Hemoglobin was prepared as described.10 Blood was drawn by cardiac puncture and centrifuged at 1250 g for 5 minutes at 4℃. The supernatant was removed and the pellet was washed, resuspended in sterile saline, and lysed by two freeze-thaw cycles. The sample was then centrifuged and the supernatant was removed. The Hb concentration was determined with an Hb assay kit (Z5030026; BioChain, Newark, CA, USA).
Cell viability assay
Cell viability was assessed with a cell proliferation reagent using a WST-1 assay kit (05015944001; Roche Diagnostics, Indianapolis, IN, USA). The NSCs were incubated in normoxia and hypoxia for 24 hours and their viability was assessed 6 and 30 hours after hypoxia using the WST-1 assay to investigate whether hypoxia enhanced cell proliferation. To examine whether hypoxic preconditioning restored cell viability, the NSCs were incubated in hypoxia for 24 hours followed by 6 hours under normoxia and treated with 20 µM Hb and 100 µM H2O2 for 24 hours.
Assessment of cell death in vitro
The NSCs were cultured on 8-well chamber slides (Thermo Fisher Scientific, Waltham, MA, USA) and were treated with 20 µM Hb for 24 hours. The NSCs were then washed with phosphate-buffered saline (PBS) and incubated with 4 µM ethidium homodimer-1 and 2 µM calcein AM for 15 minutes. Cell death was assessed by LIVE/DEAD Viability/Cytotoxicity assay kit (L3224; Molecular Probes, Grand Island, NY, USA).
Detection of paracrine factors
Growth media were collected for analysis 30 hours after hypoxic preconditioning of the NSCs in culture. In the in vivo studies, fresh brain tissue was removed 5 and 14 days after ICH. Whole cell lysate samples from the dissected striatum of the NSC-transplanted side were used. Vascular endothelial growth factor (VEGF) (RRV00; R&D Systems, Minneapolis, MN, USA) ELISA kits were used to quantify VEGF in each sample.
Western blot analysis in vitro
To investigate whether hypoxic preconditioning induces changes in hypoxia-inducible factor α (HIF-1α) and the phosphorylated serine threonine kinase, phospho-Akt (pAkt), in NSCs, Western blotting was performed. The NSCs, with or without hypoxic preconditioning, were exposed to Hb and treated with cell lysis buffer (Cell Signaling Technology, Beverly, MA, USA) and used as whole cell lysate samples. Protein concentrations were examined by comparison with a known concentration of bovine serum albumin using a kit (Thermo Fisher Scientific). Equal amounts of the samples (20 µg) were loaded per lane and analyzed by sodium dodecyl sulfate-polyacrylamide-gel electrophoresis on a 10% NuPAGE Bis-Tris gel (Invitrogen) and then immunoblotted. The primary antibodies were a 1:500 dilution of rabbit polyclonal anti-HIF-1α (molecular weight: ∼115 kDa) (Novus Biologicals, Littleton, CO, USA), a 1:500 dilution of rabbit polyclonal anti-pAkt (Ser473) (molecular weight: ∼60 kDa) (Cell Signaling Technology), a 1:2000 dilution of rabbit polyclonal anti-Akt (molecular weight: ∼60 kDa) (Cell Signaling Technology), and a 1:100000 dilution of mouse monoclonal anti-β-actin (molecular weight: ∼42 kDa) (Sigma-Aldrich). After incubation with horseradish peroxidase-conjugated anti-mouse immunoglobulin G (Cell Signaling Technology) or anti-rabbit immunoglobulin G (Cell Signaling Technology), the antigen was detected by SuperSignal West Pico Substrates (Thermo Fisher Scientific). Images were captured with a GS-700 imaging densitometer (Bio-Rad, Hercules, CA, USA) and the results were quantified using MultiAnalyst software (Bio-Rad).
Inhibition of phosphatidylinositol 3-kinase–Akt pathway with LY294002
Akt was blocked by LY294002 (9901; Cell Signaling Technology), a known selective inhibitor of phosphatidylinositol 3-kinase (PI3K) or by transfection of small interfering RNA (siRNA). The NSCs were incubated with different doses of LY294002 from 0 to 30 hours after hypoxia. The NSCs were transfected with 200 nM Akt siRNA (6211; Cell Signaling Technology) or nonfunctioning negative control siRNA (6201; Cell Signaling Technology), and with 200 nM HIF-1α siRNA (sc-35562; Santa Cruz Biotechnology, Santa Cruz, CA, USA) or nonfunctioning negative control siRNA (sc-36869; Santa Cruz Biotechnology) using an oligofectamine reagent (122521-011; Invitrogen) according to the manufacturer’s protocol.
Intracerebral hemorrhage model with autologous blood infusion
We used an experimental ICH procedure, described previously.10 Male C57BL/6 mice (14 to 15 weeks old, 25 to 30 g) were anesthetized with 2.0% isoflurane in 30% oxygen and 70% nitrous oxide and placed in a stereotactic frame. A midline scalp incision was made and a hole was drilled in the right side of the skull (0.0 mm anterior and 2.5 mm lateral to the bregma). Blood (20 µl) was collected from the tail tip, without the use of an anticoagulant, for injection. A 30-gauge needle attached to a 50-µl Hamilton microsyringe was inserted 3.5 mm ventral from the surface of the skull, with adjustment of the stereotaxic arm to a point 5° medially relative to the vertical axis. After waiting 2 minutes, the needle was withdrawn 0.5 mm, and left in place for another 5 minutes. Ten microliters of blood was injected over 10 minutes using a microsyringe pump. The remaining 10 µl of blood was injected using the same procedure. After infusion, the needle was left in place for 25 minutes and then slowly removed. All animals underwent behavioral testing 1 day after ICH and the animals with low behavioral scores were excluded from the experiment (cylinder test < 0.6, corner turn test < 0.8, 40% of all animals were excluded).
Intracerebral transplantation
Two 1.0 -µl deposits of NSCs (1 × 105 cells/µl) were transplanted into the striatum 3 days after ICH at two coordinates: (1) anterior-posterior (A-P), 0.5; medial-lateral (M-L), 2.5; dorsal-ventral (D-V), 3.0; (2) A-P, -0.75; M-L, 2.5; D-V, 3.0. These targets approximated the edge of the ICH. The control group was injected with PBS without NSCs. In the non-preconditioned group, the mice were injected with non-preconditioned NSCs, and in the hypoxic preconditioned group, the mice were injected with hypoxic preconditioned NSCs.
Quantification of survival of the transplanted green fluorescent protein-positive neural stem cells
To quantify survival of the grafted cells, they were perfused with PBS followed by 4% paraformaldehyde in PBS 35 days after ICH. For cryosectioning, fixed tissue was cryoprotected in 10% sucrose in PBS overnight, then in 20% sucrose in PBS overnight, and embedded in Tissue-Tek O.C.T. compound (Sakura Finetek, Torrance, CA, USA). Cryostat sections (20 mm) were cut and affixed to glass slides. The transplanted GFP-positive cells were counted on 12 serial coronal sections per brain (0.25 mm apart) using unbiased computational stereology (fractionator method, using STEREOINVESTIGATOR software [MicroBrightfield, Inc., Williston, VT, USA]), as described.10
Assessment of neural stem cell proliferation and differentiation
After culturing in differentiation medium containing 1% fetal bovine serum for 5 days, the NSCs were stained with a proliferation marker (Ki-67) or lineage-specific marker (microtubule associated protein 2 [MAP2], and glial fibrillary acidic protein [GFAP]) to assess NSC proliferation and differentiation in vitro. The proportion of grafted cells was also determined by staining with Ki-67 or MAP2 and with GFAP and 4′,6 diamidino-2-phenylindole (DAPI).
Measurement of striatum atrophy
The brain sections were stained with cresyl violet. The striatum size was estimated as a percentage of the contralateral striatum. The area of both sides of the striatum was measured on nine serial coronal sections per brain (0.25 mm apart) and the size of the striatum was analyzed by an observer blinded to the experimental cohort.10
Quantification of neuronal survival
The brain sections were stained with NeuN (a neuronal marker) and DAPI as described above. Surviving neurons were analyzed in a blinded manner by counting the number of cells or profiles in six defined regions of interest per striatum, measuring 62,500 µm2, both ipsilateral and contralateral to the ICH using unbiased computational stereology.10
Behavioral analysis
A cylinder test and a corner turn test were used for behavioral analysis.16,17 The animals were ranked based on their post-day 1 results and assigned to one of three groups: the control group, the non-preconditioned group, and the preconditioned group with an equal post-ICH behavior score. The animals were re-tested just before cell transplantation and at 7, 14, 21, 28, and 35 days after ICH. Forelimb use during exploratory activity was analyzed in a transparent cylinder. The behavior was scored according to the independent use of the left or right forelimb for contacting the wall while the animals were standing on their hindlimbs to initiate a weight-shifting movement and simultaneous use of both the left and right forelimbs to contact the wall. Behavior was quantified by determining the number of times the ipsilateral (unimpaired) forelimb (I), contralateral forelimb (C), and both forelimbs (B) were used as a percentage of the total number of limb usage. A single, overall limb-use asymmetry score was calculated as follows: Forelimb-Use Asymmetry Score [I/(I + C + B)] − [C/(I + C + B)]. The second behavioral analysis involved a corner turn test. The mouse was allowed to proceed into a corner, the angle of which was 30°. To exit the corner, the animal could turn either to the left or right. When the mouse turned, its choice of direction was recorded. This was repeated 10 to 15 times and the percentage of right turns was calculated.
Statistical analysis
Behavioral data were assessed using repeated measures ANOVA followed by the Tukey test. For other experimental data, comparisons among multiple groups were performed with one-way ANOVA, followed by the Tukey test. Comparisons between two groups were achieved with Student’s unpaired t-test. Data are expressed as the mean ± SD. Significance was accepted with p < 0.05.
Results
Effects of hypoxic preconditioning on cell proliferation and differentiation in vitro
Neural stem cells were isolated from fetal GFP Tg mice and grown as adherent, self-renewing and multipotent cultures in differentiation medium containing 1% fetal bovine serum for 6 days. These cells were then split into two groups: a group subjected to hypoxic preconditioning and a control group. Compared with the control group, the hypoxic preconditioned group had a significantly increased number of cells positive for the proliferation marker Ki-67 (5.2% ± 1.2% and 8.9% ± 0.6%, n = 4) (Supplementary Figure 1a), a decreased number of cells positive for the neuronal marker MAP2 (17.6% ± 4.3% and 8.3 ± 1.6%, n = 5), and no significant changes in cells positive for the astrocyte marker GFAP (56.5% ± 9.1% and 50.0% ± 15.1%, n = 5) (Supplementary Figure 1b).
Hypoxic preconditioning enhances cell viability and reduces cell death in hemoglobin cytotoxicity
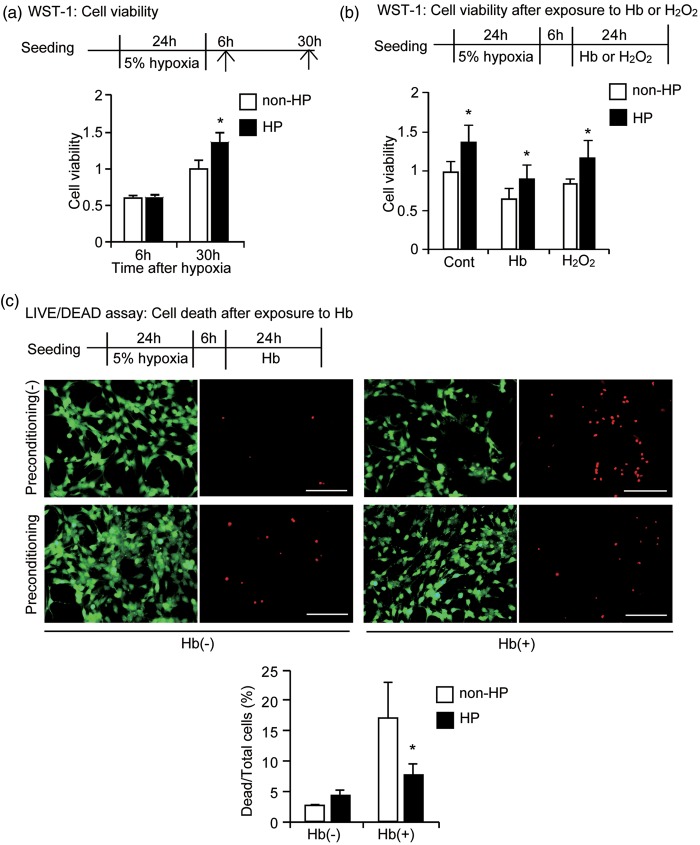
To investigate whether hypoxic preconditioning could enhance NSC viability after exposure to Hb, we first examined whether hypoxic preconditioning enhances cell viability under normal conditions using a WST-1 assay. There was no difference between the hypoxic preconditioned and control groups 6 hours after preconditioning, but 30 hours after hypoxia, the hypoxic preconditioned group had significantly increased viability compared with the control group (n = 5, p < 0.05) (Figure 1a). Subsequently, the cells were treated with Hb or H2O2, for 24 hours and as expected, both Hb and H2O2 treatment decreased cell viability; however, hypoxic preconditioning increased viability in all three groups, Hb, H2O2, and control (n = 5, p < 0.05) (Figure 1b). This cytoprotective effect of hypoxic preconditioning in the NSCs was confirmed by a LIVE/DEAD assay, where there was a significant decrease in death in the preconditioned NSCs compared with the non-preconditioned NSCs after 24 hours of exposure to Hb (n = 5, p < 0.05) (Figure 1c).
Figure 1.
Increased cell viability after hypoxic preconditioning. NSCs were exposed to 5% hypoxia for 24 hours. (a) WST-1 assay. Six hours after preconditioning, there were no differences in cell viability between hypoxic preconditioned NSCs and non-preconditioned NSCs, but the viability significantly increased 30 hours after hypoxia. (b) After 24 hours of exposure to Hb and H2O2, cell viability was significantly higher in the hypoxic preconditioned NSCs compared with the non-preconditioned NSCs (n = 5). (c) NSCs analyzed by LIVE/DEAD assay. Live cells (green) and dead cells (red) after 24 hours of exposure to 20 μM Hb showed increased death in the non-preconditioned NSCs. The increase was significantly decreased by hypoxic preconditioning (n = 5). HP: hypoxic preconditioned; Cont: control. Bars = 100 µm. *p < 0.05.
Phospho-Akt expression in neural stem cells after exposure to hemoglobin with hypoxic preconditioning in vitro
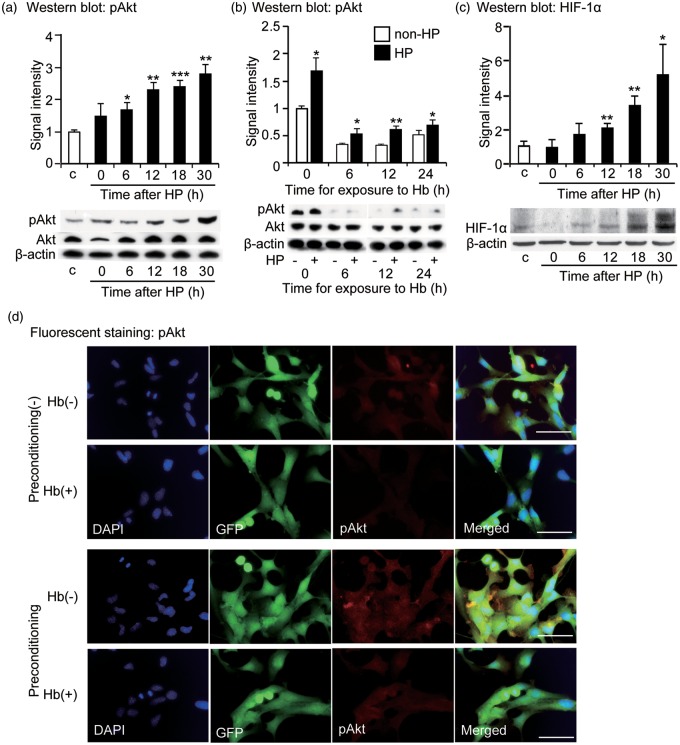
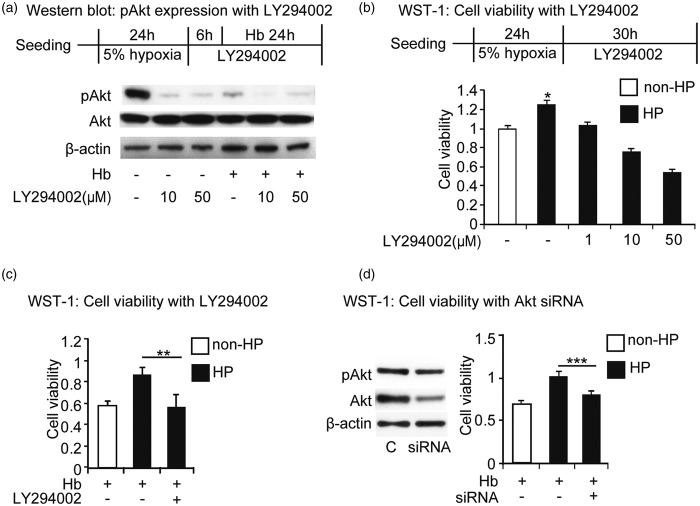
To investigate the signaling mechanisms through which hypoxic preconditioning enhances cell viability in vitro, we examined the HIF-1α and pAkt pathways. Western blot analysis revealed a time-dependent increase in pAkt expression after exposure to hypoxia (n = 4) (Figure 2a), while Hb decreased pAkt expression (n = 4) (Figure 2b). Additionally, HIF-1α expression also increased after exposure to hypoxia (n = 4) (Figure 2c). pAkt signal intensity was decreased after exposure to Hb and brighter signal intensity was seen in the hypoxic preconditioned NSCs compared with the non-preconditioned NSCs with and without Hb exposure (Figure 2d). Incubation with the PI3K inhibitor, LY294002 (10 µm or 50 µm), for 24 hours decreased the viability of stem cells 30.0% ± 4.0% and 49.7% ± 1.0 % (n = 4). LY294002 also significantly decreased pAkt expression in hypoxic preconditioned NSCs, both with and without Hb treatment, but did not change the levels of Akt expression (n = 4) (Figure 3a). We also observed a dose-dependent decrease in cell viability with LY294002 that diminished the enhanced viability conferred by hypoxic preconditioning whether Hb was or was not present (n = 4) (Figure 3b and c). These results were supported by use of HIF-1α siRNA, which decreased HIF-1α as well as downstream pAkt expression after hypoxic preconditioning (but there was no change in Akt levels). Treatment of NSCs with HIF-1α siRNA also reduced viability after Hb exposure and hypoxic preconditioning (Supplementary Figure 2a and 2b).
Figure 2.
pAkt expression in NSCs after exposure to hypoxia. (a) Western blot analysis revealed that pAkt expression significantly increased 6 to 30 hours after exposure to preconditioning (n = 4). (b) Hb decreased pAkt expression in the NSCs. Hypoxic preconditioned NSCs showed higher levels of pAkt 0 to 24 hours after exposure to Hb compared with the non-preconditioned NSCs on Western blot (n = 4). (c) HIF-1α expression increased 12 to 30 hours after preconditioning (n = 4). (d) Fluorescent staining with DAPI (blue), GFP (green), and pAkt (red). pAkt signal intensity was decreased after exposure to Hb. Brighter signal intensity was seen in the hypoxic preconditioned NSCs compared with the non-preconditioned NSCs with and without Hb exposure. C: control; HP: hypoxic preconditioned. Bars = 50 µm. *p < 0.05, **p < 0.01, ***p < 0.001.
Figure 3.
Inhibition of the PI3K-Akt pathway with LY294002. (a) pAkt expression in Western blot. Incubation with 10 μM or 50 μM of LY294002 decreased pAkt expression in hypoxic preconditioned NSCs with and without Hb exposure (n = 4). (b) WST-1 assay showed that LY290042 counteracted upregulation of viability of hypoxic preconditioned NSCs (n = 4). (c) LY290042 significantly decreased enhancement of cell viability induced by hypoxic preconditioning after exposure to Hb for 24 hours (n = 4). (d) siRNA experiment. Western blot analysis showed a decrease in pAkt and Akt in NSCs. WST-1 assay demonstrated Akt siRNA significantly decreased viability of hypoxic preconditioned NSCs after exposure to Hb for 24 hours (n = 4). HP: hypoxic preconditioned; C: control. *p < 0.05, **p < 0.01, ***p < 0.001.
Increased survival of grafted neural stem cells with hypoxic preconditioning in vivo
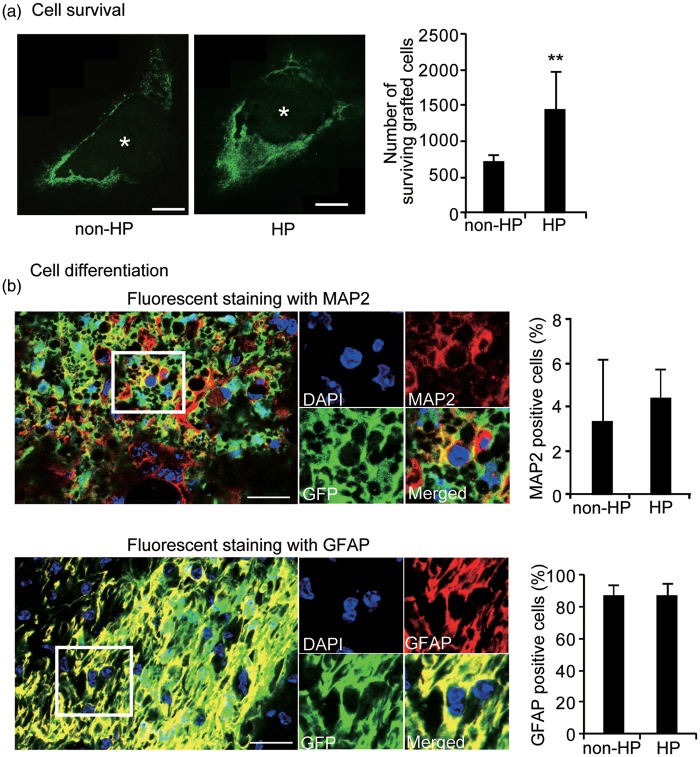
To explore whether these in vitro results could be replicated in vivo, we transplanted hypoxic preconditioned NSCs and non-preconditioned NSCs into the perihematomal striatum 3 days after ICH. GFP imaging revealed that more cells survived in the hypoxic preconditioned group than in the non-preconditioned group, and that more of these grafted cells surrounded the ICH lesion in both the hypoxic preconditioned and the non-preconditioned groups 35 days after ICH (n = 9, p < 0.01) (Figure 4a). As in previous reports, no signs of tumor formation caused by transplanted NSCs were present 35 days after ICH in the mice from either group. Fluorescent staining of GFP, MAP2, and GFAP revealed that grafted NSCs differentiated into some neurons, but mostly astrocytes, 35 days after ICH (n = 4) (Figure 4b). No cells were positive for Ki-67 by 35 days after ICH.
Figure 4.
Increased cell survival in vivo. (a) Fluorescent staining with GFP (green) revealed that the grafted cells surrounded the ICH lesion 35 days afterwards. The images were taken at a × 40 objective and were put together to make an entire image of the grafted cells and ICH using the STEREOINVESTIGATOR system (MicroBrightfield, Inc.). * represents ICH. Bars = 200 µm. The number of surviving grafted cells significantly increased with hypoxic preconditioning compared with no preconditioning 35 days after ICH (n = 9). (b) Fluorescent staining with GFP (green), DAPI (blue), and MAP2 (red) or GFAP (red) revealed that the grafted NSCs differentiated into neurons (MAP2+) and astrocytes (GFAP+) 32 days after transplantation. There were no differences in cell differentiation between no preconditioning and hypoxic preconditioning (n = 4). Bars = 25 µm. HP: hypoxic preconditioning. **p < 0.01.
Hypoxic preconditioning enhances vascular endothelial growth factor expression
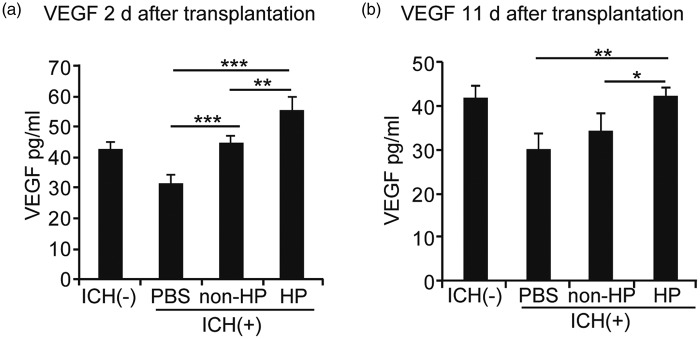
Hypoxic preconditioned NSCs had a fivefold increase in VEGF expression compared with non-preconditioned NSCs in vitro, as determined by ELISA for VEGF (Supplementary Figure 2c). HIF-1α siRNA treatment of NSCs decreased this increase in VEGF expression (Supplementary Figure 2d). To assess VEGF levels in vivo, we measured VEGF in the striatum using ELISA at day 2 and day 11 after transplantation (or day 5 and 15 after ICH). VEGF was significantly increased in the hypoxic preconditioned NSC group compared with the non-preconditioned NSC and PBS control groups (n = 5) (Figure 5a and b).
Figure 5.
Induction of paracrine factors in the striatum in vivo. (a) ELISA of the striatum revealed a significant elevation of VEGF in both the non-preconditioned NSC group and the hypoxic preconditioned NSC group compared with the PBS group 2 days after transplantation. The hypoxic preconditioned NSCs also showed a significant increase in VEGF compared with the non-preconditioned NSCs (n = 5). (b) ELISA of the striatum revealed a significant elevation in VEGF in the hypoxic preconditioned NSCs compared with the non-preconditioned NSCs and PBS 11 days after transplantation (n = 5). There was no significant difference between the non-preconditioned and PBS groups (n = 5). HP: hypoxic preconditioning. *p < 0.05, **p < 0.01, ***p < 0.001.
Hypoxic preconditioned neural stem cells enhance functional recovery
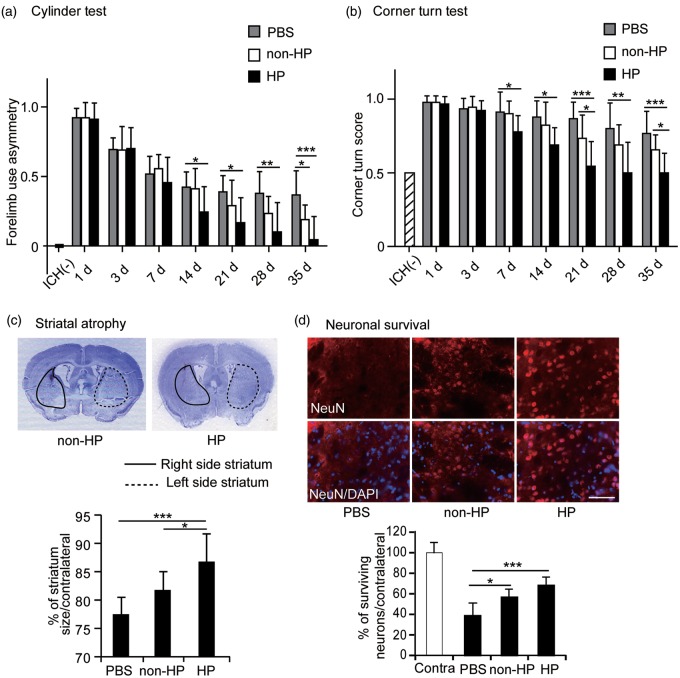
We analyzed behavioral performance using the cylinder test and the corner turn test. Mice transplanted with hypoxic preconditioned NSCs showed significant functional improvement with the cylinder test from 14 to 35 days after ICH compared with the PBS control group. In contrast, the non-preconditioned NSC group showed significant improvement 35 days after ICH compared with the PBS control group (n = 9) (Figure 6a). The hypoxic preconditioned NSC group showed significantly greater improvement compared with the PBS control group 7 to 35 days after ICH, and greater improvement compared with the non-preconditioned group at 21 and 35 days in the corner turn test (n = 9) (Figure 6b).
Figure 6.
Behavioral analysis and neuroprotection. (a) Transplantation of the hypoxic preconditioned NSCs showed greater improvement from 14 to 35 days compared with the non-transplanted PBS group, and the greatest behavioral improvement compared with the non-transplanted PBS group and the non-preconditioned NSCs 35 days after ICH in the cylinder test. The non-preconditioned NSCs had improved behavioral recovery compared with the non-transplanted PBS group 35 days after ICH in the cylinder test (n = 9). (b) The hypoxic preconditioned NSC group showed significantly greater improvement compared with the PBS control group 7 to 35 days after ICH, and greater improvement compared with the non-preconditioned group at 21 and 35 days in the corner turn test (n = 9). (c) Brain sections stained with cresyl violet showed striatal atrophy 35 days after ICH. The hypoxic preconditioned NSCs attenuated striatal atrophy compared with the PBS group and the non-preconditioned NSCs 35 days after ICH (n = 9). (d) Enhanced neuronal survival with transplantation of the hypoxic preconditioned NSCs. Fluorescent staining of NeuN (red) and DAPI (blue) and quantification of NeuN-positive cells in the striatum were significantly increased in the hypoxic preconditioned NSCs and in the non-preconditioned NSCs 35 days after ICH compared with the PBS controls (n = 6). Bar = 50 µm. HP: hypoxic preconditioning; Contra: contralateral striatum. *p < 0.05, **p < 0.01, ***p < 0.001.
Neural stem cells promote perilesional tissue protection
Hypoxic preconditioned NSC transplantation significantly prevented striatal atrophy compared with either the non-preconditioned NSC or PBS groups (n = 9) (Figure 6c). Furthermore, immunohistochemical staining for the neuronal marker NeuN revealed that neuronal survival was significantly increased in both the hypoxic preconditioned NSC group and in the non-preconditioned NSC group 35 days after ICH compared with the PBS control group, although there was no significant difference between the hypoxic preconditioned group and the non-preconditioned group (n = 6) (Figure 6d).
Discussion
Hypoxic preconditioning, or exposure to sublethal levels of hypoxia, has been demonstrated to enhance cell proliferation and viability in the ischemic stroke model;18,19 however, the effects of hypoxic preconditioning on NSCs prior to transplantation in the ICH model are yet to be explored. In this present study, we demonstrate for the first time that (1) mild hypoxia can enhance NSC proliferation and resilience against Hb cytotoxicity via Akt activation; (2) hypoxic preconditioning upregulates pAkt via HIF-1α and enhances VEGF production in NSCs, which subsequently increases expression of VEGF found around the bleed after transplantation; (3) hypoxic preconditioning increases survival of transplanted cells; and (4) transplantation of hypoxic preconditioned NSCs enhances neuroprotection and facilitates functional recovery in mice with ICH.
Blood leaking into the brain parenchyma after a vessel rupture exposes the brain to physiological and molecular factors that lead to cell death. Specifically, reactive oxygen species (ROS) produced by Hb in the parenchyma are a key factor causing secondary damage in the ICH brain. In animal models, injection of lysed erythrocytes, Hb, and iron into the brain causes significant cell death and produces a hostile environment that impairs survival of transplanted cells, thereby limiting cell transplants as potential therapy for ICH.10,20,21 However, some studies also suggest that low levels of ROS contribute to stem cell proliferation, self-renewal, and neurogenesis.22,23 This suggests that the amount of ROS is important for regulating cell fate. Indeed, in a recent study, we demonstrated that overexpression of copper-zinc superoxide dismutase, which converts ROS into the less harmful H2O2, improves survival of transplanted cells and enhances recovery.10 This proof-of-concept study demonstrates that genetically modifying cells prior to transplant can improve their therapeutic potential.10 However, genetically modifying stem cells can drastically alter their identity and tumorigenicity. Thus, another method, hypoxic preconditioning, is examined in this study.
We demonstrate that Hb inactivates pAkt, which may cause the decreased viability of NSCs. pAkt is well-known for contributing to self-renewal and differentiation in stem cells. Thus, reducing pAkt levels may directly contribute to the reduction in survival of transplanted cells.22 Since pAkt levels are decreased without significant changes in basal Akt levels, we believe that Hb-induced Akt inactivation works at the post-translational level, rather than at the epigenetic or gene expression levels. Whether pAkt is inactivated by a decrease in kinase activity or an increase in phosphatase activity is unknown.
Hypoxic preconditioning upregulates pAkt expression in NSCs, an effect diminished by the application of LY294002 or Akt siRNA. LY294002 is a potent inhibitor of PI3K. pAkt and Akt siRNA both decrease levels of pAkt expression, which may contribute to reduced cell survival. Taken together, these results suggest that hypoxic preconditioning upregulates pAkt expression through increased PI3K activity, but they do not discount the role of phosphatase activity. The results are supported by a recent study by Lee et al.8 where they demonstrate that overexpression of Akt1 in NSCs prior to transplant enhanced transplanted cell survival and functional recovery in the mouse striatal collagenase-induced ICH model. However, it is important to note that simply overexpressing Akt1 does not imply enhanced levels of pAkt expression. Interestingly, ROS have been shown to play a role in the PI3K/Akt signaling pathway in NSCs and to enhance cell proliferation and differentiation.22 According to this idea, Hb might also enhance Akt activity through ROS production, potentially supporting the idea that the amount of ROS is critical in activating/inhibiting specific pathways. However, the precise mechanism through which Hb regulates Akt signaling in NSCs remains unclear as there might be other mechanisms that Hb may act on independent of ROS.
As expected, we also observed an increased expression in the transcription factor HIF-1α after hypoxia in NSCs. This increase was abolished after exposure to HIF-1α siRNA. Interestingly, we also observed a decrease in pAkt expression with HIF-1α siRNA, suggesting that hypoxic preconditioning may increase cell proliferation and viability by acting through the HIF-1α/pAkt pathway. Earlier studies have shown that HIF-1α induces Akt.24 Furthermore, downstream of the HIF-1α pathway, VEGF was upregulated after hypoxic preconditioning in vitro. VEGF is released in response to hypoxia and promotes cell survival through anti-apoptotic pathways.25 Many studies have shown that transplanted stem cells secrete VEGF, which helps in cytoprotection of the brain after stroke.26,27 Predictably, we saw a high level of VEGF around the hematoma after ICH. However, it is unknown whether the NSCs triggered changes in the endogenous neurovascular niche to enhance host VEGF production. Nevertheless, this increased expression of VEGF around the hematoma may account for the decreased striatal atrophy after transplantation of hypoxic preconditioned NSCs. These results are consistent with previous findings showing that the HB1.F3 gene modified human NSCs.11 Additionally, our earlier study showed that copper-zinc superoxide dismutase overexpression in NSCs produces a much higher level of VEGF at the transplantation site, which provides neuroprotection, reduces atrophy, and leads to functional recovery in the mouse ICH model.10
The precise fate of transplanted NSCs remains unknown. While some of these cells may die, given the lack of Ki-67–positive cells and the presence of GFP-positive cells, our data suggest that the NSCs are still alive but have differentiated into mature cells. Most GFP-positive cells express the astrocytic marker GFAP, but some express the neuronal marker MAP2, suggesting that they have developed a neuronal phenotype. However, it is unknown whether these cells have functionally integrated into the circuit. There was no observable difference in cell differentiation between the hypoxic preconditioned and non-preconditioned transplant groups. The preconditioning increased Ki-67–positive and decreased MAP-2–positive cells in vitro. Hypoxic preconditioning could maintain the immature state of cells longer than normal conditions via Akt activation in differentiation medium, which might cause a reduction in mature neurons (the decrease of MAP-2–positive cells) in vitro. This suggests that the additional pro-recovery effects in the hypoxic preconditioned group are not due to the presence of more neurons or astrocytes, but perhaps due to production of VEGF, thereby providing cytoprotection and resulting in improvement in the outcome of behavioral tests.
It is important to note that this study is proof-of-concept and that precise parameters of hypoxic preconditioning, transplanted cell numbers, and route of transplantation have not been optimized. While there have been previous reports using NSC transplantation in ICH, most of these studies transplanted cells at later time points to study their effects on functional recovery.5,6,8 In our study, we focused on the effects of transplants during the acute stage to examine the ability of these cells to survive during this critical period, as well as the potential for neuroprotection.
In conclusion, in addition to the lack of any observable tumor formation, the lack of Ki-67–positive cells suggests that hypoxic preconditioning may be a more efficacious method to modify stem cells because of the reduced risk of tumor formation, but further research is necessary to reduce risks and to optimize the study of the mechanisms of this approach.
Supplementary Material
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: PHC was supported by grant RO1 NS025372 from the National Institutes of Health, and by the James R Doty Endowment.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
Takuma Wakai: substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; Purnima Narasimhan: substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; Hiroyuki Sakata: substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; Eric Wang: drafting the article or revising it critically for important intellectual content; Hideyuki Yoshioka: drafting the article or revising it critically for important intellectual content; Hiroyuki Kinouchi: drafting the article or revising it critically for important intellectual content; Pak H Chan: final approval of the version to be published.
Supplementary material
Supplementary material for this paper can be found at http://jcbfm.sagepub.com/content/by/supplemental-data
References
- 1.Godoy DA, Piñero G, Di Napoli M. Predicting mortality in spontaneous intracerebral hemorrhage. Can modification to original score improve the prediction? Stroke 2006; 37: 1038–1044. [DOI] [PubMed] [Google Scholar]
- 2.Dennis MS. Outcome after brain haemorrhage. Cerebrovasc Dis 2003; 16(Suppl 1): 9–13. [DOI] [PubMed] [Google Scholar]
- 3.Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet 2009; 373: 1632–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katsu M, Niizuma K, Yoshioka H, et al. Hemoglobin-induced oxidative stress contributes to matrix metalloproteinase activation and blood–brain barrier dysfunction in vivo. J Cereb Blood Flow Metab 2010; 30: 1939–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee HJ, Kim KS, Kim EJ, et al. Brain transplantation of immortalized human neural stem cells promotes functional recovery in mouse intracerebral hemorrhage stroke model. Stem Cells 2007; 25: 1204–1212. [DOI] [PubMed] [Google Scholar]
- 6.Wang Z, Cui C, Li Q, et al. Intracerebral transplantation of foetal neural stem cells improves brain dysfunction induced by intracerebral haemorrhage stroke in mice. J Cell Mol Med 2011; 15: 2624–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li F, Liu Y, Zhu S, et al. Therapeutic time window and effect of intracarotid neural stem cells transplantation for intracerebral hemorrhage. Neuroreport 2007; 18: 1019–1023. [DOI] [PubMed] [Google Scholar]
- 8.Lee HJ, Kim MK, Kim HJ, et al. Human neural stem cells genetically modified to overexpress Akt1 provide neuroprotection and functional improvement in mouse stroke model. PLoS One 2009; 4: e5586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andres RH, Guzman R, Ducray AD, et al. Cell replacement therapy for intracerebral hemorrhage. Neurosurg Focus 2008; 24: E16. [DOI] [PubMed] [Google Scholar]
- 10.Wakai T, Sakata H, Narasimhan P, et al. Transplantation of neural stem cells that overexpress SOD1 enhances amelioration of intracerebral hemorrhage in mice. J Cereb Blood Flow Metab 2014; 34: 441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee HJ, Kim KS, Park IH, et al. Human neural stem cells over-expressing VEGF provide neuroprotection, angiogenesis and functional recovery in mouse stroke model. PLoS One 2007; 2: e156 doi:110.1371/journal.pone.0000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee HJ, Park IH, Kim HJ, et al. Human neural stem cells overexpressing glial cell line-derived neurotrophic factor in experimental cerebral hemorrhage. Gene Ther 2009; 16: 1066–1076. [DOI] [PubMed] [Google Scholar]
- 13.Jäderstad J, Brismar H, Herlenius E. Hypoxic preconditioning increases gap-junctional graft and host communication. Neuroreport 2010; 21: 1126–1132. [DOI] [PubMed] [Google Scholar]
- 14.Oh JS, Ha Y, An SS, et al. Hypoxia-preconditioned adipose tissue-derived mesenchymal stem cell increase the survival and gene expression of engineered neural stem cells in a spinal cord injury model. Neurosci Lett 2010; 472: 215–219. [DOI] [PubMed] [Google Scholar]
- 15.Horie N, So K, Moriya T, et al. Effects of oxygen concentration on the proliferation and differentiation of mouse neural stem cells in vitro. Cell Mol Neurobiol 2008; 28: 833–845. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura T, Xi G, Hua Y, et al. Intracerebral hemorrhage in mice: model characterization and application for genetically modified mice. J Cereb Blood Flow Metab 2004; 24: 487–494. [DOI] [PubMed] [Google Scholar]
- 17.Rynkowski MA, Kim GH, Komotar RJ, et al. A mouse model of intracerebral hemorrhage using autologous blood infusion. Nat Protoc 2008; 3: 122–128. [DOI] [PubMed] [Google Scholar]
- 18.Liu J, Narasimhan P, Lee Y-S, et al. Mild hypoxia promotes survival and proliferation of SOD2-deficient astrocytes via c-Myc activation. J Neurosci 2006; 26: 4329–4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Theus MH, Wei L, Cui L, et al. In vitro hypoxic preconditioning of embryonic stem cells as a strategy of promoting cell survival and functional benefits after transplantation into the ischemic rat brain. Exp Neurol 2008; 210: 656–670. [DOI] [PubMed] [Google Scholar]
- 20.Qu Y, Chen J, Benvenisti-Zarom L, et al. Effect of targeted deletion of the heme oxygenase-2 gene on hemoglobin toxicity in the striatum. J Cereb Blood Flow Metab 2005; 25: 1466–1475. [DOI] [PubMed] [Google Scholar]
- 21.Wu J, Hua Y, Keep RF, et al. Oxidative brain injury from extravasated erythrocytes after intracerebral hemorrhage. Brain Res 2002; 953: 45–52. [DOI] [PubMed] [Google Scholar]
- 22.Le Belle JE, Orozco NM, Paucar AA, et al. Proliferative neural stem cells have high endogenous ROS levels that regulate self-renewal and neurogenesis in a PI3K/Akt-dependant manner. Cell Stem Cell 2011; 8: 59–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pervaiz S, Taneja R, Ghaffari S. Oxidative stress regulation of stem and progenitor cells. Antioxid Redox Signal 2009; 11: 2777–2789. [DOI] [PubMed] [Google Scholar]
- 24.Hellwig-Bürgel T, Stiehl DP, Wagner AE, et al. Hypoxia-inducible factor-1 (HIF-1): a novel transcription factor in immune reactions. J Interferon Cytokine Res 2005; 25: 297–310. [DOI] [PubMed] [Google Scholar]
- 25.Wang J-a, Chen T-l, Jiang J, et al. Hypoxic preconditioning attenuates hypoxia/reoxygenation-induced apoptosis in mesenchymal stem cells. Acta Pharmacol Sin 2008; 29: 74–82. [DOI] [PubMed] [Google Scholar]
- 26.Sakata H, Niizuma K, Yoshioka H, et al. Minocycline-preconditioned neural stem cells enhance neuroprotection after ischemic stroke in rats. J Neurosci 2012; 32: 3462–3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakata H, Narasimhan P, Niizuma K, et al. Interleukin 6-preconditioned neural stem cells reduce ischaemic injury in stroke mice. Brain 2012; 135: 3298–3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.