Abstract
Several studies indicate that the immune system can be subjected to classical conditioning. Acute ethanol intoxication significantly modulates several pro-inflammatory cytokines (e.g. interleukins-1 and 6 [IL-1β and IL-6, respectively] and tumor necrosis factor alpha [TNFα])) in several brain areas, including amygdala (AMG), paraventricular nucleus (PVN), and hippocampus (HPC). It is unknown, however, whether cues associated with ethanol can elicit conditioned alterations in cytokine expression. The present study analyzed, in male Sprague-Dawley rats, whether ethanol-induced changes in the central cytokine response may be amenable to conditioning. In Experiments 1 and 2, the rats were given one or two pairings between a distinctive odor (conditional stimulus, CS) and the post-absorptive effects of a high (3.0 or 4.0 g/kg, Experiments 1 and 2, respectively) ethanol dose. Neither of these experiments revealed conditioning of IL-6, IL-1β, or TNFα, as measured via mRNA levels. Yet, re-exposure to the lemon-odor CS in Experiment 1 significantly increased C-Fos levels in the PVN. In Experiment 3, the rats were given four pairings between an odor CS and a moderate ethanol dose (2.0 g/kg), delivered intraperitoneally (i.p.) or intragastrically (i.g.). Re-exposure to the odor CS significantly increased IL-6 levels in HPC and AMG, an effect only evident in paired rats administered ethanol i.p. Overall, this study suggests that ethanol exposure can regulate the levels of IL-6 at HPC and AMG via classical conditioning mechanisms. These ethanol-induced, conditioned alterations in cytokine levels may ultimately affect the intake and motivational effects of ethanol.
Impact statement
This study examines, across three experiments, whether odor cues associated with ethanol exposure can condition changes in cytokine expression. The analysis of ethanol-induced conditioning of immune responses is a novel niche that can help understand the transition from social drinking to alcohol abuse and dependence. Ethanol-induced conditioning of the immune system could likely exacerbate neuroinflammation and drug-related toxicity, which in turn may facilitate further engagement in ethanol intake. The main new finding of the present study was that, after four pairings of ethanol’s unconditioned effects and a distinctive odor, the latter CS increased IL-6 levels in HPC and AMG. This suggests that ethanol’s effects upon IL-6 in HPC and AMG may come under conditioned control, particularly after repeated pairings between distinctive odor cues and ethanol’s effects. This article advances our knowledge of conditioned increases in cytokine responses, which should help understand the mechanisms underlying alcohol use, abuse, and relapse.
Keywords: Ethanol, conditioning, neuroimmune, cytokine, hippocampus, amygdala
Introduction
Seminal studies on cyclophosphamide-mediated Pavlovian conditioning of the immune response1 propelled interest in understanding the interactions between the brain and the immune system that involve adaptive learning responses. The usual protocol involves pairing a neutral stimulus (technically referred to as the conditioned stimulus or CS) with a drug (unconditional stimulus, US) likely to modulate immune functionality. Food or flavors are the most often used CSs,2 probably because the well-documented evolutionary preparedness of food to predict internal malaise facilitates taste-immune associations. Immunosuppressants are, in turn, the most used USs. The endpoint of these experiments generally involves measuring the ability of the CS, applied alone, to induce a reduced functionality of the immune system, likely an alteration in cytokine or chemokine signaling. The latter are proteins that regulate the functionality of leukocytes or lymphocytes.3
Exton et al.,4 for instance, gave rats pairings of cyclosporine A and a tastant. Re-exposure to the gustatory CS induced alterations in splenocyte proliferation and a significant inhibition of interleukin (IL)-2 release from those cells. Similar results were subsequently found in mice5 and in humans.6 These studies are important for the analysis of responses to cancer therapies, unwanted immune responses, and placebo reactions.7 The conditioned responses in the rat study were associated with significantly less rejection of a heart allograft, and were dependent on sympathetic innervations to the spleen.4
Immune responses are also called upon by exposure to ethanol (EtOH) and other drugs of abuse. Most studies have found modulation of several cytokines (e.g. IL-1 and 6 [IL-1β and IL-6, respectively] and tumor necrosis factor alpha [TNFα])),8 in the hypothalamus and other areas, after acute or chronic ethanol exposure, both in human and in animal models.9,10 For instance, Knapp and colleagues11 found that chronic alcohol exposure increased TNFα, IL-1β, and toll-like receptor 4 (TLR4) mRNAs in the cerebral cortex. Chronic ethanol also increased IL-1β and the chemokine (C-C motif) ligand 2 (CCL2) in the hypothalamus. A recent work12 reported greater levels of IL-10 in Sprague Dawley rats, measured 60 min after the administration of a relatively high ethanol dose (5 g/kg). A study from our lab, in turn, indicated significant increases in IL-6 and IκBα gene expression in the hippocampus (HPC), paraventricular nucleus of the hypothalamus (PVN), and amygdala (AMG) of Sprague-Dawley rats, 3 h after an acute intoxication with 4 g/kg ethanol, while TNFα and IL-1β were generally decreased.13 A subsequent study14 replicated these acute effects and found that they persisted when ethanol was given daily, for up to 4–6 days, but not when the drug was given every other day. Doremus-Fitzwater et al.15 also observed blunted IL-6 response to ethanol in the PVN after chronic (i.e. 10 week) intermittent ethanol intake.
The previous studies indicate that, in rats or mice, acute and chronic ethanol exposure alters the levels of cytokines involved in the regulation of immune responses. Similar increases in pro-inflammatory proteins have been observed in human alcoholics.16,17 Yet alterations in chemokine or cytokine levels can, in turn, affect the intake and motivational effects of ethanol. Mice deficient in chemokines or chemokine receptors drank less ethanol and exhibited reduced ethanol-induced aversion.18 Conversely, Long-Evans rats given chronic infusions of the chemokine monocyte chemoattractant protein-1 (MCP-1) exhibited heightened ethanol intake in a two-bottle intake test.19 Prenatal ethanol, a treatment known to facilitate ethanol-induced appetitive conditioning20 and reduce ethanol aversion21 also stimulated the chemokine CCL2 in the hypothalamus.22 Moreover, there is an extensive body of literature demonstrating cytokine and chemokine expression in the brain in fetal alcohol syndrome models.23–25
In conjunction, these studies indicate that ethanol activates specific aspects of the immune response and that this can alter the reinforcing effect of the drug and, ultimately, shape ethanol intake and preference. It is unknown, however, if ethanol can induce conditioning of the immune system, although this possibility seems likely, given its conspicuous unconditional effects upon pro-inflammatory cytokines10,12,13 and significant ability to transfer motivational information to tastes and exteroceptive cues.26 Ethanol-induced conditioning of the immune system could likely exacerbate neuroinflammation and drug-related toxicity, which in turn may facilitate further engagement in ethanol intake.27 The present set of experiments examined, in male Sprague Dawley rats, whether ethanol-induced changes in the central cytokine response may be subjected to conditioning. Experiments 1 and 2 employed single- and two-trial procedures, respectively, whereas Experiment 3 gave the rats multiple pairings between the conditional stimuli and ethanol, while varying the mode of ethanol administration.
In these experiments, the brain areas examined were the PVN, AMG, and HPC, as all are known to show acute cytokine response to ethanol administration.14 Moreover, the PVN is involved in the stress response28 and in neuroinflammatory activity29 after chronic ethanol exposure. The HPC and AMG were selected due to their roles in memory and aversive processes, respectively, as well as their roles in providing limbic input to the PVN. Finally, we utilized RT-PCR as a rapid, sensitive, and cost-effective tool to assess multiple cytokines in discrete brain samples, and because unconditioned effects of ethanol on neuroimmune genes are well-established.
Material and methods
General procedures
Subjects
One-hundred and fifty-two Sprague-Dawley (300–375 g) male rats, purchased from Harlan (Frederick, MD), were used (Experiment 1: 38 animals; Experiment 2: 50 animals; Experiment 3: 64 animals). The animals were pair-housed and given continuous ad libitum access to water and food, at one of the vivariums of the Psychology Department of Binghamton University (Binghamton, USA), which is an AAALAC-accredited facility. The colony was kept at 22 ± 1℃ with lights on and off at 8:00 and 20:00, respectively. The procedures were approved by the Institutional Animal Care and Use Committee of Binghamton University, and animals were treated in accordance with PHS policy.
Drug preparation and administration procedures
Ethanol (95%) was diluted fresh daily to make a 20% (v/v) stock solution. Following previous studies,15,20 tap water was used as vehicle for the intragastric (i.g.) intubation, whereas sterile physiological saline (TEKnova, Hollister, CA) was the vehicle for the intraperitoneal (i.p.) injection. In all cases, cage mates were assigned to the same experimental conditions.
Neural tissue collection and processing
Brains were harvested after rapid unanaesthetized decapitation. Brains were placed in ice-cold saline, sliced into 2-mm sections, and then placed in RNAlater (Qiagen, Valencia, CA). They were kept at 4℃ for 24 h and then moved to −20℃. Key structures (i.e. PVN, HPC, and AMG) were identified with a brain atlas30 and micro-dissected using a dissecting scope.
After the dissection, the tissue was placed in 2.0 ml Eppendorf tube containing 500 µL Trizol® RNA reagent and 5 mm stainless steel beads. The tissue was homogenized using a Qiagen Tissue Lyser II and the RNA was extracted using Qiagen’s RNeasy mini kit, according to manufacturer’s instructions (Qiagen). RNA was separated from the supernatant through chloroform extraction (12,000 g for 15 min at 4℃). An equal volume of 70% ethanol was added to the collected RNA, which was purified through RNeasy mini columns. Columns were washed and eluted with 30 µL of RNase-free water (65℃). Total RNA yield and purity were determined using NanoDrop (Thermo Scientific). RNA was stored at −80℃ prior to cDNA synthesis.13,31
Real-time polymerase chain reaction (RT-PCR)
The synthesis of cDNA was performed on 0.1–1.0 µg of normalized total RNA from each sample using the QuantiTect ® Reverse Transcription Kit (Cat No. 205313, Qiagen, Valencia, CA), which included a DNase treatment step. All cDNA was stored at −20℃ until time of assay. Probed cDNA amplification was performed in a 10-μl reaction consisting of 5 μl IQ SYBR Green Supermix (Bio-Rad), 0.5 μl primer (final concentration 250 nM), 0.5 μl cDNA template, and 4 μl RNase-free water run in triplicate on a 384-well plate (Bio-Rad) and captured in real-time using the CFX384 Real-Time PCR Detection System (Bio-Rad, #185-5485), similar to Blandino, Barnum.32 Primers were designed using NCBI primer blast (see Table 1 for primer sequences and accession numbers). Across all experiments, the housekeeper gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was initially analyzed as a separate target to ensure its stability across experimental conditions. The corresponding statistical analyses indicated the absence of between-group differences in the levels of this transcript. Once the stability of GAPDH expression was verified in this manner, all data were adjusted relative to GAPDH using the 2–ΔΔCT transformation.33 All gene expression data were then adjusted relative to its ultimate control group (i.e. the unmanipulated home cage control [HCC] group [Experiments 1 and 2] or the unpaired-vehicle group matching each mode of administration [Experiment 3]) to serve as the baseline level of gene expression.
Table 1.
Primer sequences and accession numbers employed in the RT-PCR assays for the assessment of gene expression.
| Primer | Accession numbers | Oligo | Sequence |
|---|---|---|---|
| GAPDH | NM_017008 | Forward | 5′-ATG ACT CTA CCC ACG GCA AG-3′ |
| Reverse | 5′-AGC ATC ACC CCA TTT GAT GT-3′ | ||
| C-Fos | NM_022197.2 | Forward | 5′-CCA AGC GGA GAC AGA TCA AC-3′ |
| Reverse | 5′-AAG TCC AGG GAG GT CACA GA-3′ | ||
| IL-1 | NM_031512 | Forward | 5′-AGG ACC CAA GC ACCT TCT TT-3′ |
| Reverse | 5′-AGA CAG CAC GAG GCA TTT TT-3′ | ||
| IL-6 | NM_012589 | Forward | 5′-TAG TCC TTC CTA CCC CAA CTT CC-3′ |
| Reverse | 5′-TTG GTC CTT AGC CAC TCC TTC-3′ | ||
| TNF-α | NM_012675 | Forward | 5′-GGG GCC ACC ACG CTC TTC TG -3′ |
| Reverse | 5′-CGA CGT GGG CTA CGG GT TG-3′ |
GAPDH: glyceraldehyde 3-phosphate dehydrogenase; IL-1: interleukin-1 beta; IL-6: interleukin-6; TNF-α: tumor necrosis factor alpha.
Specific procedures
Specific procedures for Experiment 1
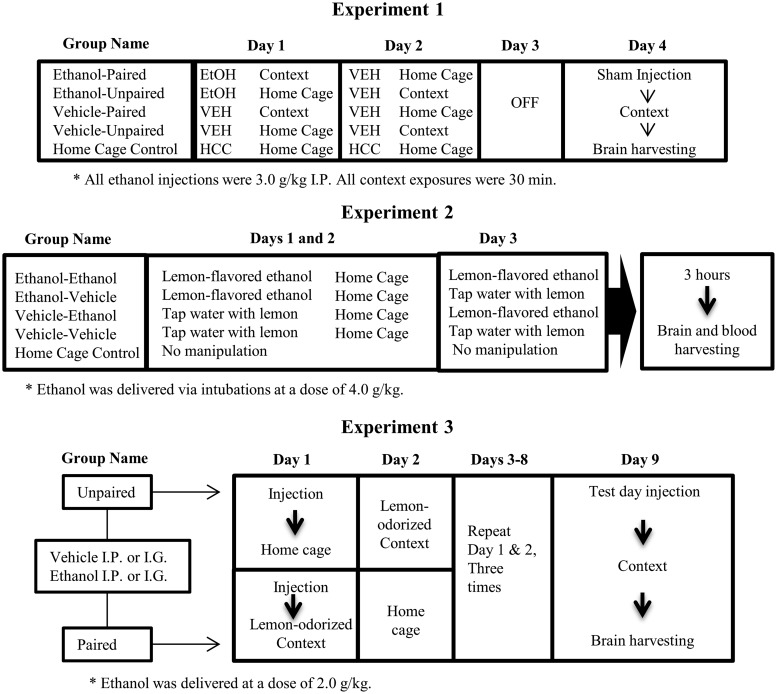
On experimental day 1, the rats were injected with ethanol (3.0 g/kg, i.p.) or vehicle. Immediately after injection, they were returned to home cage or placed into a novel context (unpaired and paired group, respectively). The novel context was a standard-sized Plexiglas holding cage devoid of bedding material, featuring a cotton ball soaked in alcohol-free lemon oil (300 μl per ball) suspended in a tea infuser within the chamber. The cages of the paired rats were located in a separate, sound-attenuating, chamber for the duration of the conditioning trial in a room adjacent to the home colony. The unpaired rats were kept in the home cage, which was located in the home colony. Rats remained in the context for 30 min and were then returned to their home cages. Rats in the HCC group remained unmanipulated in the home cage. On day 2, all animals except the HCC (home cage control) group received an injection of saline and were placed either back in home cage (paired group) or into the context odorized with lemon (unpaired group) for 30 min. During day 3, the animals were not manipulated. On day 4 (test), all animals were given a sham injection (i.e. the needle was inserted into the peritoneum without release of fluid) and immediately placed in context for 30 min, after which brains were immediately harvested. mRNA levels of C-Fos, IL-6, IL-1β, and TNFα were assessed using RT-PCR, in the PVN, AMG, and HPC. These experimental procedures are described in Figure 1 (upper section), via a representative diagram.
Figure 1.
Methods for the analysis of acute and ethanol-mediated, conditioned immune responses, in Experiments 1, 2, and 3 (upper, middle, and lower sections, respectively). On day 1 of Experiment 1, the rats were injected intraperitoneally (IP) with ethanol or vehicle and then were returned to home cage or placed into a novel context (unpaired and paired group, respectively). On day 2, the animals received an injection of saline and were placed either back in home cage (paired group) or into the context odorized with lemon (unpaired group). On day 4 (test), all animals were given a sham injection before being placed in context for 30 min, after which brains were immediately harvested. In Experiment 2, the rats were given, during days 1 and 2, intragastric (i.g.) administration of lemon-flavored ethanol or vehicle (tap water flavored with a non-alcoholic lemon extract). On day 3 (test), the rats were given intubations of lemon-flavored water or ethanol (flavored with lemon). All rats were sacrificed 3 h after the final exposure, and brain tissue was harvested and blood samples were collected. In Experiment 3, the animals were given four paired or unpaired exposures to lemon odor and the post-absorptive effects of ethanol (2.0 g/kg; i.p. or i.g.) or vehicle. During test day, all animals were given 0.5 g/kg ethanol followed by a 30 min exposure to lemon odor, after which brains were immediately harvested. Experiments 1 and 2 also included unmanipulated, home cage controls (HCC).
Specific procedures for Experiment 2
Several changes were introduced in Experiment 2, relative to Experiment 1, to favor the expression of ethanol-induced conditioned release of cytokines. Length of training, CS exposure at test, and ethanol dose were increased, and ethanol was delivered via intubations (see Figure 1, middle section).
The use of ethanol intubation was meant to increase the temporal and spatial contiguity between the CS (lemon odor) and the US (the pharmacological, post-absorptive effects of ethanol). We took advantage of the fact that a minor, yet significant (i.e. circa 10%) amount of intubated ethanol is eliminated, without any metabolic breakdown, via panting, salivation, and respiration.34 This results in perception of ethanol’s odor during the intoxication. These odor cues, and those probably resulting from hematogenic olfaction, can be employed as a CS in classical conditioning preparations.35,36 Molina and Chotro36 found conditioned aversion to the odor of ethanol in rats that had been intubated 24-h earlier with a high dose of the drug (3.0 g/kg). Based on these data, the expectation was that the lemon present in the smell and taste would become a reliable signal of the ongoing, pharmacological effects of the drug.
In experimental days 1 and 2, the rats were given i.g. administration of lemon-flavored ethanol (4.0 g/kg, i.g) or vehicle (tap water flavored with a non-alcoholic lemon extract [1%]). The expectation was that lemon (CS) would be paired with the unconditional effects of ethanol on the neuroimmune system (US). The animals were then immediately placed back into home cage. On day 3 (test), the rats were given intubations of lemon-flavored water or 4.0 g/kg ethanol (flavored with lemon). Four groups were thus formed, as a function of treatment during conditioning and testing: VEH-VEH, VEH-ETOH, ETOH-VEH, and ETOH-ETOH. The VEH-VEH was the baseline control group; whereas the VEH-ETOH was an acute exposure group, meant to provide a measure of the acute, unconditional effects of ethanol upon the immune system. An additional HCC group received no manipulation and remained in the home cage for the duration of the experiment.
All rats were sacrificed 3 h after the final exposure, and brain tissue was harvested and blood samples were collected. mRNA levels of C-Fos, IL-6, IL-1β, or TNFα were determined in the PVN, AMG, and HPC, via RT-PCR. We expected conditioned cytokine expression in the ETOH-VEH, when compared to HCC or VEH-VEH groups. It was possible, however, that exposure to the lemon CS at test also altered ethanol-induced cytokine expression in the ETOH-ETOH group. The latter condition also controlled for the possibility of a generalization decrement between conditioning and testing (i.e. ETOH-VEH animals were exposed to ethanol odor + lemon during conditioning, yet only to the lemon odor during testing).
Trunk blood was collected into EDTA-coated Vacutainers. Plasma was separated through refrigerated centrifugation (15 min at 3220 g), then aliquoted and stored at −20℃ until time of corticosterone (CORT) and blood ethanol concentrations (BEC) assays. It should be noted that the samples were collected 3 h post-ethanol administration, whereas peak BEC measurements are usually found within 20 to 60 min post-administration.37 The measurement reported, therefore, is not a peak BEC. BECs were determined in 5-μl aliquots using an Analox AM-1 alcohol analyzer (Analox Instruments, Lunenburg, MA). The machine was calibrated every 15 samples using a 100 mg% industry standard, with BECs recorded in milligram per deciliter (mg%). Quantitative determination of plasma CORT was assessed with commercially available enzyme-immunoassay (EIA) according to manufacturer’s (Assay Designs; Ann Arbor, MI) instructions, with the exception that samples were heat-inactivated to denature corticosteroid binding globulin (CBG) by immersion in 75℃ water for 60 min.31 The CORT assay had a sensitivity of 27.0 pg/ml and inter-assay variability of 7.8%.
Specific procedures for Experiment 3
Experiment 3 used an extended (i.e. 4 every-other-day pairings) version of the odor conditioning paradigm of Experiment 1, while varying the mode of ethanol administration (i.e. i.p. or i.g.). Previous studies indicate that the conditioning effects of ethanol are significantly modulated by mode of administration.38 Also, the training dose was lowered to 2.0 g/kg. Figure 1 (lower section) describes the experimental procedures via a representative diagram.
On day 1 of the Experiment, the rats were injected with ethanol (2.0 g/kg, i.p. or i.g.) or vehicle. Paired rats were immediately placed in the conditioning chamber, whereas unpaired rats were placed in the home cage. The conditioning chamber was the novel, lemon-odorized context that had been used in Experiment 1. Paired animals were returned to the home cage 3 h after the ethanol administration. In day 2, unpaired animals were given 3 h of exposure to lemon in the conditioning chamber, whereas paired counterparts remained in the home cage. The procedures of days 1 and 2 were repeated 3 times, across days 3 to 8, to complete four paired or unpaired presentations of the lemon odor CS.
On day 9 (test day, 48 h after the last ethanol administration), all animals were given a priming dose of 0.5 g/kg ethanol (i.p. or i.g., depending on the mode of ethanol administration used during conditioning). The aim was to recreate the interoceptive context of the conditioning trial. Ethanol induces a distinctive interoceptive context39 and the more similar conditioning and testing contexts are, the more likely the possibility of emission of conditioned responses. All rats were then placed into the lemon-odorized chambers for 3 h and sacrificed thereafter. Brains were obtained and mRNA levels of C-Fos, IL-6, IL-1β, and TNFα were determined in the PVN, AMG, and HPC.
Experimental designs and data analysis
Experiments 1 and 2 employed a 2 × 2 factorial design. Each had an additional, non-manipulated, HCC group, which remained in the home cage for the duration of the experiment. The first factor of Experiment 1 refers to the treatment given to the animals (0.0 [saline vehicle] or 3.0 g/kg ethanol, i.p.), whereas the second indicates if animals were exposed to a context featuring a distinctive lemon odor immediately following ethanol administration (paired groups) or 24 h after ethanol administration (unpaired groups). In Experiment 2, the animals were given ethanol or vehicle during conditioning (treatment at conditioning) and ethanol or vehicle at test (treatment at test). Each group was composed of 8 or 10 subjects (Experiments 1 and 2, respectively).
Experiment 3 employed a 2 × 2 × 2 factorial design. The animals were given ethanol (2.0 g/kg) or vehicle (i.p. injections or i.g. intubations) paired or unpaired with a 3-h exposure to a distinctive context (CS). Each of the four groups was composed of eight subjects.
Gene expression data were adjusted to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as housekeeper, and expressed as percent change from the baseline control group (i.e. the HCC group [Experiments 1 and 2] or the unpaired-vehicle group corresponding to each mode of administration [Experiment 3]). ANOVAs indicated that, across experiments, the levels of the housekeeping gene were not affected by Drug treatment or Learning condition. Data were lost in each of the datasets, due to errors during the processing of the samples. These data were not replaced. Despite this, under no circumstance did a group have less than 7 data points for a given measurement.
In Experiment 1 and 2, gene expression (i.e. mRNA levels of C-Fos, IL-6, IL-1β, or TNFα) was separately analyzed in each brain structure via a two-way ANOVA with an isolated control condition. The between-group factors in Experiment 1 were Drug treatment (ethanol or vehicle) and Learning condition (paired or unpaired), whereas Treatment during conditioning (ethanol or vehicle) and testing (ethanol or vehicle) served as between-group factors in Experiment 2. The HCC was included in the ANOVA model as an isolated (i.e. “hanging”) control condition in both experiments. This allows this group to be taken into account for the calculation of the error sums of squares, thus improving the fitness and predictive value of the statistical model. Similar ANOVAs were used to analyze BECs and CORT levels in Experiment 2. A three-way ANOVA (between factors: Drug treatment, Learning condition, and Mode of administration) was employed in Experiment 3.
Significant main effects or interactions were subsequently analyzed using follow-up ANOVAs and Fisher LSD’s post-hoc test. Planned comparisons involving the HCC group were also conducted when justified by a priori hypothesis and after finding significant main effects or interactions in the ANOVAs. The α level was set at ≤0.05. The partial eta square (η2p) was used to estimate effect size.
Results
Experiment 1
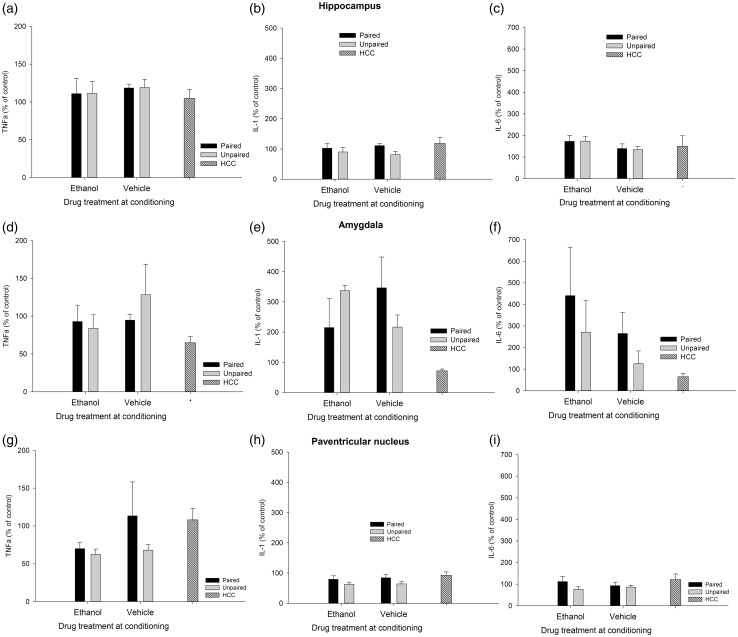
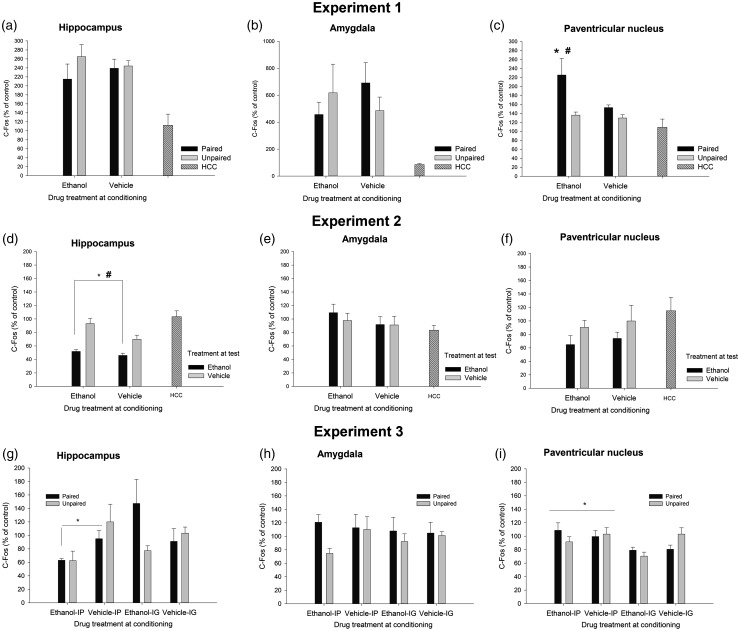
The factorial ANOVAs indicated that levels of C-Fos, IL-6, IL-1β, and TNFα were not affected by Drug or Learning condition in the HPC or AMG. Similarly, IL-1β, IL-6, and TNFα levels were not affected by these factors or their interaction in the PVN. Yet, the ANOVA for C-Fos induction in the PVN revealed significant main effects of Drug and Learning condition (F(1.29) = 6.15, p ≤ .05, η2p = 0.18; F(1.29) = 12.52, p ≤ .001, η2p = 0.30; respectively). The Drug × Learning condition also achieved significance (F(1.29) = 4.32, p ≤ .05, η2p = 0.13). The post-hoc tests revealed significantly greater C-Fos expression in the ethanol-paired group than in any of the remaining groups. A planned comparison indicated that level of C-Fos expression was significantly greater in the ethanol-paired group than in the unmanipulated HCC. IL-6, IL-1β, and TNFα are depicted in Figure 2, whereas Figure 3 (panels A, B, and C) presents mean ± SEM for C-Fos scores.
Figure 2.
Cytokine expression (mRNA levels of tumor necrosis factor alpha [TNFα], interleukin-1β [IL-1], and interleukin-6 [IL-6], obtained via RT-PCR) in the hippocampus, amygdala, and paraventricular nucleus of the hypothalamus of male Sprague Dawley rats given a single paired or unpaired exposure to lemon odor and the post-absorptive effects of ethanol (3.0 g/kg, delivered intraperitoneally) or vehicle. During test day, all animals were given a sham injection followed by a 30 min exposure to lemon odor, after which brains were immediately harvested. Rats in the home cage condition (HCC) remained unmanipulated in home cage throughout these procedures. All data were normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and expressed relative to the HCC control group. The ANOVAs indicated, across structures, that cytokine expression was not affected by Drug or Learning treatment. Results are expressed as mean, plus or minus SEM
Figure 3.
C-Fos expression (% from baseline control of each experiment) as a function of conditioning and testing treatments in the hippocampus, amygdala, and paraventricular nucleus of the hypothalamus of Sprague Dawley rats, in Experiments 1 to 3. The baseline control of Experiments 1 and 2 was an unmanipulated, home cage control (HCC) group. The baseline control of Experiment 3 was the unpaired-vehicle group corresponding to each mode of administration (i.e. intragastric [i.p.] intubation or intraperitoneal injection [i.p.]). The asterisk in panel C indicates significantly greater C-Fos expression in the PVN, in the ethanol-paired group compared to any of the remaining paired [i.g.] or unpaired groups. The pound sign indicates a significant difference in this structure between the ethanol-paired group and the unmanipulated HCC group. The asterisk and the pound sign in panel D indicate that animals given ethanol at test exhibited significantly less C-Fos in the HPC than those given vehicle at test or than HCC controls, respectively. The asterisk in panel G indicates that animals given chronic ethanol injections exhibited significantly less C-Fos levels in the HPC than those given vehicle injections. The asterisk in panel I indicates that C-Fos activity in the PVN was greater after i.p. than after i.g. administration. Results are expressed as mean ± SEM
Experiment 2
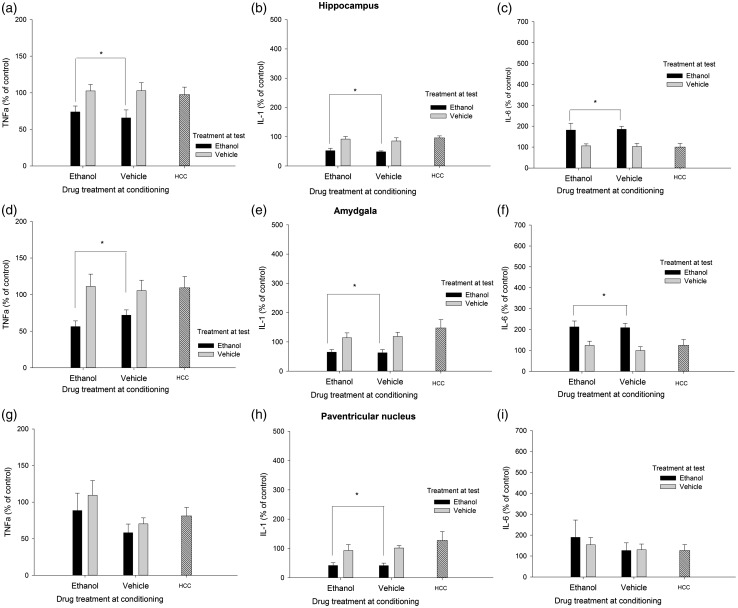
Rats administered vehicle or ethanol during conditioning exhibited similar BECs at test (171.75 ± 19.92 and 156.10 ± 14.66, respectively). CORT levels were significantly higher in rats given ethanol at test than in rats given vehicle (F(1.44) = 16.65, p ≤ .001, η2p = 0.27) or than in HCC controls. Mean and SEM CORT were as follows: 3.1 ± 1.1 (VEH-VEH), 13.8 ± 3.1 (VEH-ETOH), 3.8 ± 0.9 (ETOH-VEH), 11.0 ± 19.9 (ETOH-ETOH), and 5.1 ± 1.5 (HCC controls).
Figure 4 depicts the levels of TNFα, IL-1β, and IL-6 in HPC, AMG, and PVN, whereas C-Fos data are in Figure 3. The ANOVAs for C-Fos, TNFα, and IL-1β levels in the HPC yielded a significant main effect of treatment at test (F(1.41) = 25.10, p ≤ .001, η2p = 0.38; F(1.42) = 11.24, p ≤ .005, η2p = 0.21; F(1.41) = 18.79, p ≤ .001, η2p = 0.31). Animals given ethanol at test exhibited significantly less of these transcripts than those given vehicle or, as revealed by planned comparisons, than HCC controls. IL-6 levels, in turn, were increased by ethanol treatment at test (F(1.41) = 15.16, p ≤ .001, η2p = 0.27). Animals given ethanol at test also exhibited significantly greater IL-6 in the HPC than HCC rats.
Figure 4.
Cytokine expression (mRNA levels of tumor necrosis factor alpha [TNFα], Interleukin-1β [IL-1], and interleukin-6 [IL-6], obtained via RT-PCR) in the hippocampus, amygdala, and paraventricular nucleus of the hypothalamus of male Sprague Dawley rats that, during conditioning, were given two administrations of lemon-flavored ethanol (4.0 g/kg, intragastrically) or vehicle (tap water flavored with a non-alcoholic lemon extract [1%]). At test, the rats were sacrificed 3 h after an intubation with lemon-flavored water or 4.0 g/kg ethanol (flavored with lemon). An additional home cage control (HCC) group received no manipulation and remained in home cage for the duration of the experiment. All data are normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and expressed relative to the HCC control group . The asterisks in Panels A, B, D, E, and H indicate that ethanol treatment at test significantly reduced TNFα or IL-1β levels, when compared to the levels of these transcript found in vehicle-treated or HCC animals (p < 0.05). The asterisks in Panels C and F indicate that ethanol treatment at test significantly enhanced IL-6 levels, when compared to the levels observed in vehicle-treated or HCC animals (p < 0.05). The error bar indicates SEM
C-Fos levels in the AMG were not affected by either treatment, whereas ethanol administration at test significantly reduced TNFα (F(1.40) = 11.43, p ≤ .005, η2p = 0.22) and IL-1β (F(1.40) = 7.13, p ≤ .05, η2p = 0.15) levels, yet significantly enhanced IL-6 mRNA in this structure (F(1.40) = 15.92, p ≤ .001, η2p = 0.28). Planned comparisons indicated that the levels of these transcripts in animals injected with ethanol at test were significantly different than those of HCC rats.
Levels of C-Fos, IL-6, and TNFα in the PVN were not affected by either treatment, whereas subjects given ethanol at test exhibited a significant reduction of IL-1β levels in the PVN (F(1.36) = 8.45, p ≤ .01, η2p = 0.19).
Experiment 3
Mean and SEM across conditions, for each transcript measured in HPC, AMG, and PVN, can be found in Figure 5. A detailed description of the statistical analyses conducted and the significant differences found follows.
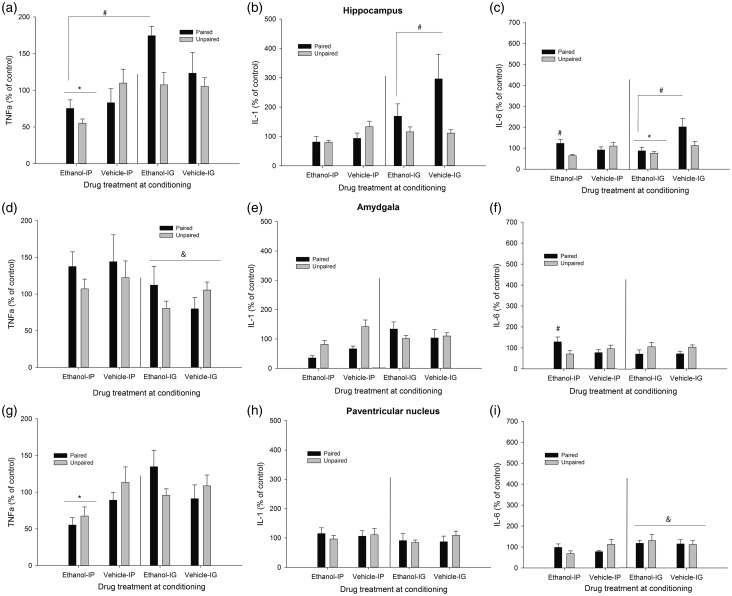
Figure 5.
Cytokine expression (mRNA levels of tumor necrosis factor alpha [TNFα], Interleukin-1β [IL-1], and interleukin-6 [IL-6], obtained via RT-PCR) in the hippocampus, amygdala, and paraventricular nucleus of the hypothalamus of male Sprague Dawley rats. During the conditioning, the rats were given four pairings between lemon odor and the post-absorptive effects of ethanol (2.0 g/kg, either intraperitoneally [i.p.] or intragastrically [i.g.]) or vehicle. Unpaired controls were given unrelated exposure between these stimuli. On test day, all animals were given 0.5 g/kg ethanol (i.p. or i.g., depending on the mode of ethanol administration used during conditioning) before a 3-h exposure to the lemon odor. Brain samples were taken thereafter. All data are normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and expressed relative to the unpaired-vehicle group corresponding to each mode of administration (i.e. i.p. or i.g). The pound signs in Panels C and F indicate that paired IP animals exhibited significantly greater mRNA levels of IL-6 at hippocampus and amygdala than unpaired counterparts (p < 0.05). The pound sign in Panel A indicates that, independent of the mode of administration, ethanol-treated paired rats exhibited greater TNFα in test at hippocampus than ethanol-treated unpaired rats. The pound sign in Panel B indicates that paired i.g. rats, ethanol- and vehicle-treated, exhibited significantly greater IL-1β levels in the hippocampus than the rest of the groups. The asterisk sign in Panel A indicates that TNFα levels in hippocampus were lower in ethanol-treated animals given the drug i.p. than in those given the drug. i.g. The ampersand sign in Panel D indicates that TNFα levels in amydgala were lower in rats, paired or unpaired and given ethanol or vehicle, that were given i.g. administrations. The ampersand sign in Panel I indicates greater IL-6 mRNA in those rats, paired or unpaired and treated with ethanol or vehicle, given i.g. intubations. The asterisk in panel G indicates that intraperitoneal, but not intragastric, ethanol treatment significantly decreased TNFα levels in the paraventricular nucleus. The error bar indicates SEM
HPC
Chronic treatment with ethanol significantly reduced C-Fos levels in the HPC (see Figure 3, panel G), but only in those given i.p. administration (significant Drug × Mode of administration interaction: F(1.49) = 4.33, p ≤ .005, η2p = 0.08). Similarly, TNFα levels in the HPC were lower in ethanol-treated animals given the drug i.p. than in those given the drug. i.g. (significant main effect of Mode of administration, and significant Drug × Mode of administration interaction: F(1.52) = 15.04, p ≤ .001, η2p = 0.22 and F(1.52) = 5.75, p ≤ .05, η2p = 0.10, respectively), although the post-hoc tests indicated that none of these ethanol-treated groups differed from their corresponding vehicle-treated controls. The analysis of TNFα levels in the HPC revealed a significant Conditioning × Drug interaction (F(1.52) = 3.97, p ≤ .05, η2p = 0.07). The post-hoc tests revealed that, independent of the mode of administration, ethanol-treated paired rats exhibited greater TNFα at test than control (i.e. unpaired) rats. Conditioning did not affect TNFα levels in vehicle-treated animals.
The ANOVA for IL-1β levels indicated a significant main effect of mode of administration and a significant mode of administration × conditioning interaction (F(1.49) = 7.78, p ≤ .01, η2p = 0.14 and F(1.49) = 6.47, p ≤ .05, η2p = 0.12, respectively). The post-hoc tests indicated that rats exposed to the odorized context after i.g. intubations (i.e. paired i.g. rats) exhibited significantly greater IL-1β levels than the rest of the groups. This apparent conditioned response was, however, similar in ethanol- and vehicle-administered animals.
The ANOVA for IL-6 levels in the HPC revealed significant main effects of Drug and Conditioning (F(1.49) = 7.78, p ≤ .01, η2p = 0.14 and F(1.49) = 6.47, p ≤ .05, η2p = 0.12, respectively), and a significant Drug × Mode of administration conditioning (F(1.49) = 7.78, p ≤ .01, η2p = 0.14). The Conditioning × Drug × Mode of administration interaction (F(1.49) = 7.78, p ≤ .01, η2p = 0.14 and F(1.49) = 6.47, p ≤ .05, η2p = 0.12, respectively) also achieved significance. Follow-up Drug × Conditioning ANOVAs, one for each mode of administration, were conducted to dissect the three-way interaction. The follow-up ANOVA for i.g.-administered animals revealed a significant main effect of drug administration (F(1.27) = 9.03, p ≤ .01, η2p = 0.25) and a significant main effect of Conditioning (F(1.27) = 4.07, p ≤ .05, η2p = 0.13). Chronic ethanol significantly decreased IL-6 levels to a similar extent in paired or unpaired rats. Paired rats, in turn, exhibited higher IL-6 levels than unpaired counterparts, yet this effect was statistically similar in ethanol or vehicle rats.
The follow-up ANOVA for i.p.-injected animals indicated a significant Drug treatment × Conditioning interaction (F(1.25) = 6.30, p ≤ .05, η2p = 0.20). As depicted in Figure 4 and confirmed by the post-hoc tests, levels of IL-6 mRNA were similar in vehicle-injected paired and unpaired animals. Conditioning, on the other hand, exerted a significant effect among ethanol-injected animals: ethanol-paired rats exhibited significantly higher levels of IL-6 than unpaired controls given unrelated exposure to ethanol and lemon odor.
Amygdala
The corresponding ANOVAs indicated that C-Fos or IL-1β activity in the AMG was not affected by either factor, whereas TNFα levels were significantly reduced in the animals, paired or unpaired and treated with ethanol or vehicle, given i.g. intubations (significant main effect of Mode of administration: F(1.54) = 4.89, p ≤ .05, η2p = 0.08). The ANOVA for IL-6 levels indicated a significant Mode of administration × Conditioning interaction (F(1.52) = 4.67, p ≤ .05, η2p = 0.08), yet the post-hoc tests failed to reveal significant differences. Inspection of the graph suggested greater expression of IL-6 in paired than in unpaired rats given i.p. ethanol, but not in paired vs. unpaired rats given i.p. vehicle. Guided by our a priori hypotheses, we conducted planned comparisons between these groups, which confirmed these impressions (F(1.52) = 5.61, p ≤ .05 and F(1.52) = 0.54, p ≤ .40, respectively).
PVN
C-Fos activity in the PVN (Figure 3, panel I) was greater after i.p. than after i.g. administration (significant main effect of Mode of administration: F(1.53) = 8.88, p ≤ .005, η2p = 0.14), yet similar among paired and unpaired rats treated with ethanol or vehicle. The ANOVA and corresponding post-hoc tests indicated that i.p. but not i.g. ethanol treatment significantly decreased TNFα levels in the PVN (significant main effect of Mode of administration and significant Mode of administration × Drug treatment interaction: F(1.51) = 5.78, p ≤ .05, η2p = 0.10 and F(1.51) = 6.38, p ≤ .05, η2p = 0.11, respectively). The ANOVA for IL-1β levels in the PVN revealed no significant main effects nor significant interactions, whereas IL-6 levels were greater after i.g. than after i.p. administration, yet this main effect of Mode of administration (F(1.52) = 4.60, p ≤ .05, η2p = 0.08) did not significantly interact with the remaining factors.
Discussion
We assessed the possibility of ethanol inducing central conditioned responses in a set of cytokines. The study was based upon previous studies from our lab indicating potent acute, unconditional effects of ethanol in Sprague Dawley rats. Specifically, ethanol heightened IL-6 and IκBα, and decreased TNFα and IL-1β gene expression in HPC, PVN and AMG14,15 at the time of intoxication. Our hypothesis was that these unconditional effects of ethanol would endow seemingly neutral stimuli with the ability to modulate the activity of these pro-inflammatory cytokines.
The main new finding was that after four pairings of ethanol’s unconditional effects and a distinctive odor, relatively long (i.e. 3 h) re-exposure to this CS was sufficient to increase IL-6 levels in HPC and AMG (Experiment 3). This effect was not evident in animals given unpaired exposure to both stimuli (i.e. ethanol intoxication and odor). This effect was observed in rats administered ethanol i.p. but not in those receiving i.g. intubations. This result is in agreement with prior studies indicating significant differences in ethanol’s unconditional effects as a function of mode of administration. Nizhnikov et al.38 found conditioned place preference by ethanol after i.g., but not after i.p., ethanol administration, after equating the level of intoxication induced by the treatments. Similarly, Ciccocioppo, Panocka40 found conditioned place preference in the genetically selected Marchigian Sardinian alcohol-preferring rats after ethanol administration via a permanent i.g. catheter, but not when using the i.p. or i.g. route of administration. A follow-up study from the same group revealed conditioned taste aversion after 0.7 g/kg i.p. ethanol, yet this learning was very mild after i.g. ethanol, even when using a dose of 1.5 g/kg.41
There were some indications of conditioning of the immune response in i.g. treated animals, yet it seemed that most of these effects were driven by the manipulations inherently associated with the intubations, and not with a pharmacological effect of ethanol. Specifically, among i.g. treated rats of Experiment 3, the conditioning resulted in greater TNFα and IL-6 in the HPC, yet these effects were observed both in ethanol and in vehicle-intubated animals. Another interesting result of Experiment 3 was that, among ethanol rats, there was significantly greater expression of TNFα in paired vs. unpaired subjects, whereas the conditioning did not affect vehicle-administered rats. This suggests that the ethanol-paired CS acquired the ability to alter TNFα expression, although the enthusiasm brought by this conclusion is somewhat lessened by the fact that levels of this cytokine were not different from those found in vehicle rats.
Experiment 2 replicated the unconditional, acute ethanol-induced changes in the central cytokine response. Three hours after the injection, ethanol intoxication (4.0 g/kg, Experiment 2) significantly decreased the levels of TNFα and IL-1β in the HPC and AMG, and IL-1β in the PVN. Conversely, IL-6 in the HPC and AMG was significantly increased by acute ethanol administration at test. These results fit well with previous reports from our lab indicating ethanol-induced upregulation of brain IL-6 mRNA, yet ethanol-induced downregulation of central IL-1β and TNFα.13 This pattern of cytokine changes is associated with the initial time-frame of the intoxication. Had the second experiment measured cytokine expression at ≥6 h post-intoxication, we would probably have found an overall increase in cytokine expression, as reported by Emanuelle et al.8
For the most part, C-Fos patterns did not mirror those of cytokine response. In Experiment 1, no conditioned cytokine response was evident, yet re-exposure to the lemon-odor CS significantly increased C-Fos levels in the PVN of conditioned rats. C-Fos is a general marker of neural activity and the PVN is a key structure in the regulation of autonomic responses to stress and aversive stimulation, and also shows changes after chronic ethanol exposure. Keshavarzy et al.42 and Acevedo et al.43 observed significant Fos immunoreactivity in the PVN after a single exposure to restraint stress, whereas Lee et al.44 found increased levels of NGFIB, a protein involved in inflammatory processes, after chronic vapor ethanol exposure. Chronic ethanol treatment in Experiment 3 did not induce specific changes in C-Fos activity in the PVN, but reduced C-Fos levels in HPC, when given intraperitoneally. In Experiment 3, we used a single dose of ethanol delivered via i.p. and i.g. administration. This likely resulted in very different BECs depending on the mode of administration, and ethanol’s effects on C-Fos have been shown to be dependent on the BEC.45 A correspondence between ethanol’s effects on C-Fos and on cytokine levels was evident in Experiment 2. Acute ethanol treatment in that experiment significantly reduced C-Fos, TNFα, and IL-1β levels in the HPC.
It could be called into question the relevance of sub-chronic ethanol (i.e. given for only a few occasions, as in Experiment 3) in inducing conditioned immune responses. Lengthy ethanol exposure causes widely known, dramatic pro-inflammatory effects.46–48 For instance, Alfonso Loeches et al.49 found that long-term (i.e. 5-month) self-administration of ethanol upregulated GFAP immunoreactivity, an indicator of neurotoxicity, in the brain cortex. This effect seems to be mediated by TLR4, since ethanol-induced glial fibrillary acidic protein (GFAP) immunoreactivity was significantly blunted in mice lacking TLR4 receptors. Another study administered mice with 6 g/kg ethanol for 10 days50 and found increased GFAP immunostaining, greater levels of the chemokine CCL2/MCP-1 in HPC, cerebellum, and cerebral cortex, and greater levels of IL-6 in the cerebellum. On the other hand, the conditioned effects reported here are subtle. Yet it is important to remark that very brief increases in pro-inflammatory cytokines have been associated with enhanced anxiety response. For instance, Rossi et al.51 observed that mice given either a single intracerebroventricular injection of IL-1β or social defeat stress exhibited decreased time spent in the center of an open field and in the open arms of an elevated plus maze (both signs of increased anxiety response), an effect that was dependent on a CB1-dependent inhibition of GABAergic activity in the striatum. This implies that transient fluctuations in the level of pro-inflammatory cytokines can enhance anxiety patterns, which in turn are significant modulators of ethanol intake.
A recent study observed that rats with higher levels of inborn anxiety, as assessed via a behavioral screening that included the elevated plus maze test, drank more ethanol than average-anxiety counterparts.28 Another study has shown greater sensitivity to the rewarding effect of ethanol in mice deficient in pleiotrophin, a cytokine that significantly modulates dopaminergic function.52 It is possible, therefore, that ethanol-induced conditioned activity of the immune system facilitates initiation or escalation of alcohol consumption. Of course, conditioned increases of IL-6 (as shown in Experiment 3) may also facilitate the acute activation of this cytokine during acute ethanol exposure (as shown in Experiment 2). Future studies should assess these hypotheses.
Important information can be extracted by analyzing the cytokine pattern found in the ethanol-administered animals of Experiment 3, regardless of their learning (i.e. paired or unpaired) condition. These rats provide a measure of the effects of sub-chronic, moderate, and intermittent ethanol exposure (i.e. 4 intubations or injections of 2.0 g/kg ethanol, spread across 8 days), upon brain cytokine induction. These ethanol-administered rats exhibited significantly decreased IL-6 and TNFα levels in the HPC, although the latter effect was only found after i.p. administration. The i.p., but not the i.g., repeated ethanol administration also decreased the TNFα levels in the PVN. A comparison can be drawn between these results and those reported by Zahr et al.53 In the latter work, continuous exposure to binge ethanol doses (5.0 g/kg first dose, 3.0 g/kg subsequent doses, separated by 8 h across 4 days) did not alter brain levels of several cytokines, including TNF-α, IFN-γ, IL-1β, and IL-4. This negative finding contrasts with the significant changes observed in Experiment 3 and in other work54 that employed intermittent, usually every-other day, ethanol treatment.
The present study has important limitations. We did not assess if the conditioned changes in cytokine activity were associated with cognitive disruption. It has been suggested that the activation of the immune system is one of the factors underlying the disruption in cognitive function associated with chronic ethanol intake.55 The relevance of this possibility should not be underestimated: a recent study (reviewed in Ward et al.56) observed a pro-inflammatory pattern in blood samples of university students reporting heavy drinking in the last 2 years. Another caveat of the present study is that the acute and conditioned effects of ethanol upon the immune system were evaluated at the transcription (i.e. mRNA of IL-1, IL-6, etc.) level, but not confirmed at the protein level. The relationship between mRNA and protein abundance is affected by several factors, including protein stability and translational processes, and it has been suggested that mRNA scores explain roughly 40% of protein concentration.57 Thus, future studies in our lab will be directed toward assessments of cytokine protein levels under varying conditions. Another limitation is that we treated AMG and HPC as homogeneous structures. These structures, however, can be divided in sub-regions with differential roles.58
A third limitation is that all animals in Experiment 3 were tested under the effects of 0.5 g/kg ethanol. We aimed, by using this relatively low ethanol dose, at recreating part of the post-absorptive, interoceptive, state that the rats were experiencing during the conditioning trial. It has been shown that rats discriminate the state of intoxication induced by 0.5 g/kg ethanol, from a non-drug state,59 and that the reinstatement of the toxic effect facilitates the emission of conditioned responses.60 Yet the lack of a control group for this manipulation in Experiment 3 (i.e. a group untreated or given only vehicle before testing) makes it difficult to discern whether this manipulation was essential for the emergence of ethanol-mediated IL-6 responses in the HPC and AMG. Again, these issues are being addressed by ongoing studies in our lab that will replicate and extend the findings reported here. It is also important to note that BECs were derived from samples collected 3 h post-ethanol administration. This is not a time associated with peak BEC.37
It may also come as a surprise that high doses of ethanol were chosen in the present study, which aimed at finding conditioned responses. Doses of ethanol ≥ 1.5 g/kg can impair learning under certain conditions, particularly in preparations that involve the HPC. For instance, a 1.5 g/kg ethanol dose disrupted trace, but not delay, context conditioning in Sprague–Dawley rats.61 It should be taken into consideration, however, that ethanol doses ≥ 3.0 g/kg yield reliable ethanol-mediated orosensory61 or place62 conditioning in rats.
In summary, the present study replicates previous findings indicating that acute ethanol induces significant effects upon the inflammatory cytokines TNFα, IL-6, and IL-1β. The study also suggests that ethanol’s effects upon IL-6 in HPC and AMG may come under conditioned control, particularly after repeated pairings between distinctive odor cues and ethanol’s effects. Indeed, the observation that a growing number of ethanol-CS pairings (when examined across experiments 1–3) led to an apparent emergence of a conditioned cytokine response suggests that 4 ethanol-CS pairings might represent a threshold at which conditioned cytokine responses become evident. Thus, one might anticipate that, as ethanol consumption becomes habitual in human alcohol drinkers and the number of ethanol-CS pairings escalates, so will the development of conditioned cytokine and other immune-related alterations. In this way, conditioned increases in cytokine responses may have important future implications for our understanding of pathophysiological mechanisms underlying the escalation of alcohol use and abuse, relapse, and other functional consequences of chronic alcohol exposure.
Authors’ contribution
AG and TDF ran all the experiments. TD had the original research idea, designed the studies, and supervised the running of the experiments. RMP conducted the statistical analyses, graphed the data, and wrote the early draft and the final version of the article. All authors participated in the design of the experiments. All authors revised the final version of the article.
Acknowledgements
This work was supported by NIH grant number P50AA017823 to T.D. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the above stated funding agencies.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Ader R, Cohen N. Behaviorally conditioned immunosuppression. Psychosom Med 1975; 37: 333–40. [DOI] [PubMed] [Google Scholar]
- 2.Lebonville CL, Jones ME, Hutson LW, Cooper LB, Fuchs RA, Lysle DT. Acquisition of heroin conditioned immunosuppression requires IL-1 signaling in the dorsal hippocampus. Brain Behav Immun 2016; 56: 325–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schedlowski M, Pacheco-Lopez G. The learned immune response: Pavlov and beyond. Brain Behav Immun 2010; 24: 176–85. [DOI] [PubMed] [Google Scholar]
- 4.Exton MS, von Horsten S, Schult M, Voge J, Strubel T, Donath S, Steinmuller C, Seeliger H, Nagel E, Westermann J, Schedlowski M. Behaviorally conditioned immunosuppression using cyclosporine A: central nervous system reduces IL-2 production via splenic innervation. J Neuroimmunol 1998; 88: 182–91. [DOI] [PubMed] [Google Scholar]
- 5.Niemi MB, Pacheco-Lopez G, Kou W, Harting M, del Rey A, Besedovsky HO, Schedlowski M. Murine taste-immune associative learning. Brain Behav Immun 2006; 20: 527–31. [DOI] [PubMed] [Google Scholar]
- 6.Goebel MU, Trebst AE, Steiner J, Xie YF, Exton MS, Frede S, Canbay AE, Michel MC, Heemann U, Schedlowski M. Behavioral conditioning of immunosuppression is possible in humans. FASEB J 2002; 16: 1869–73. [DOI] [PubMed] [Google Scholar]
- 7.Albring A, Wendt L, Benson S, Witzke O, Kribben A, Engler H, Schedlowski M. Placebo effects on the immune response in humans: the role of learning and expectation. PloS One 2012; 7: e49477–e49477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emanuele NV, LaPaglia N, Kovacs EJ, Emanuele MA. The impact of burn injury and ethanol on the cytokine network of the mouse hypothalamus: reproductive implications. Cytokine 2005; 30: 109–15. [DOI] [PubMed] [Google Scholar]
- 9.Pascual M, Montesinos J, Marcos M, Torres JL, Costa-Alba P, Garcia-Garcia F, Laso FJ, Guerri C. Gender differences in the inflammatory cytokine and chemokine profiles induced by binge ethanol drinking in adolescence. Addic Biol. Epub ahead of print 4 October 2016. DOI: 10.1111/adb.12461. [DOI] [PubMed] [Google Scholar]
- 10.Montesinos J, Alfonso-Loeches S, Guerri C. Impact of the innate immune response in the actions of ethanol on the central nervous system. Alcohol Clin Exp Res 2016; 40: 2260–70. [DOI] [PubMed] [Google Scholar]
- 11.Knapp DJ, Harper KM, Whitman BA, Zimomra Z, Breese GR. Stress and withdrawal from chronic ethanol induce selective changes in neuroimmune mRNAs in differing brain sites. Brain Sci 2016; 6: E25–E25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suryanarayanan A, Carter JM, Landin JD, Morrow AL, Werner DF, Spigelman I. Role of interleukin-10 (IL-10) in regulation of GABAergic transmission and acute response to ethanol. Neuropharmacology 2016; 107: 181–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doremus-Fitzwater TL, Buck HM, Bordner K, Richey L, Jones ME, Deak T. Intoxication- and withdrawal-dependent expression of central and peripheral cytokines following initial ethanol exposure. Alcohol Clin Exp Res 2014; 38: 2186–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gano A, Doremus-Fitzwater TL, Deak T. Sustained alterations in neuroimmune gene expression after daily, but not intermittent, alcohol exposure. Brain Res 2016; 1646: 62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doremus-Fitzwater TL, Gano A, Paniccia JE, Deak T. Male adolescent rats display blunted cytokine responses in the CNS after acute ethanol or lipopolysaccharide exposure. Physiol Behav 2015; 148: 131–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He J, Crews FT. Increased MCP-1 and microglia in various regions of the human alcoholic brain. Exp Neurol 2008; 210: 349–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hill DB, Marsano LS, McClain CJ. Increased plasma interleukin-8 concentrations in alcoholic hepatitis. Hepatology 1993; 18: 576–80. [PubMed] [Google Scholar]
- 18.Blednov YA, Bergeson SE, Walker D, Ferreira VM, Kuziel WA, Harris RA. Perturbation of chemokine networks by gene deletion alters the reinforcing actions of ethanol. Behav Brain Res 2005; 165: 110–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valenta JP, Gonzales RA. Chronic intracerebroventricular infusion of monocyte chemoattractant protein-1 leads to a persistent increase in sweetened ethanol consumption during operant self-administration but does not influence sucrose consumption in long-Evans rats. Alcohol Clin Exp Res 2016; 40: 187–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pautassi RM, Nizhnikov ME, Spear NE, Molina JC. Prenatal ethanol exposure leads to greater ethanol-induced appetitive reinforcement. Alcohol 2012; 46: 585–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fabio MC, Macchione AF, Nizhnikov ME, Pautassi RM. Prenatal ethanol increases ethanol intake throughout adolescence, alters ethanol-mediated aversive learning, and affects mu but not delta or kappa opioid receptor mRNA expression. Eur J Neurosci 2015; 41: 1569–79. [DOI] [PubMed] [Google Scholar]
- 22.Chang GQ, Karatayev O, Leibowitz SF. Prenatal exposure to ethanol stimulates hypothalamic CCR2 chemokine receptor system: Possible relation to increased density of orexigenic peptide neurons and ethanol drinking in adolescent offspring. Neuroscience 2015; 310: 163–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Terasaki LS, Schwarz JM. Effects of moderate prenatal alcohol exposure during early gestation in rats on inflammation across the maternal-fetal-immune interface and later-life immune function in the offspring. J Neuroimmune Pharmacol 2016; 11: 680–92–680–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drew PD, Johnson JW, Douglas JC, Phelan KD, Kane CJ. Pioglitazone blocks ethanol induction of microglial activation and immune responses in the hippocampus, cerebellum, and cerebral cortex in a mouse model of fetal alcohol spectrum disorders. Alcohol Clin Exp Res 2015; 39: 445–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Topper LA, Valenzuela CF. Effect of repeated alcohol exposure during the third trimester-equivalent on messenger RNA levels for interleukin-1beta, chemokine (C-C motif) ligand 2, and interleukin 10 in the developing rat brain after injection of lipopolysaccharide. Alcohol 2014; 48: 773–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pautassi RM, Nizhnikov ME, Spear NE. Assessing appetitive, aversive, and negative ethanol-mediated reinforcement through an immature rat model. Neurosci Biobehav Rev 2009; 33: 953–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pascual M, Boix J, Felipo V, Guerri C. Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. J Neurochem 2009; 108: 920–31. [DOI] [PubMed] [Google Scholar]
- 28.Acevedo MB, Fabio MC, Fernandez M, Pautassi RM. Anxiety response and restraint-induced stress differentially affect ethanol intake in female adolescent rats. Neuroscience 2016; 334: 259–74–259–74. [DOI] [PubMed] [Google Scholar]
- 29.Lee S, Schmidt D, Tilders F, Cole M, Smith A, Rivier C. Prolonged exposure to intermittent alcohol vapors blunts hypothalamic responsiveness to immune and non-immune signals. Alcohol Clin Exp Res 2000; 24: 110–22. [PubMed] [Google Scholar]
- 30.Paxinos G, Watson C. The rat brain in stereotaxic coordinates, San Diego, CA: Academic Press, 2007. [Google Scholar]
- 31.Buck HM, Hueston CM, Bishop C, Deak T. Enhancement of the hypothalamic-pituitary-adrenal axis but not cytokine responses to stress challenges imposed during withdrawal from acute alcohol exposure in Sprague-Dawley rats. Psychopharmacology 2011; 218: 203–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blandino P, Jr., Barnum CJ, Solomon LG, Larish Y, Lankow BS, Deak T. Gene expression changes in the hypothalamus provide evidence for regionally-selective changes in IL-1 and microglial markers after acute stress. Brain Behav Immun 2009; 23: 958–68. [DOI] [PubMed] [Google Scholar]
- 33.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001; 25: 402–8. [DOI] [PubMed] [Google Scholar]
- 34.Winger G, Hofmann F, Woods JH. A handbook on drug and alcohol abuse. The biomedical aspects, 3rd ed New York: Oxford University Press, 1992. [Google Scholar]
- 35.Molina JC, Chotro MG. Acute alcohol-intoxication paired with appetitive reinforcement - Effects upon ethanol intake in infant rats. Behav Neural Biol 1989; 51: 326–45. [DOI] [PubMed] [Google Scholar]
- 36.Molina JC, Chotro G, Spear NE. Early (preweanling) recognition of alcohol’s orosensory cues resulting from acute ethanol intoxication. Behav Neural Biol 1989; 51: 307–25. [DOI] [PubMed] [Google Scholar]
- 37.Walker BM, Ehlers CL. Age-related differences in the blood alcohol levels of Wistar rats. Pharmacol Biochem Behav 2009; 91: 560–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nizhnikov ME, Pautassi RM, Truxell E, Spear NE. Opioid antagonists block the acquisition of ethanol-mediated conditioned tactile preference in infant rats. Alcohol 2009; 43: 347–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shelton KL, Balster RL. Ethanol drug discrimination in rats: substitution with GABA agonists and NMDA antagonists. Behav Pharmacol 1994; 5: 441–51. [PubMed] [Google Scholar]
- 40.Ciccocioppo R, Panocka I, Froldi R, Quitadamo E, Massi M. Ethanol induces conditioned place preference in genetically selected alcohol-preferring rats. Psychopharmacology 1999; 141: 235–41. [DOI] [PubMed] [Google Scholar]
- 41.Ciccocioppo R, Angeletti S, Chhada M, Perfumi M, Froldi R, Massi M. Conditioned taste aversion induced by ethanol in alcohol-preferring rats: influence of the method of ethanol administration. Pharmacol Biochem Behav 1999; 64: 563–6. [DOI] [PubMed] [Google Scholar]
- 42.Keshavarzy F, Bonnet C, Behzadi G, Cespuglio R. Expression patterns of c-Fos early gene and phosphorylated ERK in the rat brain following 1-h immobilization stress: concomitant changes induced in association with stress-related sleep rebound. Brain Struct Funct 2015; 220: 1793–804. [DOI] [PubMed] [Google Scholar]
- 43.Acevedo MB, Fabio MC, Fernandez MS, Pautassi RM. Anxiety response and restraint-induced stress differentially affect ethanol intake in female adolescent rats. Neuroscience 2016; 334: 259–74. [DOI] [PubMed] [Google Scholar]
- 44.Lee S, Schmidt D, Tilders F, Rivier C. Increased activity of the hypothalamic-pituitary-adrenal axis of rats exposed to alcohol in utero: role of altered pituitary and hypothalamic function. Mol Cell Neurosci 2000; 16: 515–28. [DOI] [PubMed] [Google Scholar]
- 45.Fabio MC, Vivas LM, Pautassi RM. Prenatal ethanol exposure alters ethanol-induced Fos immunoreactivity and dopaminergic activity in the mesocorticolimbic pathway of the adolescent brain. Neuroscience 2015; 301: 221–34. [DOI] [PubMed] [Google Scholar]
- 46.Lieber CS. The discovery of the microsomal ethanol oxidizing system and its physiologic and pathologic role. Drug Metab Rev 2004; 36: 511–29. [DOI] [PubMed] [Google Scholar]
- 47.Ren Z, Yang F, Wang X, Wang Y, Xu M, Frank JA, Ke ZJ, Zhang Z, Shi X, Luo J. Chronic plus binge ethanol exposure causes more severe pancreatic injury and inflammation. Toxicol Appl Pharmacol 2016; 308: 11–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Montesinos J, Pascual M, Pla A, Maldonado C, Rodriguez-Arias M, Minarro J, Guerri C. TLR4 elimination prevents synaptic and myelin alterations and long-term cognitive dysfunctions in adolescent mice with intermittent ethanol treatment. Brain Behav Immun 2015; 45: 233–44. [DOI] [PubMed] [Google Scholar]
- 49.Alfonso-Loeches S, Pascual-Lucas M, Blanco AM, Sanchez-Vera I, Guerri C. Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. J Neurosci 2010; 30: 8285–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kane CJ, Phelan KD, Douglas JC, Wagoner G, Johnson JW, Xu J, Phelan PS, Drew PD. Effects of ethanol on immune response in the brain: region-specific changes in adolescent versus adult mice. Alcohol Clin Exp Res 2014; 38: 384–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rossi S, Sacchetti L, Napolitano F, De Chiara V, Motta C, Studer V, Musella A, Barbieri F, Bari M, Bernardi G, Maccarrone M, Usiello A, Centonze D. Interleukin-1beta causes anxiety by interacting with the endocannabinoid system. J Neurosci 2012; 32: 13896–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vicente-Rodríguez M, Pérez-García C, Ferrer-Alcón M, Uribarri M, Sánchez-Alonso MG, Ramos MP, Herradón G. Pleiotrophin differentially regulates the rewarding and sedative effects of ethanol. J Neurochem 2014; 131: 688–95. [DOI] [PubMed] [Google Scholar]
- 53.Zahr NM, Luong R, Sullivan EV, Pfefferbaum A. Measurement of serum, liver, and brain cytokine induction, thiamine levels, and hepatopathology in rats exposed to a 4-day alcohol binge protocol. Alcohol Clin Exp Res 2010; 34: 1858–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pascual M, Blanco AM, Cauli O, Minarro J, Guerri C. Intermittent ethanol exposure induces inflammatory brain damage and causes long-term behavioural alterations in adolescent rats. Eur J Neurosci 2007; 25: 541–50. [DOI] [PubMed] [Google Scholar]
- 55.Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun 2011; 25: 181–213. [DOI] [PubMed] [Google Scholar]
- 56.Ward RJ, Lallemand F, de Witte P. Influence of adolescent heavy session drinking on the systemic and brain innate immune system. Alcohol Alcohol 2014; 49: 193–7. [DOI] [PubMed] [Google Scholar]
- 57.Vogel C, Marcotte EM. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat Rev Genet 2012; 13: 227–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R, Biag J, Dong HW, Deisseroth K, Callaway EM, Fanselow MS, Luthi A, Anderson DJ. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature 2010; 468: 270–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hunt PS, Molina JC, Spear LP, Spear NE. Ethanol-mediated taste aversions and state-dependency in preweanling (16-day-old) rats. Behav Neural Biol 1990; 54: 300–22. [DOI] [PubMed] [Google Scholar]
- 60.Fernandez-Vidal JM, Spear NE, Molina JC. Adolescent rats discriminate a mild state of ethanol intoxication likely to act as an appetitive unconditioned stimulus. Alcohol 2003; 30: 45–60. [DOI] [PubMed] [Google Scholar]
- 61.Pautassi RM, Myers M, Spear LP, Molina JC, Spear NE. Ethanol induces second-order aversive conditioning in adolescent and adult rats. Alcohol 2011; 45: 45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hill KG, Alva H, Blednov YA, Cunningham CL. Reduced ethanol-induced conditioned taste aversion and conditioned place preference in GIRK2 null mutant mice. Psychopharmacology (Berl) 2003; 169: 108–14. [DOI] [PubMed] [Google Scholar]