Abstract
The age-associated decline of the neurological and cognitive functions becomes more and more serious challenge for the developed countries with the increasing number of aged populations. The morphological and biochemical changes in the aging brain are the subjects of many extended research projects worldwide for a long time. However, the crucial role of the blood–brain barrier (BBB) impairment and disruption in the pathological processes in age-associated neurodegenerative disorders received special attention just for a few years. This article gives an overview on the major elements of the blood–brain barrier and its supporting mechanisms and also on their alterations during development, physiological aging process and age-associated neurodegenerative disorders (Alzheimer's disease, multiple sclerosis, Parkinson's disease, pharmacoresistant epilepsy). Besides the morphological alterations of the cellular elements (endothelial cells, astrocytes, pericytes, microglia, neuronal elements) of the BBB and neurovascular unit, the changes of the barrier at molecular level (tight junction proteins, adheres junction proteins, membrane transporters, basal lamina, extracellular matrix) are also summarized. The recognition of new players and initiators of the process of neurodegeneration at the level of the BBB may offer new avenues for novel therapeutic approaches for the treatment of numerous chronic neurodegenerative disorders currently without effective medication.
Keywords: Aging, blood–brain barrier, endothelial cells, neurodegenerative disorders, transport systems
Structure of the blood–brain barrier (BBB)
Homeostasis of the extracellular microenvironment in the neural tissue of the brain as well as its protection against neurotoxic compounds and variations in the composition of the blood are important for normal function of the neurons. It is warranted by a structure formed between blood and brain, which is therefore called the BBB.1 Clear evidence for the existence of this permeability barrier in the brain emerged in 1909 with the demonstration by Edwin Goldman (a South African-German) that a dye injected into the blood stream of a rat stained the whole body – except for the brain and spinal cord. The opposite was also true: injection of the dye into the cerebral ventricles stained the brain and spinal cord but not the rest of the body.2,3 This occurs because most organs of the body are perfused by capillaries lined with endothelial cells (ECs) that have small pores (fenestrations) to allow for the rapid movement of small molecules into the organ interstitial fluid from the circulation. However, the capillary endothelium of the brain and spinal cord lack these pores because the ECs of brain capillary are connected to each other by continuous tight junctions (TJs), produced by the interaction of several transmembrane proteins that project into and seal the paracellular pathway.3–5 The interaction of these junctional proteins effectively blocks the free diffusion of polar solutes from blood along these potential paracellular pathways and so denies access to brain interstitial fluid. Thus, the BBB significantly impedes entry from blood to brain of virtually all molecules, except those that are small and lipophilic or those that enter the brain through an active transport mechanism, particularly with essential nutrients, precursors and cofactors. The other difference between the peripheral and central capillaries is that the brain capillaries are surrounded by pericytes, basal membrane and astrocyte end feet, which form a covering layer, that are missing at the peripheral microvessels. It is well established that in contrast to leaky vessels in peripheral organs,6 the BBB restricts entry of polar molecules into the brain,7–9 but can be transported into the brain via specific transporters expressed in the brain endothelium under physiological or pathological conditions.10,11
There are five basic mechanisms by which solute molecules move across membranes. First is simple diffusion, which proceeds from high to low concentrations (e.g. sucrose and ethanol). Second is facilitated diffusion (glucose via GLUT-1), a form of carrier-mediated endocytosis, in which solute molecules bind to specific membrane protein carriers, also from high to low concentration. Third is simple diffusion through an aqueous channel, formed within the membrane. Fourth is active transport through a protein carrier (e.g. P-gp and BCRP) with a specific binding site that undergoes a change in affinity. Active transport requires ATP hydrolysis and conducts movement against the concentration gradient. The fifth is movement between cells is referred to as paracellular diffusion. The BBB has a number of highly selective mechanisms for transport of nutrients into the brain.
Several BBB peptide transport mechanisms (i.e. receptor-mediated, absorptive-mediated, carrier-mediated and non-specific passive diffusion), as well as non-transport processes (i.e. endocytosis without transcytosis, absorption and metabolism) are known.12
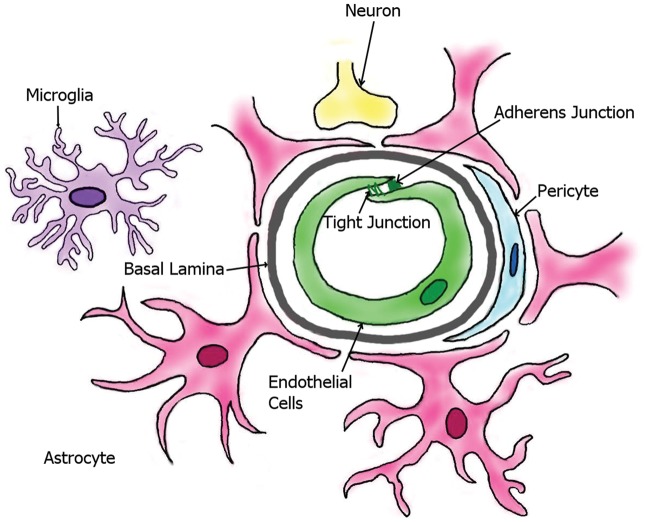
The main cellular elements of BBB are (a) the brain capillary ECs, and these cells are associated with (b) pericytes that are responsible for the feeding of the ECs, (c) end feet of the astrocytes covering the capillaries, and (d) the interacting microglial cells and (e) neurons (Figure 1). The collective name of these cell types as a collaborative system in the brain is neurovascular unit (NVU). Beside the cellular elements, BBB comprises extracellular matrix and basal lamina as the parts of the protective system. All the cell types have special structures to ensure the regulation of the penetration of xenobiotics to the brain tissue.
Figure 1.
The most important cellular elements of blood–brain barrier (modified from http://www.hcvpi.bham.ac.uk/staff/NF.html).13
In the first part of this review, we will discuss the barrier function of these cellular elements in interaction with each other and supporting mechanisms. In the second part, the alterations of the protective molecular mechanisms during the process of maturation and aging will be summarized. In the final part, the age-related neurodegenerative disorders and the role of BBB disruption in their pathologies will be presented.
Endothelial barrier
Compared with peripheral vasculature, BBB ECs are characterized by increased mitochondrial content, exhibit minimal pinocytotic activity, and lack fenestrations.14–17. Increased mitochondrial content is essential for these cells to maintain various active transport mechanisms such as those utilized to transport ions, nutrients and waste products into and out of brain parenchyma, thus contributing to precise regulation of the CNS microenvironment and ensuring proper neuronal function. The high concentration of mitochondria in cerebrovascular ECs might account for the sensitivity of the BBB to oxidant stressors. Furthermore, the physiology and pathophysiology of ECs are closely linked to the functioning of their mitochondria, and mitochondrial dysfunction is another important mediator of disease pathology in the brain.18
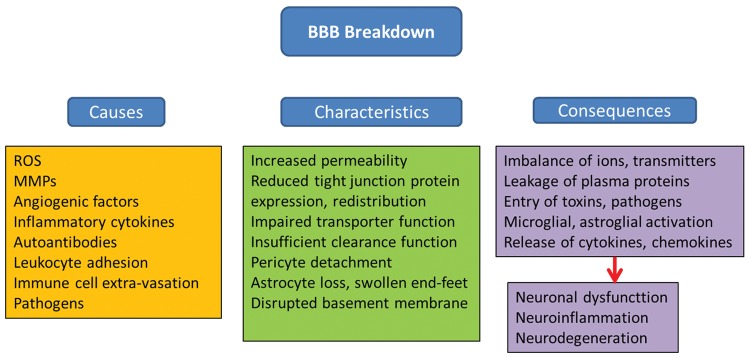
The brain endothelium is highly reactive because it serves as both a source of, and a target for, inflammatory proteins and reactive oxygen species (ROS). Neuroinflammation and oxidative stress lead to BBB breakdown, which are implicated in the pathogenesis of central nervous system (CNS) disease. Cell polarity of ECs is ascribed to differing functional expression of transporter proteins and metabolic enzymes that are differentially expressed on the luminal (or apical) and abluminal (or basolateral) membranes, which further contribute to the high selectivity of the BBB.19–21 Of the many transporters expressed at the BBB endothelium, several have been implicated in influx and/or efflux of drugs into the CNS. Decreased expression and/or defective function of transporters, leads to increased BBB permeability, toxic adverse effects of drugs and can be an early event in the pathology of several disorders. Factors that can disrupt the BBB are varied, ranging from secreted elements to immune cells and pathogens. Compromised BBB integrity manifests mainly as increased barrier permeability. In addition to direct effects on ECs, other members of the NVU can be affected, that is pericytes, astrocytes and basement membrane (BM), which in turn aggravate impairment of BBB functions. Consequences vary from dysregulated molecular and ionic flux across the damaged BBB to the initiation of a central inflammatory response. Despite manifold causes, characteristics and consequences, BBB breakdown generally culminates in neuronal dysfunction, neuroinflammation and neurodegeneration. Downstream pathological outcomes and potential for recovery are diverse22 (Figure 2).
Figure 2.
Causes, characteristics and consequences of BBB breakdown (modified from Obermeier et al.22).
Membrane transporters
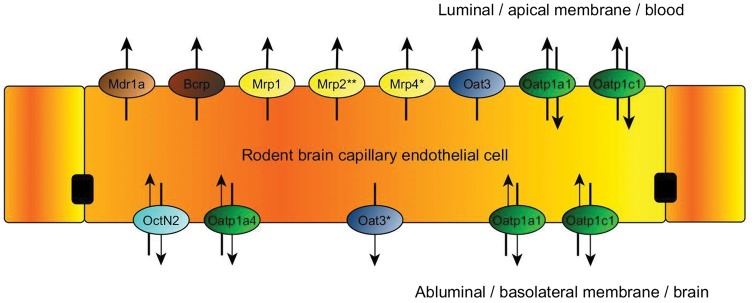
Efflux transporters include P-glycoprotein (P-gp), Breast Cancer Resistance Protein (BCRP in humans; Bcrp in rodents) and Multidrug Resistance Proteins (MRPs in humans; Mrps in rodents) in the brain. Transporters that facilitate drug entry into the brain (uptake or influx transporters) include organic anion transporting polypeptides (OATPs in humans; Oatps in rodents), organic anion transporters (OATs in humans; Oats in rodents), organic cation transporters (OCTs in humans; Octs in rodents), nucleoside transporters, monocarboxylate transporters (MCTs in humans; Mcts in rodents) and putative transport systems for peptide transport. The localization of the main membrane transporter proteins in the apical and basolateral surface of rodent ECs is presented in Figure 3.
Figure 3.
Localization of membrane transporters in the endothelial cells23,24 at the BBB in rodents (modified from the website of Solvo Biotechnology25).
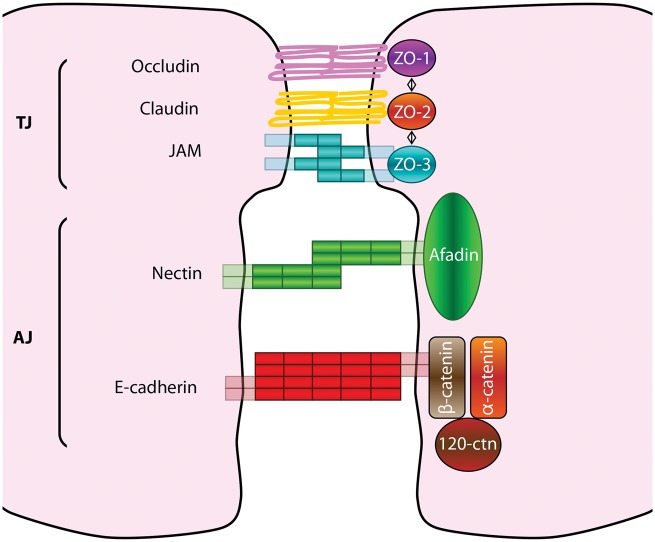
The restricted paracellular permeability of capillary EC layer is warranted by two intercellular molecular binding systems: the adherens junctions (AJ) and the TJ (Figure 4).
Figure 4.
Tight and adherens junctions components. Tight junctions (TJs) are the most apical intercellular junctions in ECs. Their major transmembrane components are the claudins, occludin and adhesion molecules (JAMs), whereas their cytoplasmic components are zonula occludens (ZOs) proteins. Adherens junctions (AJs) are localized just below TJs; their transmembrane components are cadherins and nectins that complex respectively the cytoplasmic components β-catenin and afadin.
Adherens junctions
AJs are found throughout the CNS microvasculature and are responsible for intercellular adherence between adjacent ECs.15,26 AJs are composed of multiple protein components including vascular endothelium (VE) cadherin, actinin and catenin.21 Cell–cell adhesion is mediated by homophilic interactions of VE-cadherin expressed on adjacent ECs. Such interactions mediate calcium-dependent cell adhesion by binding to the actin cytoskeleton. Cytoskeletal binding occurs via catenin accessory proteins. Specifically, β-catenin links VE-cadherin to α-catenin, an interaction that induces the direct binding to actin.14,20
Tight junctions
Although disruption of AJs can result in increased BBB permeability, TJs are primarily responsible for restricting paracellular permeability at the BBB.26,27 TJs form the primary physical barrier component of the BBB and function to greatly restrict paracellular entry of various endogenous and exogenous substances that can potentially be neurotoxic. Such TJs impart a high trans-endothelial electrical resistance (TEER) across the BBB (1500–2000 Ω cm2) that restricts free flow of ions and solutes.28 TJs are dynamic complexes of multiple protein constituents including junctional adhesion molecules (JAMs), occludin, claudins (i.e. claudin-1, -3 and -5) and membrane-associated guanylate kinase (MAGUK)-like proteins (i.e. ZO-1, -2 and -3).26
Basal membrane
BMs (it is also called as basal lamina) are considered to be uniform, thin extracellular matrix sheets that serve as a substrate for ECs. To find out whether BMs maintain their ultrastructure, protein composition and biophysical properties throughout life the natural aging history of the human inner limiting membranes (ILM) was investigated by Candiello et al.29 Transmission electron microscopy showed that the ILM steadily increases in thickness from 70 nm at foetal stages to several microns at age 90. Furthermore, the relative concentrations of collagen IV and argin increase, and the concentration of laminin decreases with age. Force-indentation measurements by atomic force microscopy also showed that ILMs become increasingly stiffer with advancing age.29
Extracellular matrix
The extracellular matrix of the basal lamina serves as an anchor for the cerebral microvascular endothelium. The anchoring function of the extracellular matrix is mediated via interactions between endothelial integrin receptors, lamin and other matrix proteins. Disruption of extracellular matrix is associated with loss of barrier function, resulting in increased permeability. Additionally, matrix proteins have been shown to influence the expression of TJ proteins, such as occludin, suggesting that the extracellular matrix plays a role in maintaining TJ protein integrity.15,26,30
Astrocytes
Astrocytes are the most abundant cell type in the brain and display a fibroblast-like morphology within grey matter15,31; however, this morphology can be influenced by their CNS location and associations with other cell types that are in close proximity.32 Astrocyte end-feet cover over 99% of cerebral capillaries,33 leading to critical cell–cell interactions that directly modulate and regulate BBB characteristics.32 Several studies have demonstrated that astrocytes play a vital role in maintenance, and perhaps induction, of BBB characteristics.
Astrocytes are fundamental for homeostasis, defence and regeneration of the CNS. Loss of astroglial function and astroglial reactivity contributes to the aging of the brain and to neurodegenerative diseases. Changes in astroglia in aging and neurodegeneration are highly heterogeneous and region-specific.34
Several inducing factors secreted by astrocytes have been identified, including TGF-β, GDNF and BFGF, which are involved in induction and regulation of the BBB phenotype. Additionally, astrocytes can regulate brain microvascular permeability via Ca2+ signalling involving astrocyte-endothelial gap junctions and purinergic transmission.15,26,30,32 Astrocytes play a critical role in preventing excitotoxicity induced by acute elevations of glutamate in the brain. This is mediated via expression of astrocyte glutamate transporters excitatory amino acid transporters (EAAT1 and EAAT2) that are responsible for glutamate uptake into the astrocyte cell, thus reducing glutamate levels in the parenchyma.35
Lipoproteins in plasma transport lipids between tissues, however, only high-density lipoproteins (HDL) appear to traverse the BBB; thus, lipoproteins found in the brain must be produced within the CNS. Apolipoproteins E (ApoE) and ApoJ are the most abundant apolipoproteins in the brain, which are mostly synthesized by astrocytes.36 ApoE has an important role in the pathology and BBB injury during the development of Alzheimer's disease (see more details later in this article).
There is also evidence that astrocytes progressively accumulate in the normal aging brain, increasing in both number and size. These astrocyte changes in normal brain aging may, in the event of an injury, contribute to the exacerbated injury response and poorer outcomes observed in older traumatic brain injury survivors.37 Astrocyte morphology changes and GFAP expression increases progressively during aging in humans and rodents.38,39
Pericytes
Pericytes are BBB associated cells. During aging, pericytes show ultrastructural changes such as vesicular and lipofuscin-like inclusions1,40–43; an increased size of mitochondria44 and a foamy transformation.45 Ueno et al.46 reported on membranous inclusions within the basal lamina or the ECM of the microvessels and suggested the degeneration of pericytes. de Jong et al.47 in the aging rat and Stewart et al.48 in the aging human brain showed the degeneration and loss of pericytes. Burns et al.49,50 described the reduction of the cross-sectional area of pericytes in aging monkey and rat brain. However, Peters et al.42 found no change in the number of pericytes in monkey, and Heinsen and Heinsen51 and Peinado et al.41 showed an increase in the number of pericytes in the rat aging brain. The obvious discrepancies in the literature could be explained by considering the degeneration and the proliferation of pericytes as distinct age-related mechanisms. Accordingly, Scheibel and Fried52 could show that pericytes in aging brain begin to lose their contact to the EC and begin to migrate. This behaviour seems to be a response of the pericytes to a yet unidentified age-related effect. The PDGF released by ECs is known to bind to the PDGF receptor expressed by pericytes and to attract pericytes to ECs.53,54 If this ligand-receptor system is disturbed, the functional integrity of the vessel is impaired.53,55 With age, this system could deteriorate as well, but data are not available as yet.
Montagne and coworkers showed that pericyte injury and possibly early degeneration correlates with increased BBB permeability within the hippocampus,56 a region known to be affected by pericyte loss and BBB breakdown on post-mortem tissue analysis in Alzheimers' disease.57
Microglia
Microglia, the primary immune cells of the brain, are ubiquitously distributed in the CNS and are activated in response to systemic inflammation, trauma and several CNS pathophysiologies.15,58–60 This cell type is not the basic element of the BBB, but it has an impact on the BBB function and integrity. Microglia present with a ramified morphology that is characterized by a small soma and fine cellular processes during their “resting state”. Microglial activation in response to pathophysiological stressors can trigger changes cell morphology, which include reduced complexity of cellular processes and transition from a ramified morphology to an amoeboid appearance.59 Activated microglia produces high levels of neurotoxic and proinflammatory mediators such as nitric oxide, peroxide, TNF-α and proteases, all of which result in cell injury and neuronal death,30 As immune cells microglia scavenge apoptotic cells, tissue debris after trauma, or microbes.30 They can also act as scavengers of extracellular molecules such as amyloid-β.59,60 Activation of microglia is associated with altered TJ protein expression and increased BBB permeability.61
Briefly the major physiological protective effector functions of microglia: (a) Proliferation, (b) morphological transformation, (c) motility and migration, (d) intercellular communication, (e) phagocytosis and (f) proteostasis. With aging, microglia shift their morphology and may display diminished capacity for normal functions related to migration, clearance and the ability to shift from a proinflammatory to an anti-inflammatory state to regulate injury and repair.62
Neurons
There is considerable evidence for direct innervation of both brain microvessel ECs and associated astrocyte processes via distinct connections with noradrenergic,15,62,63 serotonergic,64 cholinergic65,66 and GABAergic67 neurons. Therefore, neurons are in connection with the BBB and have influence on the behaviour of capillary endothelial barrier. For example, studies have shown that loss of direct noradrenergic input from the locus coeruleus results in increased BBB susceptibility to effects of acute hypertension, resulting in significantly increased permeability to 125-I labelled albumin.68
With aging, cell senescence can contribute to BBB compromise. The compromised BBB allows an influx of inflammatory cytokines to enter the brain. These cytokines lead to neuronal and glial damage. Several factors contribute to the deterioration of synaptic plasticity with age and one of these factors appears to be a heightened level of activation of microglia, which may reflect impairment in the homeostatic ability of these cells with age, or an increase in responsiveness to modulatory molecules. The age-related decrease in tissue perfusion, together with the increase in BBB permeability may alter the microenvironment in the brain; this, combined with the proposed age-related compromised homeostatic capability of microglia, may be a significant factor in maintaining the neuroinflammatory changes, which have been described in the aged brain and which exert a negative impact on synaptic function. These combined changes may contribute to the deficit in long-term potentiation (LTP) in perforant path-granule cell synapses of aged animals.69 Ultimately, the functional neuronal changes within the brain can cause age-related disease.
Changes in the BBB with physiological aging
How to define physiological aging? It is evident even from casual observation of physical activities, such as walking, that elderly people exhibit a deterioration of physiological processes. Moreover, the inability of athletes to continue peak performance when they reach their 30s or 40s indicates that deterioration begins at a relatively young age and progresses in severity from that point on. Indeed, many studies have confirmed that most physiological processes deteriorate progressively after about 30 years of age, some functions more severely affected than others. Diseases that do not occur until, or increase in frequency at, advanced ages are called age-associated diseases. Coronary heart disease, stroke, many types of cancer, osteoporosis, Alzheimer's disease, Parkinson's disease (PD) and other neurodegenerative disorders are examples of such diseases commonly found in elderly people. Indeed, age-associated disease underlies much of the physiological deterioration of old age. However, many studies focus on subjects who are free of discernible disease in what they refer to as the study of ‘normal aging or physiological aging’ (Edward J Masoro, Medicine Encyclopedia, Aging Healthy). Concerning aging of the brain and BBB, physiological aging can be defined as a deterioration of the functions without cognitive decline and dementia.
Dysfunction of BBB appears and it is associated with inflammation and loss of TJs without leukocyte recruitment at adult period and aging. Cell damage is characterized by nuclear DNA damage and can contribute to aging either indirectly by increasing apoptosis or cellular senescence or directly by increasing cell dysfunction.
The matured immune system is also entering into function to regulate the passage of cells and molecules from the periphery to the CNS through BBB. For example, ECs selectively regulate trans-endothelial migration of T-helper lymphocytes (Th1 and Th2) suppressing the initiation of CNS autoimmune.70 TJ barriers of ECs prevent the free entry of blood-derived substances; thereby maintain the extracellular environment of the brain. Astrocytes express TJ proteins and densely and tightly surround mature neurons to protect them from blood-derived neurotoxic substances.71
The main changes at the BBB during physiological aging are presented in Table 1.
Table 1.
Alterations of BBB constituents during physiological aging.
| BBB elements | Properties | References |
|---|---|---|
| ECs | Capillary wall thickness: increased in humans decreased in rats decreased in monkeys Number of ECs: decreased in humans Number of mitochondria: decreased | Hunziker et al.72 Hicks et al.44 Burns et al.49 Hunziker et al.72 Burns et al.49,50 Grammas et al.18 |
| Tight junctions | Expression of tight junction proteins: decreased | Elahy et al.,71 Enciu et al.73 |
| Basal lamina | Thickness of basement membrane: Increased Concentration of collagen IV and argin: Increased Concentration of laminin: decreased | Candiello et al.,29 Ravens74 Candiello et al.29 Candiello et al.29 |
| Astrocytes | Astrocyte proliferation: Increased number and size GFAP expression: Increased | Rodriguez-Arellano et al.,34 Harris et al.37 Chisholm and Sohrabji,38 Middeldorp and Hol39 |
| Microglia | Changes to amoeboid morphology Production of neurotoxic proinflammatory mediators | Kettenmann et al.59 Ronaldson and Davis30 |
| Pericytes | Number of pericytes: Degeneration and loss of pericytes Ultrastructural changes: vesicular and lipofuscin-like inclusions, increased size of mitochondria, foamy transformation | Stewart et al.,48 Burns et al.49,50 Rascher and Wolburg1 Hicks et al.44 Sturrock45 |
| Neurons | Deterioration of synaptic plasticity Deficit in long-term potentiation Impaired neurogenesis Increased apoptosis Neuronal damage due cytokine release | Blau et al.69 Blau et al.69 Lucke-Wold et al.75 Lucke-Wold et al.,75 Cerbai et al.76 Buschini et al.77 |
Changes at the BBB associated with age-related neurodegenerative pathologies
An important component of age-related pathology is neurodegeneration. It can be defined as progressive loss of neuronal structure and function, finally leading to neuronal cell death. Most neurodegenerative diseases have their onset in mid-life and can be characterized by motor and/or cognitive symptoms that progressively worsen with age and may reduce life expectancy.
Neurodegenerative diseases and changes in BBB functionality
In neurodegeneration, changes in functionality of the BBB and supporting cells (NVU) lead to BBB dysfunction in a more progressed state of the disease process27,32,78–81 (Figure 2). Previously, Zhao et al. reviewed the establishment and dysfunction of the BBB, with associations between BBB breakdown and pathogenesis of inherited monogenic neurological disorders and complex multifactorial diseases, including Alzheimer's disease.82 Moreover, brain vascular damage can cause BBB dysfunction and/or reduced brain blood perfusion and hypoxia. These processes lead to neuronal injury and neurodegeneration.
Below changes in BBB functioning in Alzheimer's disease, multiple sclerosis, PD and pharmacoresistant epilepsies are presented in a more disease specific manner.
BBB in Alzheimer's disease
Alteration of the BBB plays an important role in Alzheimer's disease.79,83,84 While BBB breakdown is an early event in the aging human brain that begins in the hippocampus and may contribute to cognitive impairment.56 With mild cognitive impairment, the BBB breakdown in the hippocampus worsens and is correlated with injury to BBB-associated pericytes, as shown by cerebrospinal fluid (CSF) analysis.56 Cellular and molecular mechanisms in cerebral blood vessels and the pathophysiological events that lead to cerebral blood flow dysregulation and disruption of the NVU and the BBB may contribute to the onset and progression of dementia and Alzheimer's disease. Also, there is a link between neurovascular dysfunction and neurodegeneration (including the effects of Alzheimer's disease genetic risk factors on cerebrovascular functions and clearance of amyloid-β (Aβ)), and the impact of vascular risk factors, environment and lifestyle on cerebral blood vessels, which in turn may affect synaptic, neuronal and cognitive functions, as reviewed by Nelson et al.85
Tight junctions
Both the components and functioning of TJs proteins are affected by neurodegenerative processes.79 TJ proteins include occludin and claudins (for example, claudin-3, -5 and -12). Occludin is vulnerable to be attacked by matrix metalloproteinases (MMPs) and MMPs seem to have implications in Alzheimer's disease.86,87 Furthermore, connection of adherens and TJs to the actin cytoskeleton is influenced by tau and may result in tau-induced neurotoxicity.88
Without knowing the exact mechanisms, TJs seem to be involved in receptor for advanced glycation end-products (RAGE)-mediated Aβ cytotoxicity to the brain microvascular ECs, resulting in damaged BBB structural integrity. RAGE is a multiligand membrane receptor and is the main factor mediating Aβ cytotoxicity in AD. It has interaction with Aβ stimulating activation of proinflammatory cytokines, release of ROS, which leads to neuron damage and BBB dysfunction.89
Astrocytes
Astrocytes normally regulate BBB functionality, and abnormal astrocytic activity coupled to vascular instability has been observed in Alzheimer's disease models.90 It seems that astrocyte properties are affected upon development of amyloid deposits.79,91
Pericytes
Loss of pericytes may damage the BBB due to an associated decrease in cerebral capillary perfusion, blood flow and blood flow responses to brain activation. This will lead to more chronic perfusion problems like hypoxia, while BBB breakdown may further lead to brain accumulation of blood proteins and several macromolecules with toxic effects on the vasculature and brain parenchyma, ultimately leading to secondary neuronal degeneration.79,92
Pericytes are uniquely positioned within the NVU between ECs of brain capillaries, astrocytes and neurons. Winkler et al. have reviewed the concept of the NVU and neurovascular functions of CNS pericytes, discussing vascular contributions to Alzheimer's disease and new roles of pericytes in the pathogenesis of Alzheimer's disease such as vascular-mediated Aβ-independent neurodegeneration, regulation of Aβ clearance and contributions to tau pathology, neuronal loss and cognitive decline.93 Pericytes demonstrate large numbers of intracellular inclusions, pinocytotic vesicles, large lipid granules and mitochondrial abnormalities suggesting cellular dysfunction and/or degeneration.94,95 Also, pericyte degenerative changes are associated with capillary reductions and gross dilatation and tortuosity of surviving vessels.95 In post-mortem tissue of individual AD subjects, reductions in pericyte coverage inversely correlate with evidence of BBB disruption, such as leakage of the plasma proteins including immunoglobulin G and fibrin.57 This indicates that pericyte dysfunction and/or loss are associated with key attributes of AD vascular pathology – vascular regression and disrupted vascular permeability.93
Furthermore, APOEX genetic background and pericyte degeneration seem to be related. Halliday et al.96 have shown that accelerated pericyte degeneration in Alzheimer's disease APOE4 carriers > AD APOE3 carriers > non-Alzheimer's disease controls, correlates with the magnitude of BBB breakdown to immunoglobulin G and fibrin. Also, accumulation of the proinflammatory cytokine CypA and of MMP-9 in pericytes and ECs in Alzheimer's disease (APOE4 > APOE3) has been shown to lead to BBB breakdown in transgenic APOE4 mice. This indicates that APOE4 leads to accelerated pericyte loss and enhanced activation of LRP1-dependent CypA–MMP-9 BBB-degrading pathway in pericytes and ECs, which can mediate a greater BBB damage in AD APOE4 compared with Alzheimer's disease APOE3 carriers.96
Pericytes also have a role in brain cholesterol homeostasis as they express the cholesterol efflux regulatory protein (CERP), also known as ABCA1, which mediates the efflux of cholesterol and phospholipids to lipid-poor apolipoproteins (apo-A1 and apoE), as well as the nuclear liver X receptors (LXRs). In the brain, excess cholesterol is metabolized into 24S-hydroxycholesterol (24S-OH-chol), which is an endogenous ligand for LXR.97
Shimizu et al. demonstrated that the GDNF, secreted from the brain and peripheral nerve pericytes, is one of the key molecules responsible for the up-regulation of claudin-5 expression and the permeability in the BBB.98
Furthermore, pericytes are able to internalize the amyloid-β peptides, which accumulate in brain of Alzheimer's disease patients.97 With amyloid deposits detected within degenerating pericytes in the brains of patients with Alzheimer's disease, it seems that pericyte dysfunction may also play a role in cerebral hypoperfusion and impaired amyloid β-peptide clearance in Alzheimer's disease.99
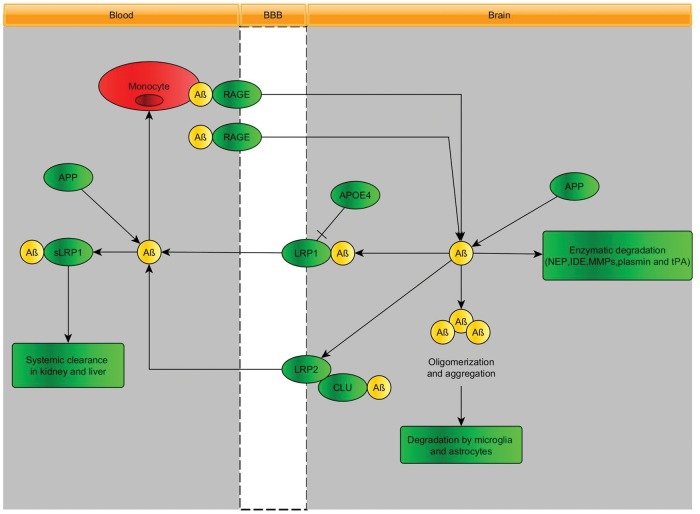
Transport and elimination of amyloid-β (Aβ) at the BBB
Active transport of Aβ across the BBB seems to occur by a number of transporters seems to occur by a number of transporters that control the level of the soluble isoform of Aβ in brain.
P-glycoprotein
P-glycoprotein (P-gp, MDR1, ABCB1) contributes to the efflux of brain-derived Aβ into blood.92,100–105 It seems that, in addition to the age-related decrease of P-gp expression, Aβ1-42 itself downregulates the expression of P-gp and other Aβ-transporters, which could exacerbate the intracerebral accumulation of Aβ and thereby accelerate neurodegeneration in Alzheimer's disease and cerebral β-amyloid angiopathy (Figure 5). Defects in P-gp-mediated Aβ clearance from the brain are thought to be triggered by systemic inflammation by lipopolysaccharide, leading to increased brain accumulation of Aβ.106 Recently, reduction of P-gp expression and transport activity has been found in isolated capillaries, as a result of Aβ40 mediated P-gp ubiquitination, internalization and proteasome-dependent degradation.107
Figure 5.
The role of blood–brain barrier transport in brain homeostasis of amyloid-β. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders.79
Low-density lipoprotein receptor-related protein 1 (LRP1)
Aβ is produced from the amyloid-β precursor protein (APP), both in the brain and in peripheral tissues. In plasma, a soluble form of LRP1 (sLRP1) is the major transport protein for peripheral Aβ. sLRP1 maintains a plasma ‘sink’ activity for Aβ through binding of peripheral amyloid-β, which in turn inhibits reentry of free plasma amyloid-β into the brain. LRP1 in the liver mediates systemic clearance of amyloid-β. LRP1 at the BBB contributes to the clearance of amyloid-β from the brain. LRP1 mediates rapid efflux of a free, unbound form of amyloid-β and of amyloid-β bound to apolipoprotein E2 (APOE2), APOE3 or α2-macroglobulin from the brain's interstitial fluid into the blood, and APOE4 inhibits such transport.108 In Alzheimer's disease, LRP1 expression at the BBB is reduced and amyloid-β binding to circulating sLRP1 is compromised by oxidation.103 Moreover, amyloid-β damages its own LRP1 mediated transport by oxidating LRP1.109 Defects in LRP-1-mediated Aβ clearance from the brain are thought to be triggered by systemic inflammation by lipopolysaccharide, leading to increased brain accumulation of amyloid-β.110,111
Low-density lipoprotein receptor-related protein 2 (LRP2)
LRP2 mediated transcytosis112 eliminates amyloid-β that is bound to clusterin (also known as apolipoprotein J) by transport across the BBB, and shows a preference for the 42-amino-acid form of this peptide.
RAGE
RAGE provides the key mechanism for influx of peripheral amyloid-β into the brain across the BBB either as a free, unbound plasma-derived peptide and/or by amyloid-β-laden monocytes. Faulty vascular clearance of amyloid-β from the brain and/or an increased re-entry of peripheral amyloid-β across the blood vessels into the brain can elevate amyloid-β levels in the brain parenchyma and around cerebral blood vessels. At pathophysiological concentrations, amyloid-β forms neurotoxic oligomers and also self-aggregates, which leads to the development of cerebral β-amyloidosis and cerebral amyloid angiopathy.79 More insight into the molecular mechanisms underlying amyloid-β-RAGE interaction-induced alterations in the BBB have been provided by Kook et al.113 They found that Aβ1-42 induces enhanced permeability, disruption of zonula occludin-1 (ZO-1) expression in the plasma membrane and increased intracellular calcium and MMP secretion in cultured ECs in vitro, and disrupted microvessels near amyloid-β plaque-deposited areas, elevated RAGE expression and enhanced MMP secretion in microvessels of the brains of 5XFAD mice, an animal model for Alzheimer's disease. The BBB transport mechanisms for amyloid-β are displayed in Figure 5.
Amyloid-β-degrading enzymes
Cerebral ECs, pericytes, vascular smooth muscle cells, astrocytes, microglia and neurons express different amyloid-β-degrading enzymes, including neprilysin, insulin-degrading enzyme, tissue plasminogen activator and MMPs, which contribute to amyloid-β clearance. In the circulation, amyloid-β is bound mainly to soluble LRP1 (sLRP1), which normally prevents its entry into the brain. Systemic clearance of amyloid-β is mediated by its removal by the liver and kidneys.
Other transporters at the BBB
Glutamate transporters
It has been suggested that glutamate excitotoxicity plays a role in the neurodegenerative processes in Alzheimer's disease.114 Strict control L-glutamate concentration in the brain extracellular fluid is important to maintain neurotransmission and avoid excitotoxicity. The role of astrocytes in handling L-glutamate transport and metabolism is well known; Na (+)-dependent transporters for glutamate exist on astrocytes (EAAT1 and EAAT2) and neurons (EAAT3). These transporters presumably assist in keeping the glutamate concentration low in the extracellular fluid of brain. Glutamate transporters (EAAT1, EAAT2 and EAAT3) at the BBB determine the levels of brain extracellular glutamate and are essential to prevent excitotoxicity,114 prompting the question whether changes in these transporters may contribute to glutamate excess and excitotoxicity. High affinity concentrative uptake of L-glutamate occurs from the brain extracellular fluid into the capillary ECs. The mechanisms in between L-glutamate uptake in the ECs and L-glutamate appearing in the blood may involve a luminal transporter for L-glutamate, metabolism of L-glutamate and transport of metabolites or a combination of the two.115 Furthermore, Na(+)-dependent glutamate transport was described on the abluminal membrane of the BBB116 that help in lowering brain extracellular glutamate concentrations.117 The Na+-dependent cotransporters of the abluminal membrane are in a position to actively transport amino acids from the extracellular fluid into the ECs of the BBB. These active transporters couple with the energy of the Na+-gradient to move glutamate and glutamine into ECs, whereupon glutamate can exit to the blood on the luminal facilitative glutamate transporter. Glutamine may also exit the brain via separate facilitative transport system that exists on the luminal membranes, or glutamine can be hydrolysed to glutamate within the BBB, thereby releasing ammonia that is freely diffusible. The γ-glutamyl cycle participates indirectly by producing oxoproline (pyroglutamate), which stimulates almost all secondary active transporters yet discovered in the abluminal membranes of the BBB.
Glucose transporters
Also facilitative glucose transport in the brain is affected in different pathophysiological conditions including Alzheimer's disease.118 Protein expression of the glucose transporter GLUT1 is reduced in brain capillaries in Alzheimer's disease, without changes in GLUT1 mRNA structure119 or levels of GLUT1 mRNA transcripts.120 Furthermore, a reduction in CNS energy metabolites has been seen in several Positron Emission Tomography (PET) scanning studies of Alzheimer's patients using fluoro-deoxy-glucose (FDG),121–123 likely because the surface area at the BBB available for glucose transport is substantially reduced in Alzheimer's disease.120,124
Furthermore, GLUT1 deficiency in mice overexpressing amyloid β-peptide precursor protein leads to early cerebral microvascular degeneration, blood flow reductions and dysregulation and BBB breakdown, and to accelerated amyloid β-peptide pathology, reduced amyloid β clearance, diminished neuronal activity, behavioural deficits and progressive neuronal loss and neurodegeneration that develop after initial cerebrovascular degenerative changes. Moreover, GLUT1 deficiency in endothelium, but not in astrocytes, initiates the vascular phenotype as shown by BBB breakdown. This indicates that reduced BBB GLUT1 expression worsens Alzheimer's disease cerebrovascular degeneration, neuropathology and cognitive function.125
Possible relationships between BBB dysfunction (measured by serum/CSF albumin index (Qalb) and IgG index) with glucose consumption as indicated by F-FDG PET studies in a selected population with by Alzheimer disease, indicated a significant negative correlation between the increase of serum/CSF albumin index and F-FDG uptake in the left superior temporal gyrus, with higher values of Qalb being related to a reduced glucose consumption in these areas. But, no significant relationships have been found between brain glucose consumption and IgG index. This suggest that BBB dysfunction is related to reduction of cortical activity in the left temporal cortex in AD subjects.126
Miscellaneous
MMP-2 and MMP-9
MMPs are a family of enzymes able to degrade components of the extracellular matrix, which is important for normal BBB function. MMP-2 and MMP-9 have been implicated in the physiological catabolism of Alzheimer's amyloid-β. Conversely, their association with vascular amyloid deposits, BBB disruption and haemorrhagic transformations after ischemic stroke also highlights their involvement in pathological processes.127 MMP function is regulated by tissue inhibitors of matrix metalloproteinases (TIMPs). Specifically, the metalloproteinases MMP-2 and MMP9 have been associated in BBB breakdown in neurodegenerative diseases, including Alzheimer's disease.128,129 Chemokines in the brain can recruit immune cells from the blood or from within the brain130 to secrete MMP-2 and MMP-9 that increase BBB permeability.131 Inhibition of this process is linked to more rapid disease progression.132 Their increasing prevalence with an increasing number of risk factors (hypertension, hyperlipidemia, diabetes, male sex and advanced age)133,134 and lower levels of TIMPs in Alzheimer's disease patients with microbleeds suggest less MMP inhibition in patients with concurrent cerebral microbleeds.128
APOE4 homozygosity
While it is well appreciated that APOE4 homozygosity is associated with an increased risk of sporadic Alzheimer's disease, its effects on the brain microvasculature and BBB have been less appreciated. Interestingly, APOE (4,4) is associated with thinning of the microvascular First easement membrane in Alzheimer's disease.94
First evidence that chronic intake of a high-fat diet induces a dramatic extravasation of immunoglobulins, indicating alterations in BBB functioning, in the brains of apoE-knockout mice, but not of C57Bl/6 control mice was provided by Mulder et al.135 Detoriation of the BBB was shown by IgG extravasation as well as for BBB transport of a small hydrophilic marker for BBB permeability (fluorescein). For apoE3-Leidenmice that were kept on a high-fat, high-cholesterol diet and that developed atherosclerosis to an extent similar to the apoE-knockoutmice, no signs of BBB disturbances were found, indicating the importance of ApoE type in the maintenance of the integrity of the BBB.
A recent study by Bell et al.94 suggested that CypA is a key target for treating APOE4-mediated neurovascular injury and the resulting neuronal dysfunction and degeneration; indeed, activating a proinflammatory CypA-nuclear factor-κB-MMP-9 pathway in pericytes is associated with increased susceptibility of the BBB to injury in APOE4 conditions.82
Cerebral microbleeds
Cerebral microbleeds as consequences of BBB breakdown in Alzheimer's disease are thought to represent cerebral amyloid angiopathy. Cerebral microbleeds are seen as possible predictors of intracerebral haemorrhage. It was found that the incidence of brain microbleeds positively correlates with age.133,134
BBB in multiple sclerosis
Formation of multiple sclerosis focal lesions follows extravasation of activated leukocytes from blood through the BBB into the CNS. Once the activated leukocytes enter the CNS environment, they propagate massive destruction to finally result in the loss of both myelin/oligodendrocyte complex and neurodegeneration. Also, the activated leukocytes locally release inflammatory cytokines and chemokines leading to focal immune activation of the brain ECs, and loss of the normal functioning of the BBB.136,137 While peripheral blood leukocyte infiltration plays an essential role in lesion development, there is also evidence suggesting that BBB dysfunction precedes immune cell infiltration. Recent evidence suggest that immune-mediated activation (or damage) of the various BBB cellular components significantly contributes to lesion development and progression.138,139 Chemokines seem to play an important role in the cascade of leukocyte extravasation. Chemokines displayed along the endothelial lumen bind chemokine receptors on circulating leukocytes, initiating intracellular signalling that culminates in integrin activation, leukocyte arrest and extravasation.140
TJs
Increased permeability of the BBB is associated with decreased expression of TJ and AJ proteins in the brain capillary ECs. Dephosphorylation of occludin in a multiple sclerosis mouse model precedes visible signs of disease, before changes in the BBB permeability were observed.141 Tight junctional abnormalities are most common in active lesions, but are present in inactive lesions in normal-appearing white matter. Tight junctional abnormalities are positively associated with leakage of the serum protein fibrinogen, which has recently been shown to be an activator of microglia.139,142
MMPs
Also multiple sclerosis MMPs seem to have implications,86,87 as increased activity of MMPs may attack the TJ protein occludin. An increase in MMP-9 activity has been demonstrated at sites of BBB disruption showing leukocyte infiltration. Moreover, the timing of MMP activity in pial and parenchymal vessels correlated with the timing of increased BBB permeability.142
Active transporters
P-glycoprotein (p-gp), multidrug resistance related protein-1 (MRP1), breast cancer resistance protein (BCRP)
P-gp functionality seems to be impaired in neuroinflammation and may play a role in immunomodulation.143,144 P-gp expression and function are strongly decreased during neuroinflammation. In vivo, the expression and function of brain endothelial P-gp in experimental allergic encephalomyelitis (EAE), an animal model for multiple sclerosis, were significantly impaired. Strikingly, vascular P-gp expression was decreased in both multiple sclerosis and EAE lesions and its disappearance coincided with the presence of perivascular infiltrates consisting of lymphocytes.143
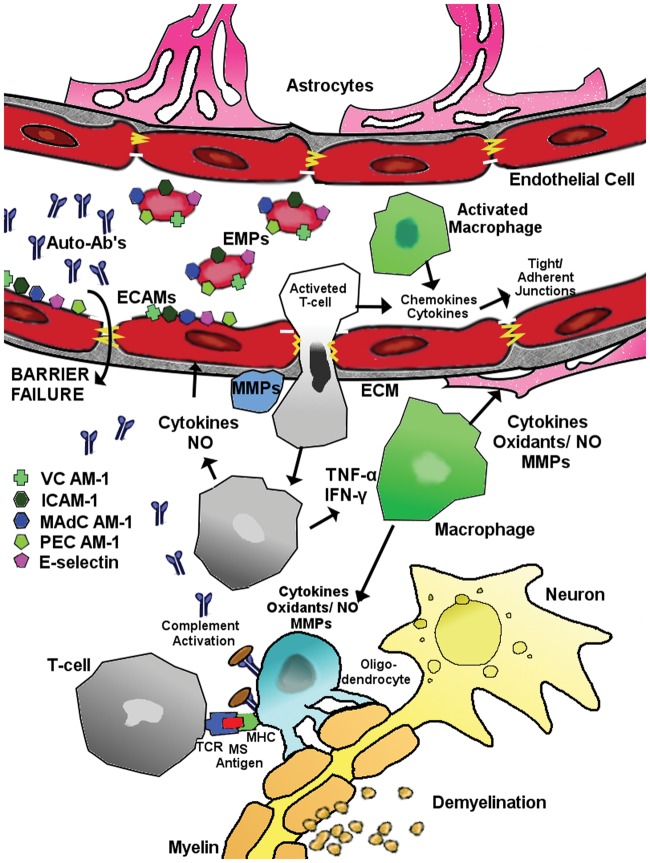
Cerebrovascular expression of P-glycoprotein was decreased in both active and chronic inactive multiple sclerosis lesions. Moreover, foamy macrophages in active multiple sclerosis lesions showed enhanced expression of MRP-1 and BCRP.145 Also, CD8(+) T lymphocyte trafficking into the brain is dependent on the activity of P-gp146 (Figure 6).
Figure 6.
Possible mechanisms leading to BBB disruption in MS. For explanation, please see the main text (modified from Minagar and Alexander147).
Transendothelial migration of immune cells
Following exposure of genetically susceptible individual to as yet unknown environmental factor(s), leukocytes are activated against CNS antigens. Activated leukocytes, mainly CD4 T lymphocytes and macrophages set in motion cerebral endothelial activation and injury by releasing proinflammatory cytokines (e.g. IFN-g and TNF-a) and chemokines. Expression of endothelial cell adhesion molecules (ECAMs) such as VCAM-1, ICAM-1, MAdCAM-1, E-selectin and PECAM-1 by activated cerebral ECs is upregulated. Some ECAMs are shed as vesicles from endothelial membranes carrying ECAMs from the parent EC known as ‘endothelial microparticles’ (EMP). The molecular organization of the TJs/AJs of the BBB is also possibly altered by effects of proinflammatory cytokines (particularly IFN-g) on expression of occludin and other tight junctional elements. Activated leukocytes also secrete MMPs, which may promote transendothelial migration of these cells into the CNS by degrading extracellular matrix macromolecules. Binding of putative multiple sclerosis antigen(s) within the CNS to CD4 T lymphocyte receptor and MHC (trimolecular complex) further promotes the inflammatory cascade against bound antigen(s).
MicroRNAs (miRNAs)
While the complex network of molecular players that leads to BBB dysfunction in multiple sclerosis is yet to be fully elucidated, recent studies indicated a critical role for miRNAs in controlling the function of the barrier endothelium in the brain.148 Emerging evidence that indicates that brain endothelial miRNAs regulate barrier function and orchestrate various phases of the neuroinflammatory response, including endothelial activation in response to cytokines as well as restoration of inflamed endothelium into a quiescent state.108
BBB in Parkinson’s disease
Using histologic markers of serum protein, iron and erythrocyte extravasation, it has been shown significantly increased permeability of the BBB in part of the caudate putamen of PD patients.149
It has been established that the process of reactive gliosis is a common feature of astrocytes during BBB disruption, which may have implications of astrocyte functions in the protection of the BBB, and in the development of PD.150 It seems that alterations in BBB permeability can also be reflected by changes in CSF/blood albumin, although changes in CSF turnover might also be responsible for such findings. Significant differences in albumin CSF/serum ratios (AR), based on samples obtained from non-demented subjects with idiopathic PD and age-matched control subjects, were found between patients with advanced disease, and both early-stage and unaffected groups. Conversely, early-phase patients did not differ from healthy subjects. Additionally, dopaminergic treatment seems to exert a possible effect on AR values. This indicates possible dysfunction of the BBB (and/or blood-CSF-barrier) in PD progression.151
Tight junctions
In a intracerebral rotenone model of PD in rats, using fluorescein as tight junctional BBB permeability marker, no changes were detected.106
Active transporters
P-glycoprotein
Brain distribution studies of paradigm compounds selected based upon their differential transport across the BBB (l-3,4-dihydroxyphenylalanine, carbamazepine, quinidine, lovastatin and simvastatin) was studied in healthy and MPTP-treated macaques. Only changes in brain distribution of Quinidine were found, indicating changes in P-gp functionality.152
In contrast, studies performed by Hou et al.153 suggest that P-gp inhibition increases BBB permeability to N-[2-(4-hydroxy-phenyl)-ethyl]-2-(2,5-dimethoxy-phenyl)-3-(3-methoxy-4-hydroxy-phenyl)-acrylamide (FLZ), a novel synthetic squamosamide derivative and potential anti-PD agent. But, no significant differences were observed in the brain distribution of FLZ between normal and PD model rats, suggesting no significant change in P-gp in PD.153
Bartels et al.154,155 investigated in vivo BBB P-gp function in patients with parkinsonian neurodegenerative syndromes, using [11C]-verapamil PET in PD patients. Advanced PD patients had increased [11C]-verapamil uptake in frontal white matter regions compared with controls; while de novo PD patients. The authors concluded that lower [11C]-verapamil uptake in midbrain and frontal regions of de novo PD patients could indicate a regional up-regulation of P-gp function.154
However, in a later study of this group, the decreased BBB P-gp function in early stage PD patients could not be confirmed.155
LRP1
Alpha-synuclein (α-Syn), being one of the dominant proteins found in Lewy Bodies in brains of PD, has been found in body fluids, including blood and CSF, and is likely produced by both peripheral tissues and the CNS. Radioactively labelled α-Syn has been found to cross the BBB in both the brain-to-blood and the blood-to-brain directions at rates consistent with saturable mechanisms. LRP-1 (but not P-gp) seems to be involved in α-Syn efflux.156,157
Large neutral amino acid transporter (LNAA)
BBB transport of L-DOPA transport in conjunction with its intra-brain conversion was studied in both control and diseased cerebral hemispheres in the unilateral rat rotenone model of PD. PD-like pathology, indicated by a huge reduction of tyrosine hydroxylase as well as by substantially reduced levels and higher elimination rates of DOPAC and HVA, does not result in changes in BBB transport of L-DOPA.158
MMPs
Like for Alzheimers disease and multiple sclerosis, MMPs seem to have implications in PD,86,87 and are associated in neurodegeneration of dopaminergic neurons.86
Altogether it can be concluded that there is much controversy on the role of the BBB in PD.
BBB in pharmacoresistant epilepsy
Older studies already indicated that seizures induce BBB transport changes.159,160 Focal epilepsies are often associated with BBB leakage. For example, BBB leakage to albumin-bound Evans blue has been found in PTZ induced epilepsy, with the location and pattern depending on the rat strain.161
When a normal brain develops epilepsy (epileptogenesis), immunoglobulin G (IgG) leakage across the BBB and neuronal IgG uptake increase concomitantly with the occurrence of seizures. IgG-positive neurons show signs of neurodegeneration, such as shrinkage and eosinophilia. This may suggest that IgG leakage across the BBB is related to neuronal impairment and may be a pathogenic mechanism in epileptogenesis and chronic epilepsy.162,163 The information on pharmacoresistant epilepsy associated changes in the BBB are relatively sparse.
Tight junctions
Claudin-8
Selective downregulation of claudin-8 by kindling epilepsy164 suggests that selective modulation of claudin expression in response to abnormal neuronal synchronization may lead to BBB breakdown and brain oedema.
Transport systems
Facilitative glucose transport
Facilitative glucose transport in the brain is affected in different pathophysiological conditions including epilepsy. It has been shown that GLUT1 mediates BBB transport of some neuroactive drugs, such as glycosylated neuropeptides, low molecular weight heparin and D-glucose derivatives.118
P-glycoprotein
A role for ABC transporters in the pathogenesis and treatment of pharmacoresisant epilepsy has been proposed.165–167 A positive association between the polymorphism in the MDR1 gene encoding P-gp (/ABCB1) and pharmacoresistant epilepsy has been reported in a subset of epilepsy patients.168 However, the follow-up association genetics studies did not support a major role for this polymorphism.169,170 Then, an increased expression of P-gp at the BBB has been reported, which was determined in epileptogenic brain tissue of patients with pharmacoresistant epilepsy171 as well as in rodent models of temporal lobe epilepsy, including the pilocarpine model. Other studies point to a profound role of seizure-induced neuronal cyclooxygenase-2 (COX-2) expression in neuropathologies that accompany epileptogenesis172 and it is thought that epileptic seizures drive expression of the BBB efflux transporter P-gp via a glutamate/COX-2 mediated signalling pathway.
Discussion and outlook
In summary, this review highlights several important key points of the disruption of BBB during the process of aging. First, the main players of the barrier formation at the blood-brain interface are introduced. Second, the possible multifactorial nature of maturation of BBB and NVU during embryonic development and postnatal period is presented. Then the changes in phenotypes and functionality in physiological aging and the consequent neurological impairment are summarized. Third, the review focuses on the cellular and molecular events and associated cerebrovascular factors contributing pathologically to different neurodegenerative disorders. Although the timing of disease symptoms and progression is very important in e.g. Alzheimer's disease, often patients present with significant tau and Aβ load by the time the symptoms begin. Given the contributions of vascular impairments at certain points during the disease process, it is important to investigate the vascular pathology and BBB permeability conditions before the disease becomes too severe to reverse. Finally, it is clear that the majority of dementia types involve an underlying small-vessel disease or cerebrovascular/BBB dysfunction at some points during disease progression. However, the causality dilemma still exists in identifying the circular cause and consequences between vascular dysfunction and the clinical signs of different dementias and age associated neurodegenerative processes, which is an important exploratory topic of future research.
The recognition of the role of efflux and uptake transporters in the pathology of Alzheimer's and PDs and many other CNS diseases might offer the avenue for new therapeutic intervention strategies for the pharmacological and clinical drug research for the therapy of currently untreatable chronic neurodegenerative disorders.
Supplementary Material
Acknowledgements
The authors thank the Faculty of Information Technology, Bionics, Pázmány Péter Catholic University, Budapest for the support of the publication costs of this article. Furthermore, special thanks to Tímea Rosta for editing the list of references and Szimonetta Tamás, Csaba Kriston and Dávid Berkecz for the preparation of the figures.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors' contributions
Franciska Erdő (structure of the BBB; changes in the BBB with physiological aging) 40%; László Denes (BBB during embryonic development; BBB in postnatal period) 20%; Elizabeth de Lange (changes at the BBB in connection with age-related pathologies) 40%.
Supplementary material
Supplementary material for this paper can be found at http://jcbfm.sagepub.com/content/by/supplemental-data
References
- 1.Rascher G, Wolburg H. The blood-brain barrier in the aging brain. In: Vellis JSd. (ed). Neuroglia in the aging brain, Totowa, NJ: Humana Press Inc., 2002, pp. 305–320. . [Google Scholar]
- 2.Clarke E, O'Malley CD. The human brain and spinal cord. A historical study illustrated by writings from antiquity to the twentieth century, Berkeley: University of California Press, 1968. [Google Scholar]
- 3.Alavijeh MS, Chishty M, Qaiser MZ, et al. Drug metabolism and pharmacokinetics, the blood-brain barrier, and central nervous system drug discovery. NeuroRx 2005; 2: 554–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis 2004; 16: 1–13. [DOI] [PubMed] [Google Scholar]
- 5.Abbott NJ. Dynamics of CNS barriers: evolution, differentiation, and modulation. Cell Mol Neurobiol 2005; 25: 5–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mann GE, Zlokovic BV, Yudilevich DL. Evidence for a lactate transport system in the sarcolemmal membrane of the perfused rabbit heart: kinetics of unidirectional influx, carrier specificity and effects of glucagon. Biochim Biophys Acta 1985; 819: 241–248. [DOI] [PubMed] [Google Scholar]
- 7.Zlokovic BV, Segal MB, Begley DJ, et al. Permeability of the blood-cerebrospinal fluid and blood-brain barriers to thyrotropin-releasing hormone. Brain Res 1985; 358: 191–199. [DOI] [PubMed] [Google Scholar]
- 8.Zlokovic BV, Begley DJ, Chain-Eliash DG. Blood-brain barrier permeability to leucine-enkephalin, D-alanine2-D-leucine5-enkephalin and their N-terminal amino acid (tyrosine). Brain Res 1985; 336: 125–132. [DOI] [PubMed] [Google Scholar]
- 9.Zlokovic BV, Apuzzo ML. Cellular and molecular neurosurgery: pathways from concept to reality–part I: target disorders and concept approaches to gene therapy of the central nervous system. Neurosurgery 1997; 40: 789–803. discussion 803–804. [DOI] [PubMed] [Google Scholar]
- 10.Zlokovic BV, Lipovac MN, Begley DJ, et al. Transport of leucine-enkephalin across the blood-brain barrier in the perfused guinea pig brain. J Neurochem 1987; 49: 310–315. [DOI] [PubMed] [Google Scholar]
- 11.Zlokovic BV, Hyman S, McComb JG, et al. Kinetics of arginine-vasopressin uptake at the blood-brain barrier. Biochim Biophys Acta 1990; 1025: 191–198. [DOI] [PubMed] [Google Scholar]
- 12.Zlokovic BV. Cerebrovascular permeability to peptides: manipulations of transport systems at the blood-brain barrier. Pharm Res 1995; 12: 1395–1406. [DOI] [PubMed] [Google Scholar]
- 13.Nicola Fletcher, http://www.hcvpi.bham.ac.uk/staff/NF.html (2015, accessed 1 November 2016).
- 14.Oldendorf WH, Cornford ME, Brown WJ. The large apparent work capability of the blood-brain barrier: a study of the mitochondrial content of capillary endothelial cells in brain and other tissues of the rat. Ann Neurol 1977; 1: 409–417. [DOI] [PubMed] [Google Scholar]
- 15.Sanchez-Covarrubias L, Slosky LM, Thompson BJ, et al. Transporters at CNS barrier sites: obstacles or opportunities for drug delivery? Curr Pharm Des 2014; 20: 1422–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takakura Y, Audus KL, Borchardt RT. Blood-brain barrier: transport studies in isolated brain capillaries and in cultured brain endothelial cells. Adv Pharmacol 1991; 22: 137–165. [DOI] [PubMed] [Google Scholar]
- 17.Fenstermacher J, Gross P, Sposito N, et al. Structural and functional variations in capillary systems within the brain. Ann NY Acad Sci 1988; 529: 21–30. [DOI] [PubMed] [Google Scholar]
- 18.Grammas P, Martinez J, Miller B. Cerebral microvascular endothelium and the pathogenesis of neurodegenerative diseases. Expert Rev Mol Med 2011; 13: e19. [DOI] [PubMed] [Google Scholar]
- 19.Betz AL, Firth JA, Goldstein GW. Polarity of the blood-brain barrier: distribution of enzymes between the luminal and antiluminal membranes of brain capillary endothelial cells. Brain Res 1980; 192: 17–28. [DOI] [PubMed] [Google Scholar]
- 20.Sanchez del Pino MM, Peterson DR, Hawkins RA. Neutral amino acid transport characterization of isolated luminal and abluminal membranes of the blood-brain barrier. J Biol Chem 1995; 270: 14913–14918. [DOI] [PubMed] [Google Scholar]
- 21.Vorbrodt AW, Dobrogowska DH. Molecular anatomy of intercellular junctions in brain endothelial and epithelial barriers: electron microscopist's view. Brain Res Brain Res Rev 2003; 42: 221–242. [DOI] [PubMed] [Google Scholar]
- 22.Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood-brain barrier. Nat Med 2013; 19: 1584–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bauer B, Hartz AM, Lucking JR, et al. Coordinated nuclear receptor regulation of the efflux transporter, Mrp2, and the phase-II metabolizing enzyme, GSTpi, at the blood-brain barrier. J Cereb Blood Flow Metab 2008; 28: 1222–1234. [DOI] [PubMed] [Google Scholar]
- 24.Ose A, Ito M, Kusuhara H, et al. Limited brain distribution of [3R,4R,5S]-4-acetamido-5-amino-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxylate phosphate (Ro 64-0802), a pharmacologically active form of oseltamivir, by active efflux across the blood-brain barrier mediated by organic anion transporter 3 (Oat3/Slc22a8) and multidrug resistance-associated protein 4 (Mrp4/Abcc4). Drug Metab Dispos 2009; 37: 315–321. [DOI] [PubMed] [Google Scholar]
- 25.Krajcsi P. Blood-brain-barrier, http://www.solvobiotech.com/barriers/blood-brain-barrier (2016, accessed 1 November 2016).
- 26.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev 2005; 57: 173–185. [DOI] [PubMed] [Google Scholar]
- 27.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 2008; 57: 178–201. [DOI] [PubMed] [Google Scholar]
- 28.Butt AM, Jones HC, Abbott NJ. Electrical resistance across the blood-brain barrier in anaesthetized rats: a developmental study. J Physiol 1990; 429: 47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Candiello J, Cole GJ, Halfter W. Age-dependent changes in the structure, composition and biophysical properties of a human basement membrane. Matrix Biol 2010; 29: 402–410. [DOI] [PubMed] [Google Scholar]
- 30.Ronaldson PT, Davis TP. Blood-brain barrier integrity and glial support: mechanisms that can be targeted for novel therapeutic approaches in stroke. Curr Pharm Des 2012; 18: 3624–3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raff MC, Abney ER, Cohen J, et al. Two types of astrocytes in cultures of developing rat white matter: differences in morphology, surface gangliosides, and growth characteristics. J Neurosci 1983; 3: 1289–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 2006; 7: 41–53. [DOI] [PubMed] [Google Scholar]
- 33.Kacem K, Lacombe P, Seylaz J, et al. Structural organization of the perivascular astrocyte endfeet and their relationship with the endothelial glucose transporter: a confocal microscopy study. Glia 1998; 23: 1–10. [PubMed] [Google Scholar]
- 34.Rodriguez-Arellano JJ, Parpura V, Zorec R, et al. Astrocytes in physiological aging and Alzheimer's disease. Neuroscience 2016; 323: 170–182. [DOI] [PubMed] [Google Scholar]
- 35.Yi JH, Hazell AS. Excitotoxic mechanisms and the role of astrocytic glutamate transporters in traumatic brain injury. Neurochem Int 2006; 48: 394–403. [DOI] [PubMed] [Google Scholar]
- 36.Wang H, Eckel RH. What are lipoproteins doing in the brain? Trends Endocrinol Metab 2014; 25: 8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harris JL, Choi IY, Brooks WM. Probing astrocyte metabolism in vivo: proton magnetic resonance spectroscopy in the injured and aging brain. Front Aging Neurosci 2015; 7: 202, . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chisholm NC, Sohrabji F. Astrocytic response to cerebral ischemia is influenced by sex differences and impaired by aging. Neurobiol Dis 2016; 85: 245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Middeldorp J, Hol EM. GFAP in health and disease. Prog Neurobiol 2011; 93: 421–443. [DOI] [PubMed] [Google Scholar]
- 40.Knox CA, Yates RD, Chen I, et al. Effects of aging on the structural and permeability characteristics of cerebrovasculature in normotensive and hypertensive strains of rats. Acta Neuropathol 1980; 51: 1–13. [DOI] [PubMed] [Google Scholar]
- 41.Peinado MA, Quesada A, Pedrosa JA, et al. Quantitative and ultrastructural changes in glia and pericytes in the parietal cortex of the aging rat. Microsc Res Tech 1998; 43: 34–42. [DOI] [PubMed] [Google Scholar]
- 42.Peters A, Josephson K, Vincent SL. Effects of aging on the neuroglial cells and pericytes within area 17 of the rhesus monkey cerebral cortex. Anat Rec 1991; 229: 384–398. [DOI] [PubMed] [Google Scholar]
- 43.Tigges J, Herndon JG, Rosene DL. Mild age-related changes in the dentate gyrus of adult rhesus monkeys. Acta Anat (Basel) 1995; 153: 39–48. [DOI] [PubMed] [Google Scholar]
- 44.Hicks P, Rolsten C, Brizzee D, et al. Age-related changes in rat brain capillaries. Neurobiol Aging 1983; 4: 69–75. [DOI] [PubMed] [Google Scholar]
- 45.Sturrock RR. A comparative quantitative and morphological study of ageing in the mouse neostriatum, indusium griseum and anterior commissure. Neuropathol Appl Neurobiol 1980; 6: 51–68. [DOI] [PubMed] [Google Scholar]
- 46.Ueno M, Akiguchi I, Hosokawa M, et al. Ultrastructural and permeability features of microvessels in the olfactory bulbs of SAM mice. Acta Neuropathol 1998; 96: 261–270. [DOI] [PubMed] [Google Scholar]
- 47.de Jong GI, Horvath E, Luiten PG. Effects of early onset of nimodipine treatment on microvascular integrity in the aging rat brain. Stroke 1990; 21(12 Suppl): IV113–IV116. [PubMed] [Google Scholar]
- 48.Stewart PA, Magliocco M, Hayakawa K, et al. A quantitative analysis of blood-brain barrier ultrastructure in the aging human. Microvasc Res 1987; 33: 270–282. [DOI] [PubMed] [Google Scholar]
- 49.Burns EM, Kruckeberg TW, Gaetano PK. Changes with age in cerebral capillary morphology. Neurobiol Aging 1981; 2: 283–291. [DOI] [PubMed] [Google Scholar]
- 50.Burns EM, Kruckeberg TW, Gaetano PK, et al. Morphological changes in cerebral capillaries with age. In: Cervos-Navarro J, Sarkander H-I. (eds). Brain Aging: Neuropathology and neuroharmacology, New York: Raven Press, 1983, pp. 115–132, . [Google Scholar]
- 51.Heinsen H, Heinsen YL. Cerebellar capillaries. Qualitative and quantitative observations in young and senile rats. Anat Embryol (Berl) 1983; 168: 101–116. [DOI] [PubMed] [Google Scholar]
- 52.Scheibel AB, Fried I. Age-related changes in the peri-capillary environment of the brain. In: Alger SD, Gershon A, Grimm S, Toffano VE. (eds). Aging of the brain 1983; Vol 22, New York: Raven Press. [Google Scholar]
- 53.Benjamin LE, Hemo I, Keshet E. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development 1998; 125: 1591–1598. [DOI] [PubMed] [Google Scholar]
- 54.Li J, Zhang M, Rui YC. Tumor necrosis factor mediated release of platelet-derived growth factor from bovine cerebral microvascular endothelial cells. Zhongguo Yao Li Xue Bao 1997; 18: 133–136. [PubMed] [Google Scholar]
- 55.Lindahl P, Johansson BR, Leveen P, et al. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science 1997; 277: 242–245. [DOI] [PubMed] [Google Scholar]
- 56.Montagne A, Barnes SR, Sweeney MD, et al. Blood-brain barrier breakdown in the aging human hippocampus. Neuron 2015; 85: 296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sengillo JD, Winkler EA, Walker CT, et al. Deficiency in mural vascular cells coincides with blood-brain barrier disruption in Alzheimer's disease. Brain Pathol 2013; 23: 303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harry GJ. Microglia during development and aging. Pharmacol Ther 2013; 139: 313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kettenmann H, Hanisch UK, Noda M, et al. Physiology of microglia. Physiol Rev 2011; 91: 461–553. [DOI] [PubMed] [Google Scholar]
- 60.Kofler J, Wiley CA. Microglia: key innate immune cells of the brain. Toxicol Pathol 2011; 39: 103–114. [DOI] [PubMed] [Google Scholar]
- 61.Huber JD, Campos CR, Mark KS, et al. Alterations in blood-brain barrier ICAM-1 expression and brain microglial activation after lambda-carrageenan-induced inflammatory pain. Am J Physiol Heart Circ Physiol 2006; 290: H732–H740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ben-Menachem E, Johansson BB, Svensson TH. Increased vulnerability of the blood-brain barrier to acute hypertension following depletion of brain noradrenaline. J Neural Transm 1982; 53: 159–167. [DOI] [PubMed] [Google Scholar]
- 63.Cohen Z, Molinatti G, Hamel E. Astroglial and vascular interactions of noradrenaline terminals in the rat cerebral cortex. J Cereb Blood Flow Metab 1997; 17: 894–904. [DOI] [PubMed] [Google Scholar]
- 64.Cohen Z, Bonvento G, Lacombe P, et al. Serotonin in the regulation of brain microcirculation. Prog Neurobiol 1996; 50: 335–362. [DOI] [PubMed] [Google Scholar]
- 65.Tong XK, Hamel E. Regional cholinergic denervation of cortical microvessels and nitric oxide synthase-containing neurons in Alzheimer's disease. Neuroscience 1999; 92: 163–175. [DOI] [PubMed] [Google Scholar]
- 66.Vaucher E, Hamel E. Cholinergic basal forebrain neurons project to cortical microvessels in the rat: electron microscopic study with anterogradely transported Phaseolus vulgaris leucoagglutinin and choline acetyltransferase immunocytochemistry. J Neurosci 1995; 15: 7427–7441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vaucher E, Tong XK, Cholet N, et al. GABA neurons provide a rich input to microvessels but not nitric oxide neurons in the rat cerebral cortex: a means for direct regulation of local cerebral blood flow. J Comp Neurol 2000; 421: 161–171. [PubMed] [Google Scholar]
- 68.Berezowski V, Landry C, Dehouck MP, et al. Contribution of glial cells and pericytes to the mRNA profiles of P-glycoprotein and multidrug resistance-associated proteins in an in vitro model of the blood-brain barrier. Brain Res 2004; 1018: 1–9. [DOI] [PubMed] [Google Scholar]
- 69.Blau CW, Cowley TR, O'Sullivan J, et al. The age-related deficit in LTP is associated with changes in perfusion and blood-brain barrier permeability. Neurobiol Aging 2012; 33: 1005 e23–e35. [DOI] [PubMed] [Google Scholar]
- 70.Biernacki K, Prat A, Blain M, et al. Regulation of Th1 and Th2 lymphocyte migration by human adult brain endothelial cells. J Neuropathol Exp Neurol 2001; 60: 1127–1136. [DOI] [PubMed] [Google Scholar]
- 71.Elahy M, Jackaman C, Mamo JC, et al. Blood-brain barrier dysfunction developed during normal aging is associated with inflammation and loss of tight junctions but not with leukocyte recruitment. Immun Ageing 2015; 12: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hunziker O, Abdel'Al S, Frey H, et al. Quantitative studies in the cerebral cortex of aging humans. Gerontology 1978; 24: 27–31. [DOI] [PubMed] [Google Scholar]
- 73.Enciu AM, Gherghiceanu M, Popescu BO. Triggers and effectors of oxidative stress at blood-brain barrier level: relevance for brain ageing and neurodegeneration. Oxid Med Cell Longev 2013; 2013: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ravens JR. Vascular changes in the human senile brain. Adv Neurol 1978; 20: 487–501. [PubMed] [Google Scholar]
- 75.Lucke-Wold BP, Logsdon AF, Turner RC, et al. Aging, the metabolic syndrome, and ischemic stroke: redefining the approach for studying the blood-brain barrier in a complex neurological disease. Adv Pharmacol 2014; 71: 411–449. . [DOI] [PubMed] [Google Scholar]
- 76.Cerbai F, Lana D, Nosi D, et al. The neuron-astrocyte-microglia triad in normal brain ageing and in a model of neuroinflammation in the rat hippocampus. PLoS One 2012; 7: e45250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Buschini E, Piras A, Nuzzi R, et al. Age related macular degeneration and drusen: neuroinflammation in the retina. Prog Neurobiol 2011; 95: 14–25. [DOI] [PubMed] [Google Scholar]
- 78.Zlokovic BV. Neurodegeneration and the neurovascular unit. Nat Med 2010; 16: 1370–1371. [DOI] [PubMed] [Google Scholar]
- 79.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat Rev Neurosci 2011; 12: 723–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Freeman LR, Keller JN. Oxidative stress and cerebral endothelial cells: regulation of the blood-brain-barrier and antioxidant based interventions. Biochim Biophys Acta 2012; 1822: 822–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Al Ahmad A, Gassmann M, Ogunshola OO. Involvement of oxidative stress in hypoxia-induced blood-brain barrier breakdown. Microvasc Res 2012; 84: 222–225. [DOI] [PubMed] [Google Scholar]
- 82.Zhao Z, Nelson AR, Betsholtz C, et al. Establishment and dysfunction of the blood-brain barrier. Cell 2015; 163: 1064–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Miyakawa T. Vascular pathology in Alzheimer's disease. Psychogeriatrics 2010; 10: 39–44. [DOI] [PubMed] [Google Scholar]
- 84.Baloyannis SJ. Brain capillaries in Alzheimer's disease. Hell J Nucl Med 2015; 18(Suppl 1): 152. [PubMed] [Google Scholar]
- 85.Nelson AR, Sweeney MD, Sagare AP, et al. Neurovascular dysfunction and neurodegeneration in dementia and Alzheimer's disease. Biochim Biophys Acta 2016; 1862: 887–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rosenberg GA, Yang Y. Vasogenic edema due to tight junction disruption by matrix metalloproteinases in cerebral ischemia. Neurosurg Focus 2007; 22: E4. [DOI] [PubMed] [Google Scholar]
- 87.Yang Y, Rosenberg GA. MMP-mediated disruption of claudin-5 in the blood-brain barrier of rat brain after cerebral ischemia. Methods Mol Biol 2011; 762: 333–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fulga TA, Elson-Schwab I, Khurana V, et al. Abnormal bundling and accumulation of F-actin mediates tau-induced neuronal degeneration in vivo. Nat Cell Biol 2007; 9: 139–148. [DOI] [PubMed] [Google Scholar]
- 89.Wan W, Chen H, Li Y. The potential mechanisms of Abeta-receptor for advanced glycation end-products interaction disrupting tight junctions of the blood-brain barrier in Alzheimer's disease. Int J Neurosci 2014; 124: 75–81. [DOI] [PubMed] [Google Scholar]
- 90.Takano T, Tian GF, Peng W, et al. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci 2006; 9: 260–267. [DOI] [PubMed] [Google Scholar]
- 91.Yang J, Lunde LK, Nuntagij P, et al. Loss of astrocyte polarization in the tg-ArcSwe mouse model of Alzheimer's disease. J Alzheimers Dis 2011; 27: 711–722. [DOI] [PubMed] [Google Scholar]
- 92.Bell RD, Zlokovic BV. Neurovascular mechanisms and blood-brain barrier disorder in Alzheimer's disease. Acta Neuropathol 2009; 118: 103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Winkler EA, Sagare AP, Zlokovic BV. The pericyte: a forgotten cell type with important implications for Alzheimer's disease? Brain Pathol 2014; 24: 371–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bell RD, Winkler EA, Singh I, et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature 2012; 485: 512–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Baloyannis SJ, Baloyannis IS. The vascular factor in Alzheimer's disease: a study in Golgi technique and electron microscopy. J Neurol Sci 2012; 322: 117–121. [DOI] [PubMed] [Google Scholar]
- 96.Halliday MR, Rege SV, Ma Q, et al. Accelerated pericyte degeneration and blood-brain barrier breakdown in apolipoprotein E4 carriers with Alzheimer's disease. J Cereb Blood Flow Metab 2016; 36: 216–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Saint-Pol J, Vandenhaute E, Boucau MC, et al. Brain pericytes ABCA1 expression mediates cholesterol efflux but not cellular amyloid-beta peptide accumulation. J Alzheimers Dis 2012; 30: 489–503. [DOI] [PubMed] [Google Scholar]
- 98.Shimizu F, Sano Y, Saito K, et al. Pericyte-derived glial cell line-derived neurotrophic factor increase the expression of claudin-5 in the blood-brain barrier and the blood-nerve barrier. Neurochem Res 2012; 37: 401–409. [DOI] [PubMed] [Google Scholar]
- 99.Dalkara T, Gursoy-Ozdemir Y, Yemisci M. Brain microvascular pericytes in health and disease. Acta Neuropathol 2011; 122: 1–9. [DOI] [PubMed] [Google Scholar]
- 100.Kuhnke D, Jedlitschky G, Grube M, et al. MDR1-P-glycoprotein (ABCB1) mediates transport of Alzheimer's amyloid-beta peptides–implications for the mechanisms of Abeta clearance at the blood-brain barrier. Brain Pathol 2007; 17: 347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Brenn A, Grube M, Peters M, et al. Beta-amyloid downregulates MDR1-P-glycoprotein (Abcb1) expression at the blood-brain barrier in mice. Int J Alzheimers Dis 2011; 2011: 690121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sharma HS, Castellani RJ, Smith MA, et al. The blood-brain barrier in Alzheimer's disease: novel therapeutic targets and nanodrug delivery. Int Rev Neurobiol 2012; 102: 47–90. [DOI] [PubMed] [Google Scholar]
- 103.Sagare AP, Deane R, Zlokovic BV. Low-density lipoprotein receptor-related protein 1: a physiological Abeta homeostatic mechanism with multiple therapeutic opportunities. Pharmacol Ther 2012; 136: 94–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vogelgesang S, Jedlitschky G, Brenn A, et al. The role of the ATP-binding cassette transporter P-glycoprotein in the transport of beta-amyloid across the blood-brain barrier. Curr Pharm Des 2011; 17: 2778–2786. [DOI] [PubMed] [Google Scholar]
- 105.Hartz AM, Miller DS, Bauer B. Restoring blood-brain barrier P-glycoprotein reduces brain amyloid-beta in a mouse model of Alzheimer's disease. Mol Pharmacol 2010; 77: 715–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ravenstijn PG, Merlini M, Hameetman M, et al. The exploration of rotenone as a toxin for inducing Parkinson's disease in rats, for application in BBB transport and PK-PD experiments. J Pharmacol Toxicol Methods 2008; 57: 114–130. [DOI] [PubMed] [Google Scholar]
- 107.Hartz AM, Zhong Y, Wolf A, et al. Abeta40 reduces P-glycoprotein at the blood-brain barrier through the ubiquitin-proteasome pathway. J Neurosci 2016; 36: 1930–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lopez-Ramirez MA, Reijerkerk A, de Vries HE, et al. Regulation of brain endothelial barrier function by microRNAs in health and neuroinflammation. FASEB J 2016; 30: 2662–2672. [DOI] [PubMed] [Google Scholar]
- 109.Owen JB, Sultana R, Aluise CD, et al. Oxidative modification to LDL receptor-related protein 1 in hippocampus from subjects with Alzheimer disease: implications for Abeta accumulation in AD brain. Free Radic Biol Med 2010; 49: 1798–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Erickson MA, Hartvigson PE, Morofuji Y, et al. Lipopolysaccharide impairs amyloid beta efflux from brain: altered vascular sequestration, cerebrospinal fluid reabsorption, peripheral clearance and transporter function at the blood-brain barrier. J Neuroinflammation 2012; 9: 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bulbarelli A, Lonati E, Brambilla A, et al. Abeta42 production in brain capillary endothelial cells after oxygen and glucose deprivation. Mol Cell Neurosci 2012; 49: 415–422. [DOI] [PubMed] [Google Scholar]
- 112.Chun HS, Son JJ, Son JH. Identification of potential compounds promoting BDNF production in nigral dopaminergic neurons: clinical implication in Parkinson's disease. Neuroreport 2000; 11: 511–514. [DOI] [PubMed] [Google Scholar]
- 113.Kook SY, Hong HS, Moon M, et al. Abeta(1)(-)(4)(2)-RAGE interaction disrupts tight junctions of the blood-brain barrier via Ca(2)(+)-calcineurin signaling. J Neurosci 2012; 32: 8845–8854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lipton SA. The molecular basis of memantine action in Alzheimer's disease and other neurologic disorders: low-affinity, uncompetitive antagonism. Curr Alzheimer Res 2005; 2: 155–165. [DOI] [PubMed] [Google Scholar]
- 115.Cederberg HH, Uhd NC, Brodin B. Glutamate efflux at the blood-brain barrier: cellular mechanisms and potential clinical relevance. Arch Med Res 2014; 45: 639–645. [DOI] [PubMed] [Google Scholar]
- 116.O'Kane RL, Martinez-Lopez I, DeJoseph MR, et al. Na(+)-dependent glutamate transporters (EAAT1, EAAT2, and EAAT3) of the blood-brain barrier. A mechanism for glutamate removal. J Biol Chem 1999; 274: 31891–31895. [DOI] [PubMed] [Google Scholar]
- 117.Hawkins RA, Viña JR. How glutamate is managed by the blood-brain barrier. Biology 2016; 5: E37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Guo X, Geng M, Du G. Glucose transporter 1, distribution in the brain and in neural disorders: its relationship with transport of neuroactive drugs through the blood-brain barrier. Biochem Genet 2005; 43: 175–187. [DOI] [PubMed] [Google Scholar]
- 119.Mooradian AD, Chung HC, Shah GN. GLUT-1 expression in the cerebra of patients with Alzheimer's disease. Neurobiol Aging 1997; 18: 469–474. [DOI] [PubMed] [Google Scholar]
- 120.Wu Z, Guo H, Chow N, et al. Role of the MEOX2 homeobox gene in neurovascular dysfunction in Alzheimer disease. Nat Med 2005; 11: 959–965. [DOI] [PubMed] [Google Scholar]
- 121.Samuraki M, Matsunari I, Chen WP, et al. Partial volume effect-corrected FDG PET and grey matter volume loss in patients with mild Alzheimer's disease. Eur J Nucl Med Mol Imaging 2007; 34: 1658–1669. [DOI] [PubMed] [Google Scholar]
- 122.Mosconi L, De Santi S, Li J, et al. Hippocampal hypometabolism predicts cognitive decline from normal aging. Neurobiol Aging 2008; 29: 676–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mosconi L, Sorbi S, de Leon MJ, et al. Hypometabolism exceeds atrophy in presymptomatic early-onset familial Alzheimer's disease. J Nucl Med 2006; 47: 1778–1786. [PubMed] [Google Scholar]
- 124.Bailey TL, Rivara CB, Rocher AB, et al. The nature and effects of cortical microvascular pathology in aging and Alzheimer's disease. Neurol Res 2004; 26: 573–578. [DOI] [PubMed] [Google Scholar]
- 125.Winkler EA, Nishida Y, Sagare AP, et al. GLUT1 reductions exacerbate Alzheimer's disease vasculo-neuronal dysfunction and degeneration. Nat Neurosci 2015; 18: 521–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chiaravalloti A, Fiorentini A, Ursini F, et al. Is cerebral glucose metabolism related to blood-brain barrier dysfunction and intrathecal IgG synthesis in Alzheimer disease?: A 18F-FDG PET/CT study. Medicine (Baltimore) 2016; 95: e4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hernandez-Guillamon M, Mawhirt S, Blais S, et al. Sequential amyloid-beta degradation by the matrix metalloproteases MMP-2 and MMP-9. J Biol Chem 2015; 290: 15078–15091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Duits FH, Hernandez-Guillamon M, Montaner J, et al. Matrix metalloproteinases in Alzheimer's disease and concurrent cerebral microbleeds. J Alzheimers Dis 2015; 48: 711–720. [DOI] [PubMed] [Google Scholar]
- 129.Weekman EM, Wilcock DM. Matrix metalloproteinase in blood-brain barrier breakdown in dementia. J Alzheimers Dis 2015; 49: 893–903. [DOI] [PubMed] [Google Scholar]
- 130.Britschgi M, Wyss-Coray T. Immune cells may fend off Alzheimer disease. Nat Med 2007; 13: 408–409. [DOI] [PubMed] [Google Scholar]
- 131.Feng MR, Turluck D, Burleigh J, et al. Brain microdialysis and PK/PD correlation of pregabalin in rats. Eur J Drug Metab Pharmacokinet 2001; 26: 123–128. [DOI] [PubMed] [Google Scholar]
- 132.Dimitrijevic OB, Stamatovic SM, Keep RF, et al. Absence of the chemokine receptor CCR2 protects against cerebral ischemia/reperfusion injury in mice. Stroke 2007; 38: 1345–1353. [DOI] [PubMed] [Google Scholar]
- 133.Shams S, Martola J, Granberg T, et al. Cerebral microbleeds: different prevalence, topography, and risk factors depending on dementia diagnosis-the Karolinska Imaging Dementia Study. AJNR Am J Neuroradiol 2015; 36: 661–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yates PA, Desmond PM, Phal PM, et al. Incidence of cerebral microbleeds in preclinical Alzheimer disease. Neurology 2014; 82: 1266–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mulder M, Blokland A, van den Berg DJ, et al. Apolipoprotein E protects against neuropathology induced by a high-fat diet and maintains the integrity of the blood-brain barrier during aging. Lab Invest 2001; 81: 953–960. [DOI] [PubMed] [Google Scholar]
- 136.Bartholomaus I, Kawakami N, Odoardi F, et al. Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature 2009; 462: 94–98. [DOI] [PubMed] [Google Scholar]
- 137.Greenwood J, Heasman SJ, Alvarez JI, et al. Review: leucocyte-endothelial cell crosstalk at the blood-brain barrier: a prerequisite for successful immune cell entry to the brain. Neuropathol Appl Neurobiol 2011; 37: 24–39. [DOI] [PubMed] [Google Scholar]
- 138.Alvarez JI, Cayrol R, Prat A. Disruption of central nervous system barriers in multiple sclerosis. Biochim Biophys Acta 2011; 1812: 252–264. [DOI] [PubMed] [Google Scholar]
- 139.McQuaid S, Cunnea P, McMahon J, et al. The effects of blood-brain barrier disruption on glial cell function in multiple sclerosis. Biochem Soc Trans 2009; 37(Pt 1): 329–331. [DOI] [PubMed] [Google Scholar]
- 140.Holman DW, Klein RS, Ransohoff RM. The blood-brain barrier, chemokines and multiple sclerosis. Biochim Biophys Acta 2011; 1812: 220–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Morgan L, Shah B, Rivers LE, et al. Inflammation and dephosphorylation of the tight junction protein occludin in an experimental model of multiple sclerosis. Neuroscience 2007; 147: 664–673. [DOI] [PubMed] [Google Scholar]
- 142.Persidsky Y, Ramirez SH, Haorah J, et al. Blood-brain barrier: structural components and function under physiologic and pathologic conditions. J Neuroimmune Pharmacol 2006; 1: 223–236. [DOI] [PubMed] [Google Scholar]
- 143.Kooij G, van Horssen J, de Lange EC, et al. T lymphocytes impair P-glycoprotein function during neuroinflammation. J Autoimmun 2010; 34: 416–425. [DOI] [PubMed] [Google Scholar]
- 144.Kooij G, Backer R, Koning JJ, et al. P-glycoprotein acts as an immunomodulator during neuroinflammation. PLoS One 2009; 4: e8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kooij G, Mizee MR, van Horssen J, et al. Adenosine triphosphate-binding cassette transporters mediate chemokine (C-C motif) ligand 2 secretion from reactive astrocytes: relevance to multiple sclerosis pathogenesis. Brain 2011; 134(Pt 2): 555–570. [DOI] [PubMed] [Google Scholar]
- 146.Kooij G, Kroon J, Paul D, et al. P-glycoprotein regulates trafficking of CD8(+) T cells to the brain parenchyma. Acta Neuropathol 2014; 127: 699–711. [DOI] [PubMed] [Google Scholar]
- 147.Minagar A, Alexander JS. Blood-brain barrier disruption in multiple sclerosis. Mult Scler 2003; 9: 540–549. [DOI] [PubMed] [Google Scholar]
- 148.Kamphuis WW, Derada Troletti C, Reijerkerk A, et al. The blood-brain barrier in multiple sclerosis: microRNAs as key regulators. CNS Neurol Disord Drug Targets 2015; 14: 157–167. [DOI] [PubMed] [Google Scholar]
- 149.Gray MT, Woulfe JM. Striatal blood-brain barrier permeability in Parkinson's disease. J Cereb Blood Flow Metab 2015; 35: 747–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cabezas R, Avila M, Gonzalez J, et al. Astrocytic modulation of blood brain barrier: perspectives on Parkinson's disease. Front Cell Neurosci 2014; 8: 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Pisani V, Stefani A, Pierantozzi M, et al. Increased blood-cerebrospinal fluid transfer of albumin in advanced Parkinson's disease. J Neuroinflammation 2012; 9: 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Thiollier T, Wu C, Contamin H, et al. Permeability of blood-brain barrier in macaque model of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson disease. Synapse 2016; 70: 231–239. [DOI] [PubMed] [Google Scholar]
- 153.Hou J, Liu Q, Li Y, et al. An in vivo microdialysis study of FLZ penetration through the blood-brain barrier in normal and 6-hydroxydopamine induced Parkinson's disease model rats. Biomed Res Int 2014; 2014: 850493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bartels AL, Willemsen AT, Kortekaas R, et al. Decreased blood-brain barrier P-glycoprotein function in the progression of Parkinson's disease, PSP and MSA. J Neural Transm (Vienna) 2008; 115: 1001–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bartels AL, van Berckel BN, Lubberink M, et al. Blood-brain barrier P-glycoprotein function is not impaired in early Parkinson's disease. Parkinsonism Relat Disord 2008; 14: 505–508. [DOI] [PubMed] [Google Scholar]
- 156.Bates CA, Zheng W. Brain disposition of alpha-Synuclein: roles of brain barrier systems and implications for Parkinson's disease. Fluids Barriers CNS 2014; 11: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sui YT, Bullock KM, Erickson MA, et al. Alpha synuclein is transported into and out of the brain by the blood-brain barrier. Peptides 2014; 62: 197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ravenstijn PG, Drenth HJ, O'Neill MJ, et al. Evaluation of blood-brain barrier transport and CNS drug metabolism in diseased and control brain after intravenous L-DOPA in a unilateral rat model of Parkinson's disease. Fluids Barriers CNS 2012; 9: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sahin D, Ilbay G, Ates N. Changes in the blood-brain barrier permeability and in the brain tissue trace element concentrations after single and repeated pentylenetetrazole-induced seizures in rats. Pharmacol Res 2003; 48: 69–73. [PubMed] [Google Scholar]
- 160.Padou V, Boyet S, Nehlig A. Changes in transport of [14C] alpha-aminoisobutyric acid across the blood-brain barrier during pentylenetetrazol-induced status epilepticus in the immature rat. Epilepsy Res 1995; 22: 175–183. [DOI] [PubMed] [Google Scholar]
- 161.Ates N, Esen N, Ilbay G. Absence epilepsy and regional blood-brain barrier permeability: the effects of pentylenetetrazole-induced convulsions. Pharmacol Res 1999; 39: 305–310. [DOI] [PubMed] [Google Scholar]
- 162.Michalak Z, Lebrun A, Di Miceli M, et al. IgG leakage may contribute to neuronal dysfunction in drug-refractory epilepsies with blood-brain barrier disruption. J Neuropathol Exp Neurol 2012; 71: 826–838. [DOI] [PubMed] [Google Scholar]
- 163.Ndode-Ekane XE, Hayward N, Grohn O, et al. Vascular changes in epilepsy: functional consequences and association with network plasticity in pilocarpine-induced experimental epilepsy. Neuroscience 2010; 166: 312–332. [DOI] [PubMed] [Google Scholar]
- 164.Lamas M, Gonzalez-Mariscal L, Gutierrez R. Presence of claudins mRNA in the brain. Selective modulation of expression by kindling epilepsy. Brain Res Mol Brain Res 2002; 104: 250–254. [DOI] [PubMed] [Google Scholar]
- 165.Marchi N, Hallene KL, Kight KM, et al. Significance of MDR1 and multiple drug resistance in refractory human epileptic brain. BMC Med 2004; 2: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bankstahl JP, Bankstahl M, Kuntner C, et al. A novel positron emission tomography imaging protocol identifies seizure-induced regional overactivity of P-glycoprotein at the blood-brain barrier. J Neurosci 2011; 31: 8803–8811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Loscher W, Luna-Tortos C, Romermann K, et al. Do ATP-binding cassette transporters cause pharmacoresistance in epilepsy? Problems and approaches in determining which antiepileptic drugs are affected. Curr Pharm Des 2011; 17: 2808–2828. [DOI] [PubMed] [Google Scholar]
- 168.Siddiqui A, Kerb R, Weale ME, et al. Association of multidrug resistance in epilepsy with a polymorphism in the drug-transporter gene ABCB1. N Engl J Med 2003; 348: 1442–1448. [DOI] [PubMed] [Google Scholar]
- 169.Sisodiya SM, Mefford HC. Genetic contribution to common epilepsies. Curr Opin Neurol 2011; 24: 140–145. [DOI] [PubMed] [Google Scholar]
- 170.Tate SK, Sisodiya SM. Multidrug resistance in epilepsy: a pharmacogenomic update. Expert Opin Pharmacother 2007; 8: 1441–1449. [DOI] [PubMed] [Google Scholar]
- 171.Dombrowski SM, Desai SY, Marroni M, et al. Overexpression of multiple drug resistance genes in endothelial cells from patients with refractory epilepsy. Epilepsia 2001; 42: 1501–1506. [DOI] [PubMed] [Google Scholar]
- 172.Serrano GE, Lelutiu N, Rojas A, et al. Ablation of cyclooxygenase-2 in forebrain neurons is neuroprotective and dampens brain inflammation after status epilepticus. J Neurosci 2011; 31: 14850–14860. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.