Abstract
Respiratory depression is the major cause of death in opioid overdose. We have previously shown that prolonged treatment of mice with morphine induces profound tolerance to the respiratory-depressant effects of the drug (Hill et al., 2016). The aim of the present study was to investigate whether tolerance to opioid-induced respiratory depression is mediated by protein kinase C (PKC) and/or c-Jun N-terminal kinase (JNK). We found that although mice treated for up to 6 days with morphine developed tolerance, as measured by the reduced responsiveness to an acute challenge dose of morphine, administration of the brain-penetrant PKC inhibitors tamoxifen and calphostin C restored the ability of acute morphine to produce respiratory depression in morphine-treated mice. Importantly, reversal of opioid tolerance was dependent on the nature of the opioid ligand used to induce tolerance, as these PKC inhibitors did not reverse tolerance induced by prolonged treatment of mice with methadone nor did they reverse the protection to acute morphine-induced respiratory depression afforded by prolonged treatment with buprenorphine. We found no evidence for the involvement of JNK in morphine-induced tolerance to respiratory depression. These results indicate that PKC represents a major mechanism underlying morphine tolerance, that the mechanism of opioid tolerance to respiratory depression is ligand-dependent, and that coadministration of drugs with PKC-inhibitory activity and morphine (as well as heroin, largely metabolized to morphine in the body) may render individuals more susceptible to overdose death by reversing tolerance to the effects of morphine.
Introduction
In mice, prolonged exposure to opioid drugs, such as morphine and methadone, results in the development of tolerance to their respiratory-depressant effects, but the tolerance to respiratory depression develops more slowly than that to antinociception (Hill et al., 2016). We have reported previously that tolerance to the respiratory-depressant effects of morphine could be reversed by acute administration of a low dose of ethanol, whereas that to methadone was unaffected (Hill et al., 2016). This may indicate that different cellular mechanisms underlie the tolerance to these two opioid ligands. In the present study, we have sought to determine the mechanism(s) underlying tolerance to opioid-induced respiratory depression.
Morphine, the prototypic opioid analgesic drug and a major active metabolite of heroin, has relatively low agonist intrinsic efficacy at μ opioid receptor (MOPr) for both G protein activation and arrestin recruitment, but it does not show overt bias for one over the other of these effector pathways relative to most other MOPr agonists (McPherson et al., 2010). Morphine’s agonist efficacy is still sufficient for it to induce both profound analgesia and potentially lethal respiratory depression in humans. We have previously reported that for low intrinsic efficacy agonists such as morphine, MOPr rapid desensitization and tolerance induced in single neurons by prolonged opioid exposure are mediated in large part by protein kinase C (PKC) (Bailey et al., 2004, 2009a,b; Johnson et al., 2006). Levitt and Williams (2012) have suggested that there are two components to the tolerance induced in locus coeruleus neurons following prolonged opioid exposure, a rapidly reversible PKC-mediated component and a slowly reversible component of an as yet unknown mechanism. Tolerance to the antinociceptive actions of morphine is mediated by a PKC-dependent mechanism, probably involving PKC α, γ, and ε isoforms (Smith et al., 2007). In contrast, for high intrinsic efficacy opioid agonists, MOPr desensitization, cellular tolerance, and tolerance to antinociception appear to involve G protein–coupled receptor kinases (GRK) (Terman et al., 2004; Johnson et al., 2006; Bailey et al., 2009a; Hull et al., 2010; Lowe et al., 2015).
In addition to PKC and GRK, other kinases have also been implicated in opioid tolerance (for review, see Williams et al., 2013). The idea of agonist-selective tolerance mechanisms has been extended by the observation that acute antinociceptive tolerance to morphine and buprenorphine in mice can be blocked by the c-Jun N-terminal kinase (JNK) inhibitor, SP600125, whereas that to methadone was insensitive to JNK inhibition (Melief et al., 2010).
In the present experiments, we have used brain-penetrant kinase inhibitors to examine the role of PKC and JNK in tolerance to the respiratory-depressant effects of three opioids that are important with regard to the abuse and maintenance treatment of heroin addiction: morphine, methadone, and buprenorphine. We have examined in detail the effects of tamoxifen, which, in addition to being a selective modulator of estrogen receptors (Alexander et al., 2015a), is also a potent, brain-penetrant inhibitor of PKC (O’Brian et al., 1985; Saraiva et al., 2003; de Medina et al., 2004). We have compared the effect of tamoxifen on opioid-induced tolerance to respiratory depression with that of calphostin C, another brain-penetrant drug that inhibits both conventional and novel isoforms of PKC (Kobayashi et al., 1989). To examine the role of JNK in opioid-induced tolerance to respiratory depression, we have used the JNK inhibitor SP600125 (Bennett et al., 2001).
Materials and Methods
Mice.
Male CD-1 mice (Harlan Laboratories, Bicester, UK) weighing approximately 30 g were maintained at 22°C on a reversed 12-hour dark:light cycle with food and water available ad libitum. All experiments were performed in the dark (active) phase. Mice were randomly ascribed to treatment groups with the experimenter blinded to the drug treatment. All procedures were performed in accordance with the UK Mice (Scientific Procedures) Act 1986, the European Communities Council Directive (2010/63/EU), and the University of Bristol ethical review document.
Measurement of Respiration.
Respiration was measured in freely moving mice using plethysmography chambers (EMKA Technologies, Paris, France) supplied with a 5% CO2 in air mixture (BOC Gas Supplies, Manchester, UK), as described previously (Hill et al., 2016). Rate and depth of respiration were recorded and converted to minute volume. Group mean minute volume data for before and during each drug treatment are given in Table 1. It can be seen from the data in Table 1 that, during the course of this project, the minute volume values obtained for mice before they had received any drug varied from group to group. For this reason, when graphically representing the change in respiration induced by drug treatment, the change in minute volume following acute drug administration was calculated for each mouse as the percentage of a 15-minute predrug baseline, and then the group mean and S.E.M. were calculated.
TABLE 1.
Effect of drug treatments on minute volume in mice breathing 5% CO2 in air
All values are mean ± S.E.M. of 5-minute averages. The predrug baseline values are taken from the 5-minute time bin just preceding the drug injection. Postdrug values are taken from the 15- to 20-minute time bin after the injection.
| Drug Treatment | Predrug Baseline | 15 Minutes Postdrug | n |
|---|---|---|---|
| Minute Volume (ml/min) | Minute Volume (ml/min) | ||
| Saline | 157.3 ± 19.0 | 144.7 ± 13.3 | 6 |
| Morphinea | 229.3 ± 8.8 | 137.8 ± 9.9* | 6 |
| Morphineb | 148.4 ± 7.8 | 90.5 ± 8.5* | 7 |
| Morphinec | 156.4 ± 4.5 | 87.6 ± 5.6* | 7 |
| Tamoxifen | 152.6 ± 25.1 | 144.2 ± 26.8 | 6 |
| Mor pellet—day 6 | 160.5 ± 6.8 | 148.1 ± 9.7 | 6 |
| + morphine | |||
| Mor pellet—day 6 | 141.8 ± 9.7 | 140.8 ± 11.7 | 6 |
| + tamoxifen | |||
| Mor pellet—day 6 | 154.2 ± 12.3 | 96.8 ± 10.0* | 6 |
| + tamoxifen | |||
| + morphine | |||
| Mor pump—day 6 | 134.2 ± 11.8 | 132.3 ± 6.4 | 7 |
| + morphine | |||
| Mor pump—day 6 | 147.2 ± 4.7 | 99.0 ± 3.6* | 7 |
| + tamoxifen | |||
| + morphine | |||
| Meth pump—day 6 | 158.8 ± 7.3 | 147.7 ± 14.1 | 7 |
| + morphine | |||
| Meth pump—day 6 | 143.7 ± 12.8 | 130.8 ± 5.9 | 7 |
| + tamoxifen | |||
| + morphine | |||
| Bup pump—day 6 | 161.0 ± 10.7 | 158.7 ± 13.5 | 7 |
| + morphine | |||
| Bup pump—day 6 | 158.9 ± 14.1 | 162.1 ± 12.2 | 7 |
| + tamoxifen | |||
| + morphine | |||
| Calphostin C | 142.5 ± 18.1 | 145.1 ± 9.8 | 7 |
| SP600125 | 158.9 ± 9.6 | 163.4 ± 11.8 | 7 |
Bup, buprenorphine; Meth, Methadone; Mor, morphine.
Indicate data for the respiratory-depressant effect of morphine in naive mice that were performed on three separate occasions—at the same time as the experiments to study amorphine pellet,/methadone pump/buprenorphine pump, bmorphine pump, and ccalphostin C/SP600125. Unless otherwise stated, there was no significant change from predrug baseline levels. Values were compared using a paired two-way Student’s t test.
Indicates a significant change (P < 0.05) from predrug baseline values.
Measurement of Locomotion.
Locomotor activity was measured using a beam break rig (Linton Instrumentation, Diss, UK) and an automated data logging suite (AMON Lite; Linton Instrumentation), as described previously (Hill et al., 2016).
Induction of Opioid Tolerance.
Morphine tolerance was induced by the following: 1) s.c. implantation of a 75 mg morphine alkaloid pellet on the dorsal flank for 6 days (see Hill et al., 2016); 2) priming with three i.p. injections of 100 mg/kg morphine at 12-hour intervals, followed by s.c. implantation of an osmotic mini-pump (ALZET, Charles River, Margate, UK) containing 56.25 mg/ml morphine (to deliver 45 mg/kg/d) on the dorsal flank for 6 days; or 3) three i.p. injections of 10 mg/kg morphine 4 hours apart. For buprenorphine and methadone, osmotic mini-pumps containing either buprenorphine (6.25 mg/ml to deliver 5 mg/kg/d) or methadone (75 mg/ml to deliver 60 mg/kg/d) were implanted on the dorsal flank (see Hill et al., 2016). To enhance the induction of tolerance to methadone, mice received priming injections of 5 mg/kg and 7.5 mg/kg methadone 12 hours apart on the day prior to, and a priming injection of 7.5 mg/kg methadone on the morning of, pump implantation. Priming doses of buprenorphine were not administered. Implantation of pellets and osmotic mini-pumps was done under isoflurane general anesthesia.
Assessment of Opioid Tolerance.
To assess the level of tolerance induced by the different opioid drug treatment regimens, mice were injected with a challenge dose of morphine (10 mg/kg i.p.), and respiration was monitored for 30 minutes. The degree of respiratory depression induced by the morphine challenge observed in opioid-treated mice was compared with that observed in control mice that were either untreated or had been treated with saline rather than opioid drug and that had been challenged with morphine (10 mg/kg i.p.).
Data Analysis.
Area under the curve was determined using a 100% baseline, as described previously (Hill et al., 2016). Overall changes from a single factor were analyzed using a one-way analysis of variance (ANOVA) with Bonferroni’s post-test. Interaction between prolonged drug treatment and challenge drug was analyzed using a two-way ANOVA in a two-by-two factorial. Changes in groups over time with repeat measurements were analyzed using a two-way repeated-measures ANOVA with Bonferroni’s post-test to analyze drug effect over time. GraphPad Prism 4 was used for all statistical analyses. All data are displayed as mean ± S.E.M.
Drugs and Chemicals.
Buprenorphine hydrochloride (Tocris, Bristol, UK), methadone hydrochloride (Sigma-Aldrich, Irvine, UK), and morphine hydrochloride (Macfarlan Smith, Edinburgh, UK) were dissolved in sterile saline. Calphostin C, G1 [(±)-1-[(3aR*,4S*,9bS*)-4-(6-bromo-1,3-benzodioxol-5-yl)-3a,4,5,9b-tetrahydro-3H-cyclopenta[c]quinolin-8-yl]-ethanone], and SP600125 (anthra[1-9-cd]pyrazol-6(2H)-one) (all from Tocris) were initially dissolved in dimethylsulfoxide and then diluted in sterile saline (final dimethylsulfoxide concentration was 0.1%). Tamoxifen (Sigma-Aldrich) was dissolved in 10% propylene glycol. Morphine alkaloid pellets containing 75 mg morphine base were obtained from the National Institute on Drug Abuse (Bethesda, MD).
Results
Induction of Morphine Tolerance Using Osmotic Mini-Pump Administration.
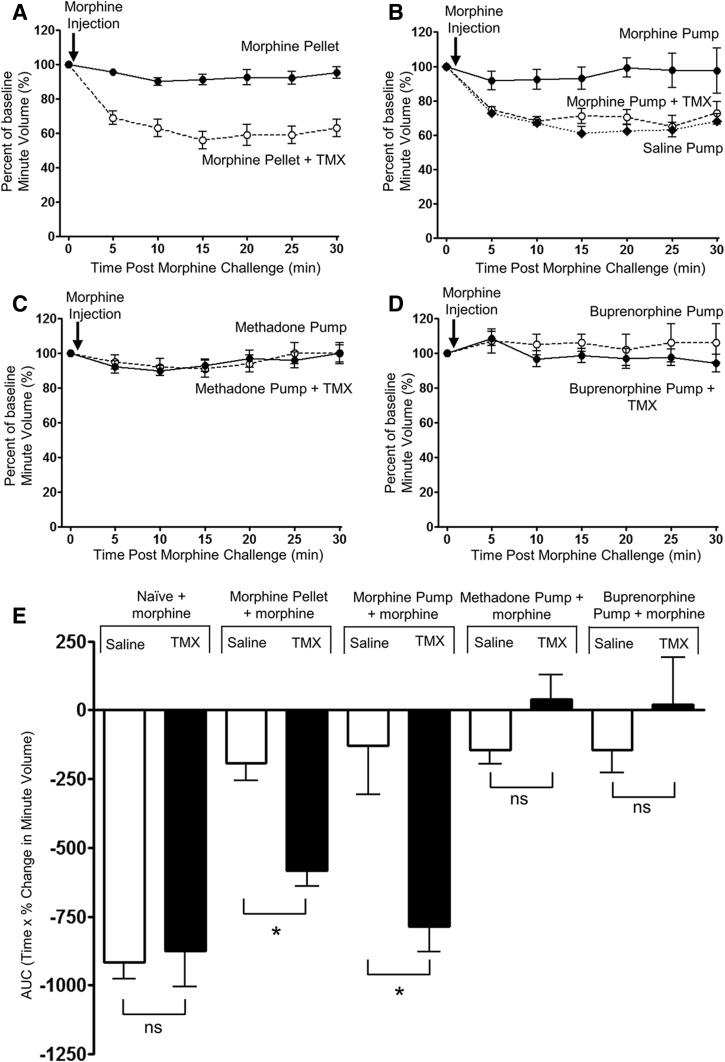
We have previously demonstrated that, in mice implanted with a 75 mg morphine pellet, tolerance develops to the respiratory-depressant effect of morphine over 6 days (Hill et al., 2016). However, the release of morphine from the pellet is not constant over time (Patrick et al., 1975). Furthermore, other opioids such as methadone and buprenorphine are not available in pellet form, although prolonged administration can be achieved using osmotic mini-pumps. We therefore sought to develop a protocol to induce morphine tolerance using osmotic mini-pump administration in order that we could compare the tolerance induced by different opioid drugs administered in the same way. Use of osmotic mini-pumps to administer morphine is somewhat limited by the relative insolubility of the drug. However, we have found that by first giving priming doses of morphine (3 × 100 mg/kg i.p. at 12-hour intervals), followed by implantation of a s.c. morphine pump delivering 45 mg/kg/d for 6 days, then the level of tolerance induced to the respiratory-depressant effect of morphine, measured as the decrease in response to an acute challenge dose of morphine (10 mg/kg i.p.), was comparable to that obtained following morphine pellet implantation (Fig. 1, A, B, and E; Table 1). In saline pump–implanted mice, the response to the acute morphine challenge was the same as in unimplanted mice (compare response to acute morphine challenge in Figs. 1B and 2A).
Fig. 1.
Differential reversal by tamoxifen of the level of tolerance induced by prolonged treatment with either morphine, methadone, or buprenorphine. In all experiments, the level of tolerance was assessed by the response to an acute injection of morphine (10 mg/kg i.p.). In mice that received prolonged treatment with morphine by either implantation of a morphine pellet (A) or implantation of an osmotic mini-pump (B) for 6 days, acute injection of morphine produced significantly greater depression of respiration when the mice had been administered tamoxifen (TMX; 0.6 mg/kg i.p., 30 minutes prior to the morphine challenge) than in mice that had not received tamoxifen. In mice that had received either prolonged methadone (C) or buprenorphine (D) treatment by implantation of an osmotic mini-pump for 6 days, treatment with TMX (0.6 mg/kg i.p., 30 minutes prior to the morphine challenge) did not alter the response to an acute injection of morphine. (E) The area under the curve (AUC) for the percentage change in minute volume has been determined for each individual animal in (A–D) and Fig. 2A before the mean AUC has been calculated and compared with that observed in mice that had not received prolonged opioid treatment. Data are expressed as mean ± S.E.M. and were analyzed using two-way ANOVA and Bonferroni post hoc test. *P < 0.05; n = 6 for morphine pellet experiments; and n = 7 for opioid pump experiments.
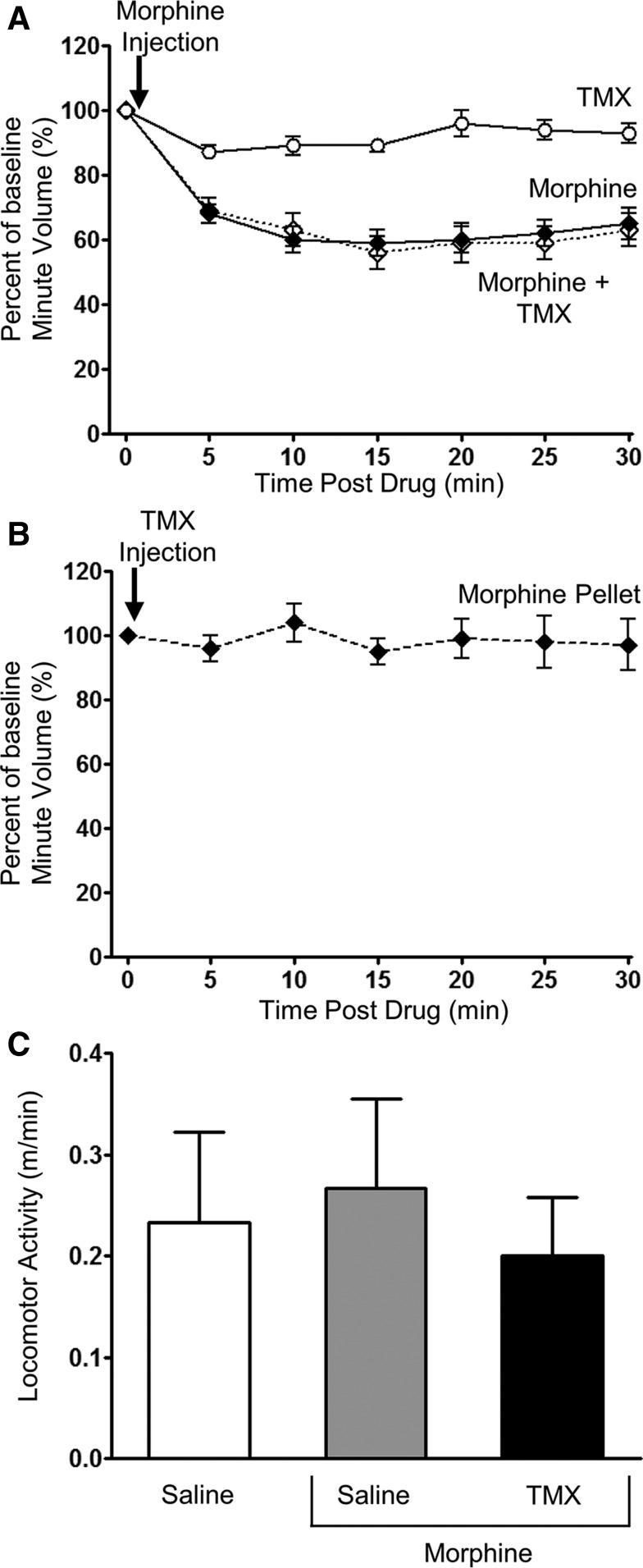
Fig. 2.
Lack of effect of tamoxifen on respiration and locomotor activity. (A) In naive mice, tamoxifen (TMX; 0.6 mg/kg i.p.) alone did not depress respiration, nor did it alter the degree of respiratory depression induced by morphine (10 mg/kg i.p.) when administered 30 minutes before in the opposite side of the peritoneal cavity. (B) TMX (0.6 mg/kg i.p.) did not depress respiration in mice that had received prolonged treatment with morphine by implantation of a morphine pellet for 6 days. (C) There was no observed change in locomotor activity in mice that had received prolonged treatment with morphine by implantation of a morphine pellet for 6 days and then received either saline or TMX (0.6 mg/kg i.p.) and 30 minutes later received saline or morphine (10 mg/kg i.p.). Locomotor activity was monitored for 30 minutes following morphine injection. Data are expressed as mean ± S.E.M. and were compared using one-way ANOVA and Bonferroni post hoc test. n = 6 for all groups.
Reversal of Morphine Tolerance by Tamoxifen.
In control mice, tamoxifen (0.6 mg/kg i.p.) alone did not alter respiration, nor did it exert any effect on the depression of respiration produced by an acute challenge dose of morphine (10 mg/kg i.p.) (Figs. 1E and 2A; Table 1). When administered to mice that had received 6-day treatment with morphine, tamoxifen (0.6 mg/kg i.p.) alone had no effect on respiration (Fig. 2B; Table 1). However, in the prolonged morphine-treated mice that had received tamoxifen 30 minutes previously, a subsequent acute challenge with morphine (10 mg/kg) resulted in significant depression of respiration, whereas in those mice that had not received tamoxifen, the acute challenge with morphine produced little respiratory depression (Fig. 1, A, B, and E; Table 1). To exclude the possibility that changes in respiration were being affected by changes in locomotor activity, we monitored the locomotor activity of prolonged morphine-treated mice following morphine challenge with and without tamoxifen. There was no change in locomotor activity in prolonged morphine-treated mice that received either morphine (10 mg/kg i.p.) or morphine (10 mg/kg i.p.) plus tamoxifen (0.6 mg/kg i.p.) compared with saline-injected controls (Fig. 2C). These results are consistent with tamoxifen reversing morphine tolerance in that tamoxifen-treated mice showed significantly greater respiratory depression in response to the acute challenge with morphine.
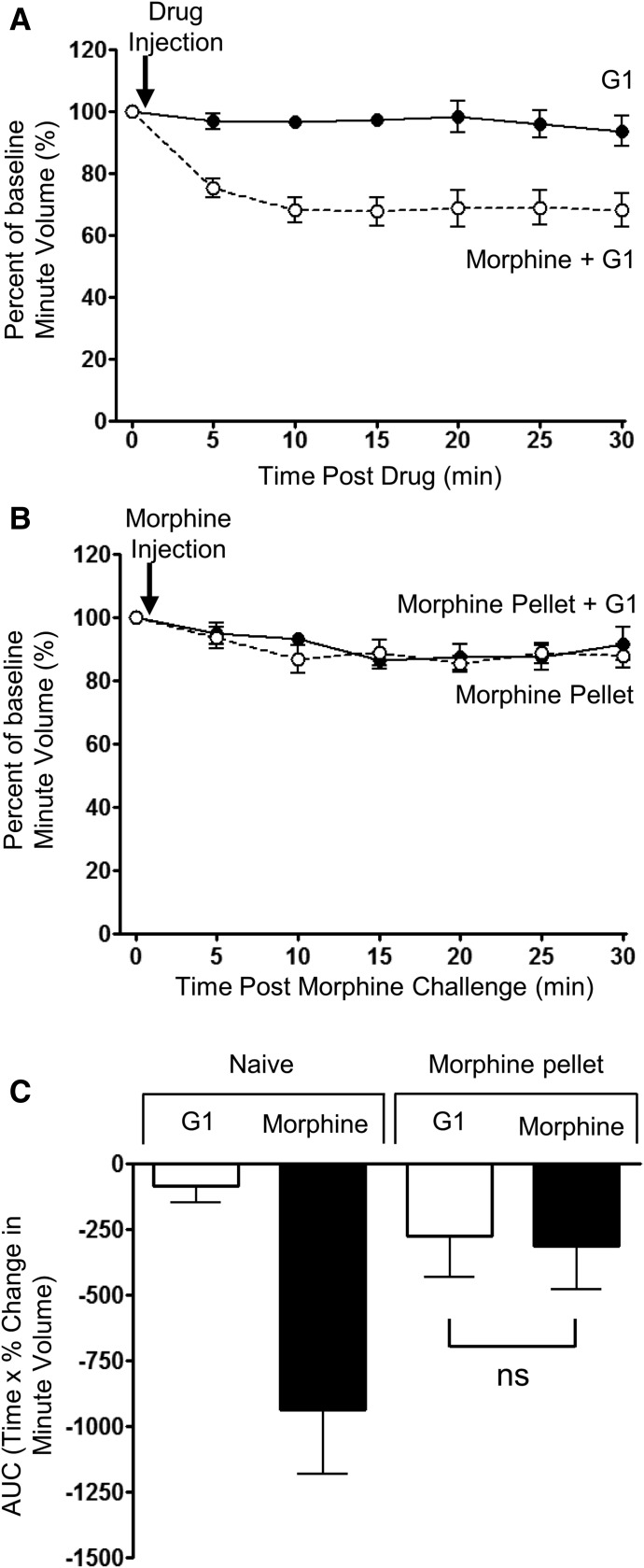
In addition to being an antagonist at nuclear estrogen receptors and a PKC inhibitor, tamoxifen also exhibits agonist activity at the G protein–coupled estrogen receptor (GPER), which is a non-nuclear receptor that localizes to the cell surface and endoplasmic reticulum (Alexander et al., 2015b). To exclude that tamoxifen reversed morphine tolerance through activation of GPER, we examined the effects of another GPER agonist, G1 (Bologa et al., 2006). G1 (0.2 mg/kg i.p.) was injected into control and prolonged morphine-treated mice, 30 minutes before an acute morphine challenge. G1 alone had no effect on respiration (Fig. 3A) and had no effect on the respiratory depression induced by the acute morphine challenge in control mice (compare depression of respiration following morphine challenge in Figs. 2A and 3A). Furthermore, G1 did not significantly alter the level of tolerance to respiratory depression in prolonged morphine-treated mice (Fig. 3, B and C). Therefore, the actions of tamoxifen to reverse morphine tolerance do not appear to be mediated through activation of GPER, as similar reversal of tolerance was not observed with G1.
Fig. 3.
Lack of effect of G1, a GPER agonist, on tolerance to respiratory depression induced by prolonged morphine treatment. (A) G1 (0.2 mg/kg i.p.) alone did not depress respiration, nor when administered 30 minutes prior did it alter the degree of respiratory depression induced by morphine (10 mg/kg i.p.). (B) In mice that had received prolonged morphine treatment by implantation of morphine pellet for 6 days, administration of G1 (0.2 mg/kg i.p.) did not alter the response to an acute injection of morphine. (C) The area under the curve (AUC) for the percentage change in minute volume has been determined for each individual animal in (A) and (B) before the mean AUC has been calculated. Data are expressed as mean ± S.E.M. and were compared using two-way ANOVA and Bonferroni post hoc test. n = 6 for all groups.
Lack of Effect of Tamoxifen in Mice Receiving Prolonged Treatment with Methadone or Buprenorphine.
We have previously reported that ethanol can reverse the tolerance to respiratory depression induced by prolonged morphine treatment, but not tolerance induced by prolonged methadone treatment, nor the blockade of morphine-induced respiratory depression produced by prolonged treatment with buprenorphine (Hill et al., 2016). We have now examined whether tamoxifen could reverse the effects of prolonged methadone or buprenorphine treatment. Mice were treated with methadone for 6 days, as described in Materials and Methods. The level of tolerance induced by prolonged methadone treatment was assessed by the reduction in the response to an acute morphine (10 mg/kg i.p.) challenge (Fig. 1, C and E; Table 1). In prolonged methadone-treated mice, the effect of acute morphine challenge was significantly reduced over that observed in control mice. However, treatment of mice with tamoxifen (0.6 mg/kg i.p.) 30 minutes prior to the acute morphine challenge failed to reverse the tolerance induced by prolonged methadone treatment. In mice that had been treated with buprenorphine for 6 days, the response to an acute morphine challenge was significantly reduced over that observed in control mice. As buprenorphine is a MOPr partial agonist that dissociates slowly from the receptors, we cannot distinguish between buprenorphine’s antagonist activity occluding the effect of the morphine challenge and the development of tolerance. In prolonged buprenorphine-treated mice, treatment with tamoxifen (0.6 mg/kg i.p.) 30 minutes prior to the acute morphine challenge failed to modify the reduced response to the acute morphine challenge (Fig. 1, D and E; Table 1).
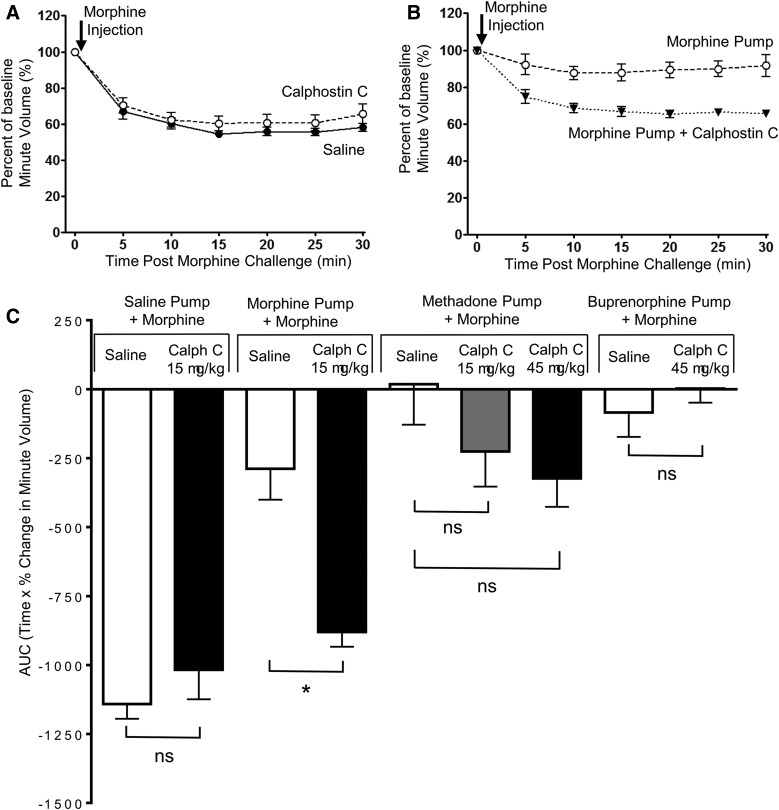
Reversal by Calphostin C of Tolerance Induced by Morphine but Not That Induced by Methadone or the Protection Afforded by Buprenorphine.
The brain-penetrant PKC inhibitor calphostin C (15 µg/kg i.p.) by itself had no direct effect on respiration in naive mice (Table 1), nor when given 30 minutes prior did it alter the acute respiratory-depressant effect of morphine (10 mg/kg) (Fig. 4, A and C). However, in mice rendered tolerant by prolonged morphine treatment, an acute injection of calphostin C (15 µg/kg i.p.) 30 minutes prior to a subsequent acute challenge with morphine (10 mg/kg) resulted in significant depression of respiration, whereas in those mice that had not received calphostin C, the acute challenge with morphine produced little respiratory depression (Fig. 4, B and C). In mice rendered tolerant to methadone, an acute injection of calphostin C (15 µg/kg i.p.) 30 minutes prior to the acute challenge with morphine (10 mg/kg) did not result in a statistically significant (P = 0.6) increase in depression of respiration (Fig. 4C). To examine whether reversal of methadone tolerance required greater inhibition of PKC, we increased the dose of calphostin threefold to 45 µg/kg, but this did not result in any greater increase in the depression of respiration over that seen with 15 µg/kg (Fig. 4C). However, from our data we cannot completely exclude the possibility that there is a small PKC component of methadone tolerance that our experiments were not powered to observe. In mice pretreated with buprenorphine, administration of calphostin C (15 or 45 µg/kg i.p.) 30 minutes prior to the acute morphine challenge failed to modify the reduced response to the acute morphine challenge (Fig. 4C). These results are consistent with calphostin C reversing tolerance induced by morphine but not tolerance induced by methadone or the protection afforded by prolonged buprenorphine treatment.
Fig. 4.
Reversal by calphostin C of tolerance to respiratory depression induced by prolonged morphine treatment, but not by prolonged methadone or buprenorphine treatment. (A) Calphostin C (15 μg/kg i.p.) administered 30 minutes previously did not alter the degree of respiratory depression induced by morphine (10 mg/kg i.p.) in naive mice. (B) In mice that had received prolonged morphine, treatment administration of calphostin C (15 μg/kg i.p.) enhanced the response to an acute injection of morphine. (C) The area under the curve (AUC) for the percentage change in minute volume has been determined for animals implanted with saline, morphine, methadone, or buprenorphine pumps and administered calphostin C (15 or 45 μg/kg i.p.) 30 minutes before morphine challenge. Data are expressed as mean ± S.E.M. and were compared using a two-way ANOVA and Bonferroni post hoc test comparing with saline controls. Calph C, calphostin C. *P < 0.05; n = 7 for all groups.
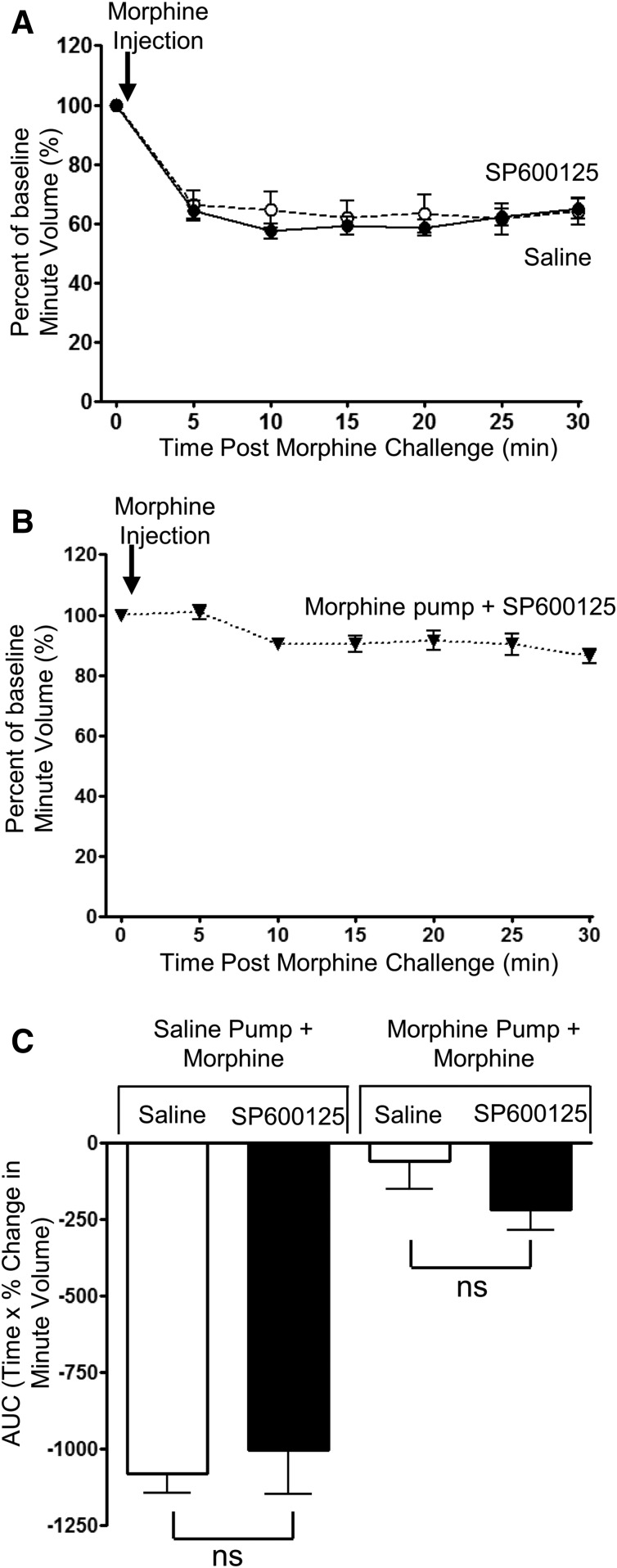
Lack of Morphine Tolerance Reversal by a JNK Inhibitor.
The JNK inhibitor SP600125 (40 mg/kg i.p.) by itself had no direct effect on respiration in naive mice (Table 1), nor when given 30 minutes prior did it alter the acute respiratory-depressant effect of morphine (10 mg/kg) (Fig. 5, A and C). Furthermore, in mice rendered tolerant to morphine, an acute injection of SP600125 (40 mg/kg i.p.) 30 minutes prior to an acute challenge with morphine (10 mg/kg) did not alter the attenuated response to morphine challenge, demonstrating that SP600125 did not reduce the level of tolerance (Fig. 5, B and C).
Fig. 5.
Lack of effect of SP600125, a JNK inhibitor, on tolerance to respiratory depression induced by prolonged morphine treatment. (A) SP600125 (40 mg/kg i.p.) administered 30 minutes previously did not alter the degree of respiratory depression induced by morphine (10 mg/kg i.p.). (B) In mice that had received prolonged morphine, treatment administration of SP600125 (40 mg/kg i.p.) did not enhance the response to an acute injection of morphine. (C) The area under the curve (AUC) for the percentage change in minute volume has been determined for each individual animal in (A) and (B) before the mean AUC has been calculated. Data are expressed as mean ± S.E.M. and were compared using a two-way ANOVA and Bonferroni post hoc test. n = 7 for all groups.
Discussion
In this study, we show that tolerance to the respiratory-depressant effect of morphine involves a PKC-dependent mechanism, as morphine tolerance is reversed by two inhibitors of PKC, tamoxifen and calphostin C. These inhibitors reversed tolerance that had been induced over a prolonged period, indicating that ongoing PKC activity is required to maintain morphine tolerance. Given that tolerance to the analgesic effect of morphine has also been shown by us and others (Smith et al., 2007; Hull et al., 2010) to be PKC-dependent, then it appears likely that PKC activation represents a major mechanism underlying morphine tolerance in the brain.
Neither tamoxifen nor calphostin C depressed respiration in prolonged morphine-treated mice as might be expected if they reversed tolerance and thus revealed a respiratory-depressant effect of the morphine still present in the brain at that time. We have previously observed the same lack of effect with ethanol (Hill et al., 2016). At present, we do not have a definitive explanation for this, but we suggest that, perhaps with long-term exposure, a significant amount of morphine is sequestered in brain tissue, such as membrane lipid, and therefore is not available for receptor activation.
Although tamoxifen is better known as an estrogen receptor modulator used in the early treatment of breast cancer (Early Breast Cancer Trialists' Collaborative Group, 1998), it can also block PKC activity (O’Brian et al., 1985; Saraiva et al., 2003; de Medina et al., 2004). The importance of our findings with tamoxifen resides in the possibility of being able to use this drug as a relatively safe and brain-penetrant PKC inhibitor to explore mechanisms underlying opioid tolerance in the intact organism, including humans.
In mice morphine-induced respiratory depression is mediated by activation of MOPr, as it is absent in MOPr knockout mice (Romberg et al., 2003). On prolonged agonist exposure, neuronal MOPr desensitize, and this desensitization contributes to the loss of responsiveness, that is, tolerance (Bailey et al., 2009b; Levitt and Williams 2012). The mechanisms underlying MOPr desensitization are agonist-specific (Johnson et al., 2006; Bailey et al., 2009a; Williams et al., 2013). Desensitization induced by morphine, a relatively low-efficacy opioid agonist, is primarily mediated by PKC (Bailey et al., 2006). However, for high-efficacy agonists, a significant proportion of MOPr desensitization involves receptor phosphorylation by GRK2/3 and arrestin binding (Lowe et al., 2015). We and others have also shown that tolerance to the antinociceptive effects of morphine is mediated in large part by PKC (Inoue and Ueda, 2000; Bohn et al., 2002; Smith et al., 2002, 2007; Hull et al., 2010), but that tolerance to high-efficacy agonists is mediated by GRKs (Terman et al., 2004; Hull et al., 2010); however, this does not definitively prove that MOPr desensitization contributes to in vivo tolerance, but only that the same kinases are involved in both phenomena. Our observation of a differential effect of PKC inhibition on tolerance to respiratory depression induced by prolonged morphine and methadone treatment further illustrates that the mechanisms of opioid tolerance, like those of MOPr desensitization, are agonist-specific.
Respiration is controlled by a complex pontine-medullary network. Within this network, there are several sites at which opioids act to depress respiration, including the pre-Bötzinger complex, the Kölliker–Fuse nucleus, and the recently described postinhibitory complex (Anderson et al., 2016). In this study, we have not sought to investigate the specific isoforms of PKC that are involved in tolerance to morphine depression of respiration, as it is unlikely to be only one isoform, as previously reported for antinociception tolerance (Smith et al., 2007), nor have we sought to define the anatomic site of tolerance development. Tolerance to the antinociceptive effects of morphine has been reported to involve PKCα and PKCγ, with some contribution from PKCε (Smith et al., 2007). That tolerance to the respiratory-depressant effect of morphine develops more slowly than that to its antinociceptive actions (Hill et al., 2016) may reflect either that there is a larger MOPr reserve that needs to be inactivated to observe tolerance to respiratory depression or that there are lower levels of PKC activity in neurons that control respiration. Indeed, expression of constitutively active PKCα or PKCε in the pre-Bötzinger complex, neurons involved in the generation of respiratory rhythm, increased the development of tolerance to respiratory depression induced by daily doses of morphine, an effect that afforded increased protection from death by overdose (Lin et al., 2012). However, in the same study, small interfering RNA knockdown of PKCα and PKCε in pre-Bötzinger complex neurons did not inhibit the development of morphine tolerance, which may indicate either that other PKC isoforms may be involved in tolerance development in pre-Bötzinger neurones and/or the effect of PKC may occur in other neurons that control respiration other than those in the pre-Bötzinger complex.
The precise mechanism underlying PKC-dependent desensitization of MOPr responsiveness is currently unknown, but PKC has clearly been shown to be activated following addition of morphine to cells and neurons expressing MOPr (Chu et al., 2010; Halls et al., 2016), and PKC activity may also be raised in vivo by concomitant activation of other G protein-coupled receptors that couple through Gq (Bailey et al., 2009a) or N-methyl-D-aspartate receptors to elevate intracellular calcium (Trujillo and Akil, 1995). The results also suggest that our previous finding that ethanol reverses morphine tolerance (Hill et al., 2016) could be mediated by ethanol or its metabolites somehow reversing the PKC-dependent mechanism. However, the molecular details of this, if it is indeed the case, remain to be worked out. Apart from PKC, it has been suggested that JNK2 plays a role in acute antinociception tolerance to morphine (Melief et al., 2010; Kuhar et al., 2015). However, using the JNK inhibitor SP600125, we and others have been unable to observe any involvement of JNK isoforms in MOPr desensitization and morphine-induced cellular tolerance in locus coeruleus neurons (Levitt and Williams 2012; Lowe et al., 2015) or, as reported in this work, in the maintenance of tolerance to morphine-induced respiratory depression. The involvement of JNK in morphine tolerance may therefore be response-dependent.
In summary, we show that tolerance to morphine-induced respiratory depression can be reversed by inhibition of PKC. However, this effect is agonist-dependent, as methadone-induced respiratory depression is not reversed by PKC inhibition, underlining the agonist-dependent nature of MOPr tolerance (Bailey et al., 2006).
Implications.
Drugs that block PKC may enhance the possibility of opioid overdose causing death, due to reversal of morphine tolerance to respiratory depression. In addition, PKC inhibition may prolong the analgesic effects of morphine.
Abbreviations
- ANOVA
analysis of variance
- GPER
G protein–coupled estrogen receptor
- GRK
G protein–coupled receptor kinase
- JNK
c-Jun N-terminal kinase
- MOPr
μ opioid receptor
- PKC
protein kinase C
Authorship Contributions
Participated in research design: Withey, Hill, Dewey, Kelly, Henderson.
Conducted experiments: Withey, Hill, Lyndon.
Performed data analysis: Withey, Hill, Lyndon.
Wrote or contributed to the writing of the manuscript: Withey, Hill, Kelly, Henderson.
Footnotes
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grant RO1036975-01].
References
- Alexander SP, Cidlowski JA, Kelly E, Marrion N, Peters JA, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Southan C, et al. (2015a) The Concise Guide to PHARMACOLOGY 2015/16: nuclear hormone receptors. Br J Pharmacol 172:5956–5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Southan C, et al. (2015b) The Concise Guide to PHARMACOLOGY 2015/16: G protein-coupled receptors. Br J Pharmacol 172:5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson TM, Garcia AJ, Baertsch NA, Pollak J, Bloom JC, Wei AD, Rai KG, Ramirez JM. (2016) A novel excitatory network for the control of breathing. Nature 536:76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CP, Kelly E, Henderson G. (2004) Protein kinase C activation enhances morphine-induced rapid desensitization of μ-opioid receptors in mature rat locus ceruleus neurons. Mol Pharmacol 66:1592–1598. [DOI] [PubMed] [Google Scholar]
- Bailey CP, Llorente J, Gabra BH, Smith FL, Dewey WL, Kelly E, Henderson G. (2009b) Role of protein kinase C and μ-opioid receptor (MOPr) desensitization in tolerance to morphine in rat locus coeruleus neurons. Eur J Neurosci 29:307–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CP, Oldfield S, Llorente J, Caunt CJ, Teschemacher AG, Roberts L, McArdle CA, Smith FL, Dewey WL, Kelly E, et al. (2009a) Involvement of PKC alpha and G-protein-coupled receptor kinase 2 in agonist-selective desensitization of μ-opioid receptors in mature brain neurons. Br J Pharmacol 158:157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CP, Smith FL, Kelly E, Dewey WL, Henderson G. (2006) How important is protein kinase C in μ-opioid receptor desensitization and morphine tolerance? Trends Pharmacol Sci 27:558–565. [DOI] [PubMed] [Google Scholar]
- Bennett BL, Sasaki DT, Murray BW, O’Leary EC, Sakata ST, Xu W, Leisten JC, Motiwala A, Pierce S, Satoh Y, et al. (2001) SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci USA 98:13681–13686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn LM, Lefkowitz RJ, Caron MG. (2002) Differential mechanisms of morphine antinociceptive tolerance revealed in β arrestin-2 knock-out mice. J Neurosci 22:10494–10500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bologa CG, Revankar CM, Young SM, Edwards BS, Arterburn JB, Kiselyov AS, Parker MA, Tkachenko SE, Savchuck NP, Sklar LA, et al. (2006) Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol 2:207–212. [DOI] [PubMed] [Google Scholar]
- Chu J, Zheng H, Zhang Y, Loh HH, Law PY. (2010) Agonist-dependent μ-opioid receptor signaling can lead to heterologous desensitization. Cell Signal 22:684–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Médina P, Favre G, Poirot M. (2004) Multiple targeting by the antitumor drug tamoxifen: a structure-activity study. Curr Med Chem Anticancer Agents 4:491–508. [DOI] [PubMed] [Google Scholar]
- Early Breast Cancer Trialists’ Collaborative Group (1998) Tamoxifen for early breast cancer: an overview of the randomised trials: Early Breast Cancer Trialists’ Collaborative Group. Lancet 351:1451–1467. [PubMed] [Google Scholar]
- Halls ML, Yeatman HR, Nowell CJ, Thompson GL, Gondin AB, Civciristov S, Bunnett NW, Lambert NA, Poole DP, Canals M. (2016) Plasma membrane localization of the μ-opioid receptor controls spatiotemporal signaling. Sci Signal 9:ra16. [DOI] [PubMed] [Google Scholar]
- Hill R, Lyndon A, Withey S, Roberts J, Kershaw Y, MacLachlan J, Lingford-Hughes A, Kelly E, Bailey C, Hickman M, et al. (2016) Ethanol reversal of tolerance to the respiratory depressant effects of morphine. Neuropsychopharmacology 41:762–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull LC, Llorente J, Gabra BH, Smith FL, Kelly E, Bailey C, Henderson G, Dewey WL. (2010) The effect of protein kinase C and G protein-coupled receptor kinase inhibition on tolerance induced by μ-opioid agonists of different efficacy. J Pharmacol Exp Ther 332:1127–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Ueda H. (2000) Protein kinase C-mediated acute tolerance to peripheral μ-opioid analgesia in the bradykinin-nociception test in mice. J Pharmacol Exp Ther 293:662–669. [PubMed] [Google Scholar]
- Johnson EA, Oldfield S, Braksator E, Gonzalez-Cuello A, Couch D, Hall KJ, Mundell SJ, Bailey CP, Kelly E, Henderson G. (2006) Agonist-selective mechanisms of μ-opioid receptor desensitization in human embryonic kidney 293 cells. Mol Pharmacol 70:676–685. [DOI] [PubMed] [Google Scholar]
- Kobayashi E, Ando K, Nakano H, Iida T, Ohno H, Morimoto M, Tamaoki T. (1989) Calphostins (UCN-1028), novel and specific inhibitors of protein kinase C. I. Fermentation, isolation, physico-chemical properties and biological activities. J Antibiot 42:1470–1474. [DOI] [PubMed] [Google Scholar]
- Kuhar JR, Bedini A, Melief EJ, Chiu YC, Striegel HN, Chavkin C. (2015) μ opioid receptor stimulation activates c-Jun N-terminal kinase 2 by distinct arrestin-dependent and independent mechanisms. Cell Signal 27:1799–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt ES, Williams JT. (2012) Morphine desensitization and cellular tolerance are distinguished in rat locus ceruleus neurons. Mol Pharmacol 82:983–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HY, Law PY, Loh HH. (2012) Activation of protein kinase C (PKC)α or PKCε as an approach to increase morphine tolerance in respiratory depression and lethal overdose. J Pharmacol Exp Ther 341:115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe JD, Sanderson HS, Cooke AE, Ostovar M, Tsisanova E, Withey SL, Chavkin C, Husbands SM, Kelly E, Henderson G, et al. (2015) Role of G protein-coupled receptor kinases 2 and 3 in μ-opioid receptor desensitization and internalization. Mol Pharmacol 88:347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson J, Rivero G, Baptist M, Llorente J, Al-Sabah S, Krasel C, Dewey WL, Bailey CP, Rosethorne EM, Charlton SJ, et al. (2010) μ-opioid receptors: correlation of agonist efficacy for signalling with ability to activate internalization. Mol Pharmacol 78:756–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melief EJ, Miyatake M, Bruchas MR, Chavkin C. (2010) Ligand-directed c-Jun N-terminal kinase activation disrupts opioid receptor signaling. Proc Natl Acad Sci USA 107:11608–11613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brian CA, Liskamp RM, Solomon DH, Weinstein IB. (1985) Inhibition of protein kinase C by tamoxifen. Cancer Res 45:2462–2465. [PubMed] [Google Scholar]
- Patrick GA, Dewey WL, Spaulding TC, Harris LS. (1975) Relationship of brain morphine levels to analgesic activity in acutely treated mice and rats and in pellet implanted mice. J Pharmacol Exp Ther 193:876–883. [PubMed] [Google Scholar]
- Romberg R, Sarton E, Teppema L, Matthes HW, Kieffer BL, Dahan A. (2003) Comparison of morphine-6-glucuronide and morphine on respiratory depressant and antinociceptive responses in wild type and μ-opioid receptor deficient mice. Br J Anaesth 91:862–870. [DOI] [PubMed] [Google Scholar]
- Saraiva L, Fresco P, Pinto E, Gonçalves J. (2003) Isoform-selectivity of PKC inhibitors acting at the regulatory and catalytic domain of mammalian PKC-alpha, -betaI, -delta, -eta and -zeta. J Enzyme Inhib Med Chem 18:475–483. [DOI] [PubMed] [Google Scholar]
- Smith FL, Gabra BH, Smith PA, Redwood MC, Dewey WL. (2007) Determination of the role of conventional, novel and atypical PKC isoforms in the expression of morphine tolerance in mice. Pain 127:129–139. [DOI] [PubMed] [Google Scholar]
- Smith FL, Javed R, Elzey MJ, Welch SP, Selley D, Sim-Selley L, Dewey WL. (2002) Prolonged reversal of morphine tolerance with no reversal of dependence by protein kinase C inhibitors. Brain Res 958:28–35. [DOI] [PubMed] [Google Scholar]
- Terman GW, Jin W, Cheong YP, Lowe J, Caron MG, Lefkowitz RJ, Chavkin C. (2004) G-protein receptor kinase 3 (GRK3) influences opioid analgesic tolerance but not opioid withdrawal. Br J Pharmacol 141:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo KA, Akil H. (1995) Excitatory amino acids and drugs of abuse: a role for N-methyl-D-aspartate receptors in drug tolerance, sensitization and physical dependence. Drug Alcohol Depend 38:139–154. [DOI] [PubMed] [Google Scholar]
- Williams JT, Ingram SL, Henderson G, Chavkin C, von Zastrow M, Schulz S, Koch T, Evans CJ, Christie MJ. (2013) Regulation of μ-opioid receptors: desensitization, phosphorylation, internalization, and tolerance. Pharmacol Rev 65:223–254. [DOI] [PMC free article] [PubMed] [Google Scholar]