Abstract
We recently showed that ischemia/reperfusion (I/R) of the heart causes CD38 activation with resultant depletion of the cardiac NADP(H) pool, which is most marked in the endothelium. This NADP(H) depletion was shown to limit the production of nitric oxide by endothelial nitric oxide synthase (eNOS), which requires NADPH for nitric oxide production, resulting in greatly altered endothelial function. Therefore, intervention with CD38 inhibitors could reverse postischemic eNOS-mediated endothelial dysfunction. Here, we evaluated the potency of the CD38 inhibitor luteolinidin, an anthocyanidin, at blocking CD38 activity and preserving endothelial and myocardial function in the postischemic heart. Initially, we characterized luteolinidin as a CD38 inhibitor in vitro to determine its potency and mechanism of inhibition. We then tested luteolinidin in the ex vivo isolated heart model, where we determined luteolinidin uptake with aqueous and liposomal delivery methods. Optimal delivery methods were then further tested to determine the effect of luteolinidin on postischemic NAD(P)(H) and tetrahydrobiopterin levels. Finally, through nitric oxide synthase–dependent coronary flow and left ventricular functional measurements, we evaluated the efficacy of luteolinidin to protect vascular and contractile function, respectively, after I/R. With enhanced postischemic preservation of NADPH and tetrahydrobiopterin, there was a dose-dependent effect of luteolinidin on increasing recovery of endothelium-dependent vasodilatory function, as well as enhancing the recovery of left ventricular contractile function with increased myocardial salvage. Thus, luteolinidin is a potent CD38 inhibitor that protects the heart against I/R injury with preservation of eNOS function and prevention of endothelial dysfunction.
Introduction
Myocardial ischemia/reperfusion (I/R) injury causes increased oxidative stress and inflammation through the formation of reactive oxygen species (Zweier et al., 1989; Ferrari et al., 1990; Zweier and Talukder, 2006). Important contributors to reactive oxygen species formation in the heart include the electron transport chain of mitochondria, NADPH oxidase, xanthine oxidase, and uncoupled endothelial nitric oxide synthase (eNOS) within endothelial cells (Zweier et al., 1988; Dumitrescu et al., 2007; Loukogeorgakis et al., 2010; De Pascali et al., 2014). eNOS dysfunction occurs secondary to oxidative depletion of its cofactor tetrahydrobiopterin (BH4) and oxidation and glutathionylation of critical enzyme cysteines that cause eNOS to switch from production of nitric oxide (NO) to superoxide (Dumitrescu et al., 2007; Chen et al., 2010, 2011).
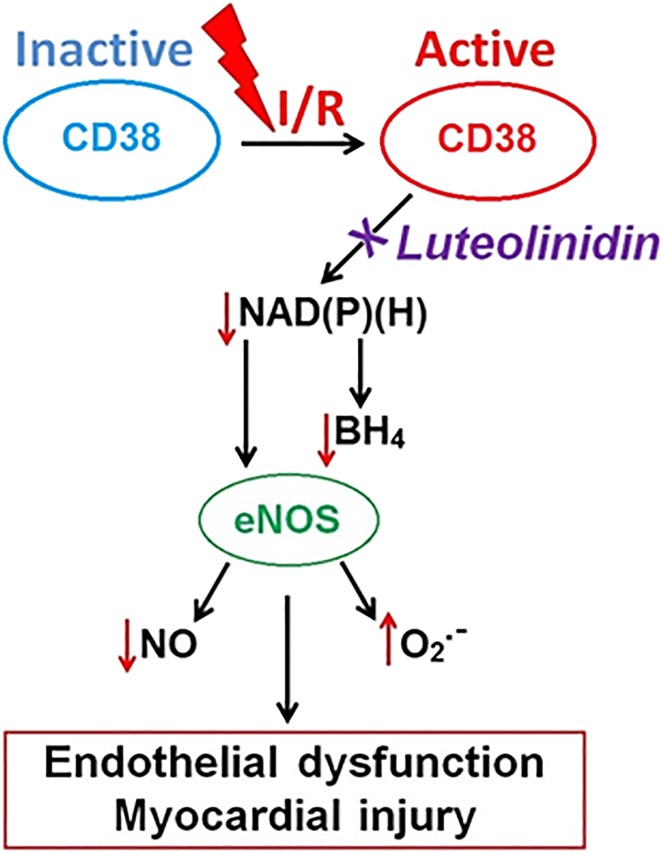
Oxidative stress accompanying I/R injury also signals the activation of degradative enzymatic pathways, including the recently identified postischemic process of CD38 activation, that can deplete the NADP(H) and NAD(H) pools (Fig. 1). CD38, an ectoenzyme endowed with NAD(P)+ase and ADP-ribosyl cyclase activity, is activated in the heart after I/R, causing severe enzymatic depletion of the myocardial and endothelial NADP(H) pools (Reyes et al., 2015). In the postischemic heart, NADPH, which is the reducing substrate required for eNOS to produce vasorelaxant and anti-inflammatory NO (Palmer et al., 1987), limits NO production from eNOS (Reyes et al., 2015), causing impaired vasodilation and decreased myocardial perfusion (see Fig. 1). Until recently, there was a lack of known highly effective CD38 inhibitors. Relatively nonspecific inhibitors, such as α-NAD or nicotinamide, had been used and require high dosage levels. Thus, there is a need to identify and characterize potent biocompatible inhibitors of CD38.
Fig. 1.
Schematic depicting role of CD38 activation with I/R injury. After I/R, depletion of the NAD(P)(H) pools occurs by enzymatic degradation of NAD(P)+ by the NAD(P)+ase CD38. Low NADPH levels in turn contribute to low BH4 levels due to the presence of NADPH-dependent enzymes in both the de novo synthesis and recycling pathways for BH4. Together, low NADPH and BH4 levels contribute to eNOS uncoupling where production of NO is lost and generation of superoxide (O2.-) occurs, leading to endothelial dysfunction and myocardial injury.
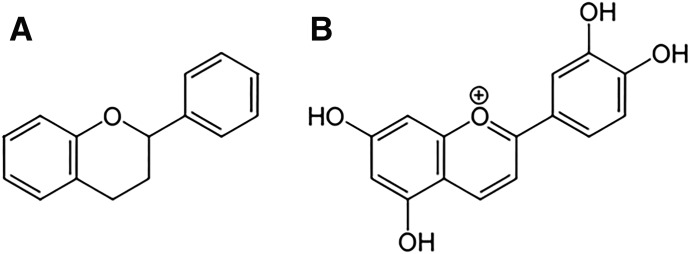
Flavonoid anthocyanidin compounds were recently shown to inhibit CD38 with relatively high potency compared with previously used inhibitors, with luteolinidin found to be the most potent of the flavonoids tested (Kellenberger et al., 2011; Escande et al., 2013). Anthocyanidins, such as luteolinidin, are a subgroup of the broad family of flavonoids, which has thousands of known compounds (Nijveldt et al., 2001) (Fig. 2). They have been shown to function as potent antioxidants and radical scavengers in vitro. Many studies have shown that increased dietary intake of flavonoid-rich foods decreases myocardial I/R injury in vivo (Hung et al., 2004; Ikizler et al., 2007; Toufektsian et al., 2008; Yamazaki et al., 2008), with many others showing protection by direct addition of flavonoids to the blood or perfusate in ex vivo isolated hearts (Amorini et al., 2003; Aneja et al., 2004; Fantinelli et al., 2005; Hirai et al., 2007; Scarabelli et al., 2009).
Fig. 2.
General flavonoid and luteolinidin structures. (A) General flavonoid structure showing a 15-carbon skeleton consisting of two phenyl rings and a middle heterocyclic ring. (B) Luteolinidin, a member of the 3-deoxyanthocyanidins.
In this study, we first tested the potency of luteolinidin as a CD38 inhibitor using a high-throughput, fluorescence-based activity assay. We then performed Lineweaver–Burk and nonlinear regression analysis to determine the mode of inhibition of CD38 by luteolinidin. High-performance liquid chromatography (HPLC) measurements were then performed to determine the cellular uptake of luteolinidin using different delivery methods to the isolated heart. The optimal delivery method was then used to determine the ability of the treatment to protect postischemic levels of the NAD(P)(H) pools. These studies were then followed by measurements of myocardial cGMP and BH4 levels and NOS-dependent coronary flow (CF) in the perfused heart as a means of testing NOS-dependent vasodilatory function. Finally, the dose-dependent cardioprotective abilities of luteolinidin were tested with measurements of left ventricular functional recovery and infarct size. It was shown that luteolinidin enhanced postischemic myocardial function and decreased infarction.
Materials and Methods
All chemicals were purchased from Sigma (St. Louis, MO), except recombinant human CD38 (rCD38) (provided by Hon-Cheung Lee and Yong Juan Zhao, Shenzhen, China; Munshi et al., 1997), BH4 and dihydrobiopterin (BH2) (Schircks Laboratories, Jona, Switzerland), and luteolinidin (Extrasynthese, Lyon, France).
CD38 Activity Assay.
rCD38 was used for measuring CD38 activity in vitro. rCD38 (0.1 μg/ml), truncated of its single-pass transmembrane domain, N-glycosylation sites, and N-terminal tail, was added to a 100-μl reaction mixture containing varying concentrations (5–200 μM) of CD38 substrate nicotinamide 1,N6-ethenoadenine dinucleotide (ε-NAD) and/or CD38 inhibitor luteolinidin (1–50 μM). Fluorescence was monitored at emission and excitation wavelengths of 300 nm and 410 nm, respectively, for the conversion of ε-NAD to the strongly fluorescent product ε-ADP-ribose on a plate reader (SpectraMax M5; Molecular Devices, Sunnyvale, CA). Lineweaver–Burk analysis was performed, in conjunction with a nonlinear regression program (GraphPad Prism; GraphPad Software Inc., La Jolla, CA), to determine the mode of enzyme inhibition and estimates of Vmax, Km, and Ki.
Langendorff-Perfused Heart.
Male Sprague-Dawley rats (Envigo, Indianapolis, IN) weighing 275–300 g were used for these experiments. Animals were given an intraperitoneal injection of ketamine (100 mg/kg) and xylazine (15 mg/kg) and hearts were excised and cannulated via the aorta and perfused in a retrograde manner with Krebs-Henseleit buffer (KHB) (119 mM NaCl, 17 mM glucose, 25 mM NaHCO3, 5.9 mM KCl, 1.2 mM MgCl2, 2.5 mM CaCl2, and 0.5 mM NaEDTA). A small latex balloon connected to a pressure transducer (AD Instruments, Colorado Springs, CO) was placed in the left ventricle and measured left ventricular developed pressure (LVDP), end systolic pressure, end diastolic pressure, and heart rate. A flow probe (Transonic, Ithaca, NY) was placed in-line to measure CF. Animal protocols were approved by the Institutional Animal Care and Use Committee of The Ohio State University and conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Drug Delivery.
Both aqueous solution (dissolved in KHB) and liposomal formulations were tested for effective delivery of luteolinidin. Liposomes were prepared from l-α-phosphatidylcholine. One-hundred milligrams of solid l-α-phosphatidylcholine was dissolved in 2 ml chloroform and placed into a glass rolling jar. Under argon, the chloroform was fully evaporated while the glass jar was slowly rolled, leaving a thin film of l-α-phosphatidylcholine around the side of the jar. The desired concentration of luteolinidin dissolved in 2.5 ml KHB (pH 8.5) was then added to the jar. The jar was then sealed and rolled on a jar roller (755RMV; US Stoneware, East Palestine, OH) for 1 hour. After multilaminar layers were formed, they were filtered through an extruder system (Avestin, Ottawa, ON, Canada) to form liposomes of approximately 200 nm. Liposomal formulations containing luteolinidin were then diluted 1:1 with KHB to achieve the final desired luteolinidin concentration. The liposomal luteolinidin preparation of 5 mL volume was infused by sidearm at 1/20 of CF until completely infused. In the case of aqueous solution delivery, luteolinidin was dissolved in KHB and infused at 1/20 of CF. At the end of infusion, the 30-minute ischemia time course began.
Experimental Protocols.
After 20 minutes of aerobic perfusion and equilibration, hearts were subjected to treatment with either 5, 15, 25, or 50 µM luteolinidin in liposomes or with empty liposome vehicle control. At the end of the infusion, 30-minute ischemia was initiated. At the end of 30-minute ischemia, hearts were reperfused for either an additional 30 minutes, at which time hearts were frozen in liquid nitrogen for further analysis, or 120 minutes for measurements of infarction (Dumitrescu et al., 2007; Reyes et al., 2015).
HPLC Analysis of Luteolinidin.
To measure luteolinidin uptake in hearts, reversed-phase HPLC (Atlantis T3 column; Waters, Milford, MA) coupled to a UV-visible detector set at 480 nm was used. Heart tissue was ground in liquid nitrogen and transferred to a dounce homogenizer. Then, 2 parts cold methanol and 0.8 parts cold water were added, and the powdered tissue was homogenized. Then, sequentially, 1 part chloroform, 1 part water, and, lastly, 1 part chloroform were added to the homogenizer, with brief homogenization between each step. The resulting mixture contained, by ratio, 2:2:1.8 methanol/chloroform/water. The mixture was sonicated, vortexed, and centrifuged for 20 minutes at 20,000g to create an upper layer of water/methanol and a lower layer of chloroform. The supernatant (water/methanol mixture) was removed, diluted 1:1 with 50% methanol/0.5% HCl (to acidify the sample), and injected into the HPLC system. This step was critical to ensure a consistent absorbance maximum for luteolinidin. Gradient elution of luteolinidin was performed at 1 ml/min with a mobile phase A of 10% acetic acid in water and a mobile phase B (MPB) of 10% acetic acid in 50% acetonitrile. The gradient was performed as follows: 0–2 minutes, 12% MPB isocratic; 2–5.5 minutes, 12%–30% MPB; 5.5–8 minutes, 30% MPB, 8–11 minutes, 30%–40% MPB; 11–15 minutes, 40% MPB; 15–20 minutes, 40% to 12% MPB; and 20–23 minutes, 12% MPB.
HPLC Analysis of NAD(P)(H).
Pyridine nucleotides were measured by HPLC with fluorescence detection as detailed previously (Reyes et al., 2015). In this method, cyanide ion from potassium cyanide is used to derivatize NAD+ and NADP+ to stable, fluorescent analytes allowing for measurement of both the oxidized and reduced nucleotides in one chromatographic run (Klaidman et al., 1995). Heart tissue from isolated rat heart experiments was ground with a mortar and pestle in liquid nitrogen and homogenized in a buffer consisting of 200 mM potassium cyanide, 60 mM KOH, and 1 mM diethylenetriaminepentaacetic acid. The resulting homogenate was centrifuged for 10 minutes at 15,000g. The resulting supernatant was then filtered using a 3000 molecular weight cutoff regenerated cellulose filter (Millipore, Billerica, MA). Filtrates were injected onto a Waters Atlantis T3 column (25 cm × 4.6 mm × 5 µm) with mobile phase A of 200 mM ammonium acetate (pH 5.8) and MPB of 50% methanol. Separation was achieved with an initial flow rate of 1.0 ml/min consisting of 8% MPB and a linear methanol gradient (0.4% per minute for 25 minutes). Analytes were detected via fluorescence spectroscopy (excitation wavelength of 330 nm; emission wavelength of 460 nm).
cGMP Measurements.
Isolated hearts subjected to control treatment, I/R with the empty liposome vehicle control, and I/R with luteolinidin treatment were assayed for cGMP content (Li et al., 2012). At the end of each protocol, hearts were treated with 1 μM acetylcholine to determine the ability of eNOS to increase cGMP. Heart tissue was homogenized in 5 volumes of 0.1 N HCl and centrifuged at 10,000g. The supernatant was collected and used for enzyme-linked immunosorbent assay detection of cGMP (Enzo Life Sciences, Farmingdale, NY). The acetylated assay protocol was used to improve sensitivity. Acetylation was performed by 1:20 addition of the acetylating reagent (1:2 acetyl anhydride/triethylamine) to the samples, which were then subjected to the enzyme-linked immunosorbent assay according to the manufacturer’s instructions, with endpoint measurements performed on a Molecular Devices SpectraMax M5 at a wavelength of 405 nm. Myocardial cGMP levels were expressed as picomoles per milligram of protein in homogenates.
HPLC Analysis of BH4/BH2.
The HPLC analysis of pteridines BH4 and BH2 was performed as previously reported using fluorescence detection with an excitation wavelength of 348 nm and an emission wavelength of 444 nm (De Pascali et al., 2014). This method was chosen for its sensitivity and selectivity in the detection of BH4 and BH2 after controlled oxidation to pterin and biopterin, respectively. The HPLC analysis of pteridines was carried out using a Waters Atlantis T3 reversed-phase column (4.6 × 150 mm). Isocratic elution of pteridines was performed at a flow rate of 1.2 ml/min using a buffer consisting of 100 mM KH2PO4, 6 mM citric acid, 2.5 mM sodium octyl sulfate, and 2% methanol, pH 2.5. Peaks were assigned by coelution with analytical standards, and quantitation was performed with use of standard curves prepared from analytical standards.
Infarct Size Measurements.
At the end of 120-minute reperfusion, hearts were collected and infarct size was measured by 2,3,5-triphenyltetrazolium chloride staining in a manner similar to that reported previously (Reyes et al., 2015). After the hearts were blot-dried and then frozen for 30 minutes at −20°C, they were placed in a heart matrix (Zivic Laboratories, Portersville, PA) and sliced with steel blades. Slices were then stained in 1.5% 2,3,5-triphenyltetrazolium chloride in phosphate-buffered saline for 20 minutes. Slices were then incubated overnight in 10% neutral-buffered formalin and digitally photographed on both sides for planimetry using ImageJ 1.43 software (National Institutes of Health, Bethesda, MD).
Statistical Analyses.
Results are expressed as means ± S.E.M. Statistical significance was determined by analysis of variance (followed by the Holm–Sidak test) for multiple groups. Paired or unpaired t tests were used for comparison between two groups. In the case of time-dependent data, analysis of variance with two-way repeated measures was used to determine significance.
Results
Luteolinidin Inhibits CD38.
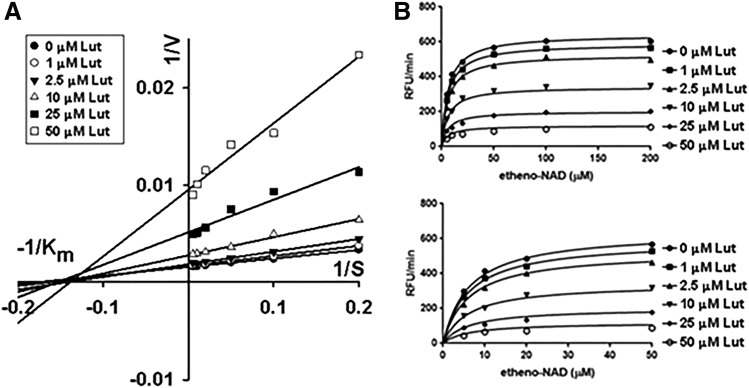
Flavonoid luteolinidin (Fig. 2) was initially tested in vitro to characterize its potency as an inhibitor of CD38. rCD38 (0.1 μg/ml) was incubated with varying concentrations of substrate ε-NAD (5–200 μM) and luteolinidin (1–50 μM). The conversion of ε-NAD to ε-ADP-ribose was monitored by increase in fluorescence. Reactions were allowed to proceed for 10 minutes. Initial rates of reaction were determined for the first minute of each reaction, and the mode of inhibition (competitive versus noncompetitive) of CD38 by luteolinidin was assessed by two methods. First, Lineweaver–Burk analysis was performed to determine the mode of CD38 inhibition by luteolinidin. This graphical representation allows for estimates of Km and Vmax, which can provide insights into the mode of inhibition of an inhibitor. Modeling the data in this manner showed an unchanging Km and decreasing Vmax with increasing inhibitor concentration, consistent with noncompetitive inhibition (Fig. 3A) (Crane and Sols, 1954). The Km and Vmax values obtained were 6.2 μM and 32 nmol/min per μg protein (64,000 RFU/min per μg), respectively. With Lineweaver–Burk analysis strongly showing a noncompetitive relationship, we attempted to fit the enzyme inhibition data to a noncompetitive model using nonlinear regression. Fitting the data this way yielded well-fit curves for a noncompetitive equation (Fig. 3B), with a calculated inhibitory constant (Ki) of 11.4 μM.
Fig. 3.
Luteolinidin as a CD38 inhibitor. (A) Lineweaver–Burk plot showing the effect of changing inhibitor concentration on 1/Vmax (y-intercept) and −1/Km (x-intercept) of rCD38 conversion of ε-NAD to ε-ADP-ribose. Luteolinidin decreased Vmax with an unchanging substrate Km for the reaction, consistent with noncompetitive enzyme inhibition. (B) Nonlinear regression using GraphPad software using a noncompetitive model. The top graph shows the full range of substrate concentrations (0–200 μM), whereas the bottom graph shows the 0–50 μM ε-NAD concentration range only. This model predicted a Ki of 11.4 μM (95% confidence interval). Each data point was the average of six to eight independent experiments.
Tissue Uptake of Luteolinidin.
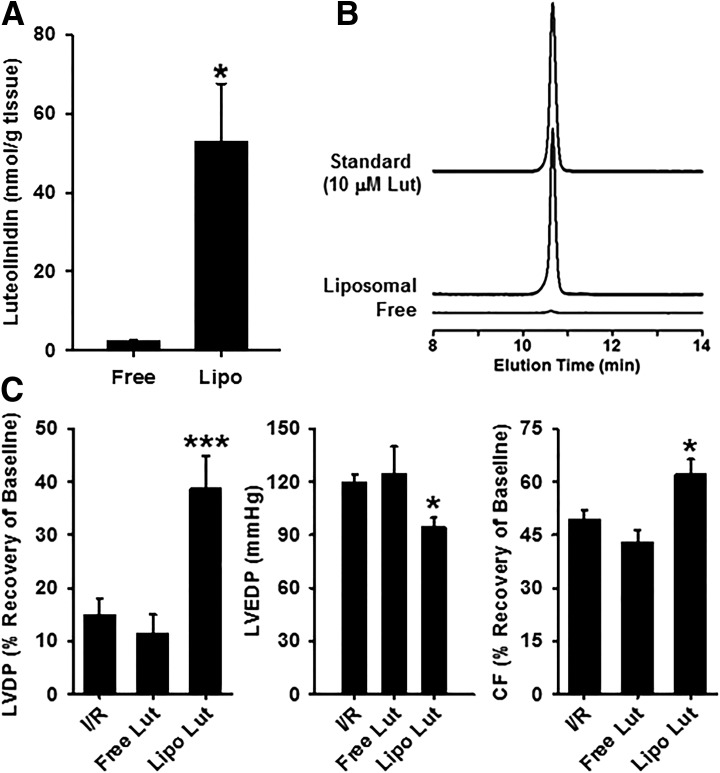
Once we determined the inhibitory potency of luteolinidin on CD38, methods were devised for acute delivery of luteolinidin to isolated, perfused rat hearts. These results would help determine the optimum strategy for testing effects of luteolinidin on recoveries of NAD(P)(H) and cardiac function after I/R. Initially, tests were made to deliver luteolinidin by dissolution and delivery in KHB, but neither tissue accumulation (Fig. 4, A and B) nor improvement of postischemic function (Fig. 4C) occurred in a pilot group of hearts. This is likely due to the polar nature of the polyphenolic structure of luteolinidin, which makes intracellular delivery slow in aqueous solvent. Instead, liposomes, which are spherical vesicles composed of a lipid bilayer surrounding aqueous solution, were used as previously described for myocardial delivery of polar compounds (Dumitrescu et al., 2007; Reyes et al., 2015). Unlike aqueous luteolinidin delivery, liposomal delivery was effective in elevating levels of luteolinidin in the heart (Fig. 4, A and B). Compared with aqueous delivery with infusion of 25 μM luteolinidin for 3 minutes, which resulted in low tissue levels of 2.43 ± 0.17 nmol/g tissue, comparable delivery of 25 μM luteolinidin in liposomal formulations resulted in far higher tissue levels of 52.84 ± 14.72 nmol/g tissue. With the assumption that approximately 75% of the heart tissue is water (Aliev et al., 2002), a concentration of approximately 70 μM luteolinidin is achieved in our model. Based on the inhibition data (Fig. 3), this would be expected to be sufficient to fully inhibit CD38.
Fig. 4.
Tissue uptake of luteolinidin and postischemic protection. (A) The levels of luteolinidin in heart tissue after 25 μM luteolinidin delivery in KHB (aqueous) or liposomal solution. Although aqueous delivery of luteolinidin failed to efficiently raise tissue luteolinidin concentration, liposomal delivery was highly effective. *P < 0.05 (mean ± S.E.M., n = 3). (B) Chromatograms showing the elution profile of luteolinidin from homogenates of hearts receiving aqueous and liposomal luteolinidin (25 μM) and from a 10 μM standard of luteolinidin. (C) Functional recovery in untreated hearts subjected to I/R (I/R), hearts treated with 25 μM luteolinidin in Krebs buffer (Free Lut), and hearts treated with 25 μM liposomal luteolinidin (Lipo Lut). Luteolinidin delivered in Krebs buffer (Free Lut) had no effect on the recovery of LVDP, LVEDP, or CF after I/R, whereas liposomal luteolinidin provided significant protection. *P < 0.05; ***P < 0.005 (Lipo Lut versus I/R or Free Lut versus I/R; mean ± S.E.M., n = 3–10).
Luteolinidin Preserves NADP(H) and NAD(H) Levels in the Isolated Postischemic Rat Heart.
To determine how luteolinidin treatment affects postischemic recovery of NADP(H) and NAD(H), hearts were subjected to either a 20-minute period of control perfusion or to I/R with either empty liposomes (vehicle control) or liposomal formulations containing luteolinidin (25 μM). At the end of the 30-minute reperfusion period, hearts were snap frozen in liquid nitrogen for HPLC measurements of the pyridine nucleotide pools [NADP(H) and NAD(H)].
Levels of NADPH and NADP+ in control hearts were 27.84 ± 1.40 and 5.57 ± 0.23 nmol/g tissue, whereas the levels of NADH and NAD+ were 29.34 ± 10.09 and 137.12 ± 22.52 nmol/g tissue. In the vehicle treatment I/R group, levels of NADPH and NADP+ declined sharply to 9.91 ± 0.59 and 1.98 ± 0.12 nmol/g tissue, with levels of NADH and NAD+ of 29.03 ± 4.32 and 52.31 ± 4.26 nmol/g tissue. With luteolinidin pretreatment, NADPH and NADP+, as well as NAD+, were significantly higher, with levels of 21.36 ± 1.35 and 4.26 ± 0.48 nmol/g tissue for NADPH and NADP+ and 43.85 ± 11.46 and 108.79 ± 14.24 nmol/g tissue for NADH and NAD+ (Fig. 5). Overall, the data show an impressive ability of luteolinidin to protect postischemic levels of NADP(H) and NAD(H) to levels close to preischemic levels.
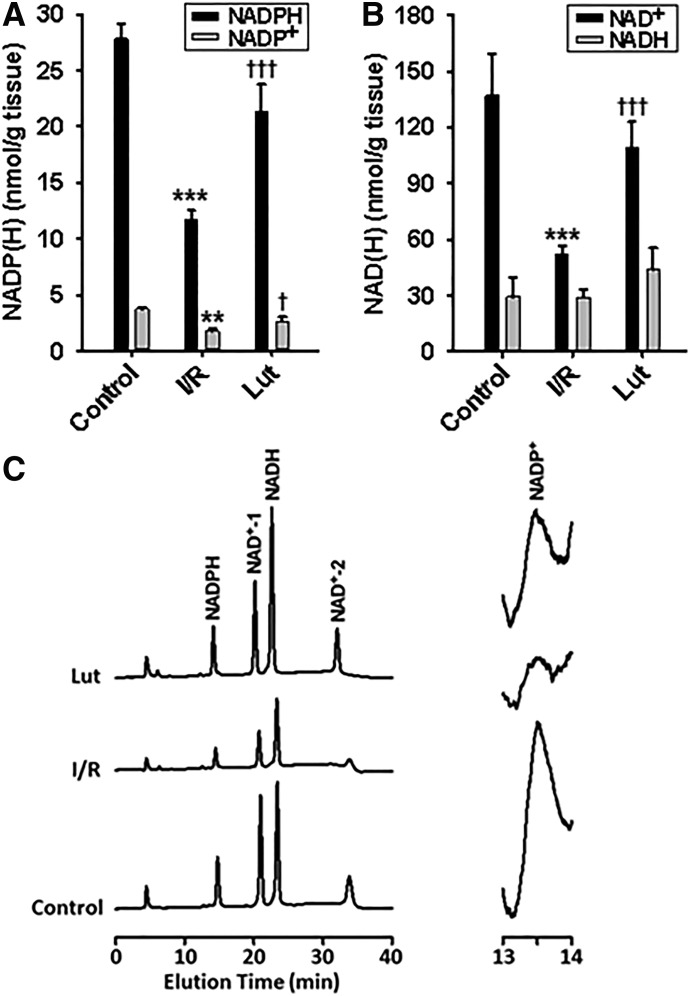
Fig. 5.
NADP(H) and NAD(H) levels in hearts. Hearts were subjected to 20-minute control perfusion (control), 30-minute ischemia/30-minute reperfusion (I/R) with preischemic vehicle control (empty liposomes), and 30-minute ischemia/30-minute reperfusion with preischemic luteolinidin treatment (Lut). (A) Luteolinidin treatment preserved levels of NADPH and NADP+ compared with vehicle-treated I/R controls. (B) In the same hearts, the levels of NAD+ were also preserved with luteolinidin treatment compared with vehicle-treated I/R controls. **P < 0.01; ***P < 0.001; †P < 0.05; †††P < 0.001 (mean ± S.E.M., n = 5–7). (C) Chromatograms depicting the enhanced recovery of NAD(P)(H) with luteolinidin treatment prior to I/R. Two reaction products occur for NAD+ with potassium cyanide treatment due to addition of cyanide ion to different parts of the nicotinamide ring.
Luteolinidin Dose-Dependently Preserves Total and NOS-Dependent CF after Ischemia.
With findings of enhanced recovery of NADP(H) and BH4 in reperfusion, experiments testing postischemic vascular function were performed to determine the physiologic significance of the findings. In these experiments, four doses of luteolinidin (5, 15, 25, and 50 μM) were tested to determine the dose dependence of the observed protection. At the 30-minute reperfusion endpoint, the percent recovery of total CF was 49.36% ± 2.63% in vehicle-treated hearts, 55.36% ± 4.85% in 5 μM luteolinidin–treated hearts, 64.03% ± 2.00% in 15 μM luteolinidin–treated hearts, 62.12% ± 4.24% in 25 μM luteolinidin–treated hearts, and 67.19% ± 4.18% in 50 μM luteolinidin–treated hearts, demonstrating significant protection of total CF after ischemia by luteolinidin above 5 μM (Fig. 6A).
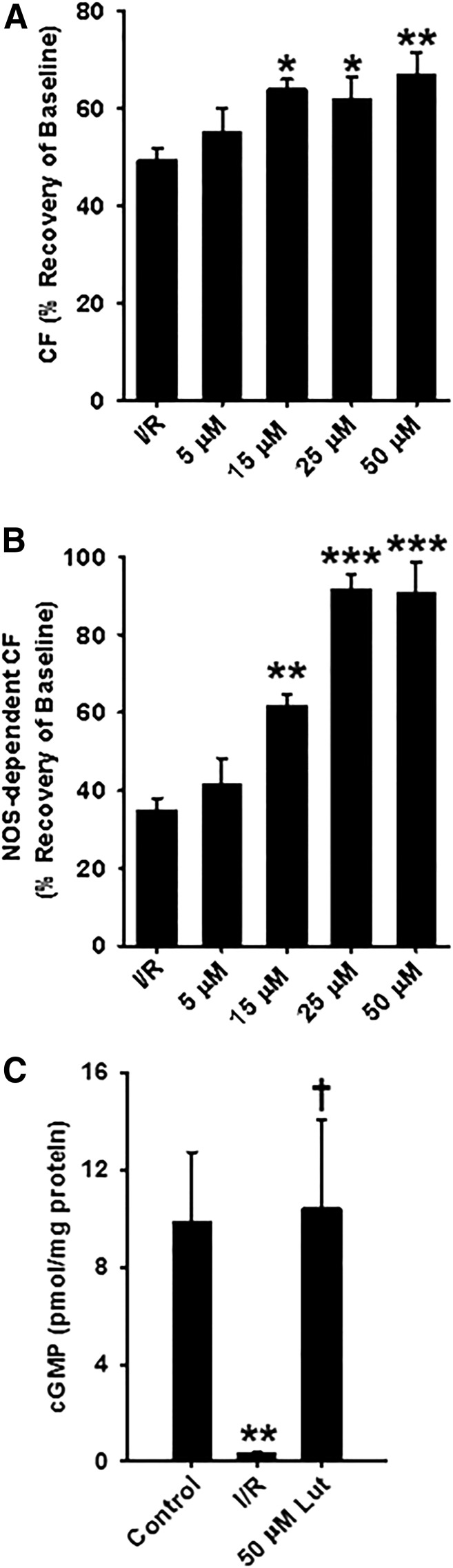
Fig. 6.
Total CF, NOS-dependent CF, and cGMP levels in luteolinidin-treated and untreated hearts. (A) After 30-minute reperfusion, total CF was significantly higher in hearts treated preischemia with 15, 25, and 50 μM luteolinidin compared with hearts receiving vehicle treatment (I/R). (B) NOS-dependent CF was also higher in hearts pretreated with 15, 25, and 50 μM luteolinidin. Luteolinidin preserved eNOS functionality, as much higher recovery of NOS-dependent CF was seen in hearts treated with luteolinidin. (C) Consistent with higher NOS-dependent CF, cGMP levels after stimulation with 1 μM acetylcholine were higher in luteolinidin-treated hearts (50 μM) compared with vehicle-treated I/R (I/R), which showed significant impairment of acetylcholine-induced cGMP production. For (A) and (B), *P < 0.05; **P < 0.01; ***P < 0.001 (with respect to I/R; mean ± S.E.M., n = 6–10). For (C), **P < 0.01; †P < 0.01 (with respect to control and I/R, respectively; mean ± S.E.M., n = 7).
This prompted experiments to determine NOS-dependent CF with Nω-nitro-l-arginine methyl ester (l-NAME; NOS inhibitor) infusions. NOS-dependent CF is defined as the amount of CF depletion in response to a 10-minute infusion of l-NAME (1 mM) (Dumitrescu et al., 2007; Reyes et al., 2015). Initially, these measurements were made in five hearts prior to ischemia to establish a baseline value for NOS-dependent CF (approximately 20% of baseline CF). The same measurements were made in I/R hearts after either preischemic vehicle or 5, 15, 25, or 50 μM luteolinidin treatment. Results were shown as the percent recovery of NOS-dependent CF using the data from control, nonischemic hearts as the baseline value. Vehicle-treated hearts undergoing I/R had severely depleted NOS-dependent CF (approximately 35% recovery). However, hearts pretreated with luteolinidin showed enhanced NOS-dependent CF recovery in a dose-dependent manner. After 30-minute reperfusion, the percent recoveries of NOS-dependent CF were 41.93% ± 6.45%, 62.06% ± 2.77%, 91.83% ± 3.72%, and 90.82% ± 7.82%, respectively, for 5, 15, 25, and 50 μM luteolinidin (Fig. 6B). This shows that NOS-dependent vasodilatory function is protected with CD38 inhibition.
Luteolinidin Protects Acetylcholine-Induced cGMP Production after I/R.
Myocardial cGMP levels were measured after acetylcholine infusion (1 μM) in hearts subjected to control perfusion or I/R with either vehicle or luteolinidin (50 μM) treatment as a means to measure the ability of the endothelium to produce NO. Levels of cGMP in control hearts after acetylcholine infusion were 9.90 ± 2.83 pmol/mg protein. These levels fell to 0.45 ± 0.15 pmol/mg protein after I/R but were protected in hearts subjected to I/R that were pretreated with 50 μM luteolinidin (10.42 ± 3.64 pmol/mg protein) (Fig. 6C).
Luteolinidin Preserves BH4 after I/R.
We have previously shown BH4 to be a highly redox-sensitive eNOS cofactor that is severely depleted by the oxidative stress that occurs following the onset of ischemia. The recovery of BH4 after ischemia may be regulated by levels of NADPH, as there are NADPH-dependent enzymes in both the de novo synthesis and recycling pathways (Bailey and Ayling, 2009; Gao et al., 2009). De novo synthesis begins with GTP cyclohydrolase I–catalyzed conversion of GTP to 7,8-dihydroneopterin triphosphate, continues with conversion of 7,8-dihydroneopterin triphosphate to 6-pyruvoyl-BH4 and ends with NADPH-dependent conversion of 6-pyruvoyl-BH4 to BH4 by sepiapterin reductase. In the recycling pathway, the reversibly oxidized BH4 product, BH2, is converted by dihydrofolate reductase in an NADPH-dependent step to BH4.
BH4 levels after I/R were approximately 70% lower than in control hearts. With luteolinidin treatment, however, BH4 levels were approximately 2-fold higher than in vehicle-treated hearts subjected to I/R (Fig. 7). Thus, luteolinidin treatment partially preserved BH4 levels, possibly due to protected function of the NADPH-dependent enzymes sepiapterin reductase and dihydrofolate reductase with conserved NADPH levels.
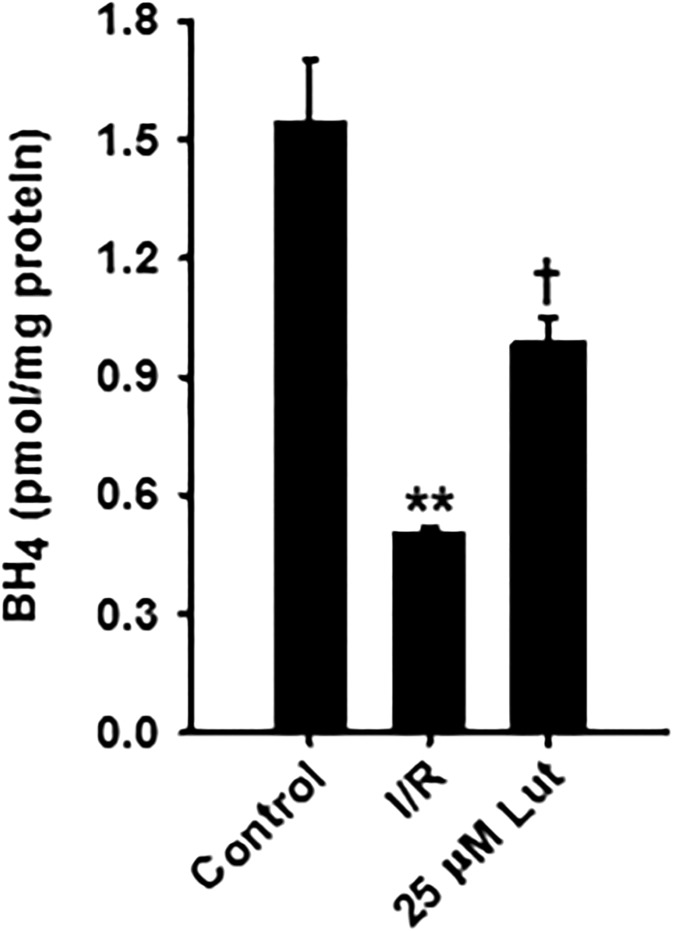
Fig. 7.
BH4 levels in control nonischemic hearts and vehicle-treated or luteolinidin-treated hearts after I/R. After I/R, BH4 levels were higher in liposomal luteolinidin-treated hearts (25 μM Lut) compared with hearts receiving vehicle of empty liposome-treatment (I/R). **P < 0.01; †P < 0.05 (with respect to nonischemic control and I/R, respectively; mean ± S.E.M., n = 3–7).
Luteolinidin Dose-Dependently Preserves Cardiac Function after Ischemia.
After vehicle or liposomal luteolinidin (5, 15, 25, or 50 μM) infusion, hearts were subjected to 30-minute global ischemia and 30-minute reperfusion. Hearts that were treated with luteolinidin showed partially enhanced recovery of contractile function throughout the reperfusion time course compared with hearts receiving vehicle alone. Although the 5-μM luteolinidin treatment group showed no significant difference in the recovery of LVDP and rate-pressure product (RPP) compared with the vehicle control group, hearts treated with 15, 25, or 50 μM luteolinidin prior to ischemia showed significant improvement. At 30-minute reperfusion, hearts treated with vehicle had recoveries of LVDP and RPP of 14.95% ± 3.02% and 14.81% ± 3.39%, respectively, whereas hearts treated with 5, 15, 25, or 50 μM luteolinidin had recoveries of 26.51% ± 6.35% and 21.76% ± 4.37%, 30.22% ± 2.12% and 32.16% ± 3.80%, 38.76% ± 6.15% and 34.04% ± 5.48%, and 40.95% ± 5.19% and 40.06% ± 5.16% for LVDP and RPP, respectively (Fig. 8, A and B). In addition, hearts treated with luteolinidin displayed lower left ventricular end diastolic pressure (LVEDP) after ischemia compared with the control, indicating preserved myocardial relaxation. After 30-minute reperfusion, hearts treated with the vehicle control had an average LVEDP of 119.8 ± 4.7 mmHg. LVEDP was significantly lower in hearts treated with 15, 25, and 50 μM luteolinidin, with values of 94.7 ± 2.5 mmHg, 94.6 ± 5.4 mmHg, and 83.1 ± 7.9 mmHg, respectively (Fig. 8C). The 5-μM luteolinidin treatment group had an unchanged LVEDP, as it was not significantly different statistically from the control. As described above, preservation of total CF after ischemia was also seen with all luteolinidin treatment doses above 5 μM (Fig. 8D).
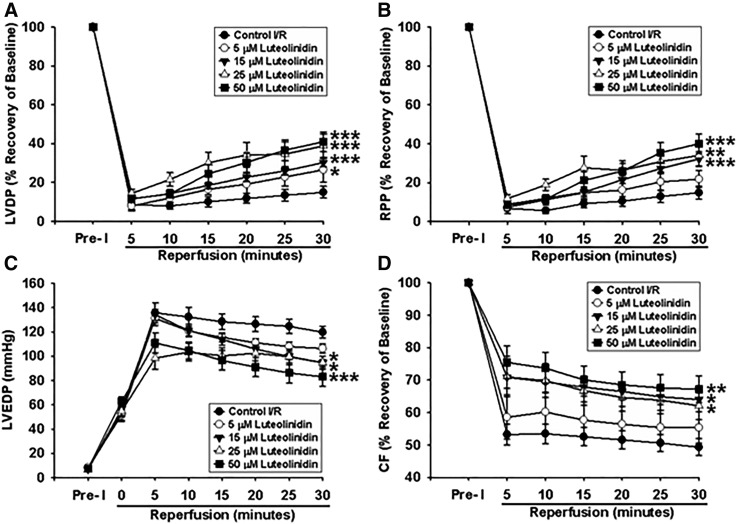
Fig. 8.
Postischemic myocardial recovery after 30-minute global ischemia and 30-minute reperfusion (I/R) with or without luteolinidin. Control hearts were given similar vehicle control (empty liposomes). (A, B, and D) LVDP (A), RPP (B), and CF (D) are expressed as the percent recovery of the preischemic (Pre-I) baseline value (100%). (C) LVEDP is expressed in mmHg. *P < 0.05; **P < 0.01; ***P < 0.001 (with respect to control I/R; mean ± S.E.M., n = 6–10).
We also evaluated the effect of vehicle infusion versus that of luteolinidin-containing liposomes with a normal perfusion protocol of sham I/R. At a time 15 minutes after vehicle or luteolinidin treatment, LVDP values were not significantly changed from baseline, with values of 100.7% ± 5.9% versus 101.3% ± 1.3% of baseline, respectively. Similarly, RPP values of 97.3% ± 3.5% versus 97.6% ± 3.3% of baseline, respectively, were observed. For CF, values of 96.2% ± 3.8% versus 95.1% ± 1.3% of baseline were seen. No significant differences were seen between the vehicle and luteolinidin treatment groups with sham I/R out to 60 minutes post-treatment.
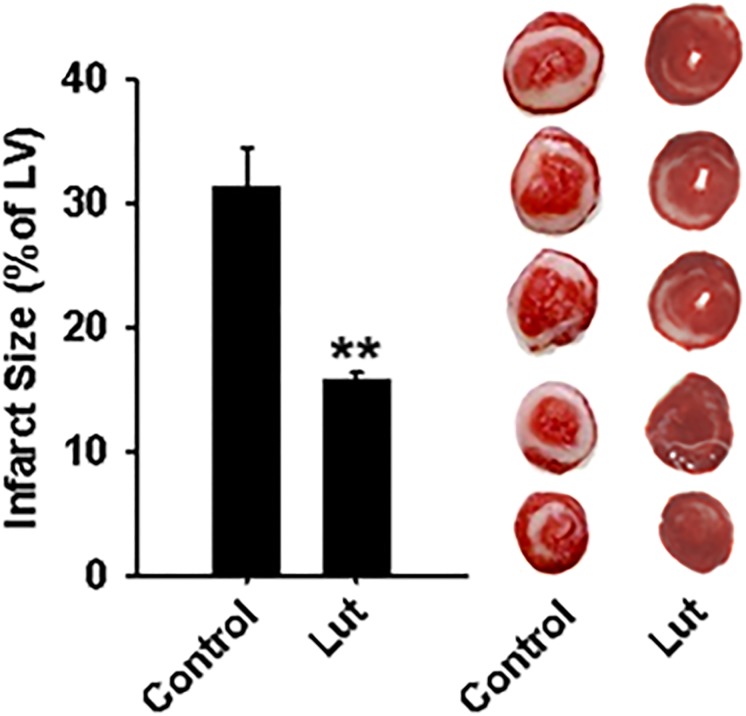
Luteolinidin Lowers Infarct Size after I/R.
Luteolinidin (50 μM) was tested for its ability to lower infarct size after 30-minute ischemia and 120-minute reperfusion. The 50-μM dose was used because it was most effective in increasing cardiac contractile functional recovery. The percent infarct of the left ventricle was greater than 30% in vehicle-treated hearts but less than 16% in luteolinidin-treated hearts (Fig. 9).
Fig. 9.
Infarct size in vehicle-treated and luteolinidin-treated hearts. After 30-minute ischemia and 120-minute reperfusion, hearts were serially sectioned and stained with 2,3,5-triphenyltetrazolium chloride. Liposomal luteolinidin-treated hearts (Lut) had significantly less infarction than liposomal vehicle-treated hearts (control) after I/R. **P < 0.01 (mean ± S.E.M., n = 6–8).
Discussion
Postischemic endothelial function in the heart is severely impaired due to dysregulation of eNOS. While the levels of eNOS are largely unaffected by I/R (Giraldez et al., 2000), major depletion of the requisite cofactor, BH4, and reducing substrate, NADPH, cause impaired eNOS function (Dumitrescu et al., 2007; Reyes et al., 2015). Experiments investigating the mechanism of NAD(P)+ consumption elucidated that CD38 is activated by I/R (Reyes et al., 2015), leading to NAD(P)(H) depletion and impaired postischemic endothelial function. CD38 activation was blocked by the CD38 inhibitor α-NAD, and this preserved postischemic NADP(H) levels and enhanced functional recovery after I/R. However, α-NAD was effective only in the low millimolar range, either due to weak inhibition or bioavailability, making it a poor candidate for further in vivo application. Several potent and naturally occurring CD38 inhibitors were recently discovered, including flavonoids (Kellenberger et al., 2011) (e.g., luteolinidin) and anthranoids (Blacher et al., 2015), and others with even higher potency have been developed, including some with inhibition in the submicromolar range (Moreau et al., 2013; Wang et al., 2014; Becherer et al., 2015; Haffner et al., 2015). The naturally occurring flavonoid CD38 inhibitors are particularly promising because of their biocompatibility with minimal toxicity as well as their wide availability in common agricultural foods (Awika et al., 2004).
Initial in vitro work confirmed the potency of the flavonoid luteolinidin as a CD38 inhibitor (Fig. 3). A Ki of approximately 11 μM for luteolinidin inhibition of CD38 was estimated. With Lineweaver–Burk analysis, we found that CD38 noncompetitively inhibits CD38 with decreasing Vmax and unchanging Km with increasing inhibitor concentration. This is in contrast with prior studies that identified luteolinidin as a competitive inhibitor of CD38 (Kellenberger et al., 2011; Escande et al., 2013). Consistent with noncompetitive inhibition, the decreasing Vmax cannot be overcome by increasing substrate concentration, unlike with competitive inhibition (Michaelis et al., 2011). Although both types of inhibitors theoretically increase the intracellular pool of substrate NAD(P)(H) for CD38, noncompetitive inhibitors are more effective at decreasing the amount of product. This is because the increased intracellular substrate concentration, which occurs with enzyme inhibition in general, can eventually outcompete a competitive inhibitor. In the case of noncompetitive inhibition, increasing the amount of substrate does not affect the level of inhibition. This difference may be important in considering the optimal inhibitor of CD38, since CD38 catalyzes reactions that lead to a wide range of second messengers, some of which are potent calcium (Ca2+) mobilizers (Lee, 2012; Gul et al., 2016). It remains possible that increased production of these second messengers contributes to ischemia-induced Ca2+ overload in myocytes (Zimmerman and Hülsmann, 1966; Hausenloy and Yellon, 2013).
At the substrate level, CD38 inhibition leads to both higher NADP(H) and NAD(H) levels. While NAD(H) is important for cellular bioenergetics, NADP(H) is important for providing the reducing equivalents for reductive biosynthetic reactions, such as generating reduced glutathione from its oxidized form (Winkler et al., 1986). NADPH is also critical in the production of NO from eNOS, which catalyzes the flavin-mediated electron transfer from the bound NADPH to the heme.
With I/R of the heart, we found marked depletion of myocardial NADP(H) and a similar effect on NAD(H). With preischemic luteolinidin treatment, both NADP(H) and NAD(H) were salvaged (Fig. 5). Interestingly, we previously found that CD38 inhibition with α-NAD had a greater effect on the NADP(H) pools relative to the NAD(H) pools, probably due to the significantly lower Km and higher Vmax of the CD38 reaction with NADP+ as substrate compared with NAD+ (Vu et al., 1996). Luteolinidin may provide a greater preservation of the NAD(H) pool than α-NAD because the liposomal formulations used provide a much more potent inhibition of CD38 with good uptake throughout the heart. Alternatively, luteolinidin could exert some additional effects on inhibition of other pathways such as poly(ADP-ribose) polymerase, which has been shown to cause severe depletion of the myocardial NAD+ pool in I/R (Pieper et al., 2000; Liaudet et al., 2001; Szabó et al., 2004; Geraets et al., 2007; Maeda et al., 2014; Boesten et al., 2015).
In parallel with the NADP(H) depletion with I/R, NOS-dependent CF was severely impaired (Fig. 6B). With the finding that CD38 inhibition with luteolinidin protected NADP(H) levels, we hypothesized that it may also protect NOS-dependent vasodilatory function. By administering l-NAME after I/R, we tested the dose-dependent effect of luteolinidin on the preservation of NOS-dependent CF. Recovery of NOS-dependent CF after I/R was approximately 35% in untreated hearts but was nearly fully recovered in hearts pretreated with 25 or 50 μM luteolinidin prior to the onset of ischemia (Fig. 6B). This suggests that NOS-dependent endothelial function is limited by low NADPH levels in I/R and can be prevented by CD38 inhibition.
Our previous work also highlighted the importance of BH4 for endothelial function after ischemia (Dumitrescu et al., 2007). However, BH4 supplementation alone does not result in complete recovery of NOS-dependent CF, although tandem replenishment including NADPH does (Reyes et al., 2015). A recent clinical study testing the effect of oral BH4 on endothelial function in patients with coronary artery disease found that systemic and vascular oxidation of BH4 limited its protective effects (Cunnington et al., 2012). Considering this finding, it is possible that NADPH-dependent reactions that either salvage BH4 from its reversible oxidation products through dihydrofolate reductase (Bailey and Ayling, 2009) or complete its de novo synthesis through sepiapterin reductase (Gao et al., 2009) are limited in I/R injury, perhaps by diminished cellular NADPH levels. Consistent with this, we see that luteolinidin-mediated inhibition of CD38, which preserves NADPH levels after I/R, also preserves BH4. This was also true with the previously tested CD38 inhibitor α-NAD but over 100-fold higher levels were required (Reyes et al., 2015). Thus, NADPH depletion may trigger an inability to maintain BH4 levels, and this can be reversed by CD38 inhibition with luteolinidin.
We also observed with luteolinidin treatment that the recovery of total CF was higher than in untreated hearts (Figs. 6A and 8D). Furthermore, the greatly enhanced recovery of NOS-dependent CF with luteolinidin treatment contributed to the increased total CF recovery observed after I/R. Recovery of left ventricular contractile function was also higher through reperfusion in luteolinidin-treated hearts compared with the control. Recovery of LVDP and RPP increased dose dependently with the concentration of luteolinidin administered, whereas LVEDP decreased in a similar dose-dependent fashion (Fig. 8, A–C). We showed previously that CD38 inhibition protected the heart from I/R in wild-type hearts, but that these effects were severely blunted in hearts from eNOS-deficient mice (Reyes et al., 2015). Although luteolinidin protects the heart through its potent inhibition of CD38, it could also exert other beneficial effects. It has been suggested that flavonoids can be protective due to their antioxidant activity (Necas et al., 2006; Chang et al., 2007). In our experiments, the dose dependence of the protection (from 5 to 50 μM) argues for relatively high potency and low toxicity for luteolinidin. At the highest tested dose of 50 μM, no negative effects were observed in the acute phase of dosing, and the protective effects were maintained after I/R.
Evidence that vegetarian diets promote cardiovascular health can be attributed to a multitude of factors. The favorable macromolecular breakdown of plant-based diets, compared with the common Western diet, is the most compelling reason for its health-promoting effect. The presence of high levels of biologically active polyphenolic compounds, such as the flavonoids, is a likely health-promoting factor. Flavonoids have repeatedly been shown to improve cardiovascular diseases including hypertension, ischemic heart disease, and stroke (Hertog et al., 1993; Keli et al., 1996; Perez-Vizcaino et al., 2009). It has been further shown that flavonoids protect and improve endothelial function (Engler et al., 2004; Vita, 2005; Hodgson, 2006; Grassi et al., 2013). This is of considerable importance because endothelial dysfunction is a hallmark of most types of cardiovascular disease, including hypertension, diabetes, atherosclerosis, and ischemic heart disease (Hadi et al., 2005; Vita, 2005; Deanfield et al., 2007). Factors capable of improving endothelial dysfunction may prove beneficial in each of these major disorders. While several of these studies have shown the beneficial effect of polyphenols on reducing oxidative stress, it is probable that polyphenols effect their biologic actions through multiple distinct mechanisms and the effects on the CD38 pathway may be of particular importance (Williamson and Manach, 2005).
In summary, we demonstrate that luteolinidin inhibits CD38 in vitro and that treatment of the ex vivo isolated heart results in increased postischemic salvage of NADP(H) and NAD(H) pools, and that this is directly correlated with improved vascular and cardiac contractile function. Liposomal delivery achieved rapid cardiac uptake and conferred marked functional protection, accompanied by increased myocardial salvage. There was no toxicity seen even at doses approximately 4-fold above the minimally effective dose. As such, liposomal luteolinidin delivery could provide a potent therapeutic approach to prevent postischemic injury in the heart and other organs. In the future, it will be important to translate these findings to in vivo models of I/R and evaluate the effects of pre- versus postischemic treatment.
Abbreviations
- BH2
dihydrobiopterin
- BH4
tetrahydrobiopterin
- CF
coronary flow
- ε-NAD
nicotinamide 1,N6-ethenoadenine dinucleotide
- eNOS
endothelial nitric oxide synthase
- HPLC
high-performance liquid chromatography
- I/R
ischemia/reperfusion
- KHB
Krebs-Henseleit buffer
- l-NAME
Nω-nitro-l-arginine methyl ester
- LVDP
left ventricular developed pressure
- LVEDP
left ventricular end diastolic pressure
- MPB
mobile phase B
- NO
nitric oxide
- rCD38
recombinant human CD38
- RPP
rate-pressure product
Authorship Contributions
Participated in research design: Boslett, Hemann, Zweier.
Conducted experiments: Boslett.
Contributed new reagents or analytic tools: Zhao, Lee.
Performed data analysis: Boslett, Hemann, Zweier.
Wrote or contributed to the writing of the manuscript: Boslett, Hemann, Zweier.
Footnotes
This research was supported by the National Institutes of Health National Heart, Lung, and Blood Institute [Grants R01-HL131941 and R01-HL135648] and the National Institutes of Health National Institute of Biomedical Imaging and Bioengineering [Grant R01-EB0169096].
References
- Aliev MK, Dos Santos P, Hoerter JA, Soboll S, Tikhonov AN, Saks VA. (2002) Water content and its intracellular distribution in intact and saline perfused rat hearts revisited. Cardiovasc Res 53:48–58. [DOI] [PubMed] [Google Scholar]
- Amorini AM, Lazzarino G, Galvano F, Fazzina G, Tavazzi B, Galvano G. (2003) Cyanidin-3-O-beta-glucopyranoside protects myocardium and erythrocytes from oxygen radical-mediated damages. Free Radic Res 37:453–460. [DOI] [PubMed] [Google Scholar]
- Aneja R, Hake PW, Burroughs TJ, Denenberg AG, Wong HR, Zingarelli B. (2004) Epigallocatechin, a green tea polyphenol, attenuates myocardial ischemia reperfusion injury in rats. Mol Med 10:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awika JM, Rooney LW, Waniska RD. (2004) Properties of 3-deoxyanthocyanins from sorghum. J Agric Food Chem 52:4388–4394. [DOI] [PubMed] [Google Scholar]
- Bailey SW, Ayling JE. (2009) The extremely slow and variable activity of dihydrofolate reductase in human liver and its implications for high folic acid intake. Proc Natl Acad Sci USA 106:15424–15429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becherer JD, Boros EE, Carpenter TY, Cowan DJ, Deaton DN, Haffner CD, Jeune MR, Kaldor IW, Poole JC, Preugschat F, et al. (2015) Discovery of 4-amino-8-quinoline carboxamides as novel, submicromolar inhibitors of NAD-hydrolyzing enzyme CD38. J Med Chem 58:7021–7056. [DOI] [PubMed] [Google Scholar]
- Blacher E, Ben Baruch B, Levy A, Geva N, Green KD, Garneau-Tsodikova S, Fridman M, Stein R. (2015) Inhibition of glioma progression by a newly discovered CD38 inhibitor. Int J Cancer 136:1422–1433. [DOI] [PubMed] [Google Scholar]
- Boesten DM, von Ungern-Sternberg SN, den Hartog GJ, Bast A. (2015) Protective pleiotropic effect of flavonoids on NAD+ levels in endothelial cells exposed to high glucose. Oxid Med Cell Longev 2015:894597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang WT, Shao ZH, Yin JJ, Mehendale S, Wang CZ, Qin Y, Li J, Chen WJ, Chien CT, Becker LB, et al. (2007) Comparative effects of flavonoids on oxidant scavenging and ischemia-reperfusion injury in cardiomyocytes. Eur J Pharmacol 566:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CA, Lin CH, Druhan LJ, Wang TY, Chen YR, Zweier JL. (2011) Superoxide induces endothelial nitric-oxide synthase protein thiyl radical formation, a novel mechanism regulating eNOS function and coupling. J Biol Chem 286:29098–29107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CA, Wang TY, Varadharaj S, Reyes LA, Hemann C, Talukder MA, Chen YR, Druhan LJ, Zweier JL. (2010) S-glutathionylation uncouples eNOS and regulates its cellular and vascular function. Nature 468:1115–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane RK, Sols A. (1954) The non-competitive inhibition of brain hexokinase by glucose-6-phosphate and related compounds. J Biol Chem 210:597–606. [PubMed] [Google Scholar]
- Cunnington C, Van Assche T, Shirodaria C, Kylintireas I, Lindsay AC, Lee JM, Antoniades C, Margaritis M, Lee R, Cerrato R, et al. (2012) Systemic and vascular oxidation limits the efficacy of oral tetrahydrobiopterin treatment in patients with coronary artery disease. Circulation 125:1356–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pascali F, Hemann C, Samons K, Chen CA, Zweier JL. (2014) Hypoxia and reoxygenation induce endothelial nitric oxide synthase uncoupling in endothelial cells through tetrahydrobiopterin depletion and S-glutathionylation. Biochemistry 53:3679–3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deanfield JE, Halcox JP, Rabelink TJ. (2007) Endothelial function and dysfunction: testing and clinical relevance. Circulation 115:1285–1295. [DOI] [PubMed] [Google Scholar]
- Dumitrescu C, Biondi R, Xia Y, Cardounel AJ, Druhan LJ, Ambrosio G, Zweier JL. (2007) Myocardial ischemia results in tetrahydrobiopterin (BH4) oxidation with impaired endothelial function ameliorated by BH4. Proc Natl Acad Sci USA 104:15081–15086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler MB, Engler MM, Chen CY, Malloy MJ, Browne A, Chiu EY, Kwak HK, Milbury P, Paul SM, Blumberg J, et al. (2004) Flavonoid-rich dark chocolate improves endothelial function and increases plasma epicatechin concentrations in healthy adults. J Am Coll Nutr 23:197–204. [DOI] [PubMed] [Google Scholar]
- Escande C, Nin V, Price NL, Capellini V, Gomes AP, Barbosa MT, O’Neil L, White TA, Sinclair DA, Chini EN. (2013) Flavonoid apigenin is an inhibitor of the NAD+ ase CD38: implications for cellular NAD+ metabolism, protein acetylation, and treatment of metabolic syndrome. Diabetes 62:1084–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantinelli JC, Schinella G, Cingolani HE, Mosca SM. (2005) Effects of different fractions of a red wine non-alcoholic extract on ischemia-reperfusion injury. Life Sci 76:2721–2733. [DOI] [PubMed] [Google Scholar]
- Ferrari R, Alfieri O, Curello S, Ceconi C, Cargnoni A, Marzollo P, Pardini A, Caradonna E, Visioli O. (1990) Occurrence of oxidative stress during reperfusion of the human heart. Circulation 81:201–211. [DOI] [PubMed] [Google Scholar]
- Gao L, Pung YF, Zhang J, Chen P, Wang T, Li M, Meza M, Toro L, Cai H. (2009) Sepiapterin reductase regulation of endothelial tetrahydrobiopterin and nitric oxide bioavailability. Am J Physiol Heart Circ Physiol 297:H331–H339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraets L, Moonen HJ, Brauers K, Wouters EF, Bast A, Hageman GJ. (2007) Dietary flavones and flavonoles are inhibitors of poly(ADP-ribose)polymerase-1 in pulmonary epithelial cells. J Nutr 137:2190–2195. [DOI] [PubMed] [Google Scholar]
- Giraldez RR, Panda A, Zweier JL. (2000) Endothelial dysfunction does not require loss of endothelial nitric oxide synthase. Am J Physiol Heart Circ Physiol 278:H2020–H2027. [DOI] [PubMed] [Google Scholar]
- Grassi D, Desideri G, Di Giosia P, De Feo M, Fellini E, Cheli P, Ferri L, Ferri C. (2013) Tea, flavonoids, and cardiovascular health: endothelial protection. Am J Clin Nutr 98(Suppl):1660S–1666S. [DOI] [PubMed] [Google Scholar]
- Gul R, Park DR, Shawl AI, Im SY, Nam TS, Lee SH, Ko JK, Jang KY, Kim D, Kim UH. (2016) Nicotinic acid adenine dinucleotide phosphate (NAADP) and cyclic ADP-ribose (cADPR) mediate Ca2+ signaling in cardiac hypertrophy induced by β-adrenergic stimulation. PLoS One 11:e0149125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadi HA, Carr CS, Al Suwaidi J. (2005) Endothelial dysfunction: cardiovascular risk factors, therapy, and outcome. Vasc Health Risk Manag 1:183–198. [PMC free article] [PubMed] [Google Scholar]
- Haffner CD, Becherer JD, Boros EE, Cadilla R, Carpenter T, Cowan D, Deaton DN, Guo Y, Harrington W, Henke BR, et al. (2015) Discovery, synthesis, and biological evaluation of thiazoloquin(az)olin(on)es as potent CD38 inhibitors. J Med Chem 58:3548–3571. [DOI] [PubMed] [Google Scholar]
- Hausenloy DJ, Yellon DM. (2013) Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest 123:92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D. (1993) Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet 342:1007–1011. [DOI] [PubMed] [Google Scholar]
- Hirai M, Hotta Y, Ishikawa N, Wakida Y, Fukuzawa Y, Isobe F, Nakano A, Chiba T, Kawamura N. (2007) Protective effects of EGCg or GCg, a green tea catechin epimer, against postischemic myocardial dysfunction in guinea-pig hearts. Life Sci 80:1020–1032. [DOI] [PubMed] [Google Scholar]
- Hodgson JM. (2006) Effects of tea and tea flavonoids on endothelial function and blood pressure: a brief review. Clin Exp Pharmacol Physiol 33:838–841. [DOI] [PubMed] [Google Scholar]
- Hung LM, Su MJ, Chen JK. (2004) Resveratrol protects myocardial ischemia-reperfusion injury through both NO-dependent and NO-independent mechanisms. Free Radic Biol Med 36:774–781. [DOI] [PubMed] [Google Scholar]
- Ikizler M, Erkasap N, Dernek S, Kural T, Kaygisiz Z. (2007) Dietary polyphenol quercetin protects rat hearts during reperfusion: enhanced antioxidant capacity with chronic treatment. Anadolu Kardiyol Derg 7:404–410. [PubMed] [Google Scholar]
- Keli SO, Hertog MG, Feskens EJ, Kromhout D. (1996) Dietary flavonoids, antioxidant vitamins, and incidence of stroke: the Zutphen study. Arch Intern Med 156:637–642. [PubMed] [Google Scholar]
- Kellenberger E, Kuhn I, Schuber F, Muller-Steffner H. (2011) Flavonoids as inhibitors of human CD38. Bioorg Med Chem Lett 21:3939–3942. [DOI] [PubMed] [Google Scholar]
- Klaidman LK, Leung AC, Adams JD., Jr (1995) High-performance liquid chromatography analysis of oxidized and reduced pyridine dinucleotides in specific brain regions. Anal Biochem 228:312–317. [DOI] [PubMed] [Google Scholar]
- Lee HC. (2012) Cyclic ADP-ribose and nicotinic acid adenine dinucleotide phosphate (NAADP) as messengers for calcium mobilization. J Biol Chem 287:31633–31640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Hemann C, Abdelghany TM, El-Mahdy MA, Zweier JL. (2012) Characterization of the mechanism and magnitude of cytoglobin-mediated nitrite reduction and nitric oxide generation under anaerobic conditions. J Biol Chem 287:36623–36633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liaudet L, Yang Z, Al-Affar EB, Szabó C. (2001) Myocardial ischemic preconditioning in rodents is dependent on poly (ADP-ribose) synthetase. Mol Med 7:406–417. [PMC free article] [PubMed] [Google Scholar]
- Loukogeorgakis SP, van den Berg MJ, Sofat R, Nitsch D, Charakida M, Haiyee B, de Groot E, MacAllister RJ, Kuijpers TW, Deanfield JE. (2010) Role of NADPH oxidase in endothelial ischemia/reperfusion injury in humans. Circulation 121:2310–2316. [DOI] [PubMed] [Google Scholar]
- Maeda J, Roybal EJ, Brents CA, Uesaka M, Aizawa Y, Kato TA. (2014) Natural and glucosyl flavonoids inhibit poly(ADP-ribose) polymerase activity and induce synthetic lethality in BRCA mutant cells. Oncol Rep 31:551–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis L, Menten ML, Johnson KA, Goody RS. (2011) The original Michaelis constant: translation of the 1913 Michaelis-Menten paper. Biochemistry 50:8264–8269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau C, Liu Q, Graeff R, Wagner GK, Thomas MP, Swarbrick JM, Shuto S, Lee HC, Hao Q, Potter BV. (2013) CD38 structure-based inhibitor design using the N1-cyclic inosine 5′-diphosphate ribose template. PLoS One 8:e66247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munshi CB, Fryxell KB, Lee HC, Branton WD. (1997) Large-scale production of human CD38 in yeast by fermentation. Methods Enzymol 280:318–330. [DOI] [PubMed] [Google Scholar]
- Necas J, Bartosíková L, Florian T, Klusáková J, Suchý V, Naggar EM, Janostíková E, Bartosík T, Lisková M. (2006) [Protective effects of the flavonoids osajin and pomiferin on heart ischemia-reperfusion]. Ceska Slov Farm 55:168–174. [PubMed] [Google Scholar]
- Nijveldt RJ, van Nood E, van Hoorn DE, Boelens PG, van Norren K, van Leeuwen PA. (2001) Flavonoids: a review of probable mechanisms of action and potential applications. Am J Clin Nutr 74:418–425. [DOI] [PubMed] [Google Scholar]
- Palmer RM, Ferrige AG, Moncada S. (1987) Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 327:524–526. [DOI] [PubMed] [Google Scholar]
- Perez-Vizcaino F, Duarte J, Jimenez R, Santos-Buelga C, Osuna A. (2009) Antihypertensive effects of the flavonoid quercetin. Pharmacol Rep 61:67–75. [DOI] [PubMed] [Google Scholar]
- Pieper AA, Walles T, Wei G, Clements EE, Verma A, Snyder SH, Zweier JL. (2000) Myocardial postischemic injury is reduced by polyADPripose polymerase-1 gene disruption. Mol Med 6:271–282. [PMC free article] [PubMed] [Google Scholar]
- Reyes LA, Boslett J, Varadharaj S, De Pascali F, Hemann C, Druhan LJ, Ambrosio G, El-Mahdy M, Zweier JL. (2015) Depletion of NADP(H) due to CD38 activation triggers endothelial dysfunction in the postischemic heart. Proc Natl Acad Sci USA 112:11648–11653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarabelli TM, Mariotto S, Abdel-Azeim S, Shoji K, Darra E, Stephanou A, Chen-Scarabelli C, Marechal JD, Knight R, Ciampa A, et al. (2009) Targeting STAT1 by myricetin and delphinidin provides efficient protection of the heart from ischemia/reperfusion-induced injury. FEBS Lett 583:531–541. [DOI] [PubMed] [Google Scholar]
- Szabó G, Liaudet L, Hagl S, Szabó C. (2004) Poly(ADP-ribose) polymerase activation in the reperfused myocardium. Cardiovasc Res 61:471–480. [DOI] [PubMed] [Google Scholar]
- Toufektsian MC, de Lorgeril M, Nagy N, Salen P, Donati MB, Giordano L, Mock HP, Peterek S, Matros A, Petroni K, et al. (2008) Chronic dietary intake of plant-derived anthocyanins protects the rat heart against ischemia-reperfusion injury. J Nutr 138:747–752. [DOI] [PubMed] [Google Scholar]
- Vita JA. (2005) Polyphenols and cardiovascular disease: effects on endothelial and platelet function. Am J Clin Nutr 81 (Suppl):292S–297S. [DOI] [PubMed] [Google Scholar]
- Vu CQ, Lu PJ, Chen CS, Jacobson MK. (1996) 2′-Phospho-cyclic ADP-ribose, a calcium-mobilizing agent derived from NADP. J Biol Chem 271:4747–4754. [PubMed] [Google Scholar]
- Wang S, Zhu W, Wang X, Li J, Zhang K, Zhang L, Zhao YJ, Lee HC, Zhang L. (2014) Design, synthesis and SAR studies of NAD analogues as potent inhibitors towards CD38 NADase. Molecules 19:15754–15767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson G, Manach C. (2005) Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am J Clin Nutr 81 (Suppl):243S–255S. [DOI] [PubMed] [Google Scholar]
- Winkler BS, DeSantis N, Solomon F. (1986) Multiple NADPH-producing pathways control glutathione (GSH) content in retina. Exp Eye Res 43:829–847. [DOI] [PubMed] [Google Scholar]
- Yamazaki KG, Romero-Perez D, Barraza-Hidalgo M, Cruz M, Rivas M, Cortez-Gomez B, Ceballos G, Villarreal F. (2008) Short- and long-term effects of (-)-epicatechin on myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 295:H761–H767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman AN, Hülsmann WC. (1966) Paradoxical influence of calcium ions on the permeability of the cell membranes of the isolated rat heart. Nature 211:646–647. [DOI] [PubMed] [Google Scholar]
- Zweier JL, Kuppusamy P, Lutty GA. (1988) Measurement of endothelial cell free radical generation: evidence for a central mechanism of free radical injury in postischemic tissues. Proc Natl Acad Sci USA 85:4046–4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweier JL, Kuppusamy P, Williams R, Rayburn BK, Smith D, Weisfeldt ML, Flaherty JT. (1989) Measurement and characterization of postischemic free radical generation in the isolated perfused heart. J Biol Chem 264:18890–18895. [PubMed] [Google Scholar]
- Zweier JL, Talukder MA. (2006) The role of oxidants and free radicals in reperfusion injury. Cardiovasc Res 70:181–190. [DOI] [PubMed] [Google Scholar]