Abstract
Background
The variability of visit-to-visit (VVV) in systolic blood pressure (SBP) and diastolic blood pressure (DBP) is proved as a predictor of renal function deterioration in patients with non-diabetic chronic kidney disease. The purpose of this study was to investigate the relationship of the variability in SBP and the magnitude of renal function impairment for normal renal function patients in the first 10-years diagnosed with type II diabetes mellitus (DM).
Methods
We retrospectively reviewed the electronic medical records of 789 patients who were first diagnosed with diabetes mellitus during 2000–2002 and regularly followed for 10 years with a total of 53,284 clinic visits. The stages of Chronic Kidney Disease (CKD) of every patient were determined using estimated glomerular filtration rate. The occurrence of nephropathy was defined in those patients whose CKD stages elevated equal or larger than three.
Results
Patients were categorized according to the VVV of systolic and diastolic BP into three groups. Patients with high VVV of both SBP and DBP had a 2.44 fold (95% CI: 1.88–3.17, p < 0.001) increased risk of renal function impairment compared with patients with low VVV of both SBP and DBP. Risk of renal function impairment for patients with high VVV of either SBP or DBP had a 1.43-fold increase (95% CI: 1.08–1.89, p = 0.012) compared with patients with low VVV of both SBP and DBP. Cox regression analysis also demonstrated that every 1-year increase of DM diagnosed age significantly raised the risk of renal function impairment with a hazard ration of 1.05 (95% CI: 1.04–1.06, p < 0.001).
Conclusions
Not only VVV of SBP but also VVV in DBP is correlated with diabetic nephropathy in the first decade for patients diagnosed with type 2 DM.
Keywords: Blood pressure control, Chronic kidney disease, Electronic medical record, Hypertension
Background
Diabetes mellitus (DM) is the primary cause of end stage renal disease [1]. In adults aged 18 years or older with DM, 71% are reported to have hypertension [1], a major risk of microvascular complications and cardiovascular mortality [2]. In patients with DM, several risk factors including mean blood pressure (BP), albuminuria, high hemoglobin A1c and serum cholesterol have been shown to accelerate the progression of chronic kidney disease (CKD) [3, 4].
In diabetic patients with CKD, the decline of the glomerular filtration rate (GFR) is highly variable, ranging from 2 to 20 mL/min/year [3]. The risk factors for losing filtration power, such as hypertension, proteinuria, glycemic control and lipids, have not been studied extensively. Controversy existed as some of these factors contributed to renal function impairment in diabetic patients [5]. To identify the risk factors of renal function deterioration is important for development of prevention modalities in diabetic patients’ treatment. In clinical diabetic treatment guideline [6], absolute BP is used as a therapeutic target to prevent clinical stroke and heart disease, as well as CKD with paucity of evidence [7].
Recently, the visit-to-visit variability (VVV) of systolic BP (SBP) has been shown to be a novel risk factor for development of renal function decline in non-diabetic CKD [8], progression of albuminuria and nephropathy in patients with type II DM [9, 10], and deterioration of renal function for stage 3–4 diabetic CKD patients [4]. Although it is widely known that average blood pressure is related to renal function deterioration. However, little is known about the long-term association of VVV of SBP and diastolic BP (DBP) with renal function impairment in patients with normal renal function at the diagnosis of DM. The association between VVV of BP and CKD generally consider SBP measurements at a few time points and in a short to medium follow-up period, limiting the appreciation of the full impact of SBP and DBP on CKD. BP fluctuation across long periods and its effect on renal function impairment in diabetic patients with normal renal function are typically not considered. Therefore, we evaluated the long-term relationship between the VVV of SBP and DBP and the change of the CKD stage in patients from the beginning of diagnosed with type 2 DM.
Methods
Patients and study design
We retrospectively collect the 10-year measurements of blood pressure, body weight, body height, and laboratory datas at every outpatient clinic visit of 789 patients who were first diagnosed with type 2 DM during 2000–2002 at Chang Gung Memorial Hospital, Keelung. Type 2 DM was diagnosed in accordance with the criteria of American Diabetes Association [11]. Body mass index (BMI) was defined as weight (kilograms) divided by height (meters) squared. Patients were classified as nonsmokers, former smokers, or current smokers according to the electronic medical record. Patients with advanced renal dysfunction (serum Cr more than 2.0 mg/dL) before diagnosed with DM were excluded from this study. Cardiovascular disease (CVD) included coronary artery disease or myocardial infarction, and ischemic stroke or transient ischemic attack [12] that resulted from atherosclerosis after type II diabetes was diagnosed. The coronary artery disease was confirmed by coronary angiography and the ischemic stroke or transient ischemic attack was confirmed by computed tomography or clinical symptoms. The definition of dyslipidemia was either total cholesterol >200 mg/dL, low density lipoprotein cholesterol >100 mg/dL, low density lipoprotein cholesterol <50 mg/dL in female and <40 mg/dL in male, or triglyceride >150 mg/dL which were based on the standards of the laboratory in our hospital. Hypertension was defined as systolic pressure ≥130 mmHg or diastolic pressure ≥ 80 mmHg in diabetic patients [13].
We then evaluated relationships of variability in blood pressure to change of CKD stage during the 10-year follow-up period. The study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of Chang Gung Memorial Hospital; informed consent was waived. Blood pressure measurements at every outpatient clinic visit throughout the follow-up period were recorded. Fasting serum total cholesterol, low-density lipoprotein, high-density lipoprotein, and triglyceride concentrations were assessed using standard enzymatic methods. Hemoglobin A1c was assayed using high-performance liquid chromatography and expressed with the unit defined by the National Glycohemoglobin Standardization Program.
Definition of BP variability
Throughout the 10-year consecutive visits from the beginning of the observation period, the mean office BP and the VVV of SBP and DBP (expressed as within-individual standard deviation (SD) were calculated. The BP instability indice was expressed as the delta BP, which was defined as a difference between the maximum and the minimum BP, through all 10-year visits [14].
Definition of CKD and renal function impairment
Serial serum creatinine data were collected and eGFR was determined by the abbreviated CKD Epidemiology Collaboration equation [15]. CKD was defined as a decreased estimated glomerular filtration rate (eGFR) (<60 ml/min per 1.73 m2) for 3 months. CKD stage was defined in accordance with the guideline of National Kidney Foundation [16], which stage 1, 2, 3, 4, and 5 had a eGFR of ≥ 90, 60 to 90, 30 to 59, 15–29, and < 15 ml/min per 1.73 m2 or commencement of dialysis therapy, respectively. Renal function impairment was defined as two or more CKD stages (from stage 1 or 2 to stage 3–5 or 4–5, respectively) deterioration without recovery.
Statistical analysis
Means and frequencies of potential confounding variables were calculated. The relationships between variability in SBP and DBP, as well as other variables, and renal function impairment were examined by Pearson’s correlation analyses. To examine the effects of various factors on the deterioration of renal function, the following factors were considered simultaneously as independent variables for Cox multiple regression analysis: age of DM diagnosed, sex, BMI, average SBP and DBP, SD of SBP and DBP, hemoglobin A1c, total cholesterol, triglyceride, smoking status, presence of CVD, hypertension and dyslipidemia. All continuous variables are presented as the mean ± SD or absolute number. A P value <0.05 was considered statistically significant. The area under each receiver operating curve (ROC) and 95% confidence intervals (CI) were estimated to compare the relative ability of SD of SBP and DBP to identify risk of renal function impairment in diabetic patients. Optimal cut-off points for SD of SBP and DBP indicator were determined [17]. The collinearity among average SBP and DBP, SD of SBP and DBP, and delta SBP and DBP was estimated using variance inflation factor [18].
Results
Eight hundred and twenty-five patients were first diagnosed with DM from 2000 to 2002. Thirty-six patients who were died or loss of follow-up were excluded. None of these patients died from renal failure. The characteristics of the 789 patients, who were first diagnosed with DM from 2000 to 2002 and followed for 10 years, enrolled in this study are shown in Table 1. The total number of measurements of BP, BMI, HbA1c, lipid profile, and serum creatinine throughout 10- year of data collection was 49739, 35432, 27424, 9327, and 14123, respectively. The characteristics of the study patients were shown in Table 1. The overall mean age of the patients diagnosed with DM was 53.3 ± 10.5 years. At baseline, the mean initial serum creatinine was 0.93 ± 0.45 mg/dL, the mean initial eGFR was 88.6 ± 22.7 mL/min per 1.73 m2, and the mean office SBP and DBP was 136.6 ± 10.1 and 73.5 ± 6.3 mm Hg, respectively. The median observation period was 4451 ± 453 days. At the end of the observation period, the mean serum creatinine level was 1.10 ± 0.81 mg/dL and the mean eGFR was 75.1 ± 27.6 mL/min per 1.73 m2. The 10 year mean change of CKD stage was 1.2 ± 0.8.
Table 1.
Patients’ demographic and clinical characteristics
| Patients (n) | 789 |
| Age at diabetes diagnosis (years) | 53.3 ± 10.5 |
| Sex (male/female) | 373/416 |
| Smoking (none/former/current) | 598/45/146 |
| Hypertension (%) | 597 (75.7) |
| Hyperlipidemia (%) | 758 (96.1) |
| Body mass index (kg/m2) | 26.8 ± 3.9 |
| Mean number of measurements | 45.0 ± 24.5 |
| Mean SBP (mmHg) | 137 ± 10 |
| Mean number of measurement | 63.0 ± 28.9 |
| SD of SBP (mmHg) | 14.7 ± 3.6 |
| Delta SBP (mmHg) | 71.7 ± 25.0 |
| Mean DBP (mmHg) | 73.5 ± 6.3 |
| SD of DBP (mmHg) | 7.4 ± 2.0 |
| Delta DBP (mmHg) | 37.7 ± 14.3 |
| Hemoglobin A1c (%) (mmol/mol) |
7.6 ± 1.0 (60.0 ± 10.9) |
| Mean number of measurements | 34.8 ± 10.9 |
| Total cholesterol (mg/dL) | 193.4 ± 28.6 |
| Mean number of measurements | 11.9 ± 5.8 |
| High-density lipoprotein (mg/dL) | 38.5 ± 10.7 |
| Low-density lipoprotein (mg/dL) | 118.7 ± 20.3 |
| Triglyceride (mg/dL) | 149.8 ± 113.8 |
| Initial eGFR (mL/min/1.73 m2) | 88.6 ± 22.7 |
| Mean number of measurements | 17.9 ± 6.7 |
| Final eGFR (mL/min/1.73 m2) | 75.1 ± 27.6 |
| Clinical Events during10-year follow-up | |
| CVD a (%) | 115 (14.6) |
| Interval from diabetes diagnosis (years) | 5.2 ± 3.1 |
| Change in CKD stage | 1.2 ± 0.7 |
| Renal function impairment (%) | 309 (39.2) |
| CKD stage 4 or 5 (%) | 83 (10.5) |
| Total follow-up period (days) | 4451 ± 453 |
Abbreviations: SBP systolic blood pressure, DBP diastolic blood pressure, SD standard deviation, eGFR estimated glomerular filtration rate, CVD cardiovascular disease, CKD chronic kidney disease
aDefined as coronary artery disease or myocardial infarction, and ischemic stroke or transient ischemic attack
Cox regression analyses revealed that the SD of SBP was positively correlated with the occurrence of renal function impairment (P < 0.001, Hazard ratio (HR) = 1.063, 95% CI = 1.028–1.100), as well as the SD of DBP (P < 0.024, HR = 1.081, 95% CI = 1.010–1.156). The age of DM first diagnosed had also positively correlated with the occurrence of renal function impairment after 10-year follow-up (P < 0.001, HR = 1.048, 95% CI = 1.036–1.060). Our results found that maximum, minimum or delta of SBP and DBP had no significant independent correlations between renal function impairment after 10-year of DM diagnosed. And multiple regression analysis demonstrated that other factors, such as mean or SD of hemoglobin A1c, BMI, total cholesterol, low-density lipoprotein, high-density lipoprotein, triglyceride, were not independently correlated with the occurrence of renal function impairment, as shown in Table 2.
Table 2.
Multivariate Cox regression analyses of renal function impairment in 789 patients after 10-year diabetes diagnosis
| Independent variable | β | P Value |
|---|---|---|
| Sex (female = 0) | 0.250 | 0.081 |
| Age of DM diagnosed | 0.047 | <0.001 |
| Non-smoking | ||
| Former smoker | 0.004 | 0.990 |
| Current smoker | 0.098 | 0.560 |
| Hypertension | 0.687 | 0.001 |
| Dyslipidemia | 0.119 | 0.772 |
| Mean SBP | 0.023 | 0.435 |
| SD of SBP | 0.062 | <0.001 |
| CV of SBP | 2.414 | 0.944 |
| Delta of SBP | −0.005 | 0.227 |
| Mean DBP | −0.048 | 0.269 |
| SD of DBP | 0.077 | 0.024 |
| CV of DBP | −0.047 | 0.856 |
| Delta of DBP | 0.002 | 0.787 |
| Mean BMI | 0.84 | 0.047 |
| SD of BMI | −0.293 | 0.119 |
| Mean HbA1c | −0.167 | 0.251 |
| SD of HbA1c | 0.214 | 0.485 |
| Mean serum cholesterol | 0.004 | 0.482 |
| SD of serum cholesterol | −0.008 | 0.111 |
| Mean serum LDL | −0.013 | 0.053 |
| SD of serum LDL | −0.001 | 0.860 |
| Mean serum HDL | 0.024 | 0.357 |
| SD of serum HDL | 0.006 | 0.726 |
| Mean serum triglyceride | −0.004 | 0.047 |
| SD of serum triglyceride | 0.002 | 0.509 |
Abbreviations: HbA1c hemoglobin A1c, SBP systolic blood pressure, DBP diastolic blood pressure, SD standard deviation, CV coefficient of variation, BMI body mass index, LDL low density lipoprotein, HDL high density lipoprotein
For clinical application, we calculated the area under the ROC curves for the SD of SBP (0.87 ± 0.02) and DBP (0.85 ± 0.03) and categorized patients into high or low SD of SBP or DBP. The best cut-point BP was calculated based on the Youden Index [19], which was calculated as sensitivity + specificity − 1. Cut-off points of SD of SBP and DBP, where sensitivity approximates specificity for renal function impairment, are 16.3 and 7.6 mmHg, respectively. Patients with SD of SBP and DBP higher than the cut-off values were defined as high VVV of SBP and DBP, respectively. Patients were grouped as low VVV of SBP and DBP, high VVV of SBP or DBP, and high VVV of SBP and DBP. The characteristics of patients in these three groups were shown in Table 3. Using univariate analysis, the age of DM diagnosed, hypertension history, BMI, mean SBP and DBP, SD of SBP and DBP, delta SBP and DBP, and mean hemoglobin A1c, and initial eGFR were significantly different between these three groups of patients.
Table 3.
Demographic and clinical characteristics compared between patients with low VVV of SBP and DBP, high VVV of SBP or DBP, and high VVV of SBP and DBP
| Low VVV of SBP and DBP group (n = 370) | High VVV of SBP or DBP group (n = 241) | High VVV of SBP and DBP group (n = 178) | P | |
|---|---|---|---|---|
| Age at diabetes diagnosis (years) | 52.8 ± 10.1 | 51.5 ± 10.4 | 56.6 ± 10.6 | <0.001 |
| Sex (male/female) | 172 (46.5) | 120 (49.8) | 81 (47.3) | 0.629 |
| Smoking (none/former/current) | 287/17/66 | 179/18/44 | 132/10/36 | 0.594 |
| Hypertension (%) | 237 (64.1) | 199 (82.6) | 161 (90.4) | <0.001 |
| Hyperlipidemia (%) | 358 (96.8) | 227 (94.2) | 173 (97.2) | 0.191 |
| Body mass index (kg/m2) | 26.4 ± 3.6 | 27.3 ± 4.2 | 27.0 ± 3.9 | 0.007 |
| Mean SBP (mmHg) | 133.9 ± 9.6 | 137.3 ± 9.2 | 141.2 ± 10.6 | <0.001 |
| Mean number of measurements | 60.4 ± 26.0 | 63.8 ± 29.9 | 67.8 ± 32.6 | 0.019 |
| SD of SBP (mmHg) | 12.3 ± 2.0 | 14.9 ± 2.6 | 19.3 ± 2.8 | <0.001 |
| Delta SBP (mmHg) | 59.3 ± 15.1 | 73.3 ± 23.0 | 95.2 ± 26.6 | <0.001 |
| Mean DBP (mmHg) | 71.6 ± 5.5 | 75.3 ± 5.9 | 74.8 ± 7.2 | <0.001 |
| SD of DBP (mmHg) | 6.0 ± 0.9 | 8.1 ± 1.4 | 9.6 ± 1.7 | <0.001 |
| Delta DBP (mmHg) | 29.4 ± 6.7 | 41.7 ± 13.4 | 49.5 ± 16.6 | <0.001 |
| HbA1c (%) (mmol/mol) |
7.4 ± 0.9 (57.0 ± 9.8) |
7.8 ± 1.0 (62.0 ± 10.9) |
7.7 ± 1.1 (61.0 ± 12.0) |
<0.001 |
| Mean number of measurements | 36.1 ± 10.8 | 34.5 ± 10.5 | 32.3 ± 11.2 | 0.001 |
| Total cholesterol (mg/dL) | 191.8 ± 26.7 | 193.0 ± 27.0 | 197.1 ± 33.9 | 0.132 |
| Mean number of measurements | 12.1 ± 5.9 | 11.9 ± 5.7 | 11.2 ± 5.8 | 0.220 |
| High-density lipoprotein (mg/dL) | 38.9 ± 10.6 | 38.1 ± 10.8 | 38.4 ± 10.7 | 0.678 |
| Low-density lipoprotein (mg/dL) | 118.3 ± 19.2 | 119.3 ± 20.9 | 118.8 ± 21.6 | 0.832 |
| Triglyceride (mg/dL) | 141.0 ± 117.4 | 155.6 ± 90.3 | 160.1 ± 132.4 | 0.119 |
| Initial eGFR (mL/min/1.73 m2) | 81.4 ± 26.0 | 77.5 ± 25.9 | 59.0 ± 27.0 | 0.003 |
| Mean number of measurements | 17.4 ± 6.4 | 17.6 ± 6.4 | 19.3 ± 7.5 | 0.005 |
| Final eGFR (mL/min/1.73 m2) | 88.6 ± 26.6 | 76.0 ± 26.6 | 55.9 ± 27.6 | <0.001 |
| Clinical Events during10-year follow-up | ||||
| CVD a (%) | 28 (7.6) | 43 (17.8) | 44 (24.7) | <0.001 |
| Interval from diabetes diagnosis (years) | 5.8 ± 2.9 | 5.1 ± 2.7 | 4.8 ± 3.5 | 0.425 |
| Change in CKD stage | 1.0 ± 0.6 | 1.1 ± 0.6 | 1.5 ± 0.8 | <0.001 |
| Renal function impairment (%) | 110 (29.3) | 92 (36.8) | 125 (62.8) | <0.001 |
| Interval from diabetes diagnosis (days) | 3899 ± 1214 | 3710 ± 1285 | 3079 ± 1468 | <0.001 |
| CKD stage 4 or 5 (%) | 35 (9.3) | 29 (11.6) | 22 (11.1) | 0.620 |
| Interval from diabetes diagnosis (days) | 4366 ± 620 | 4311 ± 676 | 4272 ± 730 | 0.267 |
| Total follow-up (days) | 4488 ± 400 | 4424 ± 474 | 4413 ± 521 | 0.102 |
Abbreviations: HbA1c hemoglobin A1c, SBP systolic blood pressure, DBP diatolic blood pressure, SD standard deviation, eGFR estimated glomerular filtration rate, CVD cardiovascular disease, CKD chronic kidney disease
aDefined as coronary artery disease or myocardial infarction, and ischemic stroke or transient ischemic attack
All significant change with p<0.05 had been italicized
After 10 years of DM diagnosis, the patients with high VVV of both SBP and DBP had the highest percentage of peripheral artery disease, cerebrovascular disease, coronary artery disease or myocardial infarction, transient ischemic attack or stroke, and the highest percentage of patients with renal function impairment, which all were significantly different among these three groups. Cox multivariate regression revealed that only the age of DM diagnosed and the group of VVV of SBP and DBP were significant risk factors for development of renal function impairment after 10-year follow-up, as shown in Table 4. All the variance inflation factors among mean, SD and delta of SBP and DBP were less than three, which excluded the collinearity between these factors. The risk of renal function impairment in patients with high VVV of both SBP and DBP significantly increased 2.773 fold (p < 0.001, 95% CI = 2.128–3.612) compared that of patients with low VVV of both SBP and DBP. Whereas the risk of renal function impairment in patients with wither high VVV of SBP or DBP increased 1.587 fold (p = 0.001, 95% CI = 1.195–2.107) compared that of patients with low VVV of both SBP and DBP. The renal function intact survival curve for these three groups of patients was shown in Fig. 1.
Table 4.
Multivariable Cox regression analysis of renal function impairment
| Hazard Ratio | 95% CI | P | |
|---|---|---|---|
| Age at diabetes diagnosis (+1 year) | 1.046 | 1.034–1.058 | <0.001 |
| Low VVV of SBP and DBP | 1 | ||
| High VVV of SBP or DBP | 1.587 | 1.195–2.107 | 0.001 |
| High VVV of SBP and DBP | 2.773 | 2.128–3.612 | <0.001 |
Abbreviations: SBP systolic blood pressure, DBP diastolic blood pressure, SD standard deviation, CI confidence interval
Fig. 1.
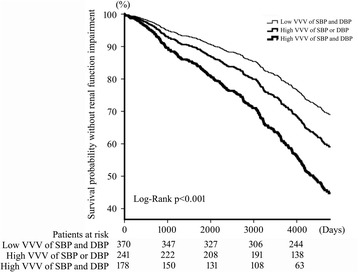
Kaplan-Meier plot of renal function impairment in newly diagnosed type II diabetic patients for 10-year follow-up. Patients was grouped into low VVV of SBP and DBP, high VVV of SBP or DBP, and high VVV of SBP and DBP
Discussion
This study showed that VVV of both SBP and DBP were significantly associated with the change to CKD stage in the first decade of patients diagnosed with DM, whereas mean office SBP/DBP, delta SBP/DBP, mean serum lipid profile, mean hemoglobin A1c concentration, and SD of hemoglobin A1c concentration were not correlated with the occurrence of renal function impairment. To the best of our knowledge, this is the first study to explore the association of the VVV of SBP and DBP and renal function decline in the first decade of patients with DM.
In the present study, not only the VVV of SBP was significantly associated with the change of CKD stage, but the VVV of DBP was also significantly associated with the occurrence of renal function impairment in our study patients group. For the first time, our result demonstrated that a VVV of SBP higher than 16.3 mmHg or a VVV of DBP higher than 7.6 mmHg would significantly increase the risk of decline of renal function in the first decade of patients diagnosed with DM. Furthermore, with both high VVV of SBP and DBP would even increase the risk of renal function impairment to 2.773 fold compared with those patients with both low VVV of SBP and DBP.
Our results are in accordance with other study findings, which showed a significant association between VVV of BP and progression of nondiabetic CKD [8, 9]. However, in contrast with our findings, the other study with a small sample size (69 patients) and short study period (32 months) from Yokota et al. reported negative association between VVV of BP and renal function deterioration in CKD patients [4]. The other large cohort study included 114,900 adults with CKD stage 3–4 followed for 180 days revealed that the highest quintile of the SD would increase the risk of end-stage renal disease by 1.45-fold (CI 1.02–2.05) [20].
Many studies proved that an increase in VVV of SBP was one of the factors which may contribute to the progression of renal function deterioration [9, 10]. Mancia et al. suggested that a steeper rate of blood pressure oscillations, in addition to blood pressure levels, was related to end-organ damage in hypertensive patients [21]. In hypertensive individuals, large arteries lose their compliance and became stiff in hypertensive patients which resulted in less buffering of blood pressure changes [22] and wider blood pressure oscillation for any given change in the stroke volume, which would be detected with the fluctuations of blood pressure [23]. The initiation and progression of atherosclerosis of the renal vasculature, resulted from the oscillatory shear stress, could contribute to the impairment of renal function [24]. However, these studies did not prove that high VVV of DBP might be another factor result in the renal function impairment in DM patients. Our 10-year long-term follow-up results revealed that high VVV of DBP had detrimental effects on the renal function of diabetic patients. The impact of DBP in renal function ad been proved by many studies. Brazy et al. stressed that reduced DBP, instead of SBP or mean BP, to less than 90 mmHg could slow down the rate of renal function deterioration [25]. Wight et al. reported a positive correlation was observed between proteinuria and DBP [26]. The discrepancy of our results from the other VVV-related studies might come from the duration of the study design. Most related reports included diabetic patients in CKD stage 3 or higher and followed less than 5 years. Our study included intact renal function patients who were in the first decade of DM diagnosed. The long-term period of our study might be the most important factor that made high VVV of DBP stand out as a risk factor of renal function impairment.
The retrospective nature of the present study and the sample size are two of the limitations of the present study. The possibility of type 2 error existed. Other limitations are the standardized procedure of blood pressure measurement and the medication prescription record through 10-year follow-up period. Certain classes of antihypertensive regimens, such as non-β-blocker-based and non-rennin-angiotensin system-based [27] or calcium channel blockers [28], had been prove in reduction of the VVV of blood pressure with preservation of end-organ damage in hypertensive patients. The anti-hypertensive drugs in each patient were not consistent throughout 10 years. It is very difficult to clarify the effect of VVV amelioration of each category of anti-hypertensive drugs, such as calcium channel blockers, which had been proved to blunt the association between the VVV of BP and renal function decline [4, 28]. Another limitation of the present study is the absence of data regarding antihypertensive prescription fill data and patients’ adherence to medication regimens. However, low antihypertensive medication adherence explained only a small proportion of VVV of BP [29], which implied that the absence of medication adherence data does not have a major impact on the result of the present study.
Nevertheless, this study has several strengths including the one-decade follow-up of patients initially diagnosed with DM with CKD and available information on demographic, clinical, and long-term BP data. In addition, the use of the electronic medical record database provided real-world evidence on the status of hypertension control in the first decade renal function prognosis of diabetic patients and minimizes selection bias related to self selection into the study. Our results prove that VVV of SBP and DBP has significant prognostic value. However, prospective investigations in stabilization of VVV of SBP and DBP are in need to identify the optimal therapeutic strategies and potentially modify the clinical practice in hypertensive patients with type II DM.
Conclusions
In conclusion, the present study showed, in patients with intact renal function, significant association between high VVV of SBP and DBP with renal function decline in the first decade of DM diagnosed.
Acknowledgement
None
Funding
This study was sponsored by Chang Gung Medical Research Program grant of Chang Gung Memorial Hospital (CMRPG2C0101, CMRPG2F0161, and CMRPG2F0041).
Availability of data and materials
The data that support the findings of this study are available from Chang Gung Biological Databases but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission from the Institutional Review Board of Chang Gung Memorial Hospital.
Authors’ contributions
CHY wrote manuscript, researched data. HCY researched data. TYH, PFH, YCW, TPC, and SYY contributed to conception and design of data, critically revised manuscript. All authors read and approved the final manuscript.
Competing interests
There authors declare that they have no competing interests.
Consent for publication
Not Applicable
Ethics approval and consent to participate
The study was conducted in accord with the Declaration of Helsinki and was approved by the Chang Gung Medical Foundation Institutional Review Board (102-4558C). Administrative permission was granted to access the patient database. Since all patient data were anonymous, patients’ signed informed consent was waived.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- BMI
Body mass index
- BP
Blood pressure
- CKD
Chronic kidney disease
- CVD
Cardiovascular disease
- DBP
Diastolic blood pressure
- eGFR
estimated Glomerular filtration rate
- GFR
Glomerular filtration rate
- HbA1c
Hemoglobin A1c
- HR
Hazard ratio
- SBP
Systolic blood pressure
- SD
Standard deviation
- VVV
Visit-to-visit variability
Contributor Information
Chi-Hsiao Yeh, Phone: 886-2-24313131, Email: yehccl@cgmh.org.tw.
Hsiu-Chin Yu, Email: yu4009np@gmail.com.
Tzu-Yen Huang, Email: s201018@gmail.com.
Pin-Fu Huang, Email: pinfu@cgmh.org.tw.
Yao-Chang Wang, Email: ycwang@cloud.cgmh.org.tw.
Tzu-Ping Chen, Email: kkl3490@yahoo.com.tw.
Shun-Ying Yin, Email: yinsten@cloud.cgmh.org.tw.
References
- 1.Centers for Disease Control and Prevention . National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. Atlanta: U.S. Department of Health and Human Services; 2014. [Google Scholar]
- 2.Hsieh YT, Tu ST, Cho TJ, Chang SJ, Chen JF, Hsieh MC. Visit-to-visit variability in blood pressure strongly predicts all-cause mortality in patients with type 2 diabetes: a 5.5-year prospective analysis. Eur J Clin Invest. 2012;42:245–53. doi: 10.1111/j.1365-2362.2011.02574.x. [DOI] [PubMed] [Google Scholar]
- 3.Hovind P, Rossing P, Tarnow L, Smidt UM, Parving HH. Progression of diabetic nephropathy. Kidney Int. 2001;59:702–9. doi: 10.1046/j.1523-1755.2001.059002702.x. [DOI] [PubMed] [Google Scholar]
- 4.Yokota K, Fukuda M, Matsui Y, Kario K, Kimura K. Visit-to-visit variability of blood pressure and renal function decline in patients with diabetic chronic kidney disease. J Clin Hypertens (Greenwich) 2014;16:362–6. doi: 10.1111/jch.12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rossing P. Promotion, prediction and prevention of progression of nephropathy in type 1 diabetes mellitus. Diabet Med. 1998;15:900–19. doi: 10.1002/(SICI)1096-9136(1998110)15:11<900::AID-DIA709>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 6.American Diabetes Association Cardiovascular disease and risk management. Sec. 8. In Standards of Medical Care in Diabetes 2016. Diabetes Care. 2016;39 Suppl 1:S60–71. doi: 10.2337/dc16-S011. [DOI] [PubMed] [Google Scholar]
- 7.Cooper-DeHoff RM, Gong Y, Handberg EM, Bavry AA, Denardo SJ, Bakris GL, Pepine CJ. Tight blood pressure control and cardiovascular outcomes among hypertensive patients with diabetes and coronary artery disease. JAMA. 2010;304:61–8. doi: 10.1001/jama.2010.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yokota K, Fukuda M, Matsui Y, Hoshide S, Shimada K, Kario K. Impact of visit-to-visit variability of blood pressure on deterioration of renal function in patients with non-diabetic chronic kidney disease. Hypertens Res. 2013;36:151–7. doi: 10.1038/hr.2012.145. [DOI] [PubMed] [Google Scholar]
- 9.Okada H, Fukui M, Tanaka M, Matsumoto S, Mineoka Y, Nakanishi N, Asano M, Yamazaki M, Hasegawa G, Nakamura N. Visit-to-visit blood pressure variability is a novel risk factor for the development and progression of diabetic nephropathy in patients with type 2 diabetes. Diabetes Care. 2013;36:1908–12. doi: 10.2337/dc12-2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okada H, Fukui M, Tanaka M, Inada S, Mineoka Y, Nakanishi N, Senmaru T, Sakabe K, Ushigome E, Asano M, Yamazaki M, Hasegawa G, Nakamura N. Visit-to-visit variability in systolic blood pressure is correlated with diabetic nephropathy and atherosclerosis in patients with type 2 diabetes. Atherosclerosis. 2012;220:155–9. doi: 10.1016/j.atherosclerosis.2011.10.033. [DOI] [PubMed] [Google Scholar]
- 11.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37 suppl 1:S81–90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- 12.Mendis S, Puska P, Norrving B, editors. Global atlas on cardiovascular disease prevention and control- Policies, strategies and interventions. Switzerland: World Health Organization; 2011. [Google Scholar]
- 13.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jones DW, Materson BJ, Oparil S, Wright JT, Roccella EJ, The National High Blood Pressure Education Program Coordinating Committee The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 14.Nagai M, Hoshide S, Ishikawa J, Shimada K, Kario K. Visit-to-visit blood pressure variations: new independent determinants for carotid artery measures in the elderly at high risk of cardiovascular disease. J Am Soc Hypertens. 2011;5:184–92. doi: 10.1016/j.jash.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Kidney Foundation K/DOQI Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification and Stratification. Am J Kidney Dis. 2002;39(suppl 1):S1–266. [PubMed] [Google Scholar]
- 17.Van der Schouw YT, Verbeek AL, Ruijs JH. ROC curves for the initial assessment of new diagnostic tests. Fam Pract. 1992;9:506–11. doi: 10.1093/fampra/9.4.506. [DOI] [PubMed] [Google Scholar]
- 18.O’brien RM. A caution regarding rules of thumb for variance inflation factors. Qual Quant. 2007;41:673. doi: 10.1007/s11135-006-9018-6. [DOI] [Google Scholar]
- 19.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–5. doi: 10.1002/1097-0142(1950)3:1<32::AID-CNCR2820030106>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 20.Chang TI, Tabada GH, Yang J, Tan TC, Go AS. Visit-to-visit variability of blood pressure and death, end-stage renal disease, and cardiovascular events in patients with chronic kidney disease. J Hypertens. 2016;34:244–52. doi: 10.1097/HJH.0000000000000779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mancia G, Parati G, Castiglioni P, et al. Daily life blood pressure changes are stepper in hypertensive than in normotensive subjects. Hypertension. 2003;42:277–82. doi: 10.1161/01.HYP.0000084632.33942.5F. [DOI] [PubMed] [Google Scholar]
- 22.Glasser SP. On arterial physiology, pathophysiology of vascular compliance and cardiovascular disease. Heart Dis. 2000;2:375–9. [PubMed] [Google Scholar]
- 23.Manios E, Tsagalis G, Tsivgoulis G, et al. Time rate of blood pressure variation is associated with impaired renal function in hypertensive patients. J Hypertens. 2009;27:2244–8. doi: 10.1097/HJH.0b013e328330a94f. [DOI] [PubMed] [Google Scholar]
- 24.Chappell DC, Varner SE, Nerem RM, Medford RM, Alexander RW. Oscillatory shear stress stimulates adhesion molecule expression in cultured human endothelium. Circ Res. 1998;82:532–9. doi: 10.1161/01.RES.82.5.532. [DOI] [PubMed] [Google Scholar]
- 25.Brazy PC, Stead WW, Fitzwilliam JF. Progression of renal insufficiency: role of blood pressure. Kidney Int. 1989;35:670–4. doi: 10.1038/ki.1989.37. [DOI] [PubMed] [Google Scholar]
- 26.Wight JP, Salzano S, Brown CB, el Nahas AM. Natural history of chronic renal failure: a reappraisal. Nephrol Dial Transplant. 1992;7:379–83. doi: 10.1093/ndt/7.12.1185. [DOI] [PubMed] [Google Scholar]
- 27.Shafi T, Sozio SM, Bandeen-Roche KJ, DEcIDE Network Patient Outcomes in End Stage Renal Disease Study Investigators et al. Predialysis systolic BP variability and outcomes in hemodialysis patients. J Am Soc Nephrol. 2014;25:799–809. doi: 10.1681/ASN.2013060667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rothwell PM, Howard SC, Dolan E, ASCOT-BPLA and MRC Trial Investigators et al. Effects of beta blockers and calcium-channel blockers on within-individual variability in blood pressure and risk of stroke. Lancet Neurol. 2010;9:469–80. doi: 10.1016/S1474-4422(10)70066-1. [DOI] [PubMed] [Google Scholar]
- 29.Muntner P, Levitan EB, Joyce C, Holt E, Mann D, Oparil S, Krousel-Wood M. Association between antihypertensive medication adherence and visit-to-visit variability of blood pressure. J Clin Hypertens (Greenwich) 2013;15:112–7. doi: 10.1111/jch.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from Chang Gung Biological Databases but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission from the Institutional Review Board of Chang Gung Memorial Hospital.


