Abstract
Dual specificity mitogen-activated protein kinase (MAPK) phosphatases [dual specificity phosphatase/MAP kinase phosphatase (DUSP-MKP)] have been hypothesized to maintain cancer cell survival by buffering excessive MAPK signaling caused by upstream activating oncogenic products. A large and diverse body of literature suggests that genetic depletion of DUSP-MKPs can reduce tumorigenicity, suggesting that hyperactivating MAPK signaling by DUSP-MKP inhibitors could be a novel strategy to selectively affect the transformed phenotype. Through in vivo structure-activity relationship studies in transgenic zebrafish we recently identified a hyperactivator of fibroblast growth factor signaling [(E)-2-benzylidene-5-bromo-3-(cyclohexylamino)-2,3-dihydro-1H-inden-1-one (BCI-215)] that is devoid of developmental toxicity and restores defective MAPK activity caused by overexpression of DUSP1 and DUSP6 in mammalian cells. Here, we hypothesized that BCI-215 could selectively affect survival of transformed cells. In MDA-MB-231 human breast cancer cells, BCI-215 inhibited cell motility, caused apoptosis but not primary necrosis, and sensitized cells to lymphokine-activated killer cell activity. Mechanistically, BCI-215 induced rapid and sustained phosphorylation of extracellular signal-regulated kinase (ERK), p38, and c-Jun N-terminal kinase (JNK) in the absence of reactive oxygen species, and its toxicity was partially rescued by inhibition of p38 but not JNK or ERK. BCI-215 also hyperactivated MKK4/SEK1, suggesting activation of stress responses. Kinase phosphorylation profiling documented BCI-215 selectively activated MAPKs and their downstream substrates, but not receptor tyrosine kinases, SRC family kinases, AKT, mTOR, or DNA damage pathways. Our findings support the hypothesis that BCI-215 causes selective cancer cell cytotoxicity in part through non-redox-mediated activation of MAPK signaling, and the findings also identify an intersection with immune cell killing that is worthy of further exploration.
Introduction
Mitogen-activated protein kinase phosphatases (MKPs) are a subgroup of the dual specificity phosphatase (DUSP) family that has recently been termed DUSP-MKPs to reconcile both current gene nomenclature and historical denominations (Kidger and Keyse, 2016). DUSP-MKPs dephosphorylate and inactivate the mitogen-activated protein kinases (MAPKs) extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK)/stress-activated protein kinase (SAPK), and p38 on tyrosine and threonine residues, thereby regulating duration and amplitude of mitogenic and survival signaling (reviewed in Farooq and Zhou, 2004). A large body of literature, which has been subject to several excellent reviews, supports a role of DUSP-MKPs in cancer (Keyse, 2008; Nunes-Xavier et al., 2011; Kidger and Keyse, 2016). The prototypic DUSP-MKP, DUSP1/MKP-1, is overexpressed in prostate, gastric, breast, pancreatic, ovarian, non-small-cell lung, and metastatic colorectal cancer, and has been associated with decreased progression-free survival (Denkert et al., 2002; Montagut et al., 2010). Genetic depletion of MKP-1 by siRNA enhances sensitivity of cancer cells to clinically used antineoplastic agents (Wu et al., 2005; Liu et al., 2014), whereas its overexpression promotes chemoresistance (Small et al., 2007). In mice, genetic ablation of DUSP1/MKP-1 limits the tumorigenicity of pancreatic cancer cells (Liu et al., 2014) and inhibits non-small-cell lung cancer tumorigenesis and metastasis (Moncho-Amor et al., 2011). Small molecule inhibitors of DUSP-MKPs could therefore provide novel approaches to treat cancer.
The discovery of potent and selective inhibitors of DUSPs has been hindered by a high degree of conservation between their active sites, a shallow and feature-poor topology (Farooq and Zhou, 2004), and the presence of a reactive, active site cysteine, which is critical for enzymatic activity but sensitive to oxidation. Perhaps not too surprisingly, in vitro screens for DUSP inhibitors have yielded agents that were reactive chemicals or lacked biologic activity (Lazo et al., 2002; Johnston et al., 2007). The utility of DUSP-MKP inhibitors as therapeutics is also disputed because of the varied roles that DUSP-MKPs play in physiology and pathophysiology, and their overlapping substrate specificities (Farooq and Zhou, 2004). Consequently, this class of enzymes is often thought of as undruggable.
Using a zebrafish live reporter for fibroblast growth factor activity we discovered a biologically active, allosteric inhibitor of zebrafish Dusp6/Mkp3, (E)-2-benzylidene-3-(cyclohexylamino)-2,3-dihydro-1H-inden-1-one (BCI) (Molina et al., 2009). Subsequent in vivo structure-activity relationship studies in zebrafish embryos coupled with mammalian cell-based assays for inhibition of DUSP1/MKP-1 and DUSP6/MKP-3 using 33 structural congeners identified an analog (BCI-215) that retained fibroblast growth factor hyperactivating and cellular DUSP6/MKP-3 and DUSP1/MKP-1 inhibitory activity but was nontoxic to zebrafish embryos and an endothelial cell line (Korotchenko et al., 2014). Prior studies to address the question of whether DUSP-MKPs could be targeted with small molecules for cancer treatment had been limited by chemical reactivity of existing inhibitors (Vogt et al., 2005, 2008), whereas the favorable toxicity profile of BCI-215 allowed us to ask for the first time whether DUSP-MKP inhibition with small molecules could selectively affect transformed cells but spare normal cells and tissues. We found that BCI-215 inhibited survival and motility of MDA-MB-231 human breast cancer cells but did not affect viability of cultured hepatocytes. BCI-215 activated MAPK signaling pathways, caused apoptosis but not primary necrosis that was partially dependent on p38 but not ERK or JNK activity, and sensitized cells to lymphokine-activated killer (LAK) cell activity. To investigate potential mechanisms for the differential toxicity, we quantified a generation of reactive oxygen species (ROS) and found that, in contrast to previously identified DUSP-MKP inhibitors, BCI-215 did not generate ROS in cancer cells, hepatocytes, or zebrafish embryos. Kinase phosphorylation arrays revealed that BCI-215 selectively activated MAPK signaling downstream from growth factor or stress receptors. The data support BCI-215 as the first cell-active inhibitor of DUSP-MKPs that causes selective cancer cell cytotoxicity, and the data also identify an intriguing intersection with immune cells that may be further exploited.
Materials and Methods
Compounds and Chemicals.
BCI-215 (Korotchenko et al., 2014) has been described previously. Sanguinarine, menadione, NSC95397, BCI, JNK-IN-8, doxorubicin, and cisplatin were purchased from Sigma-Aldrich (St. Louis, MO). CellTracker Green (Molecular Probes, Eugene, OR C2925), chloromethyl fluorescein diacetate, acetyl ester (Molecular Probes C6827), tetramethylrhodamine ethyl ester (TMRE) (Molecular Probes T-669), and dihydroethidium (DHE) (Molecular Probes D1168) were purchased from Thermo Fisher (Pittsburgh, PA). Other MAPK inhibitors were purchased from Selleckchem (Houston, TX) (SCH772984, SP600125, and SB203580). Ficoll-Paque was purchased from GE Healthcare Life Sciences (Marlborough, MA). Interleukin 2 was a generous gift of Prometheus Laboratories, Inc. (San Diego, CA) The Annexin V/PI Apoptosis Detection Kit FITC was obtained from eBioscience (San Diego, CA).
Hepatocyte Mitochondrial Function.
Rat hepatocytes were isolated using standard two-step collagenase digestion (McQueen, 1993) and subcultivated at 14,000 hepatocytes/well in collagen-1-coated 384-well plates in Williams E Media (Gibco, Frederick, MD) supplemented with 10% fetal bovine serum, 2 mM l-glutamine and 50 U/ml penicillin and streptomycin. After 4 hours medium was decanted and replaced with Hepatocyte Maintenance Media (Williams E supplemented with 1.25 mg/ml bovine serum albumin, 6.25 µg/ml human insulin, 100 nM dexamethasone, 6.25 µg/ml human transferrin, 6.25 ng/ml selenous acid, 2 mM l-glutamine, 15 mM HEPES, 100 U/ml penicillin, and 100 U/ml streptomycin). After overnight culture, cells were treated with concentration gradients of test agents. One hour after compound addition, cells were labeled with 200 nM TMRE and 4 µg/ml Hoechst 33342 (Invitrogen, Carlsbad, CA) for 45 minutes, imaged live on an ArrayScan VTI (Thermo Fisher Cellomics, Pittsburgh, PA) using a 10X objective, and the images were analyzed with the Compartmental Bioapplication (Thermo Fisher Cellomics, Pittsburgh, PA). Mitochondria were identified as cytosolic spots by size and brightness. The final parameter was %HIGH_RingSpotAvgIntenCh2 (i.e., the percentage of cells with TMRE puncta in the cytoplasm based on a threshold set with vehicle-treated cells.
ROS Generation in Hepatocytes.
Cells were cultured as described previously and labeled 4 hours following addition of drug with 4 µM DHE for 2 hours. Hoechst 33342 was added to a 4 µg/ml final concentration during the final hour of incubation. In the presence of ROS, DHE was oxidized to a red fluorescent dye (oxyethidium). Cells were imaged as described previously and the percentage of oxyethidium-nuclear positive cells was calculated based on a threshold set with vehicle-treated cells.
Five-Day Hepatocyte Toxicity.
A gelling solution of 1.25 mg/ml rat tail collagen type I in pH 7.2 90:10 (v/v) Williams E media/10X Hanks’ balanced salt solution was overlaid onto the rat hepatocytes. The collagen gel was incubated for 1 hour at 37°C, 5% CO2. The collagen sandwich cultures were then challenged for 5 days to test compounds in Hepatocyte Maintenance Media, without refeeding. A 10X solution of 40 µg/ml Hoechst 33342 was added during the final 2 hours of incubation followed by a 10X solution of 20 µg/ml propidium iodide (PI) for 1 hour. Cells were imaged and the percentage of PI positive cells calculated as described previously.
Zebrafish.
All procedures involving zebrafish were reviewed and approved by the University of Pittsburgh Institutional Animal Care and Use Committee. Wild-type AB* embryos were kept in E3 medium (5 mM NaCl, 0.17 mM KCl, 0.33 mM CaCl2, 0.33 mM MgSO4). At 48 hours postfertilization, embryos were arrayed into the wells of a 96-well microplate and treated with vehicle [0.5% dimethylsulfoxide (DMSO)] or test agents. After a 30-minute preincubation, embryos were labeled with a solution of 10 µM DHE and 40 μg/ml MS222 (tricaine methanesulfonate, Sigma-Aldrich) in E3 to restrict movement during imaging. Six hours after DHE loading, embryos were imaged on an ArrayScan II using a 2.5X objective. Images were analyzed for oxyethidium fluorescence with the TargetActivation Bioapplication (Thermo Fisher Cellomics, Pittsburgh, PA) using the MEAN_ObjectAvgIntenCh1 parameter.
Cell Culture.
MDA-MB-231 and BT-20 breast cancer and HeLa cervical cancer cell lines were obtained in 1997, 2013, and 2000, respectively, from the American Culture Type Collection (Manassas, VA) and maintained as recommended. MDA-MB-231 and HeLa cells were reauthenticated in 2011 by The Research Animal Diagnostic Laboratory at the University of Missouri (Columbia, MO) using a polymerase chain reaction–based method that detects nine short tandem repeat loci, followed by comparison of results to the American Culture Type Collection short tandem repeat database. Original American Culture Type Collection stocks and reauthenticated cells were cryopreserved in liquid nitrogen and maintained in culture for no more than 10 passages or 3 months, whichever was shorter, after which cells were discarded and a new vial thawed.
High-Content Analysis (HCA) of Apoptosis and ERK Phosphorylation.
MDA-MB-231 cells (10,000/well) were treated with identical concentration gradients of test agents on the right and left halves of a 384-well microplate for later assessment of potential compound autofluorescence. After 24 hours, cells were fixed, permeabilized with 0.2% Triton X-100, blocked with 1% bovine serum albumin/phosphate-buffered saline (PBS), and immunostained with anti-phospho-ERK (E10, Cell Signaling technologies, Beverly, MA (CST)/AlexaFluor488 and anti-cleaved caspase-3 (CST)/AlexaFluor594 primary/secondary antibody pairs. Plates were imaged on the ArrayScan II using a 10X 0.5 numerical aperture objective. Each well was background corrected by subtracting mean phospho-ERK and cleaved caspase-3 intensities from wells that had received secondary antibody only.
Detection and quantitation of ROS in cancer cells was performed exactly as described previously (Vogt et al., 2008). Briefly, MDA-MB-231 cells were labeled with Hoechst 33342, loaded with chloromethyl fluorescein diacetate, acetyl ester (5 µM, 15 minutes), and treated with test agents for 10 minutes. After two washes, cells were analyzed for Hoechst and ROS/ fluorescein isothiocyanate (FITC) fluorescence on the ArrayScan II. Cells were classified as positive for ROS if their average FITC intensity exceeded a threshold defined as the average FITC intensity plus one S.D. from DMSO-treated wells. HCA of cell motility, cytotoxicity, and colony formation in three-dimensional matrigel culture are described in the Supplemental Material.
Western Blotting.
Western blotting was performed as described previously (Vogt et al., 2008). Antibodies were: pERK (T202/Y204, CST9101), total ERK (CST9102), pJNK, (T183/Y185, CST9251), total JNK, (CST9252), pp38 (T180/Y182, CST9215), total p38 (CST9212), (pMEK1/2 (S217/221, CST9121), total MEK1/2 (CST9122), pMKK4/SEK1 (S257, CST4514), MKK4/SEK1 (CST3346), and GAPDH (Abcam, Cambridge, MA, cat# 8245). Antibodies were used at 1:1000 dilution except pJNK (1:500) and GAPDH (1:2000).
Phospho-kinase profiling was performed with a commercially available assay kit containing membrane-immobilized, phosphorylation-specific antibodies against 43 human kinases spotted in duplicate (R&D Systems, Minneapolis, MN). MDA-MB-231 cells were grown to near confluency on 100 mm cell culture dishes, treated with vehicle (0.1% DMSO) or 20 µM BCI-215 for 30 minutes, harvested by scraping into cold PBS, pelleted at 500g for 3 minutes, and lysed in 400 µl of supplied lysis buffer. Protein content was determined by Bradford assay. Each membrane was incubated overnight at 4°C with equal amounts of lysate protein (300–400 µg) and processed as per manufacturer’s instructions. Phosphorylation signals were visualized by chemiluminescence on a Fuji LAS 4000 imager, and quantified in Developer (Definiens AG, Munich, Germany) using a mapping template supplied by the manufacturer. Spot intensities were normalized to reference signals on each membrane before calculating ratios between vehicle-treated and BCI-215-treated spots.
Toxicity Reversal.
Cells were pretreated (30 minutes for SCH772984, SP600125, and SB203580, and 3 hours for JNK-IN8) with identical concentration gradients of MAPK inhibitors on the right and left halves of a 384-well microplate. After preincubation, half of the microplate was treated with vehicle (DMSO), the other with a proapoptotic concentration of BCI-215 (25 µM). To eliminate potential bias through plate/edge effects, an independent plate was prepared in parallel where vehicle and BCI-215 treatments were reversed. Twenty-four hours thereafter, plates were stained with Hoechst 33342, washed once, and imaged on the ArrayScan II using a 10X objective for analysis for cell numbers and nuclear morphology. Plates were subsequently immunostained with a cleaved caspase-3/Cy5-conjugated secondary antibody pair and analyzed for apoptosis on an ArrayScan VTI using a 20X 0.75 numerical aperture objective. Four independent readouts were extracted and correlated: cell density (SelectedObjectCountPerValidField), nuclear condensation (MEAN_ObjectAvgIntenCh1), nucleus rounding (MEAN_ObjectShapeLWRCh1), and average cellular cleaved caspase-3 intensity (MEAN_AvgIntenCh2). For each parameter, data were normalized to vehicle (maximum rescue) and BCI-215 (no rescue) as % rescue = 1 − [(data point + DMSO)/(DMSO + BCI-215)] × 100.
Immune Cell Killing.
Peripheral blood mononuclear cells were obtained from healthy volunteers with an established Institutional Review Board approved protocol, and separated from heparinized blood on Ficoll-hypaque (GE Healthcare, Chicago) gradients as previously reported (Buchser et al., 2012). Cells were cultured in RPMI 1640 supplemented with 10% fetal calf serum, 1% glutamine, and 1% penicillin/streptomycin, and stimulated with 6,000 IU of Interleukin 2 for 24 hours. After incubation, cells were washed with PBS and counted. In parallel, MDA-MB-231 cells were pretreated in a 384-well plate with vehicle or BCI-215 (3 µM). After 24 hours in culture, medium was replaced and peripheral blood mononuclear cells (PBMCs) added in 2-fold serial dilutions starting with a 50-fold excess of PBMCs in triplicate. After 24 hours of coculture, cells were fixed with formaldehyde/Hoechst 33342, washed twice with PBS, and imaged on the ArrayScan II. Cancer cells were identified by their larger nuclei compared with PBMCs, setting a size gate in the Hoechst channel. In experiments with chemotherapeutics, cells carrying a biosensor consisting of a mitochondrial targeting sequence derived from cytochrome c oxidase VIII linked to GFP that is a surrogate for cytochrome c release from mitochondria (Senutovitch et al., 2015) were pretreated for 24 hours with cisplatin (2 µM) or doxorubicin (400 nM), exposed to LAK as described previously, and cancer cells were identified and quantified by green fluorescence. Cell densities were normalized to those in the absence of PBMCs. Mean cell densities from multiple independent experiments were averaged and plotted in GraphPad Prism version 7.00 (La Jolla, CA).
Flow Cytometry.
Flow cytometric analysis was performed on a C6 flow cytometer (Accuri Cytometers, Ann Arbor, MI) instrument within the University of Pittsburgh Cancer Institute Flow and Imaging Cytometry Core Facility and analyzed using FlowJo software (Tree Star Inc., Ashland, OR). Single cell suspensions were stained with annexin/PI (eBioscience) according to the manufacturer’s protocol. Cells were identified via forward and side scatter and gated accordingly. All assessments were performed immediately after 30 minutes of incubation at 37°C. Necrotic, early, and late apoptotic cells were defined as cells that stained positive for PI only, annexin V only, or PI and annexin V, respectively.
Statistical Analysis.
Multiple data points were analyzed in GraphPad Prism by one-way analysis of variance using Dunnett’s multiple comparisons test. The half-maximal effective/inhibitory concentration (EC50/IC50) values were obtained from at least three independent experiments by nonlinear regression using a four-parameter logistic equation. The IC50, S.E., and 95% confidence intervals were calculated in GraphPad.
Results
BCI-215 Lacks Oxidative Toxicity to Rat Hepatocytes.
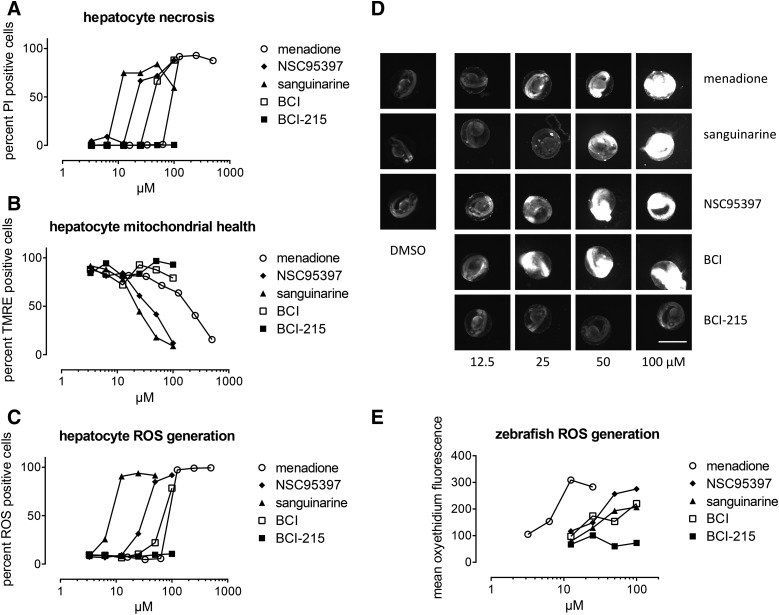
We first extended our previous studies of developmental toxicity to a clinically relevant cell type. Freshly isolated rat hepatocytes were plated into 96-well plates and treated with 2-fold concentration gradients of BCI-215, three previously described DUSP inhibitors (sanguinarine (Vogt et al., 2005), NSC95397 (Vogt et al., 2008), BCI (Molina et al., 2009), or menadione as a positive control for hepatotoxicity (Supplemental Fig. 1). Toxicity was assessed by a live cell, high-content assay counting PI positive cells after 5-day exposure, and through TMRE staining of mitochondria, which predicts hepatotoxicity due to mitochondrial damage in the clinic with high concordance (Pereira et al., 2012). NSC95397, sanguinarine, and menadione caused cell death that correlated with loss of mitochondrial membrane integrity (Fig. 1, A and B). BCI caused cell death but did not affect mitochondrial potential. BCI-215 was completely devoid of hepatocyte toxicity up to 100 µM, suggesting low hepatic toxicity if developed into a potential therapeutic.
Fig. 1.
BCI-215 is nontoxic to rat hepatocytes and developing zebrafish embryos. (A–C) Rat hepatocytes were treated in 96-well plates with 10-point concentration gradients of DUSP inhibitors and menadione as a positive control for hepatotoxicity. Sanguinarine, NSC95397, BCI, and menadione, but not BCI-215 produced dose-dependent cell death in rat hepatocytes as measured by (A) PI uptake and (B) loss of mitochondrial membrane integrity. (C) Hepatocyte toxicity correlated with production of ROS. (D and E) In contrast to other DUSP inhibitors, BCI-215 did not generate ROS in developing zebrafish embryos. Data and images are from a single experiment that has been repeated once. Scale bar, 500 µm.
BCI-215 Does Not Generate ROS in Hepatocytes or in Developing Zebrafish Larvae.
We next quantified generation of ROS by DHE staining. Similar to mitochondrial membrane potential, ROS generation is one of the best predictors of clinical hepatotoxicity (Pereira et al., 2012). From a mechanistic perspective, compounds that generate ROS can lead to nonspecific, irreversible inactivation of protein tyrosine phosphatases and DUSPs. The active sites of all protein tyrosine phosphatases and DUSPs contain a nucleophilic cysteine that is extremely sensitive to oxidation, and while mild reversible oxidation is a physiologic mechanism to regulate activity (Seth and Rudolph, 2006), oxidation past the sulfinic acid stage is irreversible (Bova et al., 2004). Irreversible oxidation is expected for the naphthoquinone NSC95397, which generates ROS in MDA-MB-231 breast cancer cells (Vogt et al., 2008), and sanguinarine, which depletes glutathione levels (Debiton et al., 2003). We found that with the exception of BCI-215, all agents generated ROS in hepatocytes (Fig. 1C), providing both a mechanism for BCI-215’s lack of toxicity and eliminating the possibility of nonselective oxidative phosphatase inactivation. All agents that caused ROS in hepatocytes also caused ROS in zebrafish embryos, although their IC50 values were slightly different in the two models, possibly reflecting differences in compound uptake or metabolism (Fig. 1, D and E). These findings document that the cellular activities of BCI-215 in zebrafish are not mediated by nonselective oxidative processes.
BCI-215 Has Antimigratory and Proapoptotic Activities in Breast Cancer Cells that Correlate with Induction of ERK Phosphorylation.
We next investigated whether BCI-215 was toxic to cancer cells. MDA-MB-231 cells were plated in an ORIS Pro 384 cell migration plate (Platypus Technologies, Madison, WI) and treated with 10-point concentration gradients of NSC95397, BCI, or BCI-215. Forty-eight hours following treatment, cells were stained live with PI and Hoechst 33342, and the percentage of PI positive cells was quantified on an ArrayScan II (Thermo Fisher) high-content reader. All agents inhibited cell motility and attachment, and showed nuclear shrinkage with IC50 values between 7 and 15 µM (Supplemental Fig. 2). As expected for a chemically reactive structure, NSC95397 caused necrosis at antimigratory concentrations (Supplemental Fig. 2A, % PI positive cells). Necrosis was reduced with BCI, and BCI-215 showed no signs of necrosis at antimigratory or proapoptotic concentrations (Supplemental Figs. 2, B and C, and 3A). BCI-215 also inhibited colony formation in the matrigel-on-top model, where cells are seeded at low densities, recapitulating an initial dormancy-like state followed by clonal outgrowth (Shibue et al., 2012). MDA-MB-231 cells were transduced with a mitochondrial-targeted, GFP-labeled cytochrome c biosensor (Senutovitch et al., 2015) to enable continuous live monitoring of colony growth, plated on a layer of matrigel, and treated 24 hours later with various concentrations of BCI-215. Following 2 days of exposure, drug was removed and cells were allowed to expand for an additional 4–6 days. At the end of the study, cells were incubated with PI and analyzed for cell numbers and PI positivity by HCA. In contrast to the short-term two-dimensional assay, BCI-215-treated cells showed pronounced cell lysis in the longer-term three-dimensional matrigel assay (Supplemental Figs. 2, D and E, and 3B).
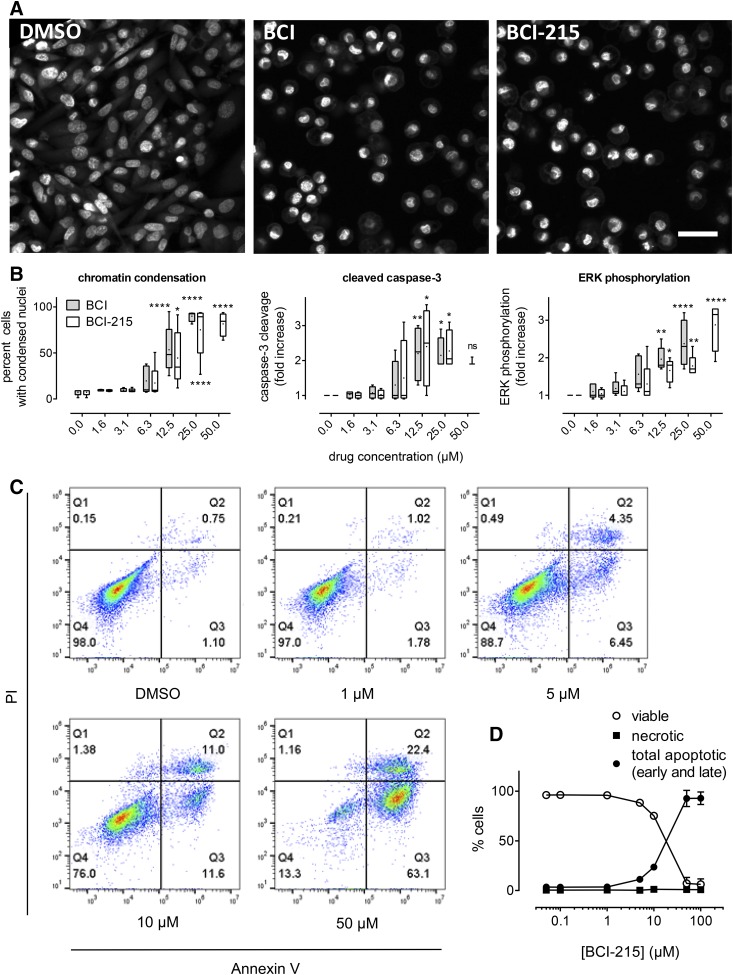
To probe the mechanisms of BCI-215-induced cell death, we performed multiplexed HCA of nuclear morphology, caspase-3 cleavage (apoptosis), and ERK phosphorylation as a pharmacodynamic biomarker for DUSP-MKP inhibition. Figure 2A shows that BCI and BCI-215 produced shrunken, condensed nuclei that resembled pyknosis, an early apoptotic event (Elmore, 2007). Simultaneous quantitation of condensed nuclei, caspase-3 cleavage, and ERK phosphorylation revealed that both agents caused apoptosis that correlated with ERK phosphorylation (Fig. 2B). Based on their IC50 values, BCI and BCI-215 were equipotent (Supplemental Table 1); however, at the highest concentration tested (50 µM) BCI’s nonspecific toxicity impaired specific cellular measurements. Flow cytometric analysis confirmed apoptotic death and documented that PI positivity was a result of secondary cell membrane permeability, occurring only in annexin V positive cells (Fig. 2, C and D).
Fig. 2.
BCI and BCI-215 cause apoptotic cell death at concentrations that induce ERK phosphorylation. MDA-MB-231 cells were treated with vehicle (DMSO), BCI, or BCI-215 and stained with Hoechst 33342 and anti-phospho-ERK and anti-cleaved caspase-3 antibodies, respectively. (A) Fluorescence micrographs show pyknotic nuclei indicative of early apoptosis. Images are maximum projections of a 10-plane, 0.25 µm each, z-series acquired using a 60X objective on a Molecular Devices ImageXpress Ultra High Content Reader (Sunnyvale, CA). BCI and BCI-215 were at 22 µM. Scale bar, 30 µm. (B) Multiparametric analysis of chromatin condensation, caspase-3 cleavage, and ERK phosphorylation by HCA. Each box plot is the aggregate of four (caspase) or five (nuclear condensation and ERK phosphorylation) independent experiments. Boxes show upper and lower quartiles; whiskers, range; dot, mean. *P < 0.05; **P < 0.01; ****P < 0.001 versus DMSO by one-way analysis of variance with Dunnett’s multiple comparisons test. The last data point for cleaved caspase is n = 3 for 50 µM BCI-215 with two of the three values being identical. (C and D) Confirmation of apoptosis with secondary cell lysis by flow cytometry. Data in (D) are the average ± S.E.M. values of three independent flow cytometry experiments. Early apoptosis, Q3, annexin V positive and PI negative; late apoptosis, Q2, annexin V and PI positive; necrosis, Q1, PI positive and annexin V negative.
BCI-215 Sensitizes Cancer Cells to Immune Cell Killing.
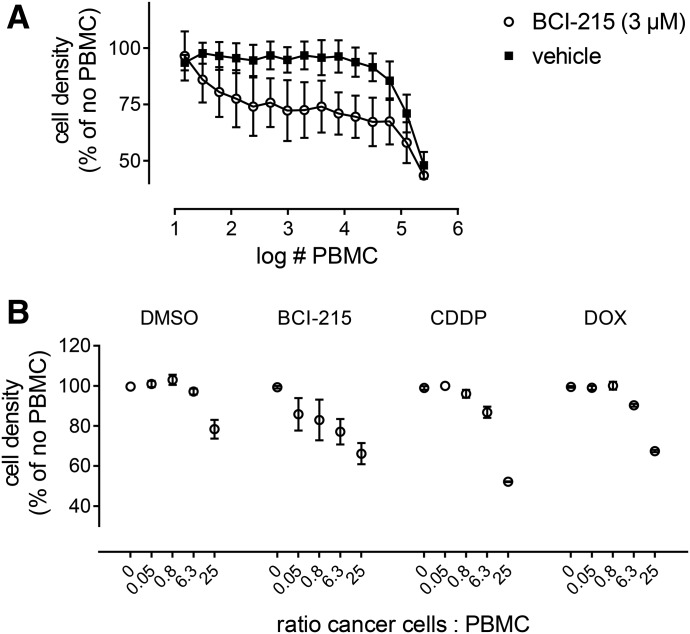
Immune system–targeted therapies are perhaps the greatest advance in cancer treatment in the last 50 years. Despite the spectacular success with immune checkpoint inhibitors, the majority of patients do not respond (Topalian et al., 2015). Thus, there is an urgent need to develop effective therapies for those patients that do not achieve durable responses, and other mechanisms of resistance should be considered including the lymphoplegic effects of damage-associated molecular pattern molecule release (Lotfi et al., 2016). A promising approach to harness the immune system in the response to small molecules is immunogenic cell death (ICD) (Kroemer et al., 2013). In ICD, tumor cells undergoing apoptosis display and secrete factors that recruit immune cells to the tumor bed and enhance cell killing activity. To test whether BCI-215 might sensitize cancer cells to immune cell kill, MDA-MB-231 cells were treated with vehicle or a mildly toxic concentration of BCI-215 (3 µM) for 24 hours followed by addition of Interleukin 2–activated PBMCs. After an additional incubation of 24 hours, cells were fixed, stained with Hoechst 33342, and imaged on the ArrayScan II. Cancer cell nuclei were gated by their larger size compared with PBMCs. Figure 3A shows the dose-response curves of activated PBMCs added to cells pretreated with vehicle or BCI-215, averaged from three separate experiments. In the presence of vehicle alone, cells were relatively insensitive to the immune cell kill; a maximal effect was obtained with a 20-fold excess of LAK; the EC50 value was about a 10-fold excess (50,000 LAK/well). In the presence of BCI-215, the kill curve was shifted dramatically to lower numbers of PBMCs, with maximal sensitization seen with as few as 1000 LAK/well, and the EC50 values of as few as 100 LAK/well, well over three log differences in killing. We then compared the effects of BCI-215 to two clinically used chemotherapeutic agents, doxorubicin and cisplatin, which have previously been reported to increase LAK activity in cell culture (Yamaue et al., 1991; Wennerberg et al., 2013). All agents sensitized cells to LAK activity; however, BCI-215 consistently showed sensitization at lower effector ratios than cisplatin or doxorubicin (Fig. 3B).
Fig. 3.
BCI-215 sensitizes breast cancer cells to immune cell kill. (A) MDA-MB-231 cells were treated overnight in 384-well plates with vehicle or 3 µM BCI-215 followed by washout. Cells were subsequently exposed to various ratios of PBMC-derived LAK. After 24 hours, cells were fixed and stained with Hoechst 33342. Cells were imaged on the ArrayScan II, cancer cell nuclei identified and gated by their larger size compared with PBMC, and enumerated. Cell densities were normalized to vehicle or BCI-215 in the absence of activated immune cells, respectively. Data are the average ± S.E.M. values from four independent experiments, each performed in triplicate. (B) Comparison of BCI-215 versus clinically used antineoplastic agents, doxorubicin (DOX) and cisplatin (CDDP). MDA-MB-231 cells were either stained with CellTracker green or transduced with a mitochondrial-targeted, GFP-labeled cytochrome c biosensor, and processed and analyzed as in (A) except that cancer cells were specifically identified by green fluorescence instead of nucleus size gating. Each data point represents the mean ± S.E.M. value of three independent experiments, each performed in triplicate.
BCI-215 Induces Mitogenic and Stress Signaling in Cancer Cells without Generating ROS.
DUSP-MKPs have unique but overlapping substrate specificities. For example, DUSP6/MKP-3 is specific for ERK, whereas DUSP1/MKP1 dephosphorylates ERK, JNK/SAPK, and p38 (Farooq and Zhou, 2004). To establish a MAPK pathway activation profile and to corroborate the results from the immunofluorescence analysis, we performed western blot analysis of the kinetics of p-ERK, p-JNK/SAPK, and p-p38 induction in MDA-MB-231 cells at cytotoxic concentrations of BCI and BCI-215 (20 µM). Figure 4A shows that both agents activated all three kinases with identical kinetics. Similar activation of signaling pathways was observed in a second triple-negative breast cancer line with different mutational profile and morphology (BT-20) and a non-breast cancer line (HeLa) (Fig. 4B). We included doxorubicin as a negative control that requires several hours for MAPK activation because of transcriptional downregulation of DUSP1/MKP-1 (Small et al., 2003). Unexpectedly, BCI-215 also activated MEK1 and MKK4/SEK1, which are upstream of ERK (Zheng and Guan, 1993) and p38/JNK, respectively (Brancho et al., 2003) (Fig. 4, A and B). While MEK1 activation was minor and not observed in all cell lines, MKK4/SEK1 phosphorylation was elevated in all three lines (Fig. 4B). To probe if activation of MAPK signaling was a cause of nonselective oxidative stress, we analyzed MDA-MB-231 cells for generation of ROS in the presence of DUSP-MKP inhibitors. Cells were prelabeled for 15 minutes with Hoechst 33342 and dichloromethyl-fluorescein diacetate, acetyl ester as previously described (Vogt et al., 2008), and treated with various concentrations of NSC95397, BCI, or BCI-215. At 30 minutes, and 1, 2, 3, and 5 hours, cells were imaged live on an ArrayScan II in the Hoechst and FITC channels. Figure 4C shows that, as expected from our previous study (Vogt et al., 2008), the para-quinone NSC95397 generated ROS within 30 minutes, with an EC50 value of about 3–5 µM (Fig. 4D). This response was diminished with BCI (EC50: 20 µM). BCI-215, at 50 µM (more than 5× the EC50 value for apoptosis and p-ERK induction), did not generate ROS in MDA-MB-231 cells (Fig. 4D). Collectively, the data indicate BCI-215 induces a stress response that is not dependent on oxidation.
Fig. 4.
BCI-215 activates mitogen-activated and SAPK cascades in the absence of oxidative stress. (A) Activation kinetics. MDA-MB-231 human breast cancer cells were treated with BCI or BCI-215 (20 µM) for the indicated time points and analyzed for phosphorylation of the DUSP1/MKP-1 and DUSP6/MKP-3 substrates, ERK, JNK/SAPK, and p38, as well as their upstream activators MEK1 and MKK4/SEK1 by Western blot. (B) Activation of kinase cascades in three different cell lines. Cells were treated for 1 hour with vehicle (DMSO), 20 µM BCI-215 (215), or 5 µM doxorubicin (DOX). Data in (A) and (B) are from a single experiment that was repeated once. (C and D) ROS generation. MDA-MB-231 cells were prelabeled with Hoechst 33342 and chloromethyl-fluorescein diacetate, acetyl ester (CM-H2-DCFDA) for 30 minutes followed by treatment with test agents for up to 5 hours. (C) At the indicated time points, cells were imaged and the percentage of ROS positive cells enumerated. (D) Concentration response at the 2-hour time point. Each data point is the mean of four wells ± S.E.M. from a single experiment that was repeated twice.
Inhibition of p38, but Not ERK or JNK/SAPK, Partially Reverses BCI-215 Toxicity.
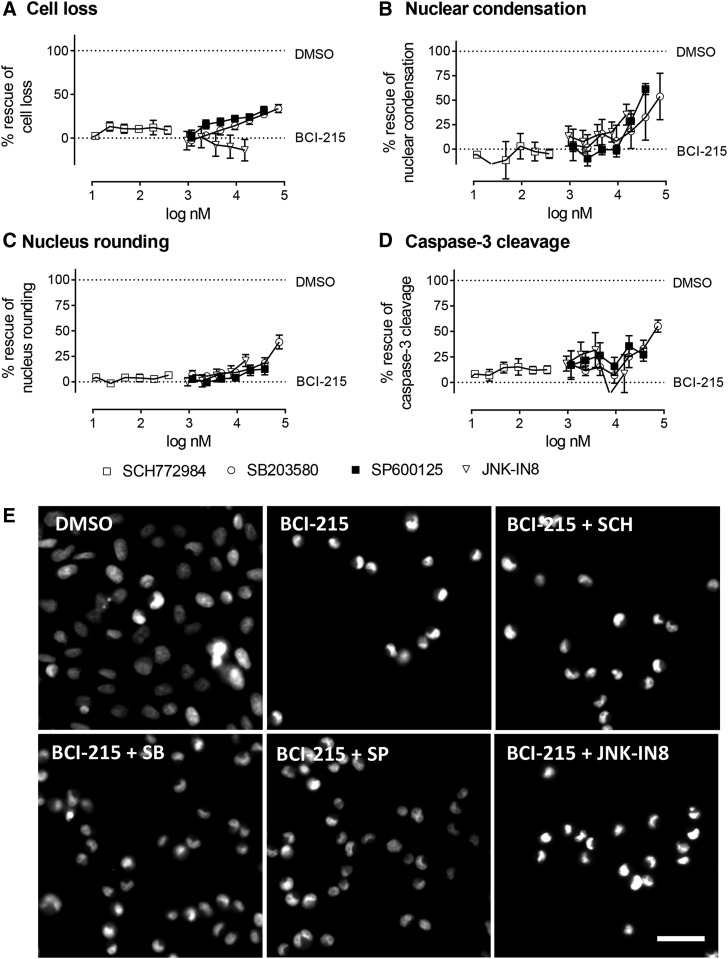
We next asked whether activation of MAPK signaling contributed to BCI-215 cytotoxicity. All three MAPKs can autophosphorylate (Mingo-Sion et al., 2004; Kim et al., 2005; Morris et al., 2013), allowing us to use MAPK inhibitors to probe pathway involvement. Cells were treated on two halves of a 384-well plate with identical concentration gradients of selective ERK, JNK, and p38 inhibitors (SCH772984, JNK-IN8, and SB203580), and a multitargeted inhibitor of JNK (SP600125), respectively, bracketed around published concentrations reported to inhibit cellular MAPK activity: SCH771984, 30 nM (Morris et al., 2013); SP600125, 10 µM (Mingo-Sion et al., 2004); SB203580, 10 µM (Kim et al., 2005), and JNK-IN8, 0.5 µM (Zhang et al., 2012). After a 30-minute preincubation (3 hours for JNK-IN8), one-half of the microplate was treated with vehicle (DMSO) and the other half was treated with a proapoptotic concentration of BCI-215 (25 µM). After 24-hour exposure, plates were stained with Hoechst 33342, and analyzed for cell numbers and nuclear morphology on the ArrayScan II. Plates were subsequently immunostained with a cleaved caspase-3 antibody. Figure 5 shows that p38 and nonselective JNK inhibition partially reversed BCI-215-induced cell loss, nuclear morphology changes, and apoptosis (Fig. 5), whereas specific inhibition of ERK or JNK had no effect. The partial rescue of toxicity indicates that either both p38 and JNK inhibition is necessary for full reversal of toxicity or that MAPK-unrelated pathways also contribute to BCI-215 cytotoxicity.
Fig. 5.
Effect of MAPK inhibition of BCI-215 toxicity. MDA-MB-231 cells were pretreated with concentration gradients of MAPK inhibitors followed by vehicle or a proapoptotic concentration of BCI-215 (25 µM). After 24 hours, cells were stained with Hoechst 33342 and an antibody against cleaved caspase-3, and analyzed for (A) cell density, (B and C) nuclear morphology, and (D) caspase cleavage. Data on graphs depict percent rescue from BCI, calculated as 1 − [(data point + DMSO)/(DMSO + BCI-215)] × 100. Images in (E) illustrate cell loss and nuclear morphology with vehicle (DMSO) and BCI-215 alone, or of BCI-215 in the presence of SCH771984 (375 nM), SB203580 (18 µM), SP600125 (18 µM), or JNK-IN8 (1.8 µM). Data are the averages of 4–7 independent experiments ± S.E.M., each performed in quadruplicate. Images are from an ArrayScan VTI using a 20X objective. Scale bar, 30 µm.
BCI-215 Selectively Activates MAPK Signaling.
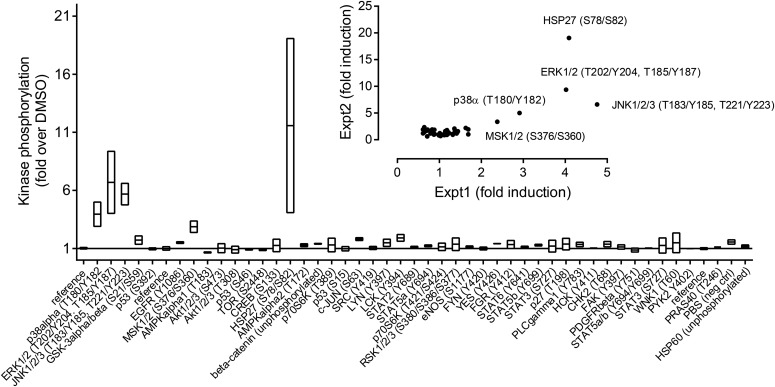
Both the partial rescue of cellular toxicity by MAPK inhibitors and the activation of MKK4/SEK1 left open the possibility that BCI-215 caused a general stress response independent of MAPKs. To probe pathway specificity, we analyzed cellular lysates of MDA-MB-213 cells for phosphorylation of 43 signal transduction kinases using a commercially available antibody array. Figure 6 shows that after a brief (30-minute) exposure, BCI-215 selectively activated MAPKs (p38, ERK, and JNK), as well as their downstream substrates, MSK1/2 (Deak et al., 1998) and HSP27 (Landry et al., 1992). No activation of receptor tyrosine kinases (the epidermal growth factor receptor and platelet-derived growth factor receptor), SRC family kinases, AKT, metabolic enzymes (mTOR and AMPK), DNA damage-activated pathways (p53 and CHK2), or inflammatory mediators (STAT) was observed. Collectively, the results are consistent with a catalytic mechanism involving elimination of MAPK signaling negative feedback downstream from the growth factor or stress receptors.
Fig. 6.
Phospho-kinase profiling of BCI-215 in MDA-MB-231 cells. Lysates from cells treated with vehicle (0.1% DMSO) or BCI-215 (20 µM) for 30 minutes were analyzed for phosphorylation levels of 43 human kinases. Bar graph shows mean ± range of two independent repeats; inset shows correlation of two independent replicate runs.
Discussion
It has long been proposed that overexpression of DUSP-MKPs represents a dependency of cancer cells, but to date, efforts to target DUSP-MKPs with small molecules have failed. The drug-ability of DUSP-MKPs has been questioned based on the feature-poor nature of their catalytic site, sensitivity to oxidation, and a high degree of conservation between members of the DUSP-MKP family. It is also being argued that even if it were possible to selectively inhibit individual DUSP-MKPs, off-target effects would invariably pose a problem because of overlapping substrate specificities. Recent studies from our laboratory and findings presented in this paper suggest that these views may be too simplistic. BCI-215 inhibits at least two DUSPs (DUSP1/MKP-1 and DUSP6/MKP-3) and yet is completely devoid of normal cell and developmental toxicity. Because BCI-215’s biologic activities are not obscured by toxicity, this compound is the first to permit testing the hypothesis that it is possible to pharmacologically target DUSP-MKPs as a dependency of cancer cell survival. We found that BCI-215 selectively killed cancer cells but spared cultured hepatocytes. In contrast to previously identified DUSP-MKP inhibitors, BCI-215 did not generate ROS. BCI-215 caused apoptosis but not primary necrosis, suggesting a physiologic form of cell kill that in clinical settings might avoid the complication of tumor lysis syndrome and the resultant inactivation of immune cells (Howard et al., 2011).
BCI-215 sensitized cancer cells to LAK activity. The precise molecular mechanisms for the remarkable shift in LAK potency are currently under investigation but are likely due to enhanced expression or secretion of stress ligands by treated cells, activating immune cells and causing ICD (Kroemer et al., 2013). The presence of immune cells in the tumor bed is one of the most powerful prognostic indicators of patient survival (Galon et al., 2006). Only a few chemotherapies induce ICD, and for those that do better clinical outcomes have been observed (reviewed in Kroemer et al., 2013). ICD involves induced expression of stress ligands on tumor cells (Miyashita et al., 2015), enabling recognition of tumor cells and facilitating enhanced interactions between tumor cells and immune effectors, release of IFN gamma and HMGB1, enhanced survival/autophagy in responding cells, and lytic elimination of tumor cells unable of responding temporally in an effective manner. Specific candidate mechanisms for ICD worthy of investigation are NKG2D (one of 12 unique natural killer receptors not expressed in lymphoblastoid cell lines) and STING. Particularly innate immune cells (Feng et al., 2016) but also T-cells (Deng et al., 2014) express NKG2D as a stress receptor sensitive to stressed cells. NKG2D ligand expression is positively correlated with a longer relapse-free period in breast cancer patients (de Kruijf et al., 2012). Furthermore, the mechanism of chemotherapy induced stress ligand expression likely involves the STING pathway (Woo et al., 2014) induced by DNA damage or other means to activate STING. An alternative notion is that such chemotherapy promotes immune cell attraction through enhanced recognition of altered self with diminished expression of molecules in stressed cells (Fine et al., 2010).
BCI-215 sensitized cancer cells to LAK activity despite showing little cell lysis in two-dimensional culture. This could suggest that display of phosphatidylserine (annexin V stain) and a relatively modest amount of secondary necrosis, which is necessary for soluble ligand release, are sufficient for the observed level of sensitization. Alternatively, cells grown in microenvironments that more closely resemble in vivo conditions might be even more susceptible to BCI-215. Experiments in long-term (1 week) three-dimensional matrigel culture documented that BCI-215 prevented colony outgrowth and resulted in much higher levels of cell lysis compared with short-term monolayer culture. This opens up the exciting possibility that BCI-215 could cause enhanced ICD in microenvironments resembling the metastatic niche.
While our LAK experiments initially focused on sensitization of cancer cells, it might also be possible to directly exploit DUSP-MKP inhibition to boost immune responses. Elevated levels of DUSP1/MKP-1 have been found in peripheral T lymphocytes in women with breast cancer (Kurt et al., 1998), possibly impairing T-cell function. In aging patients, BCI enhanced the activity of T-cells by restoring defective ERK signaling caused by increased DUSP6/MKP-3 expression (Li et al., 2012). Thus, it is conceivable that BCI-215 could directly activate PBMCs or augment Interleukin 2 activity, which is dependent on MAPK activation.
The effects of BCI and BCI-215 were not limited to MDA-MB-231 cells. BCI-215 activated MAPK signaling in BT20 and HeLa cells. BCI has been tested in the NCI 60 cell line panel (NSC150117) with a mean GI50 of 1.84 µM and a preference for leukemia cells (last tested June 2016). Consistent with this, Müschen’s group demonstrated BCI selectively induced cell death in patient-derived pre-B acute lymphoblastic leukemia cells, likely through inhibition of DUSP6/MKP3, which they showed to be essential for oncogenic transformation in mouse models of pre-B acute lymphoblastic leukemia (Shojaee et al., 2015).
To what extent the effects of BCI-215 on cancer cell toxicity are mediated by DUSPs presently cannot be answered definitively but the majority of results are consistent with DUSP inhibition. We know from prior studies that BCI analogs are bona fide inhibitors of at least some DUSPs. In zebrafish embryos, BCI restores fibroblast growth factor target gene expression in the presence of overexpressed Dusp6 but not Dusp5 or sprouty (Molina et al., 2009). BCI and BCI-215 override the effects of ectopic DUSP6/MKP-3 and DUSP1/MKP-1 expression in HeLa cells (Korotchenko et al., 2014). Thus, BCI-215 is a valuable, nontoxic chemical probe for specific DUSP-mediated biologies. In cancer cells, which express multiple redundant DUSPs, the evidence is not yet definitive but it is most consistent with negative feedback inhibition. BCI-215 rapidly and persistently activated MAPKs, which is different from the fast but transient response of growth factors or the delayed but persistent response by radiation, death ligands (Dhanasekaran and Reddy, 2008), or doxorubicin (Small et al., 2003), arguing against ligand-like or transcriptional mechanisms.
BCI-215 activated MKK4/SEK1, which is a stress-activated kinase upstream of MAPKs. This result was unexpected since SEK1 is not dephosphorylated by DUSPs but by serine/threonine phosphatases. The mechanism by which BCI-215 activates MKK4/SEK1 is currently unclear but could be interpreted as DUSP inhibition being the trigger of cellular stress. This would be consistent with the hypothesis that DUSPs are stress-adaptive genes that allow cancer cells to tolerate the effects of oncogenic transformation since removal of such an adaptive protection is expected to expose cells to unmitigated oncogenic signaling. An alternative explanation would be that BCI-215 has additional actions independent of DUSP inhibition and/or MAPK activation. To address this possibility, we performed phospho-kinase profiling, which documented that BCI-215 selectively activated MAPKs and their downstream substrates, but not receptor tyrosine kinases, SRC family kinases, AKT, mTOR, or metabolic, inflammatory, or DNA damage pathways. These data further support the notion that BCI-215 has specific cellular activities resulting from MAPK activation.
Whether DUSPs are the sole targets of BCI-215—and if so, whether a combination of multiple DUSPs is needed for cancer-selective cell killing and immune cell sensitization—remains to be seen and will require a comprehensive analysis of BCI-215’s molecular mechanism(s) of action through an array of orthogonal assays including functional genomics, target engagement studies, and chemical proteomics. However, we posit that the unique properties of this intriguing molecule make those studies worth pursuing, not only to advance BCI-215 as a complement to cancer immunotherapy, but also to possibly uncover novel mechanisms for immunogenic cell kill.
Acknowledgments
We thank Nina Senutovich for the viral stocks of the biosensor consisting of EGFP with a mitochondrial targeting sequence derived from cytochrome c oxidase subunit VIII. We also thank Dr. Herbert Zeh and Dr. Daolin Tang at the Center for Damage Associated Molecular Pattern Biology, University of Pittsburgh Cancer Institute, for collegiality and insights.
Abbreviations
- BCI
(E)-2-benzylidene-3-(cyclohexylamino)-2,3-dihydro-1H-inden-1-one
- CST
Cell signaling technologies
- DHE
dihydroethidium
- DMSO
dimethylsulfoxide
- DUSP
dual specificity phosphatase
- ERK
extracellular signal-regulated kinase
- FITC
fluorescein isothiocyanate
- HCA
high-content analysis
- ICD
immunogenic cell death
- JNK
c-Jun N-terminal kinase
- LAK
lymphokine-activated killer cell activity
- MAPK
mitogen-activated protein kinase
- MKP
mitogen-activated protein kinase phosphatase
- PBMC
peripheral blood mononuclear cell
- PBS
phosphate-buffered saline
- PI
propidium iodide
- ROS
reactive oxygen species
- SAPK
stress-activated protein kinase
- TMRE
tetramethylrhodamine ethyl ester
Authorship Contributions
Participated in research design: Kaltenmeier, Vernetti, Day, Tsang, Lotze, Vogt.
Conducted experiments: Kaltenmeier, Vollmer, Vernetti, Caprio, Davis, Korotchenko, Vogt.
Contributed new reagents or analytic tools: Hulkower, Korotchenko.
Performed data analysis: Kaltenmeier, Vernetti, Caprio, Davis, Lotze, Vogt.
Wrote or contributed to the writing of the manuscript: Kaltenmeier, Vollmer, Vernetti, Caprio, Davis, Hulkower, Day, Tsang, Lotze, Vogt.
Footnotes
This project was supported in part by the National Institutes of Health National Cancer Institute [Grants CA147985 and CA181450]; the Kennedy Shriver National Institute of Child Health and Human Development [Grant HD053287]; DARPA Big Mechanism Proposal [DARPA-BAA-14-14]; and Automated Integration of Mechanisms in Cancer [AIMCancer Award W911NF-14-1-0422]. K.D. was supported by the Doris Duke Foundation Academy for Clinical Research, University of Pittsburgh (M.T.L.). This project used the University of Pittsburgh Cancer Institute Chemical Biology and Flow and Imaging Cytometry Core Facilities that are supported in part by Award P30CA047904.
Part of this work was presented as follows: Vollmer L, Vernetti L, Bakan A, Korotchenko V, Bahar I, Day B, Tsang M, and Vogt A (2014) A non-redox reactive allosteric inhibitor of MAPK phosphatases with selective toxicity to human cancer cells. AACR Annual Meeting; 2014 Apr 5–9; San Diego, CA. American Association for Cancer Research, Philadelphia, PA.
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Bova MP, Mattson MN, Vasile S, Tam D, Holsinger L, Bremer M, Hui T, McMahon G, Rice A, Fukuto JM. (2004) The oxidative mechanism of action of ortho-quinone inhibitors of protein-tyrosine phosphatase alpha is mediated by hydrogen peroxide. Arch Biochem Biophys 429:30–41. [DOI] [PubMed] [Google Scholar]
- Brancho D, Tanaka N, Jaeschke A, Ventura JJ, Kelkar N, Tanaka Y, Kyuuma M, Takeshita T, Flavell RA, Davis RJ. (2003) Mechanism of p38 MAP kinase activation in vivo. Genes Dev 17:1969–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchser WJ, Laskow TC, Pavlik PJ, Lin HM, Lotze MT. (2012) Cell-mediated autophagy promotes cancer cell survival. Cancer Res 72:2970–2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deak M, Clifton AD, Lucocq LM, Alessi DR. (1998) Mitogen- and stress-activated protein kinase-1 (MSK1) is directly activated by MAPK and SAPK2/p38, and may mediate activation of CREB. EMBO J 17:4426–4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debiton E, Madelmont JC, Legault J, Barthomeuf C. (2003) Sanguinarine-induced apoptosis is associated with an early and severe cellular glutathione depletion. Cancer Chemother Pharmacol 51:474–482. [DOI] [PubMed] [Google Scholar]
- de Kruijf EM, Sajet A, van Nes JG, Putter H, Smit VT, Eagle RA, Jafferji I, Trowsdale J, Liefers GJ, van de Velde CJ, et al. (2012) NKG2D ligand tumor expression and association with clinical outcome in early breast cancer patients: an observational study. BMC Cancer 12:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, Li XD, Mauceri H, Beckett M, Darga T, et al. (2014) STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity 41:843–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denkert C, Schmitt WD, Berger S, Reles A, Pest S, Siegert A, Lichtenegger W, Dietel M, Hauptmann S. (2002) Expression of mitogen-activated protein kinase phosphatase-1 (MKP-1) in primary human ovarian carcinoma. Int J Cancer 102:507–513. [DOI] [PubMed] [Google Scholar]
- Dhanasekaran DN, Reddy EP. (2008) JNK signaling in apoptosis. Oncogene 27:6245–6251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore S. (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35:495–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooq A, Zhou MM. (2004) Structure and regulation of MAPK phosphatases. Cell Signal 16:769–779. [DOI] [PubMed] [Google Scholar]
- Feng H, Dong Y, Wu J, Qiao Y, Zhu G, Jin H, Cui J, Li W, Liu YJ, Chen J, et al. (2016) Epirubicin pretreatment enhances NK cell-mediated cytotoxicity against breast cancer cells in vitro. Am J Transl Res 8:473–484. [PMC free article] [PubMed] [Google Scholar]
- Fine JH, Chen P, Mesci A, Allan DS, Gasser S, Raulet DH, Carlyle JR. (2010) Chemotherapy-induced genotoxic stress promotes sensitivity to natural killer cell cytotoxicity by enabling missing-self recognition. Cancer Res 70:7102–7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, et al. (2006) Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313:1960–1964. [DOI] [PubMed] [Google Scholar]
- Howard SC, Jones DP, Pui CH. (2011) The tumor lysis syndrome. N Engl J Med 364:1844–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston PA, Foster CA, Shun TY, Skoko JJ, Shinde S, Wipf P, Lazo JS. (2007) Development and implementation of a 384-well homogeneous fluorescence intensity high-throughput screening assay to identify mitogen-activated protein kinase phosphatase-1 dual-specificity protein phosphatase inhibitors. Assay Drug Dev Technol 5:319–332. [DOI] [PubMed] [Google Scholar]
- Keyse SM. (2008) Dual-specificity MAP kinase phosphatases (MKPs) and cancer. Cancer Metastasis Rev 27:253–261. [DOI] [PubMed] [Google Scholar]
- Kidger AM, Keyse SM. (2016) The regulation of oncogenic Ras/ERK signalling by dual-specificity mitogen activated protein kinase phosphatases (MKPs). Semin Cell Dev Biol 50:125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim L, Del Rio L, Butcher BA, Mogensen TH, Paludan SR, Flavell RA, Denkers EY. (2005) p38 MAPK autophosphorylation drives macrophage IL-12 production during intracellular infection. J Immunol 174:4178–4184. [DOI] [PubMed] [Google Scholar]
- Korotchenko VN, Saydmohammed M, Vollmer LL, Bakan A, Sheetz K, Debiec KT, Greene KA, Agliori CS, Bahar I, Day BW, et al. (2014) In vivo structure-activity relationship studies support allosteric targeting of a dual specificity phosphatase. ChemBioChem 15:1436–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G, Galluzzi L, Kepp O, Zitvogel L. (2013) Immunogenic cell death in cancer therapy. Annu Rev Immunol 31:51–72. [DOI] [PubMed] [Google Scholar]
- Kurt RA, Urba WJ, Smith JW, Schoof DD. (1998) Peripheral T lymphocytes from women with breast cancer exhibit abnormal protein expression of several signaling molecules. Int J Cancer 78:16–20. [DOI] [PubMed] [Google Scholar]
- Landry J, Lambert H, Zhou M, Lavoie JN, Hickey E, Weber LA, Anderson CW. (1992) Human HSP27 is phosphorylated at serines 78 and 82 by heat shock and mitogen-activated kinases that recognize the same amino acid motif as S6 kinase II. J Biol Chem 267:794–803. [PubMed] [Google Scholar]
- Lazo JS, Nemoto K, Pestell KE, Cooley K, Southwick EC, Mitchell DA, Furey W, Gussio R, Zaharevitz DW, Joo B, et al. (2002) Identification of a potent and selective pharmacophore for Cdc25 dual specificity phosphatase inhibitors. Mol Pharmacol 61:720–728. [DOI] [PubMed] [Google Scholar]
- Li G, Yu M, Lee WW, Tsang M, Krishnan E, Weyand CM, Goronzy JJ. (2012) Decline in miR-181a expression with age impairs T cell receptor sensitivity by increasing DUSP6 activity. Nat Med 18:1518–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Gore AJ, Wilson JL, Korc M. (2014) DUSP1 is a novel target for enhancing pancreatic cancer cell sensitivity to gemcitabine. PLoS One 9:e84982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotfi R, Kaltenmeier C, Lotze MT, Bergmann C. (2016) Until death do us part: necrosis and oxidation promote the tumor microenvironment. Transfus Med Hemother 43:120–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen CA. (1993) Isolation and culture of hepatocytes from different laboratory species, in Methods in Toxicology (Tyson CA, Frazier JM. eds) pp 255–261, Academic Press, San Diego, CA. [Google Scholar]
- Mingo-Sion AM, Marietta PM, Koller E, Wolf DM, Van Den Berg CL. (2004) Inhibition of JNK reduces G2/M transit independent of p53, leading to endoreduplication, decreased proliferation, and apoptosis in breast cancer cells. Oncogene 23:596–604. [DOI] [PubMed] [Google Scholar]
- Miyashita T, Miki K, Kamigaki T, Makino I, Nakagawara H, Tajima H, Takamura H, Kitagawa H, Fushida S, Ahmed AK, et al. (2015) Low-dose gemcitabine induces major histocompatibility complex class I-related chain A/B expression and enhances an antitumor innate immune response in pancreatic cancer. Clin Exp Med 17:19–31. [DOI] [PubMed] [Google Scholar]
- Molina G, Vogt A, Bakan A, Dai W, Queiroz de Oliveira P, Znosko W, Smithgall TE, Bahar I, Lazo JS, Day BW, et al. (2009) Zebrafish chemical screening reveals an inhibitor of Dusp6 that expands cardiac cell lineages. Nat Chem Biol 5:680–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncho-Amor V, Ibañez de Cáceres I, Bandres E, Martínez-Poveda B, Orgaz JL, Sánchez-Pérez I, Zazo S, Rovira A, Albanell J, Jiménez B, et al. (2011) DUSP1/MKP1 promotes angiogenesis, invasion and metastasis in non-small-cell lung cancer. Oncogene 30:668–678. [DOI] [PubMed] [Google Scholar]
- Montagut C, Iglesias M, Arumi M, Bellosillo B, Gallen M, Martinez-Fernandez A, Martinez-Aviles L, Cañadas I, Dalmases A, Moragon E, et al. (2010) Mitogen-activated protein kinase phosphatase-1 (MKP-1) impairs the response to anti-epidermal growth factor receptor (EGFR) antibody cetuximab in metastatic colorectal cancer patients. Br J Cancer 102:1137–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris EJ, Jha S, Restaino CR, Dayananth P, Zhu H, Cooper A, Carr D, Deng Y, Jin W, Black S, et al. (2013) Discovery of a novel ERK inhibitor with activity in models of acquired resistance to BRAF and MEK inhibitors. Cancer Discov 3:742–750. [DOI] [PubMed] [Google Scholar]
- Nunes-Xavier C, Romá-Mateo C, Ríos P, Tárrega C, Cejudo-Marín R, Tabernero L, Pulido R. (2011) Dual-specificity MAP kinase phosphatases as targets of cancer treatment. Anticancer Agents Med Chem 11:109–132. [DOI] [PubMed] [Google Scholar]
- Pereira CV, Nadanaciva S, Oliveira PJ, Will Y. (2012) The contribution of oxidative stress to drug-induced organ toxicity and its detection in vitro and in vivo. Expert Opin Drug Metab Toxicol 8:219–237. [DOI] [PubMed] [Google Scholar]
- Senutovitch N, Vernetti L, Boltz R, DeBiasio R, Gough A, Taylor DL. (2015) Fluorescent protein biosensors applied to microphysiological systems. Exp Biol Med (Maywood) 240:795–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth D, Rudolph J. (2006) Redox regulation of MAP kinase phosphatase 3. Biochemistry 45:8476–8487. [DOI] [PubMed] [Google Scholar]
- Shibue T, Brooks MW, Inan MF, Reinhardt F, Weinberg RA. (2012) The outgrowth of micrometastases is enabled by the formation of filopodium-like protrusions. Cancer Discov 2:706–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shojaee S, Caeser R, Buchner M, Park E, Swaminathan S, Hurtz C, Geng H, Chan LN, Klemm L, Hofmann WK, et al. (2015) Erk negative feedback control enables pre-B cell transformation and represents a therapeutic target in acute lymphoblastic leukemia. Cancer Cell 28:114–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small GW, Shi YY, Higgins LS, Orlowski RZ. (2007) Mitogen-activated protein kinase phosphatase-1 is a mediator of breast cancer chemoresistance. Cancer Res 67:4459–4466. [DOI] [PubMed] [Google Scholar]
- Small GW, Somasundaram S, Moore DT, Shi YY, Orlowski RZ. (2003) Repression of mitogen-activated protein kinase (MAPK) phosphatase-1 by anthracyclines contributes to their antiapoptotic activation of p44/42-MAPK. J Pharmacol Exp Ther 307:861–869. [DOI] [PubMed] [Google Scholar]
- Topalian SL, Drake CG, Pardoll DM. (2015) Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 27:450–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt A, McDonald PR, Tamewitz A, Sikorski RP, Wipf P, Skoko JJ, 3rd, Lazo JS. (2008) A cell-active inhibitor of mitogen-activated protein kinase phosphatases restores paclitaxel-induced apoptosis in dexamethasone-protected cancer cells. Mol Cancer Ther 7:330–340. [DOI] [PubMed] [Google Scholar]
- Vogt A, Tamewitz A, Skoko J, Sikorski RP, Giuliano KA, Lazo JS. (2005) The benzo[c]phenanthridine alkaloid, sanguinarine, is a selective, cell-active inhibitor of mitogen-activated protein kinase phosphatase-1. J Biol Chem 280:19078–19086. [DOI] [PubMed] [Google Scholar]
- Wennerberg E, Sarhan D, Carlsten M, Kaminskyy VO, D’Arcy P, Zhivotovsky B, Childs R, Lundqvist A. (2013) Doxorubicin sensitizes human tumor cells to NK cell- and T-cell-mediated killing by augmented TRAIL receptor signaling. Int J Cancer 133:1643–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo SR, Fuertes MB, Corrales L, Spranger S, Furdyna MJ, Leung MY, Duggan R, Wang Y, Barber GN, Fitzgerald KA, et al. (2014) STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity 41:830–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Pew T, Zou M, Pang D, Conzen SD. (2005) Glucocorticoid receptor-induced MAPK phosphatase-1 (MPK-1) expression inhibits paclitaxel-associated MAPK activation and contributes to breast cancer cell survival. J Biol Chem 280:4117–4124. [DOI] [PubMed] [Google Scholar]
- Yamaue H, Tanimura H, Noguchi K, Iwahashi M, Tsunoda T, Tani M, Tamai M, Hotta T, Mizobata S, Arii K. (1991) Cisplatin treatment renders tumor cells more susceptible to attack by lymphokine-activated killer cells. J Clin Lab Immunol 35:165–170. [PubMed] [Google Scholar]
- Zhang T, Inesta-Vaquera F, Niepel M, Zhang J, Ficarro SB, Machleidt T, Xie T, Marto JA, Kim N, Sim T, et al. (2012) Discovery of potent and selective covalent inhibitors of JNK. Chem Biol 19:140–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng CF, Guan KL. (1993) Cloning and characterization of two distinct human extracellular signal-regulated kinase activator kinases, MEK1 and MEK2. J Biol Chem 268:11435–11439. [PubMed] [Google Scholar]