Abstract
This study aims to investigate the effect as well as mechanism of ginsenoside Rg1 (Rg1) on premature ovarian failure (POF) induced by d-galactose (d-gal) in mice. C57BL/6 female mice were divided into four groups randomly, which were the saline group, the d-gal group, the d-gal + Rg1 group, and the Rg1 group. Body weight was recorded. Overall ovarian function including estrous cycles, sex hormone secretion, ovarian follicle development, and ovarian morphology was analyzed by H&E staining and ELISA. Effect of Rg1 on aging was determined by analyzing the activities of oxidation-associated biomarkers, pro-inflammatory cytokine secretion, expression of senescence-associated proteins, and fertility. Compared with the d-gal group, in Rg1 + d-gal group, body weight was increased significantly, estrous cycle block was released, and fertility and the morphology of ovaries were restored. And, Rg1 treatment after d-gal administration significantly reduced senescence-associated protein expression, increased the activity of total superoxide dismutase and glutathione peroxidase from bovine erythrocyte, and induced higher follicle stimulating hormone receptor protein expression. Additionally, the expression levels of malondialdehyde, interleukin-1β, tumor necrosis factor-α, and interleukin-6 were significantly decreased. Together, Rg1 improves mouse fertility and reduces ovarian pathological damage in d-gal-induced POF model possibly through enhancing anti-inflammatory and antioxidant capacities and reducing expression of senescence signal pathway proteins.
Impact statement
Ginsenoside Rg1 (Rg1) is a kind of natural estrogen and it has antioxidation and antiaging effects. However, whether Rg1 has effects on premature ovarian failure (POF) is still not clear. In this study, aging model induced by d-galactose was used to mimic POF. The effect and possible mechanism of Rg1 on ovary aging was investigated. We found that Rg1 treatment up-regulated the expression of follicle stimulating hormone receptor and down-regulated senescence-associated protein expression in granule cells of POF mice. Particularly, Rg1 improved fertility ability and reduced ovarian pathological damages by its antioxidative and anti-inflammation capacity. Thus, Rg1 enhances the antiaging ability of ovary and fertility ability of POF mice through enhancing the anti-inflammatory and antioxidant capacities of ovary.
Keywords: Premature ovarian failure, ginsenoside Rg1, d-galactose, aging, senescence, follicle stimulating hormone receptor
Introduction
Premature ovarian failure (POF), a condition affecting 1% of women in the general population, causes amenorrhea and hypergonadotropic hypoestrogenism before the age of 40 years.1 Previously, it is thought that POF was an irreversible disease; however, it has been shown later that in POF, residual ovarian function may remain despite the presence of elevated gonadotrophins.1 Incomplete understanding to the pathogenesis of POF is the major hurdle for the development of effective therapeutic options for this disease.2 Except for hormone replacement therapy (HRT) and follicle donation, no effective therapy for POF is currently available. However, the application of HRT has been limited in the clinical treatment because HRT was confirmed to promote coronary heart disease, endometrial cancer, and breast cancer in women with POF.3 Therefore, understanding the mechanism for POF development is critical for the clinical treatment of this disease. However, there are restrictions in performing thorough studies in humans.
Estrous cycle of C57BL/6 female mice is similar with that of humans in spite that estrous cycle of mice is shorter than that of humans. It is reported that women with galactosemia eventually develop POF.4 d-galactose (d-gal) administration can cause free radicals accumulation, which gradually damages the function of ovary during natural aging. In rodents, d-gal administration simulates the normal ovary aging process in many aspects. Hence, d-gal-induced aging model is widely used to study the mechanisms underlying ovary aging.
It has been proposed that galactosemic women and other women may develop POF through similar clinical pathways.2 Therefore, studies on galactosemic subjects are liable to reveal the mechanisms for POF development.5 Ginsenoside Rg1 (Rg1) is a kind of natural estrogen6 and it has numerous pharmacological effects in antioxidation and antiaging.7 Enhancing endogenous antioxidants is nowadays considered as an striking therapy for treatment of mitochondrial oxidative stress.8–10 Rg1 can also up-regulate Bcl-2 and Bax protein expression in the ovary of rats with POF.11 However, whether Rg1 can delay senescence in ovaries in d-gal-induced mouse aging model through enhancing antioxidant content is still not clear.
Here in this study, the role of Rg1 in the d-gal-induced aging rat model was analyzed. The fertility and histopathological damages of ovary were evaluated. The weight, estrous cycles, follicle-stimulating hormone receptor (FSHr) protein expression, fertility, biomarkers of oxidative stress and inflammation, and senescence-associated protein expression were examined. The results found that Rg1 treatment up-regulated the expression of FSHr and down-regulated expression of senescence-associated protein in granule cells of POF mice. Particularly, Rg1 is able to improve fertility ability, reduce ovarian pathological damages by its antioxidative and anti-inflammation capacity.
Materials and methods
Animals
A total of 100 C57BL/6 female mice, and 20 male mice for mating trials aged three months with normal estrus were included in this study. They were provided by the Medical and Laboratory Animal Center of Chongqing (China). The mice were kept in a standard condition with free access to food and water. All animal procedures were performed according to the approval by the Institutional Animal Care and Use Committee of Chongqing Medical University, and according to the National Research Council Guide for Care and Use of Laboratory Animals.
The female mice were divided into four groups randomly with 25 mice in each group: the saline group, the Rg1 + d-gal group, the Rg1 group, and the d-gal group. In the d-gal group, d-gal (200 mg/kg/d) was injected subcutaneously (s.c.) daily into mice for 42 days. In the Rg1 group, equal volume of saline was injected s.c. daily for 42 days. At day 15 after first saline injection, daily intraperitoneal injection (i.p.) of Rg1 (20 mg/kg/d) was performed for 28 days. Mice in the Rg1 + d-gal group received daily s.c. injection of d-gal (200 mg/kg/d) for 42 days and from day 15 of first d-gal injection, received daily i.p. injection of Rg1 (20 mg/kg/d) for 28 days. The mice in the saline group received equal volume of saline both s.c. and i.p. at the same time point. The body weight was recorded every week.
Mating trials
At the last day of drug administration (day 42), 10 mice from each group were kept with fertile males at a ratio of one male:two females for three months. The offspring number per litter was recorded.
Reagents
Rg1 (RSZD-121106, Purity = 98.3%) was from Xi’an Haoxuan Biological Technology Co., Ltd (Xi’an, China). The Rg1 was dissolved in ddH2O to 20 mg/mL and sterilized by filter. Mouse Estradiol 2 (E2) Assay Kit, Anti-Mullerian hormone (AMH) kit, follicle-stimulating hormone (FSH) kit, total superoxide dismutase (T-SOD) assay kit, malondialdehyde (MDA) kit, and GSH/glutathione peroxidase from bovine erythrocyte (GSH-px) kit were bought from Jiancheng Bio-engineering Institute (Nanjing, China). ELISA kits for interleukin-6 (IL-6) and interleukin-1β (IL-1β) and goat anti-rabbit secondary antibody were obtained from Boster Bio-engineering Co., Ltd (Wuhan, China). ELISA Kit for tumor necrosis factor-α (TNF-α) was from Uscn Life Science Inc. (Wuhan, China). BCA kit was purchased from Beyotime Institute of Biotechnology (Shanghai, China). Rabbit anti-mouse polyclonal FSHr, P21, P19, and P16 were purchased from Immunoway Biotechnology Co. (Plano, TX, USA). Mouse anti-rabbit nuclear P53 monoclonal antibody was purchased from Sigma Chemical Co. (St. Louis, MO, USA). Rabbit anti-GFAP antibody was purchased from Beyotime Institute of Biotechnology (Shanghai, China).
H&E staining
At 8:00–9:00 AM every day and for seven days, vaginal smears were obtained. The estrous cycle was analyzed by HE staining according to previous description.12,13
At the last day of drug administration (day 42) and mating trials, animals were euthanized under anesthesia. Organs (livers, lungs, spleens, kidneys, and ovaries) from these mice were collected. After fixation with 4% paraformaldehyde overnight at 4℃, the samples were dehydrated, transparentized with xylene, and embedded in paraffin. The embedded organs were continuously cut into 5 µm thickness sections and then stained with hematoxylin and eosin.
The histological examinations were performed under Image-Pro Plus 6.0 (Media Cybernetics Inc., Atlanta, GA) (magnification, ×200).The ovarian follicles were detected and classified as primary follicles, secondary follicles, antral follicles, and corpus luteum according to the previous description and definition.14,15 Only the follicles that contain a clearly visible nucleus and an oocyte were counted.
Chemical colorimetric analysis
At the last day of drug administration (day 42), ovaries were sampled and lysed for 30 min on ice. After centrifugation at 12,000 rpm, 4℃ for 10 min, the supernatant was collected. Chemical colorimetric analysis was performed to measure the activity or content of T-SOD, MDA, and GSH-px.
ELISA
For detection of sex hormones (E2, FSH, and AMH), blood samples were obtained from mice under anesthesia by retro-orbital puncture at the last day of drug administration (day 42). After incubation at room temperature for 1 h, serum was collected following centrifugation at 5000 rpm for 10 min. The concentration of the sex hormone was determined by ELISA kits following the manufacturer’s instructions.
For detection of pro-inflammatory cytokines, the ovarian supernatant was collected as described above. The IL-1β, IL-6, and TNF-α levels in the ovaries were detected by the corresponding ELISA kits. The cytokine level was presented as the level of each cytokine per milligram tissue lysates.
Immunohistochemistry
Immunohistochemical analysis was performed to detect FSHr expression as previously described.16 Briefly, ovaries were washed with PBS for three times, fixed for 30 min with 4% paraformaldehyde, dehydrated with graded ethanol, transparentized with xylene, and embedded in paraffin. The samples were made into 5 µm serial sections. After rinsing with 3% phosphate buffer, microwave antigen retrieval was performed. After blocking, the primary polyclonal antibodies (1:100 dilution) were added and incubated at 37℃ for 45 min. After washing, the sections were incubated with horseradish peroxidase conjugated secondary antibody at 37℃ for 45 min. For color development, the avidin–biotin complex chromogenic reagent (Beyotime Institute of Biotechnology, Shanghai, China) was used. For negative control, PBS (pH 7.4) was used instead of primary antibody. Five fields of each view (magnification, ×400) were randomly selected from each tissue section. Intel® Integrated Performance Primitives software Version 6.0 (Media Cybernetics, Rockville, MD, USA) was used for analysis.
Western blotting
Ovaries were collected at the last day of drug administration. Total protein was extracted by RIPA buffer (Mengbio, Chongqing, China), and the protein concentrations were detected by the BCA method (Beyotime Institute of Biotechnology, Shanghai, China). Proteins were separated by SDS-PAGE and then transferred to PVDF membranes. After blocking with 5% non-fat mild, the membranes were incubated with the primary antibodies of anti-FSHr, P53, P21, P19, P16, and GAPDH (diluted at 1:100) overnight at 4℃. After that, the secondary antibody (1:1000 dilution) was added for incubation at room temperature for 1 h. The enhanced chemiluminescence detection system (Thermofisher, Waltham, MA, USA) was used for color development. GAPDH was used as an internal reference. Quantity One (Bio Rad) was used for quantification.
Statistical analysis
SPSS 18.0 software was used for data analysis. Data were presented as mean ± SD. One-way ANOVA was used to compare the differences in mean values among the groups. LSD test was conducted for multiple comparisons. A P value less than 0.05 was considered statistically significant.
Results
Rg1 increases body weight and improves estrous cycles
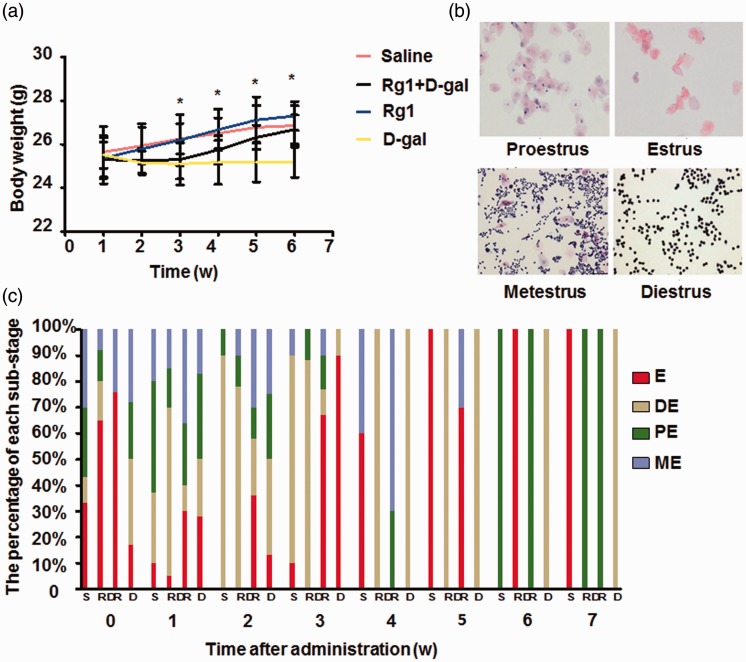
To examine the effect of Rg1 on mouse POF model, body weight was analyzed after d-gal administration. d-gal inhibited the increase of body weight from day 7 postadministration compared with saline group, and injection of Rg1 (Rg1 + d-gal group) released this inhibition from day 21, resulting in continuously increased weight (Figure 1(a)). No significant difference was found between the Rg1 group and saline group. Next vaginal smears were stained by H&E to evaluate estrous cycles. From two weeks after d-gal administration in the d-gal group, estrous cycles were shorter compared with saline group and remained at diestrus from week 4. In contrast, mice in the other three groups had normal diestrus, proestrus, estrus, and metestrus cycle stages throughout the experiment (Figure 1(b) and (c)).
Figure 1.
Effect of Rg1 on body weight and estrous cycles. d-gal (200 mg/kg/d) was injected s.c. daily for 42 days and Rg1 (20 mg/kg/d) was injected i.p. daily for 28 days from day 15 post first d-gal injection. Equal volume of saline was injected as control. (a) Body weight was monitored during the 42-day period. (b) Vaginal smears were obtained at 8:00–9:00 AM daily every seven days, and the estrous cycles were determined by H&E staining. Representative staining of diestrus (DE), proestrus (PE), estrus (E), and metestrus (ME) were shown. (c) The percentages of substages of diestrus (DE), proestrus (PE), estrus (E), and metestrus (ME) was counted. Data were presented as mean ± SD. *P < 0.05, compared with d-gal group. Rg1: ginsenoside Rg1
Rg1 increases serum levels of gonadotrophins, E2 and AMH
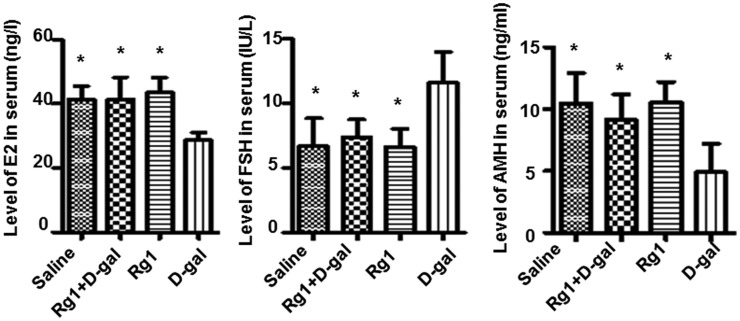
Next we investigated whether hormone levels were affected by Rg1 treatment in POF model. Mice in d-gal group had significantly higher FSH as well as lower E2 and AMH levels at 42 days after administration compared with mice in the other three groups (Figure 2). The Rg1 + d-gal group, conversely, had similar levels of these hormones compared with saline or Rg1 group (Figure 2). These results indicate that Rg1 has antiaging effect in ovary-aged mouse model.
Figure 2.
ELISA analysis of sex hormones. At the last day of drug administration (day 42), serum was collected for ELISA analysis of FSH, E2, and AMH. Data were presented as mean ± SD. *P < 0.05, compared with the d-gal group. AMH: Anti-Mullerian hormone; FSH: follicle-stimulating hormone
Rg1 affects ovarian follicular development
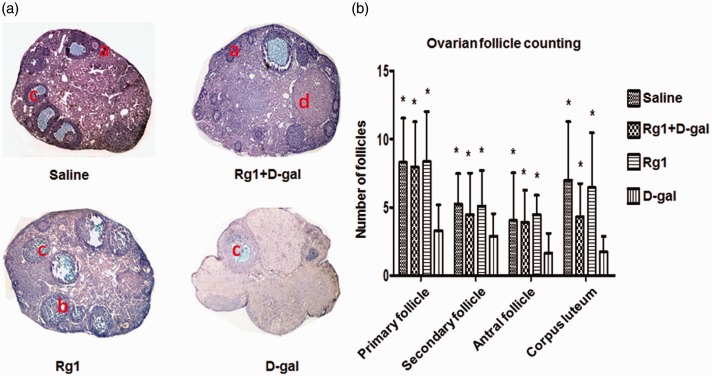
Results above prompted us to further examine the effect of Rg1 on the maturation of ovarian follicles. Using H&E staining, we found that ovaries of mice in the saline, Rg1+ d-gal, and Rg1 groups had follicles that were at different stages of maturation as well as corpus luteum (CL) at day 42 post d-gal administration. In contrast, d-gal group exhibited different architecture of the ovaries. Ovaries from d-gal group had reduced ovarian mass and no antral follicles, CL, or small follicles (Figure 3(a)). Results of ovarian follicle counting showed that mice from d-gal + Rg1 group had significantly higher numbers of growing follicles, antral follicles, and CL compared with the d-gal group, and there was no significant difference in the number of any kind of follicles between Rg1+ d-gal group, Rg1 group, and saline group (Figure 3(b)).
Figure 3.
Histopathological examination of ovarian follicles. At the last day of drug administration (day 42), ovaries were collected for H&E staining. (a) Representative staining of ovaries in each group (×100). a: primary follicle, b: secondary follicle, c: antral follicle, d: corpus luteum. (b) Number of follicles were counted and shown. Data were presented as mean ± SD. *P < 0.05, compared with the d-gal group
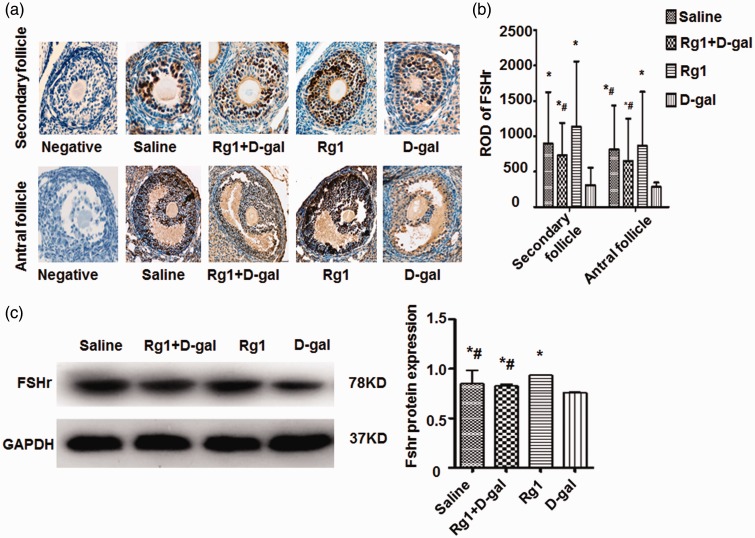
To determine the effect of Rg1 on cellular localization of FSHr within the ovaries, immunohistochemical analysis was performed. In the granule cells of mice ovaries, FSHr expression was detectable only in the secondary and antral follicles (Figure 4(a)). The intensity of immunohistochemical staining of FSHr in the follicles was assessed by relative optical density (ROD) values. The ROD for FSHr was significantly higher in the Rg1+ d-gal group compared to the d-gal group. In addition, the ROD was the highest in the Rg1 group compared with other groups (Figure 4(b)). Moreover, Western blotting analysis showed that the d-gal + Rg1 group had significantly higher levels of FSHr than the d-gal group, but not the saline group or the Rg1 group. The results demonstrate that Rg1 increases FSHr protein expression in the granule cells of secondary and antral follicles.
Figure 4.
Effect of Rg1 on FSHr protein expression. At the last day of drug administration (day 42), ovaries were collected for H&E staining. (a) FSHr expression was analyzed by immunohistochemical staining. Representative staining of FSHr expression in secondary follicles and antral follicles were shown. Original magnification, ×400. (b) The relative optical density (ROD) values of FSHr in the secondary follicles and antral follicles were calculated. (c) FSHr expression in ovaries was analyzed by Western blotting. Expression of GAPDH was used as internal control. Data were presented as mean ± SD. *P < 0.05, compared with the d-gal group. # P < 0.05, compared with the Rg1 group. FSHr: follicle-stimulating hormone receptor; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; Rg1: ginsenoside Rg1.
The antioxidative effect of Rg1 on ovaries
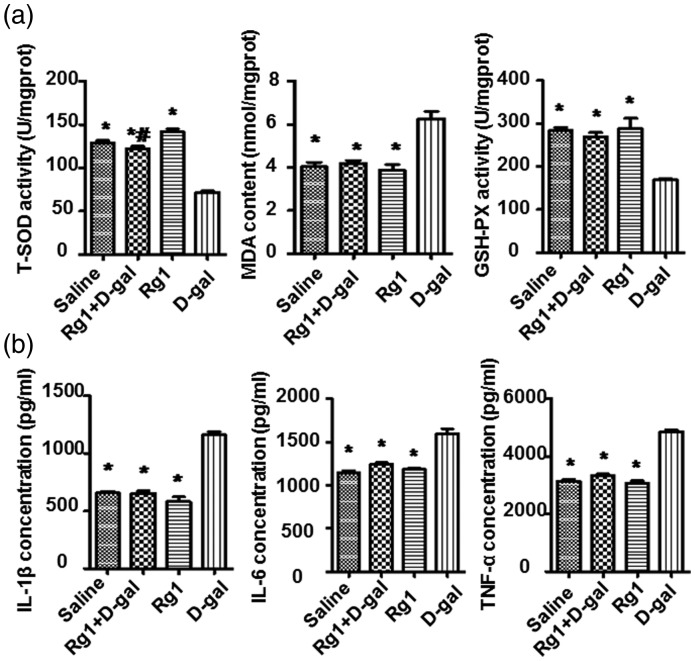
GSH-px is an important enzyme that removes free radical, and MDA is widely used as an oxidative stress biomarker. T-SOD and GSH-Px are critical antioxidative enzymes that remove the oxidative stress produced during aging. Thus, we evaluated the T-SOD activity, MDA contents, and GSH-Px activity in the ovaries. Compared with the saline group, activity of T-SOD and GSH-Px was decreased significantly and the MDA was up-regulated remarkably in the ovaries of d-gal group (P < 0.05). Nevertheless, in the d-gal+ Rg1 group, Rg1 treatment rescued the reduction of both T-SOD and GSH-Px activity levels significantly, and reduced the MDA level (Figure 5(a)). These data suggest that Rg1 has antioxidant effects against oxidative stress, possibly by improving the activity of endogenous antioxidative defense enzymes.
Figure 5.
Effect of Rg on oxidative stress, antioxidant activity, and pro-inflammatory cytokine expression. At the last day of drug administration (day 42), ovaries were collected and ovarian supernatant was acquired. (a) T-SOD activity, MDA contents, and GSH-Px activity in the ovaries were evaluated by chemical colorimetric analysis. (b) IL-1β, IL-6, and TNF-α expression in ovaries were determined by ELISA. Data were presented as mean ± SD. * P < 0.05, compared with the d-gal group; # P < 0.05, compared with the Rg1 group. GSH-Px: glutathione peroxidase from bovine erythrocyte; IL-1β: interleukin-1β; IL-6: interleukin-6; MDA: malondialdehyde; Rg1: ginsenoside Rg1; TNF-α: tumor necrosis factor-α; T-SOD: total superoxide dismutase
Rg1 decreases pro-inflammatory cytokine levels
Natural aging and POF are related to chronic inflammation in the ovary. Increased pro-inflammatory cytokines including IL-1β, IL-6, and TNF-α were found in inflammatory tissues and are associated with aged ovary. ELISA was performed to detect levels of IL-1β, IL-6, and TNF-α. The results showed that levels of the three cytokines were significantly reduced in the d-gal + Rg1 group compared with the d-gal group (Figure 5(b)), suggesting that Rg1 treatment alleviates inflammation in aged ovary induced by d-gal.
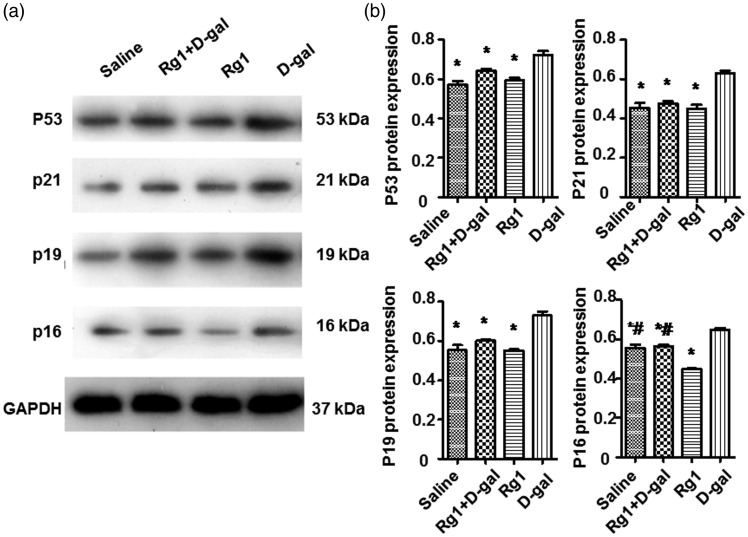
Rg1 affects expression of proteins related to aging
Expression levels of proteins in the p19–p53–p21–p16 pathway were analyzed by Western blotting. As shown in Figure 6, expression of p53, p19, p21, and p16 proteins in the d-gal + Rg1 group was significantly lower than that of the d-gal group. No significant difference between the saline group and the Rg1 group was observed. These results indicate that Rg1 can decrease the expression levels of senescence-associated proteins in the aging ovaries induced by d-gal.
Figure 6.
Effect of Rg1 on the expression of senescence-associated proteins. (a) p53, p21, p19, and p16 protein expression was determined by Western blotting analysis. Expression of GAPDH was used as internal control. (b) The integral optical density (IOD) values for p53, p21, p19, and p16 protein expression normalized to GAPDH were calculated. Data were presented as mean ± SD. * P < 0.05, compared with the d-gal group; # P < 0.05, compared with the Rg1 group. GAPDH: glyceraldehyde-3-phosphate dehydrogenase; Rg1: ginsenoside Rg1.
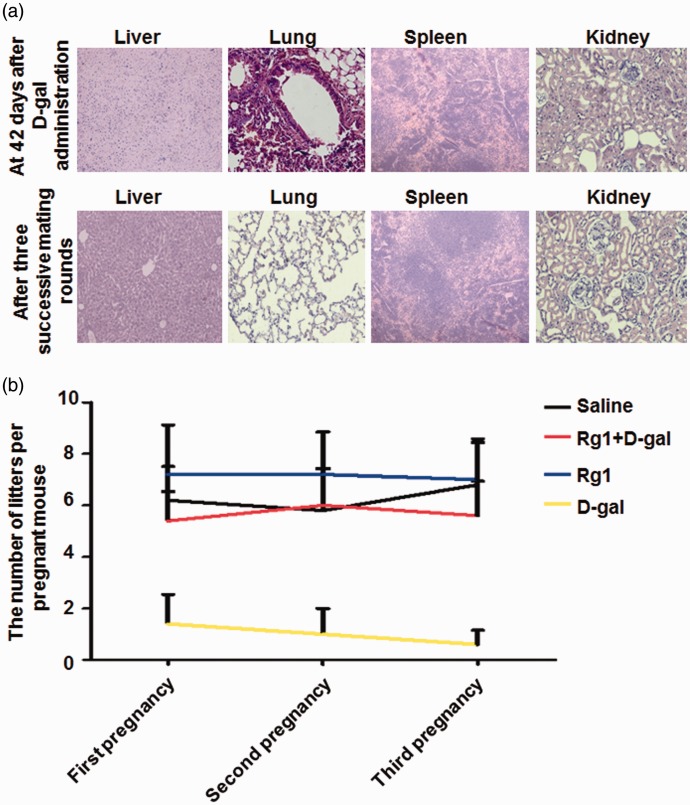
Effect of d-gal on organ morphology and fertility
The changes of major organ morphology affected by d-gal administration were evaluated by H&E staining. The livers, lungs, spleens, and kidneys were damaged at 42 days in the d-gal group (Figure 7(a)). The hepatocytes were swelling with disruption of membranes and contraction of the nucleus in livers of d-gal group. Destruction of the alveoli structure and inflammatory cell infiltration was observed in the lungs. In addition, the normal borders between the white pulp and red pulp were vanished and replaced by a diffused form of pulps in the spleens, and glomerular decrease, congestion, and atrophy were observed in the kidneys. However, after three successive mating rounds, organ inflammation was alleviated to various degrees (Figure 7(a)). Three successive mating rounds were performed to evaluate the reproductive outcomes. There were more pups per mouse in the sterilized female mice treated with Rg1 than d-gal group, and the number of pups was almost similar between saline, d-gal + Rg1, and Rg1 groups (Figure 7(b)). These results indicate that although organ inflammation was alleviated after three successive mating rounds, ovary damage induced by d-gal administration was serious and irreversible, which can be alleviated by Rg1 treatment.
Figure 7.
Effect of Rg1 on major organ morphology and fertility. (a) d-gal (200 mg/kg/d) was injected s.c. daily for 42 days and at the last day, livers, lungs, spleens, and kidneys were collected for H&E staining. After three successive mating rounds after d-gal administration, these organs were also collected for staining. Representative staining was shown. Magnification for the liver and spleen: ×100. Magnification for the lung and kidney: ×400. (b) Rg1 (20 mg/kg/d) was injected i.p. daily for 28 days from day 15 post first d-gal injection. Reproductive outcomes were assessed over three successive mating rounds in different groups. Data were presented as mean ± SD. * P < 0.05, compared with the d-gal group. Rg1: ginsenoside Rg19
Discussion
Premature or primary ovarian insufficiency is a range of disorders leading to ovarian malfunction. The clinical criteria for POF are secondary amenorrhea that lasts for at least four consecutive, with onset prior to the age of 40, low estradiol, and elevated FSH. POF could cause infertility, climacteric syndrome, osteoporosis, and senile dementia in females, which seriously affects women’s health.17 However, the pathogenesis for this disease is still unknown and treatments are inadequate.1
Our previous study has established a d-gal aging model in mice.18 In this study, we used this model to study the possible antiaging effect of Rg-1 on POF. Inflammation and edema were observed in livers, lungs, spleens, and kidneys 42 days after d-gal, which could be alleviated after three successive matings. Conversely, ovaries with d-gal treatment had severe damage, thus resulting in sterility. In addition, d-gal administration significantly reduced body weight, sex hormone secretion, ovarian follicle numbers, and FSHr protein expression level. These data demonstrate that d-gal administration mainly affects the function of ovary in female mice, inducing ovarian failure. However, after Rg1 treatment in d-gal-administrated mice, increased weight and normal estrous cycles were observed, demonstrating a potential ovarian functional recovery. In addition, Rg1 treatment delayed granular cell senescence in ovary via its anti-inflammation and antioxidant ability.
By challenging testing, about 75–96% of all females with galactosemia have dysfunctional gonads, mainly resulting in hypergonadotropic hypogonadism.2This is because that galactose wakens FSH bioactivity and exerts direct ovotoxic effects.19 Serum FSH is an ancillary indicator of reduced ovarian function.19 AMH, a hormone produced by primary granulosa cells, late preantral and preovulatory follicles,20 may serve as a predictor of ovarian function in classic galactosemia.21 Here we found that serum FSH significantly increased, but serum E2 and AMH were significantly reduced in the d-gal-treated mice, which were then restored after Rg1 treatment to the level similar as normal mice (saline group). These results show the ability of Rg1 to delay senescence in the d-gal mouse model.
Macroscopic ovarian appearance was reported to parallel the FSH levels.22 Chen et al.23 found that female rats had more than two-third decreases in the number of oocytes after exposure to high levels of galactose during gestation. Meyer et al.24 have demonstrated that ovulation and follicular maturation failures are associated with galactitol levels. Our results also showed that follicle numbers at all levels were reduced in the d-gal-treated mice, but similar to normal mice after Rg1 treatment in the d-gal + Rg1-treated mice.
Higher serum FSH values but failure of ovarian follicle development in POF patients suggested that the function of FSH receptors on the granule cells may be attenuated or impaired, resulting in insensitivity to the stimulation of FSH and inhibition of the follicle growth.25 Results in our study showed that expression of FSHr in ovaries was significantly increased in the Rg1+ d-gal group compared with that in the d-gal group. These results demonstrate that d-gal administration reduces the expression of FSHr and this reduction is reversed by Rg1 treatment.
Accumulation of galactitol in galactosemia tends to reduce free radical protection by impairing free radical scavenging and interfering with the glutathione reductase.2 Therefore, it may be practicable to administer antioxidant therapy to patients with galactosemia in order to minimize the ovarian damage. Our results showed that Rg1 treatment could protect the ovary against oxidative stress induced by d-gal through improving the activities of T-SOD and GSH-Px and reducing the activities of MDA. These results together indicate that Rg1 successfully attenuates oxidative damage induced by d-gal in the ovary, perhaps by eradicating free radicals and stimulating antioxidant enzymes. We should also note that the T-SOD activities were extraordinarily increased in the Rg1 single-treated mice, which is consistent with previous studies,26,27 and confirms the antioxidative effect of Rg1.
Some factors promote oocyte apoptosis, such as Fas ligands, androgens, pro-inflammatory cytokines, and gonadal GnRH-like proteins.19 In this study, the attenuated pro-inflammatory cytokines of IL-1β, IL-6, and TNF-α in ovaries after Rg1 treatment in Rg1+ d-gal group showed both the oocyte-promoting ability and the anti-inflammatory of Rg1.
The p16 pathway and the p53–p21–p19 pathway are signal pathways that plays important roles in aging. Recent study documented that p53-mediated intrinsic death pathway is key to the induction of follicular atresia,28 and the increased P53 protein can activate p21 at transcriptional level.29,30 In this study, the protein levels of p53, p19, p21, and p16 were increased in d-gal group but reduced by Rg1, indicating that Rg1 could down-regulate the expression of senescence proteins and delay ovary aging.
In clinical practice, HRT is currently used for the treatment of POF. However, HRT has great side effects and may induce coronary heart disease, endometrial cancer, and breast cancer.31 In contrast, Rg1 is demonstrated as a kind of natural estrogen that could significantly improve the symptoms of menopause with minimal side effects. Therefore, Rg1 may become a promising drug to replace HRT in the treatment of POF.
Taken together, Rg1 enhances the antiaging ability of ovary and fertility ability of POF mice through improving the anti-inflammatory and antioxidant capacities of ovary. Further studies are warranted to examine the mechanisms underlying the effect of Rg1 on apoptosis-related pathways in ovary.
Acknowledgements
This work is supported by National Natural Science Foundation of China Grants (No. 81671415) and a grant from Guizhou Provincial Administration of traditional Chinese Medicine and Science and Technology Research of traditional Chinese Medicine (No. GZYY-2016-078).
Authors’ contributions
Conceived and designed the experiments: LH and ZX. Performed the experiments: LH. Analyzed the data: LH, LL, TW. Contributed reagents/materials/analysis tools: LH, YW. Wrote the paper: LH, ZX. The final manuscript was read and approved by all the authors.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Bandyopadhyay S, Chakrabarti J, Banerjee S, Pal AK, Goswami SK, Chakravarty BN, Kabir SN. Galactose toxicity in the rat as a model for premature ovarian failure: an experimental approach readdressed. Hum Reprod 2003; 18: 2031–8. [DOI] [PubMed] [Google Scholar]
- 2.Liu G, Hale GE, Hughes CL. Galactose metabolism and ovarian toxicity. Reprod Toxicol 2000; 14: 377–84. [DOI] [PubMed] [Google Scholar]
- 3.Deady J. Clinical monograph: hormone replacement therapy. J Manag Care Pharm 2004; 10: 33–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaufman FR, Xu YK, Ng WG, Donnell GN. Correlation of ovarian function with galactose-1-phosphate uridyl transferase levels in galactosemia. J Pediatr 1988; 112: 754–6. [DOI] [PubMed] [Google Scholar]
- 5.Twigg S, Wallman L, McElduff A. The resistant ovary syndrome in a patient with galactosemia: a clue to the natural history of ovarian failure. J Clin Endocrinol Metab 1996; 81: 1329–31. [DOI] [PubMed] [Google Scholar]
- 6.Xu L, Liu LX, Chen WF, Xie JX, Huang WX. The protective effect of ginsenoside Rgl on dopaminergic neurons of substantia nigra in ovariectomized rat model of Parkinson’s disease. Chin J Appl Physiol 2008; 24: 1–5.. [PubMed] [Google Scholar]
- 7.Zhu J, Mu X, Zeng J, Xu C, Liu J, Zhang M, Li C, Chen J, Li T, Wang Y. Ginsenoside Rg1 prevents cognitive impairment and hippocampus senescence in a rat model of d-galactose induced aging. PLoS One 2014; 9: e101291–e101291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng Y, Shen LH, Zhang JT. Anti-amnestic and anti-aging effects of ginsenoside Rg1 and Rb1 and its mechanism of action. Acta Pharmacol Sin 2005; 26: 143–9. [DOI] [PubMed] [Google Scholar]
- 9.Chen X, Zhang J, Fang Y, Zhao C, Zhu Y. Ginsenoside Rg1 delays tertbutyl hydroperoxide-induced premature senescence in human WI-38 diploid fibroblast cells. J Gerontol A Biol Sci Med Sci 2008; 63: 253–64. [DOI] [PubMed] [Google Scholar]
- 10.Peng B, Wang CL, Feng L, Wang YP. The effects and the underlying mechanisms of Ginsenoside Rg1 to regulate neural stem cell senescence. Chinese J Cell Biol 2011; 33: 1116–22. [Google Scholar]
- 11.Tang ZY, Jiang W, Li XM, Huang QS, Zhao HH, Lai H. Protective effects of ginsenoside Rb1 on cyclophosphamide induced POF in rats. Chinese J Histochem Cytochem 2014; 23: 60–3. [Google Scholar]
- 12.Maeda K, Ohkura S, Tsukamura H. Physiology of reproduction. The laboratory rat, Switzerland: Academic, 2000. [Google Scholar]
- 13.White YA, Woods DC, Takai Y, Ishihara O, Seki H, Tilly JL. Oocyte formation by mitotically active germ cells purified from ovaries of reproductive-age women. Nat Med 2012; 18: 413–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Myers M, Britt KL, Wreford NG, Ebling FJ, Kerr JB. Methods for quantifying follicular numbers with in the mouse ovary. Reproduction 2004; 127: 569–80. [DOI] [PubMed] [Google Scholar]
- 15.Song D, Zhong Y, Qian C, Zou Q, Ou J, Shi Y, Gao L, Wang G, Liu Z, Li H, Ding H, Wu H, Wang F, Wang J, Li H. Human umbilical cord mesenchymal stem cells therapy in cyclophosphamide induced POF rat model. Biomed Res Int 2016; 2016: 2517514–2517514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu TE, Zhang L, Wang S, Chen C, Zheng J. Tripterygium glycosides induce premature ovarian failure in rats by promoting p53 phosphorylation and activating the serine/threonine kinase 11-p53-p21 signaling pathway. Exp Ther Med 2015; 10: 12–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fridovich-Keil JL, Gubbels CS, Spencer JB, Sanders RD, Land JA, Rubio-Gozalbo E. Ovarian function in girls and women with GALT-deficiency galactosemia. J Inherit Metab Dis 2011; 34: 357–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu XX, Wang HD, Ni WP. The experimental studies of Wu Bie granules to premature ovarian failure mice that caused by D-galactose. Chinese Arch Traditional Chinese Med 2011; 10: 2179–81. [Google Scholar]
- 19.Banerjee S, Chakraborty P, Saha P, Bandyopadhyay SA, Banerjee S, Kabir SN. Ovotoxic effects of galactose involve attenuation of follicle-stimulating bioactivity and upregulation of granulosa cell p53 expression. PLoS One 2012; 7: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.La Marca A, Volpe A. Anti-Mullerian hormone (AMH) in female reproduction: is measurement of circulating AMH a useful tool? Clin Endocrinol 2006; 64: 603–10. [DOI] [PubMed] [Google Scholar]
- 21.Sanders RD, Spencer JB, Epstein MP, Pollak SV, Vardhana PA, Lustbader JW, Fridovich-Keil JL. Biomarkers of ovarian function in girls and women with classic galactosemia. Fertil Steril 2009; 92: 344–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gibson JB. Gonadal function in galactosemics and in galactose-intoxicated animals. Eur J Pediatr 1995; 154: 14–20. [DOI] [PubMed] [Google Scholar]
- 23.Chen YT, Mattison DR, Feigenbaum L, Fukui H, Schulman JD. Reduction in oocyte number following prenatal exposure to a diet high in galactose. Science 1981; 214: 1145–7. [DOI] [PubMed] [Google Scholar]
- 24.Meyer WR, Doyle MB, Grifo JA, Lipetz KJ, Oates PJ, DeCherney AH, Diamond MP. Aldose reductase inhibition prevents galactose-induced ovarian dysfunction in the Sprague-Dawley rat. Am J Obstet Gynecol 1992; 167: 1837–43. [DOI] [PubMed] [Google Scholar]
- 25.Liu JY, Gromoll J, Cedars MI. Identification of allelic variants in the follicle-stimulating hormone receptor genes of females with or without hypergonadotropic amenorrhea. Fertil Steril 1998; 70: 326–9. [DOI] [PubMed] [Google Scholar]
- 26.Kim JH. Cardiovascular diseases and Panax ginseng: a review on molecular mechanisms and medical applications. J Ginseng Res 2012; 36: 16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen C, Mu XY, Zhou Y, Shun K, Geng S, Liu J, Wang JW, Chen J, Li TY, Wang YP. Ginsenoside Rg1 enhances the resistance of hematopoietic stem/progenitor cells to radiation induced aging in mice. Acta Pharmacol Sin 2014; 35: 143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hussein MR. Apoptosis in the ovary: molecular mechanisms. Hum Reprod Update 2005; 11: 162–78. [DOI] [PubMed] [Google Scholar]
- 29.el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell 1993; 75: 817–25. [DOI] [PubMed] [Google Scholar]
- 30.Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 Cdk interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell 1993; 75: 805–16. [DOI] [PubMed] [Google Scholar]
- 31.O’Donnell RL, Clement KM, Edmondson RJ. Hormone replacement therapy after treatment for a gynaecological malignancy. Curr Opin Obstet Gynecol 2016; 28: 32–41. [DOI] [PubMed] [Google Scholar]