Abstract
The human HOXB13 gene encodes a 284 amino acid transcription factor belonging to the homeobox gene family containing a homeobox and a HoxA13 N-terminal domain. It is highly linked to hereditary prostate cancer, the majority of which is manifested as a result of a Single Nucleotide Polymorphism (SNP). In silico analysis of 95 missense SNP’s corresponding to the non-homeobox region of HOXB13 predicted 21 nsSNP’s to be potentially deleterious. Among 123 UTR SNPs analysed by UTRScan, rs543028086, rs550968159, rs563065128 were found to affect the UNR_BS, GY-BOX and MBE UTR signals, respectively. Subsequent analysis by PolymiRTS revealed 23 UTR SNPs altering the miRNA binding site. The complete HOXB13_M26 protein structure was modelled using MODELLER v9.17. Computational analysis of the 21 nsSNP’s mapped into the HOXB13_M26 protein revealed seven nsSNP’s (rs761914407, rs8556, rs138213197, rs772962401, rs778843798, rs770620686 and rs587780165) seriously resulting in a damaging and deleterious effect on the protein. G84E, G135E, and A128V resulted in increased, while, R215C, C66R, Y80C and S122R resulted in decreased protein stability, ultimately predicted to result in the altered binding patterns of HOXB13. While the genotype-phenotype based effects of nsSNP’s were assessed, the exact biological and biochemical mechanism driven by the above predicted SNPs still needs to be extensively evaluated by in vivo and GWAS studies.
Prostate Cancer has been recorded as the leading cause of cancer deaths among men around the globe and the most common cause of cancer-associated deaths among men in the Republic of Korea1,2,3,4. Even though there has been a substantial decrease in the morbidity of prostate cancer, the incidence rate has been increasing over the last few decades2,5. Researchers have identified that several factors contribute to the development of prostate cancer, such as food style, sedentary lifestyle, etc.6,7,8. The hereditary predisposition for prostate cancer seems to be the major cause1,7,9,10 and almost 5–10% of the prostate cancer incidences have roots in genetic predisposition9,11,12,13.
The prostate cancer susceptibility locus in the human genome was identified as 17q21-227,14 with the help of numerous GWAS studies and linkage analysis12,15. The human HOXB13 gene consists of two exons and three introns and encodes a 284 amino acid protein (NCBI: NP_006352.2) belonging to the homeobox gene family16. It encodes a transcription factor which plays a major role in normal prostate development17,18. HOXB13 is encoded by a single gene (NCBI: NM_006361.5) and contains a DNA binding homeobox domain of 60 amino acids (216–275 AA) and an HoxA13 N-terminal domain. The former binds to the DNA and is involved in transcription (see Supplementary Fig. S1). It is also associated with the increased risk of inherited prostate cancer17. In early 2012, HOXB13 was found to be the most important hereditary prostate cancer susceptible gene15. Subsequent advances in genetic studies have proved that HOXB13 has a major role in prostate cancer susceptibility, but the exact mechanism and mode of action remains undiscovered17,19.
The major genetic cause of Prostate cancer is the occurrence of Single Nucleotide Polymorphisms (SNPs)9,20,21. Among the various types of SNPs, non-synonymous SNPs22 (nsSNP’s) resulting in the change of amino acid23,24 is crucial and is associated with most hereditary prostate cancer9,25. These nsSNP’s might have deleterious24,26 and seriously damaging effects on protein structure, stability, activity, and function27,28,29. Peer researchers have confirmed that almost half of the hereditary cancers are associated with at least one form of SNP7,22,30,31.
Since the 3D structure for the complete human HOXB13 is not available in the PDB (Protein Data Bank), the complete HOXB13 protein structure needs to be modelled using MODELLER v9.1732 based on homology modelling. Comparative homology modelling constructs a 3D model by aligning the query sequence (NP_006352.2) known as the target against the closely related template structures obtained from the PDB33,34,35. Comparative modelling involves several computational steps34 (see Supplementary Fig. S2), and finally generates the best model for the query sequence which will be further evaluated for its validity36.
The complete list of SNPs for human HOXB13 was retrieved from the dbSNP37,38 and Ensembl database39. Those SNPs were analyzed with SIFT, PolyPhen, PANTHER, PROVEAN, nsSNPAnalyzer, PhD-SNP, etc., to screen the most deleterious nsSNP’s affecting the protein structure and function. Further, the screened nsSNP’s were mapped onto the protein structure for structure-based functional analysis. The mapping of nsSNP’s to the given protein structure was done with the help of “mutation” tool in the SWISSPDB Viewer. Since the complete protein structure of the HOXB13 protein was not available in the PDB, a complete HOXB13 protein model was developed by comparative homology modelling32,33 by comparing and aligning the query sequence (HOXB13 - NP_006352.2) against similar template 3D structures obtained from the PDB. The modelling was done with the help of MODELLER v9.17. The best HOXB13 protein model constructed was selected and was then validated by Ramachandran Plot and PDBsum. The best protein model generated by MODELLER was considered as the native protein structure of HOXB13 and was then subjected to single point mutation with the help of the “mutation tool” in SwissPDBViewer40,41. Energy minimization was done for both the native and the mutated proteins using NomadRefServer42. The RMSD values were then calculated, and the stability of the mutant protein structure was analyzed by the I-Mutant server22. Finally, all the energy minimized native and mutant protein structures were subjected to dihedral angle analysis of the atoms present in the amino acid residues by Ramachandran Plot36,43,44 to ascertain the conclusive effect of the encompassed nsSNP’s in the mutated protein structure (Figs 1 and 2).
Figure 1. Overall scheme.
Schematic representation of the overall work and the outcome of in silico analysis of nsSNP’s.
Figure 2. Overall workflow.
Entire workflow for the in silico analysis of HOXB13 non-homeobox region nsSNP’s.
Results and Discussion
Retrieval of functional HOXB13 SNPs (Dataset)
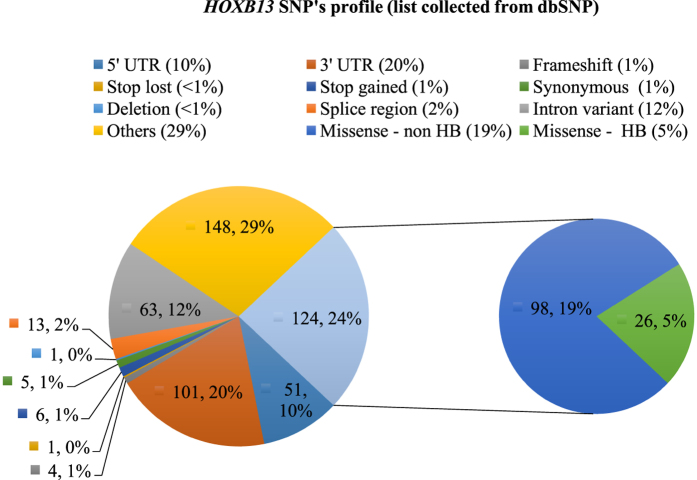
The human HOXB13 gene contained a total of 517 SNPs retrieved from the NCBI dbSNP database37 and were validated using Ensembl and HGVBase. Among the retrieved SNPs, missense, UTR, synonymous, and intronic SNPs accounted for about 123, 158, 57, and 63 (Fig. 3), respectively. The HOXB13 protein had a functional homeobox binding domain (216–275 AA) accounting for about 4% of the missense SNPs (23 SNPs), whereas the remaining 95 missense SNPs fell in the non-homeobox region. In this study, the missense SNPs of the non-homeobox region of HOXB13 were subjected to subsequent analysis for predicting their effects on the protein structure, stability, and function.
Figure 3. A statistical representation of the distribution of SNPs in the HOXB13 gene.
Distribution of SNPs in the HOXB13 gene with insight into the homeobox (HB) and non-homeobox (non-HB) region missense SNPs (info collected from dbSNP database). The numerical figures (ex. 148, 63, 101) denote the no. of SNPs as recorded in the dbSNP database, whereas, the % (ex. 29%, 20%, 12%) represents the corresponding percent of the SNP variation among the overall SNPs.
Functionally deleterious nsSNP’s predicted by SIFT program (Sequence-based homology)
From a total of 95 non-homeobox region nsSNP’s, 39 nsSNP’s were predicted to be functionally deleterious and were marked as “Affects protein structure” by the SIFT server (Table 1). Among those, 18 non-homeobox region nsSNP’s were marked as deleterious with a tolerance index of 0.00. The remaining 56 variants were predicted as “Tolerated” by the SIFT program. The detailed analysis of the effect of nsSNP’s on the entire non-homeobox region (1–215 and 276–284 AA) of HOXB13 by the SIFT program can be found as Supplementary Fig. S3. The statistical representation of the results is given in Fig. 4. The complete SIFT prediction results can be found as Supplementary Table S1.
Table 1. List of nsSNP’s predicted by SIFT as deleterious.
| Variant ID | NCBI AC.No | Nucleotide Variation | AA Variation | SIFT Score | SIFT Tolerance Index |
|---|---|---|---|---|---|
| rs761914407 | NP_006352 | C-T | R215C | 0 | Affects Protein Function |
| rs779330626 | NP_006352 | A-G | Q188R | 0 | Affects Protein Function |
| rs772349818 | NP_006352 | T-C | M182T | 0.05 | Affects Protein Function |
| rs570681642 | NP_006352 | A-G | Q181R | 0 | Affects Protein Function |
| rs777986934 | NP_006352 | G-T | G177V | 0 | Affects Protein Function |
| rs539086211 | NP_006352 | G-A | G176D | 0.03 | Affects Protein Function |
| rs747003841 | NP_006352 | G-C | G176R | 0.01 | Affects Protein Function |
| rs587780164 | NP_006352 | G-A | D167N | 0.01 | Affects Protein Function |
| rs751081605 | NP_006352 | C-G | S162C | 0.01 | Affects Protein Function |
| rs587780163 | NP_006352 | C-T | A154V | 0.01 | Affects Protein Function |
| rs766929278 | NP_006352 | G-T | G153V | 0 | Affects Protein Function |
| rs556045007 | NP_006352 | C-T | A141V | 0 | Affects Protein Function |
| rs575899185 | NP_006352 | G-C | Q138H | 0.01 | Affects Protein Function |
| rs770891609 | NP_006352 | A-C | Y137S | 0.01 | Affects Protein Function |
| rs769634543 | NP_006352 | G-A | G135E | 0.01 | Affects Protein Function |
| rs775273363 | NP_006352 | C-T | A128V | 0 | Affects Protein Function |
| rs201428095 | NP_006352 | G-A | R123H | 0.01 | Affects Protein Function |
| rs8556 | NP_006352 | C-A | S122R | 0 | Affects Protein Function |
| rs760111060 | NP_006352 | G-A | G117E | 0 | Affects Protein Function |
| rs533641489 | NP_006352 | G-A | G117R | 0 | Affects Protein Function |
| rs763448911 | NP_006352 | C-T | A101V | 0.04 | Affects Protein Function |
| rs757433384 | NP_006352 | C-A | L97M | 0.03 | Affects Protein Function |
| rs138213197 | NP_006352 | G-A | G84E | 0 | Affects Protein Function |
| rs772962401 | NP_006352 | A-G | Y80C | 0 | Affects Protein Function |
| rs763353615 | NP_006352 | C-T | T73M | 0 | Affects Protein Function |
| rs778843798 | NP_006352 | T-C | C66R | 0 | Affects Protein Function |
| rs199813155 | NP_006352 | G-A | C63Y | 0 | Affects Protein Function |
| rs758166293 | NP_006352 | T-G | C63G | 0.03 | Affects Protein Function |
| rs568967699 | NP_006352 | A-T | K61M | 0 | Affects Protein Function |
| rs770620686 | NP_006352 | C-T | P59L | 0.02 | Affects Protein Function |
| rs199799743 | NP_006352 | C-T | T41M | 0.03 | Affects Protein Function |
| rs773491778 | NP_006352 | C-A | A39E | 0.04 | Affects Protein Function |
| rs561048036 | NP_006352 | C-G | H30Q | 0 | Affects Protein Function |
| rs587780165 | NP_006352 | G-A | R25Q | 0.01 | Affects Protein Function |
| rs780947625 | NP_006352 | G-A | G24R | 0.01 | Affects Protein Function |
| rs539706443 | NP_006352 | G-A | G22R | 0.05 | Affects Protein Function |
| rs772484566 | NP_006352 | C-T | A21V | 0.01 | Affects Protein Function |
| rs776869015 | NP_006352 | G-T | G4V | 0 | Affects Protein Function |
| rs546307661 | NP_006352 | C-A | P3T | 0.02 | Affects Protein Function |
Figure 4. A statistical representation of the deleterious/damaging nsSNP predicted by various insilico tools.
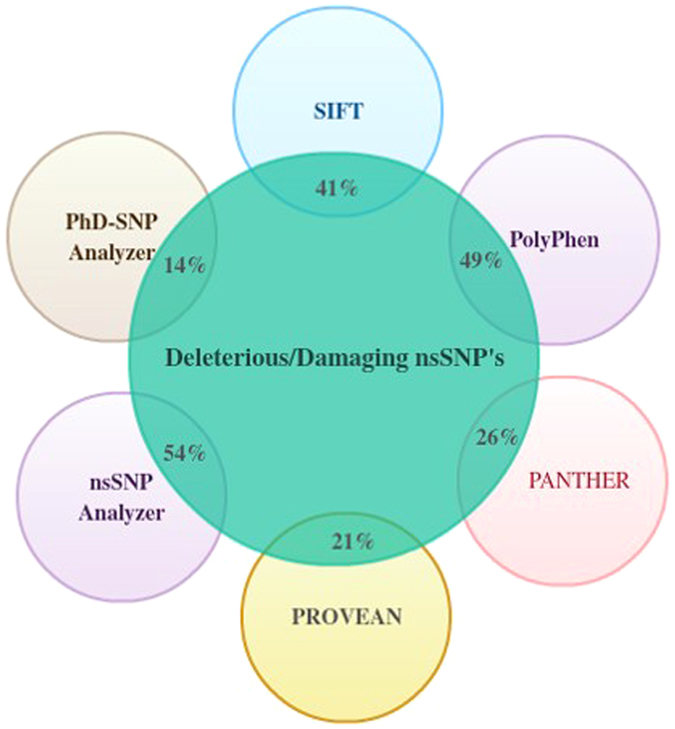
Percent deleterious nsSNP’s predicted by SIFT, PolyPhen, PANTHER, PROVEAN, nsSNPAnalyzer, PhD-SNP Analyzer. Out of 95 HOXB13 non-homeobox nsSNP’s SIFT predicted 41%, PolyPhen predicted 49%, PANTHER predicted 26%, PROVEAN 21%, nsSNPAnalyzer 54% and PhD-SNPAnalyzer 14% of the nsSNP’s to be potentially deleterious/damaging.
The functionally damaging nsSNP’s predicted by PolyPhen version 2 server (Structure-based homology)
The PolyPhen server predicted 47 non-homeobox region nsSNP’s to be functionally deleterious to the protein structure. Out of those 47 nsSNP’s, 34 nsSNP’s were predicted to be “probably damaging” with the score ranging from 0.845 to 1.00 and the remaining 13 nsSNP’s were predicted to be “possibly damaging” with the score ranging from 0.537 to 0.851 (Table 2). Interestingly, 22 nsSNP’s predicted as deleterious by SIFT were also predicted to be functionally damaging by the PolyPhen server. Therefore, the 22nsSNP’s predicted commonly by SIFT and PolyPhen were of functional importance. The statistical representation of the results are given in Fig. 4. The complete PolyPhen prediction results are available as Supplementary Table S2.
Table 2. List of nsSNP’s predicted by PolyPhen as deleterious.
| Variant ID | Nucleotide Variation | AA Variation | PolyPhen-2 |
|||
|---|---|---|---|---|---|---|
| Score | Predictions | Sensitivity | Specificity | |||
| rs191886930 | G-A | A276T | 0.998 | PD | 0.27 | 0.99 |
| rs761914407 | C-T | R215C | 1 | PD | 0 | 1 |
| rs766169510 | G-C | Q205H | 0.968 | PD | 0.77 | 0.95 |
| rs761530565 | A-G | Q205R | 0.538 | PSD | 0.88 | 0.91 |
| rs750945370 | G-T | A200S | 0.996 | PD | 0.55 | 0.98 |
| rs779330626 | A-G | Q188R | 0.985 | PD | 0.74 | 0.96 |
| rs570681642 | A-G | Q181R | 0.993 | PD | 0.7 | 0.97 |
| rs777986934 | G-T | G177V | 0.997 | PD | 0.41 | 0.98 |
| rs587780164 | G-A | D167N | 0.958 | PD | 0.78 | 0.95 |
| rs751081605 | C-G | S162C | 0.845 | PD | 0.83 | 0.93 |
| rs766929278 | G-T | G153V | 1 | PD | 0 | 1 |
| rs754280897 | G-A | G153S | 1 | PD | 0 | 1 |
| rs140373548 | C-A | L152M | 0.998 | PD | 0.27 | 0.99 |
| rs587780162 | G-A | V146M | 0.999 | PD | 0.14 | 0.99 |
| rs556045007 | C-T | A141V | 0.958 | PD | 0.78 | 0.95 |
| rs575899185 | G-C | Q138H | 0.998 | PD | 0.27 | 0.99 |
| rs770891609 | A-C | Y137S | 0.999 | PD | 0.14 | 0.99 |
| rs769634543 | G-A | G135E | 0.976 | PD | 0.76 | 0.96 |
| rs775273363 | C-T | A128V | 0.999 | PD | 0.14 | 0.99 |
| rs762666370 | C-G | P124R | 0.993 | PD | 0.7 | 0.97 |
| rs201428095 | G-A | R123H | 1 | PD | 0 | 1 |
| rs533641489 | G-A | G117R | 0.537 | PSD | 0.88 | 0.9 |
| rs370361482 | C-G | P110A | 0.906 | PSD | 0.82 | 0.94 |
| rs771674803 | T-G | L106R | 0.999 | PD | 0.14 | 0.99 |
| rs764359688 | C-A | P99T | 0.704 | PSD | 0.86 | 0.92 |
| rs751865027 | T-A | L97Q | 1 | PD | 0 | 1 |
| rs757433384 | C-A | L97M | 0.999 | PD | 0.14 | 0.99 |
| rs756135357 | G-A | R94Q | 1 | PD | 0 | 1 |
| rs778563157 | G-A | G85D | 0.827 | PSD | 0.84 | 0.93 |
| rs138213197 | G-A | G84E | 1 | PD | 0 | 1 |
| rs772962401 | A-G | Y80C | 1 | PD | 0 | 1 |
| rs763353615 | C-T | T73M | 0.905 | PSD | 0.82 | 0.94 |
| rs774579054 | G-T | G72V | 0.905 | PSD | 0.82 | 0.94 |
| rs750621041 | C-T | P70L | 0.996 | PD | 0.55 | 0.98 |
| rs370934116 | C-A | P70T | 0.996 | PD | 0.55 | 0.98 |
| rs199813155 | G-A | C63Y | 0.997 | PD | 0.41 | 0.98 |
| rs758166293 | T-G | C63G | 0.997 | PD | 0.41 | 0.98 |
| rs568967699 | A-T | K61M | 1 | PD | 0 | 1 |
| rs770620686 | C-T | P59L | 0.964 | PD | 0.78 | 0.95 |
| rs199799743 | C-T | T41M | 0.768 | PSD | 0.85 | 0.92 |
| rs550726919 | C-A | H36N | 0.925 | PSD | 0.81 | 0.94 |
| rs758169931 | C-G | S31C | 0.808 | PSD | 0.84 | 0.93 |
| rs561048036 | C-G | H30Q | 0.57 | PSD | 0.88 | 0.91 |
| rs587780165 | G-A | R25Q | 0.998 | PD | 0.27 | 0.99 |
| rs539706443 | G-A | G22R | 0.997 | PD | 0.41 | 0.98 |
| rs747307642 | C-T | T8I | 0.652 | PSD | 0.87 | 0.91 |
| rs771173385 | A-T | Y6F | 0.851 | PSD | 0.83 | 0.93 |
| PD | Probably Damaging | PSD | Possibly Damaging | |||
The functional validation of deleterious nsSNP’s by the PANTHER server
The PANTHER server predicted 25 non-homeobox region nsSNP’s to be damaging and the remaining nsSNP’s were predicted to be benign. Interestingly, 19 nsSNP’s were predicted as deleterious in common among SIFT, PolyPhen, and PANTHER server (Table 3). Additionally, two nsSNP’s (G153S, L152M) predicted by PANTHER and PolyPhen, and one nsSNP (H30Q) predicted by PANTHER and SIFT, were found to be common. The graphical representation of the results are given in Fig. 4. The complete PANTHER prediction results are available as Supplementary Table S3.
Table 3. List of nsSNP’s predicted by PANTHER as deleterious.
| Variant ID | NCBI AC.No | Nucleotide Variation | AA Variation | PANTHER Prediction |
|---|---|---|---|---|
| rs761914407 | NP_006352 | C-T | R215C | probably damaging |
| rs779330626 | NP_006352 | A-G | Q188R | probably damaging |
| rs570681642 | NP_006352 | A-G | Q181R | probably damaging |
| rs777986934 | NP_006352 | G-T | G177V | probably damaging |
| rs766929278 | NP_006352 | G-T | G153V | possibly damaging |
| rs754280897 | NP_006352 | G-A | G153S | possibly damaging |
| rs140373548 | NP_006352 | C-A | L152M | possibly damaging |
| rs575899185 | NP_006352 | G-C | Q138H | probably damaging |
| rs770891609 | NP_006352 | A-C | Y137S | probably damaging |
| rs769634543 | NP_006352 | G-A | G135E | possibly damaging |
| rs775273363 | NP_006352 | C-T | A128V | probably damaging |
| rs201428095 | NP_006352 | G-A | R123H | probably damaging |
| rs8556 | NP_006352 | C-A | S122R | probably damaging |
| rs138213197 | NP_006352 | G-A | G84E | probably damaging |
| rs772962401 | NP_006352 | A-G | Y80C | probably damaging |
| rs778843798 | NP_006352 | T-C | C66R | probably damaging |
| rs199813155 | NP_006352 | G-A | C63Y | probably damaging |
| rs758166293 | NP_006352 | T-G | C63G | probably damaging |
| rs568967699 | NP_006352 | A-T | K61M | probably damaging |
| rs770620686 | NP_006352 | C-T | P59L | probably damaging |
| rs773491778 | NP_006352 | C-A | A39E | possibly damaging |
| rs760874697 | NP_006352 | G-T | A39S | possibly damaging |
| rs561048036 | NP_006352 | C-G | H30Q | probably damaging |
| rs751338230 | NP_006352 | A-G | H30R | probably damaging |
| rs587780165 | NP_006352 | G-A | R25Q | probably damaging |
The functional impact of deleterious nsSNP’s by the PROVEAN server
The PROVEAN server predicted 20 non-homeobox region nsSNP’s to be functionally damaging out of the 95 nsSNP’s submitted for analysis (Table 4). Among those, 16 nsSNP’s were found to be in common as predicted by SIFT, PolyPhen, and PANTHER servers. One nsSNP (D167N) was found to be in common to both predicted by SIFT and PolyPhen. Two nsSNP’s (G117E and G117R) were found to be in common with the nsSNP’s predicted by SIFT program. The graphical representation of the results are given Fig. 4. The complete PROVEAN prediction results are available as Supplementary Table S4.
Table 4. List of nsSNP’s predicted by PROVEAN as deleterious.
| Variant ID | NCBI AC.No | Nucleotide Variation | AA Variation | PROVEAN SCORE | PROVEAN Prediction |
|---|---|---|---|---|---|
| rs761914407 | NP_006352 | C-T | R215C | −6.781 | Deleterious |
| rs779330626 | NP_006352 | A-G | Q188R | −3.397 | Deleterious |
| rs772349818 | NP_006352 | T-C | M182T | −2.836 | Deleterious |
| rs570681642 | NP_006352 | A-G | Q181R | −3.355 | Deleterious |
| rs777986934 | NP_006352 | G-T | G177V | −7.026 | Deleterious |
| rs587780164 | NP_006352 | G-A | D167N | −2.914 | Deleterious |
| rs766929278 | NP_006352 | G-T | G153V | −4.476 | Deleterious |
| rs575899185 | NP_006352 | G-C | Q138H | −2.605 | Deleterious |
| rs770891609 | NP_006352 | A-C | Y137S | −7.227 | Deleterious |
| rs769634543 | NP_006352 | G-A | G135E | −2.745 | Deleterious |
| rs775273363 | NP_006352 | C-T | A128V | −3.159 | Deleterious |
| rs201428095 | NP_006352 | G-A | R123H | −4.202 | Deleterious |
| rs8556 | NP_006352 | C-A | S122R | −3.662 | Deleterious |
| rs760111060 | NP_006352 | G-A | G117E | −3.229 | Deleterious |
| rs533641489 | NP_006352 | G-A | G117R | −3.205 | Deleterious |
| rs138213197 | NP_006352 | G-A | G84E | −6.485 | Deleterious |
| rs772962401 | NP_006352 | A-G | Y80C | −6.313 | Deleterious |
| rs778843798 | NP_006352 | T-C | C66R | −4.527 | Deleterious |
| rs199813155 | NP_006352 | G-A | C63Y | −4.179 | Deleterious |
| rs758166293 | NP_006352 | T-G | C63G | −4.117 | Deleterious |
The functional impact of deleterious nsSNP’s by the nsSNPAnalyzer server
Out of the 95 nsSNP’s submitted for analysis, 51 nsSNP’s were predicted to be associated with a diseased phenotype. Among those, 20 nsSNP’s were common to those predicted by the above four servers (SIFT, PolyPhen, PANTHER, and PROVEAN) (Table 5). The graphical representation of the results are given in Fig. 4. The complete nsSNPAnalyzer prediction results are available as Supplementary Table S5.
Table 5. List of nsSNP’s predicted by nsSNPAnalyzer as deleterious.
| Variant ID | Nucleotide Variation | SNP | nsSNPAnalyzer Predictions |
|||||
|---|---|---|---|---|---|---|---|---|
| Phenotype | Environ. | Area Buried | Frac. Polar | Second Str. | Scop-Link | |||
| rs761914407 | C-T | R215C | Disease | P2C | 0.177 | 0.812 | C | d1ahdp_ |
| rs779330626 | A-G | Q188R | Disease | — | — | — | — | — |
| rs748353425 | G-A | M182I | Disease | — | — | — | — | — |
| rs570681642 | A-G | Q181R | Disease | — | — | — | — | — |
| rs777986934 | G-T | G177V | Disease | — | — | — | — | — |
| rs539086211 | G-A | G176D | Disease | — | — | — | — | — |
| rs747003841 | G-C | G176R | Disease | — | — | — | — | — |
| rs587780164 | G-A | D167N | Disease | — | — | — | — | — |
| rs751081605 | C-G | S162C | Disease | — | — | — | — | — |
| rs766929278 | G-T | G153V | Disease | — | — | — | — | — |
| rs140373548 | C-A | L152M | Disease | — | — | — | — | — |
| rs556045007 | C-T | A141V | Disease | — | — | — | — | — |
| rs575899185 | G-C | Q138H | Disease | — | — | — | — | — |
| rs770891609 | A-C | Y137S | Disease | — | — | — | — | — |
| rs769634543 | G-A | G135E | Disease | — | — | — | — | — |
| rs775273363 | C-T | A128V | Disease | — | — | — | — | — |
| rs762666370 | C-G | P124R | Disease | — | — | — | — | — |
| rs201428095 | G-A | R123H | Disease | — | — | — | — | — |
| rs8556 | C-A | S122R | Disease | — | — | — | — | — |
| rs760111060 | G-A | G117E | Disease | — | — | — | — | — |
| rs533641489 | G-A | G117R | Disease | — | — | — | — | — |
| rs764401781 | C-T | T115M | Disease | — | — | — | — | — |
| rs140492479 | C-T | T105I | Disease | — | — | — | — | — |
| rs763448911 | C-T | A101V | Disease | — | — | — | — | — |
| rs778563157 | G-A | G85D | Disease | — | — | — | — | — |
| rs138213197 | G-A | G84E | Disease | — | — | — | — | — |
| rs772962401 | A-G | Y80C | Disease | — | — | — | — | — |
| rs763353615 | C-T | T73M | Disease | — | — | — | — | — |
| rs774579054 | G-T | G72V | Disease | — | — | — | — | — |
| rs750621041 | C-T | P70L | Disease | — | — | — | — | — |
| rs370934116 | C-A | P70T | Disease | — | — | — | — | — |
| rs766443552 | G-T | V69L | Disease | — | — | — | — | — |
| rs778843798 | T-C | C66R | Disease | — | — | — | — | — |
| rs199813155 | G-A | C63Y | Disease | — | — | — | — | — |
| rs568967699 | A-T | K61M | Disease | — | — | — | — | — |
| rs770620686 | C-T | P59L | Disease | — | — | — | — | — |
| rs199799743 | C-T | T41M | Disease | — | — | — | — | — |
| rs773491778 | C-A | A39E | Disease | — | — | — | — | — |
| rs587780160 | C-A | A38E | Disease | — | — | — | — | — |
| rs79344505 | C-A | L33M | Disease | — | — | — | — | — |
| rs758169931 | C-G | S31C | Disease | — | — | — | — | — |
| rs561048036 | C-G | H30Q | Disease | — | — | — | — | — |
| rs751338230 | A-G | H30R | Disease | — | — | — | — | — |
| rs587780165 | G-A | R25Q | Disease | — | — | — | — | — |
| rs780947625 | G-A | G24R | Disease | — | — | — | — | — |
| rs769323553 | G-A | G23R | Disease | — | — | — | — | — |
| rs539706443 | G-A | G22R | Disease | — | — | — | — | — |
| rs772484566 | C-T | A21V | Disease | — | — | — | — | — |
| rs747307642 | C-T | T8I | Disease | — | — | — | — | — |
| rs776869015 | G-T | G4V | Disease | — | — | — | — | — |
| rs546307661 | C-A | P3T | Disease | — | — | — | — | — |
The functional impact of deleterious nsSNP’s by the PhD-SNP server
We used the SVM based method utilizing sequence and profile information algorithm for the analysis of 95 non-homeobox region nsSNP’s of the human HOXB13 gene. The server predicted 13 nsSNP’s (Table 6) (Fig. 4) to be functionally associated with the disease and the remaining were considered benign. Among those, ten nsSNP’s were common to those predicted by the above-described servers (SIFT, PolyPhen, PANTHER, PROVEAN and nsSNPAnalyzer). The graphical representation of the results are given Fig. 4. The complete PhD-SNP prediction results are available as Supplementary Table S6.
Table 6. List of nsSNP’s predicted by PhD-SNP server as deleterious.
| Variant ID | Nucleotide Variation | AA Variation | PhD-SNP Sequence & Profile based Prediction | RI |
|---|---|---|---|---|
| rs761914407 | C-T | R215C | Disease | 7 |
| rs779330626 | A-G | Q188R | Disease | 0 |
| rs772349818 | T-C | M182T | Disease | 0 |
| rs777986934 | G-T | G177V | Disease | 8 |
| rs747003841 | G-C | G176R | Disease | 1 |
| rs766929278 | G-T | G153V | Disease | 1 |
| rs575899185 | G-C | Q138H | Disease | 6 |
| rs770891609 | A-C | Y137S | Disease | 8 |
| rs201428095 | G-A | R123H | Disease | 4 |
| rs8556 | C-A | S122R | Disease | 1 |
| rs751865027 | T-A | L97Q | Disease | 3 |
| rs138213197 | G-A | G84E | Disease | 4 |
| rs772962401 | A-G | Y80C | Disease | 7 |
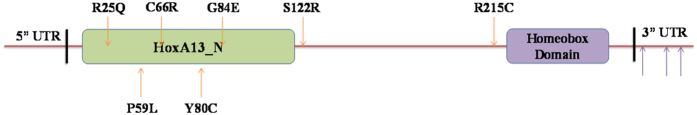
Among the 95 HOXB13 non-homeobox region nsSNP’s subjected to analysis by SIFT, PolyPhen, PANTHER, PROVEAN, nsSNPAnalyzer and PhD-SNP servers, 21 nsSNP’s were found to be functionally significant and causing damaging effects to the HOXB13 protein structure, stability, and function by the servers mentioned above. The list of those 21 nsSNP’s are as follows: rs761914407 (R215C), rs779330626 (Q188R), rs570681642 (Q181R), rs777986934 (G177V), rs587780164 (D167N), rs766929278 (G153V), rs575899185 (Q138H), rs770891609 (Y137S), rs769634543 (G135E), rs775273363 (A128V), rs201428095 (R123H), rs8556 (S122R), rs138213197 (G84E), rs772962401 (Y80C), rs778843798 (C66R), rs199813155 (C63Y), rs758166293 (C63G), rs568967699 (K61M), rs770620686 (P59L), rs561048036 (H30Q), rs587780165 (R25Q). For subsequent analysis, these 21 nsSNP’s were taken into consideration. The HOXB13 protein structure was available only for the homeobox binding domain (216–275 AA) of the complete protein and since there was no complete structure of HOXB13 in the PDB, the Homology Modelling approach was adopted for simulating the complete protein structure of HOXB13 in silico, so that we could map the above screened 21 nsSNP’s into the protein structure and could predict their effects on the protein function, stability, and bioactivity.
Functionally significant HOXB13 UTR SNPs predicted by the UTRscan server
Mutations in the untranslated region of the gene were reported very often to be linked with hereditary diseases such as cancer and various immune deficiency syndromes and also plays a key role in mRNA localization, stability, and translational efficiency45. Both the 5′UTR and the 3′UTR have important functions concerning the stability and expression of the mature mRNA. Mutations in those regions are linked with severe effects on the expression patterns of the gene at the level of mNA processing and translation46. The polymorphisms in the 5′ UTR are increasingly related to the altered patterns of ribosomal binding capacity, stability and translational regulation of mRNA, thereby influencing the RNA half-life. Whereas the polymorphisms corresponding to the 3′ UTR are highly involved in altered patterns of polyadenylation, localization, stability, translational efficiency and microRNA (miRNA) binding specificity, thereby rendering a tremendous effect on the gene expression patterns.
The UTRscan server predicts both the 5′UTR and 3′UTR SNPs. Among a total of 101 valid 3′UTR SNPs taken for evaluation, the UTRscan server predicted three SNPs (rs543028086, rs550968159, rs563065128) to be functionally significant to cause a pattern change (Table 7). However, the UTRscan server did not predict any harmful 5′ UTR SNPs.
Table 7. List of HOXB13 UTR SNPs predicted to be functionally significant by UTRscan server.
| Variant ID | Position | HGVS | Nucleotide Change | UTR region | UTR Signal | Functional Element Change |
|---|---|---|---|---|---|---|
| rs543028086 | 1484 | c.*473 G > T | G-T | 3′UTR | UNR_BS | UNR_BS - no pattern |
| rs550968159 | 1776 | c.*765 C > G | C-G | 3′UTR | GY-BOX | GY-BOX - no pattern |
| rs563065128 | 2241 | c.*1230 A > C | A-C | 3′ UTR | MBE | MBE - no pattern |
UNR (Upstream of N-ras) is a transcription factor containing five cold shock domains (CSD) that bind to single-stranded DNA and RNA47. It controls and plays a major role in transcriptional and post-transcriptional gene expression. UNR is a cytoplasmic protein known to function as an RNA chaperone and is found to be crucial in the control of cell proliferation and death48,49. The protein mainly destabilizes the c-fos mRNA and helps in the initiation and activation of cap-independent translation via the IRES for various transcripts, especially the proto-oncogene c-myc, rhinovirus, poliovirus, the cell cycle PISTLRE kinase, and pro-apoptotic factor (Apaf-1)50,51. The SNP rs543028086 was predicted to result in the disruption of the UNR Binding Site (UNR_BS) motif in the 3′UTR of human HOXB13 gene, which results in the deregulation of pro-apoptotic factor (Apaf-1), which might have a negative effect on the control of cell death.
The GY-Box is a conserved motif present in the Notch pathway target genes in Drosophila52. It is highly conserved in 3′ UTR regions that have sequence complementarity to the 5′ regions of the miRNA seed region. The result is the formation of RNA duplexes by the interaction of the 3′ UTR end of mRNA and the 5′ end of the miRNA, leading to translational repression52. The SNP rs550968159 is present in this region of the 3′UTR of human HOXB13 gene and leads to the loss of the specific GY-Box pattern, hence voiding the chance of translational repression of HOXB13 mediated by the GY-Box in the 3′UT region. Thus, an imbalance in the feedback regulation of HOXB13 expression has been predicted to result in the diseased state.
The Mushashi Binding Element (MBE) is an mRNA binding protein, which plays a very important role in the regulation of stem cell renewal process53,54 by suppressing the translation of all the mRNA coding for the proteins involved in inhibiting cell cycle progression55,56. The 3′ UTR SNP rs563065128 results in the loss of the MBE UTR motif in the human HOXB13 gene and is thereby found to lose its natural role of regulating the stem cell renewal process by suppressing the expression of cell cycle progression inhibitors, thereby predicted to result in the loss of stem cell niche. Loss of stem cell niche, in turn, leads to the unavailability of the local stem cell source to replenish the damaged cells of the tissue, thus, leading to the disease state.
These three 3′UTR SNPs were predicted to have important deleterious effects and functional significance on the expression of human HOXB13 gene.
Functionally significant HOXB13 3′ UTR SNPs predicted by the PolymiRTS Database
Out of the 95 nsSNP’s under consideration, only 23 nsSNP’s were found to have a crucial role in the 3′ UTR region (see Supplementary Table S7). Among those, five nsSNP’s (rs8064432, rs79812861, rs148791210, rs184053751 and rs183620920) were found to disrupt only the conserved (ancestral allele with support >=2) miRNA sites. Two nsSNP’s (rs116931900 and rs1056656) were exclusively found to create a new miRNA site. Whereas, the remaining 16 nsSNP’s were predicted to be involved in the disruption and creation of a new miRNA site, out of which rs61123825 (disrupting – 2 and creating – 7) and rs192244427 (disrupting – 4 and creating 5) were found to have a maximum of 9 pattern changes.
Modelling of the complete HOXB13 protein using MODELLER v9.17 (Comparative Homology Modelling)
The PDB contains only the 3D structure of the Homeobox binding domain of the human HOXB13 protein and not the complete 3D structure. In order to further analyze the effect of the above shortlisted 21 non-homeobox region missense SNPs on the HOXB13 protein structure and function, the complete protein structure was mandatory, since further analysis of those nsSNP’s demands mapping the nsSNP’s into the protein structure and thereby validating their subsequent effects on structural and functional aspects of the protein in silico. Thus, the complete HOXB13 protein structured was modelled by a technique called comparative homology modelling using the MODELLER v9.17 tool from the Andrej Sali laboratory. The complete modelling procedure and the steps performed were mentioned in the Supplementary Material.
A suitable template structure for developing the model was obtained using psiBLAST by setting PDB as the source database57 for finding the 3D structure templates. The resulting sequences of at least >30% similarity and identity were picked for comparative homology modelling. The Supplementary Table S8 shows the results of psiBLAST. PDB ID 2CRA was found to have 100% identity with the query sequence and PDB ID 2LD5 and 2L7Z showed 78% identity with the query sequence and were chosen as the template for modelling the HOXB13 protein. The respective “.pdb” files of the above-mentioned proteins were downloaded and kept in the same folder where the python script files were located. These three PDB ID structures – 2CRA, 2LD5 and 2L7Z were used as the template for modelling the complete HOXB13 protein using comparative homology modelling by MODELLER v9.17. The distance tree of the query sequence and the protein structures from the PDB computed by psiBLAST are available as Supplementary Fig. S4.
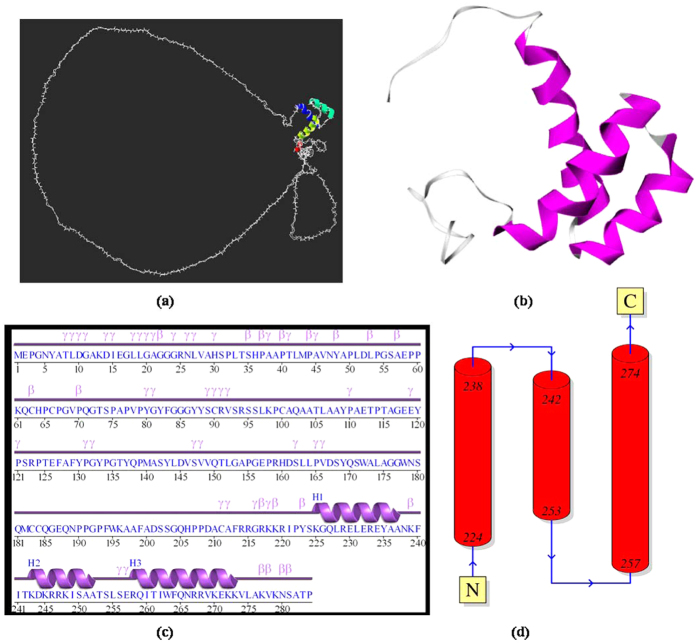
From the results, we found that 2LD5 and 2L7Z were both structurally and sequentially identical with the same crystallographic resolution of 1.0 Å (as Supplementary Fig. S5). Conversely, the structure 2CRA was found to be diversified from both 2LD5 and 2L7Z with a distance score of 63.5. Hence, the structure 2L7Z was finally selected for modelling of complete HOXB13 because of its high sequence and structural similarity to the query sequence. The alignment of the query sequence (NP_006352) with the template structure 2L7Z was done (Supplementary Fig. S6). MODELLER v9.17 was instructed to generate 30 similar models of complete HOXB13 protein based on the 2L7Z template structure and “hox13-2l7z.ali” file (Supplementary Fig. S7). There are several criteria to select the best model among the various models generated by MODELLER v9.17. The most important and widely practised criteria includes selecting the model with the lowest DOPE score58 and the highest GA341 score59. Accordingly, from the summary of the models generated (Supplementary Fig. S7.), we found that the 26th model “hoxb13.B99990026.pdb” had the lowest DOPE score of −10661.23047 and the highest GA341 score of 1.00000. Thus, the selected model “hoxb13.B99990026.pdb” was subjected to further validation of protein structure and folding properties with the help of the Ramachandran Plot and PDBsum.
Model validation by Ramachandran Plot
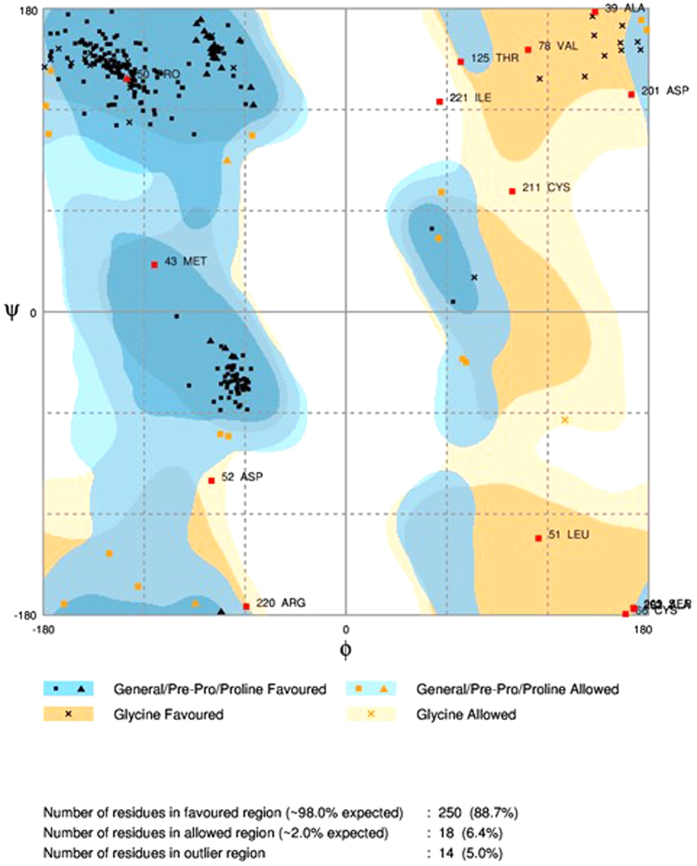
The selected HOXB13 protein model “hoxb13.B99990026.pdb” was validated and authenticated as the best-generated model and was subjected to analysis for the backbone dihedral angles (phi and psi) of the amino acid residues in the protein structure44. For a good protein structure, it is expected that there should be more than 90% of the residues in the core or favoured region of the protein36,43. The generated model “hoxb13.B99990026.pdb” was analyzed by RAMPAGE and was found to have 250 residues (88.7%) in the favored region, 18 residues (6.4%) in the allowed region and 14 residues (5.0%) in the outlier region, respectively (Fig. 5.) The model was found to be good and reliable since approximately 89% of the residues fell in the favored region and also because of the low DOPE and high GA341 score.
Figure 5. Ramachandran Plot for the generated protein model “hoxb13.B99990026.pdb”.
Almost 89% of the amino acid residues in the modelled protein “hoxb13.B99990026.pdb” occupied the favored region, 6% of the residues occupied the allowed region, and the remaining 5% of the residues occupied the outlier region, respectively.
Model validation by PDBsum
The simulated HOXB13 protein model was further validated with the help of PDBsum for information regarding the motifs, helices, strands, domains, tunnels, angles, positions, error, etc., present in the 3D structure of the proteins60. The “hoxb13.B99990026.pdb” was subjected to analysis by PDBsum and was found to have three alpha helices, three helix-helix interactions, 18 beta, and 40 gamma turns, respectively (Fig. 6). The results were in accordance with the features of the homeobox domain of the HOXB13 protein (2CRA). The complete 3D structure of the protein is given in Fig. 6(a). The complete HOXB13 protein, which was modelled using MODELLER v9.17, contained the same features and folding patterns of the homeobox domain of the HOXB13 protein (2CRA), which was clearly evident from Fig. 6(a,b). The detailed protein 3D structure features can be found as Supplementary Fig. S8.
Figure 6. Validation results of the hoxb13.B99990026.pdb modelled protein by PDBsum.
(a) 3D protein structure of the complete HOXB13 protein (hoxb13.B99990026.pdb) modelled using MODELLER v9.17 (Figure generated using SWISSPDBViewer). (b) HOXB13 (2CRA) protein 3D structure (Figure generated using PDBsum). (c) The amino acid residues contributing to the secondary structure (alpha helix and beta turns) of the complete HOXB13 protein are depicted in the topology diagram (Figure simulated using PDBsum). (d) Linear view of the modelled complete HOXB13 protein structure with alpha helices, beta and gamma turn and corresponding amino acid residues (Figure simulated using PDBsum).
Thus, the protein model “hoxb13.B99990026.pdb” generated by MODELLER v9.17 was found to be the best model based upon the DOPE and GA341 scores and was further validated to be good with the help of the Ramachandra Plot and PDBsum analysis. Hence, this model was taken as the complete human HOXB13 protein structure for further analysis of the corresponding deleterious nsSNP’s. The model “hoxb13.B99990026.pdb” was denoted as “HOXB13_M26” protein structure in the subsequent analysis.
Mapping the missense amino acid variation into the protein
Protein template for performing mutation and subsequent analysis
The HOXB13_M26 protein structure was taken as the complete native protein structure for mapping the previously predicted 21 deleterious nsSNP’s and also, for further studying their effect on the protein.
Protein mutation and Energy minimization of the native and mutated protein
The 21 nsSNP’s screened to be potentially deleterious by various servers were mapped into the HOXB13_M26 protein using the “mutation” tool in SwissPDBViewer40,61. The resulting 21 mutated proteins were denoted as “HOXB13_M26” Mutant.
In order to mimic the in vivo folding conditions and parameters of the protein, energy minimization of both the native (HOXB13_M26) and all the mutant proteins (HOXB13_M26 Mutant) was done with the help of NomadRef Gromacs Server using conjugant gradient force fields42. The resulting energy values of all of the native and the mutant structures are given in Table 8.
Table 8. RMSD, Energy Minimisation, Protein Stability, Quality and Structure Assessment of HOXB13_M26 Native and Mutant Protein Structures.
| Molecule | Variant ID | Nucleotide Variation | AA Variation | Total Energy after EM(KJ/mol) | RMSD (Å) | I-Mutant Results |
RAMPAGE (No. of residues) |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| I-Mutant Score | RI | DDG Value (Kcal/mol) | Favored | Allowed | Outlier | ||||||
| HOXB13_M26 | Nil | Nil | Nil | −4505.484 | 0 | Nil | Nil | Nil | 217 (77.2%) | 49 (17.4%) | 15 (5.3%) |
| HOXB13_M26 (Mutant) | rs761914407 | C-T | R215C | −4273.583 | 0.92 | Decrease | 5 | −0.63 | 216 (76.9%) | 51 (18.1%) | 14 (5%) |
| HOXB13_M26 (Mutant) | rs779330626 | A-G | Q188R | −4588.476 | 0.83 | Decrease | 3 | 0.51 | 220 (78.3%) | 46 (16.4%) | 15 (5.3%) |
| HOXB13_M26 (Mutant) | rs570681642 | A-G | Q181R | −4601.128 | 0.7 | Decrease | 3 | 0.67 | 219 (77.9%) | 47 (16.7%) | 15 (5.3%) |
| HOXB13_M26 (Mutant) | rs777986934 | G-T | G177V | −4564.104 | 1.56 | Decrease | 3 | 0.31 | 218 (77.6%) | 49 (17.4%) | 14 (5%) |
| HOXB13_M26 (Mutant) | rs587780164 | G-A | D167N | −4677.684 | 1.69 | Decrease | 7 | −2.08 | 220 (78.3%) | 46 (16.4%) | 15 (5.3%) |
| HOXB13_M26 (Mutant) | rs766929278 | G-T | G153V | −4575.739 | 0.8 | Decrease | 1 | −0.53 | 218 (77.6%) | 48 (17.1%) | 15 (5.3%) |
| HOXB13_M26 (Mutant) | rs575899185 | G-C | Q138H | −4376.856 | 0.71 | Decrease | 6 | −0.97 | 218 (77.6%) | 48 (17.1%) | 15 (5.3%) |
| HOXB13_M26 (Mutant) | rs770891609 | A-C | Y137S | −4456.023 | 0.72 | Decrease | 8 | −3.36 | 218 (77.6%) | 48 (17.1%) | 15 (5.3%) |
| HOXB13_M26 (Mutant) | rs769634543 | G-A | G135E | −4593.777 | 0.73 | Increase | 2 | 0.17 | 222 (79.0%) | 43 (15.3%) | 16 (5.7%) |
| HOXB13_M26 (Mutant) | rs775273363 | C-T | A128V | −4499.878 | 0.77 | Increase | 6 | 0.93 | 219 (77.9%) | 47 (16.7%) | 15 (5.3%) |
| HOXB13_M26 (Mutant) | rs201428095 | G-A | R123H | −4274.708 | 0.67 | Decrease | 6 | −0.85 | 217 (77.2%) | 50 (17.8%) | 14 (5.0%) |
| HOXB13_M26 (Mutant) | rs8556 | C-A | S122R | −4745.173 | 0.84 | Increase | 3 | 0.04 | 217 (77.2%) | 49 (17.4%) | 15 (5.3%) |
| HOXB13_M26 (Mutant) | rs138213197 | G-A | G84E | −4545.865 | 0.57 | Increase | 1 | 0.74 | 220 (78.3%) | 46 (16.4%) | 15 (5.3%) |
| HOXB13_M26 (Mutant) | rs772962401 | A-G | Y80C | −4451.958 | 1.23 | Decrease | 6 | −0.93 | 220 (78.3%) | 45 (16.0%) | 16 (5.7%) |
| HOXB13_M26 (Mutant) | rs778843798 | T-C | C66R | −4763.567 | 1.68 | Decrease | 3 | −0.83 | 222 (79.0%) | 43 (15.3%) | 16 (5.7%) |
| HOXB13_M26 (Mutant) | rs199813155 | G-A | C63Y | −4596.328 | 1.8 | Decrease | 0 | −0.04 | 218 (77.6%) | 48 (17.1%) | 15 (5.3%) |
| HOXB13_M26 (Mutant) | rs758166293 | T-G | C63Q | −4663.925 | 1.08 | Decrease | 4 | −0.97 | 219 (77.9%) | 50 (17.8%) | 12 (4.3%) |
| HOXB13_M26 (Mutant) | rs568967699 | A-T | K61M | −4516.539 | 0.71 | Decrease | 0 | −0.77 | 219 (77.9%) | 47 (16.7%) | 15 (5.3%) |
| HOXB13_M26 (Mutant) | rs770620686 | C-T | P59L | −4024.038 | 1.8 | Decrease | 5 | −0.39 | 217 (77.2%) | 48 (17.1%) | 16 (5.7%) |
| HOXB13_M26 (Mutant) | rs561048036 | C-G | H30Q | −4675.44 | 2.02 | Decrease | 5 | −0.72 | 222 (79.0%) | 45 (16.0%) | 14 (5.0%) |
| HOXB13_M26 (Mutant) | rs587780165 | G-A | R25Q | −4440.227 | 1.52 | Decrease | 5 | −0.21 | 223 (79.4%) | 43 (15.3%) | 15 (5.3%) |
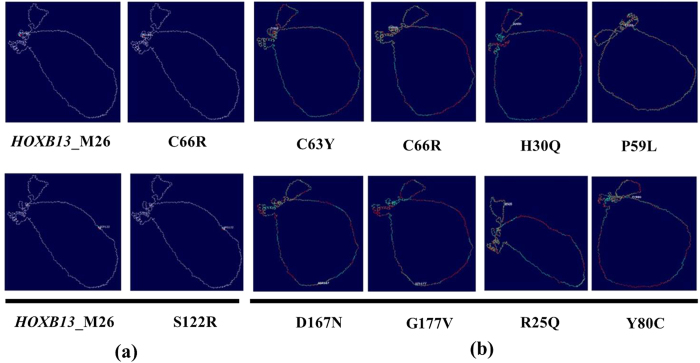
The total energy of the native protein structure HOXB13_M26 was determined to be −4505.484 KJ/mol. Among all the 21 mutants, the mutant C66R and S122R were found to have the highest energy of −4763.567 KJ/mol and −4745.173 KJ/mol, respectively even after energy minimization, when compared with the native structure. The mutants D167N (−477.684 KJ/mol), H30Q (−4675.440 KJ/mol), C63G (−4663.925 KJ/mol), Q181R (−4601.128 KJ/mol), C63Y (−596.328 KJ/mol) and Q188R (−4588.476 KJ/mol) were found to have considerably higher energy values, whereas the mutants R215C, R123H and Q138H showed very small energy values of −4273.583 KJ/mol, −4274.708 KJ/mol, −4376.856 KJ/mol, respectively, after energy minimization. These nsSNP’s with very high and low energies when compared to the native protein structure implied a possible underlying damaging effect on the protein structure, thereby affecting the protein stability and function. The other nsSNP’s were found to have near equal energy values as compared with the native structure. The electron cloud density maps of the variants C66R and S122 that had the highest energy are given in Fig. 7(a).
Figure 7. Electron Cloud densities and RMSD value calculation of the mutant proteins.
(a) Figure depicting the electron cloud density difference between the native protein structure and the mutants C66R and S122R. The images were modelled using SwissPDBViewer. (b) Superimposed native (HOXB13_M26) and mutant protein structures C63Y (1.80 Å), C66R (1.68 Å), D167N (1.69 Å), G177V (1.56 Å), H30Q (2.02 Å), P59L (1.80 Å), R25Q (1.52 Å) and Y80C (1.23 Å). The RMSD values and the image were modelled using SwissPDBViewer.
RMSD value calculation of the modelled protein
Among the 21 mutants analyzed, the variant H30Q was found to have the highest RMSD value of 2.02 Å, followed by C63Y and P59L, having 1.80 Å each, respectively (Fig. 7(b)). The mutants D167N, C66R, G177V, R25Q and Y80C, were found to have RMSD values of 1.69 Å, 1.68 Å, 1.56 Å, 1.52 Å and 1.2 Å, respectively (Table 8). The remaining mutants were found to have RMSD values of less than 1 Å. Among the mutants with high RMSD values, the mutants D167N, C66R, C63Y, P59L and H30Q were found to have both increased energies after energy minimization and RMSD values, which was of critical importance and was taken into further consideration in the subsequent analysis.
Predicting the change in stability of the mutant proteins by I-Mutant Server
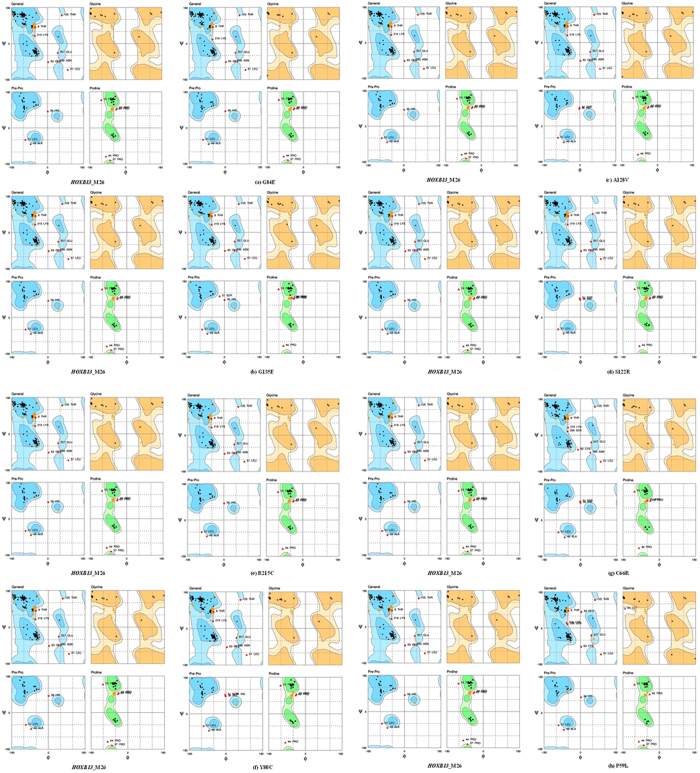
Among the 21 nsSNP’s submitted, I-Mutant predicted an increase in the stability of 4 mutants, namely rs769634543 (G135E), rs775273363 (A128V), rs8556 (S122R) and rs138213197 (G84E) (Table 8). The remaining nsSNP’s were predicted to be associated with decreased stability. Also, the four variants that were predicted to have increased stability were also found to have low RMSD values. The RMSD values and the I-Mutant results were found to be in conjunction, but the authenticity was yet to be verified by Ramachandran Plot.
Validation of the native and the mutant model using Ramachandran Plot
The energy minimized native (HOXB13_M26) and mutant (HOXB13_M26 Mutant) protein structures in.pdb format were submitted to RAMPAGE for assessment. The native (HOXB13_M26) contained 217 residues (77.2%) in the favored region, 49 residues (17.4%) in the allowed region and 15 residues (5.3%) in the outlier region, respectively (Fig. 8). Interestingly, the mutants G84E, G135E and A128V showed increased positive pattern when compared with the native protein. G84E had 220 (78.3%), 46 (16.4%), and 15 (5.3%) residues in the favored, allowed and outlier regions, respectively (Fig. 8). Three residues from the allowed regions were shifted to the favored region in the G84E mutant and resulted in a better pattern than the native protein. The mutant G135E also showed an increased and stable amino acid residue pattern with five residues shifting from the allowed region to the favored region with a total of 222 (79%), 43(15.3%) and 16 (5.7%) residues in the favored, allowed, and outlier regions, respectively (Fig. 8) (Table 8). The mutant A128V also showed a similar increased stabilizing pattern, where two residues from the allowed region became shifted to the favored region. The mutant A128V contained 219 (77.9%), 47 (16.7%) and 15 (5.3%) residues in the three regions, respectively (Fig. 8). The variant S122R, which was predicted to have increased stability by I-Mutant, showed no trace of increased pattern in the Ramachandran plot. It exactly resembled the native protein structure. The mutants Y80C, C66R and P59L, were found to have a destabilizing pattern of amino acid residues. Y80C had (220, 45, 16), C66R had (222, 43, 16), and P59L had (217, 48, 16) residues in the favored, allowed, and outlier regions, respectively. Interestingly, the mutants C66R and P59L were also found to have higher energy and RMSD values, whereas Y80C was reported to have a higher RMSD value. Among all the mutants, the mutant R215C were predicted to have the most destabilizing and damaging combination of amino acid residues with 216 (76.9%), 51 (18.1%), 14 (5.0%) residues in the favored, allowed, and outlier regions respectively. Four residues were shifted from the favored region to the allowed region. The mutant C63G was also found to have a similar destabilizing and damaging pattern to R215C. The remaining mutant models showed near similar or acceptable dihedral angles, which were predicted to confer less damaging effect to the protein when compared to the above-mentioned mutants.
Figure 8. The Ramachandran plot for the native (HOXB13_M26) & mutant (HOXB13_M26 Mutant) protein.
Each panel contains the general plot and the plots for Glycine and Proline residues. The native protein (HOXB13_M26) showed 217 (77.2%), 49 (17.4%) and 15 (5.3%) residues in the favored, allowed and outlier region, respectively (a–c) Ramachandran Plot for the mutants G84E (220, 46, 15 residues), G135E (222, 43, 16 residues), A128V (219, 47, 1 residues) respectively, showed an increased and stable amino acid residue pattern compared to the native structure. (d) The mutant S122R (217,49, 15 residues) showed the same pattern as that of the native structure. (e–h) The mutants R215C (216, 51, 14 residue), Y80C (220, 45, 16 residues), C66R (222, 43, 16 residues) and P59L (217, 48, 16 residues) showed a highly destabilizing and damaging pattern of amino acid residue distribution, respectively.
Discussion and Conclusion
The thorough computational analysis of the 21nsSNP’s mapped into the HOXB13_M26 protein model, it was predicted that 7 nsSNP’s rs761914407 (R215C), rs8556 (S122R), rs138213197 (G84E), rs772962401 (Y80C), rs778843798 (C66R), rs770620686 (P59L) and rs587780165 (R25Q) were found to have seriously damaging and deleterious effects on the HOXB13 with respect to DNA binding and function. Interestingly, the variants G84E, G135E, A128V were found to result in the increased stability of the protein structure. G84E variation was widely reported to be present in large cases of hereditary prostate cancer as epidemiologically reported elsewhere8,12,17,25,62,63,64,65 and was in agreement with the results of this study. G135E was also widely reported to be present highly among the Chinese population66 as reported elsewhere. Thus, the G84E, G135E and A128V variations were predicted to cause some severe structural changes in the protein, which renders it more stable with an increased half-life. It has also been reported that the gene RFX6 transcribed and regulated by a HOXB13 transcription factor is activated and expressed over a longer period, which results in an imbalance in the feedback mechanism5,67 that is under the vigilance of HOXB13. It has been scientifically proven that the overexpression of RFX6 helps in the prostate cancer cell migration and disease progression5,6,12,67. The variants that cause an increase in the stability of the HOXB13 were found to constitutively express the downstream genes under the influence of HOXB13 and thus it is predicted to be associated with an increase in the risk of prostate cancer, like in the case of RFX65. The mutants R215C, Y80C, C66R and P59L, were found to have highly damaging and deleterious structural and functional properties. This in turn might disturb the role of HOXB13 as a transcriptional factor in activating the genes responsible for cell cycle control and proliferation, eventually leading to the malignancy of the prostate. While G84E, G135E, and A128V were found to increase the stability of the protein structure, the other four nsSNP’s, R215C, C66R, Y80C, and S122R, were found to have decreased protein stability. The exact mechanism and the role of those predicted nsSNP’s with increased or decreased energy levels and protein stability should further be validated in vitro, since practically either the increased state or the decreased state might possibly involve in the altered patterns of protein structure, function and disease progression. Figure 9 shows the map of the predicted nsSNP’s. In addition, three 3′UTR variations rs543028086, rs550968159, and rs563065128, were found to affect the UNR_BS, GY-BOX and MBE UTR signal, respectively present in the 3′UTR of the HOXB13 gene, which was predicted to result in the deregulation of the pro-apoptotic factor (Apaf-1). This altered the pattern and regulation of the translational repression of HOXB13 via a feedback mechanism. Thus, it results in the loss of regulating the process of stem cell renewal by blocking or deregulating the cell cycle promotion inhibitors respectively, thereby causing severe damage to the HOXB13 mediated gene expression and function. Out of the 95 nsSNP’s subjected to analysis for the variation in the miRNA patterns by the PolymiRTS database five nsSNP’s (rs8064432, rs79812861, rs148791210, rs184053751 and rs183620920) were found to disrupt only the conserved (ancestral allele with support >=2) miRNA sites. Two nsSNP’s (rs116931900 and rs1056656) were exclusively found to create a new miRNA site. Conversely, the remaining 16 nsSNP’s were predicted to be involved in the disruption and creation of a new miRNA site, out of which rs61123825 (disrupting – 2 and creating – 7) and rs192244427 (disrupting – 4 and creating 5) were found to have a maximum of 9 pattern changes. Thus, the above-mentioned nsSNP’s showed up in the progression of prostate tissue malignancy either due to the increase in the stability and half-life of the HOXB13 encoded transcription factor or due to the damaging effects on the protein structure, which resulted in altered binding patterns of the transcription factor, thereby eventually leading to prostate tissue malignancy. The exact mechanism underlying the onset of hereditary prostate cancer by HOXB13 nsSNP’s needs to be evaluated and studied extensively with the help of in vivo models and GWAS studies. This study, thus, paves the gateway for future GWAS and clinical studies related to the role of SNPs in hereditary prostate cancer and also has the potential in developing a mechanism for drug targeting and biomarkers for PCa theranostic applications.
Figure 9. Mapping of the predicted SNPs to the HOXB13 protein.
Schematic representation depicting the location of seven missense and three 3′ UTR SNPs predicted as deleterious as a result of this study.
Materials and Methods
Datasets
The complete list of HOXB13 SNPs, gene and protein sequences in the FASTA format were retrieved from the dbSNP database37,38 (http://www.ncbi.nlm.nih.gov/SNP/) and Ensembl genome browser (http://asia.ensembl.org/index.html).
Prediction and Screening of deleterious nsSNP’s
The highly deleterious missense SNPs associated with the non-homeobox region of HOXB13 gene were predicted using the following in silico servers: The SIFT (Sorting Intolerant From Tolerant) program (http://sift.bii.a-star.edu.sg/www/SIFT_BLink_submit.html) predicts the deleterious or damaging nature of the missense SNPs based upon sequence homology based prediction, physical properties of amino acids and also by calculating the degree of evolutionary conservation of the sequence among various species68. The SIFT results were reliable and the scores generated by SIFT program were classified as affecting protein structure (0.00–0.05) and as tolerated (>0.05). The PolyPhen (Polymorphism and Phenotyping) server (http://genetics.bwh.harvard.edu/pph2), screens and predicts the deleterious nsSNP’s based on the observable structural changes induced by the nsSNP’s with the help of various proven algorithms69,70 (THMM, Colis2 program, SignalP program, etc.). These structural changes are in turn known to affect the protein function and stability deleteriously. The relative solvent accessibility and secondary structure details were predicted using DSSP database. The PANTHER (Protein Analysis through Evolutionary Relationships) server (http://pantherdb.org/tools/cSNPscoreForm.jsp?), calculates the duration of a given amino acid that has been evolutionary preserved among various species and predicts the effect of that specific amino acid change on the structural and functional effect on the protein71. The longer the amino acid is conserved during the course of evolution, the greater the likelihood of having functional importance in protein structure and function. The PROVEAN (Protein Variation Effect Analyzer) server (http://pantherdb.org/tools/cSNPscoreForm.jsp?) relies upon the data corresponding to the standard properties of the amino acids and protein structure, thereby predicting the effect of the amino acid variations in the protein structure, stability, and function72. The nsSNPAnalyzer (http://snpanalyzer.uthsc.edu/) is a tool to predict the phenotypic effect of the missense SNPs based on the data from MSA and three-dimensional protein structure73. The PhD-SNP (Predictor of human Deleterious Single Nucleotide Polymorphisms) server (http://SNPs.biofold.org/phd-snp/phd-snp.html) functions with the help of support vector machines based (SVM-based) and evolutionary information of the sequences20. The nsSNP’s, which are commonly predicted by more than five servers, were taken into further consideration and analysis. The UTRscan server (http://itbtools.ba.itb.cnr.it/utrscan) is a pattern match identifier that finds the UTR pattern motif match from the protein or nucleotide sequences from the UTRsite databases using UTRblast function45. The PolymiRTS (Polymorphism in microRNA and their Target Sites) database (http://compbio.uthsc.edu/miRSNP/) contains comprehensive data of all the nucleotide variations occurring in the miRNA seed regions and miRNA target sites74. The amino acid sequence of the HOXB13 protein (NP_006352) in FASTA format was fed to the server along with its corresponding amino acid variations (ex. G135E).
Modelling the complete HOXB13 protein using MODELLER v9.17
The complete HOXB13 protein was modelled using MODELLER v9.17, which is a computer program used for comparative homology modelling of protein structures. The MODELLER v9.17 was downloaded from the Andrej Sali laboratory website (https://salilab.org/modeller/). Since MODELLER v9.17 runs on Python scripts, Python was also installed along with MODELLER v9.1732,34,75. The Python scripts in MODELLER v9.17 can be executed by the command “mod9.17 script1.py”. The basic steps involved in homology modelling using MODELLER are the initial template selection using BLAST, final template selection and alignment of the query sequence with the template structure, building the model based on the final template selected, followed by model evaluation and validation using Ramachandran Plot and PDBsum. The finally validated model waas saved as the HOXB13_M26.
Mapping the missense amino acid variation on the protein structure
Template
The best model was chosen from the various models generated by MODELLER v9.17 and it was used as the template for incorporating the mutations into the protein structure and for subsequent evaluation. This template was taken as the native protein.
Mapping the nsSNP’s and Energy minimization of the modelled protein
Each mutant model (21 models) was generated using the “mutation” tool in SwissPDBViewer. Energy minimization of the native and the mutated protein was carried out using NOMAD-Ref Gromacs server (http://lorentz.immstr.pasteur.fr/gromacs/minimization_submission.php). The NOMAD-Ref Server utilizes Gromacs using conjugant gradient force fields for energy minimization according to the steepest descent, conjugate gradient, or L-BFGS methods. The conjugate gradient method was utilized in this study.
RMSD value calculation of the modelled protein
The RMSD of the atoms upon superimposing the native and the mutant protein structure was calculated using SwissPDBViewer by the “Calculate RMS” function. The extent of structural deviation between the native and the mutant protein structures was found to have an associated functional effect on the protein, which was predicted by calculating the RMSD by superimposing the native and protein structures. The higher the RMSD value, the higher the structural deviation and associated function of the protein76,77.
Predicting the change in stability of the mutant proteins
The stability study of the native and the mutant protein structure was crucial and was carried out with the help of the I-Mutant Server (http://folding.biofold.org/cgi-bin/i-mutant2.0.cgi). The stability of the protein and its structural changes were predicted by I-Mutant server based on calculating the relative solvent accessibility area, amino acid properties, evolutionary, and structural information of the protein22. The server uses the FOLD-X prediction algorithm. The input to the server was the HOXB13 protein sequence (NP_006352), and the amino acid variations were provided manually for each variation.
Validation of the native and the mutant model using Ramachandran Plot and PDBsum
The Ramachandran Plot was used to calculate the dihedral angles of the amino acid residues and to predict the energetically allowed residues based upon their phi and psi dihedral angles, thereby ascertaining the structural and functional properties of the protein structure36,43,44. The energy minimized native and the mutant protein models were validated with the online tool RAMPAGE (http://mordred.bioc.cam.ac.uk/~rapper/rampage.php). A percentage of more than 90% residues in the favored region is required for a good protein structure. PDBsum provides the 3D protein structure information regarding the motifs, domains, helices, beta sheets and strands, angles, etc. PDBsum can be accessed online at (http://www.ebi.ac.uk/thornton-srv/databases/cgi-bin/pdbsum/GetPage.pl?pdbcode=index.html).
Additional Information
How to cite this article: Chandrasekaran, G. et al. Computational Modeling of complete HOXB13 protein for predicting the functional effect of SNPs and the associated role in hereditary prostate cancer. Sci. Rep. 7, 43830; doi: 10.1038/srep43830 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
This study was financially supported by Chonnam National University, 2016 Grant Number (2015–2656). Author VKL thank the Biomedical Research Institute, Chonnam National University, Hwasun Hospital campus for the facility.
Footnotes
The authors declare no competing financial interests.
Author Contributions C.G.K. and V.K.L. conceived and designed the experiments. C.G.K. and E.C.H. performed the experiments. C.G.K. and V.K.L. analysed the data. C.G.K. and V.K.L. wrote the manuscript. V.K.L. refined the drafted manuscript. E.C.H., T.W.K., D.D.K., K.P., J.J.L. interpreted the data, reviewed, and corrected the manuscript. All the authors read and approved the final manuscript.
References
- Han H. H. et al. Epidemiology of prostate cancer in South Korea. Prostate Int. 3, 99–102 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S. K. et al. Rising prostate cancer rates in South Korea. Prostate 66, 1285–1291 (2006). [DOI] [PubMed] [Google Scholar]
- Jung K.-W. et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2012. Cancer Res. Treat. 47, 127–41 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babb C., Urban M., Kielkowski D. & Kellett P. Prostate cancer in South Africa: pathology based national cancer registry data (1986-2006) and mortality rates (1997-2009). Prostate Cancer 2014, 419801 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills I. G. HOXB13, RFX6 and prostate cancer risk. Nat. Genet. 46, 94–5 (2014). [DOI] [PubMed] [Google Scholar]
- Maia S. et al. Identification of two novel HOXB13 germline mutations in Portuguese prostate cancer patients. PLoS One 10, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eeles R. A. et al. Identification of 23 new prostate cancer susceptibility loci using the iCOGS custom genotyping array. Nat. Genet. 45, 385–91, 391e1–2 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacInnis R. J. et al. Population-Based Estimate of Prostate Cancer Risk for Carriers of the HOXB13 Missense Mutation G84E. PLoS One 8, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S. L. et al. Cumulative Association of Five Genetic Variants with Prostate Cancer. [Article]. N. Engl. J. Med. 358, 910 (2008). [DOI] [PubMed] [Google Scholar]
- Bratt O. Hereditary Prostate Cancer: Clinical Aspects. J. Urol. 168, 906–913 (2002). [DOI] [PubMed] [Google Scholar]
- Haiman C. a. et al. A common genetic risk factor for colorectal and prostate cancer. Nat. Genet. 39, 954–956 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe-Dimmer J. L. et al. The HOXB13 G84E Mutation Is Associated with an Increased Risk for Prostate Cancer and Other Malignancies. Cancer Epidemiol. Biomarkers Prev. 24, 1366–72 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Olama A. A. et al. Multiple loci on 8q24 associated with prostate cancer susceptibility. Nat. Genet. 41, 1058–1060 (2009). [DOI] [PubMed] [Google Scholar]
- Yeager M. et al. Genome-wide association study of prostate cancer identifies a second risk locus at 8q24. Nat. Genet. 39, 645–649 (2007). [DOI] [PubMed] [Google Scholar]
- Ewing C. M. et al. Germline Mutations in HOXB13 and Prostate-Cancer Risk. N. Engl. J. Med. 366, 141–149 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. C. et al. HOXB13 G84E-related familial prostate cancers: a clinical, histologic, and molecular survey. Am. J. Surg. Pathol. 38, 615–626 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H. & Cai B. G84E mutation in HOXB13 is firmly associated with prostate cancer risk: a meta-analysis. Tumour Biol. 35, 1177–82 (2014). [DOI] [PubMed] [Google Scholar]
- Xu J. et al. HOXB13 is a susceptibility gene for prostate cancer: results from the International Consortium for Prostate Cancer Genetics (ICPCG). Hum Genet 132, 5–14 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMullin R. P. et al. A FOXA1-binding enhancer regulates Hoxb13 expression in the prostate gland. Proc. Natl. Acad. Sci. USA 107, 98–103 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capriotti E., Calabrese R. & Casadio R. Predicting the insurgence of human genetic diseases associated to single point protein mutations with support vector machines and evolutionary information. Bioinformatics 22, 2729–2734 (2006). [DOI] [PubMed] [Google Scholar]
- van Oeveren J. & Janssen A. Single Nucleotide Polymorphisms. Single Nucleotide Polymorphisms 578, 73–91 (2009). [Google Scholar]
- Adzhubei I. A. et al. A method and server for predicting damaging missense mutations. Nat. Methods 7, 248–249 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramensky V., Bork P. & Sunyaev S. Human non-synonymous SNPs: server and survey. Nucleic Acids Res. 30, 3894–3900 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue P. & Moult J. Identification and analysis of deleterious human SNPs. J. Mol. Biol. 356, 1263–1274 (2006). [DOI] [PubMed] [Google Scholar]
- Kote-Jarai Z. et al. Prevalence of the HOXB13 G84E germline mutation in British men and correlation with prostate cancer risk, tumour characteristics and clinical outcomes. Ann. Oncol. 26, 756–761 (2015). [DOI] [PubMed] [Google Scholar]
- Sunyaev S. et al. Prediction of deleterious human alleles. Hum Mol Genet 10, 591–597 (2001). [DOI] [PubMed] [Google Scholar]
- Nakken S., Alseth I. & Rognes T. Computational prediction of the effects of non-synonymous single nucleotide polymorphisms in human DNA repair genes. Neuroscience 145, 1273–1279 (2007). [DOI] [PubMed] [Google Scholar]
- Kelly J. N. & Barr S. D. In silico analysis of functional single nucleotide polymorphisms in the human TRIM22 gene. PLoS One 9 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghav D. & Sharma V. An In Silico Evaluation of Deleterious Nonsynonymous Single Nucleotide Polymorphisms in the ErbB3 Oncogene. Biores. Open Access 2, 206–11 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radivojac P. et al. Identification, analysis, and prediction of protein ubiquitination sites. Proteins 78, 365–80 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doniger S. W. et al. A catalog of neutral and deleterious polymorphism in yeast. PLoS Genet. 4 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb B. & Sali A. Comparative Protein Structure Modeling Using MODELLER. Curr. Protoc. Bioinformatics 47, 5.6.1–5.6.32 (2014). [DOI] [PubMed] [Google Scholar]
- Sali a. Comparative protein modeling by satisfaction of spatial restraints. Mol. Med. Today 1, 270–277 (1995). [DOI] [PubMed] [Google Scholar]
- Eswar N. et al. Comparative protein structure modeling using MODELLER. Curr. Protoc. Protein Sci. Chapter 2, Unit 2.9 (2007). [DOI] [PubMed] [Google Scholar]
- Siezen R. J., de Vos W. M., Leunissen J. A. M. & Dijkstra B. W. Homology modelling and protein engineering strategy of subtilases, the family of subtilisin-like serine proteinases. Protein Eng. 4, 719–737 (1991). [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan K., Sowmiya G., Sheik S. S. & Sekar K. Ramachandran plot on the web (2.0). Protein Pept. Lett. 14, 669–671 (2007). [DOI] [PubMed] [Google Scholar]
- Sherry S. T. et al. dbSNP: the NCBI database of genetic variation. Nucleic Acids Res. 29, 308–11 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagwat M. Searching NCBI’s dbSNP database. Curr. Protoc. Bioinforma, doi: 10.1002/0471250953.bi0119s32 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham F. et al. Ensembl 2015. Nucleic Acids Res. 43, D662–D669 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guex N. & Peitsch M. C. SWISS-MODEL and the Swiss-PdbViewer: An environment for comparative protein modeling. Electrophoresis 18, 2714–2723 (1997). [DOI] [PubMed] [Google Scholar]
- Biasini M. et al. SWISS-MODEL: Modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 42 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl E., Azuara C., Koehl P. & Delarue M. NOMAD-Ref: Visualization, deformation and refinement of macromolecular structures based on all-atom normal mode analysis. Nucleic Acids Res. 34 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrascoza F., Zaric S. & Silaghi-Dumitrescu R. Computational study of protein secondary structure elements: Ramachandran plots revisited. J. Mol. Graph. Model. 50, 125–133 (2014). [DOI] [PubMed] [Google Scholar]
- Kleywegt G. J. & Jones T. A. Phi/Psi-chology: Ramachandran revisited. Structure 4, 1395–1400 (1996). [DOI] [PubMed] [Google Scholar]
- Grillo G. et al. UTRdb and UTRsite (RELEASE 2010): A collection of sequences and regulatory motifs of the untranslated regions of eukaryotic mRNAs. Nucleic Acids Res. 38 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesole G. et al. Structural and functional features of eukaryotic mRNA untranslated regions. In Gene 276, 73–81 (2001). [DOI] [PubMed] [Google Scholar]
- Dormoy-Raclet V. et al. Unr, a cytoplasmic RNA-binding protein with cold-shock domains, is involved in control of apoptosis in ES and HuH7 cells. Oncogene 26, 2595–605 (2007). [DOI] [PubMed] [Google Scholar]
- Elatmani H. et al. The RNA-binding protein Unr prevents mouse embryonic stem cells differentiation toward the primitive endoderm lineage. Stem Cells 29, 1504–1516 (2011). [DOI] [PubMed] [Google Scholar]
- Dormoy-Raclet V., Markovits J., Jacquemin-Sablon A. & Jacquemin-Sablon H. Regulation of Unr expression by 5′- and 3′-untranslated regions of its mRNA through modulation of stability and IRES mediated translation. RNA Biol. 2, e27–35 (2005). [PubMed] [Google Scholar]
- Hoffman B. & Liebermann D. a. Apoptotic signaling by c-MYC. Oncogene 27, 6462–72 (2008). [DOI] [PubMed] [Google Scholar]
- Hoffmann T. J. et al. Imputation of the Rare HOXB13 G84E Mutation and Cancer Risk in a Large Population-Based Cohort. PLoS Genet. 11, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai E. C., Tam B. & Rubin G. M. Pervasive regulation of Drosophila Notch target genes by GY-box-, Brd-box-, and K-box-class microRNAs. Genes Dev. 19, 1067–1080 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNicol M. C., Cragle C. E. & MacNicol A. M. Context-dependent regulation of Musashi-mediated mRNA translation and cell cycle regulation. Cell Cycle 10, 39–44 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arumugam K., Wang Y., Hardy L. L., MacNicol M. C. & MacNicol A. M. Enforcing temporal control of maternal mRNA translation during oocyte cell-cycle progression. EMBO J. 29, 387–97 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arumugam K., Macnicol M. C. & Macnicol A. M. Autoregulation of Musashi1 mRNA translation during Xenopus oocyte maturation. Mol. Reprod. Dev. 79, 553–563 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth A., Wilczynska A., Thampi P., Cox L. L. & MacNicol A. M. Musashi regulates the temporal order of mRNA translation during Xenopus oocyte maturation. EMBO J. 25, 2792–2801 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul S. F. et al. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Research 25, 3389–3402 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen M. Y. & Sali A. DOPE score-Statistical potential for assessment and prediction of protein structures. Protein Sci 15, 2507–2524 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doong S. Protein Homology Modeling with Heuristic Search for Sequence Alignment. Syst. Sci. 2007 HICSS 2007 40th Annu. Hawaii Int. Conf. 128, doi: 10.1109/HICSS.2007.453 (2007). [DOI] [Google Scholar]
- Laskowski R. A., Chistyakov V. V. & Thornton J. M. PDBsum more: New summaries and analyses of the known 3D structures of proteins and nucleic acids. Nucleic Acids Res. 33, (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold K., Bordoli L., Kopp J. & Schwede T. The SWISS-MODEL workspace: A web-based environment for protein structure homology modelling. Bioinformatics 22, 195–201 (2006). [DOI] [PubMed] [Google Scholar]
- Schroeck F. R. et al. Testing for the recurrent HOXB13 G84E germline mutation in men with clinical indications for prostate biopsy. J. Urol. 189, 849–853 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laitinen V. H. et al. HOXB13 G84E mutation in Finland: Population-based analysis of prostate, breast, and colorectal cancer risk. Cancer Epidemiol. Biomarkers Prev. 22, 452–460 (2013). [DOI] [PubMed] [Google Scholar]
- Chen Z. et al. The G84E mutation of HOXB13 is associated with increased risk for prostate cancer: Results from the REDUCE trial. Carcinogenesis 34, 1260–1264 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluźniak W. et al. The G84E mutation in the HOXB13 gene is associated with an increased risk of prostate cancer in Poland. Prostate 73, 542–548 (2013). [DOI] [PubMed] [Google Scholar]
- Lin X. et al. A novel germline mutation in HOXB13 is associated with prostate cancer risk in Chinese men. Prostate 73, 169–75 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q. et al. A prostate cancer susceptibility allele at 6q22 increases RFX6 expression by modulating HOXB13 chromatin binding. Nat. Genet. 46, 126–35 (2014). [DOI] [PubMed] [Google Scholar]
- Ng P. C. & Henikoff S. SIFT: Predicting amino acid changes that affect protein function. Nucleic Acids Res. 31, 3812–3814 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adzhubei I., Jordan D. M. & Sunyaev S. R. Predicting functional effect of human missense mutations using PolyPhen-2. Curr. Protoc. Hum. Genet, doi: 10.1002/0471142905.hg0720s76 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bava K. a. ProTherm, version 4.0: thermodynamic database for proteins and mutants. Nucleic Acids Res. 32, 120D–121 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi H., Poudel S., Muruganujan A., Casagrande J. T. & Thomas P. D. PANTHER version 10: expanded protein families and functions, and analysis tools. Nucleic Acids Res. 44, D336–42 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y. & Chan A. P. PROVEAN web server: a tool to predict the functional effect of amino acid substitutions and indels. Bioinformatics 31, btv195 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao L., Zhou M. & Cui Y. nsSNPAnalyzer: Identifying disease-associated nonsynonymous single nucleotide polymorphisms. Nucleic Acids Res. 33 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya A., Ziebarth J. D. & Cui Y. PolymiRTS Database 3.0: Linking polymorphisms in microRNAs and their target sites with human diseases and biological pathways. Nucleic Acids Res. 42 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrej Šali. MODELLER A Program for Protein Structure Modeling. Comp. protein Model. by Satisf. Spat. restraints. 779–815 (1993). [Google Scholar]
- Coutsias E. A., Seok C. & Dill K. A. Using quaternions to calculate RMSD. J. Comput. Chem. 25, 1849–1857 (2004). [DOI] [PubMed] [Google Scholar]
- Kirchmair J., Markt P., Distinto S., Wolber G. & Langer T. Evaluation of the performance of 3D virtual screening protocols: RMSD comparisons, enrichment assessments, and decoy selection - What can we learn from earlier mistakes? Journal of Computer-Aided Molecular Design 22, 213–228 (2008). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.