Abstract
The recreational use of designer drugs, including synthetic cathinones (bath salts), is associated with high levels of abuse and toxicity, and represents a growing threat to public health. 3,4-Methylenedioxypyrovalerone (MDPV) is a cocaine-like monoamine uptake inhibitor, and one of the most widely available and abused synthetic cathinones. The present study used male Sprague-Dawley rats to directly compare: (1) the acquisition of responding for MDPV and cocaine under a fixed ratio (FR) 1 schedule of reinforcement; (2) full dose-response curves for MDPV and cocaine under a FR5 schedule; and (3) progressive ratio (PR) schedules of reinforcement. Self-administration of MDPV and cocaine was acquired at comparable rates, and by a similar percentage of rats. Compared with cocaine, MDPV was ∼10-fold more potent and ∼3-fold more effective at maintaining responding (PR; final ratio completed). Unlike cocaine, for which little variability was observed among rats, the FR5 dose-response curve for MDPV was shifted ∼3-fold upward for a subset of rats (high-responders) relative to other rats with identical histories (low-responders). Compared with low-responding rats, high responders also self-administered more cocaine under the FR5 schedule, and earned significantly more MDPV, cocaine, and methamphetamine under a PR schedule of reinforcement. In addition to functioning as a significantly more effective reinforcer than either cocaine or methamphetamine, MDPV also appears to be unique in its capacity to establish an enduring phenotype in rats, characterized by unusually high levels of drug intake. Although the factors underlying this high-responder phenotype are unclear, they might be related to individual differences in human drug-taking behavior.
Introduction
The abuse of designer drugs including synthetic derivatives of cathinone represents a serious public health problem worldwide. The United Nations estimates that 25% of all new psychoactive substances are synthetic cathinones (United Nations Office of Drugs and Crime, 2014), 13 of which have been classified as Schedule I by the U.S. Drug Enforcement Administration. These compounds are marketed as bath salts or research chemicals and purported to be safe and legal alternatives to illicit stimulant drugs of abuse. Synthetic cathinones interact with monoamine transporters [for example, the dopamine transporter (DAT), norepinephrine transporter, and serotonin transporter (SERT)] and function as either cocaine-like inhibitors (inhibit uptake only), or amphetamine-like substrates (inhibit uptake and stimulate release) (e.g., Baumann et al., 2013; Simmler et al., 2013). Bath salts are primarily sold as powders or capsules and are administered by intranasal, oral, and intravenous routes for their euphoric and stimulant effects (Johnson and Johnson, 2014). Users report intense craving and frequently readminister cathinones (Winstock et al., 2011), suggesting that cathinones function as powerful reinforcers. Mirroring increases in recreational use, poison control center calls and emergency room visits related to bath salts have also increased in recent years (see the American Association of Poison Control Centers 2016 bath salts exposure cases; https://aapcc.s3.amazonaws.com/files/library/Bath_Salts_Web_Data_through_8.2016.pdf). As such, more research is urgently needed to better understand the abuse-related and toxic effects of synthetic cathinones.
Analysis of bath salts obtained in the United States suggests that 3,4-methylenedioxypyrovalerone (MDPV) is one of the most widely available and abused cathinones (Kyle et al., 2011; Spiller et al., 2011; Borek and Holstege, 2012; Murray et al., 2012; Shanks et al., 2012; Seely et al., 2013). Consistent with its capacity to inhibit monoamine uptake, MDPV is known to stimulate locomotor activity and produce discriminative stimulus effects similar to those of other stimulant drugs (e.g., cocaine, 3,4-methylenedioxymethamphetamine, and methamphetamine) in rats and mice (Huang et al., 2012; Fantegrossi et al., 2013; Gatch et al., 2013; Collins et al., 2016; Gannon et al., 2016). Based in large part on structural similarities, early studies compared the reinforcing effects of MDPV to methamphetamine (Aarde et al., 2013; Watterson et al., 2014); however, in vitro data have since indicated that MDPV is more similar to cocaine than methamphetamine, but MDPV is 100- to 500-fold more selective than cocaine for DAT relative to SERT (Baumann et al., 2013; Simmler et al., 2013).
The reinforcing effects of MDPV have been evaluated under fixed ratio (FR) and progressive ratio (PR) schedules of reinforcement, with two studies suggesting MDPV is a more effective reinforcer than methamphetamine (Aarde et al., 2013; Watterson et al., 2014), and a third study suggesting the reinforcing effects of MDPV are comparable to those of cocaine (Schindler et al., 2016). Although Schindler et al. (2016) reported that experimentally naive rats acquire responding for MDPV (0.03 mg/kg) and cocaine (0.5 mg/kg) at comparable rates, data from several behavioral assays suggest MDPV is 10-fold more potent than cocaine (Baumann et al., 2013; Gatch et al., 2013; Collins et al., 2016; Gannon et al., 2016; Schindler et al., 2016), raising the possibility that the unit dose of cocaine was too large to provide an accurate comparison. Moreover, although two studies have used PR schedules of reinforcement to suggest MDPV is a more effective reinforcer than methamphetamine (a monoamine transporter substrate) a direct comparison of the relative reinforcing effectiveness of MDPV to cocaine (another monoamine transporter inhibitor) has not been made.
Thus, the present study aimed to directly compare the following: 1) the acquisition of responding for functionally equivalent doses of MDPV (0.032 mg/kg/infusion) and cocaine (0.32 mg/kg/infusion); 2) the dose-response curves for the reinforcing effects of MDPV and cocaine under a FR5 schedule of reinforcement; and 3) a PR schedule of reinforcement (MDPV, cocaine, and methamphetamine). Together, these studies provide both qualitative and quantitative comparisons of the reinforcing effects of MDPV to two well-known stimulant drugs of abuse (cocaine and methamphetamine) and describe individual differences in drug-maintained behavior that was only observed in rats trained to respond for MDPV.
Materials and Methods
Animals
Male Sprague-Dawley rats (275–300 g) were obtained from Harlan (Indianapolis, IN) and singly housed with free access to water and rat chow in a temperature-controlled (24°C) and light-controlled (10/14-hour dark/light) environment. All procedures were conducted in accordance with the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio, and the Guide for Care and Use of Laboratory Animals (National Research Council, 2011).
Surgery
Rats were anesthetized with 2% isoflurane and prepared with chronic indwelling catheters in the left femoral vein as previously described (Collins et al., 2012a,b; Collins and France, 2015). Catheters were passed under the skin and attached to a vascular access button placed in the mid-scapular region. Immediately following surgery, rats were administered Penicillin G (60,000 U/rat) subcutaneously to prevent infection. Rats were allowed 5–7 days to recover during which time catheters were flushed daily with 0.5 ml of heparinized saline (100 U/ml). Thereafter, catheters were flushed daily with 0.2 ml of saline prior to and 0.5 ml of heparinized saline after the completion of self-administration sessions.
Apparatus
Experimental sessions were conducted in standard operant conditioning chambers (Med Associates Inc., St. Albans, VT) housed inside sound attenuating cubicles. Each chamber was equipped with two response levers located 6.8 cm above the grid floor and 1.3 cm from the right or left wall. Visual stimuli were provided by two sets of green, yellow, and red lights, one located above each of the two levers, and a white house light located at the top center of the opposite wall. Illumination of the yellow light above the active lever (left or right counterbalanced across rats) served as the discriminative stimulus. The conditioned stimulus (CS) consisted of the illumination of the green, yellow, and red lights above the active lever, as well as the illumination of the house light and was coincident with the start of the infusion. Drug solutions were delivered by a variable speed syringe pump through Tygon tubing connected to a stainless steel fluid swivel and spring tether, which was held in place by a counterbalanced arm.
Self-Administration Studies
Acquisition Procedures.
A total of 48 rats were allowed to respond for either MDPV (n = 32) or cocaine (n = 16) under a FR1/5-second timeout (TO) schedule of reinforcement during 10 daily 90-minute sessions. Doses of MDPV (0.032 mg/kg/infusion) and cocaine (0.32 mg/kg/infusion) were chosen based on their relative position on the descending limb of FR dose-response curves (Collins and Woods, 2007; Aarde et al., 2013). The discriminative stimulus signaled drug availability and completion of the response requirement resulted in a drug infusion (0.1 ml/kg over ∼1 second), presentation of the CS, and initiation of a 5-second timeout. The CS was present throughout the 5-second timeout. Responses on the inactive lever, as well as responses on either lever during timeouts were recorded but had no scheduled consequence. Acquisition criteria were as follows: ≥20 infusions for two consecutive days with ≥80% responding on the active lever. Rats that failed to acquire due to low levels of responding (<5 infusions per session) were mildly food restricted (15 g) and active levers were baited with food until responding increased (usually one to two sessions); rats that failed to acquire because of stability or accuracy were provided additional sessions at FR1 until criteria were met. Response requirements were subsequently increased to a FR5 schedule where they remained until stability criteria were met (±20% of the mean of three consecutive sessions and no increasing or decreasing trend).
Across-Session Dose-Response Curves.
Dose substitution (0.001–0.1 mg/kg/infusion) was used to generate a full FR5/5-second TO dose-response curve for MDPV in the first cohort (n = 16) of the MDPV-trained rats. The first dose was always 0.032 mg/kg/infusion, with remaining doses evaluated in random order and until stability criteria were met.
These 16 rats (n = 7, high responders; n = 9, low responders) then transitioned to a PR schedule of reinforcement under which the ratios were incremented according to the following equation: ratio = (5einfusion#*0.2)−5 (Richardson and Roberts, 1996). Sessions lasted a maximum of 12 hours but terminated if a ratio was not completed within 45 minutes (i.e., 45-minute limited hold). Ratio completion resulted in delivery of a unit dose of MDPV (0.0032–0.32 mg/kg/infusion), cocaine (0.032–1.78 mg/kg/infusion), methamphetamine (0.0032–0.178 mg/kg/infusion), or saline. Infusions were followed by a 5-second timeout signaled by CS presentation during which responding was recorded but had no scheduled consequence. MDPV was always evaluated first, but substitute drugs were evaluated in random order, with all doses for a particular drug evaluated before moving to the next; 0.032 mg/kg/infusion MDPV was re-evaluated before each substitute drug. Stability was defined as two consecutive sessions where the number of infusions obtained differed by ≤2.
Within-Session Dose-Response Curves.
The remaining 16 MDPV-trained (n = 8, high responders; n = 8, low responders) and 14 of the cocaine-trained rats transitioned to a multiple components, FR5/5-second TO schedule that comprised five 20-minute components, as described previously (Collins et al., 2012a). The discriminative stimulus signaled the start of each component, and completion of the response requirement resulted in delivery of the available unit dose, paired with CS presentation and a 5-second timeout. During the first component, completion of the response requirement resulted in the CS presentation and 5-second timeout, but no infusion. Under this procedure, the concentration of the drug in the syringe remained constant, and the infusion duration increased across components (0–5 seconds) to deliver the desired unit doses of MDPV (0.0032, 0.01, 0.032, and 0.1 mg/kg/infusion), cocaine (0.032, 0.1, 0.32, and 1.0 mg/kg/infusion), or saline (duration/volume-matched infusions). Each component was followed by a 5-minute blackout period, where all visual stimuli were extinguished. Responses during timeouts or blackouts were recorded but had no scheduled consequence. Substitution tests were completed once responding stabilized for the training drug (three consecutive sessions with <20% variance in responding during each component), with cocaine (0.032–1.0 mg/kg/infusion) substituted in MDPV-trained rats, and MDPV (0.0032–0.1 mg/kg/infusion) substituted in cocaine-trained rats; saline was substituted in both groups. Substitutes were evaluated until stability criteria were met (at least three sessions), with rats returning to their training drug between substitution tests.
Drugs
Racemic MDPV was synthesized at the Chemical Biology Research Branch of the National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Alcoholism. (−)-Cocaine hydrochloride and (+)-methamphetamine hydrochloride were provided by the National Institute on Drug Abuse Drug Supply Program. All drugs were dissolved in 0.9% physiologic saline and administered intravenously in a volume of 0.1 ml/kg, with the exception of infusion volumes for the multiple components schedule, which have been previously are described.
Data Analysis
Acquisition data are shown as the mean ± S.E.M. of the number of responses made on the active and inactive levers, as well as the percentage of rats that met acquisition criteria across the 10-day acquisition phase. Acquisition data for MDPV and cocaine were compared by two-way (drug and day) repeated-measures (day) analysis of variance (ANOVA) followed by a Holm-Sidak’s test for multiple comparisons. Mean number of days required to meet acquisition criteria between drugs were compared by the unpaired, two-tailed t test.
Data obtained during the transition from the FR1 to the FR5 schedule are presented as of the mean ± S.E.M. number of infusions of MDPV (0.032 mg/kg) or cocaine (0.32 mg/kg) obtained during the last three sessions under the FR1 schedule and the three days that satisfied stability criteria under the FR5 schedule of reinforcement. Two-way (drug and day) repeated-measures (day) ANOVA with post hoc Holm-Sidak tests were used to detect significant differences in the number of infusions obtained under FR1 and FR5 conditions for rats trained to respond for MDPV (low and high responders) and cocaine. Responding during postinfusion timeouts is expressed as the percentage of active lever responding that occurred during the timeout relative to the total number of active lever responses and plotted as a frequency distribution using 10% bins. A Pearson’s correlation test was performed to determine if percent timeout responding was related to the number of infusions (0.032 mg/kg MDPV) obtained.
Dose-response data (FR and PR) are presented as mean ± S.E.M. of the number of infusions earned for each unit dose and analyzed by two-way (phenotype and dose) repeated-measures (dose) ANOVA to detect differences between groups. The Holm-Sidak post hoc test was used to detect differences in the number of infusions between high and low responders under PR conditions.
The PR dose-response curves were used to obtain measures of reinforcing potency [dose estimated to produce a 50% effect (ED50)] and effectiveness [maximal effective level (Emax)] for individual subjects. The mean ± S.E.M. Emax value represents the dose-independent maximum number of infusions earned for a given drug and provides a measure of reinforcing effectiveness for each drug. The ED50 values were obtained for individual subjects by normalizing the dose-response curves for each drug to the Emax value (where Emax = 100%) and analyzing all of the data spanning the 20%–80% effective levels (including not more than one point above 80% and not more than one point below 20%) by linear regression. Potency data represent the mean ED50 ± 95% confidence intervals. Two-way (phenotype and drug) ANOVA followed by the Holm-Sidak test was used to detect differences in Emax values, whereas one-way ANOVA followed by the Holm-Sidak test was used to detect differences in Emax values when data were collapsed across phenotype. The ED50 values were considered statistically different if the confidence intervals did not overlap.
For the within-session studies, responding during timeouts and blackouts are presented as the mean ± S.E.M. percentage of active lever responses made during postinfusion timeouts and mean ± S.E.M. number of active lever responses made during each intercomponent blackout. Two-way (group and dose/component) repeated-measures (dose/component) ANOVA followed by the post hoc Holm-Sidak test was used to detect differences in responding among groups of rats [MDPV-trained (low and high responders) and cocaine-trained]. The Prism 6 software (GraphPad Software, Inc., La Jolla, CA) was used to generate figures and conduct statistical analyses.
Results
Acquisition.
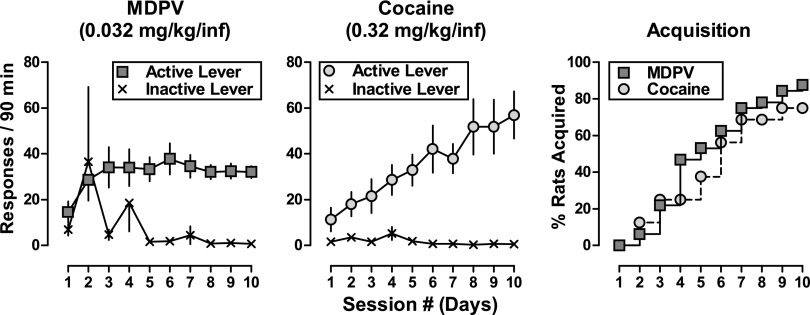
As shown in Fig. 1, rats readily acquired responding for MDPV (0.032 mg/kg/infusion) or cocaine (0.32 mg/kg/infusion), with 87.5% (28/32) of rats acquiring responding for MDPV and 75% (12/16) of rats acquiring responding for cocaine by the end of the 10-day period. The mean time to acquire was 5.1 ± 0.4 days for MDPV and 5.1 ± 0.6 days for cocaine. For both groups, the mean number of infusions earned increased as a function of day [F(9,460) = 3.5, P < 0.001], with cocaine maintaining slightly more infusions than MDPV (56.9 ± 10.3 versus 32.1 ± 2.6, respectively) by the end of the acquisition phase; however, these differences were not significant. A two-way repeated-measures ANOVA of the number of active lever responses made during acquisition revealed main effects of day [F(9,414) = 5.1, P < 0.0001] and an interaction between day and drug [F(9,414) = 2.3, P < 0.05], but no main effects of drug. When percent acquisition data were compared by two-way ANOVA, the main effects of day [F(9,9) = 48.2, P < 0.0001] and drug [F(1,9) = 7.0, P < 0.05] were identified; however, post hoc tests failed to identify group differences across the 10-day acquisition period.
Fig. 1.
Spontaneous acquisition of 0.032 mg/kg/infusion MDPV (n = 32, left) and 0.32 mg/kg/infusion cocaine (n = 16, middle) over the course of the 10-day acquisition phase in male Sprague-Dawley rats. Solid squares represent active lever responses and the X symbols represent inactive lever responses. Abscissa: numbers refer to consecutive days during the acquisition period. Ordinate: total responses emitted on each lever during the active portion of the 90-minute session. Error bars represent ±S.E.M. (Right panel) Percent of rats to acquire self-administration of 0.032 mg/kg/infusion MDPV (solid squares) and 0.32 mg/kg/infusion cocaine (solid circles) over the course of 10 days. Abscissa: numbers refer to consecutive days during the acquisition period. Ordinate: cumulative percent of rats within each training group to acquire self-administration of the training drug/dose.
Across-Session FR5.
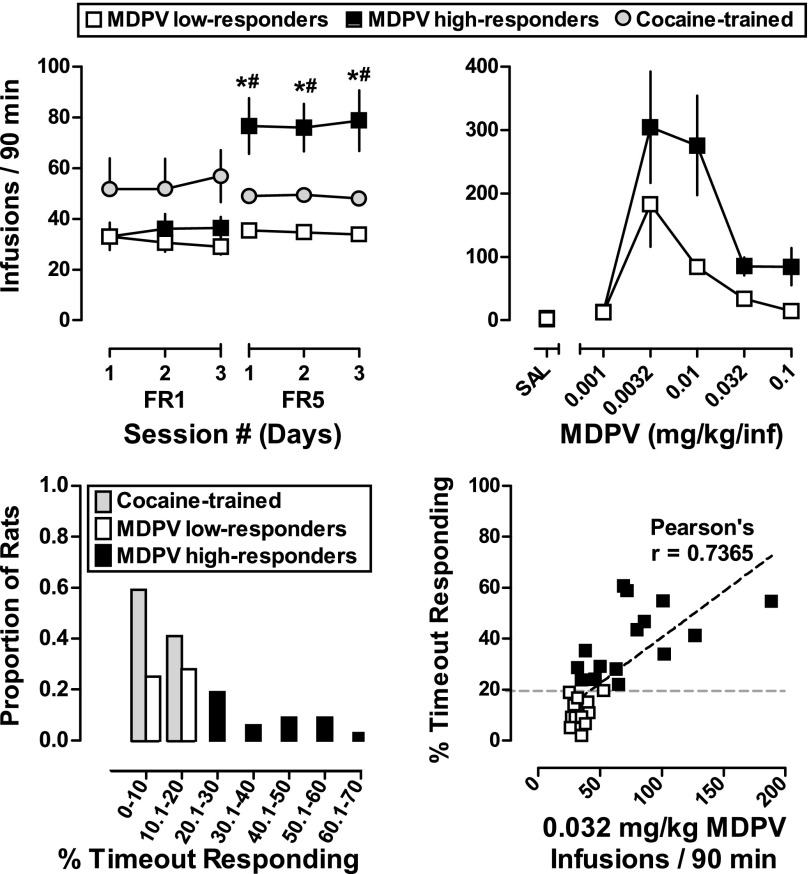
Figure 2 (upper-left panel) shows the number of infusions earned during the last three sessions in which MDPV (0.032 mg/kg) or cocaine (0.32 mg/kg) were available under the FR1/5-second TO and FR5/5-second TO schedules of reinforcement. Although the number of infusions did not vary as a function of response requirement (i.e., FR1 and FR5) when cocaine was available, a significant difference was observed when MDPV was available for infusion (P < 0.05). This difference was due to a subset of rats (Fig. 2, black squares) that significantly increased MDPV self-administration when the schedule of reinforcement changed from the FR1 to the FR5 schedule. While there were no differences in the number of infusions earned between high- and low-responding rats when MDPV was available under the FR1 schedule, post hoc tests indicated high-responding rats self-administered significantly more MDPV than low-responding rats under the FR5 schedule of reinforcement (P < 0.05). Analysis of the across-session dose-response curves (Fig. 2, upper-right panel) by two-way repeated-measures ANOVA revealed significant main effects of responder phenotype [F(1,14) = 11.6; P < 0.005] and MDPV dose [F(5,70) = 14.0; P < 0.0001] on the number of MDPV infusions obtained; however, an interaction between responder phenotype and dose was not detected.
Fig. 2.
(Upper panel, left side) Self-administration of 0.032 mg/kg/infusion MDPV (n = 32, squares) and 0.32 mg/kg/infusion cocaine (n = 16, circles) during the last 3 days of the FR1 and FR5 schedules of reinforcement. Abscissa: numbers refer to the final three sessions of FR1 and FR5 training. (Upper panel, right side) Self-administration dose-response curves obtained under the FR5 schedule of reinforcement in MDPV-trained high-responders (n = 7) and low-responders (n = 9) using dose substitution. Abscissa: SAL represents infusions of saline, while the numbers refer to dose of MDPV available during each session, expressed as mg/kg/infusion on a log scale. Ordinate: total infusions obtained during the 90-minute session. Error bars represent ±S.E.M. (Lower panel, left side) Distribution of MDPV-trained (n = 32) and cocaine-trained (n = 16) rats based on timeout responding. Abscissa: percent of total responses emitted on the active lever occurring during the postinfusion timeout, presented in 10% bins. Ordinate: proportion of MDPV- or cocaine-trained rats. (Lower panel, right side) Correlation between timeout responding and infusions obtained under FR5/5-second TO. Abscissa: average number of infusions of 0.032 mg/kg/infusion MDPV obtained over the last 3 days of FR5 conditions. Ordinate: percent responses made on the active lever during timeouts versus total active lever responses. Asterisks and pound signs indicate statistical significance: *P < 0.05 versus low-responders during same FR; #P < 0.05 versus same group during FR1.
The percentage of active lever responding that occurred during the timeouts is also shown in Fig. 2 (bottom panels). Similar to cocaine-trained rats (gray bars), approximately half of the MDPV-trained rats (white bars) made <20% of their active lever responses during postinfusion timeouts, whereas the remainder of the MDPV-trained rats (black bars) made >20% of their total active lever responses during the postinfusion timeout. Based on these data, MDPV-trained rats that made <20% of active lever responses during timeouts were operationally defined as low responders (n = 17), whereas MDPV-trained rats making >20% of their total active lever responses during timeouts were defined as high responders (n = 15). Because none of the cocaine-trained rats made >20% of their active lever responses during timeouts, they were viewed as a homogenous group (gray symbols). High rates of timeout responding were positively correlated (r = 0.7365; P < 0.0001) with the number of infusions of MDPV earned (Fig. 2, lower-right panel).
Multiple-Component FR5.
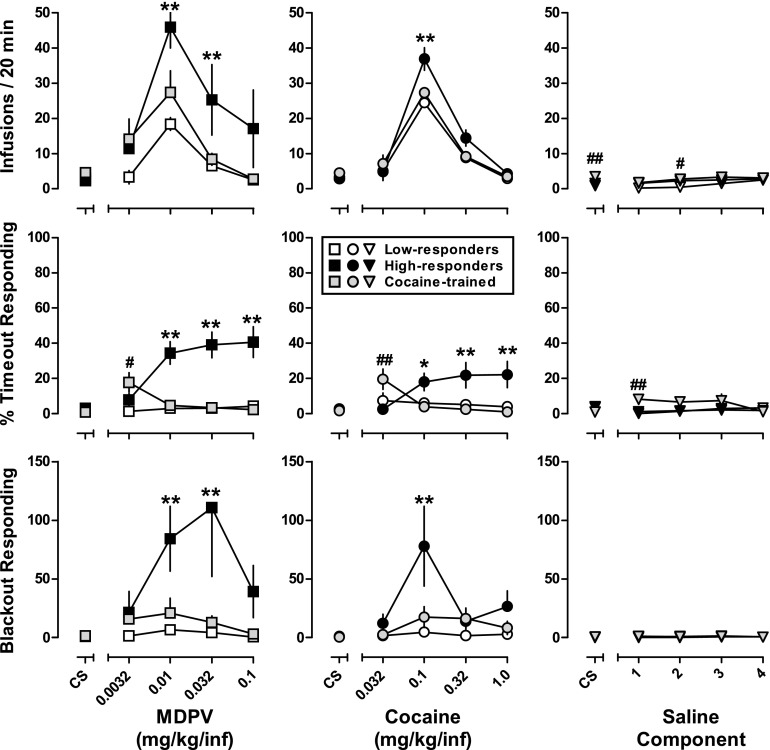
Under this paradigm, MDPV, cocaine, and saline were evaluated across five sequential 20-minute components (Fig. 3, top panel). With respect to the number of MDPV infusions, the main effects of group [F(2,27) = 3.9; P < 0.05], dose [F(4,108) = 19.1; P < 0.0001], and a group-by-dose interaction [F(8,108) = 2.3; P < 0.05] were detected. Post hoc tests revealed high responders self-administered significantly more MDPV than low responders or cocaine-trained rats at doses of 0.01 and 0.032 mg/kg/infusion; cocaine-trained rats did not differ from low responders at any dose. When cocaine was available, the main effects of group [F(2,27) = 3.4; P < 0.05], dose [F(4,108) = 179.5; P < 0.0001], as well as dose-by-group interaction [F(8,08) = 4.1; P < 0.0005] were detected for the total number of infusions. Post hoc tests revealed high responders self-administered significantly more cocaine than low responders and cocaine-trained rats at a dose of 0.1 mg/kg/infusion (Fig. 3, upper-middle panel). All three groups of rats responded at low levels when saline was available; however, post doc tests revealed that cocaine-trained rats responded more than low and high responders when the CS (alone) was presented (P < 0.05) and more than low responders (P < 0.05) during the second saline component. Although potency differences were observed between MDPV and cocaine, the relative (MDPV was 10-fold more potent than cocaine) and absolute potency of MDPV (peak at 0.01 mg/kg/infusion) and cocaine (peak at 0.1 mg/kg/infusion) to maintain responding did not differ among the groups of rats (high responders, low responders, and cocaine trained).
Fig. 3.
(Top row) Self-administration dose-response curves obtained under a multicomponent FR5 schedule of reinforcement for MDPV (left), cocaine (center), and saline (right) for high-responders (n = 8), low-responders (n = 8), and cocaine-trained (n = 16) rats. Ordinate: total infusions obtained during each 20-minute component. (Center row) MDPV-induced (left), cocaine-induced (center), and saline-induced (right) timeout responding. Ordinate: percentage of active lever responses made during postinfusion timeouts versus the active session during each 20-minute component. (Bottom row) MDPV-induced (left), cocaine-induced (center), and saline-induced (right) blackout responding. Ordinate: active lever responses made during each 5-minute intercomponent blackout period. Abscissa (for all panels): CS represents data following presentation of only the injection-paired stimuli, while numbers refer to dose of MDPV (left column) and dose of cocaine (center column) expressed as mg/kg/infusion on a log scale or saline component number (right column). Error bars represent ±S.E.M. Asterisks and pound signs indicate statistical significance: *P < 0.05 compared with cocaine-trained; **P < 0.05 compared with low-responders and cocaine-trained; #P < 0.05 compared with low-responders; ##P < 0.05 compared with low-responders and high-responders.
Similar to when a single unit dose was available throughout a 90-minute session, differences in timeout responding were also observed under the multiple components procedure (Fig. 3, middle row) with the main effects of group [MDPV: F(2,27) = 20.0, P < 0.0001; cocaine: F(2,27) = 4.3, P < 0.05], dose [MDPV: F(4,108) = 10.4, P < 0.0001; cocaine: F(4,108) = 3.0, P < 0.05], and group-by-dose interactions [MDPV: F(8,108) = 15.3, P < 0.0001; cocaine: F(8,108) = 7.0, P < 0.0001] detected when either MDPV or cocaine was available. With regard to MDPV, post hoc tests revealed that high responders made significantly more timeout responses than low responders and cocaine-trained rats at unit doses of 0.01–0.1 mg/kg/infusion (P < 0.05). Similar effects were observed when cocaine was available at unit doses of 0.1–1.0 mg/kg/infusion (P < 0.05). Although cocaine-trained rats exhibited a greater percentage of timeout responses than MDPV-trained rats when ineffective doses of MDPV (0.0032 mg/kg/infusion) and cocaine (0.032 mg/kg/infusion) or saline (second component) were available, this was likely due to the small number of total responses made under each of these conditions. With this exception, responding maintained by MDPV or cocaine did not differ between low responders and cocaine-trained rats; timeout responding when saline was available for infusion was comparable and low in all groups.
Responding during the intercomponent blackouts was also compared across rats (Fig. 4, bottom row), with a main effect of group and dose detected when either MDPV [group: F(2,27) = 4.4, P < 0.05; dose: F(4,108) = 5.5, P < 0.0005] or cocaine [group: F(2,27) = 3.6, P < 0.05; dose: F(4,108) = 7.0, P < 0.0001] was available for infusion. Interactions between dose and group were also detected when MDPV [F(8,108) = 3.3, P < 0.005] or cocaine [F(8,108) = 3.5, P < 0.005] was available. Similar to timeout responding, high responders exhibited more blackout responses than both low responders and cocaine-trained rats at unit doses of 0.01 and 0.032 mg/kg/infusion MDPV (P < 0.05) and 0.1 mg/kg/infusion cocaine (P < 0.05). When saline was available, blackout responding was low and not significantly different among the groups.
Fig. 4.
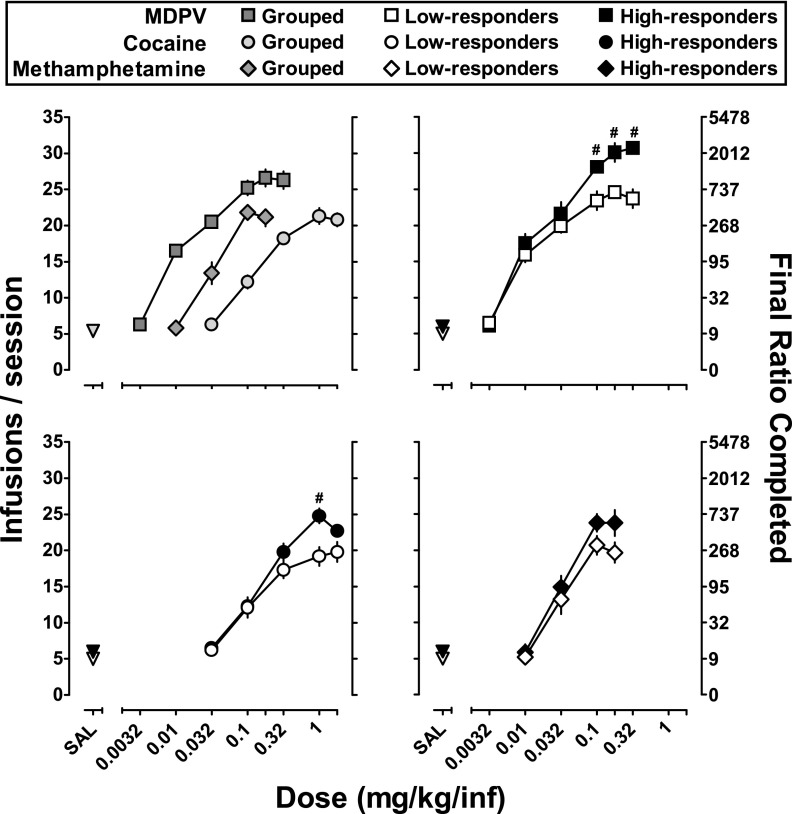
Self-administration dose-response curves for MDPV (squares), cocaine (circles), and methamphetamine (diamonds) obtained under a PR schedule of reinforcement (upper-left panel). Self-administration dose-response curves of MDPV (upper-right panel), cocaine (lower-left panel), and methamphetamine (lower-right panel) in high responders (n = 7, solid symbols) and low responders (n = 9, open symbols). Abscissa: SAL represents data obtained when saline was available for infusion, whereas doses refer to the unit dose of each drug available for infusion expressed as mg/kg/infusion on a log scale. Left ordinate: total infusions obtained during the session. Right ordinate: final ratio completed during the session. Error bars represent ±S.E.M. Pound signs indicate statistical significance compared with low responders: #P < 0.05.
Progressive Ratio.
Dose-response curves for MDPV, cocaine, and methamphetamine generated under PR conditions are shown in Fig. 4 (upper-left panel), with ED50 and Emax values provided in Table 1. The Emax values obtained for MDPV were significantly greater than cocaine (P < 0.05) or methamphetamine (P < 0.05), whereas the Emax values obtained for methamphetamine and cocaine were not significantly different. Potency differences were also observed among the drugs, with MDPV being ∼2-fold more potent than methamphetamine and ∼10-fold more potent than cocaine.
TABLE 1.
Measures of potency and reinforcing effectiveness of MDPV, cocaine, and methamphetamine
Asterisk and pound signs indicate statistical significance: *P < 0.05 compared with all other drugs, #P < 0.05 compared with low responders. Group sizes: n = 7, high responders; n = 9, low responders; and n = 16, grouped.
| Parameter | Responder | MDPV | Cocaine | Methamphetamine |
|---|---|---|---|---|
| ED50 (±95% CI) | High | 0.016* (0.01, 0.02) | 0.15* (0.08, 0.23) | 0.036* (0.03, 0.04) |
| Low | 0.019* (0.01, 0.02) | 0.17* (0.14, 0.20) | 0.036* (0.03, 0.04) | |
| Grouped | 0.017* (0.01, 0.02) | 0.16* (0.11, 0.21) | 0.036* (0.03, 0.04) | |
| Emax (±S.E.M.) | High | 31.2# ± 0.7 | 25.0# ± 0.9 | 25.4# ± 0.9 |
| Low | 26.3 ± 1.3 | 20.7 ± 1.4 | 21.2 ± 1.2 | |
| Grouped | 28.1* ± 1.0 | 22.3 ± 1.1 | 22.8 ± 1.0 |
CI, confidence interval.
Although the potency of these drugs to reinforce behavior was not affected by responder phenotype, significant differences in the Emax values were observed between high and low responders for all three drugs (P < 0.05). Although a two-way ANOVA revealed significant main effects of dose for MDPV [F(6,84) = 198.0, P < 0.0001], cocaine [F(5,70) = 144.0, P < 0.0001], and methamphetamine [F(4,56) = 98.3, P < 0.0001], a main effect of responder phenotype was only observed when MDPV was available for infusion [F(1,14) = 9.9, P < 0.01]. Interactions between dose and responder phenotype were observed for MDPV [F(6,84) = 4.1, P < 0.005] and cocaine [F(5,70) = 2.8, P < 0.05], but not methamphetamine. Post hoc tests indicated high responders self-administered more infusions of 0.1–0.32 mg/kg MDPV (P < 0.05) as well as more infusions of 1.0 mg/kg cocaine than low responders (P < 0.05).
Discussion
Synthetic cathinone abuse has increased dramatically over the past decade, and MDPV is one of the most widely abused cathinones in the United States. MDPV is readily self-administered by rats (e.g., Aarde et al., 2013, 2015; Watterson et al., 2014; Schindler et al., 2016), and mounting evidence suggests it is more reinforcing than methamphetamine (Aarde et al., 2013; Watterson et al., 2014); however, direct comparisons of its reinforcing effectiveness to cocaine, the prototypical monoamine transporter inhibitor, have not been made. As such, the present study directly compared the reinforcing effects of MDPV and cocaine using three different endpoints: 1) acquisition of responding for functionally equivalent doses; 2) dose-response curves for MDPV and cocaine under a FR5 schedule of reinforcement with cross substitution; and 3) a within-subject, quantitative analysis of PR dose-response curves for MDPV, cocaine, and methamphetamine. The results of this study not only provide additional evidence that MDPV is a more potent reinforcer, but also provide strong evidence that the relative reinforcing effectiveness of MDPV is significantly greater than cocaine or methamphetamine. Additionally, a subset of rats was identified that self-administered significantly more MDPV than all other rats, and these individual differences were also apparent when other stimulant reinforcers were available under either FR or PR schedules of reinforcement.
When evaluated under acquisition conditions, MDPV (0.032 mg/kg/infusion) and cocaine (0.32 mg/kg/infusion) did not differ with regard to the rate (days to acquire), level (number of infusions), or percentage of rats that acquired responding. These data contrast those of Schindler et al. (2016), who reported MDPV maintained significantly more infusions than cocaine during acquisition; however, because larger unit doses generally maintain lower levels of FR responding, this discrepancy likely resulted from differences in the unit dose of cocaine evaluated in the previous study (0.5 mg/kg/infusion) and the current study (0.32 mg/kg/infusion). Upon transitioning from the FR1 to the FR5 schedule, all cocaine-trained rats exhibited proportional increases in their responding such that consumption of cocaine was similar under FR1 (∼17 mg/kg/d) and FR5 (∼16 mg/kg/d) schedules of reinforcement. Although all MDPV-trained rats (n = 32) responded at rates sufficient to obtain ∼1 mg/kg day when MDPV was available under the FR1 schedule of reinforcement, this level of intake was only maintained in 17 rats after the response requirement was increased to the FR5 schedule. Rather than simply increasing their responding 5-fold to meet the new response requirement, the remaining 15 rats exhibited a disproportionate (∼12-fold) increase in responding, with daily levels of MDPV intake maintained at ∼2.7 mg/kg/d. Such increases in responding are not common when drug reinforcers are evaluated using relatively short sessions and simple FR schedules and suggest there may be something unique about the effects of MDPV in this subset of rats.
Intersubject variability in daily MDPV intake and time to meet stability criteria among these high responders was large; however, all rats met stability criteria before transitioning into subsequent experiments. Interestingly, MDPV intake positively correlated with responding during timeouts. Aarde et al. (2015) previously reported that rats who intermittently engaged in binge-like patterns of MDPV self-administration also made more timeout responses during these brief time periods; however, this observation was limited to a single dose (0.05 mg/kg/infusion MDPV) available under a single schedule of reinforcement (FR1/20-second TO). The present study extends this finding by examining patterns of responding maintained by a full range of MDPV doses, under FR5 (using both single component and multiple components sessions) and PR schedules of reinforcement in an attempt to more fully characterize factors contributing to individual differences in MDPV intake. Although high levels of timeout responding may suggest a loss of stimulus control in these rats, others have alternatively argued that responding during periods of signaled drug unavailability is indicative of a compulsive pattern of responding that may contribute to addiction in humans (Deroche-Gamonet et al., 2004).
Examining the behavior of MDPV-trained rats under a variety of conditions allowed for several important observations. First, while the minimally effective dose of MDPV (0.0032 mg/kg/infusion) was the same for both subsets of MDPV-trained rats under FR5 conditions, high responders exhibited greater rates of responding when a range of MDPV doses (0.0032–0.1 mg/kg/infusion) was available for self-administration compared with low responders. Thus, the observed differences are not the result of differential sensitivities to the reinforcing effects of MDPV but rather reflect vertical differences in the dose-response curves of MDPV between these subgroups of rats. Since the level of responding maintained by drugs under simple FR schedules of reinforcement can be influenced by a number of pharmacokinetic and pharmacodynamic properties, it is difficult to use these schedules to compare drugs with regard to their reinforcing effectiveness.
Full MDPV dose-response curves were therefore generated under a PR schedule of reinforcement that provides a more quantitative approach to assessing reinforcing effectiveness (Richardson and Roberts, 1996). Consistent with data obtained under the FR5 schedule, the potency (ED50) of MDPV to maintain PR responding did not differ between the two subsets of rats; nevertheless, the maximum number of infusions earned (Emax) by high responders was significantly greater than that of low responders, suggesting MDPV is a more effective reinforcer in high-responding relative to low-responding rats. Because drug intake during the early portion of the PR sessions (when response costs are low) may impact responding later in the session (i.e., when measures of effectiveness are determined), it will be important to demonstrate whether a similar result is obtained using behavioral economic procedures (e.g., demand-curve analyses) that have been developed to limit the potential impact of drug intake early in the session (e.g., Hursh and Silberberg, 2008).
To determine whether the differences between high and low responders were specific to behavior maintained by MDPV, a series of within-subject substitution tests were performed under both FR5 and PR schedules of reinforcement. When cross substituted for MDPV under a multiple components FR5 schedule, cocaine maintained significantly higher rates of responding in MDPV-trained high responders than in MDPV-trained low responders or cocaine-trained rats. Similar to what was observed when MDPV was available, when high responders were allowed to self-administer cocaine they made significantly more responses during postinfusion timeouts and intercomponent blackouts relative to MDPV-trained low responders and cocaine-trained rats. Together with the data obtained under standard FR5 conditions, these findings suggest that once established by MDPV self-administration, the high-responder phenotype [e.g., elevated drug intake and high rates of responding during periods of signaled unavailability (i.e., postinfusion timeouts and blackouts)] is persistent across time and transferable to other drug reinforcers. Because the current study was limited to drugs with similar mechanisms of action, whether the high-responder phenotype also transfers when responding is maintained by drugs from different pharmacological classes (e.g., opioids or ethanol) or by nondrug reinforcers will be important to determine.
In summary, acquisition of responding for MDPV (0.032 mg/kg/infusion) and cocaine (0.32 mg/kg/infusion) was comparable with regard to the number of infusions, days to meet acquisition criteria, and the proportion of rats acquiring. Consistent with potency differences reported for MDPV and cocaine based on elicited (i.e., locomotor activity) and operant behaviors (i.e., drug discrimination) in rats and mice (Marusich et al., 2012; Gatch et al., 2013; Collins et al., 2016; Gannon et al., 2016), as well as in vitro measures of inhibition of dopamine uptake (Baumann et al., 2013), MDPV was 10-fold more potent than cocaine at reinforcing responding under FR5 and PR schedules of reinforcement. In direct comparisons of reinforcing effectiveness, rats made ∼3× as many responses to obtain a single infusion of MDPV (final ratio ∼2000) relative to cocaine or methamphetamine (final ratio ∼700). These data are consistent with the literature on human patterns of MDPV use and abuse (Johnson and Johnson, 2014) and suggest MDPV is more reinforcing than two drugs widely abused by humans (cocaine and methamphetamine). Although the precise mechanisms that underlie the differences in the reinforcing effectiveness of these drugs are unclear, one explanation may be related to their selectivity for DAT over SERT [or DAT/SERT ratios; ∼300–800 (MDPV), ∼1.5–3 (cocaine), and ∼10 (methamphetamine)] (Baumann et al., 2013; Simmler et al., 2013). Evidence showing a negative correlation between extracellular serotonin levels and the reinforcing effectiveness of monoamine transporter ligands as measured by PR self-administration (Wee and Woolverton 2006) appears to support this hypothesis; however, that wild-type and SERT knockout mice self-administer cocaine at comparable levels (Thomsen et al., 2009) argues against such a role for serotonin.
The current study provides a comprehensive and direct comparison of the reinforcing effects of MDPV and cocaine and suggests MDPV is a significantly more effective reinforcer than cocaine. Additionally, MDPV appears to be unique in its capacity to establish a behavioral phenotype characterized by high levels of drug intake that are stable across time, apparent across a range of doses, and transferable to other drugs with similar mechanisms of action. Although the factors (e.g., behavioral, pharmacological, genetic, etc.) that contribute to the transition from low to high levels of MDPV self-administration are currently unknown, they may be related to individual differences in drug-taking behavior among human drug abusers.
Abbreviations
- ANOVA
analysis of variance
- CS
conditioned stimulus
- DAT
dopamine transporter
- Emax
maximal effect level
- FR
fixed ratio
- MDPV
3,4-methylenedioxypyrovalerone
- PR
progressive ratio
- SERT
serotonin transporter
- TO
timeout
Authorship Contributions
Participated in research design: Collins.
Conducted experiments: Gannon, Galindo, Collins.
Contributed new reagents or analytic tools: Rice.
Performed data analysis: Gannon, Collins.
Wrote or contributed to the writing of the manuscript: Gannon, Rice, Collins.
Footnotes
This research was supported by the National Institutes of Health National Institute on Drug Abuse [Grants R01DA039146 and T32DA031115]. The work of the Drug Design and Synthesis Section was supported by the Intramural Research Programs of the National Institutes of Health National Institute on Drug Abuse and the National Institutes of Health National Institute of Alcohol Abuse and Alcoholism.
References
- Aarde SM, Huang PK, Creehan KM, Dickerson TJ, Taffe MA. (2013) The novel recreational drug 3,4-methylenedioxypyrovalerone (MDPV) is a potent psychomotor stimulant: self-administration and locomotor activity in rats. Neuropharmacology 71:130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarde SM, Huang PK, Dickerson TJ, Taffe MA. (2015) Binge-like acquisition of 3,4-methylenedioxypyrovalerone (MDPV) self-administration and wheel activity in rats. Psychopharmacology (Berl) 232:1867–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M, Rothman RB, Goldberg SR, Lupica CR, Sitte HH, et al. (2013) Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive ‘bath salts’ products. Neuropsychopharmacology 38:552–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borek HA, Holstege CP. (2012) Hyperthermia and multiorgan failure after abuse of “bath salts” containing 3,4-methylenedioxypyrovalerone. Ann Emerg Med 60:103–105. [DOI] [PubMed] [Google Scholar]
- Collins GT, Abbott M, Galindo K, Rush EL, Rice KC, France CP. (2016) Discriminative stimulus effects of binary drug mixtures: studies with cocaine, MDPV, and caffeine. J Pharmacol Exp Ther 359:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Cunningham AR, Chen J, Wang S, Newman AH, Woods JH. (2012a) Effects of pramipexole on the reinforcing effectiveness of stimuli that were previously paired with cocaine reinforcement in rats. Psychopharmacology (Berl) 219:123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, France CP. (2015) Determinants of conditioned reinforcing effectiveness: dopamine D2-like receptor agonist-stimulated responding for cocaine-associated stimuli. Eur J Pharmacol 769:242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Narasimhan D, Cunningham AR, Zaks ME, Nichols J, Ko MC, Sunahara RK, Woods JH. (2012b) Long-lasting effects of a PEGylated mutant cocaine esterase (CocE) on the reinforcing and discriminative stimulus effects of cocaine in rats. Neuropsychopharmacology 37:1092–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Woods JH. (2007) Drug and reinforcement history as determinants of the response-maintaining effects of quinpirole in the rat. J Pharmacol Exp Ther 323:599–605. [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV. (2004) Evidence for addiction-like behavior in the rat. Science 305:1014–1017. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Gannon BM, Zimmerman SM, Rice KC. (2013) In vivo effects of abused ‘bath salt’ constituent 3,4-methylenedioxypyrovalerone (MDPV) in mice: drug discrimination, thermoregulation, and locomotor activity. Neuropsychopharmacology 38:563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon BM, Williamson A, Suzuki M, Rice KC, Fantegrossi WE. (2016) Stereoselective effects of abused “bath salt” constituent 3,4-methylenedioxypyrovalerone in mice: drug discrimination, locomotor activity, and thermoregulation. J Pharmacol Exp Ther 356:615–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Taylor CM, Forster MJ. (2013) Locomotor stimulant and discriminative stimulus effects of ‘bath salt’ cathinones. Behav Pharmacol 24:437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang PK, Aarde SM, Angrish D, Houseknecht KL, Dickerson TJ, Taffe MA. (2012) Contrasting effects of d-methamphetamine, 3,4-methylenedioxymethamphetamine, 3,4-methylenedioxypyrovalerone, and 4-methylmethcathinone on wheel activity in rats. Drug Alcohol Depend 126:168–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR, Silberberg A. (2008) Economic demand and essential value. Psychol Rev 115:186–198. [DOI] [PubMed] [Google Scholar]
- Johnson PS, Johnson MW. (2014) Investigation of “bath salts” use patterns within an online sample of users in the United States. J Psychoactive Drugs 46:369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyle PB, Iverson RB, Gajagowni RG, Spencer L. (2011) Illicit bath salts: not for bathing. J Miss State Med Assoc 52:375–377. [PubMed] [Google Scholar]
- Marusich JA, Grant KR, Blough BE, Wiley JL. (2012) Effects of synthetic cathinones contained in “bath salts” on motor behavior and a functional observational battery in mice. Neurotoxicology 33:1305–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray BL, Murphy CM, Beuhler MC. (2012) Death following recreational use of designer drug “bath salts” containing 3,4-methylenedioxypyrovalerone (MDPV). J Med Toxicol 8:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council (2011) Guide for the Care and Use of Laboratory Animals, 8th ed, National Academy Press, Washington, DC. [Google Scholar]
- Richardson NR, Roberts DC. (1996) Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods 66:1–11. [DOI] [PubMed] [Google Scholar]
- Schindler CW, Thorndike EB, Goldberg SR, Lehner KR, Cozzi NV, Brandt SD, Baumann MH. (2016) Reinforcing and neurochemical effects of the “bath salts” constituents 3,4-methylenedioxypyrovalerone (MDPV) and 3,4-methylenedioxy-N-methylcathinone (methylone) in male rats. Psychopharmacology (Berl) 233:1981–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seely KA, Patton AL, Moran CL, Womack ML, Prather PL, Fantegrossi WE, Radominska-Pandya A, Endres GW, Channell KB, Smith NH, et al. (2013) Forensic investigation of K2, Spice, and “bath salt” commercial preparations: a three-year study of new designer drug products containing synthetic cannabinoid, stimulant, and hallucinogenic compounds. Forensic Sci Int 233:416–422. [DOI] [PubMed] [Google Scholar]
- Shanks KG, Dahn T, Behonick G, Terrell A. (2012) Analysis of first and second generation legal highs for synthetic cannabinoids and synthetic stimulants by ultra-performance liquid chromatography and time of flight mass spectrometry. J Anal Toxicol 36:360–371. [DOI] [PubMed] [Google Scholar]
- Simmler LD, Buser TA, Donzelli M, Schramm Y, Dieu LH, Huwyler J, Chaboz S, Hoener MC, Liechti ME. (2013) Pharmacological characterization of designer cathinones in vitro. Br J Pharmacol 168:458–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller HA, Ryan ML, Weston RG, Jansen J. (2011) Clinical experience with and analytical confirmation of “bath salts” and “legal highs” (synthetic cathinones) in the United States. Clin Toxicol (Phila) 49:499–505. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Hall FS, Uhl GR, Caine SB. (2009) Dramatically decreased cocaine self-administration in dopamine but not serotonin transporter knockout mice. J Neurosci 29:1087–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime (2014) World Drug Report 2014 (United Nations publication, Sales No. E.14.XI.7).
- Watterson LR, Kufahl PR, Nemirovsky NE, Sewalia K, Grabenauer M, Thomas BF, Marusich JA, Wegner S, Olive MF. (2014) Potent rewarding and reinforcing effects of the synthetic cathinone 3,4-methylenedioxypyrovalerone (MDPV). Addict Biol 19:165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Woolverton WL. (2006) Self-administration of mixtures of fenfluramine and amphetamine by rhesus monkeys. Pharmacol Biochem Behav 84:337–343. [DOI] [PubMed] [Google Scholar]
- Winstock A, Mitcheson L, Ramsey J, Davies S, Puchnarewicz M, Marsden J. (2011) Mephedrone: use, subjective effects and health risks. Addiction 106:1991–1996. [DOI] [PubMed] [Google Scholar]