Abstract
The clinical data of 183 patients with hepatitic cirrhosis and portal hypertensive splenomegaly complicated by peripheral cytopenia were retrospectively analyzed to investigate the causes of peripheral cytopenia, as well as the proportion of the causes in these patients. All patients underwent splenectomy. Before operation, these patients had one or more types of peripheral cytopenia (cumulative cytopenia: 390 patient-times). After splenectomy, blood counts in 79.2% (309/390) returned to normal, while in 15.9% (62/390) they increased but failed to reach to normal levels, and in 4.9% (19/390) they became lower than before the operations. For the last group of patients (n = 19), long-term follow-up showed that blood counts returned to normal in five patients. In other words, in 80.5% [(309 + 5)/390 or 314/390] of patient-times, the peripheral cytopenia was due to hypersplenism, in 15.9% it was due to a combination of factors, and in 3.6% [14/390] it had nothing to do with the hypersplenism. Thus, hypersplenism is a major cause, but not the only cause, of peripheral cytopenia in patients with hepatic cirrhosis and portal hypertensive splenomegaly, and splenectormy is an effective treatment for these patients.
Impact statement
For a long time, the development of peripheral cytopenias as a complication to cirrhotic portal hypertension has been attributed to hypersplenism; however, this has never been fully demonstrated. Dameshek summarized that hypersplenism should be diagnosed by the presence of four conditions: (a) mono- or multi-lineage peripheral cytopenias; (b) compensatory hyperplasia of bone marrow; (c) splenomegaly; and (d) correction of cytopenias after splenectomy. We retrospectively analyzed the clinical data from 183 surgical patients, and found that 80.5% of peripheral cytopenias was caused by hypersplenism, 16% by a combination of factors, and 3.5% by other factors unrelated to hypersplenism. As the first quantitative findings in this field, our results verify that hypersplenism is a major, but not exclusive, cause of peripheral cytopenias, and provides important clinical evidence for investigating the cause of peripheral cytopenias.
Keywords: Portal hypertension, peripheral cytopenia, causes, hypersplenism, other factors
Introduction
Globally there are approximately 360 million hepatitis B virus (HBV) carriers, and more than half are in the Asia-Pacific region. China is a high prevalence area of HBV infection where the positive HBV carrier rate is 9.8%. On average, 20% of these carriers will develop into chronic hepatitis,1 and 50% of those with chronic hepatitis will develop into cirrhosis and portal hypertension. Peripheral cytopenia, defined as a peripheral white blood cell (WBC) count <4.0 × 109/L, red blood cell (RBC) count <3.5 × 1012/L, or platelet (PLT) count <100 × 109/L, is very common in patients with hepatitic cirrhosis and portal hypertensive splenomegaly.2,3 Ninety percent of these patients have one or more types of peripheral cytopenia,4,5 which affects the prognosis.
In the past, peripheral cytopenia was considered to be caused solely by hypersplenism.6,7 However, according to Kalambokis and Tsianos, this etiology has not been well documented.8 Many scholars have now reported that toxicity of hepatitis virus to bone marrow,9–11 liver hypofunction,12,13 gastrointestinal bleeding,14 immune dysfunction,15,16 drug toxicity,17–19 peripheral platelet destruction,20 hematopoietic disorders caused by vitamin and nutritional deficiency21–23 and at the same time with the blood system diseases can also lead to peripheral cytopenia. Karasu and Tekin24 stated that hypersplenism cannot be the only cause for patients with peripheral thrombopenia. Dameshek25 proposed four criteria for diagnosis of hypersplenism: (1) splenomegaly; (2) one or several types of cytopenia; (3) bone marrow normal or in hyperplastic state; (4) disappearance of pathological changes of blood cells after splenectomy. The clinical manifestations of portal hypertension include splenomegaly, and for patients with hypersplenism, peripheral cytopenia should be present and blood counts should become normal after splenectomy26 and state the hypothesis addressed in this study: for example, this study addressed the hypothesis that hypersplenism is a major cause, but there are other causes, of peripheral cytopenia in patients with hepatic cirrhosis and portal hypertensive splenomegaly. We studied the clinical data of 183 patients with posthepatitic cirrhosis and portal hypertensive splenomegaly complicated by mono- or multiple-lineage peripheral cytopenia treated in our hospital from January 1996 to December 2013, and investigated the causes of peripheral cytopenia, as well as the proportion of the causes, in these patients to facilitate the choice of treatment.
Materials and methods
Patient cohort
The inclusion criteria were patients with posthepatitic cirrhosis and portal hypertensive splenomegaly who were complicated by peripheral mono- or multiple-lineage cytopenia treated with splenectomy. Exclusion criteria included (1) incomplete clinical data and (2) cytopenia blood diseases. All patients signed an informed consent form approved by the hospital ethics committee to comply with ethical requirements. There were 141 males and 42 females, making a male to female ratio of 3.4:1. Their ages ranged from 13 to 79, with an average of 43 years. There were 150 patients with posthepatitic B cirrhosis, and 33 patients with posthepatitic C cirrhosis. The diagnoses were confirmed by histopathological examination of liver tissues. The spleens in these patients were enlarged and could all be felt under the left costal margin. In 80 patients, the spleen was felt <5 cm under the left costal margin (splenomegaly degree I), in 72 patients the spleen was felt from >5 cm under the left costal margin to the umbilicus (splenomegaly degree II), and in 31 patients the spleen was felt at the umbilicus or beyond the abdominal midline (splenomegaly degree III). Ultrasound or computed tomography (CT) showed the average size of the spleen to be 226 mm × 162 mm × 96 mm. Radiological imaging of the upper digestive tract or gastroscopy showed moderate to severe varices in the lower esophagus and gastric fundus of these patients (Figure 1). Bone marrow biopsy was carried out in 142 patients (77.6%). In 82 patients, the bone marrow was normal and in 60 patients it showed hyperplasia. Splenectomy was carried out in 97 patients for massive digestive tract bleeding (blood loss ≥ 500 ml), in 55 patients for a PLT ≤ 5 × 109/L, and in 31 patients for splenomegaly. Concomitant pericardial devascularization was carried out in 163 patients.
Figure 1.
Severe varicose veins existed in lower esophagus and gastric fundus
Statistical analysis
The SPSS 18.0 (SPSS, Chicago, IL, USA) statistical software was used. The Student’s t test was used to process numerical variables, which were expressed as mean ± standard deviation (S.D.); a P < 0.05 was considered statistically significant.
Results
The 30-day mortality was 8/183 or 4.4%. The causes of death were perioperative bleeding (n = 3), hepatic encephalopathy (n = 1), hepatorenal failure (n = 2), and serious abdominal infection (n = 2). The 90-day mortality was 11/183 or 6.0%, after three more patients died from hepatic encephalopathy. The hospital stay ranged from 15 to 119 days (mean 36 days). After operation, the patients’ peripheral venous blood was sampled at two-day intervals for cell counts. The changes in the various blood cell counts from the first blood cell examination after hospital admission to the last examination before discharge from hospital are shown in Table 1. In patients with monolineage cytopenia, the WBC and PLT counts all returned to normal after splenectomy. However, in 36 patients with erythropenia after splenectomy, RBC returned to normal only in 20 patients (55.6%), RBC did not change in one patient, and RBC was lower than before the operation in 15 patients. In patients with bi-lineage cytopenia, blood counts in the WBC + RBC group and the WBC + PLT group all returned to normal after splenectomy. However, in the RBC + PLT group and the pancytopenia group, the change in blood cells was very complex after splenectomy. Also, as the change of each of the blood cells was not synchronous, the patient-times of each of the monolineage cytopenia were counted separately in patients with multi-lineage cytopenia in our study so as to obtain more objective results.
Table 1.
Changes before and after operation in 183 patients with peripheral cytopenia (n)
| Group | Item | Cytopenia before operation (n) | Cell types | Changes after splenectomy |
||||
|---|---|---|---|---|---|---|---|---|
| Increase but not reaching normal value (n) | Higher than normal value (n) | Normal value (n) | No change (n) | Lower than before operation (n) | ||||
| Monolineage cytopenia | WBC | 3 | WBC | 0 | 3b | 0 | 0 | 0 |
| RBC | 36 | RBC | 7a | 3a | 17a | 1a | 8a | |
| PLT | 11 | PLT | 0 | 2c | 9c | 0 | 0 | |
| Bi-lineage cytopenia | WBC + RBC | 4 | WBC+RBC | 1b | 0 | 3b4a | 0 | 0 |
| WBC | 0 | 0 | 0 | 0 | 0 | |||
| RBC | 0 | 0 | 0 | 0 | 0 | |||
| WBC + PLT | 24 | WBC+PLT | 3b | 9b5c | 15b13c | 0 | 0 | |
| WBC | 0 | 0 | 0 | 0 | 0 | |||
| PLT | 0 | 3c | 0 | 0 | 0 | |||
| RBC + PLT | 31 | RBC+PLT | 3a15c | 3a4c | 18a10c | 0 | 6a1c | |
| RBC | 0 | 1a | 0 | 0 | 0 | |||
| PLT | 0 | 0 | 0 | 1c | 0 | |||
| Pancytopenia | WBC + RBC + PLT | 74 | WBC+RBC+PLT | 1b21a11c | 30b1a16c | 41b50a44c | 0 | 0 |
| WBC+RBC | 0 | 0 | 1b1a | 0 | 1a | |||
| WBC+PLT | 0 | 1b | 1c | 0 | 1c | |||
| RBC+PLT | 0 | 0 | 1c | 0 | 0 | |||
| Total | 183 | 62 | 81 | 228 | 2 | 17 | ||
RBC.
WBC.
PLT.
The preoperative accumulative peripheral cytopenia was 390 patient-times. A comparison between the postoperative and preoperative states of cytopenia is shown in Table 2. Of the 172 patients who survived >90 days, 47 patients were lost to follow-up. The follow-up of the remaining 125 patients (68.3%) ranged from 5 months to 23 years (average 6 years 8 months). During follow-up, 11 patients died of massive bleeding from the digestive tract, and two patients died of liver cancer. Of the 112 surviving patients, two received liver transplantation. The accumulated peripheral cytopenia on follow-up was 239 patient-times. A comparison was made on the various preoperation blood cells (white blood cell, red blood cells, and platelet) counts with the various blood cells counts on follow-up, the results of which are shown in Table 3.
Table 2.
Comparison between postoperative state and preoperative state in the cumulative 390 patient-times of cytopenia
| Item | Cytopenia before operation |
Blood cell count after splenectomy (n, %) |
t | P | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of cumulative patient-types | Mean ± S.D. | Increase ≥ normal value | % | Increase < normal value | % | No change | Lower | Mean ± S.D. | |||
| WBC | 105 | 2.35 ± 0.63 | 103 | 98.1 | 2 | 1.9 | 0 | 0 | 7.02 ± 2.71 | 15.21 | <0.0005 |
| RBC | 145 | 3.41 ± 0.57 | 98 | 67.6 | 31 | 21.4 | 1 | 15 | 3.87 ± 1.07 | 2.20 | <0.05 |
| PLT | 140 | 69.65 ± 20.77 | 108 | 77.1 | 29 | 20.7 | 1 | 2 | 210.18 ± 104.88 | 10.01 | <0.0005 |
| Total | 390 | 309 | 79.2 | 62 | 15.9 | 2 | 17 | ||||
Table 3.
Comparison between postoperative follow-up and preoperative state in 239 patient-times of blood cell change (mean ± SD)
| Item | Number of patient-types | Before operation | Follow-up | t Value | P |
|---|---|---|---|---|---|
| WBC | 64 | 2.35 ± 0.63 | 3.42 ± 0.81 | 13.722 | <0.0005 |
| RBC | 89 | 3.41 ± 0.57 | 3.82 ± 0.67 | 2.000 | <0.05 |
| PLT | 86 | 69.65 ± 20.77 | 7.07 ± 99.37 | 8.771 | <0.0005 |
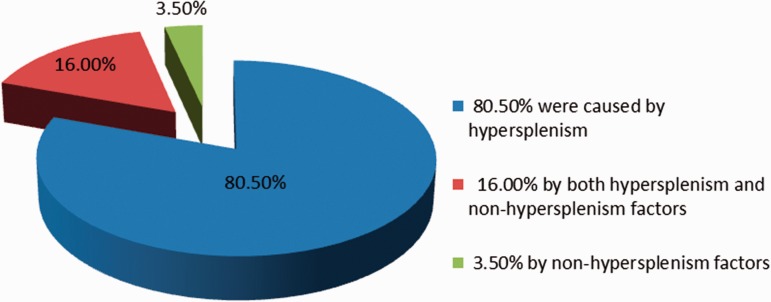
In 17 patients whose postoperative blood cells decreased rather than increased and in two patients whose postoperative blood cells did not change, isotopic scans did not show any accessory spleen. These patients were followed for 1 to 23 years (average seven years). In 15 patients with erythropenia, three died (two patients died of massive bleeding from the digestive tract, and one patient died of a stroke). In five patients, the RBC counts returned to normal (33.3%). In the remaining seven patients, the RBC counts were still low. In addition, two patients with thrombocytopenia died. For the two patients whose postoperative blood cells did not change, one patient’s RBC count returned to normal on follow-up, and one patient with postoperative unchanged PLT count died five years ago after surviving the operation for 20 years. Thus, the cytopenia was due to hypersplenism and two-thirds were related to other factors. A decrease or no change in postoperative PLT count predicted poor prognosis. The proportion of causes of peripheral cytopenias among the 183 patients is shown in Figure 2.
Figure 2.
The proportion of causes of peripheral cytopenias among the 183 patients
In about 4.9% (19/390) of patient-types, the peripheral blood cells became lower than before operation. In most of these patients, the decrease was in RBC. In 15 patients with a decrease in postoperative RBC, the decrease occurred mainly in the mono-lineage cytopenia group (n = 8) and in the bi-lineage cytopenia group (n = 6). However, in the pancytopenia group, there was only one such patient. Besides, all the preoperative RBCs were more than 3.2 × 1012/L, the WBC was >3.5 × 109/L, and the PLT > 50 × 109/L. Thus, patients with mildly low blood cells (WBC + PLT) had a decrease in postoperative red blood cells. Two patients had a decrease in postoperative PLT. One was in the bi-lineage cytopenia group and the other was the pancytopenia group. To find out the causes in these two patients, we carefully studied these patients’ spleen size, liver function, and postoperative bleeding volume and compared these patients with other patients during the same period. However, we did not find any explanation. Isotopic scans also found no accessory spleen.
Discussion
In this study, all patients underwent splenectomy carried out for massive bleeding from ruptured lower esophageal and gastric fundic varices, or severe cytopenia from hypersplenism. The vast majority of the operations were performed before 2008. With recent developments in endoscopic hemostasis,27–29 transjugular intrahepatic portosystemic shunt (TIPS),30 and hemostasis through medication,31 operation is now only considered when non-surgical treatment is unsuccessful. As less and less patients underwent operation, the clinical data of these 183 patients become valuable to provide important information to study the causes of peripheral cytopenia. Table 1 shows that peripheral cytopenias are usually multiple linage cytopenias, accounting for 72.2% of patients (133/183). The more the types of blood cells are involved, the more severe is the disease and the worse is the prognosis.32 The spleen is the target organ in hypersplenism. If peripheral cytopenia is caused by hypersplenism, the blood cells should return to normal after splenectomy.33 Otherwise, causes other than hypersplenism should be considered. In this study, of the 183 patients, the cumulative monolineage cytopenia before operation was 390 patient-times. After splenectomy, approximately 80.5% (314/390) of patient-times with peripheral cytopenia had their blood cells counts returned to normal, indicating that these peripheral cytopenias were caused by hypersplenism. Hypersplenism is related to spleen size and phagocytic capacity of splenic macrophages.34 A larger spleen size, which indicates more blood cells stored, and a higher phagocytic capacity of splenic macrophages would result in lower peripheral blood cell counts, and thus a more obvious increase in peripheral blood cell counts after splenectomy. In 15.9% (62/390) of patient-times with peripheral cytopenia, the blood cells counts increased but they did not reach normal, indicating that in addition to hypersplenism other factors were involved.23 Also, thrombocytopenia is related to thrombopoietin (TPO), and the more severe the liver cirrhosis, the worse the liver functional reserve, thus the more obvious the thrombocytopenia, and the poorer the prognosis.35 TPO is almost specially produced by liver cells. When cirrhosis develops, the number of functional liver cells secreting TPO decreases,24,36 resulting in a reduction of TPO secretion37,38 and imbalance in production and destruction of TPO.39 Therefore, even if the spleen does not store and does not destroy PLT, the PLT in circulating blood might decrease. As these factors cannot be eliminated through splenectomy, the blood cell counts in these patients with cytopenia cannot fully recover.
Kalambokis and his associates8 found an increase in incidence of peripheral cytopenia in cirrhotic patients being related to activated monocytes and promoted proinflammatory cytokines, such as serum interleukin-1, interleukin (IL)-6, tumor necrosis factor (TNF)-α, and interferon-γ, caused by hypersplenism in cirrhosis. The endotoxin produced by gut bacteria and antibiotic therapy for endotoxemia could also increase the number of blood cells in cirrhotic patients. Of the 105 patient-times of leukopenia before operation, the white cell counts returned to normal in 103 patient-types after operation. In the remaining two patient-types (1.9% or 2/105), the WBC count increased above normal. Thus, there was no patient who had a long-lasting decrease in WBC on follow-up. In 1979, Spigos et al.40 first successfully used partial splenic arterial embolization (PSE) to treat hypersplenism. Subsequently, more clinicians41–43 used PSE to treat portal hypertension, hypersplenism, and hemorrhage from esophageal and gastric fundal varices. PSE not only enhances PLT and WBC counts,44,45 but also decreases splenic size, improves pancytopenia,46 and induces immune function.44 Zhengran et al.47 reported that when the splenic artery embolization region was controlled at 60–80%, peripheral cytopenia caused by cirrhotic hypersplenism improved, portal venous blood flow, portal pressure, and degree of esophageal-gastric fundic varices reduced. Kontchou and his associates,48 on the other hand, proposed that although PSE can treat splenomegaly and hypersplenism, serious complications such as splenic infarction or splenic abscess resulted in a high risk of mortality and the indications of PSE were limited. As splenectomy, especially laparoscopic splenectomy, has few serious complications,49–51 splenectomy is still a common and effective method to treat splenomegaly and hypersplenism.52,53
For a long time, the causes of peripheral cytopenias in patients with cirrhosis and portal hypertension have been controversial. The present clinical study of 183 patients showed that hypersplenism was still a major cause of peripheral cytopenias (80.5%). However, 16% of the cases were caused by a combination of factors, and 3.5% were not related to hypersplenism at all, i.e. caused by out-of-spleen factors. This conclusion not only reveals the proportion of causes of peripheral cytopenias, but more importantly, can be used to guide the treatment and determine the treatment effect. Non-surgical treatment is recommended for patients with mild to moderate cytopenias or hypersplenism, while splenectomy may be ideal for patients with severe cytopenias or hypersplenism.54
The research statement
This research has been approved by the Ethics Committee of the Hainan Province People’s Hospital. All patients gave informed consent for their data to be used for research. All the authors have read and approved this article.
Funding
This research is supported by the China's Hainan Province Science and Technology special funds for international cooperation projects (grant no. KJHZ2015-28).
Authors’ contribution
YL conceived and designed the research; WYL took part in the design and writing of the paper; HWu, XYH, XG contributed to data collection and proofreading; NL, JY, YJL, JD, and QL took part in data statistical analysis and preparation of figures and tables.
References
- 1.Yunfu Lv. Causes of peripheral blood cytopenias in patients with liver cirrhosis portal hypertension and cilinical significances. Open J Endocrine Metab Dis 2014; 4: 85–9. [Google Scholar]
- 2.Bashour FN, Teran JC, Mullen KD. Prevalence of peripheral blood cytopenias (hypersplenism) in patients with nonalcoholic liver disease. Am J Gastroenterol 2000; 95: 2936–9. [DOI] [PubMed] [Google Scholar]
- 3.Lv Y, Li X, Han XY, Gong XG, Chang S. Peripheral blood cell variations in cirrhotic portal hypertension patients with hypersplenism. Asian Pacific J Tropic Med 2013; 6: 663–6. [DOI] [PubMed] [Google Scholar]
- 4.Yongxiang W, Zongfang L, Guowei L, Zongzheng J, Xi C, Tao W. Effects of splenomegaly and splenic macrophage activity in hypersplenism due to cirrhosis. Am J Med 2002; 113: 428–31. [DOI] [PubMed] [Google Scholar]
- 5.Lv Y, Li X, Xie X, Gong X, Tian X, Yang YJ. Portal hypertension splenomegaly is not always associated with hematocytopenia. J US–China Med Sci 2009; 6: 28–30. [Google Scholar]
- 6.Pereira J, Accatino L, Alfaro J, Brahm J, Hidalgo P, Mezzano D. Platelet autoantibodies in patients with chronic liver disease. Am J Hematol 1995; 50: 173–8. [DOI] [PubMed] [Google Scholar]
- 7.Jiang A, Zhang S, Li Z, Liang R, Ren S, Li J, Pu Y, Yang J. miR-615-3p promotes the phagocytic capacity of splenic macrophages by targeting ligand-dependent nuclear receptor corepressor in cirrhosis-related portal hypertension. Exp Biol Med (in Chinese) 2011; 236: 672–80. [DOI] [PubMed] [Google Scholar]
- 8.Kalambokis G, Tsianos EV. Endotoxaemia in the pathogenesis of cytopenias in liver cirrhosis. Could oral antibiotics raise blood counts? Med Hypoth 2011; 76: 105–9. [DOI] [PubMed] [Google Scholar]
- 9.Cudillo L. Aplastica anemia and viral hepatitis. Mediterranean J Hematol Infect Dis 2009; 1: e2009026–e2009026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Espanol I, Gallego A, Enriquez J, Rabella N, Lerma E, Hernandez A, Pujol-Moix N. Thrombocytopenia associated with liver cirrhosis and hepatitis C viral infection: role of thrombopoietin. Hepatogastroenterology 2000;47:1404–6. [PubMed]
- 11.Djordjević J, Svorcan P, Vrinić D, Dapčević B. Splenomegaly and thrombocytopenia in patients with liver cirrhosis. Vojnosanit Pregl 2010; 67: 166–9. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto M, Taniguchi H, Ohara M, Suzuki C, Naishiro Y, Ozeki I, Yamamoto H, Takahashi H, Imai K. Beneficial effect of glucocorticosteroids for esophageal varices due to idiopathic portal hypertension following systemic lupus erythematosus. Japan J Clin Immunol 2004; 27: 40–7. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Q, Zhang HY, Zhang WL, Zhong FL, Feng J, Meng QX, Yin WH, Nie LP. Clinical features and laboratory findings of adult Epstein-Barr virus associated T/NK lymphoproliferative disease. Zhongguo Shi Yan Xue Ye Xue Za Zhi (in Chinese) 2013; 21: 953–7. [DOI] [PubMed] [Google Scholar]
- 14.Srichaikul T, Punyagupta S, Kanchanapoom T, Chanokovat C, Likittanasombat K, Leelasiri A. Hemophagocytic syndrome in Dengue hemorrhagic fever with severe multiorgan complications. J Med Assoc Thailand 2008; 91: 104–9. [PubMed] [Google Scholar]
- 15.Barzaghi F, Passerini L, Bacchetta R. Immune dysregulation, polyendocrinopathy, enteropathy, x-linked syndrome: a paradigm of immunodeficiency with autoimmunity. Frontier Immunol 2012; 3: 211–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng P, Chang X, Lu Q, Liu Y. Cytopenia and autoimmune diseases: a vicious cycle fueled by mTOR dysregulation in hematopoietic stem cells. J Autoimmunity 2013; 41: 182–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh N, Yu VL, Mieles LA, Wagener MM. Beta-Lactam antibiotic-induced leukopenia in severe hepatic dysfunction: risk factors and implications for dosing in patients with liver disease. Am J Med 1993; 94: 251–6. [DOI] [PubMed] [Google Scholar]
- 18.Cobo F, De Celis G, Pereira A, Latorre X, Pujadas J, Albiol S. Oxaliplatin-induced immune hemolytic anemia: a case report and review of the literature. Anti-Cancer Drug 2007; 18: 973–6. [DOI] [PubMed] [Google Scholar]
- 19.Saif MW, Lee AM, Offer SM, McConnell K, Relias V, Diasio RB. A DPYD variant (Y186C) specific to individuals of African descent in a patient with life-threatening 5-FU toxic effects: potential for an individualized medicine approach. Mayo Clinic Proc 2014; 89: 131–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cho YG, Lee JH, Kim DS, Lee HS, Choi SI. Clinical usefulness of the simple technique to diagnose thrombocytopenia using immature platelet fraction. Korean J Lab Med 2007; 27: 1–6. [DOI] [PubMed] [Google Scholar]
- 21.Lavigne C, Lavigne E, Massenet D, Binet C, Bremond JL, Prigent D. Role of vitamin deficiency in pancytopenia in Djibouti. Findings in a series of 81 consecutive patients. Medecine tropicale: revue du Corps de sante colonial 2005; 65: 59–63. [PubMed] [Google Scholar]
- 22.Abrishami F, Golshan A. Frequency of iron deficiency anemia in girls studying in mashhad high schools. Iran J Pediatr Hematol Oncol 2013; 3: 143–8. [PMC free article] [PubMed] [Google Scholar]
- 23.Lv Y, Lau WY, Deng J, Li YJ, Dong Y. Non-hypersplenism causes of peripheral cytopenias in patients with cirrhotic portal hypertension: a review. J Hypertens: Open Access 2016; 5: 2–5. [Google Scholar]
- 24.Karasu Z, Tekin F, Ersoz G, Gunsar F, Batur Y, Ilter T. Liver fibrosis Is associated with decreased peripheral platelet count in patients with chronic hepatitis B and C. Digest Dis Sci 2007; 52: 1535–9. [DOI] [PubMed] [Google Scholar]
- 25.Dameshek W. Hypersplenism. Bull N Y Acad Med 1955; 31: 113–6. [PMC free article] [PubMed] [Google Scholar]
- 26.Lv Y, Gong X, Xie X, Wang B, Yang Y, Li Y. Clinical study on the relationship between hematocytopenia and splenomegaly caused by cirrhotic portal hypertension. Cell Biochem Biophys 2014; 70: 355–60. [DOI] [PubMed] [Google Scholar]
- 27.Villanueva C, Colomo A, Aracil C, Guarner C. Current endoscopic therapy of variceal bleeding. Best Pract Res Clin Gastroenterol 2008; 22: 261–78. [DOI] [PubMed] [Google Scholar]
- 28.Geraci G, Arnone E, Lo Nigro C, Sciuto A, Modica G, Sciumè C. Endoscopic rubber band ligation in treatment of esophageal varices bleeding. Personal experience. G Chir 2011; 32: 113–7. [PubMed] [Google Scholar]
- 29.Liu PX, Kong GQ. Endoscopic ligation in treatment of advanced schistosomiasis patients with esophageal variceal bleeding: a report of 68 cases. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi (in Chinese) 2013; 25: 112–4. [PubMed] [Google Scholar]
- 30.Garcia-Tsao G, Bosch J. Management of varices and variceal hemorrhage in cirrhosis. N Engl J Med 2010; 362: 823–32. [DOI] [PubMed] [Google Scholar]
- 31.D'Amico G, Pagliaro L, Pietrosi G, Tarantino I. Emergency sclerotherapy versus vasoactive drugs for bleeding oesophageal varices in cirrhotic patients. Cochrane Database Syst Rev 2010;3:CD002233. [DOI] [PMC free article] [PubMed]
- 32.Yunfu L. Changes of peripheral blood cells in patients with cirrhotic portal hypertension. In Garbuzenko D (ed.) Portal Hypertension - Causes and Complications. Rijeka, Croatia: InTech. 2012, pp.133–42.
- 33.Lv YF, Li XQ, Gong XG, Xie XH, Han XY, Wang BC. Effect of surgery treatment on hypersplenism caused by cirrhotic portal hypertension. Minerva Chir 2013; 68: 409–13. [PubMed] [Google Scholar]
- 34.Lv Y, Lau WY, Li YJ, Deng J, Dong Y, Han XY, Gong XG, Liu N, Wu H. Hypersplenism: history and current status(Review). Experiment Therapeut Med 2016; 12: 2377–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lv Y-F, Han X-Y, Gong X-G, Liu N, Yue J, Li Y-J, Zhang S-J, Pang Y-B. Key factors of therapeutic effects for surgery in patients with cirrhotic portal hypertension. Eur Rev Med Pharmacol Sci 2015; 19: 3492–9. [PubMed] [Google Scholar]
- 36.Eissa LA, Gad LS, Rabie AM, El-Gayar AM. Thrombopoietin level in patients with chronic liver diseases. Ann Hepatol 2008; 7: 235–44. [PubMed] [Google Scholar]
- 37.Dusheiko G. Thrombopoietin agonists for the treatment of thrombocytopenia in liver disease and hepatitis C. Clin Liver Dis 2009; 13: 487–501. [DOI] [PubMed] [Google Scholar]
- 38.Wolber EM, Ganschow R, Burdelski M, Jelkmann W. Hepatic thrombopoietin mRNA levels in acute and chronic liver failure of childhood. Hepatology 1999; 29: 1739–42. [DOI] [PubMed] [Google Scholar]
- 39.Sezai S, Kamisaka K, Ikegami F, Usuki K, Urabe A, Tahara T, Kato T, Miyazaki H. Regulation of hepatic thrombopoietin production by portal hemodynamics in liver cirrhosis. Am J Gastroenterol 1998; 93: 80–2. [DOI] [PubMed] [Google Scholar]
- 40.Spigos DG, Jonasson O, Mozes M, Capek V. Partial splenic embolization in the treatment of hypersplenism. Am J Roentgenol 1979; 132: 777–82. [DOI] [PubMed] [Google Scholar]
- 41.Sankararaman S, Velayuthan S, Vea R, Herbst J. Severe gastric variceal bleeding successfully treated by emergency splenic artery embolization. Pediatr Int 2013; 55: e42–5. [DOI] [PubMed] [Google Scholar]
- 42.Huang JH, Wu PH, Gu YK, Zhang FJ, Li CX, Gao F, Zhang L, Fan WJ, Li CJ. Study on primary hepatocellular carcinoma associated with hypersplenism treated by partial splenic embolization combined with hepatic arterial chemoembolization. Ai Zheng 2006; 25: 1003–6. [PubMed] [Google Scholar]
- 43.Chikamori F1, Inoue A, Okamoto H, Kuniyoshi N, Kawashima T, Takase Y. Hemodynamic effects of combined therapy using partial splenic embolization and transjugular retrograde obliteration for gastric varices with gastrorenal shunt. World J Surg 2010; 34: 1046–51. [DOI] [PubMed] [Google Scholar]
- 44.Noguchi H, Hirai K, Aoki Y, Sakata K, Tanikawa K. Changes in platelet kinetics after a partial splenic arterial embolization in cirrhotic patients with hypersplenism. Hepatology 1995; 22: 1682–8. [PubMed] [Google Scholar]
- 45.Harao M, Beppu T, Masuda T, Hayashi H, Okabe H, Okabe K, Imseung C, Komori H, Horino K, Baba H. The significance of combined treatment for hepatocellular carcinoma with partial splenic embolization and transcatheter arterial chemoembolization using IA call/lipiodol. Gan to kagaku ryoho Cancer Chemother 2008; 35: 2027–9. [PubMed] [Google Scholar]
- 46.Krishnan SK, Hill A, Hillmen P, Arnold LM, Brooksbank GL, Wood A. Improving cytopenia with splenic artery embolization in a patient with paroxysmal nocturnal hemoglobinuria on eculizumab. Int J Hematol 2013; 98: 716–8. [DOI] [PubMed] [Google Scholar]
- 47.Zhengran L, Hong S, Kangshun Z. Clinical quantitative study of therapeutic effect of partial splenic embolization (PSE) on portal vein hemodynamis. Chinese J Radiol 2002; 36: 913–7. [Google Scholar]
- 48.N'Kontchou G, Seror O, Bourcier V, Mohand D, Ajavon Y, Castera L. Partial splenic embolization in patients with cirrhosis: efficacy, tolerance and long-term outcome in 32 patients. Eur J Gastroenterol Hepatol 2005; 17: 179–84. [DOI] [PubMed] [Google Scholar]
- 49.Kedia S, Goyal R, Mangla V, Kumar A, SS, Das P, Pal S, Sahni P, Acharya SK. Splenectomy in cirrhosis with hypersplenism: improvement in cytopenias, Child's status and institution of specific treatment for hepatitis C with success. Ann Hepatol 2012; 11: 921–9. [PubMed] [Google Scholar]
- 50.Inagaki Y, Sugimoto K, Shiraki K, Tameda M, Kusagawa S, Nojiri K, Ogura S, Yamamoto N, Takei Y, Ito M, Mizuno S, Usui M, Sakurai H, Isaji S. The long-term effects of splenectomy and subsequent interferon therapy in patients with HCV-related liver cirrhosis. Mol Med Rep 2014; 9: 487–92. [DOI] [PubMed] [Google Scholar]
- 51.Zhan XL, Ji Y, Wang YD. Laparoscopic splenectomy for hypersplenism secondary to liver cirrhosis and portal hypertension. World J Gastroenterol 2014; 20: 5794–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bancu S, Borz C, Popescu G, Torok A, Mureşan A, Bancu L, Turcu M. Spleno-renal distal and proximal shunts for hypersplenism due to hepatic cirrhosis. Chirurgia (Bucur) 2007; 102: 665–8. [PubMed] [Google Scholar]
- 53.Kim H, Suh KS, Jeon YM, Park MS, Choi Y, Mori S, Hong G, Lee HW, Yi NJ, Lee KW. Partial splenic artery embolization for thrombocytopenia and uncontrolled massive ascites after liver transplantation. Transplant Proc 2012; 44: 755–6. [DOI] [PubMed] [Google Scholar]
- 54.Lv Y, Han X, Gong X, Ma Q, Chang S, Wu H, Li Y, Deng J. Grading of peripheral cytopenias caused by nonalcoholic cirrhotic portal hypertension and its clinical significance. Cell Biochem Biophys 2015; 71: 1141–5. [DOI] [PubMed] [Google Scholar]