Abstract
The surfaces of colloidal nanocrystals are complex interfaces between solid crystals, coordinating ligands, and liquid solutions. For fluorescent quantum dots, the properties of the surface vastly influence the efficiency of light emission, stability, and physical interactions, and thus determine their sensitivity and specificity when they are used to detect and image biological molecules. But after more than 30 years of study, the surfaces of quantum dots remain poorly understood and continue to be an important subject of both experimental and theoretical research. In this article, we review the physics and chemistry of quantum dot surfaces and describe approaches to engineer optimal fluorescent probes for applications in biomolecular imaging and sensing. We describe the structure and electronic properties of crystalline facets, the chemistry of ligand coordination, and the impact of ligands on optical properties. We further describe recent advances in compact coatings that have significantly improved their properties by providing small hydrodynamic size, high stability and fluorescence efficiency, and minimal nonspecific interactions with cells and biological molecules. While major progress has been made in both basic and applied research, many questions remain in the chemistry and physics of quantum dot surfaces that have hindered key breakthroughs to fully optimize their properties.
Keywords: semiconductor, nanoparticle, photoluminescence, CdSe, optics, ligands, bioimaging, probe, label
1. Background
Quantum dots (QDs) are nanoparticles composed of crystalline semiconductors that are among the most widely studied class of nanoscale materials [1-4]. The most useful attributes of these tiny crystals are their tunable optical and electronic properties. By precisely controlling the QD size, shape, composition, and structure, one can modulate the wavelengths of light absorption and fluorescence emission and tune the mobility and location of electronic charge carriers [5]. These particles have been widely applied as functional materials for light absorption and light emission across diverse fields, from electronics to biomedical imaging. They have been particularly useful as fluorescent probes for imaging biological molecules and cells because their bright emission, high stability, and wavelength tunability provide exceptionally high detection sensitivity, stable signals, and multiplexed molecular analysis [1, 2]. QDs are also used in light-emitting devices and displays to improve color purity and reduce power consumption due to their narrow bandwidths and high quantum yield [3, 6, 7]. In addition, they are used to improve energy harvesting in solar cells due to efficient formation of multiple electronic charges from a single photon, a process called multiexciton generation [4, 8, 9].
The success of these applications critically depends on the properties of the QD surface [10-13]. Due to their small sizes compared with bulk solids, a large fraction of the constituent atoms in a QD crystal (often 10-50%) are on their surfaces. These atoms are not bonded fully within the crystalline lattice, and thus their electronic configuration is dissimilar from the atoms within the crystal. It is critical to control these surface atoms because their distinct electronic energy states can trap electrons, quench fluorescence emission, and reduce charge transfer rates. Indeed, for nearly all applications, these surface atoms must be bonded to coordinating ligands. The ligands further dictate how the QD interacts with the surrounding medium, whether it is an electronic device or biological fluid. However the atoms and molecules comprising the surface remain poorly understood due to the difficulty of accurately measuring their subtly unique electronic properties and their heterogeneous and dynamically changing structure [14-16].
In this review article, we describe the current understanding of the QD surface and discuss recent and ongoing experimental and theoretical advances. We primarily focus on ionic CdSe-based materials synthesized as colloids dispersed in liquids, and coated with a variety of chemisorbed ligands. As depicted in Fig. 1, we discuss important molecular interactions and state-of-the-art engineering principles from the inside-out, describing the inorganic crystal structure, crystal facets, the chemistry of ligand coordination, ligand-dependent optical properties, and ligand designs for interfacing with biological systems. Our engineering goals are driven by applications in biomedical imaging and sensing, such that small sizes, high quantum yield, high stability, and inert behavior are desired and largely determined by the surface. The engineering of QD surfaces is an ongoing effort for which basic studies are still needed to reveal critical structure-function relationships.
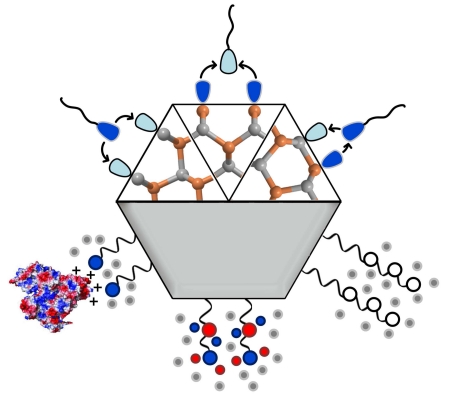
Fig. 1.
Surfaces of quantum dots, depicting interactions between (a) crystalline facets and coordinating ligands and (b) ligands and the surrounding medium. (a) Atoms on surface facets can display different combinations of electron-rich or electron-deficient orbitals that cause the facets to be electrostatically cationic, anionic, or neutral. Different classes of ligands, distinguished by their “head” groups as X, L, or Z, can bind to each facet type selectively. (b) The ligand “tail” groups dictate interactions with the surrounding medium. For a biological fluid, ligands are chosen to be polar or charged so that they interact with water to provide colloidal stability. Different tail groups interact in different ways with biological media. Strongly anionic or cationic ligands will electrostatically adsorb to locally charged regions of proteins like albumin [17], causing protein “fouling,” whereas zwitterionic and nonionic surfaces have a net neutral charge that suppress nonspecific protein binding.
2. Quantum dot crystal facets
The QD surface is a chemically heterogeneous interface between atoms of a crystalline solid and ligands coordinated to the surface atoms. Although QDs are often nearly spherical in shape, when examined closely, their surfaces are typically locally flat regions of truncated crystalline planes [5, 18]. The types of facets on the surface are initially defined during synthesis, dictated by the crystal phase and the ligands that bind to surface atoms and modulate interface energies. The ligand layer is usually considered to be dynamically chemisorbed [19] due to relatively weak bond energies (20-40 kJ mol−1, or 0.2-0.4 eV) and can be modified through post-synthetic ligand exchange to adjust the QD properties for specific applications. Here we summarize key attributes of QD surface facets to establish how ligands bind to these surfaces, as deduced from a combination of empirical and theoretical studies of inorganic crystal facets over the past two decades.
2.1 Crystal structure
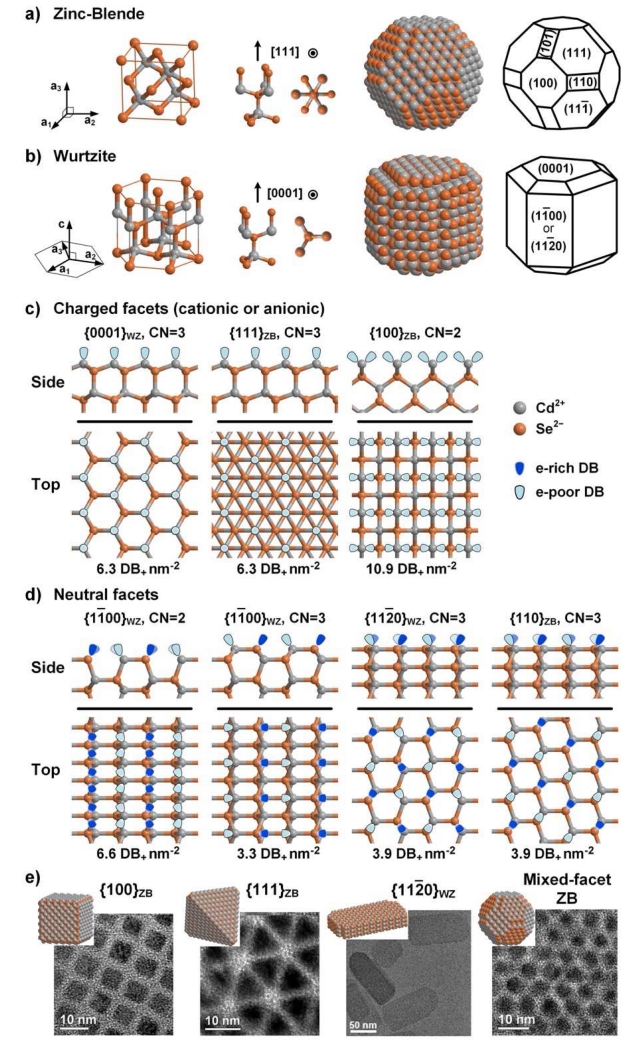
The binary semiconductor CdSe is an ionic crystal in which anionic Se2− and cationic Cd2+ atoms are each coordinated tetrahedrally by four nearest neighbor atoms of opposite charge. This type of lattice and bonding is similar throughout the II-VI chemical group (e.g. ZnS, CdSe) and III-V group (e.g. InP, GaAs). The crystal phase can either be zinc blende (ZB) or wurtzite (WZ), each of which has nearly the same anion-cation bond length and identical nearest neighbor bonding geometry. The difference between the two phases lies in the second nearest neighbors, which are either in a ‘staggered’ conformation (ZB) or ‘eclipsed’ conformation (WZ) along lattice directions specified by Miller indices [111]ZB or [0001]WZ, respectively (see unit cells in Fig. 2a and 2b).1 This single conformational difference gives the ZB structure a cubic lattice that has a high degree of three-dimensional symmetry (Td point group) whereas the WZ structure is a less symmetric (C3v) hexagonal lattice with a unique [0001]WZ axis of eclipsed atoms (called the c-axis). This phase difference yields only a small difference (~0.1%) in bond energy (approximately 100-200 kJ mol−1 or 1-2 eV per bond) [21], but profoundly impacts the atomic and ionic symmetry of the crystal, determining facet orientation and facet charge [22]. For example, due to higher crystal symmetry, ZB nanocrystals tend to be more symmetric in shape, yielding quasi-spherical or tetrahedron-shaped particles, whereas WZ nanocrystals tend to be elongated along their c-axis, with anisotropic optical and electronic properties [23].
Fig. 2.
Surface facet chemistry of ionic nanocrystals with zinc blende (ZB) or wurtzite (WZ) crystal phases, showing CdSe examples. (a,b) Bonding geometries and structures of ZB and WZ QDs. From left to right: crystal phase unit vectors, unit cell, two views of the internal bonding geometry parallel to, and perpendicular to, the [111]ZB and [0001]WZ direction, and two schematics of nanocrystals indicating atomic locations and lattice facet identifications. Cd2+ ions are grey and Se2− ions are orange. (c,d) Surface facets are depicted from two orientations (side and top views). As shown, they are “bare” without ligands and without reconstruction. Dangling bonds (DBs) are shown indicating whether they are electron-rich on Se2− or electron-poor on Cd2+. (c) Charged facets have either cationic Cd2+-rich surfaces or anionic Se2−-rich surfaces (only cation-rich surfaces are shown). (d) Neutral facets have equal numbers of cations and anions. (e) Transmission electron micrographs of four nanocrystals are shown with different facets exposed, showing QDs with all {100}ZB, all {111}ZB, all {112̄0}WZ, or mixed facets (reproduced from references [27] and [28] with permission).
2.2 Surface atom electrostatic charge
The most prevalent nanocrystal surfaces are simply truncated crystal planes generated by slicing along lattice directions that allow only bonds to be cut, without cutting through an atom; these are the high symmetry directions, yielding low Miller index facets. As shown in Fig. 2c and 2d, the low Miller index facets for ZB are {111}ZB, {100}ZB, and {110}ZB, and for WZ are {0001}WZ, {11̄00}WZ, and {112̄0}WZ. By cutting the crystal along specific directions, the surface can be terminated by cations, anions, or a combination of both. The anion-cation stoichiometry on the surface determines the electrostatic charge density on the surface, and thus the chemical reactivity with ligands. As shown in Fig. 2c the {111}ZB, {100}ZB, and {0001}WZ facets are all charged and are covered with cationic atoms (Cd2+) to yield cationic facets. Alternatively, they can be similarly terminated with anionic atoms (Se2−) to yield anionic facets. As shown in Fig. 2d the {110}ZB, {11̄00}WZ, and {112̄0}WZ facets have equal number of anionic and cationic atoms and thus are electrostatically neutral facets. The charged facets exhibit locally high electrostatic charge repulsion within the facet plane and thus have high surface energy (~1 J m−2 or ~1.3 eV per atom) compared to the neutral facets that comprise an alternating array of cations and anions (~0.5 J m−2 or ~0.5 eV per atom) [24]. The higher surface energy of charged facets makes them less favorable than neutral facets and more chemically reactive, especially toward charged ligands. Note that in reality the surface structure of a “bare” facet (without ligands) is not a perfect reflection of the truncated crystal, because it is often structurally distorted due to reconstruction of the surface atoms to different bond lengths and geometries to redistribute charge and reduce surface energy, especially for bare charged surfaces [25, 26].
2.3 Surface atom coordination number
In addition to facet charge, the coordination number (CN) of surface atoms also significantly affects the physical and chemical properties of facets. The CN of an atom in the interior of a tetrahedrally coordinated nanocrystal is 4, whereas a surface atom is missing one bond (CN=3) or two bonds (CN=2). An atom with CN=1 is usually considered to constitute an adatom, a high energy atomic configuration that will either be readily removed due to weak associations or migrate to a different set of surface facets [29], although exceptions have been observed when using chloride ion ligands with CN=1 Cd2+-terminated facets [30]. As shown in Fig. 2c and 2d, surface atoms on bare ZB and WZ facets have a CN of 3 or 2 such that there are either 1 or 2 unpassivated dangling bonds (DBs) per surface atom, respectively. When simply considering II–VI compound formation as a purely ionic neutralization between cations (Cd2+) and anions (Se2−) through tetrahedral coordination, one deficient coordination (1 DB) should leave a net partial charge of either +0.5 or −0.5 on the corresponding cation or anion (in reality it is closer to +0.3 or −0.3 based on Pauling ionicities). Hence, the CN of surface atoms indicates the magnitude of charge of the surface atoms, which in turn determines the surface charge density, surface energy, and chemical reactivity of QD facets.
The CN and surface densities of cationic DBs (DB+ nm−2) are shown along with each facet in Fig. 2c and 2d. Based on the CN and density of charge, charged {100}ZB facets (CN=2; 2 DB per atom) have a higher surface energy compared with other charged facets such as {111}ZB and {0001}WZ (CN=3; 1 DB per atom). The neutral facets are all similar with CN=3 and do not differ substantially in surface energy, although the exact orientations and surface densities of DBs are distinct, which impacts how ligands will bind. In addition, the {11̄00}WZ facet can alternate between CN=2 and CN=3 between adjacent planes, whereas the others are always CN=3.
2.4 QD shape and geometric orientation of facets
The QD shape determines the presentation of different facets geometrically around the nanocrystal. In a ZB QD, any type of facet may be distributed isotropically over the QD surface due to the underlying crystalline centrosymmetry. That is, it is possible to cover an entire ZB QD surface with any single type of {111}ZB, {100}ZB, or {110}ZB facets to yield a tetrahedron, cube, or rhombic dodecahedron, respectively. Some of these structures have been empirically demonstrated in CdSe QDs [31], as illustrated in Fig. 2e. Through a combination of multiple types of facets, it is also possible to derive a multitude of shapes [29, 32, 33]. Depending on the types of facets, the nanoparticle can be enriched overall with cations or anions, yielding a net charge on the nanocrystals and an anisotropic spatial orientation of charge. The facets present, and thus the shape, is driven by minimization of total surface energy [34], which is substantially impacted by ligands, as described below.
Multi-faceted QDs are particularly prominent for the WZ phase due to the unique c-axis; a WZ QD will always have a multi-faceted surface because the {0001}WZ facets terminating the two ends of the c-axis are oppositely charged, and thus are chemically dissimilar from the neutral facets orthogonal to the axis, {11̄00}WZ and {112̄0}WZ. This unique spatial orientation of surface charges implies that the surface properties and chemical reactivity of a WZ QD can be modified depending on aspect ratio. For example, a WZ QD with rod-like morphology will have a relatively neutral surface and lower reactivity compared to one with more disk-like morphology that will be mostly covered with the two oppositely charged surfaces (yielding a dipole) and higher reactivity. WZ and ZB domains can also exist on the same QD to yield a polytypic crystal [23], and lower symmetry facets beyond those shown in Fig. 2 can also be present such as {404̄5}WZ [30], which together provide a high degree of QD facet and shape diversity [32].
2.5 Composition dependence
While CdSe is the prototypical composition, QDs have been prepared from chemically diverse families of semiconductors to yield nanocrystals with different crystal structures (e.g. rock salt IV-VI materials), different CN (e.g. 6 for rock salt), different atomic sizes or bond lengths (e.g. CdS vs. CdTe), and different ionicities (lower for III-V, higher for IV-VI, and zero for covalent C and Si). All of these attributes are expected to strongly impact facet surface energies and chemical reactivities. For example, smaller atoms yield higher crystal bond strengths [35, 36], which will increase surface energy. Among the other materials, many experimental and theoretical studies have focused on rock salt (RS) PbSe, and it has been similarly observed for this centrosymmetric crystal (Oh) that charged facets such as {111}RS have higher surface energy than neutral facets such as {100}RS [37, 38]. Therefore cubic shapes reflecting the prevalence of the relatively stable {100}RS surface facets are commonly observed, whereas it is rare to observe tetrahedrons with {111}RS surfaces or shapes that are elongated or anisotropic [39, 40]. A wide variety of new compositions and facets are also becoming available through cation exchange processes, for which the cations of an ionic nanocrystal are exchanged with different cations to yield a new composition for which anions maintain the crystalline framework and, presumably, the original facets [39, 41].
3. Quantum dot ligand coordination
Diverse classes of ions, molecules, and polymers function as ligands on the surfaces of QDs. The nature of ligand bonding to a QD is analogous to molecular-scale coordination bonds in metal complexes [42] but also shares important similarities with self-assembled monolayers (SAMs) on macroscale metal surfaces [43]. As in these classical analogues, ligands bind selectively to surface atoms based on facet charge, alter the physical and electronic properties of the coordinated metal atoms, and determine interactions with the surrounding solvent or matrix. Here we describe the current understanding of QD ligand coordination, which is a rapidly advancing field aided by theory but still largely driven by empirical chemical analysis of chemisorbed ligands, structural analysis of ligand-bound QDs, and opto-electronic analysis of ligand impacts on QD properties.
3.1 Quantum dot ligand chemistry
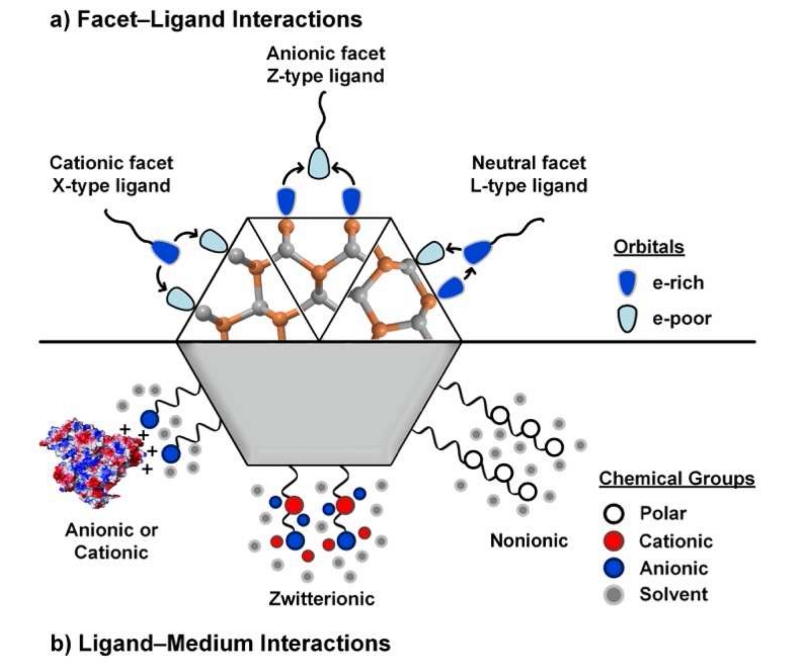
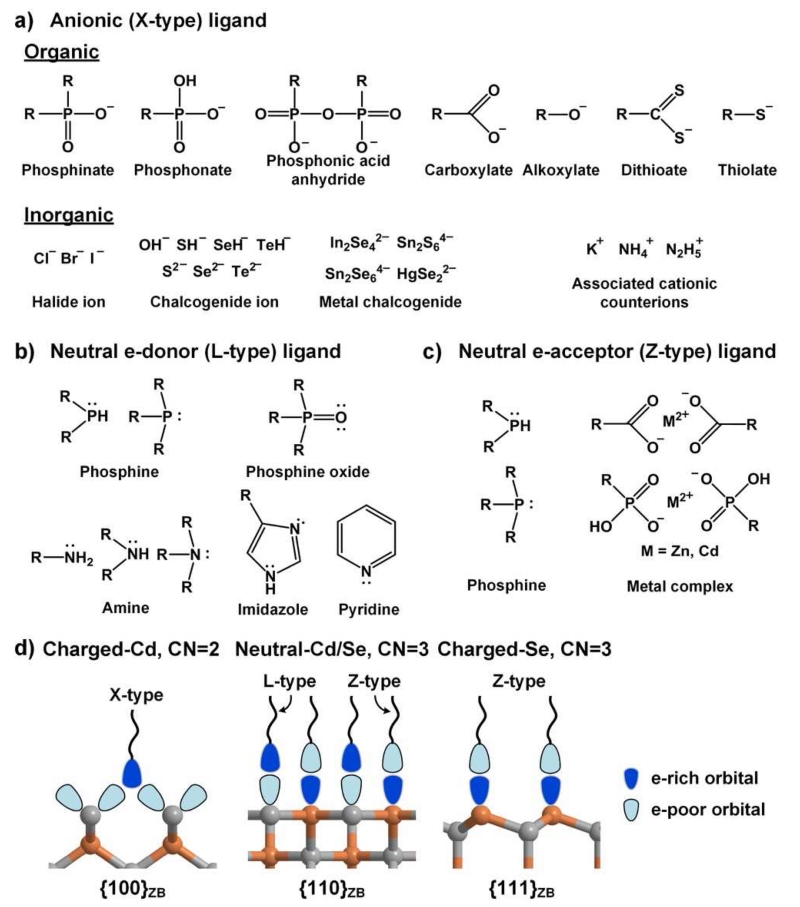
As shown in Fig. 3, the most common ligands for QD facets have electron-donating “head” groups that are either anionic (X-type) or electrostatically neutral (L-type) [42]. These types of ligands analogously form monomeric and multimeric coordination complexes with Cd2+ metal ions with coordination numbers between 2 to 8 [44, 45]. X-type ligands bind with strongest affinity to cationic surface atoms whereas L-type ligands form dative bonds with electron-deficient DBs. All of these ligands are σ-bond donors and can also be either π-bond donors or acceptors. Among the X-type ligands shown in Fig. 3a, the most common are organic carboxylates (R-COO−), phosphonates (R-P(OH)OO−), and thiolates (R-S−), where R is usually an alkyl chain with 8-18 carbons. These are all conjugate bases of weak acids. A wide range of inorganic ions have also been used that include inorganic halides [46], chalcogenide ions [47], and molecular metal-chalcogenide complexes [13, 48]. These ligands are typically introduced with counterions (e.g. K+ or ), which may remain associated with the ligand on the QD surface. As shown in Fig. 3b, L-type ligands are distinguished by one or more lone pairs of electrons or delocalized π-electrons and commonly include amine (R-NH2), phosphine (R3-P), and phosphine oxide (R3-PO) groups, in addition to the protonated versions of X-type ligands. While X- and L-type ligands selectively bind to cationic and neutral facets, anionic facets can be passivated by Z-type ligands (Fig. 3c) that are electron-withdrawing but electrostatically neutral, such as metal complexes and phosphines that are σ-donors with π-backbonding [49]. Overall it is critical that the final nanoparticle is nearly net neutral in charge in nonpolar solution [50] in which they are typically synthesized, although this is not necessarily the case in polar solvents [48] as charge screening can reduce the electrostatic repulsion between like charges in close proximity.
Fig. 3.
Common ligands for colloidal QD facets. (a) Anionic X-type ligands are anions that are either conjugate bases of weak organic acids or inorganic ions, often associated with cationic counterions. (b) Electrostatically neutral, electron-donating L-type ligands have lone pairs of electrons on heteroatoms and/or delocalized π electrons. (c) Neutral electron-accepting Z-type ligands are electron-withdrawing metal cations in complexes with X-type ligands, or L-type ligands that can also function as electron acceptors. (d) The expected preferential orientations of ligands on different types of facets, based on X-ray crystal structures and theoretical atomistic studies, with orbitals shown in shading indicating their degree of filling. X-type ligands are charged and preferentially bind to cationic facets, typically in a bridging conformation between two adjacent surface cations. Electrostatically neutral L-type and Z-type ligands can bind to neutral facets that present both electron-rich and electron-deficient DBs. Z-type ligands bind weakly to anionic surfaces.
3.2 Facet-specific ligand binding
Theoretical studies and X-ray crystallographic analyses have revealed that there are specific favorable ligand-facet configurations, some of which are shown in Fig. 3d. X-type carboxylate and thiolate ligands on CdSe QDs are expected to show strongest binding to {100}ZB facets covered with CN=2 cationic atoms, each with charge of +1 (assuming fully ionic bonding), and comparatively weaker binding to other cationic and neutral facets [51]. Other X-type ligands should similarly show strongest binding to this facet due to the highest density of positive surface charge. L-type ligands are weakly bound on all facets but typically show highest binding to cationic facets with CN=3 metal atoms and neutral facets compared with anionic facets [52]. It has been shown that while phosphines and thiols bind to the neutral {11̄00}WZ and {112̄0}WZ facets, only bind weak associations are predicted for amines, which have no π-bonding capacity [53]. In interactions between L-type ligands and neutral facets, the electronegative atoms of the ligand are positioned closest to the facet cation (e.g. for amines, R-H2N⋯Cd) and the more electropositive ligand atoms are directed toward the facet anions, possibly forming a hydrogen bond (e.g. for amines, R-HN-H⋯Se) [54]. Z-type ligand can also bind to neutral facets but have highest affinity toward anion-rich facets due to their electron withdrawing properties. The selectivity between ligands and facets is still a developing field that remains primarily theoretical, as empirical tools are lacking.
3.3 Coordinating ligands during synthesis
A typical synthesis of CdSe QDs is a reaction between cadmium and selenium precursors in a hot reaction solvent in which multiple types of ligands are present as both precursor complexes and as soluble additives [55]. These ligands end up bound to the surface facets, and can later be exchanged to modify the QD surface properties. Common cadmium precursors in CdSe QD syntheses are CdX2 complexes containing divalent metal ions (Cd2+) coordinated by two mono-anionic X-type ligands with long alkyl chains such as stearate. Selenium precursors include elemental selenium (Se), bis-silyl selenides (R3Si-Se-SiR3), and phosphine selenides (R3P-Se), containing the chalcogen in the 0 or −2 oxidation state. Reaction solvents can be either entirely composed of coordinating ligands (called coordinating solvents) [56] or contain a stoichiometric amount needed for coordination [57]. Common solvent additives include L-type ligands such as phosphine oxides, phosphines, and primary-, secondary-, or tertiary amines [23, 56], while non-coordinating components are mostly high boiling point hydrocarbons, most commonly 1-octadecene [58]. Importantly in a mixture of ligands, it may not be clear which ones will dominate binding to the surface during growth, but X-type ligands are generally dominant over competing L-types even when the latter are in large excess [59, 60]. Protic ligands can be especially tricky to predict because of their potential to switch between L-type and X-type by their protonation state; very small protic molecules like water and acetic acid can have a profound impact on the resulting nanocrystal shape [61, 62].
These syntheses typically yield QDs with a cadmium-rich nonstoichiometric composition (CdxSey, x>y) with a coordinating ligand shell containing X-type ligands that neutralize the extra positive charges [63]. The presence of X-type ligands indicates the presence of cationic facets, however L-type and Z-type ligands are also usually present on the surface, often in larger amounts, and may function to passivate anionic and neutral facets, or adsorb as outer sphere ligands [64]. Their binding strengths are much weaker and they can be easily washed away during purification [65, 66]. While cadmium-rich QDs are typical whenever X-type ligands are used, minimizing the concentration of these ligands and including excess L-type ligands can yield a Cd:Se stoichiometry near 1:1, indicating the dominance of neutral facets on the surface [67-70]. It is also possible to prepare anion-rich QDs [71], which require an excess of Z-type ligands such as phosphines for stabilization.
3.4 X-ray crystal structures including ligands
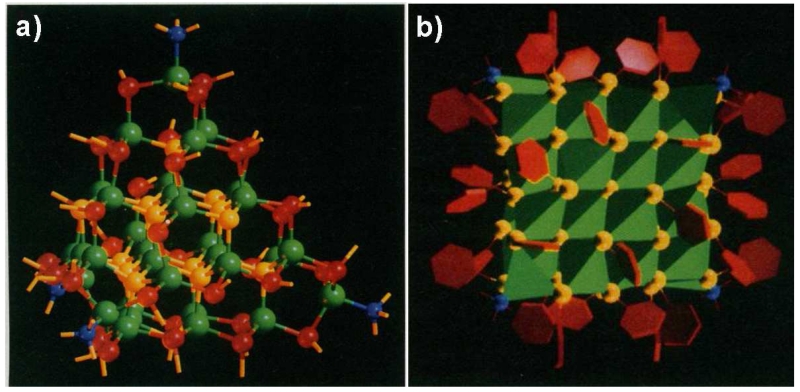
Much of what we know empirically about ligand-facet selectivity derives from analysis of X-ray crystallographic structures of “perfect” nanocrystals that are precisely homogeneous in size [72]. Whereas most QDs typically exhibit heterogeneity in size that prevents high-resolution structural analysis due to insufficient crystallinity of the macroscale solid, some clusters are inherently highly stable and can be synthesized as pure, homogeneous species. These “magic-sized” clusters have been generated for a series of QDs composed of M2+E2− (CdS, CdSe, HgS, and HgSe), synthesized to yield precise ligand-coated structures (M17E4)X26L4, (M32E14)X36L4, and [(M54E32)X48L4]4− where X is an X-type ligand (such as benzenethiolate) and L is an L-type ligand (water or dimethylformamide, DMF) [73-76]. These cation-rich non-stoichiometric crystals are faceted with a combination of {100}ZB and {111}ZB surfaces. It is remarkable that these charged facets with high surface energy dominate the surface structure, which validates the concept that selective ligand binding to these facets to minimizes the total surface energy.
The crystal structure of (Cd32Se14)X36L4 [73] is shown in Fig. 4, with the X-type ligand benzenethiolate and L-type ligand DMF. Each of the 36 thiolate ligands bind in an sp2-like bridging conformation between two adjacent CN=2 cadmiums on {100}ZB facets (as in Fig. 3d), with 6 on each of the 6 {100}ZB facets [73, 74]. This bridging structure has been widely reported to be the preferred conformation of thiolates and carboxylates on cationic facets [22, 77, 78]. This is unlike DMF, which binds through its electron-donating tertiary amine as a σ-bond donor to a single CN=3 cadmium atom on {111}ZB cationic facets, which have lower charge density. This is a common conformation observed for L-type ligands [28]. Importantly, the crystallographically opposite CN=3 {111}ZB anion atoms are unpassivated. These structures have been highly useful in the analysis of preferred ligand binding on QD facets, but unfortunately high-resolution crystal structures have yet to be generated for larger clusters (>2 nm) that are more commonly used, or for more diverse shapes and ligand types. Interestingly, other magic-sized clusters have been reported that seem to be distinct, either synthesized with X-type ligands to yield cadmium-rich QDs [79], or synthesized with excess L-type amines to yield neutral QDs (e.g. Cd33Se33) [69, 70]. However, these have not been crystallized to a level suitable for X-ray crystallography presumably due to the flexibility of alkyl chains on the ligands.
Fig. 4.
X-ray crystal structure of the (Cd32Se14)X36L4 cluster, where X is −SC6H6 (benzenethiolate) and L is N(CH3)2CHO (dimethylformamide). (a) The QD is aligned close to the {110}ZB plane orientation. Atom colors represent Cd (green), sulfide S (yellow), thiolate ligand S (red), and amine ligand N (blue). Phenyl groups are omitted for clarity. Each of the four Cd at the tetrahedral vertices can be interpreted as a single {111}ZB plane atom bound to a single L-type ligand. (b) Orientation of the ligands, with phenyl groups depicted as red hexagons. The orientation is shown looking at the {100}ZB direction. Each of the core atomic clusters is shown in reduced form as a green tetrahedron centered on each Cd and surrounded by coordinating S (yellow) and N (blue) atoms. The cluster comprises 6 faces, each with 6 thiolate ligands bound. A single unpassivated S core atom is in the center, which can be interpreted as an unpassivated anionic {111}ZB facet. Adapted with permission from reference [73].
3.5 Steric hindrance and packing
The steric dimensions of ligands are expected to impact their binding to QD surfaces. This effect is analogous to the impact of ligand size on the CN of metal complexes and the dependence of SAM packing density on the steric size of thiol molecules. Monoalkyl ligands are the primary ligands used in QD synthesis, and the alkyl “tail” groups provide the most important steric constraints to ligand packing. The surface packing density of pure alkanes (~5.5 chains nm−2) is higher than what can be achieved on flat surface SAMs (~4.5 chains nm−2) [43]. On a CdSe surface, the total surface density of DBs (6.3-10.9 DB+,− nm−2) is higher than this packing density, so theoretically it is not reasonable for each DB to be passivated by a separate ligand. For binding of X ligands with a cationic facet, the charge density is a more relevant parameter (+3.2 nm−2 to +5.5 nm−2). Interestingly, the +5.5 nm−2 charge density of {100}ZB facets closely matches the ideal alkane chain density, so these facets are particularly suitable for close packing of ligands on the surface to maximize van der Waals interactions between adjacent alkanes. The lower +3.2 nm−2 charge density on {111}ZB and {0001}WZ facets would leave gaps which, in the case of SAMs, causes the ligands to tilt on the surface to maximize inter-ligand interactions [80]. Theoretically these latter two facets are more compatible with bulkier ligands, such as oleate ligands, which have a cis-double bond that intrinsically bends the alkyl chain [81]. Importantly, the surface densities of X-type ligands on QDs measured through nuclear magnetic resonance (3.5-4.6 nm−2) are in the same range as the theoretical surface charge densities [19].
For neutral CN=3 facets, the surface density of cations is between 3.2 to 3.9 DB+ nm−2 (and equal to the anion charge density) so complete passivation of all DBs is possible if an L-type ligand can simultaneously bind to DBs of both a cation and an adjacent anion. However complete passivation may require stringent orientation of the ligand dipole on the surface to maximize interactions with DBs of opposite charge (see different orientations in Fig. 2d). For a bulky L-type ligand like trialkylphosphine oxide, it is unlikely that it can completely passivate all DBs on a facet, as its maximum packing density is near 1.8 nm−2 and has been experimentally measured at 1.3 nm−2 on QDs [19]. However in comparison with SAMs, steric repulsion is generally relaxed for nanoparticles due to the presence of nearby facet edges that provide more volume per ligand, which could allow full coordination on crowded facets [82]. This is consistent with the low degree of order in alkane chain packing observed for small nanoparticles (2-3 nm) and the nearly complete DB passivation of magic-sized clusters even with sterically bulky benzyl ligand tails [73], but this may not be possible for larger nanocrystals or for much bulkier ligands.
3.6 Ligand-directed facet and shape selection
Many studies have shown that ligands play a major role in determining the crystal phase, facet distribution (3D morphology), and shape during colloidal QD synthesis [23, 37, 83-86]. This effect is caused by the direct modulation of surface energy of specific facets by ligand chemisorption. The thermodynamically most stable QD geometry is one in which the total surface energy is minimized, so it will be quasi-spherical (to maximize CN) if all facets have similar surface energy [34]. When surface energies strongly differ over the nanocrystal or when strongly binding ligands modulate surface energies of specific facet types, it is possible to grow shapes with high surface area and high aspect ratios. Growth can also be performed under conditions that are kinetically dominated to yield anisotropic shapes. The crystalline phase of CdSe QDs is also strongly dependent on the nature of ligands used for synthesis, with strongly binding X-type phosphonate ligands yielding pure WZ and weaker X-type carboxylates yielding ZB [87]. This phase selection is due to kinetic control for which the phase is initially dictated when the QDs nucleate as small clusters that exhibit differential stabilities with these two ligand coatings. The shape is also strongly determined by ligands: rod-shaped WZ CdSe nanocrystals typically require a combination of X-type ligands to passivate cationic ends as well as L-type ligands to passivate neutral sides [88, 89]. Two distinct types of QD nanoplatelets, flat sheets with extremely large surface areas, have also been prepared by selective ligand binding during synthesis. X-type carboxylate ligands generate nanoplatelets with cationic {100}ZB facets on the top and bottom, whereas L-type amine ligands generate nanoplatelets with neutral {112̄0}WZ facets on the top and bottom [28, 90].
3.7 Post-Synthesis Ligand Exchange
After synthesis, QDs are usually coated with ligands with long alkyl chains that are hydrophobic and electronically insulating. For use in many applications such as devices and biomolecular analysis, these ligands must be replaced with hydrophilic ligands that enable dispersion in polar matrices and solvents. For efficient exchange, the incoming ligands should have stronger binding than the original ligands [19] or be introduced in large excess to drive exchange through mass action. Neutral ligands (L- and Z-type) can be easily removed due to weak surface binding [65, 66], although some exceptions have been reported for strongly bindings amine on CdTe QDs [91]. This ligand exchange process can significantly alter the nanocrystal as well, as L-type ligands can remove selenium atoms from facets with low CN [92] and cadmium-containing Z-type ligands are displaced by L-type ligands to yield neutral surfaces [65]. For full ligand exchange, X-type ligands are usually required to displace the native X-type ligands, often using thiolates with hydrophilic tail groups for biomedical applications [93, 94], or small inorganic ions for device applications requiring strong conductivity with the surrounding matrix [13].
3.8 Composition dependence
The absolute bond strengths of coordinating ligands to QDs with different compositions have not been thoroughly evaluated but it is likely that the relationships will be complex due to combined differences in facet polarizability, surface DB densities, and facet surface energies. However when comparing within chemical families of QDs (e.g. ZnS vs. ZnSe and ZnSe vs. CdSe) it is generally expected that hard and soft acid/base rules should apply [51] such that QDs containing smaller cationic metal atoms (hard acids, Zn2+) should bind more strongly to hard base ligands composed of less polarizable atoms (amines). Indeed QDs with zinc-rich facets can strongly bind to amines [95] so it should be expected that QDs with larger metal atoms (hard acids, Cd2+) will bind more strongly to ligands containing more polarizable atoms (thiols and phosphines), as observed in metal complexes [96, 97]. The dependence on the QD anion is even less clear, but one study showed that X-type thiolates bind to CdS and CdSe more strongly than CdTe QDs [98]. Ligand packing differences are expected to be substantial, as ZnS nanocrystals can support a 25% higher packing density of ligands based on surface DB density alone compared with CdSe. PbSe has been shown to have a higher ligand packing density than CdSe on charged facets [19], but it also has a broader range of DB surface densities on neutral facets (2.8-4.9 DB nm−2) compared with CdSe [37] which allow more isotropic shapes when using a wider range of ligand types.
4. Optical properties dictated by the surface
Ligand binding to a QD surface alters the electronic energy levels of the surface atoms, which can profoundly impact the optical and electronic properties of the entire nanocrystal. QDs absorb and emit light through transitions of electrons between energy levels that can shift when they mix with ligand orbitals [99], much like the effect of ligand field strength on electronic transitions of metal coordination complexes. However, changes in electronic absorption spectra due to ligand binding are usually not substantial (<50 meV shift) except in the case of in very small QDs (<2 nm) [100] and thin QD sheets (nanoplatelets) [77] for which a large fraction of atoms are on the surface. The impact is much lower for the more common larger QDs for which the energy levels of the transitioning electron primarily arise from the fully coordinated atoms within the crystal. Instead, for these larger QDs, the primary impact of ligands is on the efficiency of light emission, which has recently become highly controllable by engineering the crystalline surface and ligands.
4.1 Quantum yield
The ligands and surface states can dominate the electronically excited state of a QD, in which it emits light. The electron in an excited electronic state typically exists for ~10 nanoseconds before it returns to its ground state by emitting a fluorescent photon. The efficiency of this process, the fluorescence quantum yield (QY), can be strongly impacted by faster competing processes that largely occur at the surface. If the electron is physically moved away from its corresponding empty ground state orbital (the hole), it can no longer undergo a transition to emit light. “Trapping” of the electron or hole at surface states is thought to be the primary determinant of low QY, whereas passivation of traps is thought to be necessary for high QY. Surface traps extract the electron or hole in picoseconds when surface atoms have energy levels that differ substantially from those of the fully coordinated interior atoms, or when ligands energy levels are similar to the QD electronic levels [15]. These trap states are also thought to be the primary cause of inefficient charge transfer when QDs are used in devices, limiting their overall utility [101].
4.2 Blinking and spectral wandering
Insufficient passivation is also reflected in fast and complex processes observed at the single-QD level. When single QDs are observed in a fluorescence microscope, they randomly fluctuate between fluorescent and nonfluorescent states during continuous excitation [102]. This “blinking” phenomenon is thought to arise due to ionization of the QD or trapping of the electron or hole, and correlates with a small shift in energy of the emitted photon, a phenomenon called spectral wandering [103]. Blinking is detrimental for most applications for which continual light emission is desired and is well known to depend on the surface ligands [104].
4.3 Surface trap states
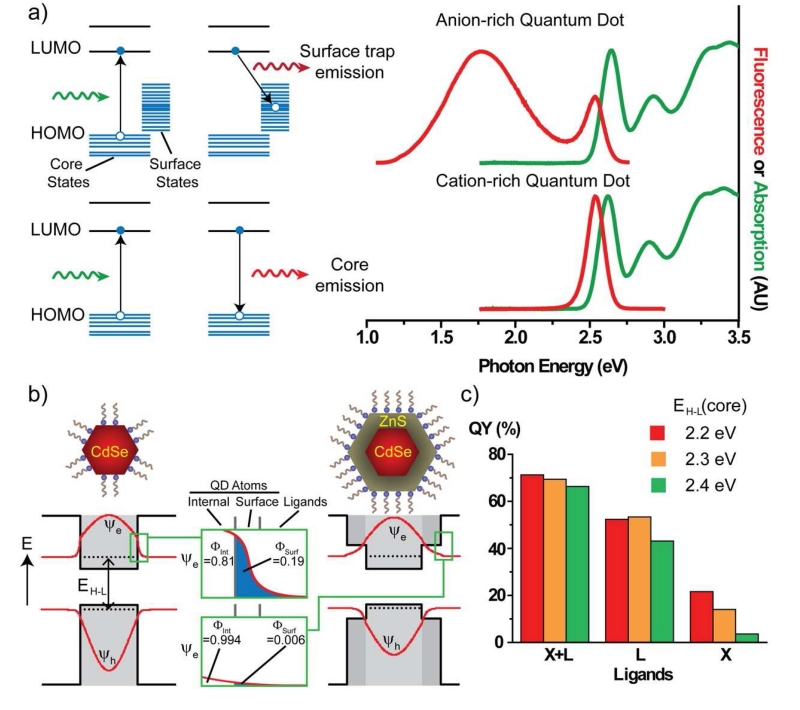
The physical and electronic nature of surface traps that reduce QY and induce blinking is of substantial current interest. A common observation is that ligand displacement, which is expected to produce surface DBs, reduces QY, presumably because electronic energy levels of unpassivated DB orbitals are lower in energy than those of the QD core. It is thought that negatively charged electrons are trapped at unpassivated DBs of cationic facets, because the lowest unoccupied molecular orbital (LUMO) electron energy states derive primarily from the Cd s-orbitals. Likewise, positively charged holes are trapped at unpassivated DB of anionic facets, as the highest occupied molecular orbital (HOMO) hole energy states derive primarily from the Se p-orbitals. These latter hole traps are thought to have greater impact [105, 106] because they arise on anionic DBs, for which weakly bound Z-type ligands are readily displaced compared with X-type and L-type ligands that passivate cationic DBs. An example of this is shown in Fig. 5a, in which a QD with a surface rich with anions exhibits broadband low energy emission called “deep trap” emission. With the addition of excess cadmium and a mixture of X- and L-type ligands, this deep trap emission is eliminated and the only emission is from the QD core LUMO→HUMO electronic transition. The anionic DBs efficiently trap holes, as depicted in the energy diagram, and their wide range of energies reflect the diverse chemical states on surface facets. Deep trap emission is not always observed when DBs are present, and instead fluorescence can be entirely quenched and the excited state electron decays through non-radiative pathways by releasing heat or infrared photons.
Fig. 5.
Surface-dependent optical properties of quantum dots. (a) Electronic energy band diagrams show the highest occupied molecular orbital (HOMO) levels and lowest unoccupied molecular orbitals (LUMO), as well as the HOMO-LUMO transitions for an anion-rich QD with a manifold of surface trap hole states. Anion-rich QDs have DBs that yield energy states on the surface that are between the core HOMO-LUMO levels to provide decay pathways that are either non-radiative, or show deep trap emission that is lower in energy than the LUMO→HOMO transition. When the surface is passivated with ligands and cations, high QY can be restored. (b) Electronic wavefunctions are depicted for the excited state electron (Ψe) and its empty orbital, the hole (Ψh). In the core nanocrystals at left, the wavefunctions penetrate into the surroundings, and the overlap integral with the surface (ΦSurf) can be a large fraction (0.19 for the electron) of the total. This overlap with the surface can be drastically reduced with the growth of a thin shell of ZnS. A 1 nm shell on a 3 nm core reduces the overlap of the electron with the surface by 30-fold and reduces the overlap of the hole with the surface by 2500-fold. (c) The experimental QY of core/shell nanocrystals is shown with 3 different core HOMO-LUMO energies (EH-L = 2.2, 2.3 and 2.4 eV) and identical shells. Each is coated with 3 different ligands that are either X-type, L-type, or a mixture of X and L in aqueous solution. QY values are similar for the three QDs using the X+L mixture ligands. X type ligands alone (thiolates) yield a very strong difference in QY that depends on the EH-L value: a large core EH-L causes quenching due to tunneling of the excited state electron and hole deeper into the shell due to their higher energies relative to the shell material. Panel (a) is reproduced from reference [5] with permission. Panel (c) reproduced from reference [107] with permission.
Many studies have focused on evaluating surface states, primarily through atomistic modeling. It is thought that only certain types of DBs and certain types of ligands function as surface traps [67], so complete passivation may not be necessary for high QY and low blinking. In particular, cadmium and selenium surface atoms with CN=3 do not necessarily yield trap states by themselves [78], although an ordered 2D array of theses on a facet could potentially localize charges. In general, it is thought that lower CN surface atoms dominate trap states. These atoms can be individual adatoms or a small island of atoms on an otherwise high CN facet or potentially an atomic vacancy in a facet. It is also thought that excess passivation can delocalize charge too substantially and trap charge carriers, as the addition of excess ligands can sometimes reduce QY [15]. It may eventually be possible to image and monitor these mysterious defect states, as recent studies using scanning tunneling microscopy and spectroscopy show that the energy states of specific surface defects such as adatoms can be potentially spatially mapped to precise locations on QDs [108].
4.4 Surface engineering to reduce surface traps
The ligand coating can be tuned to both increase QY and reduce blinking. For example, the addition of excess L-type amine ligands can drastically boost QY [12] and an excess of thiols can almost entirely eliminate blinking [109]. Unfortunately, immersion in an excess of ligands is not practical for biological imaging studies. Ligands can also have a detrimental impact, as thiolate ligands that are commonly used for biological applications entirely quench the fluorescence of CdSe nanocrystals [110, 111]. This is in stark contrast to CdTe-based QDs, which exhibit very high QY when coated with thiolates [112]. The difference derives from the energy levels of electrons in the nanocrystals and how closely they align with those of the ligands, which for CdSe yields hole-trapping energy levels on the surface [113].
The most robust way to decouple the QD fluorescence from surface traps is through the growth of an insulating crystalline shell material on the QD core using a material with higher energy electronic energy levels, functioning as an insulator [11, 114]. This attenuates the impact of surface ligand displacement in a thickness-dependent manner [95, 115], increases QY, and reduces blinking [115, 116]. The mechanism is shown in Fig. 5b. For a core-only CdSe QD the wavefunctions of the excited state electron (Ψe ) and hole (Ψh) have substantial spatial overlap with the surface atoms and surrounding medium (overlap integral Φsurf = 19% for the electron). This surface overlap is reduced by growth of an insulating shell (here 1 nm-thick ZnS shell), decreasing the electron surface overlap by 30-fold ( Φsurf = 0.6%) and reducing the hole overlap with surface states by 2500-fold. Because of this decreased overlap with the surface, this reduces the impact by surface traps and increases the probability of maintaining electron-hole co-localization and thus fluorescence emission. One downside is that thick shells cause the overall nanoparticle dimensions to increase, which usually is not desirable for applications in biology.
The electronic energy levels can have a complex interplay with ligand energy levels, even when shells are thick. To demonstrate this, we synthesized three QDs with identical ligands and identical shells (CdZnS), with cores differing only by their HOMO-LUMO energy gap (EH-L = 2.2, 2.3, or 2.4 eV) by tuning the composition of a ternary alloy HgCdSe QD core (see Fig. 5c) [107]. With a mixture of both X-type carboxylates and L-type amines, all three had similar QY values in aqueous solution. However, when they were coated with only X-type thiolates, QY dropped substantially for the ones with higher EH-L for which tunneling to the surface is highest. This suggests that surface trapping in core/shell QDs is significantly impacted by small changes in the core electronic energy levels alone. This effect was less severe with L-type imidazole ligands alone, which have a high ligand field strength that boosts QY [12, 92]. These findings suggest that the QY and blinking can be precisely controlled by simultaneous engineering of the ligands and core/shell materials together.
4.5 Surface fluctuations
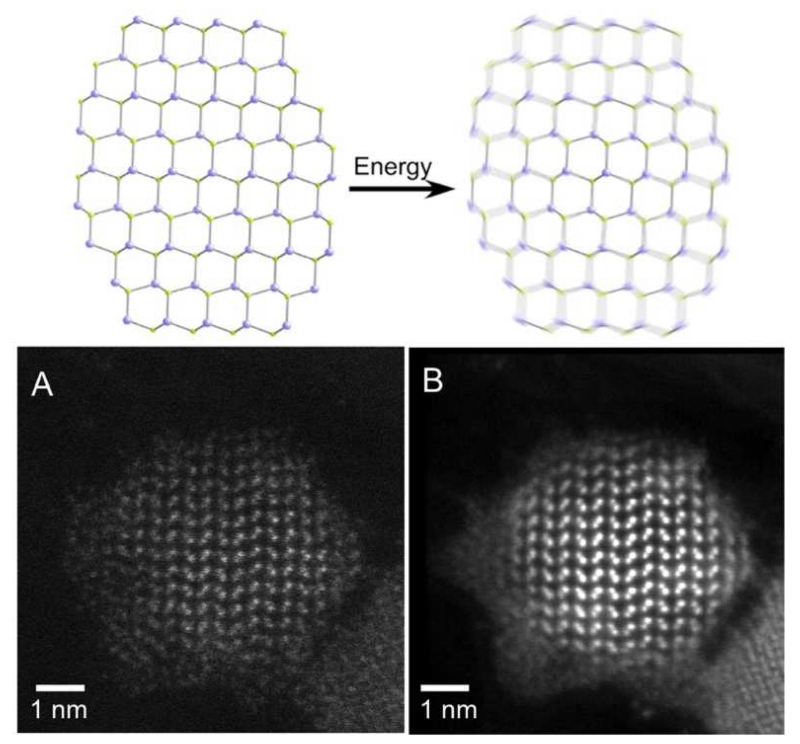
A new picture is coming into view of the nanocrystal surface as a dynamically changing, rapidly fluctuating chemical environment. Indeed even at room temperature, the rotation of alkyl chains on ligands is thermally accessible [43], and excitation of a QD with high energy light can theoretically heat a nanocrystal to 500 K, yielding energy that can be directly transferred to vibrate surface atoms and ligands [15, 117, 118]. This fluctuation occurs on the order of femtoseconds, much more rapidly than trapping or emission, so it could potentially dominate the emission dynamics. This energy could also displace or rearrange adatoms, vacancies, and weakly bound ligands so that they can reach more or less passivating states, which potentially can explain spectral wandering, blinking [119, 120], and other light-induced alterations called photoannealing and photobrightening, which are QY-boosting phenomena arising from higher power electronic excitation [121, 122]. Under electron-beam excitation it has also been observed that the surface atoms of CdSe QDs dynamically fluctuate, as shown in Fig. 6 [16], almost forming a liquid-like or amorphous state. However is not clear to what extent this phenomenon would occur in aqueous solution.
Fig. 6.
Potential surface instability of quantum dots arising from thermal fluctuations, observed by drift-corrected scanning transmission electron microscopy (STEM). (A) Single frame and (B) a frame-averaged STEM image from a movie of a 5-nm CdSe nanocrystal, illustrating blurring of the surface due to atomic fluctuations. Reproduced from reference [16] with permission.
5. Experimental characterization of quantum dot surfaces
Many analytical methods have been applied for the study of QD surfaces, including transmission electron microscopy (TEM), X-ray crystallography, nuclear magnetic resonance (NMR) spectroscopy, X-ray photoelectron spectroscopy (XPS), Fourier transform infrared (FT-IR) spectroscopy, and mass spectrometry (MS). Each of these techniques is uniquely suited to answer specific questions about QD facets and ligands, and in combination the data provide a global picture of the QD surface structure, although one that is far from complete due to the lack of direct measurement methods with high surface specificity.
5.1 Surface facet characterization
X-ray crystallography, MS, XPS, and TEM are used to analyze the inorganic surface facets of QDs. As discussed in Section 3.4, X-ray crystallography is the optical technique to definitively determine the 3D structure of atoms composing surface facets as well as ligands, however it has only been useful in rare instances when magic-sized clusters can be generated without structural heterogeneity. TEM, as a single-particle technique, is a powerful tool to directly identify structure facets (e.g. Fig. 6), especially for larger nanocrystals for which surface atoms exhibit substantial spatial periodicity, although it is low throughput, challenging to interpret when samples are heterogeneous, and not very chemically selective even when paired with electron or X-ray spectroscopies. MS has been also used to identify the exact masses of magic-sized clusters that can give insight into the surface structure [123-125], and recently it has been applied to larger colloidal nanocrystals with some degree of size dispersion [126]. XPS is also often used to characterize inorganic domains of QD surfaces because it can provide quantitative stoichiometries of anionic and cationic atoms on QD surfaces [71], probe the oxidation states of surface atoms, and provide reaction pathways for how QDs oxidatively degrade [127].
5.2 Surface ligand characterization
The analysis of surface ligands on QDs has primarily relied on NMR and FT-IR, with some use of MS and XPS. NMR spectroscopy is a key information-rich technique for surface ligand characterization because it allows direct chemical identification of ligands, quantification of ligands when using internal standards, and monitoring of dynamic interactions between ligands and QD surfaces in solution since it can distinguish between ligands that are surface-bound or free in solution. 1H, 13C, and 31P NMR have been used to characterize binding mechanisms of ligands, including carboxylic acids [128-132], thiols [46, 94, 133, 134], phosphonic acids [46, 60, 128, 135, 136], amines [137-140], and phosphines/phosphine oxides [128, 135, 136]. NMR-based ligand studies combined with elemental analysis of the total QD has been instrumental in revealing the stoichiometry of ligand binding whereby metal-rich surfaces are passivated by strongly-bound anionic X-type ligands such as carboxylates [129, 141]. NMR spectra have also been instrumental in the understanding of binding and exchange equilibrium because surface-bound ligands show broader peaks compare to those of free ligands in solution, so their individual concentrations can be spectrally deconvolved [46, 60, 130, 136, 138, 140, 142, 143]. Despite the rich information it can provide, NMR is limited by the need for relatively large quantities of highly pure samples and because it provides little insight into binding geometry. FT-IR has been employed to infer the structure of ligands bound to QD surfaces by probing their vibrational signatures. This has allowed the study of binding modes [131, 132, 144] and ligand tail conformation [145], as well as identification of chemical functionality [146], and monitoring of ligand exchange [147, 148]. While NMR and IR are particularly well suited for organic ligands, XPS has allowed the analysis of how inorganic ligands such as halide and hydroxide ions bind to QD surfaces [46, 61]. MS has also provided unique insights into ligand dynamics due to its high level of molecular specificity, even allowing monitoring of ligand loss from QDs inside cells [149]. MS can also be used as an imaging technique, providing a unique way to interrogate ligands across different regions of a material [150, 151].
6. Modeling of quantum dot surfaces
The interactions between QD surfaces and their ligands are most often simulated using first principles calculations that allow the incorporation of specific ligand chemical structures and specific QD facets. These computational studies are useful for quantitative analysis of how ligands are orientated on facets, how facet reconstruction relates to passivation, for calculating ligand binding strengths, and for understanding the influence of ligands on QD growth and optical properties [24, 26, 152]. A comprehensive review of recent methods and results for nanoparticles and clusters was recently published [153]; here we focus on the methodologies relevant to QD surface engineering for optical and physical stabilization.
6.1 Problems with scale
The biggest challenge for accurate first-principles modeling of QDs is related to the large number of atoms involved in the calculations. Atoms in a hypothetical spatial arrangement are computationally relaxed to geometric locations that minimize the total energy, which is assumed to approximate the most realistic average configuration of the QD and its ligands, and then different properties such as electronic energy levels and optical transitions are calculated. The most accurate first-principles approaches currently available to model interactions such as quantum-chemical MP2 calculations [154] can only be applied to small clusters of atoms even when modern supercomputers are used. The immense computational power needed can be reduced by using simplistic classical molecular dynamics simulations or quantum-chemical approximations, which in certain cases have been found to provide results (e.g. ligand binding energies) in good agreement with more accurate calculations and experiments [155]. Depending on computational cost of the different methods, systems between <10 up to ~1000 atoms are typically studied, which comprise only the smallest particles used in practice (usually ~200-10,000 atoms) although this does allow simulation of magic-sized clusters for which empirical atomistic structures are available [154, 155].
6.2 Modeling ligand coordination
The surface structure of QDs has been studied using bare facets and surfaces bound by theoretical ligands or ligands with varying degrees of physical relevance. Bare QDs undergo substantial atomic rearrangements and small bare WZ clusters are unstable with respect to relaxation [152]. This is consistent with our own studies of CdSe QDs, which exhibit a near-zero bandgap (i.e. metallic) in the absence of ligands or passivating atoms. For this reason, pseudo-hydrogen atoms with 0.5 and 1.5 charge can be used to passivate DBs of Cd and Se, respectively. However hydrogen passivation is not always suitable, especially for small QDs, and can lead to their disintegration [154], or yield unrealistically large relaxations of the surface atoms and destabilize the bulk-like structure of the core [156]. Incorporating realistic ligands necessitates the addition of more atoms, which increases the computational cost, so realistic ligands with alkyl chains are usually truncated to include a minimum number of carbon atoms.
An alternative strategy for systems that are too large for explicit simulations is to simulate surface slabs with different facets [24, 26, 53]. This may be a viable approximation for large QDs where surface curvature may not need to be explicitly taken into account and for nanoplatelets with very large surfaces [28, 77]. It is important to note that many assumptions enter into these calculations that are not fully supported by robust evidence, especially the use of single ligand types, as a mixture of different types are usually bound. Constructing the surface is also nontrivial since many different inputs such as bare ideal facets, reconstruction, and assumed configurations with ligands can have important effects on final calculated values. Calculations of surface energies and binding energies can therefore vary widely. Nevertheless, DFT studies have been invaluable for understanding the facet-specificity of ligand binding [51, 157], the ordering of ligand binding energies [26, 154, 155, 158], and the optimum binding geometries of ligands to specific facets [54, 77].
6.3 Modeling ligand packing
While it is reasonable that metal-coordination bonds may be realistically simulated using truncated ligands, steric effects from the tail are lost with this approximation [37]. The influence of van-der-Waals interactions between tails and ligand packing density are currently not well studied, but it has been possible to combine ligand coordination models with force-field models of the tails to determine their orientation and impact on binding affinity, considering the tails independently from surface binding groups [51, 61]. From these results, it has been observed that ligand tails significantly interplay with ligand binding to result in dense packing and conformations that can span orientations perpendicular to the surface to ones that are more bent, depending on the density and degree of hydrocarbon saturation. The ligand tails are also expected to be impacted by interactions with solvation shells, solvent adsorbates, and desorbed ligands, which are all expected to be impacted by temperature and ligand volatility. For example, it was found that carboxylate X-type ligand binding on {100}ZB surfaces is diminished roughly 3-fold in polar solvents such as acetonitrile when compared with vacuum [51, 159]. Incorporating all important constituents into a single model is a major challenge but will be necessary for providing mechanistic insights into some of the more dynamic processes thought to occur structurally on the surface.
6.4 Calculating surface-dependent optical properties
Theoretical modeling of electronic and optical properties is even more challenging than a description of the low-energy atomic configurations: It requires not only accurate atomic positions but also a reliable approach to compute optical transitions, the electronic structure, and excited states. In addition the calculation of a converged optical absorption spectrum requires a dense sampling of electronic wavefunctions in real or reciprocal space (i.e. the Brillouin zone), which makes the simulations computationally challenging. In practice it turns out that Kohn-Sham electronic orbitals from density functional theory (DFT) are well-suited to compute optical transition probabilities but they do not accurately describe the excitation energies and infamously severely underestimate the electronic bandgap. The description can be improved using either time-dependent DFT or many-body perturbation theory, but at a higher computational cost [160].
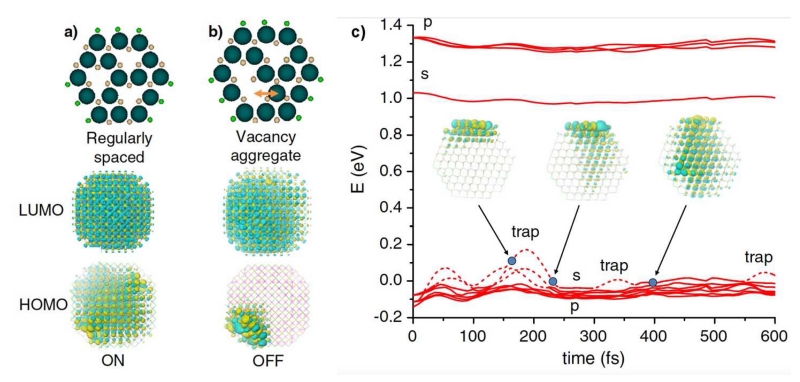
A host of interesting optical and charge transfer phenomena have been predicted and studied at the atomic level using DFT [161]. In one of the most intriguing examples (see Fig. 7) using a structure derived from cation-rich magic-sized clusters, surface fluctuations of electronic trap state energies and surface vacancies were observed that may be related to QD blinking and spectral wandering [78, 162]. The traps were observed to move at the femtosecond time scale, and vacancies moved due to thermal vibrations. In addition, these studies showed that CN=3 cationic facets produce no surface traps even when DBs are unpassivated, indicating that trapless nanocrystals may be possible even without complete DB passivation. However, CN=3 anionic facets yielded traps that could not be passivated, even by Z-type ligands. Therefore the high QY state is expected to be one in which anionic sites are removed, yielding lattice vacancies. These studies are consistent with experimental evidence for these magic-sized clusters, as they do not show vacancies and indeed, their emission is largely dominated by deep trap states [163]. Importantly, X-ray structures that provide empirical insights may simply represent the lowest energy configuration of a mobile surface, much like the crystal structures of dynamically moving proteins.
Fig. 7.
Relationship between surface vacancy defects and electronic energy states via DFT. (a) A CdSe QD with regularly spaced atomic vacancies shows no surface states in the HOMO or LUMO orbital wavefunctions. (b) If the vacancies diffuse to yield local aggregates, they form HOMO traps on the surface, which can turn the fluorescence off. (c) Shallow trap dynamics of a CdSe QD with both Cd and Se vacancies, showing that energy levels fluctuate over time as vacancies diffuse. The s and p orbitals near the 0 energy level are the HOMO levels which show fluctuations on the femtosecond time scale, randomly trapping the hole, which may indicate blinking and spectral diffusion. Figure is reproduced from reference [162] with permission.
7. Surface engineering for biomedical applications
For nearly 20 years, QDs have been used to as fluorescent probes to image and detect biological molecules such as proteins and DNA in biological fluids, living cells, and living organisms [2, 164]. For these applications, QDs must be coated in ligands compatible with aqueous biological media because bare surfaces are highly reactive and adsorb the constituents of biological solutions. These ligands are bifunctional: the headgroup chemisorbs to a surface facet and is appended through a backbone to a tailgroup that interfaces with the surrounding media, which is composed of water molecules, ions, small molecules like sugars, and macromolecules like proteins. It is critical that the surface ligands are precisely controlled because they are the primary determining factor of hydrodynamic size, optical and chemical stability, and nonspecific interactions. Therefore, ligands are a major focus of current efforts for optimizing QDs for molecular imaging and labeling in complex solutions, cells, and tissues.
7.1 Colloidal size
For most applications, it is desirable to minimize the QD size to 5-10 nm in diameter so that they minimally perturb the binding event or biological process under study. Artifacts due to steric hindrance are particularly evident in crowded media of cells and tissues, which have characteristic size thresholds above which large QDs (~15-35 nm) cannot be used to accurately measure biomolecular targets. One of these is the synaptic cell-cell junction between connected neurons, a 20-30 nm region that large QDs cannot access [165]. Large QDs are also immobile in the cellular cytoplasm [166, 167], cannot efficiently penetrate through tissue matrix [168], and cannot clear from mammalian vascular circulation through renal filtration [168-170]. However, it is a general trend that smaller QDs with thin coatings (~1-2 nm) have low stability due to ligand decomposition or ligand desorption from the surface. Long-term stability is usually only possible when the ligand coating layer is >3 nm and more often 5-10 nm. This tradeoff between size and stability has been a major focus of recent research efforts toward generating QDs that can be more widely employed in sterically crowded media.
7.2 Stability
Maximum stability is desired to provide a continuous fluorescent signal. Destabilization can be due to oxidative etching of the nanocrystal, ligand detachment, chemical decomposition of ligands, or colloidal destabilization-induced aggregation [171]. Ligand coatings are generally optimized to provide stability against chemicals present in the complex biological media in which they are used. Usually these are optimized for the pH range of normal mammalian physiology (~7.2-7.6), although some specific applications require stabilization in more extreme pH ranges of lysosomes (~5.5) or the gut (~1-10) [172], in which hydronium or hydroxide ions can alter the protonation state of X-type and L-type ligands to induce ligand desorption and surface traps [98, 173-175]. Most biological fluids are also high in salinity (~0.3 osmolar) and can either be oxidizing (e.g. arterial blood) or reducing (e.g. cellular cytosol or hypoxic tissue) [176]. Oxidizing agents can chemically oxidize thiol ligands to cause ligand desorption, and oxidize chalcogenides in the core to etch the nanocrystal [171, 177]. Reducing environments are generally more stabilizing for QDs, but small biogenic reducing compounds such as glutathione, which contains a reduced thiol, also function as ligands and may displace native ligands on QDs surface [178, 179]. Proteins in solution exhibit the most deleterious impact for current applications, as they bind to the surface and “foul” its function, as described below.
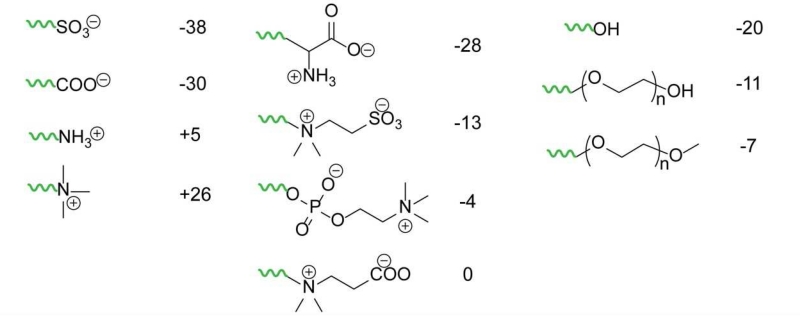
7.3 Hydrophilic ligand categories
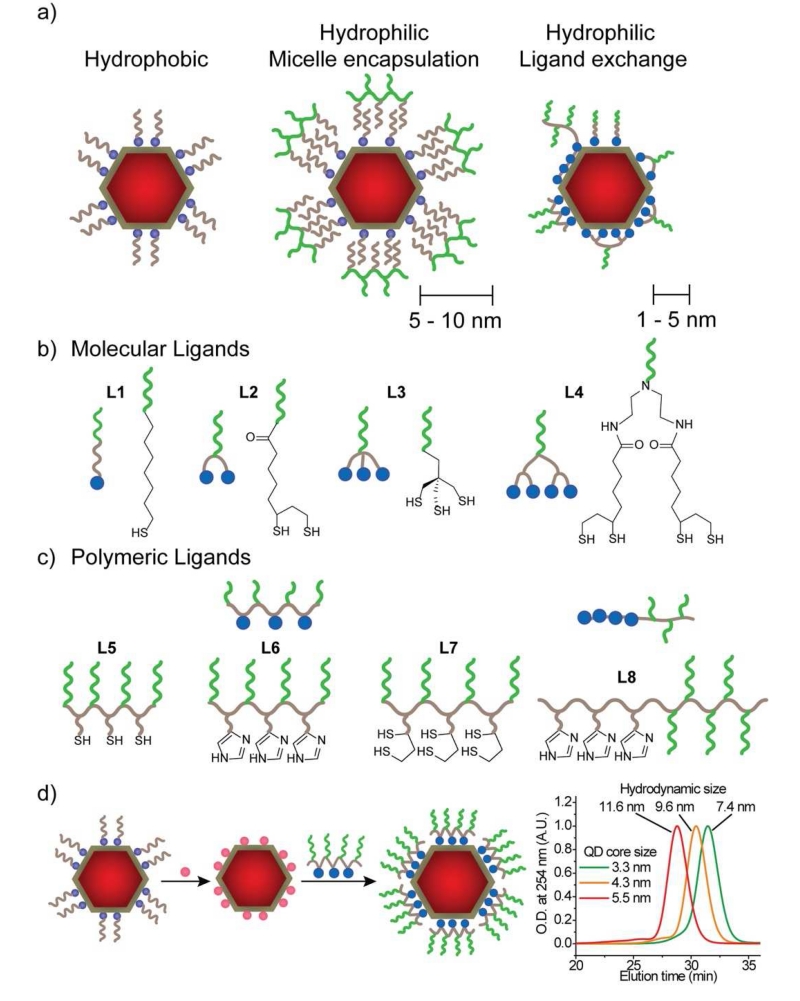
High quality QDs are initially prepared with a ligand coating such as alkylcarboxylates (e.g. oleic acid) and alkylamines (e.g. oleylamine) that yield a highly hydrophobic nanocrystal that is not dispersible in water. To render these stable in aqueous phase for use in biological environments, there are two general methodologies: micellar encapsulation and ligand exchange (shown in Fig. 8a). For micellar encapsulation, the QD and its hydrophobic ligands are together assembled into the core of a micelle composed of polymers or lipids. For ligand exchange, a wide variety of hydrophilic monodentate and multidentate ligands have been explored that displace the original hydrophobic ligands. Each of these coatings results in different combinations of size and stability, which fundamentally derive from three key parameters: ligand size, packing density on the surface, and binding affinity.
Fig. 8.
Hydrophilic ligands used for QDs in aqueous solution, distinguished by their conformation on the surface and number of binding domains. Colored circles indicate ligand head groups, brown lines indicate hydrophobic backbones (e.g. alkanes), and green lines indicate hydrophilic groups (e.g. PEG). (a) Hydrophobic QDs are coated with hydrophobic alkyl ligands, Hydrophilic micelle-encapsulated QDs are further surrounded by an amphiphilic polymer, yielding a coating that is 5-10 nm thick. Hydrophilic ligand-exchanged QDs have thinner coatings (1-5 nm) and have been designed with diverse chemistries. (b) Examples of small molecule ligands, distinguished by the number of binding groups [165, 179-183]. (c) Examples of polymeric ligands, distinguished by the types of binding groups and whether they are random copolymers (L5-L7) or block copolymers (L8) [184-188]. (d) Coating methodologies for multidentate polymeric ligands require an intermediate ligand exchange step to render the nanoparticle hydrophilic with monodentate ligands (pink circles) prior to coating with polymeric ligands to ensure homogeneous multidentate attachment and compact binding, as assessed by gel chromatograms at right, showing hydrodynamic sizes in the range of 7-12 nm for three different particle core sizes (reproduced from reference [189] with permission).
7.4 Micellar encapsulation is a process that derives from aqueous phase self-assembly of amphiphilic molecules to generate colloidal structures containing a hydrophobic core domain that can entrap a QD [190-192]. The QD ligands bound immediately after synthesis are retained on the surface, which is a tremendous advantage, as the native ligands often are chemically matched to passivate all charged and neutral surfaces. After micellar encapsulation, these ligands are associated with the hydrophobic domains of the amphiphilic encapsulants due to hydrophobic interactions, forming a densely packed hydrophobic bilayer because of short-range van der Waals interactions. Due to this environment and their intrinsically low solubility in aqueous solution, this coating structure is extraordinarily stable colloidally, allowing homogeneous dispersion sometimes for years [171, 192, 193]. This outcome is quite remarkable considering the weak binding strength between the ligand and QD facet, which is similar to that of a single hydrogen bond. These QDs are also highly chemically stable as a result of the dense packing in the alkyl bilayer that prevents access to polar compounds, hydronium ions, and many oxidizing agents [171, 177].
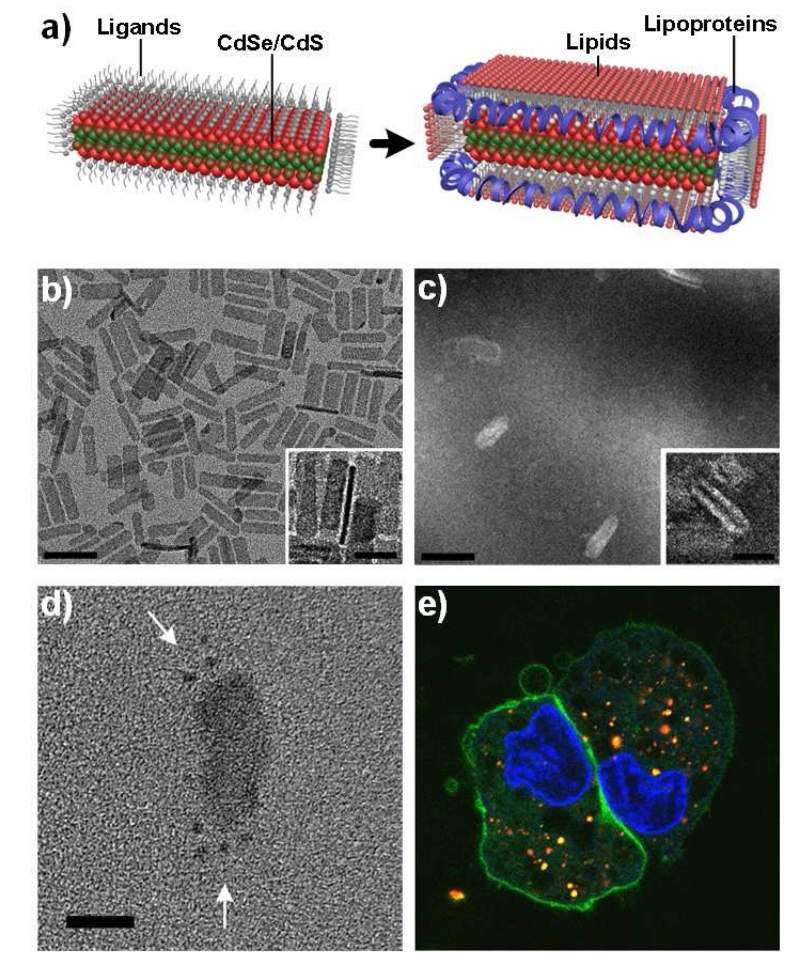
Diverse classes of amphiphilic molecules and polymers have been used for micellar encapsulation and usually contain alkyl chains that chemically match the QD ligands. For maximum stability, it is important to select amphiphiles that match the nanocrystal surface curvature such that the amphiphiles natively self-assemble in aqueous solution in geometries reflecting the nanocrystal being encapsulated. Thereby most lipids used for spherical QDs have a packing parameter greater than 1 [194], meaning that the volume of the hydrophilic group is larger than that of the hydrophobic group [195]. Phospholipids with a large head domain (e.g. polyethylene glycol, PEG) work well to entrap single QDs, whereas phospholipids with sterically small choline or serinol head groups do not work well. These latter lipids natively self-assemble into two-dimensional lamellar sheets. As shown in Fig. 9, we have recently found that these lipids efficiently encapsulate planar nanoplatelets, as they pack densely on the flat tops and bottoms of these nanostructures. However, this assembled structure is only stable when a secondary amphiphile is added that can bind to the high curvature edges. In this case, helical lipoproteins called membrane scaffolding proteins (MSPs) have been shown to efficiently bind to the sides of lamellar sheets to generate nanodisc structures that are alone highly stable [196]. This combination of phospholipids and MSPs efficiently coat all sides of a nanoplatelet, with different adsorbates localizing to different regions depending on curvature (Fig. 9c). When encapsulated in this manner, these lipoprotein-nanoplatelets (L-NPLs) are stable for weeks at room temperature and in high salt solutions (>4 M NaCl), and interestingly rapidly enter cells due to their large surface area (Fig. 9e). PEG-phospholipids also yield highly stable NPLs in solution, but their QY is entirely quenched, likely due to uncoated nanocrystal surfaces caused by low amphiphile packing density.
Fig. 9.
Micellar encapsulation of sheet-like nanoplatelets using a combination of lipids and membrane scaffold proteins (MSP). Both coatings are required to enable full coating of flat surfaces and highly curved surfaces of the nanoplatelets. (a) Schematic depiction of the nanoparticle coating process. (b) Transmission electron microscopy image of CdSe/CdS core/shell nanoplatelets. (c) Counterstained electron microscopy image of encapsulated nanoplatelets. Insets show side-views of particles. Scale bars = 50 nm in wide-field images and 20 nm in insets. (d) Encapsulated nanoplatelets after MSP conjugation to spherical gold colloids to localize the protein with respect to the nanoplatelet. Arrows indicate the gold. Scale bar = 50 nm. (e) Fluorescence image of A431 cells 24 hours after exposure to lipoprotein-nanoplatelets, shown in red. Cells are counterstained with a nuclear dye (blue) and a plasma membrane dye (green). Nanoplatelets remain brightly fluorescent and localize throughout the cells. Image reproduced with permission from reference [195].
These micellar coatings are the standard of commercially sold QDs, with excellent optical properties and stability [191] however their main drawback is their size. The combination of a hydrophobic bilayer with its hydration shell is typically at least 5 nm thick (oleic acid is about 2.3 nm outstretched). With a typical core size of 5-6 nm, these nanoparticles usually have a hydrodynamic diameter of 15-20 nm, and many polymer varieties yield a size of 30-40 nm [171, 192, 193, 197]. This has limited their use to applications where diffusion in dense matrices is not necessary.
7.5 Mono- and bi-dentate ligands have been most often employed when minimal size is desired, and are usually hydrophilic X-type thiolates (L1 and L2 in Fig. 8b). These ligands commonly contain one thiol headgroup (e.g. mercaptoacetic acid or thiol-PEG) [198] or two thiols (primarily derived from dihydrolipoic acid, DHLA) [199], and have diverse tail group chemistries. These ligands replace the original hydrophobic ligands on the surface but provide only temporary stabilization in aqueous solution because the weak binding affinity cannot fully prevent desorption from the surface, which is irreversible near neutral pH [98]. The thiol can also oxidize under fairly mild conditions to form disulfides that no longer bind as X-type ligands [133, 200]. Stability is especially problematic when the particles are highly dilute, as the ligand equilibrium shifts toward the dissociated state [201], and when multiple purification steps are needed, which repeatedly removes desorbed ligands to strip the ligands from the surface. In addition, when the particles are exposed to light, photocatalytic redox reactions on the surface can oxidize the ligands more rapidly [94].
7.5.1 The nature of the thiolate bond
Despite their low bond strength to the QD surface (25-35 kJ/mol, 10 times weaker than a C-C or C-H bond) [98], thiolate ligands are widely used and may be uniquely suited for surface passivation due to the nonpolar nature of S-H and S-C bonds [202], which makes them relatively insoluble in aqueous solution at pH values substantially lower than the pKa of a thiol (~10). This is evident from the lack of successful use of other X-type ligands (such as phosphates and carboxylates) that incorporate electronegative heteroatoms with lower pKa values. These other binding groups can have greater or similar binding constants as thiols in nonpolar solvents but have much higher solubility which shifts their equilibrium toward the desorbed state unless excess ligands are present, conditions generally not suitable for most applications. Interestingly, thiols appear to be a fairly universal way to render QDs of many chemistries and shapes water soluble, although it is not clear how they adsorb strongly to neutral facets – it is possible that a multi-faceted QD may undergo substantial rearrangements of surface atoms or may be passivated by thiolates with their associated protons or other counterions to balance the surface charge. Note that it also possible to synthesize QDs in the presence of thiolates to select for facets with higher binding affinity [203].
7.5.2 Optimized thiolate structures
The packing density of ligands on the surface is thought to be a major determining factor in stability. Much of what we know derives from extensive structure-function relationships of alkane thiolate SAMs on gold surfaces [204]. In these systems, a higher ligand surface density and longer alkyl chains yield closer alkane-alkane packing and stronger van der Waals interactions that prevent solutes from diffusing to the substrate surface that can etch or degrade the underlying nanocrystal or ligand [80, 205]. In addition, tightly packed alkane thiolates tend to stay locked together even if their coordination to the surface is broken, so dissociation from the surface is slower [206], and such additive physisorption interactions can be just as strong as ligand-mediated chemisorption [43]. To render QDs polar, alkane thiolates can be terminated with a polar tail group (e.g. mercaptohexadecanoic acid) [94]. In general, longer alkane chains yield higher stability against oxidation [94]. However single hydrophilic functional groups are not usually sufficient to prevent aggregation in high salt, and instead PEG thiolates have been widely explored as hydrophilic variants of alkane thiolates on both SAMs and nanoparticles. However the stability of PEG thiolates is diminished due to their lower packing density (~2.2 nm−2) compared with alkane thiolates (~4.5 nm−2) and their reduced inter-chain van der Waals interactions due to hydration, which favors dissociation [207]. Our groups and others have therefore focused on ligands that balance the stability imparted by hydrophobic alkanethiols and the hydrophilic capabilities of PEG [165] using alkane thiolates with PEG tail groups. The hydrophobic alkane dramatically increases the overall stability compared with PEG-thiolates from approximately one day to over a month. For these hybrid ligands, packing densities on gold nanoparticles (3.9 nm−2) and gold surface (3.2 nm−2) are substantially higher compared with PEG-thiolates [82]. It is critical to balance the hydrophobic and hydrophilic character to both minimize ligand solubility and provide hydrophilic character for colloidal stability [98], but optimal ligand designs may not be universal for all QD sizes due to the size-dependence of packing densities [208, 209].
7.6 Molecular multidentate ligands
Ligands L2-L4 in Fig. 8b depict a small subset of the diverse multidentate ligands that have been explored as small molecules. By increasing the number of ligating groups per molecule, the equilibrium constant of ligand adsorption to the QD surface can theoretically increase exponentially [210, 211]. While quantitative studies are sparse, binding strengths have been observed to be slightly higher for ligands containing two thiols to gold surfaces [212] and to QDs [98]. However in molecular ligands, the flexibility needed to maximize binding affinity [210] may be difficult to achieve and the binding groups typically take up a larger footprint area on the QD surface which can reduce ligand packing density [213]. As such, the stability of the QDs may be greater in storage, but they may be more susceptible to degradation toward solutes that can etch the QD or oxidize ligands due to their greater permeability [80]. Optimizing ligand designs can be difficult due to non-independent contributions from ligand affinity, packing density, conformation, hydrophobicity, and solubility. In addition, while much is known much from SAMs on gold surfaces, these highly stable 2D systems do not reflect more dynamic processes of colloidal QD surfaces.
7.7 Polymeric multidentate ligands have been used in recent years to overcome the limited aqueous stability of QDs coated with molecular thiolate ligands while maintaining a small size [184]. Lequeux and coworkers estimated a binding affinity increase of almost three orders of magnitude for a polymer containing just a few binding groups [201] and the resulting QDs have been shown to exhibit dramatically increased colloidal stability [179, 183, 184, 214, 215]. QDs coated with polymeric multidentate ligands are stable for months to years without ligand loss, even after extensive purification and dilution, without the need to incorporate hydrophobic domains for stabilization. Compared with molecular multidentate ligands, the backbones can be more flexible and the number of binding domains can be much greater to allow improved attachment to the surface. Moreover, the density of hydrophilic domains and binding domains can be chosen independently to tune packing parameters and to append a variety of chemistries together on the same molecular backbone, as it is challenging to achieve a chemically mixed surface using molecular thiolates that may phase separate on surfaces [212, 216].
7.7.1 Multidentate polymer structures
These ligands are usually prepared with a linear polymeric backbone decorated with groups that either bind to the QD facets or extend away from the QD surface to interact with the solvent. A relatively small range of architectures has been explored, briefly summarized as L5-L8 in Fig. 8c [184-186, 201, 217-222]. Ligating groups include both X-type thiolates as well as L-type imidazole and pyridine groups, both of which yield high stability at neutral pH. While imidazole and pyridine ligands are unstable in an acidic environment (pH < 6) due to ligand protonation caused by their high pKa (imidazole, pKa = 6 [220]; pyridine, pKa = 5.6 [219]) [185, 219], polymers containing both thiol and imidazole groups (L-8) yield QDs with high stability across a wider pH range (3-13) [220]. Note that the successful use of L-type ligands appears to require polymeric multidentate attachment, but it is not clear how these ligands passivate diverse facet chemistries; counterions may play an important role in their association with charged facets. Imidazole groups may be unique, however, as their protonated imidazolium ions may stabilize anionic facets through π interactions [223] and may also bind through one amine to cationic facets [224].
7.7.2 Optimized binding conformation
Despite their high binding affinity, it is challenging to attach multidentate polymeric ligands to QD surfaces in their most stable and compact conformation. During coating, some of these anchoring groups unbound to the surface can crosslink QDs, which can be deleterious for most applications [218, 225, 226]. Indeed the coating process is quite different compared with mass action-driven attachment of molecular ligands, as the multidentate binding affinity is so high that the quantity used for coating can be stoichiometric [184]. However multiple kinetically stable conformations may be possible due to the polymer flexibility, although the minimum energy conformation should be one with maximum number of binding groups adsorbed to the surface, forming a “loops-trains-and-tails” conformation [227-229]. Indeed it is has been widely reported that for molecules with multiple ligands, full coordination is not always achieved, especially when surfaces are not atomically flat, like in QDs, and in conditions of excess ligands [80, 230-234]. To achieve homogeneous products, it is critical to initially displace the original hydrophobic ligands on the nanocrystal with weakly bound hydrophilic ligands. We recently screened a variety of intermediate ligands and found that weakly bound X-type ions (hydroxide) [47] are optimal for yielding compact and homogeneous products (Fig. 8d) due to their high charge that stabilizes the particles in polar solvents and allows rapid displacement by multidentate polymers [189]. By heating at high temperature (110°C), small QD clusters are dissociated, the polymer is densely packed, and QY can be high, generating homogeneously coated nanocrystals with a hydrodynamic size of 7-12 nm with exceptional stable in aqueous solution in ambient conditions for more than one year without noticeable size changes [184, 214].
7.7.3 Future improvements
While these polymeric multidentate coatings are colloidally stable and yield compact products, further engineering is needed to truly minimize the coating size and to make them more chemically inert. Multidentate ligands have been widely explored on solid substrates [211, 212, 235] and it has been frequently observed that as the degree of multidentate binding increases, the ligand packing density on the surface decreases [235] causing the monolayer porosity to increase [80, 211]. Indeed, for QDs, their surfaces remain accessible to chelating solutes like dithiothreitol and EDTA that can disrupt the coating, which is problematic due to their common use for protein stabilization. Therefore, the true “ideal” structure may be imagined to be one in which a monolayer of ligands are densely packed and fully crosslinked in two dimensions across the surface. This is possible with crosslinked polymers (e.g. polyethylenimine and dendrons), however these tend to yield much bigger products due to their inability to bind in a flat conformation on the surface [177, 236, 237]. Amorphous silica (SiO2) has been widely used to provide highly crosslinked shells on nanocrystals that yield stable products, coated directly through oxide coordination or via ligand anchoring groups [238]. However despite nearly 20 years of reports, silica-coated QDs have not been widely adopted due to the difficulty in generating shells less than ~5 nm due to complex and unpredictable nature of silane chemistry [239-241].
7.8 Nonfouling stabilizing groups
The surface coating not only affects hydrodynamic dimensions and chemical stability of QDs, but also determines their colloidal stability and interactions with biological molecules such as proteins. In aqueous solution, hydrophilic domains of bifunctional ligands are hydrated by water molecules and may associate with counterion layers, which together stabilize QD dispersions through electrostatic or steric repulsion [242]. Table 1 depicts many of the ligand terminal groups in common use and the typical zeta potential of the resulting QDs at physiological pH [186, 243-250]. In general these ligands are chosen so as to minimize interactions with proteins and cell membranes. In protein-rich solutions such as blood plasma or cell culture media, QDs and other nanoparticles will rapidly adsorb a protein corona to their surfaces, a process called “fouling” that has been recently studied in detail through proteomic analysis of the adsorbed proteins [251]. This effect is undesirable as it will yield associations with proteins and cellular components that are not the intended target for study or analysis, which increases background signal, and thus decreases the signal-to-noise ratio of detection assays and imaging experiments [250].
Table 1.
Hydrophilic groups used for QD stabilization and typical zeta potential at physiological pH.
QDs with charged surfaces (e.g. carboxylate or ammonium functional groups) can be very stable as colloids due to electrostatic repulsion to prevent aggregation but are prone to adhesion to opposite charged domains of cells and proteins through electrostatic attraction [164, 252]. Therefore there has been a major focus on hydrophilic but electrostatically neutral surface coatings, and a wide diversity of chemical moieties have been explored [253]. The primary strategy is to use flexible PEG polymers, which can be tethered to the surface by one end to stabilize the QD dispersion, sterically blocking the intrinsically sticky surface of the QD and providing an entropic barrier to ligand fouling [254-256]. However, nonspecific binding resistance depends on the length and conformation of the polymer. While higher molecular weight improves fouling resistance, the packing density must be specifically balanced to prevent access to the surface and to limit coating rigidity. Unlike in the case of surface porosity, it has been found that a more sparse packing near 0.5 molecules nm−2 for short-chain PEG is optimal to yield a disordered “mushroom” conformation that more strongly interacts with water [257] to repel protein adsorption [258, 259]. Likewise increased rigidity of the coating by chain-chain interactions driven by hydrogen bonds can enhance protein fouling [260]. While these findings indicate a tradeoff between chemical stability and anti-fouling characteristics, others have not detected an upper limit for PEG packing density for minimizing fouling [261].
We have found that a densely hydroxylated surface can yield stable surfaces that are highly resistant to nonspecific binding compared with long-chain PEG coatings [250], but with a much more compact size. However hydroxyls cannot simply be appended to the termini of hydrophobic chains [258], but instead require hydrogen bond acceptors such as ether or carbonyl groups to provide adequate ligand hydration to prevent protein adsorption. Solutes and proteins can penetrate beyond the terminal group of a ligand layer and hydrophobic groups directly adjacent to the tail can favor binding to hydrophobic domains of proteins. Another strategy is to use zwitterionic coatings containing an equal mix of cations and anions so that the nanoparticle is nearly electrostatically neutral but adsorbs many counterions with their associated water molecules. Groups such as sulfobetaine, carboxybetaine, cysteine, and phosphorylcholine have been widely explored [170, 186, 215, 245, 247] and found to strongly resist protein and cell binding, in some instances with better performance than PEG-based coatings [262]. These polymer types have been investigated as both linear and brush conformations, and in general, denser engraftment of hydrophilic groups yields better resistance to nonspecific binding [253, 263, 264], but architectures to simultaneously minimize size have not yet been optimized.
8. Attachment to molecular targets
For use as molecular and cellular imaging agents, QDs are chemically coupled to functional biomolecules such nucleic acids, peptides, aptamers, and antibodies that allow binding to specific targets [265]. Ideally, conjugation methods should be efficient, minimally perturb QD optical properties, yield stable products, and retain the activity or binding affinity of the conjugated targeting agent. Conjugations are usually performed by covalent coupling between the ligand terminal groups on the QD and the biological molecule, and are usually more efficient when the linking group is tethered to a flexible chain [266]. As shown in Table 2, carboxylic acid, amine, and thiol groups can be used to generate stable amide or thioether bonds with biological molecules by using activating agents such as carbodiimides, succinimides, and maleimides [164, 267-271]. However, amide-generating reactions can be challenging to control due to the ubiquity of carboxylic acids and amines on both QDs and proteins, so conjugation products are often heterogeneous. Maleimide-thiol reactions are more controllable due to precise placement of thiols on nucleic acids and proteins, but succinimidyl thioether products can be cleaved by excess thiols such as cysteine and glutathione, which are abundant in cells [272].
Table 2.
Conjugation chemistries for attaching proteins and nucleic acids to QDs.
| Conjugation | QDs | Biomolecules | Product |
|---|---|---|---|
| Amide formation |
|
|
|
| Thioether formation |
|
|
|
| Tetrazine-norbornene cycloaddition |
|
|
|
| Azide-alkyne cycloaddition |
|
|
|
| Streptavidin-biotin binding |
|
|
|
| Antibody-Protein A/G binding |
|

|
|
| His-tag self-assembly |

|
|

|
Much more controllable reactions can result from “click” chemistry approaches that can allow the generation of conjugates without the need for coupling reagents or catalysts [270]. Norbornene-tetrazine cycloaddition reactions [217, 218] and strain-promoted azide-cyclooctyne cycloaddition reactions [189, 273] have been found to be fully compatible with QDs. These reactions are often quantitative in yield and therefore do not require purification, in contrast to conventional amide-generating coupling for which extensive purification steps are required. The reactions are also bioorthogonal, meaning that the reagents do not react with functional groups in native proteins or nucleic acids, providing high specificity [274]. However some click coupling chemistries are incompatible with QDs, such as copper-catalyzed reactions due to high affinity copper interactions with QD surfaces that results in irreversible fluorescence quenching [275, 276].
The most commonly used coupling technique is streptavidin-biotin binding due to commercial availability of streptavidin-coated QDs and the ease of introducing a biotin molecule to almost any type of biological molecule. The strong affinity (88 kJ mol−1, dissociation constant Kd = 10−15 M) yields highly stable products [277, 278], but this interaction necessarily adds additional bulk due to the ~7 nm size of streptavidin. Also streptavidin is a tetramer with 4 biotin binding sites, which yields an uncertain stoichiometry with products and potential for crosslinking targets. Monomeric streptavidin has been used to prevent cross-linking of biotinylated cell-surface proteins but generation of the monomeric streptavidin remains challenging [279]. For antibody conjugation, molecular adaptor proteins such as Protein A/G or Fc Receptor provide unique advantages due to oriented attachment to antibody regions that are not needed for target binding, leaving the target-binding domain extended away from the QD surface, without the need for covalent modification of the antibody [226, 280, 281]. Protein A and G are also oligomers that can bind to multiple proteins, whereas Fc Receptor I is monomeric and binds to a single antibody [226].
Proteins have also been engineered with metal-binding peptides to bind specifically to QDs by self-assembly. Oligo-histidine (his6-tag) binds directly as a ligand on the QD facets [226] because histidine is an amino acid with an imidazole residue, and thus his6-tag can bind in the same way as a multidentate polymer. Compared to covalent conjugation, the use of his6-tag for QD bioconjugation is a versatile technique that allows controlled orientation of proteins on the surface and high product yield so that purification is not needed. However his6-tag self-assembly efficiency is sensitive to the thickness of the surface coatings, and surfaces with a PEG coating with molecular weight higher than 500 Da can inhibit binding [282]. In this case, more porous multidentate coatings and thinner coatings are beneficial for his6-tag biomolecules attachment [265].
9. Conclusions
Over the past 30 years, substantial progress has been made toward understanding the nature and structure of QD facets, their ligand coordination chemistry, and engineering principles for practical implementation in biological media. However, surfaces still remain the critical limiting characteristic for most applications, as they are the site that yields colloidal instability and degradation, fluorescence loss, charge trapping, and nonspecific binding. Further studies are needed to experimentally determine the independent contributions and interplay between facets and ligand chemistry in aqueous environments, as much remains unknown in highly polar media in terms of their binding strength and bonding nature.
It would be of great value to study ligand binding on QDs with only single facet types, some of which can already be prepared, as it is plausible that inconsistencies in reported stability and hydrodynamic dimensions may derive from dissimilar facets generated by different preparation methods. New characterization tools are needed to validate the presence of specific facets, determine their level of reconstruction, and measure surface traps directly. Further studies also should focus on the nature of multidentate binding, which seems to be the critical technology for generating ultra-stable QDs. While polymeric ligands are challenging to assess because they are inherently polydisperse and their interactions with QDs may be heterogeneous, there is significant merit in focused explorations of precisely controlled molecular multidentate ligands, as has been performed already for SAMs on metal surfaces [212].
Further studies are also needed to understand the physicochemical mechanisms that lead to nonspecific interactions with proteins, and how to best minimize it. While extensive studies have been successful in minimizing nonspecific binding from flat surfaces [253, 283], studies are needed to understand how these effects differ on colloidally dispersed and curved surfaces. The ultimate goal for biological applications is to have a QD with minimal coating size (<1 nm thick), without nonspecific interactions, and with long stability (many hours in cells and tissues and months to years in storage). Biogenic colloids such as proteins already provide these attributes, so biologically inspired engineering approaches to emulate the surfaces of these macromolecules should provide a logical direction for these future studies.
Highlights.
In fluorescent quantum dots, the surface of the nanocrystal is the primary determining factor in the efficiency of light emission and stability, as well as binding to biological molecules and cells.
Crystalline facets determine the shape of a quantum dot as well as interactions with coordinating ligands.
New ligands are being designed for small hydrodynamic size, high stability and fluorescence efficiency, and minimal nonspecific interactions with cells and biological molecules.
Many questions remain in the chemistry and physics of quantum dot surfaces that have hindered key breakthroughs for full optimization of their properties.
Acknowledgments
The authors thank Paul Selvin and Pinghua Ge for insightful discussions and thank funding organizations who sponsored the work. The authors acknowledge support from the NIH (AMS: R00CA153914 and R21NS087413; LM: T32ES007326) and the UIUC College of Engineering Strategic Research Initiatives Program (AMS). This research is part of the Blue Waters sustained-petascale computing project, which is supported by the National Science Foundation (awards OCI-0725070 and ACI-1238993) and the state of Illinois.
Footnotes
In this review we use Miller index notations simply to distinguish facet chemical attributes – an extensive understanding of crystallography is not necessary. Miller indices in brackets, such as [100], are standard notation for crystallographic directions, whereas indices in braces, such as {100}, indicate the crystal plane orthogonal to the direction. Three numbers are used for ZB and four numbers are used for WZ, indicating the crystallographic direction in reciprocal space, as described in standard materials science texts (G. Gottstein, Physical Foundations of Materials Science, Springer, New York, NY, 2004 [20]). Unit vectors are shown in Figure 2a and 2b. A dash ‘-’ above a number indicates a negative value.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Kairdolf BA, Smith AM, Stokes TH, Wang MD, Young AN, Nie SM. Ann. Rev. Anal. Chem. 2013;6:143–162. doi: 10.1146/annurev-anchem-060908-155136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Vu TQ, Lam WY, Hatch EW, Lidke DS. Cell Tissue Res. 2015;360:71–86. doi: 10.1007/s00441-014-2087-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Talapin DV, Lee JS, Kovalenko MV, Shevchenko EV. Chem. Rev. 2010;110:389–458. doi: 10.1021/cr900137k. [DOI] [PubMed] [Google Scholar]
- [4].Nozik AJ, Beard MC, Luther JM, Law M, Ellingson RJ, Johnson JC. Chem. Rev. 2010;110:6873–6890. doi: 10.1021/cr900289f. [DOI] [PubMed] [Google Scholar]
- [5].Smith AM, Nie SM. Acc. Chem. Res. 2010;43:190–200. doi: 10.1021/ar9001069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Anikeeva PO, Halpert JE, Bawendi MG, Bulovic V. Nano Lett. 2009;9:2532–2536. doi: 10.1021/nl9002969. [DOI] [PubMed] [Google Scholar]
- [7].Salter CL, Stevenson RM, Farrer I, Nicoll CA, Ritchie DA, Shields AJ. Nature. 2010;465:594–597. doi: 10.1038/nature09078. [DOI] [PubMed] [Google Scholar]
- [8].Lee YL, Lo YS. Adv. Funct. Mater. 2009;19:604–609. [Google Scholar]
- [9].Hollingsworth JA. Coord. Chem. Rev. 2014;263:197–216. [Google Scholar]
- [10].Graetzel M, Janssen RAJ, Mitzi DB, Sargent EH. Nature. 2012;488:304–312. doi: 10.1038/nature11476. [DOI] [PubMed] [Google Scholar]
- [11].Hines MA, Guyot-Sionnest P. J. Phys. Chem. 1996;100:468–471. [Google Scholar]
- [12].Talapin DV, Rogach AL, Kornowski A, Haase M, Weller H. Nano Lett. 2001;1:207–211. doi: 10.1021/nl0155126. [DOI] [PubMed] [Google Scholar]
- [13].Kovalenko MV, Scheele M, Talapin DV. Science. 2009;324:1417–1420. doi: 10.1126/science.1170524. [DOI] [PubMed] [Google Scholar]
- [14].Bozyigit D, Volk S, Yarema O, Wood V. Nano Lett. 2013;13:5284–5288. doi: 10.1021/nl402803h. [DOI] [PubMed] [Google Scholar]
- [15].Peterson MD, Cass LC, Harris RD, Edme K, Sung K, Weiss EA. Annu. Rev. Phys. Chem. 2014;65:317–339. doi: 10.1146/annurev-physchem-040513-103649. [DOI] [PubMed] [Google Scholar]
- [16].McBride JR, Pennycook TJ, Pennycook SJ, Rosenthal SJ. ACS Nano. 2013;7:8358–8365. doi: 10.1021/nn403478h. [DOI] [PubMed] [Google Scholar]
- [17].Bujacz A. Acta Crystallogr., Sect. D: Biol. Crystallogr. 2012;68:1278–1289. doi: 10.1107/S0907444912027047. [DOI] [PubMed] [Google Scholar]
- [18].Alivisatos AP. J. Phys. Chem. 1996;100:13226–13239. [Google Scholar]
- [19].Hens Z, Martins JC. Chem. Mater. 2013;25:1211–1221. [Google Scholar]
- [20].Gottstein G. Physical Foundations of Materials Science. Springer; New York, NY: 2004. [Google Scholar]
- [21].Yeh CY, Lu ZW, Froyen S, Zunger A. Phys. Rev. B. 1992;46:10086–10097. doi: 10.1103/physrevb.46.10086. [DOI] [PubMed] [Google Scholar]
- [22].Lim SJ, Kim W, Shin SK. J. Am. Chem. Soc. 2012;134:7576–7579. doi: 10.1021/ja212205q. [DOI] [PubMed] [Google Scholar]
- [23].Mahler B, Lequeux N, Dubertret B. J. Am. Chem. Soc. 2010;132:953–959. doi: 10.1021/ja9034973. [DOI] [PubMed] [Google Scholar]
- [24].Manna L, Wang LW, Cingolani R, Alivisatos AP. J. Phys. Chem. B. 2005;109:6183–6192. doi: 10.1021/jp0445573. [DOI] [PubMed] [Google Scholar]
- [25].Berrettini MG, Braun G, Hu JG, Strouse GF. J. Am. Chem. Soc. 2004;126:7063–7070. doi: 10.1021/ja037228h. [DOI] [PubMed] [Google Scholar]
- [26].Rempel JY, Trout BL, Bawendi MG, Jensen KF. J. Phys. Chem. B. 2005;109:19320–19328. doi: 10.1021/jp053560z. [DOI] [PubMed] [Google Scholar]
- [27].Liu L, Zhuang Z, Xie T, Wang YG, Li J, Peng Q, Li Y. J. Am. Chem. Soc. 2009;131:16423–16429. doi: 10.1021/ja903633d. [DOI] [PubMed] [Google Scholar]
- [28].Son JS, Wen XD, Joo J, Chae J, Baek SI, Park K, Kim JH, An K, Yu JH, Kwon SG, Choi SH, Wang ZW, Kim YW, Kuk Y, Hoffmann R, Hyeon T. Angew. Chem. Int. Ed. 2009;48:6861–6864. doi: 10.1002/anie.200902791. [DOI] [PubMed] [Google Scholar]
- [29].Scher EC, Manna L, Alivisatos AP. Philos. Trans. R. Soc. London A. 2003;361:241–255. doi: 10.1098/rsta.2002.1126. [DOI] [PubMed] [Google Scholar]
- [30].Ghosh S, Gaspari R, Bertoni G, Spadaro MC, Prato M, Turner S, Cavalli A, Manna L, Brescia R. ACS Nano. 2015;9:8537–8546. doi: 10.1021/acsnano.5b03636. [DOI] [PubMed] [Google Scholar]
- [31].Kim Y, Song NW, Yu H, Moon DW, Lim SJ, Kim W, Yoon HJ, Shin SK. Phys. Chem. Chem. Phys. 2009;11:3497–3502. doi: 10.1039/b822351c. [DOI] [PubMed] [Google Scholar]
- [32].Lim SJ, Smith AM, Nie SM. Curr. Opin. Chem. Engin. 2014;4:137–143. doi: 10.1016/j.coche.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Peng XG, Manna L, Yang WD, Wickham J, Scher E, Kadavanich A, Alivisatos AP. Nature. 2000;404:59–61. doi: 10.1038/35003535. [DOI] [PubMed] [Google Scholar]
- [34].Wulff G. Z. Krystallogr. Minera. 1901;34:449–530. [Google Scholar]
- [35].Manca P. J. Phys. Chem. Solids. 1961;20:268–273. [Google Scholar]
- [36].Batsanov SS. Russ. J. Inorg. Chem. 2007;52:1223–1229. [Google Scholar]
- [37].Bealing CR, Baumgardner WJ, Choi JJ, Hanrath T, Hennig RG. ACS Nano. 2012;6:2118–2127. doi: 10.1021/nn3000466. [DOI] [PubMed] [Google Scholar]
- [38].Li H, Chen D, Li L, Tang F, Zhang L, Ren J. Crystengcomm. 2010;12:1127–1133. [Google Scholar]
- [39].Luther JM, Zheng H, Sadtler B, Alivisatos AP. J. Am. Chem. Soc. 2009;131:16851–16857. doi: 10.1021/ja906503w. [DOI] [PubMed] [Google Scholar]
- [40].Jain PK, Amirav L, Aloni S, Alivisatos AP. J. Am. Chem. Soc. 2010;132:9997–9999. doi: 10.1021/ja104126u. [DOI] [PubMed] [Google Scholar]
- [41].Li H, Brescia R, Povia M, Prato M, Bertoni G, Manna L, Moreels I. J. Am. Chem. Soc. 2013;135:12270–12278. doi: 10.1021/ja404694k. [DOI] [PubMed] [Google Scholar]
- [42].Green MLH. J. Organomet. Chem. 1995;500:127–148. [Google Scholar]
- [43].Schreiber F. Prog. Surf. Sci. 2000;65:151–256. [Google Scholar]
- [44].Zheng SL, Chen XM. Aust. J. Chem. 2004;57:703–712. [Google Scholar]
- [45].Vecchiosadus AM. J. Appl. Electrochem. 1993;23:401–416. [Google Scholar]
- [46].Owen JS, Park J, Trudeau P-E, Alivisatos AP. J. Am. Chem. Soc. 2008;130:12279–12281. doi: 10.1021/ja804414f. [DOI] [PubMed] [Google Scholar]
- [47].Nag A, Kovalenko MV, Lee J-S, Liu W, Spokoyny B, Talapin DV. J. Am. Chem. Soc. 2011;133:10612–10620. doi: 10.1021/ja2029415. [DOI] [PubMed] [Google Scholar]
- [48].Jin H, Choi S, Xing G, Lee J-H, Kwon Y, Chong WK, Sum TC, Jang HM, Kim S. J. Am. Chem. Soc. 2015;137:13827–13835. doi: 10.1021/jacs.5b05787. [DOI] [PubMed] [Google Scholar]
- [49].Orpen AG, Connelly NG. Organometallics. 1990;9:1206–1210. [Google Scholar]
- [50].Shevchenko EV, Talapin DV, Kotov NA, O’Brien S, Murray CB. Nature. 2006;439:55–59. doi: 10.1038/nature04414. [DOI] [PubMed] [Google Scholar]
- [51].Margraf JT, Ruland A, Sgobba V, Guldi DM, Clark T. Langmuir. 2013;29:15450–15456. doi: 10.1021/la403633e. [DOI] [PubMed] [Google Scholar]
- [52].Meyns M, Iacono F, Palencia C, Geweke J, Coderch MD, Fittschen UEA, Gallego JM, Otero R, Juarez BH, Klinke C. Chem. Mater. 2014;26:1813–1821. [Google Scholar]
- [53].Csik I, Russo SP, Mulvaney P. J. Phys. Chem. C. 2008;112:20413–20417. [Google Scholar]
- [54].Sun W, Corni S, Di Felice R. J. Phys. Chem. C. 2013;117:16034–16041. [Google Scholar]
- [55].Zhuang Z, Peng Q, Li Y. Chem. Soc. Rev. 2011;40:5492–5513. doi: 10.1039/c1cs15095b. [DOI] [PubMed] [Google Scholar]
- [56].Murray CB, Norris DJ, Bawendi MG. J. Am. Chem. Soc. 1993;115:8706–8715. [Google Scholar]
- [57].Battaglia D, Peng XG. Nano Lett. 2002;2:1027–1030. [Google Scholar]
- [58].Yu MW, Peng XG. Angew. Chem. Int. Ed. 2002;41:2368–2371. doi: 10.1002/1521-3773(20020703)41:13<2368::AID-ANIE2368>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- [59].Wang FD, Buhro WE. J. Am. Chem. Soc. 2012;134:5369–5380. doi: 10.1021/ja300135c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Kopping JT, Patten TE. J. Am. Chem. Soc. 2008;130:5689–5698. doi: 10.1021/ja077414d. [DOI] [PubMed] [Google Scholar]
- [61].Zherebetskyy D, Scheele M, Zhang Y, Bronstein N, Thompson C, Britt D, Salmeron M, Alivisatos P, Wang L-W. Science. 2014;344:1380–1384. doi: 10.1126/science.1252727. [DOI] [PubMed] [Google Scholar]
- [62].Houtepen AJ, Koole R, Vanmaekelbergh D, Meeldijk J, Hickey SG. J. Am. Chem. Soc. 2006;128:6792–6793. doi: 10.1021/ja061644v. [DOI] [PubMed] [Google Scholar]
- [63].Kopping JT, Patten TE. J. Am. Chem. Soc. 2008;130:5689–5698. doi: 10.1021/ja077414d. [DOI] [PubMed] [Google Scholar]
- [64].Morris-Cohen AJ, Frederick MT, Lilly GD, McArthur EA, Weiss EA. J. Phys. Chem. Lett. 2010;1:1078–1081. [Google Scholar]
- [65].Anderson NC, Hendricks MP, Choi JJ, Owen JS. J. Am. Chem. Soc. 2013;135:18536–18548. doi: 10.1021/ja4086758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Hassinen A, Moreels I, De Nolf K, Smet PF, Martins JC, Hens Z. J. Am. Chem. Soc. 2012;134:20705–20712. doi: 10.1021/ja308861d. [DOI] [PubMed] [Google Scholar]
- [67].Weiss EA. Acc. Chem. Res. 2013;46:2607–2615. doi: 10.1021/ar400078u. [DOI] [PubMed] [Google Scholar]
- [68].Dolai S, Nimmala PR, Mandal M, Muhoberac BB, Dria K, Dass A, Sardar R. Chem. Mater. 2014;26:1278–1285. [Google Scholar]
- [69].Kasuya A, Sivamohan R, Barnakov YA, Dmitruk IM, Nirasawa T, Romanyuk VR, Kumar V, Mamykin SV, Tohji K, Jeyadevan B, Shinoda K, Kudo T, Terasaki O, Liu Z, Belosludov RV, Sundararajan V, Kawazoe Y. Nat. Mater. 2004;3:99–102. doi: 10.1038/nmat1056. [DOI] [PubMed] [Google Scholar]
- [70].Wang Y, Zhou Y, Zhang Y, Buhro WE. Inorg. Chem. 2015;54:1165–1177. doi: 10.1021/ic502637q. [DOI] [PubMed] [Google Scholar]
- [71].Jasieniak JJ, Mulvaney P. J. Am. Chem. Soc. 2007;129:2841–2848. doi: 10.1021/ja066205a. [DOI] [PubMed] [Google Scholar]
- [72].Harrell SM, McBride JR, Rosenthal SJ. Chem. Mater. 2013;25:1199–1210. [Google Scholar]
- [73].Herron N, Calabrese JC, Farneth WE, Wang Y. Science. 1993;259:1426–1428. doi: 10.1126/science.259.5100.1426. [DOI] [PubMed] [Google Scholar]
- [74].Wang Y, Herron N. J. Phys. Chem. 1991;95:525–532. [Google Scholar]
- [75].Behrens S, Bettenhausen M, Deveson AC, Eichhofer A, Fenske D, Lohde A, Woggon U. Angew. Chem. Int. Ed. 1996;35:2215–2218. [Google Scholar]
- [76].Zheng NF, Bu XH, Lu HW, Zhang QC, Feng PY. J. Am. Chem. Soc. 2005;127:11963–11965. doi: 10.1021/ja053588o. [DOI] [PubMed] [Google Scholar]
- [77].Szemjonov A, Pauporte T, Ithurria S, Lequeux N, Dubertret B, Ciofini I, Labat F. RSC Adv. 2014;4:55980–55989. [Google Scholar]
- [78].Voznyy O. The Journal of Physical Chemistry C. 2011;115:15927–15932. [Google Scholar]
- [79].Beecher AN, Yang X, Palmer JH, LaGrassa AL, Juhas P, Billinge SJL, Owen JS. J. Am. Chem. Soc. 2014;136:10645–10653. doi: 10.1021/ja503590h. [DOI] [PubMed] [Google Scholar]
- [80].Miller MS, San Juan RR, Ferrato M-A, Carmichael TB. J. Am. Chem. Soc. 2014;136:4212–4222. doi: 10.1021/ja411140j. [DOI] [PubMed] [Google Scholar]
- [81].Yordanov GG, Gicheva GD, Bochev BH, Dushkin CD, Adachi E. Colloids Surf. A. 2006;273:10–15. [Google Scholar]
- [82].Torelli MD, Putans RA, Tan Y, Lohse SE, Murphy CJ, Hamers RJ. ACS Appl. Mater. Inter. 2015;7:1720–1725. doi: 10.1021/am507300x. [DOI] [PubMed] [Google Scholar]
- [83].Nan WN, Niu YA, Qin HY, Cui F, Yang Y, Lai RC, Lin WZ, Peng XG. J. Am. Chem. Soc. 2012;134:19685–19693. doi: 10.1021/ja306651x. [DOI] [PubMed] [Google Scholar]
- [84].Yu WW, Wang YA, Peng XG. Chem. Mater. 2003;15:4300–4308. [Google Scholar]
- [85].Peng ZA, Peng XG. J. Am. Chem. Soc. 2001;123:1389–1395. doi: 10.1021/ja003633m. [DOI] [PubMed] [Google Scholar]
- [86].Huang J, Kovalenko MV, Talapin DV. J. Am. Chem. Soc. 2010;132:15866–15868. doi: 10.1021/ja105132u. [DOI] [PubMed] [Google Scholar]
- [87].Gao Y, Peng X. J. Am. Chem. Soc. 2014;136:6724–6732. doi: 10.1021/ja5020025. [DOI] [PubMed] [Google Scholar]
- [88].Talapin DV, Nelson JH, Shevchenko EV, Aloni S, Sadtler B, Alivisatos AP. Nano Lett. 2007;7:2951–2959. doi: 10.1021/nl072003g. [DOI] [PubMed] [Google Scholar]
- [89].Carbone L, Nobile C, De Giorg M, Sala FD, Morello G, Pompa P, Hytch M, Snoeck E, Fiore A, Franchini IR, Nadasan M, Silvestre AF, Chiodo L, Kudera S, Cingolani R, Krahne R, Manna L. Nano Lett. 2007;7:2942–2950. doi: 10.1021/nl0717661. [DOI] [PubMed] [Google Scholar]
- [90].Ithurria S, Tessier MD, Mahler B, Lobo R, Dubertret B, Efros A. Nat. Mater. 2011;10:936–941. doi: 10.1038/nmat3145. [DOI] [PubMed] [Google Scholar]
- [91].Hassinen A, Gomes R, De Nolf K, Zhao Q, Vantomme A, Martins JC, Hens Z. J. Phys. Chem. C. 2013;117:13936–13943. [Google Scholar]
- [92].Kim W, Lim SJ, Jung S, Shin SK. J. Phys. Chem. C. 2010;114:1539–1546. [Google Scholar]
- [93].Chan WCW, Nie SM. Science. 1998;281:2016–2018. doi: 10.1126/science.281.5385.2016. [DOI] [PubMed] [Google Scholar]
- [94].Aldana J, Wang YA, Peng XG. J. Am. Chem. Soc. 2001;123:8844–8850. doi: 10.1021/ja016424q. [DOI] [PubMed] [Google Scholar]
- [95].Xie RG, Kolb U, Li JX, Basche T, Mews A. J. Am. Chem. Soc. 2005;127:7480–7488. doi: 10.1021/ja042939g. [DOI] [PubMed] [Google Scholar]
- [96].Mittal ML, Saxena RS, Pandey AV. J. Inorg. Nucl. Chem. 1973;35:1691–1693. [Google Scholar]
- [97].Yamasaki T, Nanjo M. Sci. Rep. Res. Tohoku A. 1969;21:45–62. [Google Scholar]
- [98].Aldana J, Lavelle N, Wang YJ, Peng XG. J. Am. Chem. Soc. 2005;127:2496–2504. doi: 10.1021/ja047000+. [DOI] [PubMed] [Google Scholar]
- [99].Giansante C, Infante I, Fabiano E, Grisorio R, Suranna GP, Gigli G. J. Am. Chem. Soc. 2015;137:1875–1886. doi: 10.1021/ja510739q. [DOI] [PubMed] [Google Scholar]
- [100].Cossairt BM, Juhas P, Billinge SJL, Owen JS. J. Phys. Chem. Lett. 2011;2:3075–3080. doi: 10.1021/jz2013769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Ip AH, Thon SM, Hoogland S, Voznyy O, Zhitomirsky D, Debnath R, Levina L, Rollny LR, Carey GH, Fischer A, Kemp KW, Kramer IJ, Ning Z, Labelle AJ, Chou KW, Amassian A, Sargent EH. Nat. Nanotech. 2012;7:577–582. doi: 10.1038/nnano.2012.127. [DOI] [PubMed] [Google Scholar]
- [102].Nirmal M, Dabbousi BO, Bawendi MG, Macklin JJ, Trautman JK, Harris TD, Brus LE. Nature. 1996;383:802–804. [Google Scholar]
- [103].Blanton SA, Hines MA, Guyot-Sionnest P. Appl. Phys. Lett. 1996;69:3905–3907. [Google Scholar]
- [104].Lane LA, Smith AM, Lian T, Nie S. J. Phys. Chem. B. 2014;118:14140–14147. doi: 10.1021/jp5064325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Hughes BK, Ruddy DA, Blackburn JL, Smith DK, Bergren MR, Nozik AJ, Johnson JC, Beard MC. ACS Nano. 2012;6:5498–5506. doi: 10.1021/nn301405j. [DOI] [PubMed] [Google Scholar]
- [106].Nguyen KA, Pachter R, Day PN, Su H. J. Chem. Phys. 2015;142:234305. doi: 10.1063/1.4922320. [DOI] [PubMed] [Google Scholar]
- [107].Lim SJ, Zahid MU, Le P, Ma L, Entenberg D, Harney AS, Condeelis J, Smith AM. Nat Commun. 2015;6:8210. doi: 10.1038/ncomms9210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Kislitsyn DA, Gervasi CF, Allen T, Palomaki PKB, Hackley JD, Maruyama R, Nazin GV. J. Phys. Chem. Lett. 2014;5:3701–3707. doi: 10.1021/jz5019465. [DOI] [PubMed] [Google Scholar]
- [109].Hohng S, Ha T. J. Am. Chem. Soc. 2004;126:1324–1325. doi: 10.1021/ja039686w. [DOI] [PubMed] [Google Scholar]
- [110].Munro AM, Ginger DS. Nano Lett. 2008;8:2585–2590. doi: 10.1021/nl801132t. [DOI] [PubMed] [Google Scholar]
- [111].Munro AM, Jen-La Plante I, Ng MS, Ginger DS. J. Phys. Chem. C. 2007;111:6220–6227. [Google Scholar]
- [112].Gaponik N, Talapin DV, Rogach AL, Hoppe K, Shevchenko EV, Kornowski A, Eychmuller A, Weller H. J. Phys. Chem. B. 2002;106:7177–7185. [Google Scholar]
- [113].Wuister S, Donega C, Meijerink A. J. Phys. Chem. B. 2004;108:17393–17397. [Google Scholar]
- [114].Dabbousi BO, Rodriguez-Viejo J, Mikulec FV, Heine JR, Mattoussi H, Ober R, Jensen KF, Bawendi MG. J. Phys. Chem. B. 1997;101:9463–9475. [Google Scholar]
- [115].Chen YF, Vela J, Htoon H, Casson JL, Werder DJ, Bussian DA, Klimov VI, Hollingsworth JA. J. Am. Chem. Soc. 2008;130:5026–5027. doi: 10.1021/ja711379k. [DOI] [PubMed] [Google Scholar]
- [116].Mahler B, Spinicelli P, Buil S, Quelin X, Hermier JP, Dubertret B. Nat. Mater. 2008;2008 doi: 10.1038/nmat2222. [DOI] [PubMed] [Google Scholar]
- [117].Pandey A, Guyot-Sionnest P. Science. 2008;322:929–932. doi: 10.1126/science.1159832. [DOI] [PubMed] [Google Scholar]
- [118].Lifshitz E. J. Phys. Chem. Lett. 2015;6:4336–4347. doi: 10.1021/acs.jpclett.5b01567. [DOI] [PubMed] [Google Scholar]
- [119].Knappenberger KL, Wong DB, Romanyuk YE, Leone SR. Nano Lett. 2007;7:3869–3874. doi: 10.1021/nl0714740. [DOI] [PubMed] [Google Scholar]
- [120].Knappenberger KL, Jr., Wong DB, Xu W, Schwartzberg AM, Wolcott A, Zhang JZ, Leone SR. ACS Nano. 2008;2:2143–2153. doi: 10.1021/nn800421g. [DOI] [PubMed] [Google Scholar]
- [121].Manna L, Scher EC, Li LS, Alivisatos AP. J. Am. Chem. Soc. 2002;124:7136–7145. doi: 10.1021/ja025946i. [DOI] [PubMed] [Google Scholar]
- [122].Oda M, Tsukamoto J, Hasegawa A, Iwami N, Nishiura K, Hagiwara I, Ando N, Horiuchi H, Tani T. J. Lumin. 2007;122:762–765. [Google Scholar]
- [123].Kasuya T. J. Magn. Magn. Mater. 1999;195:141–147. [Google Scholar]
- [124].Eichhofer A, Hampe O. Chem. Phys. Lett. 2005;407:186–191. [Google Scholar]
- [125].Wang YY, Liu YH, Zhang Y, Wang FD, Kowalski PJ, Rohrs HW, Loomis RA, Gross ML, Buhro WE. Angew. Chem. Int. Ed. 2012;51:6154–6157. doi: 10.1002/anie.201202380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Kim BH, Shin K, Kwon SG, Jang Y, Lee HS, Lee H, Jun SW, Lee J, Han SY, Yim YH, Kim DH, Hyeon T. J. Am. Chem. Soc. 2013;135:2407–2410. doi: 10.1021/ja310030c. [DOI] [PubMed] [Google Scholar]
- [127].Katari JEB, Colvin VL, Alivisatos AP. J. Phys. Chem. 1994;98:4109–4117. [Google Scholar]
- [128].Liu H, Owen JS, Alivisatos AP. J. Am. Chem. Soc. 2007;129:305–312. doi: 10.1021/ja0656696. [DOI] [PubMed] [Google Scholar]
- [129].Moreels I, Fritzinger B, Martins JC, Hens Z. J. Am. Chem. Soc. 2008;130:15081–15086. doi: 10.1021/ja803994m. [DOI] [PubMed] [Google Scholar]
- [130].Fritzinger B, Capek RK, Lambert K, Martins JC, Hens Z. J. Am. Chem. Soc. 2010;132:10195–10201. doi: 10.1021/ja104351q. [DOI] [PubMed] [Google Scholar]
- [131].Cros-Gagneux A, Delpech F, Nayral C, Cornejo A, Coppel Y, Chaudret B. J. Am. Chem. Soc. 2010;132:18147–18157. doi: 10.1021/ja104673y. [DOI] [PubMed] [Google Scholar]
- [132].Cass LC, Malicki M, Weiss EA. Anal. Chem. 2013;85:6974–6979. doi: 10.1021/ac401623a. [DOI] [PubMed] [Google Scholar]
- [133].Majetich SA, Carter AC, Belot J, McCullough RD. J. Phys. Chem. 1994;98:13705–13710. [Google Scholar]
- [134].Malicki M, Knowles KE, Weiss EA. Chem. Commun. 2013;49:4400–4402. doi: 10.1039/c2cc32895j. [DOI] [PubMed] [Google Scholar]
- [135].Wang FD, Tang R, Buhro WE. Nano Lett. 2008;8:3521–3524. doi: 10.1021/nl801692g. [DOI] [PubMed] [Google Scholar]
- [136].Gomes R, Hassinen A, Szczygiel A, Zhao QA, Vantomme A, Martins JC, Hens Z. J. Phys. Chem. Lett. 2011;2:145–152. [Google Scholar]
- [137].Fritzinger B, Moreels I, Lommens P, Koole R, Hens Z, Martins JC. J. Am. Chem. Soc. 2009;131:3024–3032. doi: 10.1021/ja809436y. [DOI] [PubMed] [Google Scholar]
- [138].Hassinen A, Moreels I, Donega CD, Martins JC, Hens Z. J. Phys. Chem. Lett. 2010;1:2577–2581. [Google Scholar]
- [139].Valdez CN, Schimpf AM, Gamelin DR, Mayer JM. ACS Nano. 2014;8:9463–9470. doi: 10.1021/nn503603e. [DOI] [PubMed] [Google Scholar]
- [140].Ji XH, Copenhaver D, Sichmeller C, Peng XG. J. Am. Chem. Soc. 2008;130:5726–5735. doi: 10.1021/ja710909f. [DOI] [PubMed] [Google Scholar]
- [141].Moreels L, Lambert K, De Muynck D, Vanhaecke F, Poelman D, Martins JC, Allan G, Hens Z. Chem. Mater. 2007;19:6101–6106. [Google Scholar]
- [142].Sachleben JR, Wooten EW, Emsley L, Pines A, Colvin VL, Alivisatos AP. Chem. Phys. Lett. 1992;198:431–436. [Google Scholar]
- [143].Donakowski MD, Godbe JM, Sknepnek R, Knowles KE, de la Cruz MO, Weiss EA. J. Phys. Chem. C. 2010;114:22526–22534. [Google Scholar]
- [144].Cooper JK, Franco AM, Gul S, Corrado C, Zhang JZ. Langmuir. 2011;27:8486–8493. doi: 10.1021/la201273x. [DOI] [PubMed] [Google Scholar]
- [145].Meulenberg RW, Strouse GF. J. Phys. Chem. B. 2001;105:7438–7445. [Google Scholar]
- [146].Tavasoli E, Guo YJ, Kunal P, Grajeda J, Gerber A, Vela J. Chem. Mater. 2012;24:4231–4241. [Google Scholar]
- [147].von Holt B, Kudera S, Weiss A, Schrader TE, Manna L, Parak WJ, Braun M. J. Mater. Chem. 2008;18:2728–2732. [Google Scholar]
- [148].Dong AG, Ye XC, Chen J, Kang YJ, Gordon T, Kikkawa JM, Murray CB. J. Am. Chem. Soc. 2011;133:998–1006. doi: 10.1021/ja108948z. [DOI] [PubMed] [Google Scholar]
- [149].Zhu ZJ, Yeh YC, Tang R, Yan B, Tamayo J, Vachet RW, Rotello VM. Nat. Chem. 2011;3:963–968. doi: 10.1038/nchem.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Min H, Kim Y, Yu H, Moon DW, Lim SJ, Yoon HJ, Lee TG, Shin SK. Chem. Eur. J. 2008;14:8461–8464. doi: 10.1002/chem.200801301. [DOI] [PubMed] [Google Scholar]
- [151].Son JG, Choi E, Piao Y, Han SW, Lee TG. Nanoscale. 2016;8:4573–4578. doi: 10.1039/c5nr07592k. [DOI] [PubMed] [Google Scholar]
- [152].Puzder A, Williamson AJ, Zaitseva N, Galli G, Manna L, Alivisatos AP. Nano Lett. 2004;4:2361–2365. [Google Scholar]
- [153].Fernando A, Weerawardene KLDM, Karimova NV, Aikens CM. Chem. Rev. 2015;115:6112–6216. doi: 10.1021/cr500506r. [DOI] [PubMed] [Google Scholar]
- [154].Yang P, Tretiak S, Masunov AE, Ivanov S. J. Chem. Phys. 2008;129 doi: 10.1063/1.2965532. [DOI] [PubMed] [Google Scholar]
- [155].Schapotschnikow P, Hommersom B, Vlugt TJH. J. Phys. Chem. C. 2009;113:12690–12698. [Google Scholar]
- [156].Azpiroz JM, De Angelis F. ACS Appl. Mater. Inter. 2015;7:19736–19745. doi: 10.1021/acsami.5b05418. [DOI] [PubMed] [Google Scholar]
- [157].Wang Y, Zhang Y, Zhang W. RSC Adv. 2014;4:19302–19309. [Google Scholar]
- [158].Chou H-L, Tseng C-H, Pillai KC, Hwang B-J, Chen L-Y. Nanoscale. 2010;2:2679–2684. doi: 10.1039/c0nr00569j. [DOI] [PubMed] [Google Scholar]
- [159].Kuznetsov AE, Beratan DN. J. Phys. Chem. C. 2014;118:7094–7109. [Google Scholar]
- [160].Onida G, Reining L, Rubio A. Rev. Mod. Phys. 2002;74:601–659. [Google Scholar]
- [161].Kaushik AR, Lukose B, Clancy P. ACS Nano. 2014;8:2302–2317. doi: 10.1021/nn405755n. [DOI] [PubMed] [Google Scholar]
- [162].Voznyy O, Sargent EH. Phys. Rev. Lett. 2014;112:157401. doi: 10.1103/PhysRevLett.112.157401. [DOI] [PubMed] [Google Scholar]
- [163].Soloviev VN, Eichhofer A, Fenske D, Banin U. J. Am. Chem. Soc. 2001;123:2354–2364. doi: 10.1021/ja003598j. [DOI] [PubMed] [Google Scholar]
- [164].Kairdolf BA, Smith AM, Stokes TH, Wang MD, Young AN, Nie S. Ann. Rev. Anal. Chem. 2013;6:143–162. doi: 10.1146/annurev-anchem-060908-155136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Cai E, Ge P, Lee SH, Jeyifous O, Wang Y, Liu Y, Wilson KM, Lim SJ, Baird MA, Stone JE, Lee KY, Davidson MW, Chung HJ, Schulten K, Smith AM, Green WN, Selvin PR. Angew. Chem. Int. Ed. 2014;53:12484–12488. doi: 10.1002/anie.201405735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Courty S, Luccardini C, Bellaiche Y, Cappello G, Dahan M. Nano Lett. 2006;6:1491–1495. doi: 10.1021/nl060921t. [DOI] [PubMed] [Google Scholar]
- [167].Smith AM, Nie S. Nat. Biotech. 2009;27:732–733. doi: 10.1038/nbt0809-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Sykes EA, Chen J, Zheng G, Chan WCW. ACS Nano. 2014;8:5696–5706. doi: 10.1021/nn500299p. [DOI] [PubMed] [Google Scholar]
- [169].Choi HS, Liu W, Liu F, Nasr K, Misra P, Bawendi MG, Frangioni JV. Nat. Nanotech. 2010;5:42–47. doi: 10.1038/nnano.2009.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Soo Choi H, Liu W, Misra P, Tanaka E, Zimmer JP, Itty Ipe B, Bawendi MG, Frangioni JV. Nat. Biotech. 2007;25:1165–1170. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Smith AM, Duan HW, Rhyner MN, Ruan G, Nie SM. Phys. Chem. Chem. Phys. 2006;8:3895–3903. doi: 10.1039/b606572b. [DOI] [PubMed] [Google Scholar]
- [172].Sorkin A, von Zastrow M. Nat. Rev. Mol. Cell Biol. 2002;3:600–614. doi: 10.1038/nrm883. [DOI] [PubMed] [Google Scholar]
- [173].Mohs AM, Duan HW, Kairdolf BA, Smith AM, Nie SM. Nano Res. 2009;2:500–509. doi: 10.1007/s12274-009-9046-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Liu W, Greytak AB, Lee J, Wong CR, Park J, Marshall LF, Jiang W, Curtin PN, Ting AY, Nocera DG, Fukumura D, Jain RK, Bawendi MG. J. Am. Chem. Soc. 2010;132:472–483. doi: 10.1021/ja908137d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Smith AM, Duan H, Rhyner MN, Ruan G, Nie S. Phys. Chem. Chem. Phys. 2006;8:3895–3903. doi: 10.1039/b606572b. [DOI] [PubMed] [Google Scholar]
- [176].Traverso N, Ricciarelli R, Nitti M, Marengo B, Furfaro AL, Pronzato MA, Marinari UM, Domenicotti C. Oxidative Medicine and Cellular Longevity. 2013;2013:1–11. doi: 10.1155/2013/972913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Mancini MC, Kairdolf BA, Smith AM, Nie SM. J. Am. Chem. Soc. 2008;130:10836–10837. doi: 10.1021/ja8040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Zhu Z-J, Yeh Y-C, Tang R, Yan B, Tamayo J, Vachet RW, Rotello VM. Nat. Chem. 2011;3:963–968. doi: 10.1038/nchem.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Gravel E, Tanguy C, Cassette E, Pons T, Knittel F, Bernards N, Garofalakis A, Duconge F, Dubertret B, Doris E. Chemical Science. 2013;4:411–417. [Google Scholar]
- [180].Liu W, Howarth M, Greytak AB, Zheng Y, Nocera DG, Ting AY, Bawendi MG. J. Am. Chem. Soc. 2008;130:1274–1284. doi: 10.1021/ja076069p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Thiry M, Boldt K, Nikolic MS, Schulz F, Ijeh M, Panicker A, Vossmeyer T, Weller H. ACS Nano. 2011;5:4965–4973. doi: 10.1021/nn201065y. [DOI] [PubMed] [Google Scholar]
- [182].Stewart MH, Susumu K, Mei BC, Medintz IL, Delehanty JB, Blanco-Canosa JB, Dawson PE, Mattoussi H. J. Am. Chem. Soc. 2010;132:9804–9813. doi: 10.1021/ja102898d. [DOI] [PubMed] [Google Scholar]
- [183].Zhan N, Palui G, Safi M, Ji X, Mattoussi H. J. Am. Chem. Soc. 2013;135:13786–13795. doi: 10.1021/ja405010v. [DOI] [PubMed] [Google Scholar]
- [184].Smith AM, Nie S. J. Am. Chem. Soc. 2008;130:11278–11279. doi: 10.1021/ja804306c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Liu WH, Greytak AB, Lee J, Wong CR, Park J, Marshall LF, Jiang W, Curtin PN, Ting AY, Nocera DG, Fukumura D, Jain RK, Bawendi MG. J. Am. Chem. Soc. 2010;132:472–483. doi: 10.1021/ja908137d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Han HS, Martin JD, Lee J, Harris DK, Fukumura D, Jain RK, Bawendi M. Angew Chem Int Ed Engl. 2013;52:1414–1419. doi: 10.1002/anie.201208331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Palui G, Na HB, Mattoussi H. Langmuir. 2011;28:2761–2772. doi: 10.1021/la203968t. [DOI] [PubMed] [Google Scholar]
- [188].Tasso M, Giovanelli E, Zala D, Bouccara S, Fragola A, Hanafi M, Lenkei Z, Pons T, Lequeux N. ACS Nano. 2015;9:11479–11489. doi: 10.1021/acsnano.5b05705. [DOI] [PubMed] [Google Scholar]
- [189].Ma L, Tu C, Le P, Chitoor S, Lim SJ, Zahid MU, Teng KW, Ge P, Selvin PR, Smith AM. J. Am. Chem. Soc. 2016;138:3382–3394. doi: 10.1021/jacs.5b12378. [DOI] [PubMed] [Google Scholar]
- [190].Dubertret B, Skourides P, Norris DJ, Noireaux V, Brivanlou AH, Libchaber A. Science. 2002;298:1759–1762. doi: 10.1126/science.1077194. [DOI] [PubMed] [Google Scholar]
- [191].Wu XY, Liu HJ, Liu JQ, Haley KN, Treadway JA, Larson JP, Ge NF, Peale F, Bruchez MP. Nat. Biotechnol. 2003;21:41–46. doi: 10.1038/nbt764. [DOI] [PubMed] [Google Scholar]
- [192].Pellegrino T, Manna L, Kudera S, Liedl T, Koktysh D, Rogach AL, Keller S, Radler J, Natile G, Parak WJ. Nano Lett. 2004;4:703–707. [Google Scholar]
- [193].Yu WW, Chang E, Falkner JC, Zhang J, Al-Somali AM, Sayes CM, Johns J, Drezek R, Colvin VL. J. Am. Chem. Soc. 2007;129:2871–2879. doi: 10.1021/ja067184n. [DOI] [PubMed] [Google Scholar]
- [194].Israelachvili JN. Intermolecular and Surface Forces. 3rd Edition Academic Press; Boston, MA: 2011. [Google Scholar]
- [195].Lim SJ, McDougle DR, Zahid MU, Ma L, Das A, Smith AM. J. Am. Chem. Soc. 2016;138:64–67. doi: 10.1021/jacs.5b11225. [DOI] [PubMed] [Google Scholar]
- [196].Shenkarev ZO, Lyukmanova EN, Paramonov AS, Panteleev PV, Balandin SV, Shulepko MA, Mineev KS, Ovchinnikova TV, Kirpichnikov MP, Arseniev AS. Acta Nat. 2014;6:84–94. [PMC free article] [PubMed] [Google Scholar]
- [197].Smith AM, Nie SM. Nat. Biotechnol. 2009;27:732–733. doi: 10.1038/nbt0809-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Chan WC, Nie S. Science. 1998;281:2016–2018. doi: 10.1126/science.281.5385.2016. [DOI] [PubMed] [Google Scholar]
- [199].Susumu K, Uyeda HT, Medintz IL, Pons T, Delehanty JB, Mattoussi H. J. Am. Chem. Soc. 2007;129:13987–13996. doi: 10.1021/ja0749744. [DOI] [PubMed] [Google Scholar]
- [200].Aldana J, Lavelle N, Wang Y, Peng X. J. Am. Chem. Soc. 2005;127:2496–2504. doi: 10.1021/ja047000+. [DOI] [PubMed] [Google Scholar]
- [201].Giovanelli E, Muro E, Sitbon G, Hanafi M, Pons T, Dubertret B, Lequeux N. Langmuir. 2012;28:15177–15184. doi: 10.1021/la302896x. [DOI] [PubMed] [Google Scholar]
- [202].Moreira NH, Domiinguez A, Frauenheim T, da Rosa AL. Phys. Chem. Chem. Phys. 2012;14:15445–15451. doi: 10.1039/c2cp42435e. [DOI] [PubMed] [Google Scholar]
- [203].Turo MJ, Macdonald JE. ACS Nano. 2014;8:10205–10213. doi: 10.1021/nn5032164. [DOI] [PubMed] [Google Scholar]
- [204].Rittikulsittichai S, Jamison AC, Lee TR. Langmuir. 2011;27:9920–9927. doi: 10.1021/la201313g. [DOI] [PubMed] [Google Scholar]
- [205].Srisombat L.-o., Park J-S, Zhang S, Lee TR. Langmuir. 2008;24:7750–7754. doi: 10.1021/la800511g. [DOI] [PubMed] [Google Scholar]
- [206].Jacob JDC, Lee TR, Baldelli S. J. Phys. Chem. C. 2014;118:29126–29134. [Google Scholar]
- [207].Smith AM, Marbella LE, Johnston KA, Hartmann MJ, Crawford SE, Kozycz LM, Seferos DS, Millstone JE. Anal. Chem. 2015;87:2771–2778. doi: 10.1021/ac504081k. [DOI] [PubMed] [Google Scholar]
- [208].Frederick MT, Achtyl JL, Knowles KE, Weiss EA, Geiger FM. J. Am. Chem. Soc. 2011;133:7476–7481. doi: 10.1021/ja200466z. [DOI] [PubMed] [Google Scholar]
- [209].Bordenyuk AN, Weeraman C, Yatawara A, Jayathilake HD, Stiopkin I, Liu Y, Benderskii AV. J. Phys. Chem. C. 2007;111:8925–8933. [Google Scholar]
- [210].Mammen M, Choi SK, Whitesides GM. Angew. Chem. Int. Ed. 1998;37:2755–2794. doi: 10.1002/(SICI)1521-3773(19981102)37:20<2754::AID-ANIE2754>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- [211].Wang W, Zhang S, Chinwangso P, Advincula RC, Lee TR. J. Phys. Chem. C. 2009;113:3717–3725. [Google Scholar]
- [212].Chinwangso P, Jamison AC, Lee TR. Acc. Chem. Res. 2011;44:511–519. doi: 10.1021/ar200020s. [DOI] [PubMed] [Google Scholar]
- [213].Lee S, Shon YS, Colorado R, Guenard RL, Lee TR, Perry SS. Langmuir. 2000;16:2220–2224. [Google Scholar]
- [214].Duan H, Kuang M, Wang YA. Chem. Mater. 2010;22:4372–4378. [Google Scholar]
- [215].Muro E, Pons T, Lequeux N, Fragola A, Sanson N, Lenkei Z, Dubertret B. J. Am. Chem. Soc. 2010;132:4556–4557. doi: 10.1021/ja1005493. [DOI] [PubMed] [Google Scholar]
- [216].Uzun O, Hu Y, Verma A, Chen S, Centrone A, Stellacci F. Chem. Commun. 2008:196–198. doi: 10.1039/b713143g. [DOI] [PubMed] [Google Scholar]
- [217].Han H-S, Devaraj NK, Lee J, Hilderbrand SA, Weissleder R, Bawendi MG. J. Am. Chem. Soc. 2010;132:7838–7839. doi: 10.1021/ja101677r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Han H-S, Niemeyer E, Huang Y, Kamoun WS, Martin JD, Bhaumik J, Chen Y, Roberge S, Cui J, Martin MR, Fukumura D, Jain RK, Bawendi MG, Duda DG. Proc. Natl. Acad. Sci. U.S.A. 2015;112:1350–1355. doi: 10.1073/pnas.1421632111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Susumu K, Oh E, Delehanty JB, Pinaud F, Gemmill KB, Walper S, Breger J, Schroeder MJ, Stewart MH, Jain V, Whitaker CM, Huston AL, Medintz IL. Chem. Mater. 2014;26:5327–5344. [Google Scholar]
- [220].Wang W, Kapur A, Ji X, Safi M, Palui G, Palomo V, Dawson PE, Mattoussi H. J. Am. Chem. Soc. 2015;137:5438–5451. doi: 10.1021/jacs.5b00671. [DOI] [PubMed] [Google Scholar]
- [221].Wang W, Ji X, Kapur A, Zhang C, Mattoussi H. J. Am. Chem. Soc. 2015;137:14158–14172. doi: 10.1021/jacs.5b08915. [DOI] [PubMed] [Google Scholar]
- [222].Tasso M, Singh M, Giovanelli E, Fragola A, Loriette V, Frugier-Regairaz M, Dautry F, Treussart F, Lenkei Z, Lequeux N, Pons T. ACS Appl. Mater. Inter. 2015;7:26904–26913. doi: 10.1021/acsami.5b09777. [DOI] [PubMed] [Google Scholar]
- [223].Xiong W-W, Li J-R, Hu B, Tan B, Li R-F, Huang X-Y. Chem. Sci. 2012;3:1200–1204. [Google Scholar]
- [224].Wu T, Bu X, Liao P, Wang L, Zheng S-T, Ma R, Feng P. J. Am. Chem. Soc. 2012;134:3619–3622. doi: 10.1021/ja210039u. [DOI] [PubMed] [Google Scholar]
- [225].Pelaz B, Jaber S, de Aberasturi DJ, Wulf V, Aida T, de la Fuente JM, Feldmann J, Gaub HE, Josephson L, Kagan CR, Kotov NA, Liz-Marzán LM, Mattoussi H, Mulvaney P, Murray CB, Rogach AL, Weiss PS, Willner I, Parak WJ. ACS Nano. 2012;6:8468–8483. doi: 10.1021/nn303929a. [DOI] [PubMed] [Google Scholar]
- [226].Kim C, Galloway JF, Lee KH, Searson PC. Bioconjug. Chem. 2014;25:1893–1901. doi: 10.1021/bc5003778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Chakraborty AK, Golumbfskie AJ. Annu. Rev. Phys. Chem. 2001;52:537–573. doi: 10.1146/annurev.physchem.52.1.537. [DOI] [PubMed] [Google Scholar]
- [228].Wang MF, Felorzabihi N, Guerin G, Haley JC, Scholes GD, Winnik MA. Macromolecules. 2007;40:6377–6384. [Google Scholar]
- [229].Wang XS, Dykstra TE, Salvador MR, Manners I, Scholes GD, Winnik MA. J. Am. Chem. Soc. 2004;126:7784–7785. doi: 10.1021/ja0489339. [DOI] [PubMed] [Google Scholar]
- [230].San Juan RR, Allan CJ, Iqbal M, Eichhorn SH, Macdonald CLB, Carmichael TB. J. Am. Chem. Soc. 2013;135:15784–15793. doi: 10.1021/ja404798q. [DOI] [PubMed] [Google Scholar]
- [231].Lim JK, Kwon O, Joo S-W. J. Phys. Chem. C. 2008;112:6816–6821. [Google Scholar]
- [232].Singhana B, Rittikulsittichai S, Lee TR. Langmuir. 2013;29:561–569. doi: 10.1021/la303101x. [DOI] [PubMed] [Google Scholar]
- [233].Fox MA, Whitesell JK, McKerrow AJ. Langmuir. 1998;14:816–820. [Google Scholar]
- [234].Yam CM, Cho J, Cai CZ. Langmuir. 2003;19:6862–6868. [Google Scholar]
- [235].Park JS, Vo AN, Barriet D, Shon YS, Lee TR. Langmuir. 2005;21:2902–2911. doi: 10.1021/la0475573. [DOI] [PubMed] [Google Scholar]
- [236].Liu YC, Kim M, Wang YJ, Wang YA, Peng XG. Langmuir. 2006;22:6341–6345. doi: 10.1021/la052747e. [DOI] [PubMed] [Google Scholar]
- [237].Nann T. Chem. Commun. 2005:1735–1736. doi: 10.1039/b414807j. [DOI] [PubMed] [Google Scholar]
- [238].Gerion D, Pinaud F, Williams SC, Parak WJ, Zanchet D, Weiss S, Alivisatos AP. J. Phys. Chem. B. 2001;105:8861–8871. [Google Scholar]
- [239].Zhang F, Lees E, Amin F, Rivera_Gil P, Yang F, Mulvaney P, Parak WJ. Small. 2011;7:3113–3127. doi: 10.1002/smll.201100608. [DOI] [PubMed] [Google Scholar]
- [240].Hu X, Gao X. ACS Nano. 2010;4:6080–6086. doi: 10.1021/nn1017044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Zhang X, Shamirian A, Jawaid AM, Tyrakowski CM, Page LE, Das A, Chen O, Isovic A, Hassan A, Snee PT. Small. 2015:1–6. doi: 10.1002/smll.201501886. [DOI] [PubMed] [Google Scholar]
- [242].Lane LA, Qian XM, Smith AM, Nie S. Annu. Rev. Phys. Chem. 2015;66:521–547. doi: 10.1146/annurev-physchem-040513-103718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].Schollbach M, Zhang F, Roosen-Runge F, Skoda MWA, Jacobs RMJ, Schreiber F. J. Colloid Interface Sci. 2014;426:31–38. doi: 10.1016/j.jcis.2014.03.052. [DOI] [PubMed] [Google Scholar]
- [244].Yeh Y-C, Saha K, Yan B, Miranda OR, Yu X, Rotello VM. Nanoscale. 2013;5:12140–12143. doi: 10.1039/c3nr04037b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Liu X, Zhu H, Jin Q, Zhou W, Colvin VL, Ji J. Adv. Healthc. Mater. 2013;2:352–360. doi: 10.1002/adhm.201200210. [DOI] [PubMed] [Google Scholar]
- [246].Tamang S, Beaune G, Texier I, Reiss P. ACS Nano. 2011;5:9392–9402. doi: 10.1021/nn203598c. [DOI] [PubMed] [Google Scholar]
- [247].Park J, Nam J, Won N, Jin H, Jung S, Jung S, Cho S-H, Kim S. Adv. Funct. Mater. 2011;21:1558–1566. [Google Scholar]
- [248].Al-Hajaj NA, Moquin A, Neibert KD, Soliman GM, Winnik FM, Maysinger D. ACS Nano. 2011;5:4909–4918. doi: 10.1021/nn201009w. [DOI] [PubMed] [Google Scholar]
- [249].Verma A, Uzun O, Hu Y, Hu Y, Han H-S, Watson N, Chen S, Irvine DJ, Stellacci F. Nat. Mater. 2008;7:588–595. doi: 10.1038/nmat2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Kairdolf BA, Mancini MC, Smith AM, Nie S. Anal. Chem. 2008;80:3029–3034. doi: 10.1021/ac800068q. [DOI] [PubMed] [Google Scholar]
- [251].Walkey CD, Chan WCW. Chem. Soc. Rev. 2012;41:2780–2799. doi: 10.1039/c1cs15233e. [DOI] [PubMed] [Google Scholar]
- [252].Sheinerman FB, Norel R, Honig B. Curr. Opin. Struct. Biol. 2000;10:153–159. doi: 10.1016/s0959-440x(00)00065-8. [DOI] [PubMed] [Google Scholar]
- [253].Lowe S, O’Brien-Simpson NM, Connal LA. Polym. Chem. 2015;6:198–212. [Google Scholar]
- [254].Åkerman ME, Chan WCW, Laakkonen P, Bhatia SN, Ruoslahti E. Proceedings of the National Academy of Sciences. 2002;99:12617–12621. doi: 10.1073/pnas.152463399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [255].Gao X, Cui Y, Levenson RM, Chung LWK, Nie S. Nat. Biotechnol. 2004;22:969–976. doi: 10.1038/nbt994. [DOI] [PubMed] [Google Scholar]
- [256].Smith AM, Nie S. Journal of visualized experiments : JoVE. 2012:e4236. doi: 10.3791/4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [257].Johnson PS, Goel M, Abbott NL, Himpsel FJ. Langmuir. 2014;30:10263–10269. doi: 10.1021/la500978s. [DOI] [PubMed] [Google Scholar]
- [258].Herrwerth S, Eck W, Reinhardt S, Grunze M. J. Am. Chem. Soc. 2003;125:9359–9366. doi: 10.1021/ja034820y. [DOI] [PubMed] [Google Scholar]
- [259].Unsworth LD, Sheardown H, Brash JL. Langmuir. 2005;21:1036–1041. doi: 10.1021/la047672d. [DOI] [PubMed] [Google Scholar]
- [260].Dobrzanska DA, Cooper AL, Dowson CG, Evans SD, Fox DJ, Johnson BR, Biggs CI, Randev RK, Stec HM, Taylor PC, Marsh A. Langmuir. 2013;29:2961–2970. doi: 10.1021/la4003719. [DOI] [PubMed] [Google Scholar]
- [261].Taylor W, Jones RAL. Langmuir. 2013;29:6116–6122. doi: 10.1021/la4005483. [DOI] [PubMed] [Google Scholar]
- [262].Yang W, Liu S, Bai T, Keefe AJ, Zhang L, Ella-Menye J-R, Li Y, Jiang S. Nano Today. 2014;9:10–16. [Google Scholar]
- [263].Gunkel G, Weinhart M, Becherer T, Haag R, Huck WTS. Biomacromolecules. 2011;12:4169–4172. doi: 10.1021/bm200943m. [DOI] [PubMed] [Google Scholar]
- [264].Colak S, Tew GN. Langmuir. 2012;28:666–675. doi: 10.1021/la203683u. [DOI] [PubMed] [Google Scholar]
- [265].Blanco-Canosa JB, Wu M, Susumu K, Petryayeva E, Jennings TL, Dawson PE, Algar WR, Medintz IL. Coord. Chem. Rev. 2014;263:101–137. [Google Scholar]
- [266].Shuster MJ, Vaish A, Gilbert ML, Martinez-Rivera M, Nezarati RM, Weiss PS, Andrews AM. J. Phys. Chem. C. 2011;115:24778–24787. [Google Scholar]
- [267].Fayi S, Warren CWC. Nanotechnology. 2011;22:494006. [Google Scholar]
- [268].Zhou M, Nakatani E, Gronenberg LS, Tokimoto T, Wirth MJ, Hruby VJ, Roberts A, Lynch RM, Ghosh I. Bioconjug. Chem. 2007;18:323–332. doi: 10.1021/bc0601929. [DOI] [PubMed] [Google Scholar]
- [269].Parak WJ, Gerion D, Zanchet D, Woerz AS, Pellegrino T, Micheel C, Williams SC, Seitz M, Bruehl RE, Bryant Z, Bustamante C, Bertozzi CR, Alivisatos AP. Chem. Mater. 2002;14:2113–2119. [Google Scholar]
- [270].Kotagiri N, Li Z, Xu X, Mondal S, Nehorai A, Achilefu S. Bioconjug. Chem. 2014;25:1272–1281. doi: 10.1021/bc500139u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [271].Mahmoud W, Rousserie G, Reveil B, Tabary T, Millot J-M, Artemyev M, Oleinikov VA, Cohen JHM, Nabiev I, Sukhanova A. Anal. Biochem. 2011;416:180–185. doi: 10.1016/j.ab.2011.05.018. [DOI] [PubMed] [Google Scholar]
- [272].Fontaine SD, Reid R, Robinson L, Ashley GW, Santi DV. Bioconjug. Chem. 2015;26:145–152. doi: 10.1021/bc5005262. [DOI] [PubMed] [Google Scholar]
- [273].Schieber C, Bestetti A, Lim JP, Ryan AD, Nguyen T-L, Eldridge R, White AR, Gleeson PA, Donnelly PS, Williams SJ, Mulvaney P. Angew. Chem. Int. Ed. 2012;51:10523–10527. doi: 10.1002/anie.201202876. [DOI] [PubMed] [Google Scholar]
- [274].Tong R, Tang L, Ma L, Tu C, Baumgartner R, Cheng J. Chem. Soc. Rev. 2014;43:6982–7012. doi: 10.1039/c4cs00133h. [DOI] [PubMed] [Google Scholar]
- [275].Binder WH, Sachsenhofer R, Straif CJ, Zirbs R. J. Mater. Chem. 2007;17:2125–2132. [Google Scholar]
- [276].Bernardin A, Cazet A, Guyon L, Delannoy P, Vinet F, Bonnaffé D, Texier I. Bioconjug. Chem. 2010;21:583–588. doi: 10.1021/bc900564w. [DOI] [PubMed] [Google Scholar]
- [277].Wu X, Liu H, Liu J, Haley KN, Treadway JA, Larson JP, Ge N, Peale F, Bruchez MP. Nat. Biotechnol. 2003;21:41–46. doi: 10.1038/nbt764. [DOI] [PubMed] [Google Scholar]
- [278].Xing Y, Chaudry Q, Shen C, Kong KY, Zhau HE, Chung LW, Petros JA, O’Regan RM, Yezhelyev MV, Simons JW, Wang MD, Nie S. Nat. Protocols. 2007;2:1152–1165. doi: 10.1038/nprot.2007.107. [DOI] [PubMed] [Google Scholar]
- [279].Howarth M, Chinnapen DJF, Gerrow K, Dorrestein PC, Grandy MR, Kelleher NL, El-Husseini A, Ting AY. Nat. Methods. 2006;3:267–273. doi: 10.1038/NMETHXXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [280].Zrazhevskiy P, Gao X. Nat. Commun. 2013;4:1619. doi: 10.1038/ncomms2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [281].Sasaki A, Tsukasaki Y, Komatsuzaki A, Sakata T, Yasuda H, Jin T. Nanoscale. 2015;7:5115–5119. doi: 10.1039/c4nr06480a. [DOI] [PubMed] [Google Scholar]
- [282].Uyeda HT, Medintz IL, Jaiswal JK, Mattoussi H. J. Am. Chem. Soc. 2005;127:3870–3878. doi: 10.1021/ja044031w. [DOI] [PubMed] [Google Scholar]
- [283].Hucknall A, Rangarajan S, Chilkoti A. Adv. Mater. 2009;21:2441–2446. doi: 10.1002/adma.200803125. [DOI] [PMC free article] [PubMed] [Google Scholar]