Abstract
The aim was to characterize the effects of magnesium sulfate, using i.v. bolus and local administration, using intrinsic signal imaging, and on electrocorticographic activity during the induction and propagation of spreading depolarizations in the gyrencephalic porcine brain. Local application of magnesium sulfate led to a complete inhibition of spreading depolarizations. One hour after washing out the topical magnesium sulfate, re-incidence of the spreading depolarizations was observed in 50% of the hemispheres. Those spreading depolarizations showed attenuation in hemodynamic characteristics and speed in intrinsic optical signal imaging. The electrical amplitude decreased through electrocorticographic activity. Intravenous magnesium therapy showed no significant effects on spreading depolarization incidence and characteristics.
Keywords: Animal models, brain imaging, cerebral blood flow measurement, intrinsic optical signal imaging, magnesium, optical imaging, spreading depression
Introduction
Magnesium (Mg2+) is an established neuroprotective agent1 that increases the voltage-dependent block of the NMDA receptor channel, and limits the overexcitation2 that may be involved in spreading depolarization (SD) initiation and/or propagation.
In addition, Mg2+ can inhibit flux through voltage-dependent Ca2+ channels in vascular smooth muscle cells,3 and could thereby limit inappropriate vasoconstrictor responses in the wake of SD in injured tissues. Prior work has shown that intracisternal Mg2+ reduces the frequency and the depolarization time of SD and it reduces lesion volume following subarachnoid hemorrhage in rats.4,5 It has been shown that intraperitoneal6 and intracarotid Mg2+ 7 reduced infarct volume in rat stroke models. However, according to MASH-2, a Phase III study8 and a meta-analysis,9 i.v. administration of Mg2+ does not improve the clinical outcome after aneurysmal subarachnoid hemorrhage (aSAH). The i.v. administration of Mg2+ did not reduce infarct growth or have any effect on the outcome in the IMAGES trial.10 Therefore, whether i.v. Mg2+ at the given concentrations could inhibit SDs effectively in those patients is still unknown.
The aim of this study is to analyze the effect of local and intravenous Mg2+ on SD susceptibility and characteristics, through intrinsic optical signal (IOS) imaging and electrocorticography (ECoG) in the gyrencephalic brain.
Material and methods
Animal preparation
The experiments were approved by the ethics commission Regierungspräsidium Karlsruhe, Karsruhe, Germany (Nr. G-167/14) according to the 2010/63/EU guidelines and following the ARRIVE guidelines for reporting in vivo experiments. Three groups of five German Landrace swine (mean weight of 29.3 kg, 3–4 months of age) were craniotomized and monitored for over 6 h, to test local and i.v. Mg2+ vs. controls. The animal handling protocol was described previously11 and in the Supplementary material. An extensive craniotomy and dura mater excision was performed to expose both hemispheres. The translucent border of a 6-contact platinum ECoG strip was placed on the cortex surface for ECoG monitoring. The ECoG was recorded in one of the two hemispheres from a subdural strip. IOS settings and recording were performed as described before.12,13
SD induction and structure of the experiment
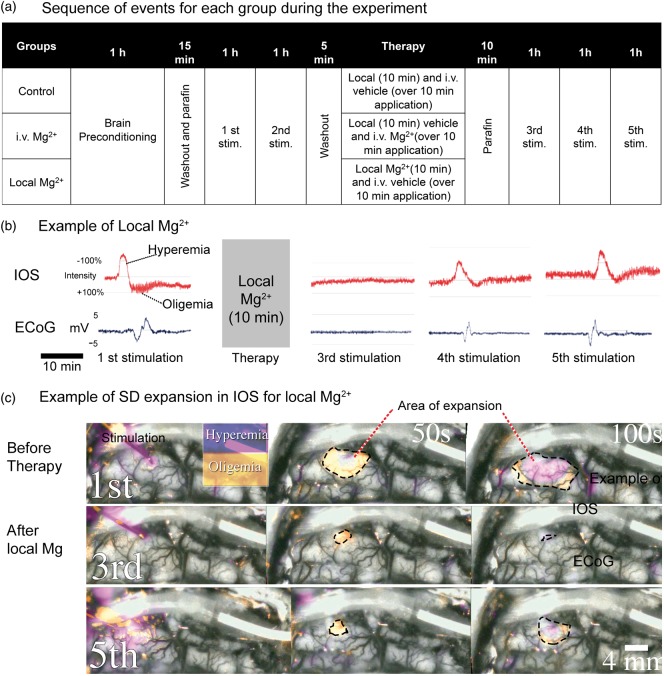
SDs were induced with a small drop (1–3 μl) of KCl (1 mol/l), which was placed directly onto the cortical surface in a central visible area, using a dull needle from an insulin syringe. The brain cortex was stimulated hourly in both hemispheres. The experiment consisted in two hourly stimulations before therapy or vehicle, followed by three hourly stimulations (Figure 1(a)). Comparison of SD from the first stimulation with the SD from the third simulation, and the SD from the second stimulation with the SD from the fourth stimulation was performed. They presented the same recovery time after washout and placement of the paraffin pool. The change of medium affects the temperature and ion concentrations and it can interfere with IOS variables, as seen in previous experiments,12 but the washout is better to maintain an adequate visualization of the SDs free of blood and detritus.
Figure 1.
a) Structure of the experiment. The control group received 0.9% NaCl local and i.v., one group received only local Mg2+ and 0.9% NaCl i.v. and the other group received 0.9% NaCl local and Mg2+ i.v. Stim.: Stimulation. (b) Effect of local Mg2+ on IOS and ECoG signals at distal ROI and ECoG electrode where the SDs showed mostly a byphasic pattern of hyperemia followed by oligemia. The ECoG signal was 0.05 Hz low-pass filtered. The scale of IOS is relative. (c) Initiation and initial expansion of SDs. The first stimulation was done before local Mg2+ application. The third stimulation was done 10 min after of 10 min local 1% MgSO4 application. The fifth stimulation was performed 2 h after local Mg2+ application. The area of expansion on a given time is marked with dotted lines. Those waves show a hypoperfusion front followed by an intense enlarged hyperemic phase in the initial 100 s.
Experimental groups
Stimulations were followed by a 1-h observation period; if more than one SD appeared in the 1-h period of observation (clusters), only the first SD was considered for analysis. All of the animals had two hourly stimulations before the first medium change occurred. One hour after the second stimulation, the medium covering the brain was changed to either NaCl 0.9% (in control and Mg2+ i.v. groups) or NaCl 0.9% with 20 mmol/l=8.12 mEq/l MgSO4 (in the local Mg2+ group). Simultaneously, an i.v. application of either 40 ml MgSO4 10% (in the Mg2+ i.v. group) or NaCl 0.9% (in the control and local Mg2+ group) was performed, according to the corresponding group. After 10 min of brain exposure, solutions were washed out and a pool of paraffin covered the brain again. After stabilization for 10 min, the brain was again stimulated (third, fourth, and fifth stimulations).
Data analysis
IOS and ECoG recordings were analyzed for SD events, according to the methods described previously14,15 using the LabChart7 software. In IOS, for each experiment, a number of six regions of interest (ROIs) of about 10×10 pixels (approximately 0.06–0.2 mm2) in size were selected and distributed along the hemispheres. Details on how ROIs were distributed and used for analysis are given in the Supplementary material. After obtaining a baseline intensity value in IOS, the total amplitude of the intensity change, the minimum of the intensity change (equal to the peak hyperemia due to increased hemoglobin concentration), and the maximum of the intensity change (equal to peak oligemia due to decreased hemoglobin tissue concentration), which are expressed as changes relative to the baseline in percantage, were compared to the wave components of the SDs without therapy. Measurements of the time to reach peak hyperemia, the duration of oligemia, the duration of the total hemodynamic response, and the number of ROIs reached per SD, were performed.
The speed was measured separately. Two dedicated ROIs separated by a distance of 6 mm were chosen in a direction perpendicular to the SD expansion, in order to calculate the time to expand the given distance.
Statistics
Descriptive statistics were calculated for all outcome variables of interest. Continuous variables were assessed for normality, using histograms and Kolmogorov–Smirnov tests. In order to test for differences in the means, medians and number of events, the (paired) Student’s t-test, the Wilcoxon-signed rank test, the Kruskal–Wallis rank sum test (ANOVA on ranks), and the one-way analysis of variance (ANOVA) were used, where appropriate. Descriptive statistics and test group differences were obtained, using the statistical analysis software SPSS 20.0. P values <0.05 were considered statistically significant.
Results
Physiological parameters and SDs before therapy
Physiological parameters showed no significant difference when comparing the mean value one hour before intervention to the mean value 1 h after intervention (paired Student’s t-test and Wilcoxon-signed rank test accordingly, Supplementary Table 1).
Before therapy administration (i.e. during the first and second stimulations), SDs could be evoked through KCl in both groups, according to the established stimulation times. SDs were viewed mostly in IOS as biphasic and/or triphasic hemodynamic changes (Figure 1(b)).
Effect of local Mg2+ on SD
A profound inhibitory effect on SD induction was observed following localized exposures to Mg2+ (Figure 1(b) and (c)). Ten minutes after exposure to localized Mg2+, SDs were not able to be elicited. SDs were completely blocked in IOS for the third stimulation. Nevertheless, we detected an electrical signal in the ECoG, in the electrode adjacent to the stimulation, which did not spread. Susceptibility to SD progressively recovered over the subsequent 2 h, such that by the fourth stimulation there were SDs in 5 of 10 hemispheres, an expansion occurred in 43% of the predefined ROIs and in the half of the cases SDs appeared to have reached 90% of the ECoG channels. After the fifth stimulation, SDs were detected in 8 of 10 hemispheres and presented an expansion in 60% of the ROIs. In 80% of the cases, SDs for this stimulation expanded to 100% of the ECoG channels (see supplementary video online).
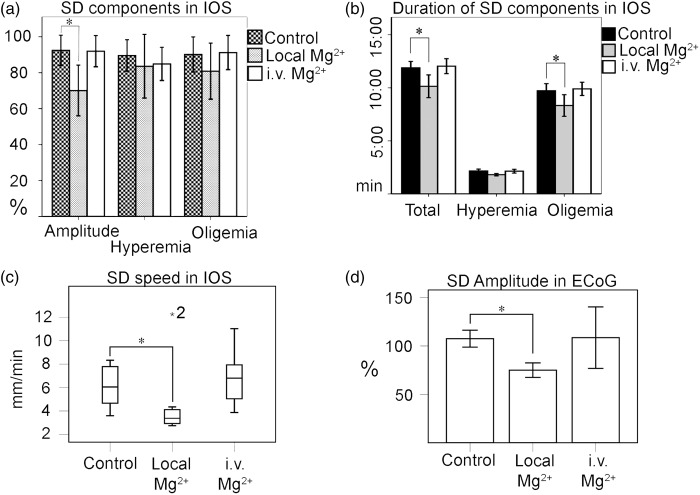
IOS characteristics for the fourth SD of the local groups showed a significant decrease in total amplitude when compared to the second SD (−22.37%, SE 7.6, p = 0.023, ANOVA with Bonferroni correction) for both the control and (−1%, SE 7.6, p = 0.027) the i.v. therapy groups. Peak hyperemia and peak oligemia showed no significant difference between groups (Figure 2(a)). The time to reach peak hyperemia showed no significant differences (ANOVA on ranks) between groups. The duration of the oligemia of the second SD was 10:04 (mm:ss), SE 00:08 and it was significantly reduced after local Mg2+ application compared to the control group (−01:34, SE 00:34, p = 0.036). The duration of the oligemia after local therapy (fourth simulation) also showed a significant difference compared to the SD from the second stimulation (01:51, SE 00:15, p = 0.019) (Figure 2(b)). The total duration of the hemodynamic response of the second SD was 12:14, SE 00:09 and it was significantly reduced after local Mg2+ application in the fourth simulation SD, compared to the control (−01:44, SE 00:35, p = 0.021) (Figure 2(b)) and compared to the SD from the second stimulation (−02:03, SE 00:30, p = 0.016). The mean speed was 4.1, SE 0.5 mm/min. The third and fourth SD showed no significant difference with the first and second SD, respectively (ANOVA on ranks). For the analysis across the groups, we found a significant decrease in speed in the SDs from the fifth stimulation (−1.7 mm/min p = 0.039) between the local Mg2+ and control group (Figure 2(c)).
Figure 2.
a) Effect of Mg2+ therapy on IOS characteristics. Means from amplitude, hyperemia, and oligemia were calculated as percentage in relation to the means of the SD before therapy (e.g. mean of the amplitude in fourth SD×100%/mean of the amplitude in second SD). Data were compared using one-way ANOVA. (b) Means from durations were calculated for the fourth SD. Data were compared using one-way ANOVA for ranks. (a) and (b) Only local Mg2+ showed a significant difference compared to the control group in relative amplitude, duration of oligemia and total duration of hemodynamic response. (c) Local Mg2+ reduces speed compared to control at the fifth stimulation. (d) Effect of local Mg2+ on depolarization signal after the third stimulation in the proximal electrode. Error bars represent standard error in all charts.
In ECoG, the SDs from the third stimulation were between local Mg2+and control groups. Given that the stimulation place was close to a strip electrode (4 mm), an electrical signal could be measured in all cases, at least in one electrode, although the expansion was blocked in coherence with the hemodynamic signal. When comparing the electrical characteristics of the depolarization wave, a difference was seen for local for amplitude (−32.5%, p = 0.023) but not for duration (−2.5 min, p = 0.89, ANOVA), (Figure 2(d)).
Effect of i.v. Mg2+ on SD
No effect was found on SD induction and expansion during i.v. Mg2+ infusion. In IOS, SDs properties were found to be unaffected after i.v. Mg2+ administration when compared with the control group. Similarly, in EcoG, no difference in amplitude was found after the intravenous administration (Figure 2(a) to (d)).
Discussion
The current study enables a systematic comparison of the effect of Mg2+ at a therapeutic dosage, based on prior studies in humans, with the goal of neuroprotection9–10 on SD induction, expansion and SD properties registered through IOS and ECoG on a gyrencephalic brain.
The most remarkable finding is that the hemodynamic and electric responses of the SDs were blocked in a sustained fashion following 10 min of local 1% MgSO4 application. This model enables the evaluation of SDs when Mg2+ reabsorbs and the concentration in the subarachnoid space decreases. When SDs reappeared over time, local Mg2+ was able to render the hemodynamic changes of the SDs milder. The Mg2+ concentration chosen in our animal experiments was 1%, based on previous reports that doses of up to 500 mg intrathecal (=6.3%) seem to be safe.16 Our data also indicate that i.v. Mg2+ therapy had no effect either on SD incidence or on SD characteristics. This may be related to reduced blood brain barrier permeability, higher astrocyte specialization, low bioavailability, and/or effective renal elimination in higher species.
Changes in SD characteristics after local Mg2+
In our experiments, local Mg2+ not only blocked the hemodynamic and electric response, but also decreased the amplitude of the hemodynamic and electric change, the total duration and the duration of the oligemic phase of the SDs. It is not known how detrimental SDs with reduced amplitude and duration in IOS and ECoG are for the neurons. SDs with less amplitude might be less harmful per se, but SDs appearing as clusters tend to reduce their amplitude also when they get closer. On the other hand, speed has a modest sensitivity and specificity in predicting a change in SD susceptibility, with positive and negative values of 84% and 56%, respectively, in a susceptibility animal model.17 Higher SD susceptibility should correlate with clusters in patients. Clusters of SDs have been correlated with worse brain metabolism and outcome.18 We speculate that the main neuroprotective effect would be the reduction of SD frequency and the effectiveness of the therapy could be indirectly estimated with the reduction of the expansion, speed, and other SD characteristics in our model.
Action mechanism of Mg2+
One of the Mg2+ action mechanisms is through an NMDA receptor that is activated by a voltage-dependent process. There is a voltage nonlinear dependence of the NMDA conductance in the presence of extracellular Mg2+ at least from 1 μl to 10 mM, as seen in whole cell recordings.19 NMDA activation depends on the Mg2+ removal of the receptor. At high Mg2+ concentrations, an NMDA receptor can be inactivated, reducing the flow of Na+, K+ and particularly the Ca2+ influx, keeping the neuron in a stable state of excitation and avoiding cellular damage during SD.
We consider that, under our experimental conditions, Mg2+ has an effect on the vascular response, independent from the effect on neuronal depolarization and glutamate release. It is well known that Mg2+ also alters the Na+, K+, and Ca2+ currents of the brain vessels via direct effects on vascular muscle and indirect effects on endothelial cells.20
Translation from basic research to clinical studies
Theoretically, in a clinical study, neuroprotection to the noxious SD effects could be achieved when SDs have been inhibited or the harmful hemodynamic components have been reduced. SDs were not monitored in IMAGES and MASH-2 trials but it is possible that the i.v. dose given in those studies would not have had an effect on SDs. This corresponds to basic studies, where, for example, the high intravenous MgSO4 dose of 720 µmol/kg had no effect on the infarct volume in rats.21,22 Intravenous Mg2+ reaches very low concentrations in cerebrospinal fluid when it is given over a long period of time. For example, inducing an hypermagnesemia (2.1–2.5 mmol/l) in brain-injured patients could increase the amount of Mg2+ in cerebrospinal fluid only by 15% (1.43 ± 0.13 mmol/l).23
This study supports the concept that Mg2+ may still benefit patients with SAH and stroke if it were applied topically, either intracisternally or intrathecally, as also supported by rat experiments.4,5,7 A recent monocentric study randomized 70 patients presenting with aneurysmal SAH (WFNS Grades 1–4, Fisher Grades 2–3). One group received standard treatment with cisternal irrigation therapy and the other with continuous infusion of MgSO4 in addition. No significant differences in outcome were observed after three months between the two groups,24 but a concentration of 1.2 mmol/l would be too low to suppress SDs in many animal models and brain slices.17,25 In our study, we applied Mg2+ at significantly higher concentrations (≈20 mmol/l).
Study limitations
This study has some limitations that should be considered. One of them is the disadvantage presented by IOS in which the visualization of SDs is not only selective for cerebral hemoglobin in a qualitative manner, but also for other not well known intracellular changes related to the mitochondrial redox status seen in histological preparations without hemoglobin.26,27
Conclusion
Our results indicate that local Mg2+ is capable of interfering with KCl-induced SD development in the swine gyrencephalic brain. In contrast, i.v. Mg2+ administration has no effect on SD incidence and characteristics. Local Mg2+ may be a therapeutically effective option to inhibit SD in patients. Still, adequate concentrations, way of administration, and duration of effectiveness need to be confirmed.
Supplementary Material
Supplementary Material
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
ES planned the project, conducted experiments, analyzed data, and wrote the manuscript; FL and HS conducted experiments and analyzed data; RSP conducted experiments, analyzed data, and critically reviewed the manuscript; CWS, AWU, and OWS critically reviewed the manuscript.
Supplementary material
Supplementary material for this paper can be found at http://jcbfm.sagepub.com/content/by/supplemental-data
References
- 1.Chang JJ, Mack WJ, Saver JL, et al. Magnesium: potential roles in neurovascular disease. Front Neurol 2014; 5: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nowak L, Bregestovski P, Ascher P, et al. Magnesium gates glutamate-activated channels in mouse central neurones. Nature 1984; 307: 462–465. [DOI] [PubMed] [Google Scholar]
- 3.Kemp PA, Gardiner SM, March JE, et al. Assessment of the effects of endothelin-1 and magnesium sulphate on regional blood flows in conscious rats, by the coloured microsphere reference technique. Br J Pharmacol 1999; 126: 621–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van den Bergh WM, Zuur JK, Kamerling NA, et al. Role of magnesium in the reduction of ischemic depolarization and lesion volume after experimental subarachnoid hemorrhage. J Neurosurg 2002; 97: 416–422. [DOI] [PubMed] [Google Scholar]
- 5.van der Hel WS, van den Bergh WM, Nicolay K, et al. Suppression of cortical spreading depressions after magnesium treatment in the rat. Neuroreport 1998; 9: 2179–2182. [DOI] [PubMed] [Google Scholar]
- 6.Izumi Y, Roussel S, Pinard E, et al. Reduction of infarct volume by magnesium after middle cerebral artery occlusion in rats. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab 1991; 11: 1025–1030. [DOI] [PubMed] [Google Scholar]
- 7.Song W, Wu Y-M, Ji Z, et al. Intra-carotid cold magnesium sulfate infusion induces selective cerebral hypothermia and neuroprotection in rats with transient middle cerebral artery occlusion. Neurol Sci Off J Ital Neurol Soc Ital Soc Clin Neurophysiol 2013; 34: 479–486. [DOI] [PubMed] [Google Scholar]
- 8.Dorhout Mees SM, Algra A, Vandertop WP, et al. Magnesium for aneurysmal subarachnoid haemorrhage (MASH-2): a randomised placebo-controlled trial. Lancet Lond Engl 2012; 380: 44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dorhout Mees SM, Algra A, Wong GKC, et al. Early magnesium treatment after aneurysmal subarachnoid hemorrhage: individual patient data meta-analysis. Stroke J Cereb Circ 2015; 46(11): 3190–3193. [DOI] [PubMed] [Google Scholar]
- 10.Muir KW, Lees KR, Ford I, et al. Magnesium for acute stroke (Intravenous Magnesium Efficacy in Stroke trial): randomised controlled trial. Lancet Lond Engl 2004; 363: 439–445. [DOI] [PubMed] [Google Scholar]
- 11.Sanchez-Porras R, Santos E, Scholl M, et al. Ketamine modulation of the haemodynamic response to spreading depolarization in the gyrencephalic swine brain. J Cereb Blood Flow Metab 2016. Epub ahead of print 28 April 2016. DOI: 10.1177/0271678X16646586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santos E, Schöll M, Sánchez-Porras R, et al. Radial, spiral and reverberating waves of spreading depolarization occur in the gyrencephalic brain. NeuroImage 2014; 99: 244–255. [DOI] [PubMed] [Google Scholar]
- 13.Sánchez-Porras R, Santos E, Schöll M, et al. The effect of ketamine on optical and electrical characteristics of spreading depolarizations in gyrencephalic swine cortex. Neuropharmacology 2014; 84: 52–61. [DOI] [PubMed] [Google Scholar]
- 14.Schöll M, Santos E, Sánchez-Porras R, et al. Large field-of-view motion-compensated intrinsic optical signal imaging for the characterization of the hemodynamic response to spreading depolarizations in large gyrencephalic brains. J Cereb Blood Flow Metab 2016. In press. DOI: 10.1177/0271678X16668988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fabricius M, Fuhr S, Bhatia R, et al. Cortical spreading depression and peri-infarct depolarization in acutely injured human cerebral cortex. Brain J Neurol 2006; 129: 778–790. [DOI] [PubMed] [Google Scholar]
- 16.Mebazaa MS, Ouerghi S, Frikha N, et al. Is magnesium sulfate by the intrathecal route efficient and safe? Ann Fr Anesthèsie Rèanimation 2011; 30: 47–50. [DOI] [PubMed] [Google Scholar]
- 17.Ayata C. Pearls and pitfalls in experimental models of spreading depression. Cephalalgia Int J Headache 2013; 33: 604–613. [DOI] [PubMed] [Google Scholar]
- 18.Sakowitz OW, Santos E, Nagel A, et al. Clusters of spreading depolarizations are associated with disturbed cerebral metabolism in patients with aneurysmal subarachnoid hemorrhage. Stroke J Cereb Circ 2013; 44: 220–223. [DOI] [PubMed] [Google Scholar]
- 19.Jahr CE, Stevens CF. Voltage dependence of NMDA-activated macroscopic conductances predicted by single-channel kinetics. J Neurosci Off J Soc Neurosci 1990; 10: 3178–3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delpiano MA, Altura BM. Modulatory effect of extracellular Mg2+ ions on K+ and Ca2+ currents of capillary endothelial cells from rat brain. FEBS Lett 1996; 394: 335–339. [DOI] [PubMed] [Google Scholar]
- 21.Campbell K, Meloni BP, Zhu H, et al. Magnesium treatment and spontaneous mild hypothermia after transient focal cerebral ischemia in the rat. Brain Res Bull 2008; 77: 320–322. [DOI] [PubMed] [Google Scholar]
- 22.Zhu H-D, Martin R, Meloni B, et al. Magnesium sulfate fails to reduce infarct volume following transient focal cerebral ischemia in rats. Neurosci Res 2004; 49: 347–353. [DOI] [PubMed] [Google Scholar]
- 23.McKee JA, Brewer RP, Macy GE, et al. Analysis of the brain bioavailability of peripherally administered magnesium sulfate: a study in humans with acute brain injury undergoing prolonged induced hypermagnesemia. Crit Care Med 2005; 33: 661–666. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto T, Mori K, Esaki T, et al. Preventive effect of continuous cisternal irrigation with magnesium sulfate solution on angiographic cerebral vasospasms associated with aneurysmal subarachnoid hemorrhages: a randomized controlled trial. J Neurosurg 2015; 124: 18–26. [DOI] [PubMed] [Google Scholar]
- 25.Dreier JP, Victorov IV, Petzold GC, et al. Electrochemical failure of the brain cortex is more deleterious when it is accompanied by low perfusion. Stroke J Cereb Circ 2013; 44: 490–496. [DOI] [PubMed] [Google Scholar]
- 26.Sun X, Wang Y, Chen S, et al. Simultaneous monitoring of intracellular pH changes and hemodynamic response during cortical spreading depression by fluorescence-corrected multimodal optical imaging. NeuroImage 2011; 57: 873–884. [DOI] [PubMed] [Google Scholar]
- 27.Yin C, Zhou F, Wang Y, et al. Simultaneous detection of hemodynamics, mitochondrial metabolism and light scattering changes during cortical spreading depression in rats based on multi-spectral optical imaging. NeuroImage 2013; 76: 70–80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.