Abstract
P-glycoprotein, an ATP-driven efflux pump, regulates permeability of the blood-brain barrier (BBB). Sphingolipids, endogenous to brain tissue, influence inflammatory responses and cell survival in vitro. Our laboratory has previously shown that sphingolipid signaling by sphingosine 1-phosphate decreases basal P-glycoprotein transport activity. Here, we investigated the potential for another sphingolipid, ceramide 1-phosphate (C1P), to modulate efflux pumps at the BBB. Using confocal microscopy and measuring luminal accumulation of fluorescent substrates, we assessed the transport activity of several efflux pumps in isolated rat brain capillaries. C1P treatment induced P-glycoprotein transport activity in brain capillaries rapidly and reversibly. In contrast, C1P did not affect transport activity of two other major efflux transporters, multidrug resistance protein 2 and breast cancer resistance protein. C1P induced P-glycoprotein transport activity without changing transporter protein expression. Inhibition of the key signaling components in the cyclooxygenase-2 (COX-2)/prostaglandin E2 signaling cascade (phospholipase A2, COX-2, multidrug resistance protein 4, and G-protein–coupled prostaglandin E2 receptors 1 and 2), abolished P-glycoprotein induction by C1P. We show that COX-2 and prostaglandin E2 are required for C1P-mediated increases in P-glycoprotein activity independent of transporter protein expression. This work describes how C1P activates a signaling cascade to dynamically regulate P-glycoprotein transport at the BBB and offers potential clinical targets to modulate neuroprotection and drug delivery to the CNS.
Introduction
The blood-brain barrier (BBB), located in the endothelium of brain capillaries, protects the central nervous system (CNS) from neurotoxins. A specialized network of microvessels, the BBB forms a chemical and structural barrier between the brain and circulatory system. Tight junction complexes restrict paracellular flow of solutes, and substrate-specific ATP-driven efflux transporters regulate levels of endogenous metabolites and xenobiotics (Begley, 2004). When modified, the activity of these efflux transporters alters the permeability of the BBB (Miller, 2010).
The most studied and highest expressed efflux transporter at the BBB is P-glycoprotein. Expressed luminally in brain endothelial cells, P-glycoprotein exports a vast range of substrates out of brain tissue and into the circulatory system, making it an essential part of neuroprotection and a redoubtable obstacle in drug delivery (Begley, 2004; Miller et al., 2008). Some therapeutic strategies are designed to decrease P-glycoprotein activity to improve drug delivery into the CNS, and others attempt to increase P-glycoprotein activity to restrict the movement of toxins across the BBB and enhance CNS protection. Specifically, in certain cases of CNS disease or injury, such as cerebral ischemia, traumatic brain injury, or subarachnoid hemorrhage, studies have proposed preserving the integrity of the BBB to protect against further cellular damage (Alfieri et al., 2011; Zhang et al., 2013).
Even without clinical intervention, P-glycoprotein transport activity is dynamic. Studies show that P-glycoprotein transport increases or decreases in response to cellular injury, such as inflammation or oxidative stress (Seelbach et al., 2007; Miller et al., 2008; Chodobski et al., 2011; Wang et al., 2014). Increased P-glycoprotein activity has also been observed in animals with certain neurologic and neuroinflammatory disorders, such as epilepsy and amyotrophic lateral sclerosis (Brandt et al., 2006; Bauer et al., 2008; Milane et al., 2010; Jablonski et al., 2012). Understanding the mechanisms that regulate P-glycoprotein and how basal P-glycoprotein is modulated will help the development of clinical targets for both enhanced neuroprotection and drug delivery.
Sphingolipids are signaling molecules that are endogenous to brain tissue and involved in inflammatory responses. However, despite observations that inflammation in brain tissue can alter BBB efflux transport, research regarding the involvement of sphingolipids at the BBB remains limited. Structurally, sphingolipids contain a sphingoid backbone acetylated at the N terminus with a fatty acid chain specific to one of many ceramide species (Maceyka and Spiegel, 2014). One of the most commonly studied sphingolipids is ceramide, which can be converted to many other species. The membrane-bound enzyme ceramide kinase (CERK) phosphorylates ceramide intracellularly to produce the proinflammatory molecule ceramide 1-phosphate (C1P) (Lamour and Chalfant, 2008). Although the physiologic role of C1P is not fully understood, in vitro studies suggest that C1P induces proinflammatory cascades, decreases apoptosis, increases cell survival, increases cell migration, and is released in high levels from damaged cells (Granado et al., 2009; Arana et al., 2010; Gómez-Muñoz et al., 2010; Kim et al., 2013).
Our laboratory has previously documented the ability of another sphingolipid, sphingosine 1-phosphate (S1P), to regulate P-glycoprotein transport activity at the BBB (Cannon et al., 2012). In this study, we investigated whether C1P could similarly regulate transport at the BBB, especially since its formative enzyme, CERK, is highly active in brain tissue (Van Overloop et al., 2006). Our study explores the ability of C1P to modify P-glycoprotein activity at the BBB. In contrast to S1P, which decreases P-glycoprotein activity, we found that exposure of rat brain capillaries to C1P rapidly increases P-glycoprotein transport activity. The effect is reversible, transporter-specific, and occurs with no change to transporter protein expression. Further characterization revealed that the effect of C1P on P-glycoprotein transport activity is mediated via the cyclooxygenase-2 (COX-2) and prostaglandin E2 (PGE2) signaling cascade. With these findings, we propose a model for C1P-mediated signaling that induces P-glycoprotein transport activity quickly and reversibly to render the BBB impermeable to toxins or drugs.
Materials and Methods
Chemicals.
C18:1 ceramide 1-phosphate (d18:1/18:1) and sphingosine 1-phosphate (d18:1) were purchased from Avanti Polar Lipids (Alabaster, AL). Stock solution of C1P was prepared in 2:1 chloroform/methanol. NBD-CSA, [N-ε-(4-nitrobenzofurazan-7-yl)-d-Lys8]cyclosporine A, was custom synthesized. PSC-833 (valspodar), a specific inhibitor of P-glycoprotein, was provided by Novartis (Basel, Switzerland). Mouse monoclonal C219 antibody to P-glycoprotein for Western blotting was purchased from Thermo Fisher Scientific (Waltham, MA). Alexa Fluor-488-conjugated goat anti-mouse IgG was purchased from Invitrogen (Carlsbad, CA). NS-398 [N-(2-cyclohexyloxy-4-nitrophenyl)methanesulfonamide] was purchased from Santa Cruz Biotechnology (Dallas, TX). Rabbit monoclonal PGE2 receptor EP2 antibody and ceefourin 1 were purchased from Abcam (Cambridge, MA). AH-6809 (9-oxo-6-propan-2-yloxyxanthene-2-carboxylic acid) was purchased from Cayman Chemical (Ann Arbor, MI). BODIPY prazosin [boron[1-(4-amino-6,7-dimethoxy-2-quinazolinyl)-4-[3-[5-[(3,5-dimethyl-2H-pyrrol-2-ylidene-κN)methyl]-1H-pyrrol-2-yl-κN]-1-oxopropyl]piperazinato]difluoro-(T-4)-175799-93-6] was purchased from Life Technologies (Carlsbad, CA). KO143 [(3S,6S,12aS)-1,2,3,4,6,7,12,12a-octahydro-9-methoxy-6-(2-methylpropyl)-1,4-dioxopyrazino-[1ʹ,2ʹ:1,6]pyrido[3,4-b]indole-3-propanoic acid 1,1-dimethylethyl ester], a specific inhibitor of breast cancer resistance protein (BCRP), was purchased from Enzo Life Sciences (Ann Arbor, MI). G-protein antagonist peptide was purchased from Tocris Bioscience (Minneapolis, MN). Ceramide from bovine spinal cord, mouse monoclonal β-actin antibody, chlorpromazine hydrochloride, Ficoll PM 400, cycloheximide, SC-51089 hydrate [3-chloro-Nʹ-(3-pyridin-4-ylpropanoyl)-6H-benzo[b][1,4]benzoxazepine-5-carbohydrazide;hydrate;hydrochloride], PF-04418948 [1-(4-fluorobenzoyl)-3-[(6-methoxynaphthalen-2-yl)oxymethyl]azetidine-3-carboxylic acid], celecoxib, NVP-231 [N-(2-benzamido-1,3-benzothiazol-6-yl)adamantane-1-carboxamide], and all other chemicals were obtained from Sigma-Aldrich (St. Louis, MO).
Animals.
All experiments were performed in compliance with the National Institutes of Health animal care and use guidelines and approved by the Animal Care and Use Committee of National Institute of Environmental Health Sciences. Sprague-Dawley male retired rats were obtained from Taconic Biosciences (Hudson, NY). COX-2-deficient mice and littermate controls on a 129B6F1 background were obtained from Dr. Artiom Gruzdev at the National Institute of Environmental Health Sciences (Morham et al., 1995). All animals were housed in temperature-controlled rooms under a 12-hour light/dark cycle with ad libitum access to food and water. The animals were euthanized by CO2 inhalation followed by decapitation. For transport assays and immunohistochemistry, brain capillaries were harvested and used immediately; for Western blot analysis, capillaries were treated and stored at −80°C for further analysis.
Brain Capillary Isolation.
The procedure for rat brain capillary isolation has been detailed elsewhere (Miller et al., 2000; Hartz et al., 2004). In summary, brains were stripped of their midbrain, meninges, choroid plexus, olfactory lobes, and white matter. The remaining brain tissue was homogenized in cold 1x phosphate-buffered saline (PBS) supplemented with glucose and sodium pyruvate (2.7 mM KCL, 1.5 mM KH2PO4, 136.9 mM NaCl, 8.1 mM Na2HPO4·7H20, 1 mM CaCl2·2H20, 1 mM MgCl2·6H20, 5 mM d-glucose, and 1 mM sodium pyruvate). An equal volume of 30% Ficoll was added to the homogenate, and the solution was centrifuged at 6800 rpm for 20 minutes at 4°C to separate the capillaries from the parenchyma. The resulting capillary pellet was resuspended in 1% bovine serum albumin (BSA) in PBS, passed over 30 µm-mesh filters (pluriStrainer; pluriSelect, Leipzig, Germany), and washed with PBS before immediate use.
Transport Assay.
Details about transport activity assays using isolated brain capillaries may be found in previous studies (Hartz et al., 2004; Bauer et al., 2007). Experiments in this study were performed at room temperature in chambered coverglass (Lab-Tek; Thermo Fisher Scientific) filled with 1x PBS. Isolated rat brain capillaries were allowed to adhere to the coverglass for at least 20 minutes in plain 1x PBS, followed by incubation for 40 minutes in a fluorescent substrate specific to one BBB efflux transporter: 2 µM NBD-CSA for P-glycoprotein; 2 µM Texas Red for multidrug resistance protein 2 (MRP2); and 2 µM BODIPY prazosin for BCRP (Hartz et al., 2004; Wang et al., 2010). Capillaries were treated with C1P plus NBD-CSA for a further 20 minutes. In select cases, capillaries were pretreated for 40 minutes with an inhibitor or antagonist plus NBD-CSA before exposure to C1P.
For each experiment, one chamber contained 10 µM PSC833, a specific P-glycoprotein inhibitor. Images of 10–20 capillaries were acquired per chamber using a Zeiss 510 confocal microscope (Carl Zeiss Inc., Thornwood, NY) with a 40× water-immersion objective. Luminal fluorescence (as a measure of substrate accumulation) was analyzed via National Institutes of Health ImageJ software (https://imagej.nih.gov/ij/). Specific P-glycoprotein activity was calculated as the difference between total luminal fluorescence and the fluorescence of capillaries exposed to PSC833.
Western Blotting.
Whole capillary isolates were collected and assayed for protein concentration using the Bradford method. Protein aliquots were mixed with 4x loading buffer, added to a 4–12% Bis-Tris gel for electrophoresis, and transferred to an Immobilon-FL membrane (EMD Millipore, Billerica, MA). Membranes were blocked in Odyssey blocking buffer (Li-Cor Biosciences, Lincoln, NE) for 30 minutes at room temperature and incubated overnight at 4°C with primary antibody: for P-glycoprotein, C219 antibody (1/200 dilution, predicted band size 180 kDal, secondary goat anti-mouse); for EP2, antiprostaglandin E receptor EP2 antibody (1/1000 dilution, predicted band size 40 kDal, secondary goat anti-rabbit). As a loading control, membranes were also immunoblotted with β-actin (1/10,000 dilution; predicted band size 42 kDa; secondary goat anti-mouse).
Before imaging, membranes were washed in PBS with 0.1% Tween 20 and treated with corresponding secondary antibody IRDye 680RD for 1 hour. Imaging was performed with the Li-Cor Odyssey Infrared Imaging System.
Immunohistochemistry.
Isolated capillaries were allowed to adhere to chambered coverglass and were then fixed with 4% paraformaldehyde/0.2% glutaraldehyde for 15 minutes. Chambers were washed with 1x PBS, permeabilized in 0.1% Triton X-100 (in PBS) for 30 minutes, and blocked in 1% BSA (in PBS) for 30 minutes. Capillaries were incubated with prostaglandin E receptor EP2 antibody (rabbit; monoclonal; 1/500 dilution) overnight at 4°C. The negative control omitted the primary antibody. Isotype control capillaries were incubated with rabbit monoclonal IgG primary antibody (1/25,000 dilution).
Before imaging, capillaries were washed in 1x PBS, followed by exposure to Alexa Fluor-488-conjugated goat anti-mouse IgG for 1 hour at 37°C. Imaging was performed by confocal microscopy using a Zeiss 510 microscope with a 40× water-immersion objective.
Statistical Analysis.
Data are expressed as mean ± standard error. Statistical analyses between groups were calculated by one-way analysis of variance with Tukey-Kramer post test (multiple comparisons) or Student’s unpaired t test with GraphPad Prism 6 software (GraphPad Software, San Diego, CA). P < 0.05 was considered a statistically significant difference between means.
Results
C1P Increases P-Glycoprotein Activity Ex Vivo.
Our laboratory has previously established a confocal microscopy-based assay to measure the activity of transporters at the BBB in isolated rat brain capillaries (Miller et al., 2000; Hartz et al., 2004). Specific transporter activity is determined by measuring the luminal accumulation of fluorescent substrates specific to a particular transporter. To assess the activity of P-glycoprotein in brain capillaries, we measured the luminal accumulation of 2 µM NBD-CSA at steady state (approximately 40 minutes of exposure). To quantify specific P-glycoprotein activity, we subtracted the nonspecific fluorescence of capillaries treated with 10 µM PSC833, a potent P-glycoprotein inhibitor.
Using this method, we determined the effect of C1P exposure on BBB efflux transporter activity by exposing freshly isolated rat brain capillaries to 250 nM C1P for 20 minutes. Figure 1A shows representative confocal images of rat brain capillaries after 1 hour of exposure to 2 μM NBD-CSA (control), 250 nM C1P (40 minutes of blank NBD-CSA followed by 20 minutes C1P concurrently with NBD-CSA), or 10 μM PSC833 (30 minutes of PSC833 pretreatment, followed by 1 hour of PSC833 concurrently with NBD-CSA). Figure 1B shows quantitatively that the luminal accumulation of PSC833-treated capillaries decreased significantly by 50%–60%. These data are consistent with prior studies that show PSC833 maximally inhibits P-glycoprotein transport of NBD-CSA; any residual fluorescence after PSC833 treatment results from nonspecific luminal entry (Hartz et al., 2004).
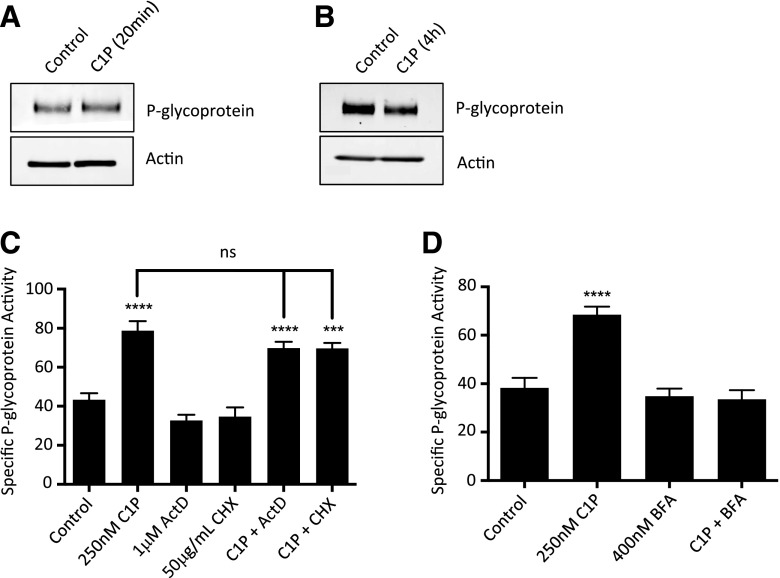
Fig. 1.
C1P induces P-glycoprotein transport activity at the blood-brain barrier. (A) Representative confocal images showing that accumulation of NBD-CSA in the lumen of isolated rat brain capillaries increases after 20 minutes of exposure to 250 nM C1P. (B) Quantification of luminal NBD-CSA fluorescence in isolated rat brain capillaries treated for 90 minutes with 10 μM PSC833 (specific inhibitor of P-glycoprotein) or for 20 minutes with 250 nM C1P. (C) PSC833-sensitive luminal fluorescence of NBD-CSA expressed as specific P-glycoprotein transport activity. Shown are mean ± S.E.M. for 10–20 capillaries from single preparation (pooled brains from 3–5 rats). ****P<0.0001, significantly different than control.
Figure 1 also shows the changes in luminal fluorescence of isolated rat brain capillaries exposed to 250 nM C1P for 20 minutes. The luminal fluorescence of capillaries exposed to C1P increased significantly by approximately 50% (Fig. 1B). The PSC833-sensitive NBD-CSA luminal fluorescence in capillaries exposed to 250 nM C1P was 2-fold higher than in the control capillaries (Fig. 1C). The PSC833-sensitive luminal fluorescence of another P-glycoprotein substrate, rhodamine 123, was also found to increase 2-fold after C1P exposure (Supplemental Fig. 1). These data show that specific P-glycoprotein transport activity doubles in response to short-term 250 nM C1P exposure.
Ceramide Is Converted to C1P via CERK to Induce P-Glycoprotein.
We tested whether ceramide, the intracellular precursor to C1P, could similarly affect P-glycoprotein activity. Exposing isolated rat brain capillaries to 250 nM ceramide increased P-glycoprotein transport activity after 20 minutes; however, compared with C1P, the effect was modest (Fig. 2A). For further comparison between ceramide and C1P, we analyzed the time course required for both sphingolipids to increase P-glycoprotein transport activity. Capillaries treated with 250 nM C1P reached maximal P-glycoprotein induction in under 5 minutes (Fig. 2B), while capillaries treated with ceramide required between 15 and 40 minutes to reach peak P-glycoprotein induction (Fig. 2C).
Fig. 2.
Exposure to C1P or ceramide induces P-glycoprotein transport activity at the blood-brain barrier. (A) Dose response of 20 minutes ceramide treatment, showing that ceramide increases specific P-glycoprotein activity in a concentration-dependent manner. Capillaries were exposed to 2 μM NBD-CSA for 40 minutes followed by 20 minutes exposure to either C1P or ceramide concurrently NBD-CSA. (B) Time course of C1P-mediated P-glycoprotein induction, showing that C1P increases P-glycoprotein activity in under 5 minutes. (C) Time course of ceramide-mediated P-glycoprotein induction, showing that ceramide requires approximately 15–40 minutes to take significant effect. (D) Inhibiting ceramide kinase with NVP-231 abolishes P-glycoprotein induction caused by ceramide. Shown are mean ± S.E.M. for 10–20 capillaries from single preparation (pooled brains from 3–5 rats). *P<0.05, **P<0.01, ****P<0.0001, significantly higher than control.
These results prompted us to analyze whether the delay in ceramide-mediated P-glycoprotein induction resulted from intracellular conversion of ceramide to C1P. Given that CERK converts ceramide into C1P, we treated isolated brain capillaries with a CERK inhibitor (50 nM NVP-231) and measured P-glycoprotein activity. We found that CERK inhibition blocked the ability of ceramide to increase P-glycoprotein activity, indicating that ceramide must first be converted to C1P to induce P-glycoprotein transport activity (Fig. 2D).
C1P Action Is Rapid, Reversible, and Transporter Specific.
To characterize the induction of P-glycoprotein activity caused by C1P, we exposed capillaries to concentrations of C1P ranging from 50 nM to 250 nM. After 20 minutes, C1P increased P-glycoprotein activity in a concentration-dependent manner, with peak induction occurring between 100 nM and 250 nM (Fig. 3A). As such, we exposed capillaries to 250 nM C1P in all subsequent experiments. To confirm that the changes in capillary fluorescence were biologic, we analyzed the interaction between C1P and NBD-CSA fluorescence in the absence of brain capillaries. An assessment of the baseline fluorescence of NBD-CSA with and without 250 nM C1P confirmed that C1P does not chemically affect the intensity of NBD-CSA (Fig. 3B). This indicates that the changes observed in the luminal fluorescence of capillaries treated with NBD-CSA and C1P result from alterations in P-glycoprotein transport rather than from a chemical interaction.
Fig. 3.
Effects of C1P exposure on efflux transporter activity in isolated rat brain capillaries. (A) C1P concentration response in regards to P-glycoprotein activity. Capillaries were exposed to 2 μM NBD-CSA for 40 minutes followed by exposure to C1P concurrently with NBD-CSA for 20 minutes. (B) C1P treatment does not affect baseline NBD-CSA fluorescence in the absence of transporters. (C) C1P exposure for 20 minutes does not affect the transport activity of MRP2 or BCRP. (D) Time course and washout of C1P action, showing that C1P induction of P-glycoprotein is rapid and reversible. Capillaries were treated with NBD-CSA for 45 minutes to reach steady state, followed by exposure to 250 nM C1P plus NBD-CSA for time shown. P-glycoprotein induction can be completely reversed 1 hour after C1P is removed from treatment solution. Shown are means ± S.E.M. for 10–20 capillaries from single preparation (pooled brains from 3–5 rats). *P<0.05, ****P<0.0001, significantly higher than control.
To investigate whether C1P affects any other efflux transporters at the BBB, we tested the effects of C1P exposure (250 nM; 20 minutes) on MRP2 and BCRP in brain capillaries. After exposure to C1P, we saw no changes in the luminal accumulation of the fluorescent substrates for these transporters: Texas Red for MRP2 and BODIPY Prasozin for BCRP. Quantitatively, we saw no change in the specific activity of these transporters (Fig. 3C). Because C1P exposure elicited no changes to these transporters, we focused exclusively on P-glycoprotein for the remainder of our study.
In time course experiments, C1P induced P-glycoprotein transport in under 5 minutes and sustained the induction for up to 90 minutes, provided that C1P remained in the capillary treatment buffer (Fig. 3D). When C1P was removed from the treatment buffer, P-glycoprotein transport activity returned to control levels in 1 hour (Fig. 3D). These results suggest that C1P acts on P-glycoprotein in a rapid and reversible manner. Our laboratory has previously shown that translational events in brain capillaries require several hours to measurably affect transporter activity (Wang et al., 2014). Hence, we hypothesized that C1P increased P-glycoprotein independently of transcription or translation. Western blots showed that neither 20 minutes nor 4 hours of exposure to C1P increased the total protein expression of P-glycoprotein in isolated rat brain capillaries (Fig. 4, A and B and Supplemental Fig. 2).
Fig. 4.
C1P does not increase overall protein levels of P-glycoprotein in isolated rat brain capillaries. (A, B) Western blots of whole capillary lysates (brain capillaries pooled from 8–10 rats) show that exposure to C1P for 20 minutes or 4 hours does not increase P-glycoprotein protein expression. (C) Pretreatment of 40 minutes with an inhibitor of transcription, 1 μM actinomycin D (ActD), or translation, 50 μg/ml cycloheximide (CHX), does not affect the ability of C1P to increase P-glycoprotein activity in 20 minutes. (D) Pretreatment of 40 minutes with an inhibitor of vesicle trafficking, 400 nM brefeldin A (BFA), blocks C1P-mediated P-glycoprotein induction. Shown are mean ± S.E.M. for 10–20 capillaries from single preparation (pooled brains from 3–5 rats). ****P<0.0001, significantly higher than control.
Further, we performed transport assays with capillaries that had been pretreated with an inhibitor of either transcription (1 μM actinomycin D) or translation (50 μg/ml cycloheximide) before treatment with C1P. Neither inhibitor blocked C1P-mediated P-glycoprotein induction (Fig. 4C). Together, these data strongly suggest that C1P increases P-glycoprotein activity without increasing overall transporter protein expression.
To explore an alternative mechanism of how P-glycoprotein is up-regulated, we pretreated capillaries with 400 nM brefeldin A, an inhibitor of vesicle formation that prevents intracellular protein trafficking (Klausner et al., 1992). Breldein A is certainly not specific for P-glycoprotein, but it has previously been shown to prevent the trafficking of intracellular P-glycoprotein to the plasma membrane (Fu et al., 2004). Our study found that pretreatment with brefeldin A blocked C1P-mediated P-glycoprotein induction (Fig. 4D). It is possible that exposure to C1P may lead to relocation of P-glycoprotein, causing increased P-glycoprotein activity at the BBB. Alternatively, vesicle trafficking may be involved elsewhere in the C1P-initiated signaling cascade.
C1P Requires Phospholipase A2 and COX-2 Signaling.
We sought to identify the signaling cascade through which C1P increases P-glycoprotein activity. Previous studies in cell lines indicate that C1P stimulates the release of arachidonic acid (AA) by activating cytosolic phospholipase A2 (PLA2) (Pettus et al., 2003, 2004; Nakamura et al., 2006). Furthermore, both AA and cyclooxygenase-2 (COX-2), the enzyme that converts AA into prostaglandins, have previously been associated with increased P-glycoprotein transport activity (Bauer et al., 2008; Zibell et al., 2009). As such, we investigated whether C1P depends on activation of an AA/COX-2-associated signaling cascade to alter P-glycoprotein activity.
We inhibited PLA2 by pretreating capillaries with chlorpromazine (200 nM; 40 minutes), which blocked the ability of C1P to increase P-glycoprotein activity (Fig. 5A). Next, we blocked COX-2 with two selective inhibitors: celecoxib (100 nM; 40 minutes) and NS-398 (5 μM; 40 minutes), which similarly blocked the action of C1P (Fig. 5, B and C).
Fig. 5.
Involvement of PLA2 and COX-2 signaling on C1P-mediated P-glycoprotein induction. (A) Inhibiting PLA2 with 200 nM chlorpromazine blocks P-glycoprotein induction caused by C1P treatment. (B) Pretreatment of 40 minutes with a COX-2 inhibitor (100 nM celecoxib) blocks the increases in P-glycoprotein activity caused by C1P treatment. (C) Another COX-2 inhibitor (5 μM NS-398) similarly blocks the increases in P-glycoprotein activity caused by C1P treatment. (D) Exposing COX-2 deficient mouse brain capillaries to 250 nM C1P for 20 minutes resulted in no induction of P-glycoprotein activity. Wild-type (WT) mouse brain capillaries exposed to 250 nM C1P for 20 minutes exhibit an increase in P-glycoprotein activity comparable to that of wild-type rat brain capillaries. Shown are mean ± S.E.M. for 10–20 capillaries from single preparation (pooled brains from 3–5 rats or 4–6 mice). ***P<0.001, ****P<0.0001, significantly higher than control.
To further confirm that COX-2 was required for C1P-mediated P-glycoprotein induction, we performed transport assays using COX-2-deficient mice. Figure 5D shows that C1P treatment on wild-type mice brain capillaries resulted in a 2-fold fluorescence increase of P-glycoprotein activity comparable to the increases observed in wild-type rat brain capillaries. C1P exposure in brain capillaries isolated from COX-2–deficient mice produced no change in the luminal accumulation of NBD-CSA (Fig. 5D). These results indicate that COX-2 is necessary for C1P to increase P-glycoprotein activity.
C1P Pathway Involves PGE2 Receptor.
Previous research in cell lines has proposed the existence of an as-yet-unidentified G-protein–coupled C1P-specific receptor (Granado et al., 2009). In our study, blocking Gi, Go, and Gs activation with a G-protein antagonist peptide prevented C1P from inducing P-glycoprotein activity (Fig. 6A). However, because no C1P-specific receptor has yet been identified in brain capillaries, we sought to investigate alternative explanations for this observation. We thus explored downstream signaling events in the PLA2/COX-2 pathway that involve G-protein–coupled receptors.
Fig. 6.
Involvement of PGE2 in C1P-mediated P-glycoprotein induction. (A) Inhibiting Gi, Go, and Gs activation with 25 μM G-protein antagonist peptide (GPAnt-2) abolishes the increase of P-glycoprotein activity caused by 20 minutes of exposure to 250 nm C1P. S1P, known to act through a G-protein–coupled receptor, is included as a positive control. (B) An inhibitor of MRP4 (1 μM ceefourin) abolishes C1P-mediated P-glycoprotein transport induction. (C) EP2 is expressed in cytosolic and membrane fractions of isolated rat brain capillaries. (D) Representative immunohistochemical images of localization of PGE2 receptor EP2 in rat brain capillaries, using prostaglandin E receptor EP2 antibody (rabbit, monoclonal, 1/500 dilution). (E) Quantification of EP2 localization in isolated rat brain capillaries shows that EP2 is found most abundantly at the luminal membrane of rat brain capillaries. (F) Targeting PGE2 receptors EP1 and EP2 with dual antagonist (835 nM AH-6809) abolishes C1P-mediated P-glycoprotein transport induction. (G) A specific antagonist of EP1 (1 μM SC-51089) partially blocks the ability of C1P to increase P-glycoprotein activity. (H) Specifically targeting the EP2 receptor with an antagonist (100 nM PF-044189448) completely reduces C1P-mediated P-glycoprotein transport induction in a dose-dependent manner. Shown are mean ± S.E.M. for 10–20 capillaries from single preparation (pooled brains from 3–5 rats). *P<0.05 ***P<0.001, ****P<0.0001, significantly higher than control.
The enzyme COX-2 produces prostaglandin H2 from AA, which is then converted to various prostaglandins, including prostaglandin E2 (PGE2). PGE2 is transported extracellularly by MRP4 where it activates four G-protein–coupled receptors: EP1, EP2, EP3, and EP4 (Coleman et al., 1994; Reid et al., 2003). Research has previously associated PGE2 production with S1P and C1P (Pettus et al., 2005), and EP1 and EP2 receptors have been associated with BBB health and maintenance (McCullough et al., 2004; Pekcec et al., 2009). More specifically, several studies have implicated the EP2 receptor in inflammation, cell migration, proliferation, apoptosis, and angiogenesis—processes associated with C1P or BBB transport (Sung et al., 2005; Kamiyama et al., 2006; Liang et al., 2008; Jiang and Dingledine, 2013).
We tested whether C1P signaled through any PGE2 receptors to increase P-glycoprotein activity. First, we targeted MRP4, the transporter that moves PGE2 from the intracellular to the extracellular matrix (Reid et al., 2003). Pretreatment with an inhibitor of MRP4, ceefourin (1 μM; 40 minutes), blocked the ability of C1P to induce P-glycoprotein activity (Fig. 6B). Second, we focused on the EP1 and EP2 receptors. Considerable levels of EP1 have already been noted in brain capillary membranes (Pekcec et al., 2009). To test whether EP2 is similarly present in brain capillaries, we performed Western blot analyses on cytosolic and membrane lysate fractions from isolated capillaries using anti-prostaglandin E receptor EP2 antibody (rabbit, monoclonal, 1/1000 dilution). EP2 protein was found to be expressed in both fractions (Fig. 6C). Immunohistochemical analysis confirmed that the EP2 receptor is expressed in rat brain capillaries and is most abundantly localized in the luminal membrane (Fig. 6D and Fig. 6E).
Transport assays revealed that pretreatment with AH-6809 (835 nM; 40 minutes), a dual-antagonist for EP1 and EP2, completely inhibited the ability of C1P to alter P-glycoprotein activity in brain capillaries (Fig. 6F). Pretreatment with an EP1-specific antagonist, SC51089 (1 μM; 40 minutes), only partially inhibited the action of C1P without also lowering control activity of P-glycoprotein (Fig. 6G). On the other hand, pretreating with an EP2-specific antagonist, PF-04418948 (10 nM and 100 nM; 40 minutes), blocked C1P action in a concentration-dependent manner, with the highest concentration completely abolishing C1P action without affecting control P-glycoprotein activity (Fig. 6H).
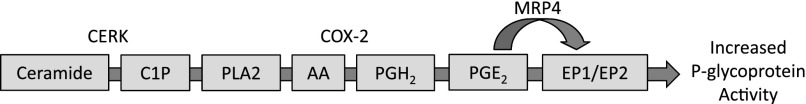
In all, these data suggest that C1P requires the action of PLA2, COX-2, PGE2, and EP2 to increase P-glycoprotein activity. To a lesser extent, C1P may also require the action of EP1. Figure 7 shows a complete working model of this proposed pathway.
Fig. 7.
Proposed signaling cascade for the induction of P-glycoprotein activity by ceramide and C1P.
Discussion
Efflux transporters at the BBB remain an obstacle to CNS pharmacotherapy yet are an effective form of neuroprotection. Understanding the biologic mechanisms that regulate the basal activity of these transporters is critical in developing clinical targets for improved drug delivery to the brain or increasing CNS protection in cases of cellular injury or stress (Miller, 2010). In this study, we demonstrate that short-term exposure to 18-carbon C1P, an endogenous sphingolipid in brain tissue, increases P-glycoprotein transport activity in isolated rat and mouse brain capillaries. Furthermore, we show the requirement for signaling through PLA2, COX-2, and PGE2. Given that C1P acts rapidly and is highly expressed in brain tissue, we speculate that C1P levels regulate a signaling pathway that rapidly increases basal activity of P-glycoprotein, regulating minute-by-minute transport at the BBB.
Exposure to long-chain (18-carbon) ceramide, the precursor for C1P, increases P-glycoprotein activity similarly to 18-carbon C1P. However, unlike C1P, ceramide requires the activity of CERK, the enzyme that converts ceramide into C1P. Furthermore, ceramide requires considerably more time than C1P to increase P-glycoprotein activity. Both observations have led us to speculate that exogenous ceramide must first undergo conversion into C1P before altering P-glycoprotein activity and that ceramide alone has no measurable, short-term effect on P-glycoprotein.
Prior research has shown that CERK is highly active in brain tissue and acts optimally on ceramides with 12-acyl carbon chains or longer (Wijesinghe et al., 2005; Van Overloop et al., 2006). Unsurprisingly, the most abundant C1P species found in brain tissue contain 16-carbon chains or longer (Yamashita et al., 2016). Therefore, CERK activity may modulate basal P-glycoprotein transport activity at the BBB by converting long-chain ceramide species into C1P.
Given that drug efflux pumps such as P-glycoprotein contribute substantially to multidrug resistance in certain diseases, these findings point to a potential role for C1P and CERK in drug resistance. Down-regulation of ceramide, an important mediator of apoptosis, has been associated with poor prognosis and multidrug resistance in tumors, possibly as a result of dysfunctional metabolism into other sphingolipid species (Senchenkov et al., 2001; Koybasi et al., 2004). As such, enzymes that metabolize ceramide, such as CERK, could be targeted for tumor therapy (Reynolds et al., 2003; Payne et al., 2014). In light of our findings, the phosphorylation of ceramide by CERK should be investigated in cases of multidrug resistance to determine whether such resistance results from elevations in P-glycoprotein activity caused by C1P.
Our experiments using fluorescent substrates for other transporters suggest that C1P acts selectively on P-glycoprotein. After C1P treatment, we observed no changes in the accumulation of luminal fluorescence of capillaries incubated with Texas Red (substrate for MRP2) or BODIPY prazosin (substrate for BCRP). These results indicate that 1) C1P does not affect the transport activity of MRP2 or BCRP and 2) the structural integrity of endothelial tight junctions remains unaffected in capillaries exposed to C1P. In the scope of our study, C1P seems to act exclusively on the transport activity of P-glycoprotein.
In time course experiments, we determined that the effect of C1P on P-glycoprotein occurs in approximately 5–15 minutes and reverses fully within 1 hour after C1P is removed from capillaries. Previously, our laboratory has shown that another sphingolipid, S1P, decreases P-glycoprotein activity in a comparably rapid and reversible manner (Cannon et al., 2012). Such immediate action suggests that endogenous C1P, S1P, and possibly other sphingolipids in brain tissue regulate minute-by-minute activity of P-glycoprotein at the BBB.
Our study also indicates that C1P acts through signaling and does not increase the overall protein expression of P-glycoprotein in rat brain capillaries. Western blot analyses revealed that the total protein expression of P-glycoprotein is not induced after treatment with C1P, and transport assays confirmed that C1P does not require the processes of transcription or translation to affect P-glycoprotein transport activity. To our knowledge, this is the first time a molecule has been shown to increase P-glycoprotein activity with no change to overall transporter protein expression. This may be an important component of basal P-glycoprotein activity regulation. In cases when the BBB must be rendered immediately impermeable to environmental toxins or harmful xenobiotics, C1P could produce a rapid increase of efflux from the BBB, resulting in greater neuroprotection.
To begin elucidating a possible mechanism that is independent of protein expression, we found that inhibiting protein trafficking blocks the ability of C1P to increase P-glycoprotein activity. P-glycoprotein has been shown to exist not only at the plasma membrane but also within intracellular compartments, including the endoplasmic reticulum, Golgi, and cytoplasmic vesicles (Bendayan et al., 2006; Fu and Arias, 2012). Some studies suggest that accumulation of intracellular P-glycoprotein caused by inhibition of protein trafficking can interfere with P-glycoprotein function (Fu et al., 2004; McCaffrey et al., 2012). Our results suggest that C1P exposure may promote trafficking of intracellular P-glycoprotein to the capillary membrane, which could increase transport activity of P-glycoprotein at the BBB without increasing overall transporter protein expression. However, because other components in the C1P signaling cascade may also be subject to trafficking, this mechanism must be explored in future studies with less general inhibitors of protein traffic.
Using specific signaling inhibitors, immunohistochemistry analysis, and COX-2–deficient mice, we have characterized the involvement of the PLA2/COX-2/PGE2 inflammatory signaling pathway in C1P-mediated P-glycoprotein induction. Previous cell models show that C1P targets and activates cytosolic PLA2 (Pettus et al., 2003; Nakamura et al., 2006), which subsequently stimulates a high release of AA (Pettus et al., 2004). Downstream in this pathway, COX-2 converts AA into prostaglandins. Studies have implicated COX-2 in P-glycoprotein up-regulation (Bauer et al., 2008; Zibell et al., 2009). Together, these findings led us to speculate that a connection exists between C1P and COX-2-mediated P-glycoprotein induction. Indeed, our results showed that COX-2 activity is necessary for C1P to increase the activity of P-glycoprotein in isolated rat and mouse brain capillaries.
We further identified that EP2, a G-protein–coupled receptor for PGE2, is involved in the induction of P-glycoprotein caused by C1P. In cell lines, C1P has been shown to increase PGE2 production, even at concentrations as low as 300 nM (Pettus et al., 2005). Additional studies have proposed the involvement of G-protein coupled receptors in C1P signaling (Granado et al., 2009). While our study did not identify any C1P-specific receptors in brain capillaries, we found that C1P action on P-glycoprotein did require the general activity of a G-protein coupled receptor. More specifically, EP2 receptor antagonists blocked the ability of C1P to increase P-glycoprotein activity. Our experiments also indicate possible involvement of EP1, although EP1 inhibitors only partially attenuated P-glycoprotein induction.
Previous studies associate EP receptors with the BBB (McCullough et al., 2004; Pekcec et al., 2009; Jiang and Dingledine, 2013). EP2, in particular, shares biologic characteristics with C1P; both EP2 and C1P have been implicated with decreased apoptosis, increased angiogenesis, and the promotion of inflammatory responses through COX-2 (Sung et al., 2005; Kamiyama et al., 2006; Liang et al., 2008; Kim et al., 2013; Rivera et al., 2015). Our results show that EP2 exists almost exclusively on the luminal membrane of rat brain capillaries, suggesting that in our model PGE2 activates its receptor in the capillary lumen. Signaling components downstream of PGE2 should be identified to determine the full pathway through which C1P increases P-glycoprotein activity.
Drug resistance in disorders such as brain cancer, epilepsy, and depression show associations with higher activity of efflux transporters at the BBB, including P-glycoprotein (Löscher and Potschka, 2005; Brandt et al., 2006). It is not uncommon for such diseases to also exhibit increased levels of the enzymes involved in C1P production, such as CERK and sphingomyelinase D. Higher levels of CERK have been associated with tumor recurrence (Payne et al., 2014), and patients suffering from depression have presented with raised levels of sphingomyelinase D (Kornhuber et al., 2005). Given the results of our study, future work should explore the associations between C1P-mediated P-glycoprotein induction and drug resistance associated with precursors of C1P. Drug resistance in certain diseases might be linked with C1P-mediated up-regulation of transporters that restrict drug access to the CNS.
On the other hand, P-glycoprotein is critical for brain homeostasis and limits the passage of harmful metabolites and xenobiotics into the CNS. Studies have shown that inflammation can change the activity of P-glycoprotein (Bauer et al., 2007; Miller et al., 2008; Chodobski et al., 2011), and some speculate that up-regulation of P-glycoprotein in response to inflammation may actually provide neuroprotection (Seelbach et al., 2007; Alfieri et al., 2011). Over recent years, targeting sphingolipids has become an attractive clinical possibility for the treatment of various health conditions; for example, studies propose targeting C1P and CERK for tissue regeneration and the treatment of inflammatory diseases, cancer, and other conditions (Zeidan and Hannun, 2007; Granado et al., 2009; Arana et al., 2010; Gómez-Muñoz et al., 2010; Kim et al., 2013; Maceyka and Spiegel, 2014; Pastukhov et al., 2014; Baudiß et al., 2016). Given our results, the neuroprotective functions of C1P, CERK, and other sphingolipids should be considered in these cases. While inhibition of C1P or CERK might be an effective therapeutic strategy certain cases, it might also have the potential to lower CNS protection against further cellular harm.
From our results, we propose that endogenous production of C1P via CERK in brain tissue increases the basal activity of P-glycoprotein and contributes to general neuroprotection in healthy brains. In cases of cellular injury or stress, it is possible that increases in C1P would protect against additional cellular damage. Conversely, in disease states wherein the levels of C1P and CERK increase, the activation of the PLA2/COX-2/PGE2 pathway may contribute to drug resistance. As such, the ability of C1P and CERK to restrict BBB transport could either be targeted for enhanced drug delivery or exploited for neuroprotection. Also important is the potential for enzymatic interconversion of the sphingolipids (C1P and S1P) and their ability to rapidly alter P-glycoprotein activity in opposite directions. Controlling the balance of C1P and S1P cellular levels would provide a mechanism for dynamic regulation of P-glycoprotein transport activity at the BBB. In all, our data support that C1P is an important regulator of P-glycoprotein activity and has the potential to be a versatile molecule for clinical manipulation.
Acknowledgments
The authors thank the members of the Miller laboratory for technical support: Dr. Gary Chan, Rebecca Evans, David Banks, and Joyce Blaisdell. The authors also thank Dr. Artiom Gruzdev and Dr. Matthew Edin for their involvement and advice, and the staff at the NIEHS animal facility for providing care to the animals used in this study.
Abbreviations
- AA
arachidonic acid
- AH-6809
9-oxo-6-propan-2-yloxyxanthene-2-carboxylic acid
- BBB
blood-brain barrier
- BCRP
breast cancer resistance protein
- BODIPY prazosin
boron[1-(4-amino-6,7-dimethoxy-2-quinazolinyl)-4-[3-[5-[(3,5-dimethyl-2H-pyrrol-2-ylidene-κN)methyl]-1H-pyrrol-2-yl-κN]-1-oxopropyl]piperazinato]difluoro-(T-4)-175799-93-6
- BSA
bovine serum albumin
- CERK
ceramide kinase
- CNS
central nervous system
- C1P
ceramide 1-phosphate
- COX-2
cyclooxygenase-2
- EP1
prostaglandin E2 receptor 1
- EP2
prostaglandin E2 receptor 2
- KO143
(3S,6S,12aS)-1,2,3,4,6,7,12,12a-octahydro-9-methoxy-6-(2-methylpropyl)-1,4-dioxopyrazino-[1ʹ,2ʹ:1,6]pyrido[3,4-b]indole-3-propanoic acid 1,1-dimethylethyl ester
- MRP
multidrug resistance protein
- NBD-CSA
[N-ε-(4-nitrobenzofurazan-7-yl)-d-Lys8]cyclosporine A
- NS-398
N-(2-cyclohexyloxy-4-nitrophenyl)methanesulfonamide
- PBS
phosphate-buffered saline
- NVP-231
N-(2-benzamido-1,3-benzothiazol-6-yl)adamantane-1-carboxamide
- PF-04418948
1-(4-fluorobenzoyl)-3-[(6-methoxynaphthalen-2-yl)oxymethyl]azetidine-3-carboxylic acid
- PGE2
prostaglandin E2
- PLA2
phospholipase A2
- PSC833
valspodar
- SC-51089 hydrate
3-chloro-Nʹ-(3-pyridin-4-ylpropanoyl)-6H-benzo[b][1,4]benzoxazepine-5-carbohydrazide
- hydrate
- hydrochloride
- S1P
sphingosine 1-phosphate
Authorship Contributions
Participated in research design: Mesev, Miller, Cannon.
Conducted experiments: Mesev.
Performed data analysis: Mesev, Cannon.
Wrote or contributed to the writing of the manuscript: Mesev, Cannon.
Footnotes
This work was supported by the Intramural Research Program of the National Institutes of Health National Institute of Environmental Health Sciences.
 This article has supplemental material available at molpharm.aspetjournals.org.
This article has supplemental material available at molpharm.aspetjournals.org.
References
- Alfieri A, Srivastava S, Siow RC, Modo M, Fraser PA, Mann GE. (2011) Targeting the Nrf2-Keap1 antioxidant defence pathway for neurovascular protection in stroke. J Physiol 589:4125–4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arana L, Gangoiti P, Ouro A, Trueba M, Gómez-Muñoz A. (2010) Ceramide and ceramide 1-phosphate in health and disease. Lipids Health Dis 9:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudiß K, de Paula Vieira R, Cicko S, Ayata K, Hossfeld M, Ehrat N, Gómez-Muñoz A, Eltzschig HK, Idzko M. (2016) C1P attenuates lipopolysaccharide-induced acute lung injury by preventing NF-κB activation in neutrophils. J Immunol 196:2319–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer B, Hartz AMS, Miller DS. (2007) Tumor necrosis factor alpha and endothelin-1 increase P-glycoprotein expression and transport activity at the blood-brain barrier. Mol Pharmacol 71:667–675. [DOI] [PubMed] [Google Scholar]
- Bauer B, Hartz AMS, Pekcec A, Toellner K, Miller DS, Potschka H. (2008) Seizure-induced up-regulation of P-glycoprotein at the blood-brain barrier through glutamate and cyclooxygenase-2 signaling. Mol Pharmacol 73:1444–1453. [DOI] [PubMed] [Google Scholar]
- Begley DJ. (2004) ABC transporters and the blood-brain barrier. Curr Pharm Des 10:1295–1312. [DOI] [PubMed] [Google Scholar]
- Bendayan R, Ronaldson PT, Gingras D, Bendayan M. (2006) In situ localization of P-glycoprotein (ABCB1) in human and rat brain. J Histochem Cytochem 54:1159–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt C, Bethmann K, Gastens AM, Löscher W. (2006) The multidrug transporter hypothesis of drug resistance in epilepsy: proof-of-principle in a rat model of temporal lobe epilepsy. Neurobiol Dis 24:202–211. [DOI] [PubMed] [Google Scholar]
- Cannon RE, Peart JC, Hawkins BT, Campos CR, Miller DS. (2012) Targeting blood-brain barrier sphingolipid signaling reduces basal P-glycoprotein activity and improves drug delivery to the brain. Proc Natl Acad Sci USA 109:15930–15935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chodobski A, Zink BJ, Szmydynger-Chodobska J. (2011) Blood-brain barrier pathophysiology in traumatic brain injury. Transl Stroke Res 2:492–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman RA, Smith WL, Narumiya S. (1994) International Union of Pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol Rev 46:205–229. [PubMed] [Google Scholar]
- Fu D, Bebawy M, Kable EP, Roufogalis BD. (2004) Dynamic and intracellular trafficking of P-glycoprotein-EGFP fusion protein: Implications in multidrug resistance in cancer. Int J Cancer 109:174–181. [DOI] [PubMed] [Google Scholar]
- Fu D, Arias IM. (2012) Intracellular trafficking of P-glycoprotein. Int J Biochem Cell Biol 44:461–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Muñoz A, Gangoiti P, Granado MH, Arana L, Ouro A. (2010) Ceramide-1-phosphate in cell survival and inflammatory signaling. Adv Exp Med Biol 688:118–130. [DOI] [PubMed] [Google Scholar]
- Granado MH, Gangoiti P, Ouro A, Arana L, González M, Trueba M, Gómez-Muñoz A. (2009) Ceramide 1-phosphate (C1P) promotes cell migration involvement of a specific C1P receptor. Cell Signal 21:405–412. [DOI] [PubMed] [Google Scholar]
- Hartz AMS, Bauer B, Fricker G, Miller DS. (2004) Rapid regulation of P-glycoprotein at the blood-brain barrier by endothelin-1. Mol Pharmacol 66:387–394. [DOI] [PubMed] [Google Scholar]
- Jablonski MR, Jacob DA, Campos C, Miller DS, Maragakis NJ, Pasinelli P, Trotti D. (2012) Selective increase of two ABC drug efflux transporters at the blood-spinal cord barrier suggests induced pharmacoresistance in ALS. Neurobiol Dis 47:194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Dingledine R. (2013) Prostaglandin receptor EP2 in the crosshairs of anti-inflammation, anti-cancer, and neuroprotection. Trends Pharmacol Sci 34:413–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiyama M, Pozzi A, Yang L, DeBusk LM, Breyer RM, Lin PC. (2006) EP2, a receptor for PGE2, regulates tumor angiogenesis through direct effects on endothelial cell motility and survival. Oncogene 25:7019–7028. [DOI] [PubMed] [Google Scholar]
- Kim C, Schneider G, Abdel-Latif A, Mierzejewska K, Sunkara M, Borkowska S, Ratajczak J, Morris AJ, Kucia M, Ratajczak MZ. (2013) Ceramide-1-phosphate regulates migration of multipotent stromal cells and endothelial progenitor cells—implications for tissue regeneration. Stem Cells 31:500–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner RD, Donaldson JG, Lippincott-Schwartz J. (1992) Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol 116:1071–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornhuber J, Medlin A, Bleich S, Jendrossek V, Henkel AW, Wiltfang J, Gulbins E. (2005) High activity of acid sphingomyelinase in major depression. J Neural Transm (Vienna) 112:1583–1590. [DOI] [PubMed] [Google Scholar]
- Koybasi S, Senkal CE, Sundararaj K, Spassieva S, Bielawski J, Osta W, Day TA, Jiang JC, Jazwinski SM, Hannun YA, et al. (2004) Defects in cell growth regulation by C18:0-ceramide and longevity assurance gene 1 in human head and neck squamous cell carcinomas. J Biol Chem 279:44311–44319. [DOI] [PubMed] [Google Scholar]
- Lamour NF, Chalfant CE. (2008) Ceramide kinase and the ceramide-1-phosphate/cPLA2alpha interaction as a therapeutic target. Curr Drug Targets 9:674–682. [DOI] [PubMed] [Google Scholar]
- Liang X, Wang Q, Shi J, Lokteva L, Breyer RM, Montine TJ, Andreasson K. (2008) The prostaglandin E2 EP2 receptor accelerates disease progression and inflammation in a model of amyotrophic lateral sclerosis. Ann Neurol 64:304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löscher W, Potschka H. (2005) Drug resistance in brain diseases and the role of drug efflux transporters. Nat Rev Neurosci 6:591–602. [DOI] [PubMed] [Google Scholar]
- Maceyka M, Spiegel S. (2014) Sphingolipid metabolites in inflammatory disease. Nature 510:58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffrey G, Staatz WD, Sanchez-Covarrubias L, Finch JD, Demarco K, Laracuente ML, Ronaldson PT, Davis TP. (2012) P-glycoprotein trafficking at the blood-brain barrier altered by peripheral inflammatory hyperalgesia. J Neurochem 122:962–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough L, Wu L, Haughey N, Liang X, Hand T, Wang Q, Breyer RM, Andreasson K. (2004) Neuroprotective function of the PGE2 EP2 receptor in cerebral ischemia. J Neurosci 24:257–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milane A, Fernandez C, Dupuis L, Buyse M, Loeffler JP, Farinotti R, Meininger V, Bensimon G. (2010) P-glycoprotein expression and function are increased in an animal model of amyotrophic lateral sclerosis. Neurosci Lett 472:166–170. [DOI] [PubMed] [Google Scholar]
- Miller DS, Nobmann SN, Gutmann H, Toeroek M, Drewe J, Fricker G. (2000) Xenobiotic transport across isolated brain microvessels studied by confocal microscopy. Mol Pharmacol 58:1357–1367. [DOI] [PubMed] [Google Scholar]
- Miller DS, Bauer B, Hartz AMS. (2008) Modulation of P-glycoprotein at the blood-brain barrier: opportunities to improve central nervous system pharmacotherapy. Pharmacol Rev 60:196–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DS. (2010) Regulation of P-glycoprotein and other ABC drug transporters at the blood-brain barrier. Trends Pharmacol Sci 31:246–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morham SG, Langenbach R, Loftin CD, Tiano HF, Vouloumanos N, Jennette JC, Mahler JF, Kluckman KD, Ledford A, Lee CA, et al. (1995) Prostaglandin synthase 2 gene disruption causes severe renal pathology in the mouse. Cell 83:473–482. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Hirabayashi T, Shimizu M, Murayama T. (2006) Ceramide-1-phosphate activates cytosolic phospholipase A2α directly and by PKC pathway. Biochem Pharmacol 71:850–857. [DOI] [PubMed] [Google Scholar]
- Pastukhov O, Schwalm S, Zangemeister-Wittke U, Fabbro D, Bornancin F, Japtok L, Kleuser B, Pfeilschifter J, Huwiler A. (2014) The ceramide kinase inhibitor NVP-231 inhibits breast and lung cancer cell proliferation by inducing M phase arrest and subsequent cell death. Br J Pharmacol 171:5829–5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne AW, Pant DK, Pan T, Chodosh LA. (2014) Ceramide kinase promotes tumor cell survival and mammary tumor recurrence. Cancer Res 74:6352–6363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekcec A, Unkrüer B, Schlichtiger J, Soerensen J, Hartz AMS, Bauer B, van Vliet EA, Gorter JA, Potschka H. (2009) Targeting prostaglandin E2 EP1 receptors prevents seizure-associated P-glycoprotein up-regulation. J Pharmacol Exp Ther 330:939–947. [DOI] [PubMed] [Google Scholar]
- Pettus BJ, Bielawska A, Spiegel S, Roddy P, Hannun YA, Chalfant CE. (2003) Ceramide kinase mediates cytokine- and calcium ionophore-induced arachidonic acid release. J Biol Chem 278:38206–38213. [DOI] [PubMed] [Google Scholar]
- Pettus BJ, Bielawska A, Subramanian P, Wijesinghe DS, Maceyka M, Leslie CC, Evans JH, Freiberg J, Roddy P, Hannun YA, et al. (2004) Ceramide 1-phosphate is a direct activator of cytosolic phospholipase A2. J Biol Chem 279:11320–11326. [DOI] [PubMed] [Google Scholar]
- Pettus BJ, Kitatani K, Chalfant CE, Taha TA, Kawamori T, Bielawski J, Obeid LM, Hannun YA. (2005) The coordination of prostaglandin E2 production by sphingosine-1-phosphate and ceramide-1-phosphate. Mol Pharmacol 68:330–335. [DOI] [PubMed] [Google Scholar]
- Reid G, Wielinga P, Zelcer N, van der Heijden I, Kuil A, de Haas M, Wijnholds J, Borst P. (2003) The human multidrug resistance protein MRP4 functions as a prostaglandin efflux transporter and is inhibited by nonsteroidal antiinflammatory drugs. Proc Natl Acad Sci USA 100:9244–9249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CP, Maurer BJ, Kolesnick RN. (2003) Ceramide synthesis and metabolism as a target for cancer therapy. Cancer Letters 206:169–180. [DOI] [PubMed] [Google Scholar]
- Rivera IG, Ordoñez M, Presa N, Gomez-Larrauri A, Simón J, Trueba M, Gomez-Muñoz A. (2015) Sphingomyelinase D/ceramide 1-phosphate in cell survival and inflammation. Toxins (Basel) 7:1457–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelbach MJ, Brooks TA, Egleton RD, Davis TP. (2007) Peripheral inflammatory hyperalgesia modulates morphine delivery to the brain: a role for P-glycoprotein. J Neurochem 102:1677–1690. [DOI] [PubMed] [Google Scholar]
- Senchenkov A, Litvak DA, Cabot MC. (2001) Targeting ceramide metabolism--a strategy for overcoming drug resistance. J Natl Cancer Inst 93:347–357. [DOI] [PubMed] [Google Scholar]
- Sung YM, He G, Fischer SM. (2005) Lack of expression of the EP2 but not EP3 receptor for prostaglandin E2 results in suppression of skin tumor development. Cancer Res 65:9304–9311. [DOI] [PubMed] [Google Scholar]
- Van Overloop H, Gijsbers S, Van Veldhoven PP. (2006) Further characterization of mammalian ceramide kinase: substrate delivery and (stereo)specificity, tissue distribution, and subcellular localization studies. J Lipid Res 47:268–283. [DOI] [PubMed] [Google Scholar]
- Wang X, Sykes DB, Miller DS. (2010) Constitutive androstane receptor-mediated up-regulation of ATP-driven xenobiotic efflux transporters at the blood-brain barrier. Mol Pharmacol 78:376–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Campos CR, Peart JC, Smith LK, Boni JL, Cannon RE, Miller DS. (2014) Nrf2 upregulates ATP binding cassette transporter expression and activity at the blood-brain and blood-spinal cord barriers. J Neurosci 34:8585–8593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijesinghe DS, Massiello A, Subramanian P, Szulc Z, Bielawska A, Chalfant CE. (2005) Substrate specificity of human ceramide kinase. J Lipid Res 46:2706–2716. [DOI] [PubMed] [Google Scholar]
- Yamashita R, Tabata Y, Iga E, Nakao M, Sano S, Kogure K, Tokumura A, Tanaka T. (2016) Analysis of molecular species profiles for ceramide-1-phosphate and sphingomyelin using MALDI-TOF mass spectrometry. Lipids 51:263–270. [DOI] [PubMed] [Google Scholar]
- Zeidan YH, Hannun YA. (2007) Translational aspects of sphingolipid metabolism. Trends Mol Med 13:327–336. [DOI] [PubMed] [Google Scholar]
- Zhang M, An C, Gao Y, Leak RK, Chen J, Zhang F. (2013) Emerging roles of Nrf2 and phase II antioxidant enzymes in neuroprotection. Prog Neurobiol 100:30–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zibell G, Unkrüer B, Pekcec A, Hartz AMS, Bauer B, Miller DS, Potschka H. (2009) Prevention of seizure-induced up-regulation of endothelial P-glycoprotein by COX-2 inhibition. Neuropharmacology 56:849–855. [DOI] [PubMed] [Google Scholar]