Abstract
Accumulating evidence suggests associations between cerebrovascular disease (CVD) and Alzheimer’s disease (AD). White matter hyperintensities of presumed vascular origin (WMHs) are increased in subjects with mild cognitive impairment (MCI) and AD, but the exact pathomechanistic link is unknown. The current study investigated effects of amyloid dysmetabolism on the microstructure of WMHs in subjects with MCI or subjective cognitive decline (N = 51), dichotomized according to pathological or normal levels of amyloid-β peptide (Aβ42) in cerebrospinal fluid (CSF). Thirty-one subjects with low CSF Aβ42 (Aβ+) and 20 subjects with normal CSF Aβ42 (Aβ−) were assessed with magnetic resonance diffusion tensor imaging (DTI), and fractional anisotropy (FA), radial diffusivity (DR), axial diffusivity (DA), and mean diffusivity (MD) were determined. There were no significant differences in WMH volume or distribution between the groups, and neither age nor WMH volume had significant impact on the DTI indices. Nevertheless, there were significantly higher DA, DR, and MD in WMHs in Aβ+ relative to Aβ−; however, no differences in FA were found. The present results suggest that amyloid accumulation is associated with impaired structural integrity (e.g. relating to more extensive demyelination and loss of axons) in WMHs putatively adding to effects of ischemia.
Keywords: Alzheimer’s, cerebrospinal fluid, cognitive impairment, diffusion tensor imaging, white matter disease
Introduction
Epidemiologic studies show that vascular disease and Alzheimer’s disease (AD) share common risk factors,1,2 and accumulating evidence suggests that cerebrovascular disease (CVD) is involved in the pathomechanism of Alzheimer’s disease.3 White matter hyperintensities of presumed vascular origin (WMHs),4 visualized on T2-weighted magnetic resonance imaging (MRI), are considered markers of small-vessel CVD and are associated with AD: studies have shown that WMH load is associated with and increased in mild cognitive impairment (MCI) and AD.5,6
Dysmetabolism of amyloid-β peptide (Aβ) is a key factor in the pathogenesis of AD and decreased Aβ levels in the cerebrospinal fluid (CSF) become apparent years before clinical symptoms.7 However, the underlying mechanism of the relationship between CVD and AD has not been fully elucidated. Cerebrovascular dysfunction could result from perivascular Aβ deposition. Cerebral amyloid angiopathy (CAA), defined as deposition of Aβ in the walls of leptomeningeal and cortical arteries, co-exists with WMHs in AD patients.8 Further, the link between CVD and AD could be partly explained by vascular damage restricting brain Aβ clearance,9 or by ischemia affecting Aβ production10 or impeding energy-dependent intra-axonal transport eliciting further Aβ accumulation and local tissue damage.11,12
To date, no disease-modifying treatment is available for AD. Early intervention strategies will rely on biomarkers to reliably diagnose early stage AD and monitor disease progression, and imaging biomarkers are becoming increasingly important. Diffusion tensor imaging (DTI) measures tissue diffusion properties and is a helpful tool to evaluate the microstructural integrity of white matter (WM) and may reveal WM changes in preclinical AD. We have previously shown that DTI is a better predictor of cognitive decline than CSF biomarkers in subjects with SCD and MCI.13 Four common DTI indices are derived, likely reflecting different microstructural properties, fractional anisotropy (FA), mean diffusivity (MD), radial diffusivity (DR), and axial diffusivity (DA). Although the neurobiological interpretation of the DTI indices is complex, increased DR is thought to reflect changes to axonal diameter, axonal density and myelination, while DA has been associated with the axonal integrity.14 FA is perhaps the most commonly used DTI index and quantifies the directionality of diffusion,15 while MD is the total diffusivity.
DTI changes in cerebral white matter have been studied previously and impaired white matter integrity, as measured by DTI, have been reported in AD patients.16 We have previously shown alterations in DTI indices in patients with pathological CSF total Tau protein (T-tau),17 and degenerative changes in WM tracts already at the SCD stage with more extensive changes at the MCI stage.18 Diffusivity measures (DA, DR, and MD) probably are more sensitive measures in early cognitive impairment and AD, while FA is the least sensitive.19,20 Further, WMHs have been associated with decreased FA,21 and amyloid-positive subjects have presented with increased DA.22 However, to our knowledge, no studies have evaluated the effects of amyloid metabolism on WMHs diffusion properties.
Hypothesizing that amyloid metabolism may interact with vascular factors, the objective of this study was to determine whether subjects with low CSF Aβ42 compared to subjects with normal CSF Aβ42 have more pronounced microstructural differences within WMHs as measured by DTI parameters. As a secondary objective, we investigated whether pathological amyloid metabolism is associated with diffusion properties in normal-appearing white matter (NAWM) and in cingulum (typically shown to be affected in MCI and early AD23,24), as a comparator to effects within WMHs.
Materials and methods
Subjects
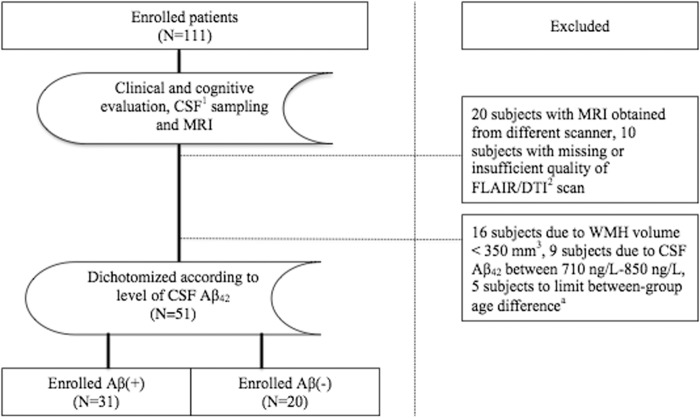
Subjects referred to a university-hospital based neurological outpatient clinic between 2005 and 2013 were routinely evaluated for study inclusion (Figure 1). Written informed consent was obtained from all participants prior to enrollment. Inclusion criteria were age 40–79 years, subjective cognitive complaints with symptoms lasting ≥6 months, and absence of dementia. Subjects included in the study were classified as MCI (mild cognitive impairment) or SCD (subjective cognitive decline) corresponding to Global Deterioration Scale (GDS) 2 or 3,25–27 respectively, as determined from clinical interview and the following screening tests: Mini-Mental State Examination (MMSE),28 the Neurobehavioral Cognitive Status Examination (Cognistat),29 and I-Flex (word fluency, interference, and numeral-letter items).27,30 SCD was defined as GDS 2, based on scores above published cut-off on all of the above-listed screening tests (≥28 for MMSE, <2 for I-Flex, above mild impairment on all the items of Cognistat). MCI was defined as GDS 3, based on scores below cut-off on one or more of the screening tests.27 Subjects classified as GDS 3/MCI will fulfill general criteria for MCI, as revised by Petersen et al.31 Further, all participants were clinically diagnosed at a consensus conference of physicians and neurologists at a university hospital. Participants with GDS >3 and Clinical Dementia Rating >0.532 were considered demented and therefore excluded from the study. Further, subjects with history of learning disabilities, significantly impaired activities of daily living, established psychiatric comorbidity, anoxic brain damage, drug abuse, or solvent exposure were excluded. All participants were submitted to a standardized clinical assessment including neurological examinations, MRI, as well as spinal puncture.
Figure 1.
Inclusions and exclusions in the cohort.
CSF: cerebrospinal fluid; DTI: diffusion tensor imaging; Aβ: amyloid-β peptide, Aβ.
aSubjects of >73 or <44 years of age were excluded to ensure that the groups were similar in age.
For the current study, 51 subjects were selected based on CSF Aβ42 <710 ng/L or >850 ng/L and WMH volume >350 mm3, of whom 29 female subjects and 22 male subjects. All 51 subjects met the above listed inclusion criteria. They were dichotomized according to the level of CSF Aβ42. CSF Aβ42 was considered as low (Aβ+) if Aβ42 <710 ng/L and normal (Aβ−) if Aβ42 >850 ng/L (used as a control group). CSF Aβ42 between 710 ng/L and 850 ng/L was considered intermediate, and these subjects were therefore excluded. Subjects with WMH volume <350 mm3 were excluded as we considered WMH volume of 350 mm3 to be the minimum volume for reasonable DTI analyses in WMHs. Thirty-one subjects were included as Aβ+, and 20 subjects as Aβ−. As age is considered a contributor to WMHs, between-group age difference was limited by excluding subjects >73 and <44 years of age.
The study was evaluated (based on the Norwegian Health and Research Act and the Helsinki Declaration of 1964, revised 2013) and approved by the Regional Committee for Medical and Health Research Ethics, South East Norway.
MRI/DTI acquisition
Magnetic resonance imaging was performed using a Siemens Espree 1.5T scanner. One 3D MP-RAGE (magnetization-prepared rapid gradient echo) T1-weighted sequence was obtained (TR/TE/inversion time/flip angle = 2400/3.65/1000/8°, matrix = 240 × 192, 160 sagittal slices, thickness = 1.2 mm, in-plane resolution of 1 mm × 1.2 mm. The protocol also included 2D axial fluid-attenuated inversion recovery (FLAIR) images with the following parameters: TR/TE/TI = 13420/121/2500, 36 slices, spaced at 3.0 and 3.9 mm thick.
The pulse sequence for DTI was as follows: b = 750, 12 directions repeated 5 times, five b0-values per slice, TR = 6100 ms, TE = 117 ms, number of slices = 30, slice thickness = 3 mm (gap = 1.9 mm), in-plane resolution = 1.2 × 1.2 mm2, bandwidth = 840 Hz/pixel.
Image processing
DTI analyses and calculations were performed using The Oxford Centre for Functional MRI of the Brain (FMRIB) Software Library (FSL) version 5.0.33,34 Initially, FMRIB’s Linear Image Registration Tool35 was used for affine registrations of each DTI volume to the low-b (b = 0) image. Motion between scans and residual eddy currents were corrected for, before creation of FA and eigenvalue maps. FA is a normalized standard deviation of the eigenvalues.36,37 DA was defined as the principal eigenvalue (L1), DR was defined as the mean of eigenvalues 2 and 3, and MD as the mean of all three eigenvalues. Tract-Based Spatial Statistics (TBSS) (part of FSL) was used for voxelwise statistical analysis of the DTI variables (FA, DR, MD and DA) in cingulum as determined by the Johns Hopkins University (JHU) white-matter tractography atlas,38 that is implemented in FSL. FMRIB’s Diffusion Toolbox was used to create FA images by fitting a tensor model to the raw diffusion data, and FSL’s Brain Extraction Tool was used for subsequent brain extraction. All subjects’ FA data were then aligned into a common space using the nonlinear registration tool FMRIB’s Nonlinear Image Registration Tool, which uses a B-spline representation of the registration warp field.39 Next, the mean FA image was created and thinned to create a mean FA skeleton that represents the centers of all tracts common to the group. Each subject’s aligned FA data were then projected onto this skeleton and the resulting data fed into voxelwise cross-subject statistics. DR, MD and DA data were then extracted from each subject according to the skeletonized FA map in cingulum as determined by the JHU white-matter tractography atlas. Further, the DTI and structural images were coregistered to high-resolution three-dimensional T1 weighted MRI scans for WMH segmentation and measurements.
WMH load was estimated by recently developed in-house object-based supervised machine learning algorithm for automated segmentation and quantification of white matter hyperintensities based on FLAIR image intensity and masks of tissue types.40 This provides an automated quantitative assessment of WMH load (Figure 2). All segmentations were manually inspected for accuracy. The reconstruction and segmentation of NAWM were performed with FreeSurfer image analysis suite version 5.3.0 (http://surfer.nmr.mgh.harvard.edu/) by subtracting WMH from the total white matter. DTI measurements were extracted using FSL. Further, using FreeSurfer definition of lobes, spatial distribution of WMHs per lobe was determined.
Figure 2.
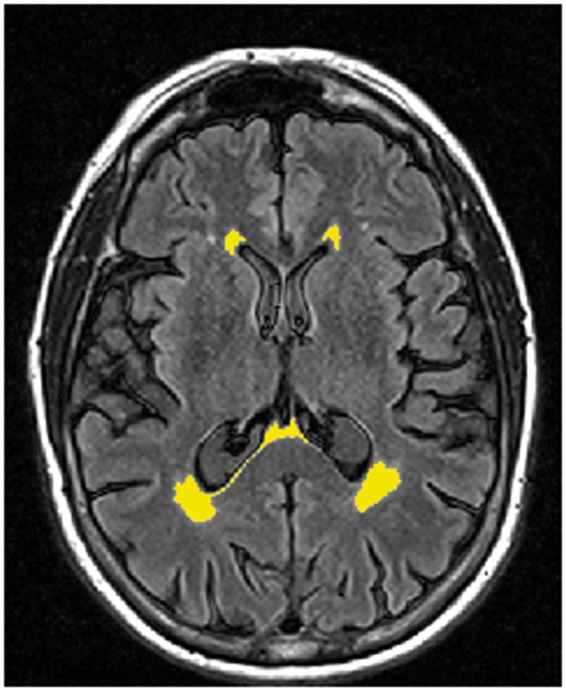
WMH segmentation. An example of the in-house WMH automated quantitative assessment of WMH load in one of the low CSF Aβ subjects.
Cerebrospinal fluid analysis
As part of the routine clinical assessment, all participants underwent lumbar puncture consecutively after inclusion. It was performed in L3/L4 or L4/L5 interspace and CSF samples were examined for levels of Aβ42, total microtubule associated protein Tau (t-tau) and Tau phosphorylated at threonine 181 (p-tau) with commercially available enzyme-linked immunosorbent assay kits (Innogenetics, Belgium, presently Fujirebio Europe).
Statistical analyses
Differences in demographic features between the two groups of participants, as well as volume of white matter lesions, were assessed using χ2 tests for categorical data and independent-samples T-test or Mann–Whitney U-test for continuous data. The WMH volume was skewed and therefore log transformed prior to this analysis.
Fractional anisotropy (FA), mean diffusivity (MD), radial diffusivity (DR), and axial diffusivity (DA) were measured from WMHs of subjects with low and normal CSF Aβ42. The DTI indices were adjusted for age and WMH volume by a linear regression model to ensure that the results would not be biased by these factors. We did not adjust for sex as linear regression analyses revealed no significant effect of sex and also to avoid overadjustment. Furthermore, the diagnoses (SCD/MCI) were not corrected for, as there was no significant difference in the diagnoses between the two groups. Although WMH volume was skewed, the resulting residuals were normally distributed as assessed by the Kolmogorov–Smirnov test and visual inspection of histograms, and thus fulfill the assumptions of linear regression. Therefore, the resulting residuals were used for further analyses. To test our hypothesis, an independent-samples T-test was conducted for each DTI index of the two groups. With the significance level set at p < 0.05, differences in DTI indices between the two groups of low and normal CSF Aβ42 were assessed.
Further, linear regression analyses were carried out to evaluate the effect of age WMH volume on the DTI indices. DTI indices were used as dependent variables and WMH volume and age as independent variables in the regression model. As stated above, although the WMH volume was skewed, the resulting residuals were normally distributed and therefore used for further analyses.
For the analyses of WMHs spatial distribution, we averaged measurements from the right and left hemispheres to reduce the number of comparisons, and the resulting measurements were normally distributed as assessed by Kolmogorov–Smirnov test and visual inspection of histograms. We compared the WMH load of the two groups in each lobe (frontal, parietal, temporal, occipital) and cingulate by conducting an independent-samples T-test for each lobe. For these comparisons, significance was determined by a p-value <0.05.
Cingulum DTI indices were calculated by averaging measurements from the left and right hemisphere cingulate gyrus and cingulum hippocampal part, as determined by the JHU white-matter tractography atlas. The resulting variables, and also the DTI indices in NAWM, were adjusted for age and WMH volume (the ensuing variables were normally distributed as assessed by Kolmogorov–Smirnov test and visual inspection of histograms). Conducting independent-samples T-tests, we compared DTI indices of the two groups of low and normal CSF Aβ42. For these comparisons, significance was determined by a p-value < 0.05.
Statistical analyses were performed with SPSS version 22 (SPSS Inc., Chicago, IL, USA).
Results
Demographic and clinical data are listed in Table 1. There were no significant differences in sex or age between the groups with low and normal CSF Aβ42. Further, there was no significant difference in WMH volume between the two groups. By design, CSF Aβ42 was significantly lower (p < 0.001) in the group of low CSF Aβ42 compared to the group with normal CSF Aβ42. In addition, there were no significant group differences in diagnoses (SCD/MCI). When comparing the included subjects (n = 51) and the excluded subjects (n = 60), no significant differences were encountered in terms of demographic or clinical data, except for CSF Aβ42, which, by design, was significantly higher in the excluded group (data not shown).
Table 1.
Demographic and clinical data.
| Total study cohort (n = 51) | Aβ ( + ) (n = 31) | Aβ (−) (n = 20) | p-value (Aβ + vs Aβ−) | |
|---|---|---|---|---|
| Age, years | 62.25 (7.18) | 63.77 (6.87) | 59.85 (7.29) | 0.058a |
| Women/men, n | 29/22 | 19/12 | 10/10 | 0.427b |
| MMSE | 27.92 (1.43) | 27.77 (1.38) | 28.15 (1.50) | 0.342c |
| Cognistat, n above/below (%)e | 18 (35.3)/33 (64.7) | 10 (32.3)/21 (67.7) | 8 (40.0)/12 (60.0) | 0.740b |
| I-flex itemsf | 0.74 (1.09) | 0.57 (0.90) | 1.06 (1.35) | 0.292c |
| SCD/MCI diagnoses, n (%) | 8 (15.7)/43 (84.3) | 4 (12.9)/27 (87.1) | 4 (20.0)/16 (80.0) | 0.445b |
| CSF Aβ42 (ng/L) | 722.14 (284.73) | 519.13 (131.43) | 1036.80 (120.82) | <0.001a,* |
| WMH volume (mm3) | 4610.89 (6491.35) | 5427.35 (7227.44) | 3345.38 (5063.31) | 0.083a,d |
Values are given in mean (standard deviation) unless otherwise indicated.
Calculated using independent-samples T-test.
Calculated using Pearson Chi-square test.
Calculated using Mann–Whitney U-test.
p-Value for log transformed WMH volume.
n (%) subjects above/below cut-off on any of the Cognistat items (orientation, attention, language, construction, memory, calculation, reasoning).
Word fluency, interference, and numeral-letter items.
Significant difference between the two groups.
CSF: cerebrospinal fluid; Aβ: amyloid-β peptide; WMH: white matter hyperintensity; MMSE: mini mental state examination; MCI: mild cognitive impairment; SCD: subjective cognitive decline.
Data were used to compare DTI indices in WMHs in Aβ± subjects (Table 2). Using age and WMH volume as covariates, Aβ+ subjects exhibited a significantly increased radial diffusivity, DR (p = 0.026), mean diffusivity, MD (p = 0.011), and axial diffusivity, DA (p = 0.005) relative to Aβ− subjects. Fractional anisotropy (FA) was not significantly different between the two groups (p = 0,269). The same tendency was noted in additional analyses without adjustment for WMH volume and age. Linear regression did not reveal significant influence on DTI indices as a function of WMH volume or age (Table 3), although a trend could be noted when considering the effects on DR and DA.
Table 2.
DTI indices in WMHs, cingulum, and NAWM.
| Low CSF Aβ42 (n = 31) | Normal CSF Aβ42 (n = 20) | p-Valuea | |
|---|---|---|---|
| FA in WMHs | 0.28 (0.04) | 0.28 (0.03) | 0.269 |
| MD in WMHs (mm2/s) | 134.00 × 10−5 (12.46) | 123.63 × 10−5 (12.31) | 0.011* |
| DR in WMHs (mm2/s) | 115.45 × 10−5 (11.60) | 106.69 × 10−5 (11.66) | 0.026* |
| DA in WMHs (mm2/s) | 171.12 × 10−5 (15.46) | 157.50 × 10−5 (14.60) | 0.005* |
| FA, cingulum | 0.54 (0.04) | 0.57 (0.06) | 0.256 |
| DR, cingulum (mm2/s) | 51.81 × 10−5 (5.76) | 47.09 × 10−5 (7.18) | 0.095 |
| MD, cingulum (mm2/s) | 75.17 × 10−5 (5.97) | 70.57 × 10−5 (6.43) | 0.074 |
| DA, cingulum (mm2/s) | 121.89 × 10−5 (7.67) | 117.53 × 10−5 (7.02) | 0.106 |
| FA, NAWM | 0.36 (0.033) | 0.38 (0.027) | 0.226 |
| DR, NAWM (mm2/s) | 72.22 × 10−5 (6.40) | 68.20 × 10−5 (3.22) | 0.110 |
| MD, NAWM (mm2/s) | 88.83 × 10−5 (5.41) | 85.29 × 10−5 (2.83) | 0.090 |
| DA, NAWM (mm2/s) | 122.04 × 10−5 (4.00) | 119.47 × 10−5 (2.68) | 0.119 |
Values are given in mean (standard deviation).
p-Values when corrected for age and WMH volume.
Significant difference between the two groups.
CSF: cerebrospinal fluid; WMHs: white matter hyperintensities; FA: fractional anisotropy; DR: radial diffusivity; MD: mean diffusivity; DA: axial diffusivity; NAWM: normal-appearing white matter.
Table 3.
Influence of WMH volume and age on DTI indices in WMHs.
| Dependent variables |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Independent variables | FA |
MD |
DR |
DA |
||||||||
| Beta | p | R 2 | Beta | p | R 2 | Beta | p | R 2 | Beta | p | R 2 | |
| Low CSF Aβ42 | ||||||||||||
| WMH volume | −0.40 | 0.04 | 0.21 | 0.31 | 0.11 | 0.12 | 0.36 | 0.06 | 0.16 | 0.23 | 0.26 | 0.06 |
| Age | −0.12 | 0.50 | 0.07 | 0.71 | 0.08 | 0.67 | 0.06 | 0.79 | ||||
| Normal CSF Aβ42 | ||||||||||||
| WMH volume | −0.24 | 0.31 | 0.18 | 0.41 | 0.07 | 0.23 | 0.39 | 0.10 | 0.18 | 0.43 | 0.05 | 0.29 |
| Age | −0.31 | 0.19 | −0.33 | 0.14 | −0.27 | 0.25 | −0.42 | 0.06 | ||||
Associations between age and WMH volume and DTI indices were determined by means of linear regression. R2 refers to adjusted R square.
CSF: cerebrospinal fluid; WMH: white matter hyperintensity; MD: mean diffusivity; DR: radial diffusivity; DA: axial diffusivity; FA: fractional anisotropy.
Furthermore, the spatial distribution of WMHs was compared in Aβ± subjects. We found no significant difference in the distribution in neither of the lobes when comparing the two groups: frontal (p = 0.638), temporal (p = 0.298), parietal (p = 0.140), occipital (p = 0.771), and cingulate (p = 0.403). Therefore we did not include this factor as a covariate.
As a secondary objective, we evaluated DTI index differences in the cingulum and NAWM (Table 2). With age and WMH volume as covariates, Aβ± subjects were compared. There were no significant differences in cingulum in FA (p = 0.256), DR (p = 0.095), DA (p = 0.106), or MD (p = 0.074). Further, no significant differences in DTI indices were found in NAWM when comparing the two groups, FA (p = 0.226), DR (p = 0.110), DA (p = 0.119), and MD (p = 0.090). When not adjusted for WMH volume and age, a trend was noted in the DTI indices in cingulum, but indices of NAWM analyses remained nonsignificant.
Discussion
Here, we find that (1) Aβ dysmetabolism is associated with increased microstructural damage in WMHs (Aβ+ > Aβ− subjects), and (2) this effect seems limited to WMHs, i.e. there are no significant differences when evaluating the effects of amyloid metabolism on DTI indices in other areas of the brain, including NAWM and the cingulum.
These findings suggest that Aβ dysmetabolism affects diffusion properties in WMHs and that there is an association between amyloid metabolism and impaired structural connectivity in WMHs. To the best of our knowledge, this is the first study to investigate the association between amyloid and diffusion properties in WMHs.
During the last years, DTI has become increasingly important when investigating structural integrity in cerebral white matter in AD. Previous studies have investigated DTI changes in association with β-amyloid in white matter tracts. A recent study compared cognitively normal subjects with low and normal CSF Aβ42 and found increased DA without changes in the remaining indices, possibly indicating axonal damage with preservation of white matter integrity in presymptomatic phases of AD.22 However, these findings were in different regions of interest (ROIs) and not within WMHs. Further, it has previously been suggested that DA is one of the first DTI changes observed in AD.41 In agreement with this, in our study we demonstrate more profound differences in DA than the remaining indices in WMHs when comparing Aβ± subjects, potentially indicating stronger effects of amyloid metabolism on DA in WMHs.
Age is a strong contributor to WMHs. To ensure our results were not biased by confounding factors, including age and WMH volume, these were used as covariates. Although statistically nonsignificant, there were slight differences in WMH volume between the two groups. However, linear regression did not reveal any significant influence on diffusion properties in WMHs as a function of WMH volume or age, although a trend could be noted when evaluating the effects on DR and DA and therefore these factors were corrected for. This indicates that WMH volume and age cannot explain the total effect, supporting the link between β-amyloid metabolism and WMHs diffusion properties.
Although evidence suggests associations between CVD and AD, the exact pathomechanistic interaction is unclear. Presence of both CVD and AD pathology is shown to have additive effects in terms of cognitive symptoms in AD,42 and we recently demonstrated correlations between WMH load and neuropsychological tests of episodic memory, which typically are impaired in early AD.43 WMHs are often considered markers of small vessel CVD and thought to originate from ischemia due to hypoperfusion.44 In this case, WMHs may function to lower the threshold for symptomatic presentation in AD or to contribute to the clinical manifestations. However, associations between WMHs and AD in patients without medical history of vascular disease45 and a putative link between vascular risk factors and AD1,2 suggest that other factors may be important in the development of WMHs, e.g. CAA. These findings support our hypothesis of β-amyloid as a putative contributor to WMHs. A recent review concludes with ischemic brain injury as a common finding in CAA,46 and it is possible to construe that WMHs may in pertinent cases reflect vascular β-amyloid deposition. This is supported by the predilection of WMHs in AD5 and CAA47 for posterior brain regions, and an increase in WMH volume over time in patients diagnosed with possible or probable CAA,48 e.g. by enhanced cytotoxicity caused by perivascular and endothelial Aβ-accumulation. Further, Zhu et al.49 demonstrated a dominant posterior distribution of WMHs in CAA when compared to hypertension-related small vessel CVD. Hence, a potential bias in the current study could be difference in the spatial distribution of WMHs between the two groups, as this could influence the observed group differences in WMHs DTI measures. However, no significant difference in the spatial distribution was found, and therefore this was not corrected for in the current study.
β-amyloid itself may affect the vascular bed. In previous studies, Aβ is shown to induce vasoconstriction of the cerebral microcirculation, potentially through effects of Aβ on vascular smooth muscle.50 Further, transgenic mice, whose cortices were exposed to Aβ, have presented with reduced resting cerebral blood flow (CBF) and impaired effect of endothelium-dependent vasodilators on CBF,51 i.e. cerebral autoregulatory dysfunction. The vascular effect of β-amyloid may also include impaired clearance of Aβ, as it has been shown impaired clearance in AD patients compared to controls, despite similar Aβ production rates.52 These data may further support our findings.
The Aβ(+) and (−) groups consist of cases with or without amyloid plaques, respectively, and the between-group differences likely reflect WMHs state differences with and without co-existing plaques, even though plaque origin in both groups are related to CVD risk factors.53 Microscopically, Aβ accumulates around perivascular cells and affects the neurovascular unit (vascular and perivascular cells, glia, and neurons). Microvascular changes in AD and vascular cognitive impairment (VCI) include hyaline degradation, thickening of capillary basement membrane and reduction in numbers of capillaries, thereby also affecting the function of the blood–brain barrier.3 These alterations add to the pool of evidence linking AD and CVD and, with the present results taken into consideration, Aβ dysmetabolism could be the initiating event.
Our additional objective was to study the effects of amyloid on diffusion properties in other areas of the brain. We focused on a ROI likely to be affected in AD and NAWM. Cingulum is connected to the temporal lobe and hippocampus54 and has shown to be affected in MCI and early AD.23,24 Therefore, the cingulum is considered to play an important role in memory function. In previous DTI studies, cingulum has presented with lower FA in MCI, with more pronounced changes in AD.55 In the current study, no effects in other areas, including cingulum and NAWM, may indicate that our observed effects of amyloid on diffusion properties in WMHs might be specific for WMHs rather than white matter in general. However, this finding is surprising seen in light of changes observed in other studies, e.g. previously demonstrated DTI changes in SCD and MCI,18 and as one would expect to detect changes even in NAWM. One possible explanation for this may be the small sample size, and consequently reduced statistical power, in our study, although when not adjusting for WMH volume and age, the DTI indices of cingulum were significantly different between the two groups.
Further, we did not adjust for vascular risk factors (e.g. hypertension) as previous studies have shown a link between WMHs and AD independent of these factors and to avoid overadjustment. However, it is possible that these variables may contribute to our findings. In addition, although the diagnosis may influence DTI indices, and both SCD and MCI are rather heterogeneous conditions (SCD more so than MCI), the subjects of the current study were selected based on biomarkers (CSF Aβ42) rather than clinical diagnosis, which we believe is a strength of our study. We have previously examined white matter DTI differences in SCD and MCI as compared to each other and controls. Although, greater differences were found between a larger MCI group and controls than between a smaller SCD group and controls, we found no statistically significant differences between the SCD and the MCI groups.18 Furthermore, in the current study, there was no significant difference between the two groups in terms of diagnosis. As such, the diagnosis (SCD/MCI) was not corrected for. In addition, we did not adjust for multiple comparisons, as the main analysis of the current study was diffusion measures in WMHs, and the DTI indices are highly correlated. Although multiple comparisons may increase the chances of type I error, the risk of type I error is reduced as the remaining analyses were complementary and the results in the current study were consistent. Therefore, we did not correct for multiple comparisons. Taken these limitations into account, our findings need to be replicated in other cohorts. Although the findings suggest a link between β-amyloid metabolism and WMHs, the more detailed pathomechanism of this link also further needs to be examined.
In conclusion, our study reveals impaired structural integrity in WMHs in subjects with low CSF Aβ42. This could be suggestive of more pronounced demyelination and reduced axonal integrity in response to amyloid dysmetabolism, and the pathomechanism of WMHs in AD may be heterogeneous, i.e. a combination of both small vessel disease, and myelin and axonal damage due to the pathological process occurring in AD.
Supplementary Material
Funding
This study has received financial support from The Research Council of Norway (grant reference number 217780/H10), South-Eastern Norway Regional Health Authority (grant reference number 2013131), EU-JPND via The Research Council of Norway (grant reference number 237250).
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
L Kalheim contributed to conception and design of the study, analyzed data, and contributed to the interpretation of the data. L Kalheim further drafted the article. A Bjornerud contributed to the conception and design of the study, acquisition and interpretation of data (notably MRI data), and revised the article. K Vegge contributed to the acquisition and interpretation of data (notably MRI data), and revised the article. T Fladby contributed to the conception and design of the study, acquisition and interpretation of data, and revised the article. P Selnes contributed to the conception and design of the study, acquisition and interpretation of the data, and revised the article. All authors approved the final version.
References
- 1.Breteler MM. Vascular risk factors for Alzheimer’s disease: An epidemiologic perspective. Neurobiol Aging 2000; 21: 153–160. [DOI] [PubMed] [Google Scholar]
- 2.Hofman A, Ott A, Breteler MM, et al. Atherosclerosis, apolipoprotein E, and prevalence of dementia and Alzheimer’s disease in the Rotterdam Study. Lancet 1997; 349: 151–154. [DOI] [PubMed] [Google Scholar]
- 3.Iadecola C. The overlap between neurodegenerative and vascular factors in the pathogenesis of dementia. Acta Neuropathol 2010; 120: 287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013; 12: 822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshita M, Fletcher E, Harvey D, et al. Extent and distribution of white matter hyperintensities in normal aging, MCI, and AD. Neurology 2006; 67: 2192–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luchsinger JA, Brickman AM, Reitz C, et al. Subclinical cerebrovascular disease in mild cognitive impairment. Neurology 2009; 73: 450–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jack CR, Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol 2010; 9: 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haglund M, Englund E. Cerebral amyloid angiopathy, white matter lesions and Alzheimer encephalopathy - a histopathological assessment. Dement Geriatr Cogn Disord 2002; 14: 161–166. [DOI] [PubMed] [Google Scholar]
- 9.Deane R, Wu Z, Sagare A, et al. LRP/amyloid beta-peptide interaction mediates differential brain efflux of Abeta isoforms. Neuron 2004; 43: 333–344. [DOI] [PubMed] [Google Scholar]
- 10.Selnes P, Blennow K, Zetterberg H, et al. Effects of cerebrovascular disease on amyloid precursor protein metabolites in cerebrospinal fluid. Cerebrospinal Fluid Res 2010; 7: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah SB, Nolan R, Davis E, et al. Examination of potential mechanisms of amyloid-induced defects in neuronal transport. Neurobiol Dis 2009; 36: 11–25. [DOI] [PubMed] [Google Scholar]
- 12.Suenaga T, Ohnishi K, Nishimura M, et al. Bundles of amyloid precursor protein-immunoreactive axons in human cerebrovascular white matter lesions. Acta Neuropathol 1994; 87: 450–455. [DOI] [PubMed] [Google Scholar]
- 13.Selnes P, Aarsland D, Bjornerud A, et al. Diffusion tensor imaging surpasses cerebrospinal fluid as predictor of cognitive decline and medial temporal lobe atrophy in subjective cognitive impairment and mild cognitive impairment. J Alzheimer’s Dis 2013; 33: 723–736. [DOI] [PubMed] [Google Scholar]
- 14.Concha L. A macroscopic view of microstructure: using diffusion-weighted images to infer damage, repair, and plasticity of white matter. Neuroscience 2014; 276: 14–28. [DOI] [PubMed] [Google Scholar]
- 15.Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. NeuroImage 2006; 31: 1487–1505. [DOI] [PubMed] [Google Scholar]
- 16.Amlien IK, Fjell AM. Diffusion tensor imaging of white matter degeneration in Alzheimer’s disease and mild cognitive impairment. Neuroscience 2014; 276: 206–215. [DOI] [PubMed] [Google Scholar]
- 17.Stenset V, Bjornerud A, Fjell AM, et al. Cingulum fiber diffusivity and CSF T-tau in patients with subjective and mild cognitive impairment. Neurobiol Aging 2011; 32: 581–589. [DOI] [PubMed] [Google Scholar]
- 18.Selnes P, Fjell AM, Gjerstad L, et al. White matter imaging changes in subjective and mild cognitive impairment. Alzheimers Dement 2012; 8: S112–121. [DOI] [PubMed] [Google Scholar]
- 19.Nir TM, Jahanshad N, Villalon-Reina JE, et al. Effectiveness of regional DTI measures in distinguishing Alzheimer’s disease, MCI, and normal aging(). NeuroImage 2013; 3: 180–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Acosta-Cabronero J, Williams GB, Pengas G, et al. Absolute diffusivities define the landscape of white matter degeneration in Alzheimer’s disease. Brain 2010; 133: 529–539. [DOI] [PubMed] [Google Scholar]
- 21.Lee DY, Fletcher E, Martinez O, et al. Regional pattern of white matter microstructural changes in normal aging, MCI, and AD. Neurology 2009; 73: 1722–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Molinuevo JL, Ripolles P, Simo M, et al. White matter changes in preclinical Alzheimer’s disease: A magnetic resonance imaging-diffusion tensor imaging study on cognitively normal older people with positive amyloid beta protein 42 levels. Neurobiol Aging 2014; 35: 2671–2680. [DOI] [PubMed] [Google Scholar]
- 23.Choo IH, Lee DY, Oh JS, et al. Posterior cingulate cortex atrophy and regional cingulum disruption in mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging 2010; 31: 772–779. [DOI] [PubMed] [Google Scholar]
- 24.Chua TC, Wen W, Slavin MJ, et al. Diffusion tensor imaging in mild cognitive impairment and Alzheimer’s disease: A review. Curr Opin Neurol 2008; 21: 83–92. [DOI] [PubMed] [Google Scholar]
- 25.Auer S, Reisberg B. The GDS/FAST staging system. Int Psychogeriatr 1997; 9: 167–171. [DOI] [PubMed] [Google Scholar]
- 26.Reisberg B, Ferris SH, de Leon MJ, et al. Global Deterioration Scale (GDS). Psychopharmacol Bull 1988; 24: 661–663. [PubMed] [Google Scholar]
- 27.Reisberg B, Gauthier S. Current evidence for subjective cognitive impairment (SCI) as the pre-mild cognitive impairment (MCI) stage of subsequently manifest Alzheimer’s disease. Int Psychogeriatr 2008; 20: 1–16. [DOI] [PubMed] [Google Scholar]
- 28.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12: 189–198. [DOI] [PubMed] [Google Scholar]
- 29.Kiernan RJ, Mueller J, Langston JW, et al. The Neurobehavioral Cognitive Status Examination: A brief but quantitative approach to cognitive assessment. Ann Intern Med 1987; 107: 481–485. [DOI] [PubMed] [Google Scholar]
- 30.Royall DR, Mahurin RK, Gray KF. Bedside assessment of executive cognitive impairment: The executive interview. J Am Geriatr Soc 1992; 40: 1221–1226. [DOI] [PubMed] [Google Scholar]
- 31.Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment–beyond controversies, towards a consensus: Report of the International Working Group on Mild Cognitive Impairment. J Intern Med 2004; 256: 240–246. [DOI] [PubMed] [Google Scholar]
- 32.Morris JC. Clinical dementia rating: A reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int Psychogeriatr 1997; 9: 173–176; discussion 177–178. [DOI] [PubMed] [Google Scholar]
- 33.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 2004; 23: S208–219. [DOI] [PubMed] [Google Scholar]
- 34.Woolrich MW, Jbabdi S, Patenaude B, et al. Bayesian analysis of neuroimaging data in FSL. NeuroImage 2009; 45: S173–186. [DOI] [PubMed] [Google Scholar]
- 35.Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal 2001; 5: 143–156. [DOI] [PubMed] [Google Scholar]
- 36.Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson 1996; 111: 209–219. [DOI] [PubMed] [Google Scholar]
- 37.Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med 1996; 36: 893–906. [DOI] [PubMed] [Google Scholar]
- 38.Mori S, Wakana S, Van Zijl PCM. MRI atlas of human white matter, 1st ed Amsterdam, The Netherlands; Boston: Elsevier, 2005. [Google Scholar]
- 39.Rueckert D, Sonoda LI, Hayes C, et al. Nonrigid registration using free-form deformations: Application to breast MR images. IEEE Trans Med Imag 1999; 18: 712–721. [DOI] [PubMed] [Google Scholar]
- 40.Rincon M, Selnes P, Larsson C, et al. Automatic WML segmentation and quantification using a machine learning approach. Proc Intl Soc Mag Reson Med 2011.
- 41.Acosta-Cabronero J, Alley S, Williams GB, et al. Diffusion tensor metrics as biomarkers in Alzheimer’s disease. PloS one 2012; 7: e49072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brickman AM, Honig LS, Scarmeas N, et al. Measuring cerebral atrophy and white matter hyperintensity burden to predict the rate of cognitive decline in Alzheimer disease. Arch Neurol 2008; 65: 1202–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Selnes P, Grambaite R, Rincon M, et al. Hippocampal complex atrophy in poststroke and mild cognitive impairment. J Cereb Blood Flow Metab 2015; 35: 1729–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fernando MS, Simpson JE, Matthews F, et al. White matter lesions in an unselected cohort of the elderly: Molecular pathology suggests origin from chronic hypoperfusion injury. Stroke 2006; 37: 1391–1398. [DOI] [PubMed] [Google Scholar]
- 45.Provenzano FA, Muraskin J, Tosto G, et al. White matter hyperintensities and cerebral amyloidosis: Necessary and sufficient for clinical expression of Alzheimer disease? JAMA Neurol 2013; 70: 455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reijmer YD, van Veluw SJ and Greenberg SM. Ischemic brain injury in cerebral amyloid angiopathy. J Cereb Blood Flow Metab 2015; 36: 55–71. [DOI] [PMC free article] [PubMed]
- 47.Shams S, Martola J, Granberg T, et al. Cerebral microbleeds: Different prevalence, topography, and risk factors depending on dementia diagnosis-the karolinska imaging dementia study. Am J Neuroradiol 2015; 36: 661–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen YW, Gurol ME, Rosand J, et al. Progression of white matter lesions and hemorrhages in cerebral amyloid angiopathy. Neurology 2006; 67: 83–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu YC, Chabriat H, Godin O, et al. Distribution of white matter hyperintensity in cerebral hemorrhage and healthy aging. J Neurol 2012; 259: 530–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Niwa K, Porter VA, Kazama K, et al. A beta-peptides enhance vasoconstriction in cerebral circulation. Am J Physiol Heart Circ Physiol 2001; 281: H2417–2424. [DOI] [PubMed] [Google Scholar]
- 51.Niwa K, Carlson GA, Iadecola C. Exogenous A beta1-40 reproduces cerebrovascular alterations resulting from amyloid precursor protein overexpression in mice. J Cereb Blood Flow Metab 2000; 20: 1659–1668. [DOI] [PubMed] [Google Scholar]
- 52.Mawuenyega KG, Sigurdson W, Ovod V, et al. Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science 2010; 330: 1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stenset V, Johnsen L, Kocot D, et al. Associations between white matter lesions, cerebrovascular risk factors, and low CSF Abeta42. Neurology 2006; 67: 830–833. [DOI] [PubMed] [Google Scholar]
- 54.Wakana S, Jiang H, Nagae-Poetscher LM, et al. Fiber tract-based atlas of human white matter anatomy. Radiology 2004; 230: 77–87. [DOI] [PubMed] [Google Scholar]
- 55.Zhang Y, Schuff N, Jahng G, et al. Diffusion tensor imaging of cingulum fibers in mild cognitive impairment and Alzheimer disease. Neurology 2007; 68: 13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.