Abstract
Adoptive cell therapy holds much promise in the treatment of cancer but results in solid tumors have been modest. The notable exception is tumor-infiltrating lymphocyte (TIL) therapy of melanoma, but this approach only works with high-dose preconditioning chemotherapy and systemic interleukin (IL)-2 postconditioning, both of which are associated with toxicities. To improve and broaden the applicability of adoptive cell transfer, we constructed oncolytic adenoviruses coding for human IL-2 (hIL2), tumor necrosis factor alpha (TNF-α), or both. The viruses showed potent antitumor efficacy against human tumors in immunocompromised severe combined immunodeficiency (SCID) mice. In immunocompetent Syrian hamsters, we combined the viruses with TIL transfer and were able to cure 100% of the animals. Cured animals were protected against tumor re-challenge, indicating a memory response. Arming with IL-2 and TNF-α increased the frequency of both CD4+ and CD8+ TILs in vivo and augmented splenocyte proliferation ex vivo, suggesting that the cytokines were important for T cell persistence and proliferation. Cytokine expression was limited to tumors and treatment-related signs of systemic toxicity were absent, suggesting safety. To conclude, cytokine-armed oncolytic adenoviruses enhanced adoptive cell therapy by favorable alteration of the tumor microenvironment. A clinical trial is in progress to study the utility of Ad5/3-E2F-d24-hTNFa-IRES-hIL2 (TILT-123) in human patients with cancer.
Keywords: adenovirus, tumor-infiltrating lymphocytes, cytokines, TNF, interleukin 2
Introduction
Immunotherapies have shown promising results in cancer types previously hard to cure, such as melanoma,1, 2, 3 non-small cell lung cancer,4 and renal cell carcinoma.5 In addition to checkpoint-inhibiting antibodies, patient-derived T cells are a potent approach because they can be re-targeted against tumors ex vivo, or they can be infused to the patient without modifications, when extracted from the tumor biopsy.6 One advantage of using biopsy-derived polyclonal tumor-infiltrating lymphocytes (TILs) is the presence of neoantigen-specific clones—an aspect absent from receptor-modified T cells.7 As a downside, TIL infusion requires high-dose preconditioning to eradicate suppressive immune cell subsets from the tumor microenvironment and postconditioning with high-dose systemic interleukin (IL)-2, both often causing severe toxicities.6, 8
Instead of systemic administration of cytokines like IL-2, it could be more attractive to deliver them locally with gene therapy vectors, such as viruses.9, 10 In particular, tumor-targeted replication-competent viruses (i.e., oncolytic viruses) enable a thousand-fold amplification of transgene expression, restricted to tumor tissue. With regard to immunotherapy, oncolytic adenovirus constitutes a personalized cancer vaccine generated for each patient in situ, due to release of tumor-associated antigens.11 Of note, virus-mediated danger signaling helps the immune system to recognize tumor cells,12 and immunostimulatory cytokines further boost this effect.3, 13, 14
We have shown that the most promising T cell-stimulating factors, in the context of adoptive cell therapy, are IL-2 and tumor necrosis factor alpha (TNF-α).15, 16, 17 Regarding prior knowledge about the recombinant proteins, IL-2 has been widely used in treating malignant melanoma and renal cell carcinoma18 and it stimulates T cell proliferation and differentiation.9, 19, 20 Like IL-2, TNF-α can activate immune cells,21 but it also induces antitumor inflammation and the production of other cytokines and chemokines.22, 23 Moreover, it directly causes cancer cell necrosis and apoptosis.23
In this study, we constructed and characterized new oncolytic adenoviruses built on a backbone of Ad5/3-E2F-d24 (OAd) carrying human IL-2 (hIL2), TNF-α, or both. Two modifications render virus replication tumor specific: an E2F promoter and a 24-base pair (bp) deletion in the constant region 2 of E1A, make the viruses selective for cells defective in the retinoblastoma/p16 pathway—including most tumor cells.24, 25 In addition, the Ad5/3 chimeric capsid featuring the Ad3 knob but Ad5 shaft and tail has demonstrated improved cancer cell transduction as well as antitumor efficacy.26 Importantly, safety of this configuration in humans has also been established.13, 27, 28 Based on the results in an immunocompetent Syrian hamster model of pancreatic cancer, these viruses emerged as strong candidates for stimulating the immune system in tumors locally, with the specific application of enabling effective and safe TIL therapy. Ad5/3-E2F-d24-hTNFa-IRES-hIL2 (TILT-123) rose as the leading candidate for human translation.
Results
Armed Oncolytic Adenoviruses Have Cell-Killing Ability, Show Synergy When Combined with TILs, and Express Biologically Active Cytokines
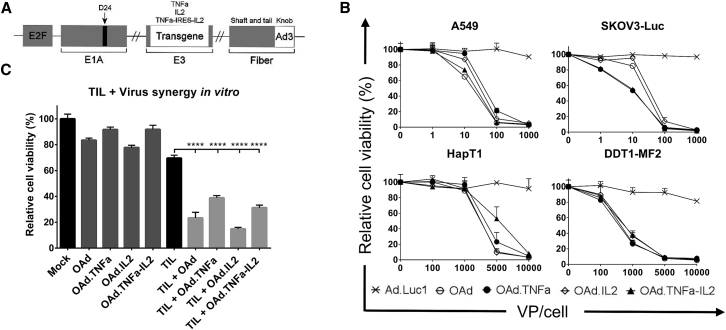
Oncolytic adenoviruses were constructed to feature a backbone carrying serotype 5 (Ad5) nucleic acid with an Ad3 fiber knob. In addition, a 24-bp deletion (d24) in the Rb-binding region of adenoviral E1A together with the E2F promoter was established to direct the replication to Rb-deficient cancer cells. The transgenes were inserted into the E3 region, to replace some superfluous adenoviral open reading frames, which links expression to virus replication (Figure 1A).
Figure 1.
Oncolytic Activity of Adenoviral Vectors in Human and Hamster Cancer Cell Lines
(A) A schematic presentation of chimeric oncolytic adenovirus with E2F promoter; 24-base-pair deletion in E1A; human TNF-α, IL-2, or TNF-α-IRES-IL2 inserted in the E3 region; and an Ad3 serotype knob in the Ad5 fiber. (B) Oncolytic activity of the viruses was shown in human lung adenocarcinoma (A549) and luciferase-expressing ovarian carcinoma (SKOV3-Luc) as well as in hamster pancreatic cancer (HapT1) and leiomyosarcoma (DDT1-MF2). The cells were incubated with the viruses for 3 days (A549 and DDT1-MF2), 5 days (SKOV3-Luc), or 6 days (HapT1) before determining cell viability. (C) Cell-killing efficacy was enhanced when combining viruses with HapT1-specific TILs. The cells were incubated 72 hr with 5,000 VPs and 24 hr with TILs. Means ± SEM are shown (n = 8). Statistical differences were evaluated with one-way ANOVA. ****p ≤ 0.0001. Ad.Luc1, replication-deficient Ad5/3-Luc1; OAd, Ad5/3-E2F-d24; OAd.TNFa, Ad5/3-E2F-d24-hTNFa; OAd.IL2, Ad5/3-E2F-d24-hIL2; OAd.TNFa-IL2, Ad5/3-E2F-d24-hTNFa-IRES-hIL2.
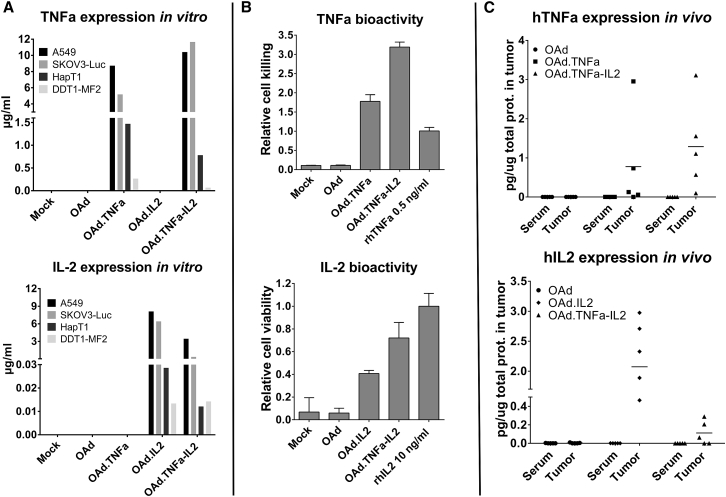
All cytokine-armed adenovirus constructs were able to kill a panel of human cancer cell lines with similar efficacy as the virus without transgenes (Figures 1B and S1). Importantly, when virus was combined with HapT1-targeting TILs, the cell-killing effect was significantly increased (Figure 1C). Both human and hamster cells were able to produce biologically active human IL-2 as well as TNF-α in vitro when infected with armed viruses (Figures 2A, 2B, and S2). Importantly, local production of cytokines was observed with all three armed viruses in vivo while systemic levels remained undetectable (Figure 2C), highlighting the feasibility of the technology from a safety perspective.
Figure 2.
Biologically Active Cytokines Are Produced from Human and Hamster Cell Lines and Expressed Locally In Vivo
(A) Human and hamster cell lines were incubated for 72 hr with 1,000 or 5,000 VPs per cell, respectively. Cytokine concentrations were measured from cell culture supernatants with a cytometric bead array. (B) Indicator cell line L929 was used in the cell-killing assay to confirm the biological activity of TNF-α, while mouse T cell line CTLL-2 was utilized in the IL-2-induced cell proliferation assay. The samples were derived from virus-infected HapT1 supernatants. Means ± SD are shown. (C) HapT1 tumors were injected with 1 × 108 VPs and collected together with blood after 48 hr. Cytokine concentrations were measured with a cytometric bead array and normalized against total protein concentration of the sample. Horizontal lines indicate mean values. OAd, Ad5/3-E2F-d24; OAd.TNFa, Ad5/3-E2F-d24-hTNFa; OAd.IL2, Ad5/3-E2F-d24-hIL2; OAd.TNFa-IL2, Ad5/3-E2F-d24-hTNFa-IRES-hIL2.
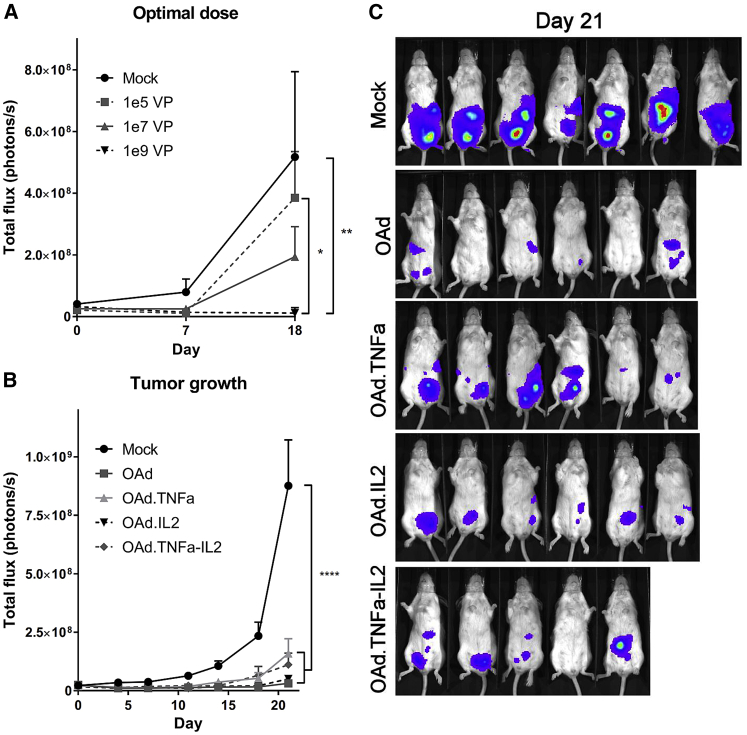
Cytokine-Armed Viruses Inhibit Tumor Growth in a Dose-Dependent Manner
To establish an optimal virus dose, immunocompromised mice bearing orthotopic human ovarian tumors (SKOV3-Luc) received three different doses of Ad5/3-E2F-d24-hTNFa-IRES-hIL2. The best efficacy was achieved with the highest dose of 1 × 109 viral particles (VPs), which was significantly different compared with the untreated control group (p = 0.0085) as well as with the lowest dose of 1 × 105 VPs (p = 0.0287) on day 18 (Figure 3A). When SKOV3-Luc tumors were treated with Ad5/3-E2F-d24-hTNFa-IRES-hIL2 and control viruses, all viruses had similar antitumor efficacy (Figures 3B and 3C), suggesting that adenovirus replication rates in vivo were comparable despite the inclusion of transgenes.
Figure 3.
Armed Oncolytic Adenoviruses Show Dose-Dependent Antitumor Efficacy in Immunocompromised Mice
(A–C) SCID mice bearing orthotopic luciferase-expressing ovarian carcinoma SKOV3-Luc were treated with (A) different doses of Ad5/3-E2F-d24-hTNFa-IRES-hIL2 (n = 3) and with (B and C) all the viruses with selected dose (1 × 109 VPs/animal, n = 5–7). Means ± SEM are shown. Statistical differences were evaluated with two-way ANOVA. *p ≤ 0.05; **p ≤ 0.01; ****p ≤ 0.0001. (C) Bioluminescent images from day 21 were merged with photographs. The color scale from violet to red represents luminescence intensity from 1 × 106 to 1 × 108 p/s/cm2/sr, respectively. OAd, Ad5/3-E2F-d24; OAd.TNFa, Ad5/3-E2F-d24-hTNFa; OAd.IL2, Ad5/3-E2F-d24-hIL2; OAd.TNFa-IL2, Ad5/3-E2F-d24-hTNFa-IRES-hIL2.
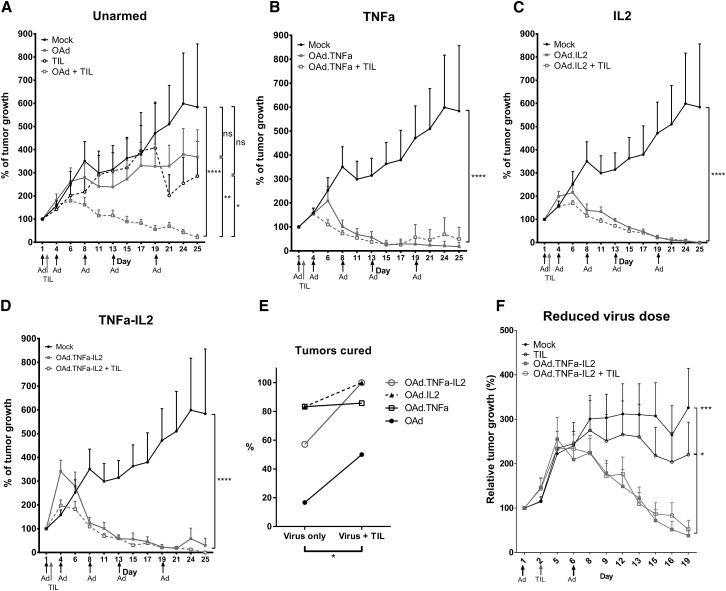
Armed Oncolytic Viruses Improve the Efficacy of Adoptive TIL Transfer
Encouraged by the ex vivo results (Figure 1C), the ability of cytokine-armed viruses to enhance TIL therapy was investigated in immunocompetent Syrian hamsters (Figure 4). The unarmed virus and TILs had only moderate antitumor effects when administered alone, but a significant improvement in efficacy was observed when they were combined (p = 0.002) (Figure 4A). The armed viruses had tremendous efficacy even as single agents (Figures 4B–4D), but the percentage of cured animals was higher in groups receiving TILs and virus compared with the virus-only groups (p = 0.034; Figure 4E). In fact, cured hamsters treated with the combination of Ad5/3-E2F-d24-hTNFa-IRES-hIL2 and TIL therapy comprised 100% (Figure 4E). The experiment was repeated with a reduced virus dose with similar results in efficacy (Figure 4F). The cured animals from the second experiment stayed tumor free for the follow-up period of 3 months.
Figure 4.
Armed Oncolytic Adenoviruses Improve the Curative Efficacy of Adoptive TIL Therapy in Immunocompetent Syrian Hamsters
(A–D) Syrian hamsters with established subcutaneous HapT1 tumors were treated five times with 1 × 109 VPs and once with 4 × 105 freshly expanded TILs, both injected intratumorally (n = 6–7). Tumors lacking neoplastic cells were considered cured. Statistical differences were evaluated with a linear mixed-effects model. (E) Complete tumor remission was evaluated before animals were euthanized or in unclear cases after histopathological analysis. The effect of TILs on cure rates was evaluated with the Wilcoxon signed-rank test. (F) The experiment was repeated with a reduced virus dose (1 × 108 VPs on days 1 and 8, n = 5–6). Differences in antitumor efficacy were analyzed with two-way ANOVA. *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001; ****p ≤ 0.0001. ns, non-significant; OAd, Ad5/3-E2F-d24; OAd.TNFa, Ad5/3-E2F-d24-hTNFa; OAd.IL2, Ad5/3-E2F-d24-hIL2; OAd.TNFa-IL2, Ad5/3-E2F-d24-hTNFa-IRES-hIL2.
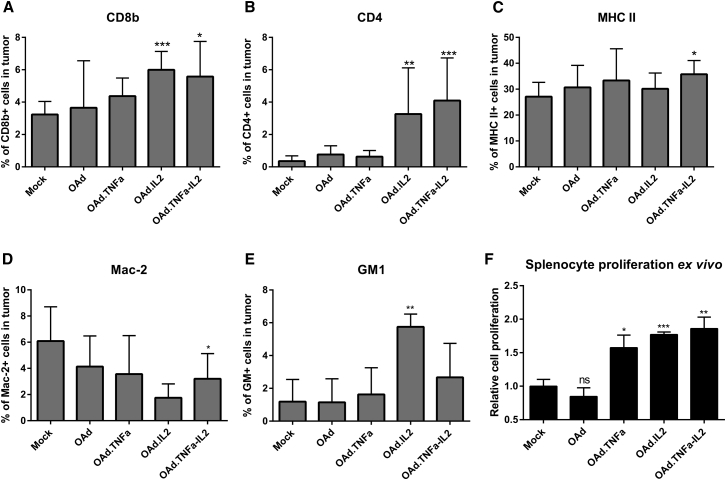
Cytokines Increase the Frequency of T Cells in Tumors and Augment Splenocyte Proliferation
To investigate immunological effects of the armed viruses, cells from tumors, spleens, and tumor-draining lymph nodes were analyzed with flow cytometry (Figures 5A–5E and S3). Natural killer (NK) cell marker GM1 and T cell markers CD8 and CD4 were more frequent in tumors treated with IL-2, whereas the cytokine combination was the only treatment capable of increasing the level of major histocompatibility complex class II (MHC II) and decreasing the Mac-2 expression in tumors (Figures 5A–5E). Differences in the cell composition in spleens and lymph nodes were minor (Figure S3). Interestingly, splenocytes exhibited greater cell proliferation ex vivo if the animals had been treated with armed viruses (Figure 5F).
Figure 5.
Adenovirus Treatments Induce Beneficial Immunological Changes in Tumor Microenvironment
(A–E) The following cells were stained from endpoint tumors with fluorochrome-conjugated antibodies and analyzed by flow cytometry: (A) CD8+, (B) CD4+, (C) MHC II+, (D) Mac-2+, and (E) GM1+. Because of the great number of cured tumors, groups with or without TILs were combined. Means ± SD are shown (n = 3–9). Differences in cell percentages were analyzed with the Student’s t test. (F) Splenocyte proliferation was determined by counting the cells. Because TILs did not affect the proliferation, groups with or without TILs were combined for simplification. Means ± SEM are shown (n = 4). Statistical significance was calculated with the non-parametric Mann-Whitney test. *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001. ns, non-significant; OAd, Ad5/3-E2F-d24; OAd.TNFa, Ad5/3-E2F-d24-hTNFa; OAd.IL2, Ad5/3-E2F-d24-hIL2; OAd.TNFa-IL2, Ad5/3-E2F-d24-hTNFa-IRES-hIL2.
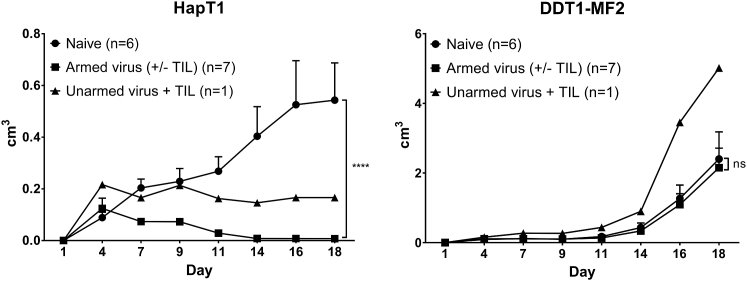
Treatments with Cytokine-Armed Viruses Induce Protection from Tumor Re-challenge
To estimate whether the viral treatment established tumor-specific immunity, cured hamsters were re-challenged with the same cancer cells as previously (HapT1). As a control, different types of cancer cells (DDT1-MF2) were implanted in the other flank of the animal (Figure 6). The animals that had previously been cured with cytokine-coding viruses rejected HapT1, whereas the animal treated with unarmed virus had a stable condition. The number of animals in these groups differs, because the curative potential of the unarmed virus was more limited. DDT1-MF2 tumor growth in cured hamsters was comparable to growth in naive animals, indicating the induction of tumor-specific antitumor immunity.
Figure 6.
Cytokine-Armed Virus Treatments Induce Tumor-Specific Immunological Memory
Hamsters previously cured of HapT1 tumors were re-challenged with the same HapT1 tumor cells or with a distinct cell line (DDT1-MF2). Hamsters previously treated with cytokine-armed viruses completely rejected the re-introduced HapT1 tumors, whereas the hamster treated with unarmed virus had a stable condition. Means ± SEM are presented. ****p ≤ 0.0001. ns, non-significant.
Virus Treatment Does Not Induce Major Histological Changes in Selected Organs
Treatment-related changes in tissue structures of the heart, lung, liver, and kidney were undetectable (Supplemental Materials and Methods). Meanwhile, spleens collected from all treatment groups showed mild and minimal lymphocyte hyperplasia, slightly expanded white pulp, and a mildly increased number of heterophils in the marginal zone or red pulp. There were no differences in the severity of the changes between any of the treatment groups including mock and other controls, suggesting a lack of systemic effects linked to the transgenes as predicted by low serum concentrations (Figure 2C).
Discussion
Immuno-oncology has made some clinical breakthroughs over the years, but, currently, a minority of patients respond and single-agent treatment modalities seldom lead to lasting remissions.1, 2, 3, 4 Thus, the utility of immunotherapy has been established on a proof-of-concept level, but much work remains to help the majority of patients with currently incurable cancer. Checkpoint-inhibiting antibodies have received much attention due to their ability to downregulate immunosuppression, but they cannot generate new immunity.29 By contrast, new immune reactions can be achieved with adoptive cell therapies and oncolytic immunotherapy, thus being complementary to the former.29 However, adoptive cell therapy of non-melanoma solid tumors has proved clinically unimpressive results, because the tumor microenvironment is able to anergize the cell graft.16, 30 This effect can be countered with the biological phenomena resulting from adenoviral oncolysis, and it can be optimized with TNF-α and IL-2.16, 31 We previously studied the effects of these cytokines with recombinant cytokines and with replication-defective vectors coding for murine cytokines.15, 16, 17 Here, we constructed clinically applicable oncolytic adenoviruses coding for human IL-2 and TNF-α and used them to boost adoptive cell transfer.
The viruses were capable of infecting and lysing a variety of human and Syrian hamster cancer cell lines. In addition, infected cells produced bioactive cytokines. After intratumoral virus administration, high cytokine concentrations were achieved in target tissue, while blood levels remained undetectable. In addition, signs of toxicity were absent in histopathological evaluation of all major organs. Taking into consideration the potential toxicity of high systemic cytokine levels,8, 32 the results suggest a safer approach for cytokine delivery. Moreover, local delivery of IL-2 could replace the need for high-dose IL-2 administration often included in clinical T cell therapy protocols,6 although detailed studies are needed to validate the concept.
To investigate the efficacy of the viruses on human cancer cells in vivo, we established an orthotopic ovarian carcinoma model in immunocompromised SCID mice. Despite the replication competence of the virus, the efficacy was dose dependent. The problem of virus infiltration into the tumor is well known; thus, intratumoral administration represents a more efficient way of delivering the virus.33 In addition, this model develops resistance to oncolytic adenovirus, seen as a reduction in antitumor efficacy at later time points. The mechanism of resistance was previously shown to be related to the induction of interferon pathways.34 Significant differences in the efficacy between the viruses were absent, which was expected because the cytokines have few effects in an immunocompromised model lacking a complete immune system.
Cytokine-armed oncolytic viruses are potential enhancers of T cells and T cell-based therapies.14, 35, 36 To investigate the synergy between our viruses and TILs, we chose Syrian hamsters as an immunocompetent animal model. Syrian hamsters provide an interesting model for studying oncolytic adenoviruses, since human adenovirus is capable of replicating in hamsters, unlike in mice.37 Conveniently, human IL-238 and human TNF-α are also bioactive in hamsters, which is evident from the hamster experiment where the unarmed virus had only moderate antitumor effects as compared with the human cytokine-coding viruses that share the same backbone construct. Furthermore, we have developed a method to extract and expand TILs from established hamster tumors, despite the limited availability of hamster-specific reagents.39 Unfortunately, specific expansion of, for example, CD8+ T cells is unfeasible and the TIL pool depends on the population present in the tumor at the time of collection.
Even in vitro, the synergistic effect of combining the viruses and TILs was evident. In vivo, the combination of unarmed virus and TILs led to improvement in efficacy. With regard to the cytokine-armed viruses, good efficacy was seen with single-agent treatment in two individual experiments. However, the combination of armed virus and TIL therapy resulted in the highest frequency of complete responses, as confirmed by histopathological analysis of the tumors. Of note, all tumors in hamsters treated with Ad5/3-E2F-d24-hTNFa-IRES-hIL2 and TILs were cured, suggesting that inclusion of adenovirally delivered immunostimulatory cytokines contributes to the curative efficacy of TIL therapy.
Immunocompetent mouse models have revealed that combining adenovirus-delivered TNF-α and IL-2 with adoptive T cell transfer decreases immunosuppressive characteristics of the tumor microenvironment and increases the number of active cytotoxic T cells in melanoma tumors.15, 17 Moreover, mouse studies have revealed that the danger signals and immunostimulation caused by adenovirus and cytokines can result in repertoire expansion by polyclonal amplification of many classes of antitumor T cells while T cell exhaustion seems to be thwarted.15, 16, 17
Mouse tumor models are useful for immunological studies because many reagents are available. However, human adenovirus does not replicate productively in mouse tissues; therefore, the hamster model has some advantages in this regard. Unfortunately, the repertoire of reagents available for hamster studies is much more limited. There are only a few hamster-specific or cross-reactive antibodies, and only limited characterization of the immune cell subsets in the tumor is possible.
Nevertheless, we saw decreased presence of macrophage marker Mac-2 in the hamster tumors, whereas the frequency of GM1+ (mostly NK) cells as well as CD4+ and CD8+ T cells was increased following intratumoral treatment with the cytokine-coding viruses. Balza et al.31 observed from immunocompetent mice that both CD8+ and CD4+ cells are essential for antitumor efficacy resulting from the combination of TNF-α and IL-2. IL-2 induces both regulatory and helper T cells;40 however, in this study, the nature of CD4+ cells could not be specified. Interestingly, the observed upregulation of MHC II (a marker for antigen-presenting cells) might indicate that the combination of both cytokines enables efficient tumor recognition by cytotoxic CD4+ T cells,39, 41 thus contributing to the overall efficacy. In addition, the presence of TNF-α stimulates the expression of IL-6, a cytokine needed for helper T cell differentiation.40, 42 Taken together, although systemic IL-2 may cause stimulation of regulatory T cells, which may not necessarily be true for local IL-2, the possibility of the CD4+ population also containing cytotoxic and helper T cells cannot be excluded.
In addition to immunological changes seen in the tumor microenvironment, further benefit from the cytokines was seen on the systemic level. Because the spleen serves as an indicator for the common status of the immune system, we investigated the proliferative capability of the splenocytes derived from the treated animals ex vivo. Interestingly, the splenocytes from the cytokine-treated animals showed increased proliferative capability compared with the controls, indicating increased adaptive cellular response. The same effect has been seen in a study with oncolytic vaccinia virus coding for granulocyte-macrophage colony-stimulating factor.10 Moreover, adenovirally encoded cytokines evidently induced the formation of immunological memory, typically mediated by T cells.43 Currently, because there are no anti-hamster antibodies available, the presence of memory-type T cells could not be verified. The animals treated with armed viruses resisted tumor recurrence unlike the animal treated with the virus without the cytokines, as also seen with other oncolytic viruses.10, 43, 44
In conclusion, we provide evidence that oncolytic adenoviruses coding for human IL-2 and TNF-α appear safe in immunocompetent hamsters. In addition to direct oncolytic effects and attractive immunological effects, these viruses seem useful for enabling successful TIL therapy of human solid tumors. Whereas the virus itself shows potent antitumor efficacy, the cytokines are useful for induction of T cells in the tumor and for immunological memory responses. The preclinical data reported here allowed TILT Biotherapeutics to initiate a human trial studying the utility of Ad5/3-E2F-d24-TNFa-IRES-hIL2 in patients with advanced cancer receiving TIL therapy.
Materials and Methods
Cell Lines
All cell lines were purchased from American Type Culture Collection (ATCC) unless otherwise stated. Human lung adenocarcinoma A549, human pancreatic cancer Panc1, human melanoma SK-MEL-28, human ovarian carcinoma OVCAR-3, mouse fibroblast L929, and mouse T lymphocyte cell line CTLL-2 were utilized in in vitro assays. Firefly luciferase-expressing ovarian adenocarcinoma SKOV3-Luc (kindly provided by Dr. Negrin, Stanford Medical School), hamster leiomyosarcoma DDT1-MF2 (a kind gift from Dr. William Wold), and hamster pancreatic cancer HapT1 (DSMZ) were used both in vitro and in vivo. All cell lines were maintained in RPMI 1640 or DMEM supplemented with 10% fetal bovine serum (FBS), 2 mM L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (all from Sigma-Aldrich) and cultured at +37°C and 5% CO2.
Generation of Viral Constructs
The experimental viruses have a backbone of Ad5/3-E2F-d24. The transgenes in Ad5/3-E2F-d24-hIL2 (OAd.IL2), Ad5/3-E2F-d24-hTNFa (OAd.TNFa), and Ad5/3-E2F-d24-hTNFa-IRES-hIL2 (OAd.TNFa-IL2, also known as TILT-123) were placed into the E3 region and they were generated with the bacterial artificial chromosome (BAC)-recombineering strategy based on the selection marker galK adapted from Warming et al.,45 Ruzsics et al.,46 and Mück-Häusl et al.47
Plasmids were propagated in ElectroMAX DH5α-E Competent Cells (Thermo Fisher Scientific/Invitrogen) and the virus genomes were released from BACs with PacI restriction enzyme (Thermo Scientific). The genomes were transfected into A549 cells with Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer’s instructions.
The viruses were purified with cesium chloride gradient centrifugation. VP concentration was determined with optical density 260 (OD260) reading and infectious units by the tissue culture infectious dose (TCID50) assay. The functionality of the viruses was confirmed by infecting human and hamster cancer cell lines and measuring cell viability with the MTS assay by adding 10% CellTiter 96 AQueous One Solution (Promega) for the cells and reading the absorbance at 490 nm after 2 hr. Functionality assays were repeated at least once.
Cytokine Expression and Biological Activity
A549, SKOV3-Luc, HapT1, and DDT1-MF2 cells were infected for 72 hr. Human IL-2 and TNF-α were detected from cell supernatants using the BD Cytometric Bead Array Human Soluble Protein Master Buffer Kit together with human IL-2 and TNF-α Flex Sets (BD) according to the manufacturer’s instructions. The beads were detected with a BD Accuri Flow Cytometer and the results were analyzed with FCAP Array software (version 3.0.1; BD Biosciences).
Biological activity of the cytokines was confirmed with IL-2-dependent CTLL-2 cells or TNF-α-sensitive L929 cells. CTLL-2 cells were cultured with filtered supernatants or recombinant human IL-2 (R&D Systems) 10 ng/mL at +37°C and 5% CO2 for 72 hr. For TNF-α activity experiments, L929 cells were incubated with cell growth media supernatants or 0.5 ng/mL recombinant human TNF-α (R&D Systems) together with 2 μg/mL actinomycin D for 24 hr. In both assays, viable cells were detected with the MTS assay.
In vivo cytokine expression was examined by injecting established HapT1 tumors with 1 × 108 VPs 48 hr before collection. Human IL-2 and TNF-α levels in serum and in homogenized tumors were quantified with a cytometric bead array and normalized to total protein content.
TIL Extraction and Ex Vivo Killing Assay
Subcutaneous HapT1 tumors established on Syrian hamsters (Mesocricetus auratus, HsdHan:AURA; Envigo) were allowed to develop for 10 days. Tumor fragments (1–3 mm3) were cultured in G-Rex10 (Wilson Wolf) in the presence of 3,000 IU/mL human IL-2 (PeproTech). Half of the medium was replaced with fresh medium containing 1 μg/mL concanavalin A (Con A) (Sigma-Aldrich) after 5 days and every other day thereafter until day 10. Fresh TILs were employed in animal experiments or in an ex vivo killing assay, where HapT1 cells were infected with 5,000 VPs per cell for 72 hr before adding 2.5 × 104 TILs extracted from HapT1 tumors. Cell viability was measured with the MTS assay 24 hr after adding TILs.
Animal Experiments
The Experimental Animal Committee of the University of Helsinki and the Provincial Government of Southern Finland approved the animal experiments performed in this study. The animals were under isoflurane anesthesia during all procedures. Immunocompromised female CB-17 SCID mice (Janvier Labs), aged 4–6 weeks, received 5 × 106 SKOV3-Luc cells intraperitoneally. To investigate the effect of different dosing of the virus, the animals were divided to four groups (n = 3) and treated with Ad5/3-E2F-d24-hTNFa-IRES-hIL2 in concentrations of 1 × 105, 1 × 107, or 1 × 109 VPs in 300 μL PBS intraperitoneally. The control group received PBS.
Animals were imaged once a week with an IVIS 100 imaging system (Xenogen). Three milligrams of D-luciferin (Synchem) in 100 μL PBS was administered intraperitoneally 8 minutes before bioluminescent imaging as previously described.48 To explore the differences in efficacy of the viruses in vivo, the mice were randomized into groups of five to seven mice and treated with 1 × 109 VPs in 300 μL PBS intraperitoneally or PBS alone once a week.
Subcutaneous HapT1 tumors (2 × 106 cells per tumor) were established in 5- to 6-week-old immunocompetent male Syrian hamsters. When the average tumor diameter reached 0.5 cm, the animals were randomized into groups of six to seven. Intratumoral injection of 1 × 109 VPs in 50 μL PBS or PBS alone was performed. Viral treatments were repeated on days 4, 8, 13, and 19. On day 2, hamsters received intratumoral administration of either 4 × 105 HapT1-derived TILs in 50 μL plain RPMI or media only. Twenty-five days after the treatments began, the animals were euthanized and tumors and selected organs were collected to evaluate the histopathological characteristics and immune cell subsets present. The experiment was repeated with reduced virus dosing (1 × 108 VPs on days 1 and 8).
Cured animals were re-challenged with the same tumors (HapT1) or immunologically distinct tumors (DDT1-MF2) (4 × 106 cells/tumor) after a 2-week rest period. Tumor growth was followed for 18 days until DDT1-MF2 tumors reached the maximum tolerated diameter (2 cm).
Flow Cytometric Analyses and Splenocyte Proliferation
CD8b+, CD4+, and MHC class II+ cells were detected from hamster tumors, spleens, and lymph nodes as described by Siurala et al.39 Polyclonal anti-Asialo-GM1 AF488 antibody (eBioscience) was used for detecting natural killer cells and a subset of monocytes/macrophages (0.5 μg per reaction) and anti-human/mouse Galectin-3 (Mac-2) PE (eBioscience) for detecting macrophages and dendritic cells (0.2 μg per reaction). In addition, hamster splenocytes from each treatment group were pooled and assessed for proliferation after 72 hr of cultivation. Cell count was determined with a BD Accuri Flow Cytometer.
Histopathology
Tumor samples along with tissue samples from the hamster heart, lung, liver, spleen, and kidney were collected for pathological evaluation. Samples were fixed in 10% formalin for 48 hr and stored in 70% ethanol until histological processing. Sections at 4-μm thickness were cut from paraffin blocks and the slides were stained with hematoxylin and eosin. A veterinary pathologist evaluated histological changes from the stained samples. Tumors lacking neoplastic cells were considered cured.
Statistical Analyses
Differences between groups were estimated with the two-tailed Student’s t test, non-parametric Mann-Whitney test, or ANOVA with GraphPad Prism software (version 6.05). IBM SSPS Statistics software (version 22.0.0.1) was utilized when analyzing log-transformed tumor volume data from hamster experiment using a linear mixed-effects model and for analyzing the curative effect of TILs with the Wilcoxon signed-rank test. Differences were considered statistically significant when p < 0.05.
Author Contributions
R.H., M.S., S.P., S.T., and A.H. designed the study. R.H., M.S., S.P., S.T. and M.B. developed the methodology. D.M.N., A.E., and A.K. provided materials for the experiments that were performed by R.H., M.S., S.G.-V.-K., S.T., J.M.S., and J.R. R.H. and P.K. analyzed the data. All authors have reviewed the manuscript.
Conflicts of Interest
R.H. has been supported by the University of Helsinki Doctoral Programme in Clinical Research. A.H. received grants from Helsinki University Central Hospital (HUCH) Research Funds, the Sigrid Juselius Foundation, Biocentrum Helsinki, Biocenter Finland, and Finnish Cancer Organizations, and he is a Jane and Aatos Erkko Professor of Oncology at the University of Helsinki. In addition, A.H. is a shareholder in Targovax ASA and an employee and a shareholder in TILT Biotherapeutics, Ltd. M.S., S.P., and J.M.S. are employees of TILT Biotherapeutics, Ltd. The other authors declare no potential conflicts of interest.
Acknowledgments
We thank the Biomedicum Imaging Unit (BIU) and FACS Core Facility of Biomedicum Helsinki for providing the facilities, technical assistance, and software for in vivo imaging and flow cytometry, respectively. The Finnish Centre for Laboratory Animal Pathology (FCLAP) at the University of Helsinki is acknowledged for histopathological sample processing and evaluation. We thank Majlen Fazer and Paul Ehrnrooth for their donation, as well as the Jane and Aatos Erkko Foundation.
Footnotes
Supplemental Information includes Supplemental Materials and Methods, three figures, and one appendix and can be found with this article online at http://dx.doi.org/10.1016/j.omto.2016.12.004.
Supplemental Information
References
- 1.Rosenberg S.A., Yang J.C., Sherry R.M., Kammula U.S., Hughes M.S., Phan G.Q., Citrin D.E., Restifo N.P., Robbins P.F., Wunderlich J.R. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin. Cancer Res. 2011;17:4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larkin J., Chiarion-Sileni V., Gonzalez R., Grob J.J., Cowey C.L., Lao C.D., Schadendorf D., Dummer R., Smylie M., Rutkowski P. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andtbacka R.H., Kaufman H.L., Collichio F., Amatruda T., Senzer N., Chesney J., Delman K.A., Spitler L.E., Puzanov I., Agarwala S.S. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J. Clin. Oncol. 2015;33:2780–2788. doi: 10.1200/JCO.2014.58.3377. [DOI] [PubMed] [Google Scholar]
- 4.Li H., Wang C., Yu J., Cao S., Wei F., Zhang W., Han Y., Ren X.B. Dendritic cell-activated cytokine-induced killer cells enhance the anti-tumor effect of chemotherapy on non-small cell lung cancer in patients after surgery. Cytotherapy. 2009;11:1076–1083. doi: 10.3109/14653240903121252. [DOI] [PubMed] [Google Scholar]
- 5.Zhao X., Zhang Z., Li H., Huang J., Yang S., Xie T., Huang L., Yue D., Xu L., Wang L. Cytokine induced killer cell-based immunotherapies in patients with different stages of renal cell carcinoma. Cancer Lett. 2015;362:192–198. doi: 10.1016/j.canlet.2015.03.043. [DOI] [PubMed] [Google Scholar]
- 6.Itzhaki O., Levy D., Zikich D., Treves A.J., Markel G., Schachter J., Besser M.J. Adoptive T-cell transfer in melanoma. Immunotherapy. 2013;5:79–90. doi: 10.2217/imt.12.143. [DOI] [PubMed] [Google Scholar]
- 7.Radvanyi L.G., Bernatchez C., Zhang M., Fox P.S., Miller P., Chacon J., Wu R., Lizee G., Mahoney S., Alvarado G. Specific lymphocyte subsets predict response to adoptive cell therapy using expanded autologous tumor-infiltrating lymphocytes in metastatic melanoma patients. Clin. Cancer Res. 2012;18:6758–6770. doi: 10.1158/1078-0432.CCR-12-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwartz R.N., Stover L., Dutcher J. Managing toxicities of high-dose interleukin-2. Oncology (Williston Park) 2002;16(Suppl 13):11–20. [PubMed] [Google Scholar]
- 9.Dummer R., Rochlitz C., Velu T., Acres B., Limacher J.M., Bleuzen P., Lacoste G., Slos P., Romero P., Urosevic M. Intralesional adenovirus-mediated interleukin-2 gene transfer for advanced solid cancers and melanoma. Mol. Ther. 2008;16:985–994. doi: 10.1038/mt.2008.32. [DOI] [PubMed] [Google Scholar]
- 10.Parviainen S., Ahonen M., Diaconu I., Kipar A., Siurala M., Vähä-Koskela M., Kanerva A., Cerullo V., Hemminki A. GMCSF-armed vaccinia virus induces an antitumor immune response. Int. J. Cancer. 2015;136:1065–1072. doi: 10.1002/ijc.29068. [DOI] [PubMed] [Google Scholar]
- 11.Turnbull S., West E.J., Scott K.J., Appleton E., Melcher A., Ralph C. Evidence for oncolytic virotherapy: where have we got to and where are we going? Viruses. 2015;7:6291–6312. doi: 10.3390/v7122938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Endo Y., Sakai R., Ouchi M., Onimatsu H., Hioki M., Kagawa S., Uno F., Watanabe Y., Urata Y., Tanaka N., Fujiwara T. Virus-mediated oncolysis induces danger signal and stimulates cytotoxic T-lymphocyte activity via proteasome activator upregulation. Oncogene. 2008;27:2375–2381. doi: 10.1038/sj.onc.1210884. [DOI] [PubMed] [Google Scholar]
- 13.Bramante S., Kaufmann J.K., Veckman V., Liikanen I., Nettelbeck D.M., Hemminki O., Vassilev L., Cerullo V., Oksanen M., Heiskanen R. Treatment of melanoma with a serotype 5/3 chimeric oncolytic adenovirus coding for GM-CSF: results in vitro, in rodents and in humans. Int. J. Cancer. 2015;137:1775–1783. doi: 10.1002/ijc.29536. [DOI] [PubMed] [Google Scholar]
- 14.Khammari A., Nguyen J.M., Saint-Jean M., Knol A.C., Pandolfino M.C., Quereux G., Brocard A., Peuvrel L., Saiagh S., Bataille V. Adoptive T cell therapy combined with intralesional administrations of TG1042 (adenovirus expressing interferon-γ) in metastatic melanoma patients. Cancer Immunol. Immunother. 2015;64:805–815. doi: 10.1007/s00262-015-1691-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tähtinen S., Blattner C., Vähä-Koskela M., Saha D., Siurala M., Parviainen S., Utikal J., Kanerva A., Umansky V., Hemminki A. T cell therapy enabling adenoviruses coding for IL2 and TNF-α induce systemic immunomodulation in mice with spontaneous melanoma. J. Immunother. 2016;39:343–354. doi: 10.1097/CJI.0000000000000144. [DOI] [PubMed] [Google Scholar]
- 16.Tähtinen S., Kaikkonen S., Merisalo-Soikkeli M., Grönberg-Vähä-Koskela S., Kanerva A., Parviainen S., Vähä-Koskela M., Hemminki A. Favorable alteration of tumor microenvironment by immunomodulatory cytokines for efficient T-cell therapy in solid tumors. PLoS ONE. 2015;10:e0131242. doi: 10.1371/journal.pone.0131242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siurala M., Havunen R., Saha D., Lumen D., Airaksinen A.J., Tahtinen S., Cervera-Carrascon V., Bramante S., Parviainen S., Vähä-Koskela M. Adenoviral delivery of tumor necrosis factor-alpha and interleukin-2 enables successful adoptive cell therapy of immunosuppressive melanoma. Mol. Ther. 2016;24:1435–1443. doi: 10.1038/mt.2016.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenberg S.A. IL-2: the first effective immunotherapy for human cancer. J. Immunol. 2014;192:5451–5458. doi: 10.4049/jimmunol.1490019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weide B., Derhovanessian E., Pflugfelder A., Eigentler T.K., Radny P., Zelba H., Pföhler C., Pawelec G., Garbe C. High response rate after intratumoral treatment with interleukin-2: results from a phase 2 study in 51 patients with metastasized melanoma. Cancer. 2010;116:4139–4146. doi: 10.1002/cncr.25156. [DOI] [PubMed] [Google Scholar]
- 20.Trudel S., Trachtenberg J., Toi A., Sweet J., Li Z.H., Jewett M., Tshilias J., Zhuang L.H., Hitt M., Wan Y. A phase I trial of adenovector-mediated delivery of interleukin-2 (AdIL-2) in high-risk localized prostate cancer. Cancer Gene Ther. 2003;10:755–763. doi: 10.1038/sj.cgt.7700626. [DOI] [PubMed] [Google Scholar]
- 21.Hirvinen M., Rajecki M., Kapanen M., Parviainen S., Rouvinen-Lagerström N., Diaconu I., Nokisalmi P., Tenhunen M., Hemminki A., Cerullo V. Immunological effects of a tumor necrosis factor alpha-armed oncolytic adenovirus. Hum. Gene Ther. 2015;26:134–144. doi: 10.1089/hum.2014.069. [DOI] [PubMed] [Google Scholar]
- 22.Balkwill F. Tumour necrosis factor and cancer. Nat. Rev. Cancer. 2009;9:361–371. doi: 10.1038/nrc2628. [DOI] [PubMed] [Google Scholar]
- 23.Mocellin S., Rossi C.R., Pilati P., Nitti D. Tumor necrosis factor, cancer and anticancer therapy. Cytokine Growth Factor Rev. 2005;16:35–53. doi: 10.1016/j.cytogfr.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Heise C., Hermiston T., Johnson L., Brooks G., Sampson-Johannes A., Williams A., Hawkins L., Kirn D. An adenovirus E1A mutant that demonstrates potent and selective systemic anti-tumoral efficacy. Nat. Med. 2000;6:1134–1139. doi: 10.1038/80474. [DOI] [PubMed] [Google Scholar]
- 25.Fueyo J., Gomez-Manzano C., Alemany R., Lee P.S., McDonnell T.J., Mitlianga P., Shi Y.X., Levin V.A., Yung W.K., Kyritsis A.P. A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene. 2000;19:2–12. doi: 10.1038/sj.onc.1203251. [DOI] [PubMed] [Google Scholar]
- 26.Kanerva A., Zinn K.R., Chaudhuri T.R., Lam J.T., Suzuki K., Uil T.G., Hakkarainen T., Bauerschmitz G.J., Wang M., Liu B. Enhanced therapeutic efficacy for ovarian cancer with a serotype 3 receptor-targeted oncolytic adenovirus. Mol. Ther. 2003;8:449–458. doi: 10.1016/s1525-0016(03)00200-4. [DOI] [PubMed] [Google Scholar]
- 27.Hemminki O., Parviainen S., Juhila J., Turkki R., Linder N., Lundin J., Kankainen M., Ristimäki A., Koski A., Liikanen I. Immunological data from cancer patients treated with Ad5/3-E2F-Δ24-GMCSF suggests utility for tumor immunotherapy. Oncotarget. 2015;6:4467–4481. doi: 10.18632/oncotarget.2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koski A., Bramante S., Kipar A., Oksanen M., Juhila J., Vassilev L., Joensuu T., Kanerva A., Hemminki A. Biodistribution analysis of oncolytic adenoviruses in patient autopsy samples reveals vascular transduction of noninjected tumors and tissues. Mol. Ther. 2015;23:1641–1652. doi: 10.1038/mt.2015.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Disis M.L. Mechanism of action of immunotherapy. Semin. Oncol. 2014;41(Suppl 5):S3–S13. doi: 10.1053/j.seminoncol.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 30.Tähtinen S., Grönberg-Vähä-Koskela S., Lumen D., Merisalo-Soikkeli M., Siurala M., Airaksinen A.J., Vähä-Koskela M., Hemminki A. Adenovirus improves the efficacy of adoptive T-cell therapy by recruiting immune cells to and promoting their activity at the tumor. Cancer Immunol. Res. 2015;3:915–925. doi: 10.1158/2326-6066.CIR-14-0220-T. [DOI] [PubMed] [Google Scholar]
- 31.Balza E., Carnemolla B., Mortara L., Castellani P., Soncini D., Accolla R.S., Borsi L. Therapy-induced antitumor vaccination in neuroblastomas by the combined targeting of IL-2 and TNFalpha. Int. J. Cancer. 2010;127:101–110. doi: 10.1002/ijc.25018. [DOI] [PubMed] [Google Scholar]
- 32.Kuei J.H., Tashkin D.P., Figlin R.A. Pulmonary toxicity of recombinant human tumor necrosis factor. Chest. 1989;96:334–338. doi: 10.1378/chest.96.2.334. [DOI] [PubMed] [Google Scholar]
- 33.Russell S.J., Peng K.W., Bell J.C. Oncolytic virotherapy. Nat. Biotechnol. 2012;30:658–670. doi: 10.1038/nbt.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liikanen I., Monsurrò V., Ahtiainen L., Raki M., Hakkarainen T., Diaconu I., Escutenaire S., Hemminki O., Dias J.D., Cerullo V. Induction of interferon pathways mediates in vivo resistance to oncolytic adenovirus. Mol. Ther. 2011;19:1858–1866. doi: 10.1038/mt.2011.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yan Y., Li S., Jia T., Du X., Xu Y., Zhao Y., Li L., Liang K., Liang W., Sun H., Li R. Combined therapy with CTL cells and oncolytic adenovirus expressing IL-15-induced enhanced antitumor activity. Tumour Biol. 2015;36:4535–4543. doi: 10.1007/s13277-015-3098-7. [DOI] [PubMed] [Google Scholar]
- 36.Nishio N., Diaconu I., Liu H., Cerullo V., Caruana I., Hoyos V., Bouchier-Hayes L., Savoldo B., Dotti G. Armed oncolytic virus enhances immune functions of chimeric antigen receptor-modified T cells in solid tumors. Cancer Res. 2014;74:5195–5205. doi: 10.1158/0008-5472.CAN-14-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomas M.A., Spencer J.F., La Regina M.C., Dhar D., Tollefson A.E., Toth K., Wold W.S. Syrian hamster as a permissive immunocompetent animal model for the study of oncolytic adenovirus vectors. Cancer Res. 2006;66:1270–1276. doi: 10.1158/0008-5472.CAN-05-3497. [DOI] [PubMed] [Google Scholar]
- 38.Gowen B.B., Judge J.W., Wong M.H., Jung K.H., Aylsworth C.F., Melby P.C., Rosenberg B., Morrey J.D. Immunoprophylaxis of Punta Toro virus (Phlebovirus, Bunyaviridae) infection in hamsters with recombinant Eimeria profilin-like antigen. Int. Immunopharmacol. 2008;8:1089–1094. doi: 10.1016/j.intimp.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siurala M., Vähä-Koskela M., Havunen R., Tähtinen S., Bramante S., Parviainen S., Mathis J.M., Kanerva A., Hemminki A. Syngeneic Syrian hamster tumors feature tumor-infiltrating lymphocytes allowing adoptive cell therapy enhanced by oncolytic adenovirus in a replication permissive setting. OncoImmunology. 2016;5:e1136046. doi: 10.1080/2162402X.2015.1136046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liao W., Lin J.X., Leonard W.J. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity. 2013;38:13–25. doi: 10.1016/j.immuni.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quezada S.A., Simpson T.R., Peggs K.S., Merghoub T., Vider J., Fan X., Blasberg R., Yagita H., Muranski P., Antony P.A. Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J. Exp. Med. 2010;207:637–650. doi: 10.1084/jem.20091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Cesaris P., Starace D., Riccioli A., Padula F., Filippini A., Ziparo E. Tumor necrosis factor-alpha induces interleukin-6 production and integrin ligand expression by distinct transduction pathways. J. Biol. Chem. 1998;273:7566–7571. doi: 10.1074/jbc.273.13.7566. [DOI] [PubMed] [Google Scholar]
- 43.Tysome J.R., Li X., Wang S., Wang P., Gao D., Du P., Chen D., Gangeswaran R., Chard L.S., Yuan M. A novel therapeutic regimen to eradicate established solid tumors with an effective induction of tumor-specific immunity. Clin. Cancer Res. 2012;18:6679–6689. doi: 10.1158/1078-0432.CCR-12-0979. [DOI] [PubMed] [Google Scholar]
- 44.Cerullo V., Pesonen S., Diaconu I., Escutenaire S., Arstila P.T., Ugolini M., Nokisalmi P., Raki M., Laasonen L., Särkioja M. Oncolytic adenovirus coding for granulocyte macrophage colony-stimulating factor induces antitumoral immunity in cancer patients. Cancer Res. 2010;70:4297–4309. doi: 10.1158/0008-5472.CAN-09-3567. [DOI] [PubMed] [Google Scholar]
- 45.Warming S., Costantino N., Court D.L., Jenkins N.A., Copeland N.G. Simple and highly efficient BAC recombineering using galK selection. Nucleic Acids Res. 2005;33:e36. doi: 10.1093/nar/gni035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruzsics Z., Wagner M., Osterlehner A., Cook J., Koszinowski U., Burgert H.G. Transposon-assisted cloning and traceless mutagenesis of adenoviruses: development of a novel vector based on species D. J. Virol. 2006;80:8100–8113. doi: 10.1128/JVI.00687-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mück-Häusl M., Solanki M., Zhang W., Ruzsics Z., Ehrhardt A. Ad 2.0: a novel recombineering platform for high-throughput generation of tailored adenoviruses. Nucleic Acids Res. 2015;43:e50. doi: 10.1093/nar/gkv031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hemminki O., Bauerschmitz G., Hemmi S., Lavilla-Alonso S., Diaconu I., Guse K., Koski A., Desmond R.A., Lappalainen M., Kanerva A. Oncolytic adenovirus based on serotype 3. Cancer Gene Ther. 2011;18:288–296. doi: 10.1038/cgt.2010.79. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.