Abstract
Diffusion-related magnetic resonance imaging parametric maps may be employed to characterize white matter of brain. We hypothesize that entropy of diffusion anisotropy may be most effective for detecting therapeutic effects of bone marrow stromal cell treatment of ischemia in type 2 diabetes mellitus rats. Type 2 diabetes mellitus was induced in adult male Wistar rats. These rats were then subjected to 2 h of middle cerebral artery occlusion, and received bone marrow stromal cell (5 × 106, n = 8) or an equal volume of saline (n = 8) via tail vein injection at three days after middle cerebral artery occlusion. Magnetic resonance imaging was performed on day one and then weekly for five weeks post middle cerebral artery occlusion. The diffusion metrics complementarily permitted characterization of axons and axonal myelination. All six magnetic resonance imaging diffusion metrics, confirmed by histological measures, demonstrated that bone marrow stromal cell treatment significantly (p < 0.05) improved magnetic resonance imaging diffusion indices of white matter in type 2 diabetes mellitus rats after middle cerebral artery occlusion compared with the saline-treated rats. Superior to the fractional anisotropy metric that provided measures related to organization of neuronal fiber bundles, the entropy metric can also identify microstructures and low-density axonal fibers of cerebral tissue after stroke in type 2 diabetes mellitus rats.
Keywords: White matter, diabetes, stroke, magnetic resonance imaging, diffusion
Introduction
White matter anatomically connects different functional domains in brain. The remodeling or reconstruction of impaired white matter after stroke, thus, plays a pivotal role in functional improvement, a primary goal of therapy for stroke patients. A non-invasive tool to dynamically monitor the rebuilding of white matter in stroke patients would be very helpful in evaluating the therapeutic efficacy of any treatment and predicting the functional recovery. Diffusion-related magnetic resonance imaging (MRI) measures provide such a means because of its sensitivity to the diffusion of water, and water accounts for 75% brain content.1
Diffusion-weighted imaging (DWI) of MRI has been employed for decades to investigate cerebral ischemic stroke.2 Apparent diffusion coefficient (ADC) of water in individual directions, derived from the corresponding directional DWI, is rapidly and sharply decreased after ischemia due to cytotoxic edema of the ischemic cerebral tissue.3,4 Thus, the mobility of water molecules, by quantifying the degree of deviation from the unrestricted water diffusion, characterized by ADC, is a sensitive measure of tissue microstructure. However, the ADC is inadequate to properly describe water diffusion in brain, since the complexity of the biological cytoarchitecture of cerebral tissue impacts the random Brownian motion of water molecules.5
Water diffusion in white matter is strongly restricted by myelinated axons.6 The movements of water molecules in the intra- and extra-cellular space of organized glial bundles surrounding the tightly packed and coherently aligned axons are hindered to a greater extent in a direction perpendicular to the axonal orientation than parallel to it.7 This restriction will cause anisotropic diffusion. Therefore, fractional anisotropy (FA) of water diffusion needs to be evaluated to characterize this cerebral white matter architecture. FA can be derived from diffusion tensor imaging (DTI),8 which extends DWI from one direction to conventional six non-coplanar directions to measure the diffusion tensor. Henceforth, ADC is derived as a tensor.
The restriction of free diffusion of water molecules in the biological cytoarchitecture also results in non-Gaussian diffusion, which prompted the development of diffusion spectrum imaging (DSI).9 DSI is a technical extension of DTI by encoding additional directions and increased diffusion gradients (i.e. multiple higher b-values) with the DWI pulse sequence. The development of DSI, in order to reduce its acquisition time, has lead to multiple MRI approaches, such as q-ball imaging, which focuses on anisotropy of diffusion with a higher resolution of angular distribution,10 and diffusion kurtosis imaging (DKI), which emphasizes the non-Gaussian features of quantitative diffusion probability distribution with multiple higher b-value shells.11 DKI produces the apparent kurtosis coefficient (AKC), a tensor as ADC, to characterize the degree of non-Gaussian diffusion of water molecules in cerebral tissue.
In addition to ADC, FA, and AKC, the axonal water fraction (AWF) and entropy of diffusion anisotropy can be derived to characterize cerebral tissue from analysis of DSI data under biological assumptions.12,13 Multiple diffusion metrics provide complementary information of cerebral tissue. For instance, FA was able to monitor reorganization of white matter after stroke in rats;14–16 q-ball imaging aims to solve the orientation distribution function of water molecules diffusion, and is used to describe the directionality of multimodal diffusion in regions with complex fiber architecture, and permits accurate detection of crossing fibers,10,17 whereas the AKC can provide additional information on neural tissue micro-architecture.18
Such complementary information may help us to further evaluate brain tissue response, particularly the white matter response, to the ischemic insult and therapeutic treatments. However, the multiple MRI diffusion approaches contain overlapping information. Therefore, to conserve time and resources for proper evaluation of white matter response to stroke and therapy, it may be more practical and efficient to employ fewer parameters, such as entropy of diffusional anisotropy.
A stroke model of type 2 diabetes mellitus (T2DM) rats was selected to compare the ability of these diffusion metrics to detect white matter damage and remodeling. T2DM may hamper the white matter reorganization involving the corpus callosum after stroke,19 and T2DM worsens the overall neurological outcomes after stroke. Therefore, by employing diffusion MRI in the present study, the temporal changes of white matter were monitored using multiple diffusion metrics weekly over five weeks after stroke in the T2DM rats with or without cell-based therapy. Since MRI diffusion-related parameters are sensitive to white matter integrity, we hypothesize in the present study that these results will permit investigation of the ability of diffusion metrics to characterize white matter remodeling after stroke with or without BMSC treatments in T2DM rats and will provide insight into the therapeutic responses of white matter with and without a cell-based restorative therapy in a co-morbid (T2DM) model of stroke. Data gleaned from the present study may provide information applicable to clinical translation of diffusion imaging for stroke in response to therapy.
Materials and methods
All experimental procedures were conducted in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of Henry Ford Health System (No. 1040), and experimental guidelines of ARRIVE (items 8, 10 to 13).
Animal model and experimental protocol
T2DM was induced in adult (175–200 g, 2–3 months) male Wistar rats (Charles River, Wilmington, MA) by feeding them a high-fat diet (40% of calories as fat) for two weeks, then intraperitoneally injecting a single low dose (35 mg/kg) of Streptozotocin (Zanosar®, Sigma Chemical Co., St. Louis, MO), a naturally occurring chemical that is particularly toxic to the insulin-producing beta cells of the pancreas in mammals, and the high-fat diet was continued for another two weeks.19–21 Blood glucose level was measured using test strips for glucose (Polymer Technology System, Indianapolis, IN) for confirmation of hyperglycemia. The rats with 8 h fasting plasma glucose of ≥300 mg/dl were considered diabetic and selected for middle cerebral artery occlusion (MCAo).19
Right middle cerebral artery was occluded for 2 h using the filament model.22 Briefly, a 4-0 monofilament nylon suture, its tip rounded by heating, was introduced into the internal carotid artery lumen through the stump of the external carotid artery and gently advanced into the internal carotid artery 19–21 mm past the common carotid artery bifurcation to block the origin of the middle cerebral artery. Reperfusion was initiated through removal of the thread and tying off the distal external carotid artery.
Rats were treated via tail vein injection at three days after MCAo with either bone marrow stromal cells (BMSC) at a dose of 5 million (5 × 106) cells or an equal volume of phosphate-buffered saline (PBS) as the vehicle. Seven rats (three from control and four from treated rats) died before going through the completion of five weeks MRI among the total 23 rats (11 for control and 12 for treated rats) in the experiment, and were excluded from the study. The treated and control T2DM animal groups were age and body weight matched.
MRI was performed prior to the surgery for MCAo, as an internal control. Then, MRI was performed on day one and then weekly for five weeks after ischemia-reperfusion for all treated and control rats. After completing MRI scans, all animals (n = 8 for the treated and n = 8 for the control) were euthanized five weeks post stroke.
MRI measurements
MRI measurements were performed with a 7T system (Bruker-Biospin, Ettlingen, Germany). A birdcage type coil was used as the transmitter and a quadrature half-volume coil as the receiver. During MRI measurements, anesthesia was maintained using medical air (1.0 L/min) with isoflurane (1.0–1.5%). Stereotactic ear bars were used to minimize movement, and rectal temperature was maintained at 37 ± 1.0℃ using a feedback controlled water bath (YSI Inc, Yellow Springs, OH).
At each MRI session, a fast gradient echo imaging sequence was used for reproducible positioning of the animal in the magnet, and a spin-echo sequence of T2-weighted imaging (T2WI) was acquired for detection of ischemic lesion in the rat brain. The T2WI contained six sets of images using echo time of 15, 30, 45, 60, 75, and 90 ms; repetition time of 4.5 s, and 32 × 32 mm2 field-of-view (FOV), 128 × 128 matrix, 1 mm slice thickness of 13 slices.
Diffusion-related measures included q-ball imaging and DKI, using a pulsed gradients (duration δ = 10 ms; interval Δ = 18 ms) spin-echo sequence and one-shot echo-planar readout with one baseline of b = 0 s/mm2. The geometric parameters of diffusion MRI were matched to T2WI with repetition time of 10 s and echo time of 50 ms. Additional MRI parameters were 64 directions of diffusion gradients with b = 1500 s/mm2 in q-ball acquisition; as for DKI, 20 directions of diffusion gradients with b = 900 and 1800 s/mm2, respectively, and two averages, were employed. Signal–noise ratio is the major concern of using relative low b-value in diffusion MRI.
Acquisition time for these imaging protocols was approximately 10 min for T2WI, 11 min for q-ball, and 13 min for DKI, respectively.
Histological staining
Rats were euthanized with ketamine (44 mg/kg, intraperitoneal) and xylazine (13 mg/kg, intraperitoneal) with the completion of MRI scan at five weeks after treatment. Brains were isolated, post-fixed in 4% paraformaldehyde for two days at room temperature, and then processed for paraffin sectioning. Coronal sections (6 µm thick) were cut from each block and double-stained with Bielschowsky’s silver and Luxol fast blue (BLFB). The BLFB staining was performed for evaluation of axons and myelin.
Data and statistical analysis
Data analysis was performed in a blind fashion. MRI image analysis was generally performed with homemade software, Eigentool.23 The diffusion MRI data analysis was first performed using Camino software,17 and then Eigentool was used after format transformation.
T2 maps were obtained from T2WI by using linear least-square fit to the plot of the natural logarithm pixel-by-pixel. The entropy map was generated from q-ball imaging using in-house software.13 The entropy, E, was calculated pixel-by-pixel using the following formula
where D is the set of all gradient directions in q-ball sequence, and p(xi,dj) is the probability of the MRI signal attenuation value being repeated throughout D due to water displacement along a certain spatial orientation dj for pixel xi, and the MRI signal attenuation value for pixel xi in diffusion direction dj is calculated by dividing its diffusion-weighted signal intensity by pixel xi’s diffusion-free (b = 0 s/mm2) signal intensity.
Using the linear least-square fitting, a diffusive ellipsoid for each pixel was calculated from the DKI data. The ADC along the longest ellipsoidal principal axis (eigenvalue λ1) was noted as the axial ADC, whereas the perpendicular or radial ADC was the mean of the other two eigenvalues (λ2 and λ3).24 FA was then calculated from these three eigenvalues.24 With a two compartment model of the intra- and extra-cellular space used for cerebral tissue, AWF and mean AKC were deduced by fitting the DKI data.12
All time points of T2 maps were coregistered to the one week T2 maps, and all diffusion-related parametric maps were coregistered to T2 maps slice by slice.23 Ischemic lesion size was demarcated with coregistered T2 maps acquired after stroke using values above mean plus two standard deviations of contralateral measurements. The infarct volume could potentially include tissues that are evolving into a cystic lesion by the five weeks after ischemia. The difference of the ischemic lesion sizes in T2 maps obtained between one day and five weeks after stroke is referred to recovery area.15 Then, the recovery areas, as the regions-of-interest (ROI), were loaded from T2 maps onto the individual diffusion metric maps for measurement. In addition to the white matter, the selected ROI includes gray matter. However, since most diffusion-related MRI metrics are more sensitive to changes of white matter than gray matter, the white matter was the primary focus of this study.
The MicroComputer Imaging Device system (Imaging Research Inc, Ontario, Canada) was used for histological measurements. With BLFB staining, four locations of each coronal section, which were located inside of the corresponding ROI of T2WI along the selected recovery areas at five weeks after stroke, were used for myelin and axonal density quantification.25 The positive stained areas of the ROI were measured under a 40× objective of optical microscope, using an average of all locations as the histological result.
MRI measurements are summarized as mean and standard deviation. Differences in the MRI data between groups were analyzed by a mixed model of analysis of variance and covariance, respectively. For the longitudinal MRI measurements, the analysis started testing the group and time (without baseline time point) interaction, followed by testing the group difference at each time point if the interaction or overall group effect was detected at the 0.05 level.
Results
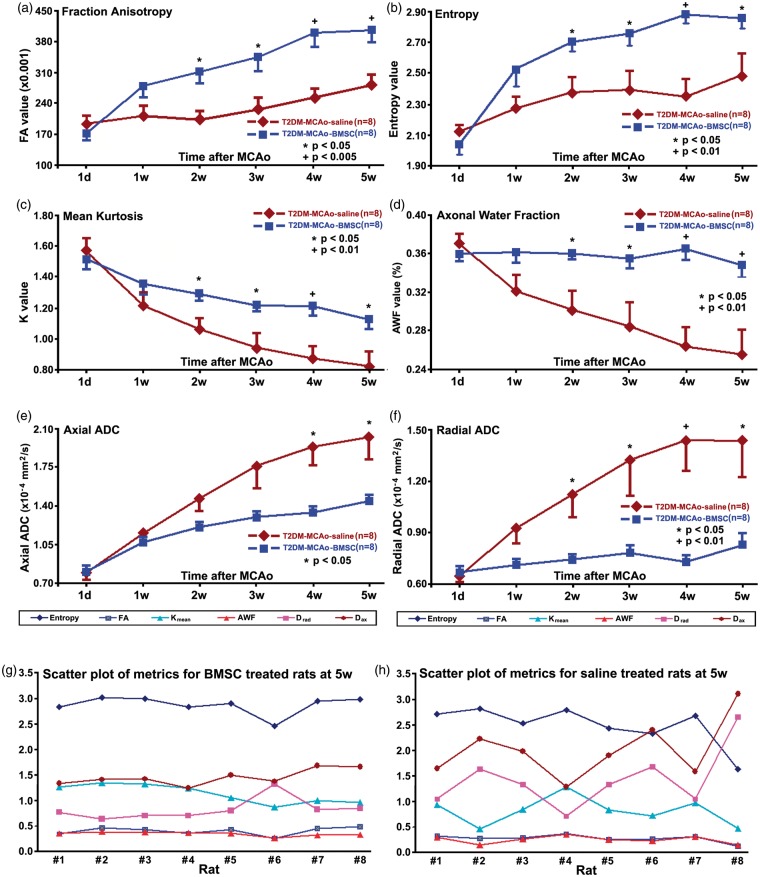
Six diffusion-related parametric maps, i.e. FA, entropy, mean AKC, AWF, axial and radial ADCs, were derived from q-ball imaging and DKI, respectively, for characterizing white matter changes from one day and weekly to five weeks after stroke in all T2DM rats. The parametric values of each map and each time point were measured from the recovery ROI in the MRI central slice, as graphed and shown in Figure 1. The recovered tissue volumes (ROI) at five weeks after stroke are, 102.4 ± 21.1 mm3 and 72.1 ± 37.3 mm3 for the BMSC (n = 8)- and saline (n = 8)-treated T2DM rats, respectively. This difference is marginally statistically significant with the p value of 0.071.
Figure 1.
Evolution of changes for the six diffusion-related parametric maps, fraction anisotropy of diffusion (a), entropy of diffusion anisotropy (b), mean kurtosis (c), axonal water fraction (d), axial (e) and radial (f) apparent diffusion coefficient in recovery ROI from one day to five weeks after stroke in T2DM rats with or without BMSC treatments. Two scatter plots of all the metrics at the five weeks after stroke were presented for both BMSC (g) and saline (h) treated rats, the diversity of diffusion metrics probably depended on initial ischemic damage after stroke for both treated and control rats.
In contrast to the PBS-treated group, FA demonstrated a monotonic increase during one day to five weeks after stroke in the BMSC-treated T2DM rats. FA exhibited significantly higher values (p < 0.05) in the BMSC-treated T2DM rats than the control T2DM rats starting from two weeks after onset of stroke (Figure 1(a)).
Shannon’s entropy of diffusion anisotropy is based on the premise that the increase of the entropy indicates the increase of diffusion anisotropy.26 The entropy of diffusion anisotropy was measured and, as the FA, exhibited significantly (p < 0.05) elevated values in the BMSC-treated T2DM rats compared with the control T2DM rats, starting from two weeks after stroke (Figure 1(b)). For the BMSC-treated T2DM rats, the temporal profiles of entropy and FA after stroke demonstrated similar increase patterns. But, in the control T2DM rats, unlike the FA (p > 0.5), entropy increased during the first two weeks after stroke (p < 0.05).
The measurements of diffusional excess kurtosis demonstrated that the mean kurtosis monotonically decreased during five weeks after stroke for both treated and control T2DM rats (Figure 1(c)). However, the mean kurtosis declined slower in the treated T2DM rats than in the control T2DM rats, leading to significantly higher (p < 0.05) mean kurtosis in the treated rats than in the controls starting at two weeks after MCAo. Since kurtosis likely arises from diffusion restricted by barriers, such as cell membranes and organelles,6 this result suggests that more microstructures of cerebral tissue remained in the ischemic brain in the BMSC-treated T2DM rats than in the control T2DM rats.
With the two non-exchanging intra-axonal and extra-axonal compartments model, AWF is the fraction of MRI visible water in the axons relative to the total visible water signal of the voxel.12 The measurements of AWF (Figure 1(d)) showed only a slight alternation through the five weeks after stroke in the BMSC-treated T2DM rats, which suggests that the treated T2DM rats may not lose water in the axons after stroke. However, the AWF of the control T2DM rats demonstrated a monotonic decrease during five weeks after stroke, and was significantly lower (p < 0.05) than that of the treated T2DM rats starting at two weeks post MCAo, which indicates a significant loss of the axonal water after MCAo in the control T2DM rats compared with the treated T2DM rats.
Axial diffusivity reflects the axonal integrity and density of fiber bundles, whereas radial diffusion metrics are assumed to reflect myelin integrity and axonal density.6 Therefore, the differences between the axial and radial ADC likely reflect differences between axonal and myelin integrity. MRI measurements of axial and radial ADC demonstrated significantly (p < 0.05) lower values for the BMSC-treated T2DM rats than the control T2DM rats, starting at four weeks (Figure 1(e)) or two weeks (Figure 1(f)) after onset of ischemia, respectively. Thus, at two weeks and three weeks after MCAo, axial ADC was not, but radial ADC was, significantly different between the treated and the control T2DM rats, which suggests that BMSC treatment of stroke significantly improves axonal integrity two weeks later than the significant improvement of myelin integrity found in T2DM rats, compared to the controls.
Typical MRI images of these six diffusion-related parametric maps, obtained from one day to five weeks after stroke, are shown in Figures 2 and 3, which presents representative PBS- and BMSC-treated T2DM rats with approximately equal ischemic lesion volumes, respectively. The corresponding T2 maps were presented at the rightest column for convenience.
Figure 2.
Demonstration of evolution of the six diffusion and one T2 maps (right column), obtained from one day to five weeks after stroke, of the representative saline-treated (c) T2DM rat. Recovery ROI for this rat is loaded onto the five weeks T2 map.
Figure 3.
Demonstration of evolution of the six diffusion and one T2 maps (right column), obtained from one day to five weeks after stroke, of the representative BMSC-treated (T) T2DM rat. Recovery ROI for this rat is loaded onto the five weeks T2 map.
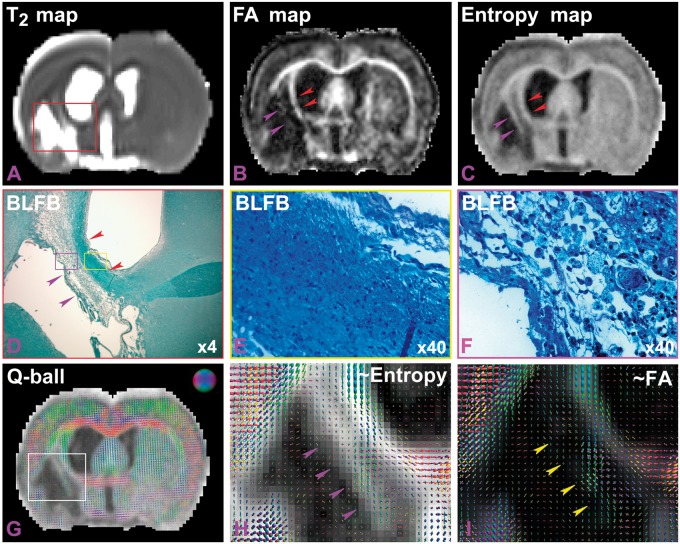
According to the quantitative measurements, FA (Figure 1(a)) appeared more sensitive than entropy (Figure 1(b)) in detecting the axonal improvement chronically after stroke between the saline and BMSC-treated T2DM rats, since the p-values were 0.005 versus 0.05 at 5 weeks post stroke. But, by pairwise comparisons of FA and entropy columns in Figures 2 and 3, entropy maps exhibited more structures of ischemic cerebral tissue within the region of infarction after stroke than FA maps, in both control and treated T2DM rat groups (Figures 2 and 3). In addition, FA and entropy maps in Figure 4 apparently confirmed this result (purple arrow heads in Figure 4(b) and (c)). However, no quantitative measurements were performed in the present study. Furthermore, Figure 4 provides a direct comparison between entropy and FA in a control T2DM rat. A T2 map acquired five weeks after stroke (Figure 4(a)) showed the location of the ischemic lesion in the section. Both FA (Figure 4(b)) and entropy (Figure 4(c)) maps, obtained at five weeks after stroke, demonstrated increased parametric values in the ischemic boundary zone along the ventricular wall, indicated with the red arrow heads in Figure 4(b) and (c). Corresponding to the red rectangular frame region in Figure 4(a), Figure 4(d) is shown at four times the magnification image of the BLFB staining section, and identifies white matter along the ventricular wall with dark blue color, as indicated with the red arrow heads in Figure 4(d), and confirms the findings of FA and entropy. A 40 times magnified BLFB staining picture (Figure 4(e)), as the part of yellow rectangle in Figure 4(d), demonstrated that the tissue with dark blue color contained increased density of axonal fibers (black) in the area. Interestingly, the FA map failed to detect some structures of cerebral tissue that the entropy map identified, as indicated with the purple arrow heads in Figure 4(b) to (d). This part of brain tissue was within the purple rectangle in Figure 4(d) and is 40 times enlarged in Figure 4(f), which shows cerebral tissue without dense axonal fibers (scarce black spots).
Figure 4.
A T2 map (a) showed the location of ischemic lesion at five weeks after stroke. The red arrow heads in both FA (b) and entropy (c) maps, obtained at five weeks after stroke, indicated higher values in the ischemic boundary, corresponding tissue with black color in BLFB staining image ((d), red arrow heads), which was the red frame part in panel A. The 40× magnified (e) picture, from yellow rectangle in (d), demonstrated relative higher density of axonal fibers in the area. The entropy map, but not FA map, identified some structures of cerebral tissue, indicated by the purple arrow heads in (b), (c), and (d). The enlarged picture (F, ×40) of the purple rectangle in (d) showed cerebral tissue without dense axonal fibers. By overlapping the orientation map on entropy maps (g), diffusion orientation in the area was not restricted along single direction. It was clearer in the zoomed map ((h), purple arrow heads). FA exhibited low values (dark), as indicated with the yellow arrow heads in the zoomed orientation map (i).
By overlapping the fiber orientation map, obtained from q-ball imaging, onto the entropy map (Figure 4(g)), due to lack of dense organized axonal fibers, it appears that the diffusion of water molecules in the area was not restricted along a single direction. Zooming in on the image in the white box in Figure 4(g), i.e. in Figure 4(h), it is apparent that the diffusion orientations crossed into voxels of brain tissue within this area (purple arrow heads). Due to the two factors of density and crossing orientations of fibers, FA exhibited low values (dark) and failed to detect the brain tissue, as indicated with the yellow arrow heads in the zoomed orientation map overlapping onto the FA map (Figure 4(i)).
Histologically, the axonal and myelin densities were measured (Table 1) at five weeks after stroke in the ischemic boundary zone of the striatum with Bielschowsky’s silver (an axon marker) and Luxol fast blue (a myelin marker) staining sections, respectively. The measurements in Luxol fast blue staining were 19.1 ± 7.4 (×103 µm2) for the control T2DM rat group versus 30.3 ± 6.5 (×103 µm2) for the BMSC-treated T2DM rats, which were significantly different (p < 0.01). With Bielschowsky’s silver staining, the measures were significantly different (p < 0.03) between the animal groups, i.e. 13.7 ± 3.9 (×103 µm2) for the control T2DM rats versus 22.2 ± 8.0 (×103 µm2) for the BMSC-treated T2DM rats. The histological BLFB measurements were consistent with the six MRI diffusion metrics.
Table 1.
Staining positive area (×103 µm2) by Bielschowsky’s silver & Luxol fast blue.
| Treatment | Staining | Rats |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| #1 | #2 | #3 | #4 | #5 | #6 | #7 | #8 | ||
| Saline | Bielschowsky | 25.0 | 12.7 | 15.2 | 11.0 | 16.0 | 28.4 | 29.9 | 14.8 |
| BMSC | Bielschowsky | 30.6 | 29.5 | 34.0 | 19.7 | 28.8 | 42.9 | 29.1 | 27.7 |
| Saline | Luxol | 15.5 | 14.3 | 15.2 | 20.4 | 10.7 | 11.8 | 14.2 | 7.2 |
| BMSC | Luxol | 17.9 | 26.3 | 16.0 | 15.7 | 23.1 | 14.3 | 26.3 | 38.0 |
BMSC: bone marrow stromal cell.
Discussion
In the clinic, the vast majority (90–95%) of diabetic patients are T2DM. Diabetes mellitus induces chronic vascular disease.27 Hyperglycemia induces biochemical changes within endothelial cells, including those in the cerebral vasculature,28 leading to vascular endothelial cell dysfunction, and increased vascular permeability in various vascular beds in humans and animal models.29 Many pathways are involved in the diabetes-related changes in the blood–brain barrier.30,31 Thus, many studies of diabetes have been focused on vascular issues. However, white matter plays a pivotal role in neurological function. In the present study, the emphasis is on the white matter changes after treatment of stroke in T2DM rats with BMSC using diffusion MRI, since MRI provides a non-invasive in vivo probe into the microstructure of biomedical materials.17 We found that diffusion metrics are sensitive to detect treatment effects, and BMSC treatment of stroke in T2DM rats significantly improves most diffusion metrics of white matter from two to five weeks, except the axial ADC which shows improvement from four to five weeks, after stroke. As indicated by measurements of axial and radial diffusivities, remyelination after BMSC treatment of T2DM stroke rats, compared with the PBS treatment, was significantly improved two weeks earlier than the improvment of the axonal remodeling. FA provided higher statistical significance, but entropy provided a more complete picture of axonal bundle information, especially for the lower density and the crossing bundles of axons. In agreement with MRI, BMSC treatment significantly improved histological results of BLFB stainings at five weeks after stroke, compared with the PBS-treated T2DM stroke rats. Although no significant correlation was found in the present study between MRI diffusion metrics and histological measurements; however, MRI demonstrated its advantage in evaluating dynamic changes of neurovascular remodeling in the same animal, which can monitor the temporal profile of neurovascular remodeling stages after stroke, avoid errors due to lesion and pathophysiological variations within different animals, and, importantly, allow dynamic evaluation and application to patients.
The FA is a popular marker of white matter integrity.32 FA is able to monitor well-reorganized white matter after stroke in rats14–16 and in humans.33 In the present study, FA demonstrated higher significance to detect the chronic differences of white matter remodeling between the control and treated T2DM rats than entropy (e.g. p < 0.005 vs. p < 0.05 at five weeks after stroke, in Figure 1(a) and (b)). However, as indicated in Figure 4, FA was unable to detect the microstructures of brain tissue without dense organized fibers (purple arrow heads in Figure 4(b) to (d)), whereas the entropy was able to detect the cerebral tissue architecture (Figure 4(c), purple arrow heads). This feature indicates that entropy is superior to FA in detecting changes of cerebral microstructures of both high and low neurofilament densities in T2DM stroke rats. Furthermore, FA may be unable to detect the crossing white matter fiber bundles,34,35 while the entropy and kurtosis were able to.13,34 In addition, the relative entropy contrast to the non-ischemia contralateral tissue, rather than the relative FA, exhibited nearly significant differences of white matter between the treated and the control T2DM rats at one week after onset of stroke (p < 0.051, data not shown) with current sample space (n = 8 for the treated and control, respectively).
In general, fiber crossing is present in the injured brain during the early stage of white matter remodeling, and therefore may be detected by low FA values accompanied by high entropy values. However, in the present study, FA and entropy did not identify the fiber crossing period in the BMSC-treated T2DM rats up to five weeks after stroke.
Both axial and radial ADCs of ischemic cerebral tissue are elevated during the chronic stage after stroke in the human,33 which may result from the breakdown and loss of ischemic cerebral tissue. Thus, lower values of axial and radial ADCs indicate less cerebral tissue damage during the chronic stage of stroke. In the present study, axial and radial ADCs exhibited increased values from 1 day to 5 weeks post stroke in T2DM rats, and were lower in the BMSC-treated T2DM rats than in the controls. Axial ADC measures exhibited a delay of two weeks, in contrast to radial ADC measures for detecting the significant differences between the treated and control T2DM rats after stroke. Since radial diffusivity is a biomarker of myelin integrity, and the combination of axial and radial diffusivities reflects axonal integrity,6 these data suggest that axonal integrity improvement follows myelin integrity improvement in the T2DM rats with BMSC treatment of stroke, compared to the control T2DM rats after stroke. In a previous study, positive axons of neurofilament-H immunostaining appeared swollen and bulbous in the ischemic striatum seven days after MCAo in saline-treated rats, and these axons were not surrounded by cyclic nucleotide phosphodiesterase immunoreactivity,36 suggesting that demyelination may precede the destruction of axons in the ischemic brain, which is consistent with the axial and radial ADC results in the present study. These results are also consistent with measurements in the present study of decreased AKC and nearly unchanged AWF early after stroke in the BMSC-treated T2DM rats.
The AWF provides a measure of the intra-axonal space water volume relative to the extra-axonal space water volume.12 The measured mean AWF values in the current study were close to 0.36 for the treated T2DM rats at five weeks after stroke, which are comparable with the mean value of 0.33 for the volume fraction of myelinated fibers of human white matter.37 However, the relatively low b-value (up to 1800 s/mm2) and a small number of diffusion directions (20 directions) in the present study limited the amount of information that can be extracted.
The non-Gaussian diffusion effects in the brain arise from diffusion restricted by barriers, such as cell membranes and organelles, as well as the presence of distinct water compartments.38 DKI metrics for the white matter are more sensitive to the highly restricted water diffusion inside the axons than the slow compartment and the less hindered diffusion in the extra-axonal space to the fast compartment.39,40 Combining AWF and kurtosis measurements may provide insight into the barrier microarchitecture of axons.
In summary, all six MRI diffusion metrics provide complementary information of white matter, including axons and myelin, and demonstrated by most of the diffusion MRI metrics starting from two to five weeks after stroke in T2DM rat, that BMSC treatment (dose of 3 × 106 cells) of stroke administered three days after 2 h of MCAo significantly improves white matter features compared with the PBS-treated control rats, as was histologically confirmed by BLFB staining at five weeks after stroke. Both FA and entropy metrics exhibit similar temporal patterns for organized neuronal fibers in recovery ROI after stroke in T2DM rats with or without BMSC treatments (Figure 1(a) and (b)). However, entropy was able to, whereas FA was unable to, detect the cerebral microstructure with low density of neurofibers and crossing white matter fiber bundles.
Funding
This work was financially supported by NIH RO1 NS083078 (JC), NS064134 (QJ), AG037506 (MC), R41 S080329 (JC), and American Heart Association (AHA) 14GRNT20460026 (JC).
Declaration of conflicting interests
No conflicts are declared for all authors. The content is solely the responsibility of the authors and does not necessary represent the official view of the National Institute of Health.
Authors’ contributions
Substantial contributions to conception and design: MC, JC and QJ. Acquisition of data, or analysis and interpretation of data: GD, LL, TY, ED-B, QL, SD. Drafting the article or revising it critically for important intellectual content: GD, MC, JC and QJ. Final approval of the version to be published: GD, MC, JC and QJ.
References
- 1.Conturo TE, Lori NF, Cull TS, et al. Tracking neuronal fiber pathways in the living human brain. Proc Natl Acad Sci U S A 1999; 96: 10422–10427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Le Bihan D, Breton E, Lallemand D, et al. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology 1986; 161: 401–407. [DOI] [PubMed] [Google Scholar]
- 3.Hoehn-Berlage M, Eis M, Back T, et al. Changes of relaxation times (T1, T2) and apparent diffusion coefficient after permanent middle cerebral artery occlusion in the rat: temporal evolution, regional extent, and comparison with histology. Magn Reson Med 1995; 34: 824–834. [DOI] [PubMed] [Google Scholar]
- 4.Ding G, Jiang Q, Zhang L, et al. Multiparametric ISODATA analysis of embolic stroke and rt-PA intervention in rat. J Neurol Sci 2004; 223: 135–143. [DOI] [PubMed] [Google Scholar]
- 5.Le Bihan D, Turner R, Douek P, et al. Diffusion MR imaging: clinical applications. AJR Am J Roentgenol 1992; 159: 591–599. [DOI] [PubMed] [Google Scholar]
- 6.Van Cauter S, Veraart J, Sijbers J, et al. Gliomas: diffusion kurtosis MR imaging in grading. Radiology 2012; 263: 492–501. [DOI] [PubMed] [Google Scholar]
- 7.Lazar M, Weinstein DM, Tsuruda JS, et al. White matter tractography using diffusion tensor deflection. Hum Brain Mapp 2003; 18: 306–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hagmann P, Jonasson L, Maeder P, et al. Understanding diffusion MR imaging techniques: from scalar diffusion-weighted imaging to diffusion tensor imaging and beyond. Radiographics 2006; 26(Suppl 1): S205–S223. [DOI] [PubMed] [Google Scholar]
- 9.Wedeen VJ, Hagmann P, Tseng WY, et al. Mapping complex tissue architecture with diffusion spectrum magnetic resonance imaging. Magn Reson Med 2005; 54: 1377–1386. [DOI] [PubMed] [Google Scholar]
- 10.Tuch DS, Reese TG, Wiegell MR, et al. Diffusion MRI of complex neural architecture. Neuron 2003; 40: 885–895. [DOI] [PubMed] [Google Scholar]
- 11.Jensen JH, Helpern JA, Ramani A, et al. Diffusional kurtosis imaging: the quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med 2005; 53: 1432–1440. [DOI] [PubMed] [Google Scholar]
- 12.Fieremans E, Jensen JH, Helpern JA. White matter characterization with diffusional kurtosis imaging. Neuroimage 2011; 58: 177–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fozouni N, Chopp M, Nejad-Davarani SP, et al. Characterizing brain structures and remodeling after TBI based on information content, diffusion entropy. PLoS One 2013; 8: e76343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang Q, Zhang ZG, Ding GL, et al. MRI detects white matter reorganization after neural progenitor cell treatment of stroke. Neuroimage 2006; 32: 1080–1089. [DOI] [PubMed] [Google Scholar]
- 15.Ding G, Jiang Q, Li L, Zhang L, et al. Magnetic resonance imaging investigation of axonal remodeling and angiogenesis after embolic stroke in sildenafil-treated rats. J Cereb Blood Flow Metab 2008; 28: 1440–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding G, Jiang Q, Li L, Zhang L, et al. Longitudinal magnetic resonance imaging of sildenafil treatment of embolic stroke in aged rats. Stroke 2011; 42: 3537–3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alexander DC. Multiple-fiber reconstruction algorithms for diffusion MRI. Ann N Y Acad Sci 2005; 1064: 113–133. [DOI] [PubMed] [Google Scholar]
- 18.Cheung MM, Hui ES, Chan KC, et al. Does diffusion kurtosis imaging lead to better neural tissue characterization? A rodent brain maturation study. Neuroimage 2009; 45: 386–392. [DOI] [PubMed] [Google Scholar]
- 19.Ding G, Yan T, Chen J, et al. Persistent cerebrovascular damage after stroke in type two diabetic rats measured by magnetic resonance imaging. Stroke 2015; 46: 507–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reed MJ, Meszaros K, Entes LJ, et al. A new rat model of type 2 diabetes: The fat-fed, streptozotocin-treated rat. Metabolism 2000; 49: 1390–1394. [DOI] [PubMed] [Google Scholar]
- 21.Srinivasan K, Viswanad B, Asrat L, et al. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacol Res 2005; 52: 313–320. [DOI] [PubMed] [Google Scholar]
- 22.Chen J, Li Y, Wang L, Zhang Z, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke 2001; 32: 1005–1011. [DOI] [PubMed] [Google Scholar]
- 23.Ding G, Jiang Q, Li L, Zhang L, et al. Angiogenesis detected after embolic stroke in rat brain using magnetic resonance T2*WI. Stroke 2008; 39: 1563–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alexander AL, Lee JE, Lazar M, et al. Diffusion Tensor Imaging of the Brain. Neurotherapeutics 2007; 4: 316–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen J, Cui X, Zacharek A, et al. White matter damage and the effect of matrix metalloproteinases in type 2 diabetic mice after stroke. Stroke 2011; 42: 445–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Metwalli NS, LaConte SM, Hu XP. An information theoretic approach characterizing diffusion anisotropy in diffusion-weighted magnetic resonance images. Conf Proc IEEE Eng Med Biol 2006; 1: 2260–2263. [DOI] [PubMed] [Google Scholar]
- 27.Ergul A, Li W, Elgebaly MM, et al. Hyperglycemia, diabetes and stroke: focus on the cerebrovasculature. Vascular Pharmacol 2009; 51: 44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001; 414: 813–820. [DOI] [PubMed] [Google Scholar]
- 29.Li W, Qu Z, Prakash R, Chung C, et al. Comparative analysis of the neurovascular injury and functional outcomes in experimental stroke models in diabetic Goto-Kakizaki rats. Brain Res 2013; 1541: 106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martini SR, Kent TA. Hyperglycemia in acute ischemic stroke: a vascular perspective. J Cereb Blood Flow Metab 2007; 27: 435–451. [DOI] [PubMed] [Google Scholar]
- 31.Mooradian AD, Haas MJ, Batejko O, et al. Statins ameliorate endothelial barrier permeability changes in the cerebral tissue of streptozotocin-induced diabetic rats. Diabetes 2005; 54: 2977–2982. [DOI] [PubMed] [Google Scholar]
- 32.Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B 1996; 111: 209–219. [DOI] [PubMed] [Google Scholar]
- 33.Lindenberg R, Zhu LL, Rüber T, et al. Predicting functional motor potential in chronic stroke patients using diffusion tensor imaging. Human brain mapping 2012; 33: 1040–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang Q, Qu C, Chopp M, et al. MRI evaluation of axonal reorganization after bone marrow stromal cell treatment of traumatic brain injury. NMR Biomed 2011; 24: 1119–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alexander DC, Barker GJ, Arridge SR. Detection and modeling of non-Gaussian apparent diffusion coefficient profiles in human brain data. Magn Reson Med 2002; 48: 331–340. [DOI] [PubMed] [Google Scholar]
- 36.Zhang L, Chopp M, Zhang RL, et al. Erythropoietin amplifies stroke-induced oligodendrogenesis in the rat. PLoS One 2010; 5: e11016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang Y, Nyengaard JR, Pakkenberg B, et al. Age-induced white matter changes in the human brain: a stereological investigation. Neurobiol Aging 1997; 18: 609–615. [DOI] [PubMed] [Google Scholar]
- 38.Assaf Y, Cohen Y. Assignment of the water slow-diffusing component in the central nervous system using q-space diffusion MRS: implications for fiber tract imaging. Magn Reson Med 2000; 43: 191–199. [DOI] [PubMed] [Google Scholar]
- 39.Assaf Y, Basser PJ. Composite hindered and restricted model of diffusion (CHARMED) MR imaging of the human brain. Neuroimage 2005; 27: 48–58. [DOI] [PubMed] [Google Scholar]
- 40.Fieremans E, Novikov DS, Jensen JH, Helpern JA. Monte Carlo study of a two-compartment exchange model of diffusion. NMR Biomed 2010; 23: 711–724. [DOI] [PMC free article] [PubMed] [Google Scholar]