Abstract
Xenin-25, a neurotensin (NT)-related anorexigenic gut hormone generated mostly in the duodenal mucosa, is believed to increase the rate of duodenal ion secretion, because xenin-induced diarrhea is not present after Roux-en-Y gastric bypass surgery. Because the local effects of xenin on duodenal ion secretion have remained uninvestigated, we thus examined the neural pathways underlying xenin-induced duodenal anion secretion. Intravenous infusion of xenin-8, a bioactive C-terminal fragment of xenin-25, dose dependently increased the rate of duodenal HCO3− secretion in perfused duodenal loops of anesthetized rats. Xenin was immunolocalized to a subset of enteroendocrine cells in the rat duodenum. The mRNA of the xenin/NT receptor 1 (NTS1) was predominantly expressed in the enteric plexus, nodose and dorsal root ganglia, and in the lamina propria rather than in the epithelium. The serosal application of xenin-8 or xenin-25 rapidly and transiently increased short-circuit current in Ussing-chambered mucosa-submucosa preparations in a concentration-dependent manner in the duodenum and jejunum, but less so in the ileum and colon. The selective antagonist for NTS1, substance P (SP) receptor (NK1), or 5-hydroxytryptamine (5-HT)3, but not NTS2, inhibited the responses to xenin. Xenin-evoked Cl- secretion was reduced by tetrodotoxin (TTX) or capsaicin-pretreatment, and abolished by the inhibitor of TTX-resistant sodium channel Nav1.8 in combination with TTX, suggesting that peripheral xenin augments duodenal HCO3− and Cl− secretion through NTS1 activation on intrinsic and extrinsic afferent nerves, followed by release of SP and 5-HT. Afferent nerve activation by postprandial, peripherally released xenin may account for its secretory effects in the duodenum.
Introduction
The duodenal mucosa, because of its strategic location between the stomach and the rest of the small intestine, senses luminal nutrients, affects appetite, and regulates duodenal anion secretion, particularly HCO3− secretion, which in turn is important for nutrient absorption and mucosal protection from gastric acid (Kaji et al., 2013). We have been investigating the contributions of enteroendocrine cells (EECs) and afferent sensory nerves that link the duodenal mucosa with mucosal defense mechanisms (Akiba and Kaunitz, 2011; Said et al., 2015). As part of this quest, we hypothesized that xenin, a homolog of amphibian xenopsin and mammalian neurotensin (NT) (Feurle et al., 1992), which is specifically generated in the duodenal mucosa, directly affects duodenal anion secretion.
Xenin as a 25-amino acid peptide is predominantly detected in human gastric and duodenal mucosa in addition to the hypothalamus (Feurle et al., 1992). Some xenin degradation fragments (e.g., xenin 9–25, 11–25, 14–25, or 18–25) are identified in some animal species and an octapeptide xenin-8 (18–25) retains the signal sequence (Hamscher et al., 1995; Martin et al., 2014). The origin of active xenin-25 in the gastric mucosa has been controversial, because xenin can be generated by peptic digestion from its precursor (proxenin), a component of the highly conserved ubiquitous vesicular coat proteins, coatomer protein complex-α (Hamscher et al., 1995). Thus, xenin peptides could be generated in vitro from homogenized gastric mucosa by pepsin (Hamscher et al., 1995).
A specific xenin antibody identified a subset of duodenal EECs that coexpressed xenin with gastric inhibitory peptide (GIP), but not 5-hydroxytryptamine (5-HT) in canine duodenum (Anlauf et al., 2000). Because GIP is released in response to luminal nutrients in the regulation of glucose homeostasis, xenin is thought to also be released from EECs by physiologic stimuli. Indeed, intravenous xenin delays gastric emptying, decreases gastric acid secretion, and potentiates insulin secretion when combined with GIP (Feurle, 1998; Wice et al., 2010). Nevertheless, the effects of xenin in the duodenal mucosa have not been previously investigated.
NT, produced by central nervous system and enteroendocrine N cells, is present predominantly in the lower ileum and released by dietary fat. NT inhibits intestinal transit and food intake (Dumoulin et al., 1998; Cooke et al., 2009) and increases electrogenic Cl− secretion in human colon in vitro (Riegler et al., 2000). The secretory effects of NT were identified in canine duodenum (Konturek et al., 1985), consistent with NT receptor expression in the duodenum (Tanaka et al., 1990). Owing to peptide sequence similarity between NT and the C terminus of xenin and the observation that the NT receptors (NTS) 1 and 2 are activated by xenin with similar affinity to that of NT (Botto et al., 1998), NTS are also thought to be xenin receptors. NTS1 and NTS2 are Gq/11-coupled G protein-coupled receptors abundantly expressed in neural tissues.
Plasma xenin concentrations are increased by food intake and by sham feeding in humans, suggesting that luminal nutrients and vagal activation release xenin from the duodenal mucosa (Feurle et al., 2003). Because nocturnal workers with high energy intake and high body fat mass have no elevation of plasma xenin after meals (Schiavo-Cardozo et al., 2013) and obese adolescents have significantly higher concentrations of plasma xenin between meals compared with healthy controls (Arslan et al., 2014), xenin-induced satiety signals appear to be disrupted in obesity. Peripheral and central injection of xenin decreases food intake in experimental animals (Alexiou et al., 1998; Cooke et al., 2009), consistent with a satiety function of xenin. The infusion of low concentrations (4–12 pmol/kg) of xenin to humans increases satiety and causes mild diarrhea by an unknown mechanism (Chowdhury et al., 2014; Gault et al., 2015). Because Roux-en-Y gastric bypass surgery diminishes xenin-associated diarrhea (Sterl et al., 2016), the duodenum may significantly contribute to the fluid and ion secretory response to xenin. We thus aimed to characterize the secretory effects of xenin in the duodenum and to investigate the neural pathways underlying xenin-induced duodenal anion secretion.
Materials and Methods
Animals.
Male Sprague-Dawley rats weighing 200–250 g (Harlan, San Diego, CA) were fed a pellet diet and water ad libitum. All studies were performed with approval of the Veterans Affairs Institutional Animal Care and Use Committee. Rats were fasted overnight with free access to water before the experiments. Animals were euthanized by terminal exsanguination under deep isoflurane anesthesia, followed by thoracotomy.
Duodenal Loop Perfusion.
Rat duodenal loops were prepared and perfused under isoflurane anesthesia as described previously (Mizumori et al., 2006). In brief, prewarmed saline was infused via the right femoral vein at 1.08 ml/h using a Harvard infusion pump (Harvard Apparatus, Holliston, MA). Under isoflurane anesthesia (2%), a polyethylene tube (diameter 5 mm) was inserted through the forestomach and tied at 0.5 cm caudal from the pyloric ring. Another polyethylene tube was inserted through the distal duodenum and ligated proximal to the ligament of Treitz. To prevent contamination from bile or pancreatic juice, the pancreaticobiliary duct was ligated just proximal to its insertion into the duodenal wall and cannulated with a PE-10 tube to drain the juice. The resultant closed proximal duodenal loop (perfused length ∼2 cm) was perfused with prewarmed saline (pH 7.0) by using a peristaltic pump (Fisher Scientific, Pittsburgh, PA) at 1 ml/min. After stabilization with continuous luminal perfusion of O2-bubbled saline (pH 7.0) for ∼30 minutes, the time was set as t = 0. The duodenal loop was perfused with saline from t = 0 minute until t = 45 minutes. After intravenous infusion of saline for 15 minutes, xenin-8 in saline was continuously infused at 3 or 10 nmol/kg/h from t = 15 minutes to t = 45 minutes. Duodenal HCO3− secretion was measured using flow-through pH and CO2 electrodes and expressed as total CO2 output, calculated from the measured pH and [CO2] in the effluent solution, as reported previously (Mizumori et al., 2006).
Immunofluorescence Staining and Real-Time Polymerase Chain Reaction.
Small pieces of intestine were immersed in Zamboni’s fixative containing 2% paraformaldehyde and 0.2% picric acid in 0.1 M phosphate buffer (pH 7.4) overnight for 4°C. The fixed tissues were then submerged in 20% sucrose in phosphate-buffered saline (PBS, pH 7.4) overnight at 4°C, and embedded in optimum cutting temperature (OCT) compound. Frozen sections of 8-μm thickness were placed on aminosilane-coated glass slides (Matsunami Glass USA Inc., Bellingham, WA). Sections were pretreated with 5% normal donkey serum in PBS containing 0.3% Triton X-100 for 1 hour, followed by incubation with primary antibodies: rabbit anti-xenin-25 (Phoenix Pharmaceuticals, Belmont, CA; 1 µg/ml), mouse anti-5-HT (dilution 1:100, MCA3190Z; AbD Serotec, Kidlington, UK), goat anti-GIP (dilution 1:200, sc-23554; Santa Cruz Biotechnology Inc., Santa Cruz, CA), goat anti-cholecystokinin (CCK) (dilution 1:200, sc-21617; Santa Cruz Biotechnology), or goat anti-glucagon-like peptide (GLP)-2 (dilution 1:200, sc-7781; Santa Cruz Biotechnology) overnight at 4°C. To confirm antibody specificity, xenin-25 antibody was preabsorbed with xenin-25 (50 µg/ml) overnight before application to tissue sections (absorption test). After rinsing in PBS, fluorescence-conjugated secondary antibodies were reacted for 2 hours at room temperature. The sections were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) and covered with EverBrite mounting medium (Biotium, Hayward, CA). Immunofluorescence was imaged and captured using a confocal laser microscope (LSM710; Carl Zeiss GmbH, Jena, Germany).
The duodenum was separated into the epithelium, interstitium-submucosa, and muscle layers under a stereomicroscope. The tissue was immersed in ice-cold pH 7.0 Krebs buffer containing 10 mM EDTA for 10 minutes, followed by stripping muscle layers by microdissection using fine forceps. The epithelial cells were separated from the interstitium by gentle scraping using a silicone-coated pipette tip. The separated duodenal samples, dorsal root ganglia (DRG), and nodose ganglia (NG) were kept in a RNA stabilization solution (RNAlater, Quiagen, Valencia, CA) at 4°C until use. Real-time polymerase chain reaction was performed as described previously (Akiba et al., 2015) with the following sense and antisense primers, respectively; NTS1 (5′-gtcaaggtcgtcatccaggt-3′; 5′-agaccacaaaggcaatgacc-3′), NTS2 (5′-ccatcgtggctgtgtatgtc-3′; 5′-agcgtgttggtcaccatgta-3′), NK1 (5′-tcctcctgccctacatcaac-3′; 5′-tgaccttgtacacgctgctc-3′), NK2 (5′-gtgaaggccatggtactggt-3′; 5′-tccagcctgtcttcctcagt-3′), and NK3 (5′-tactgccgcttccagaactt-3′; 5′-tccaacgatggtgtaggtga-3′). β-Actin was used as an internal control. The expression level was presented as the fold induction per 103 copies of β-actin by the ΔCt method.
Short-Circuit Current Measurements in Ussing-Chambered Preparations.
Mucosa-submucosa preparations were created from the duodenum as described previously (Kaji et al., 2015b). The same preparations from the midjejunum (∼20 cm from the pyloric ring), terminal ileum (5 cm from the ileocecal junction), proximal colon (5 cm from the cecum), and distal colon (5 cm from the anus) were used for comparing segmental differences. Each segment was divided longitudinally into two pieces, and each piece was mounted between two hemisliders with an aperture = 0.3 cm2 (Physiologic Instruments, San Diego, CA). The serosal Krebs-Ringer solution contained (in mM) NaCl, 117; KCl, 4.7; MgCl2, 1.2; NaH2PO4, 1.2; CaCl2, 2.5; NaHCO3, 25; glucose, 11; and bubbled with 95% O2–5% CO2 to maintain pH at 7.4. For HCO3−-free conditions, NaHCO3 was replaced with NaCl and acetazolamide (0.2 mM) was added into the serosal bath bubbled with 100% O2. For Cl−-free solutions, NaCl, KCl, and CaCl2 were replaced with sodium gluconate, potassium gluconate, and 8 mM calcium gluconate, respectively. The luminal bathing solution for small intestine contained (in mM) NaCl, 136; KCl, 2.6; CaCl2, 1.8; HEPES, 10 (pH 7.4); and mannitol, 11; bubbled with 100% O2. The luminal bathing solution for colon was Krebs-Ringer solution. Measurements of short-circuit current (Isc) and tissue conductance (Gt) were conducted as described previously (Kaji et al., 2015b). Positive values for Isc indicate a negative electrical charge flux from the serosal → luminal bath as a result of anion secretion or cation absorption. Indomethacin (10 μM) was added to the serosal bath for the small intestine or to both baths for the colon to eliminate the effects of endogenous prostaglandin production. The tissues were stabilized for 30–45 minutes before the effects of xenin and other drugs were investigated.
Peptide Synthesis.
Xenin-25 (MLTKFETKSARVKGLSFHPKRPWIL) was synthesized using solid-phase methodology according to the Fmoc-strategy using an automated peptide synthesizer (Model Pioneer, Thermo Fisher Scientific, Waltham, MA). The crude peptide was purified using reverse-phase high performance liquid chromatography (HPLC: Delta 600 HPLC System, Waters, MA) on a column of Develosil ODS-HG-5 (2 × 25 cm, Nomura Chemical Co., Ltd, Seto, Japan). The purity of each peptide was confirmed by analytical HPLC and matrix assisted laser desorption/ionization time of flight and mass spectrometry analysis.
Chemicals.
The vasoactive intestinal peptide (VIP)/pituitary adenylate cyclase-activating peptide receptor 1 (VPAC1) antagonist [Ac-His1, d-Phe2, Lys15, Arg16, Leu27]-VIP(1–7)-GRF(8–27) was purchased from Phoenix Pharmaceuticals (Burlingame, CA). Xenin-8, A803467, SR48692 (meclinertant), NTRC824, SB268262, CP96345, CP99994, MEN10376, osanetant, PSB603, MRS3777, and GR113808 were purchased from Tocris Bioscience (Pittsburgh, PA). Aprepitant was purchased from Cayman Chemical (Ann Arbor, MI). Levocabastine, ondansetron, suramin, 8-cyclopentyl-1,3-dipropylxanthine (CPX), 8-(3-chlorostyryl) caffeine (8CC), atropine, tetrodotoxin (TTX), lidocaine, capsaicin, indomethacin, Nω-nitro-l-arginine methyl ester hydrochloride (l-NAME), nifedipine, and other chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
Statistical Analysis.
Values are expressed as the mean ± S.E.M. The number of animals in each experimental group was ≥5. Statistical analysis was performed using Prism, ver. 6 (GraphPad Software Inc., La Jolla, CA) with Student’s t test or analysis of variance followed by Dunnett’s or Tukey’s multiple comparison depending on the number of experimental groups. P < 0.05 was considered statistically significant.
Results
Secretory Effect of Xenin-25 and Xenin-8 in Ussing-Chambered Duodenal Mucosa.
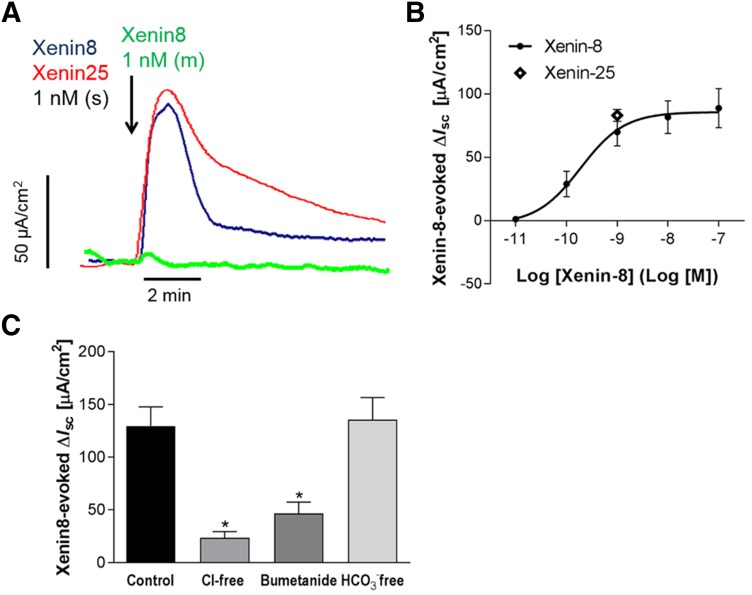
After a 30-minute incubation in the Ussing chamber, basal Isc and Gt stabilized at 38.9 ± 4.6 µA/cm2 and 41.0 ± 1.3 mS/cm2, respectively, in mucosa-submucosa preparations of distal duodenum. Serosal application of xenin-8 immediately increased Isc and Gt in a dose-dependent manner with EC50 = 0.3 nM (Fig. 1, A and B). A submaximal concentration (1 nM) was subsequently used in experiments to identify the underlying mechanisms. Serosal xenin-25 (1 nM) increased Isc (83.2 ± 4.7 µA/cm2; N = 5) with a similar time course and peak value to those of xenin-8. Mucosal application of xenin-8 (1 nM) however, did not alter Isc or Gt, suggesting that xenin receptors are present on the submucosal plexus or epithelial cell basolateral membrane rather than on the apical membrane of duodenocytes.
Fig. 1.
Effect of xenin on short-circuit current (Isc) in mucosa-submucosa preparation of rat duodenum mounted in Ussing chambers. (A) Representative traces of Isc. Serosal (s) application of xenin-8 (dark blue line) or xenin-25 (red line) at 1 nM immediately increased Isc, whereas mucosal (m) application of xenin-8 (green line) had no effect. (B) Concentration-response curve of serosal xenin-8-induced Isc increases. Effect of xenin-25 at 1 nM (unfilled diamond) was similar extent with that of xenin-8. (C) Xenin-8-induced Isc increases in the presence or absence of Cl−, HCO3−, or bumetanide in the serosal bathing solution. The serosal bath was replaced with each anion-free buffer, or bumetanide (0.1 mM) was added 15 minutes before the application of xenin-8 (1 nM). Each datum represents the mean ± S.E.M. (n = 5). *P < 0.05 versus control group by analysis of variance (ANOVA) followed by Dunnett’s test.
To confirm the nature of anion secretion in Ussing-chambered duodenal mucosa, the effects of xenin-8 were measured in the presence or absence of HCO3− or Cl− in the serosal buffer. HCO3− depletion of the buffer did not alter basal Isc or Gt (Isc, 49.3 ± 13.3 µA/cm2; Gt, 49.6 ± 4.1 mS/cm2), or the xenin-8-induced Isc increase, compared with normal conditions. Cl− depletion in the serosal bath, however, reduced basal Isc and Gt (Isc, −14.9 ± 28.6 µA/cm2; Gt, 22.3 ± 1.6 mS/cm2) and the response to xenin-8 by 80%, suggesting that the xenin-induced Isc increase is predominantly electrogenic Cl− secretion (Fig. 1C). The NKCC1 inhibitor bumetanide reduced xenin-8-evoked Isc, further supporting the presence of Cl− secretion, which depends on basolateral NKCC1 activity.
Segmental Differences in the Response to Xenin-8 in the Ussing Chamber.
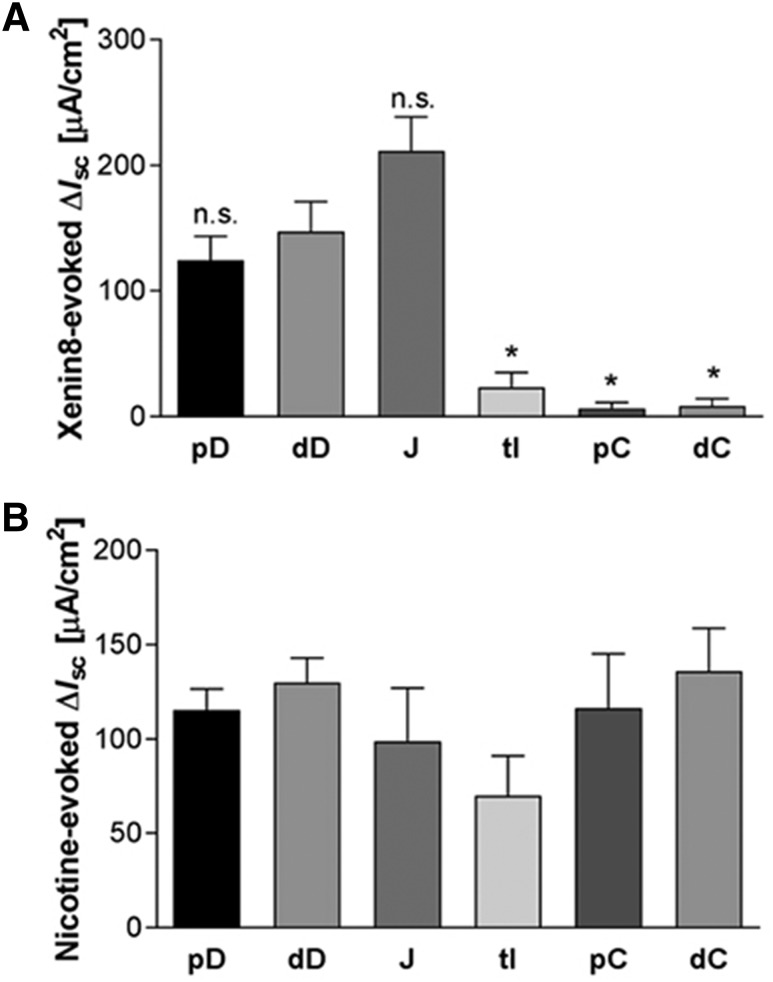
To compare the extent of secretory response to xenin-8, we measured ion secretion in mucosa-submucosa preparations from proximal duodenum, distal duodenum, midjejunum, terminal ileum, proximal colon, and distal colon. Basal Isc and Gt after stabilization differed among the segments tested (Table 1). Serosal application of xenin-8 (1 nM) increased Isc accompanied by Gt increase to the same extent in the proximal and distal duodenum. The hindgut segments had significantly lower or no response compared with the distal duodenum (Fig. 2A). The viability of the preparations was confirmed by the addition of nicotine (0.1 mM) into the serosal bath after the experiment, which increased Isc to a similar extent in all segments tested (Fig. 2B).
TABLE 1.
Segmental differences of basal Isc and Gt in-Ussing chambered intestinal segments
| Basal Isc | Basal Gt | |
|---|---|---|
| µA/cm2 | mS/cm2 | |
| Proximal duodenum | 39.6 ± 4.5 | 41.0 ± 1.9 |
| Distal duodenum | 37.2 ± 2.0 | 41.2 ± 0.8 |
| Jejunum | 48.2 ± 12.3 | 52.8 ± 2.8 |
| Terminal ileum | 42.2 ± 10.1 | 28.5 ± 3.4 |
| Proximal colon | 55.1 ± 4.65 | 18.4 ± 0.8 |
| Distal colon | 31.7 ± 5.06 | 11.5 ± 1.6 |
Fig. 2.
Segmental heterogeneity in response to xenin-8 (A) and nicotine (B) in Ussing-chambered rat intestinal segments. The Isc peak values in response to serosal xenin-8 (1 nM) or nicotine (0.1 mM) were compared among mucosa-submucosa preparations from proximal duodenum (pD), distal duodenum (dD), mid-jejunum (J), terminal ileum (tI), proximal colon (pC), and distal colon (dC). Each datum represents the mean ± S.E.M. (n > 5). *P < 0.05 versus dD, n.s. (not significant) by ANOVA followed by Dunnett’s test.
Effects of NTS Antagonists on Xenin-8-Induced Anion Secretion in the Duodenum.
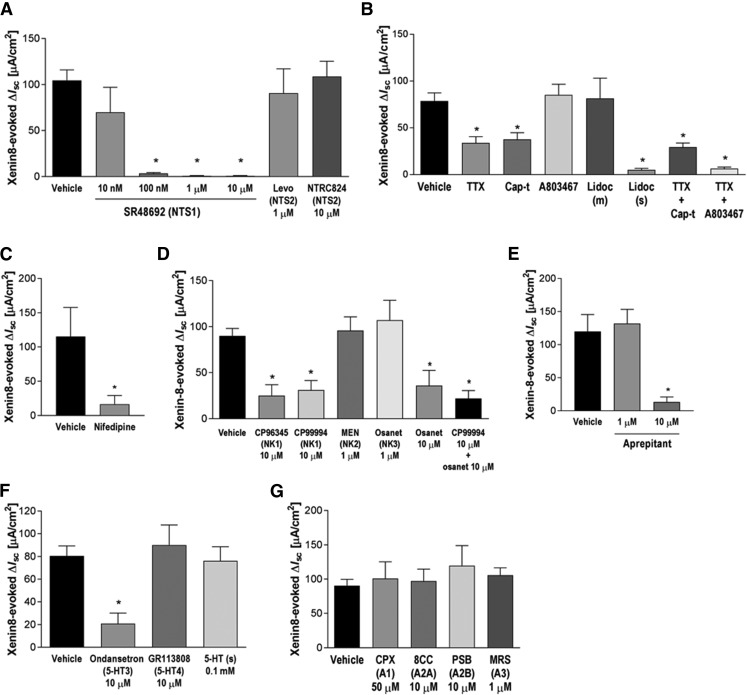
To evaluate the contribution of NTS1 and NTS2 toward xenin-8-induced anion secretion, we tested the effects of the selective antagonist SR48692 [2-[[[1-(7-chloro-4-quinolinyl)-5-(2,6-dimethoxyphenyl)-1Hpyrazol-3-yl]carbonyl]amino]-tricyclo[3.3.1.13,7]decane-2-carboxylic acid] for NTS1 and levocabastine and NTRC824 for NTS2. SR48692 pretreatment did not alter basal Isc or Gt, but dose dependently inhibited the response to xenin-8 (Fig. 3A), with an IC50 ∼30 nM, comparable to the results from in vitro binding assay (Gully et al., 1993). Neither the partial NTS2 agonist levocabastine nor the selective and potent NTS2 antagonist NTRC824 altered basal or xenin-8-induced Isc, suggesting that xenin-8-induced duodenal anion secretion is mediated by NTS1, but not by NTS2.
Fig. 3.
Effect of selective receptor antagonists and neural sodium channel inhibitors on xenin-8-induced Isc changes. (A) Neurotensin receptor (NTS) 1 antagonist SR48692 or NTS2 antagonists levocabastine (Levo) or NTRC824 was applied to the serosal bath. (B) Tetrodotoxin (TTX, 1 µM), capsaicin (Cap-t, 10 µM), and/or A803467 (1 µM) were applied to the serosal bath. Lidocaine (Lidoc, 0.5 mM) was applied to mucosal (m) or serosal (s) bath. (C) L-type Ca2+ channel blocker nifedipine (0.1 mM) was applied to serosal bath. (D) Substance P/tachykinin receptor (NK) 1 antagonist CP96345 or CP99994, NK2 antagonist MEN10376 (MEN), and/or osanetant (Osanet) were applied to the serosal bath. (E) Selective NK1 antagonist aprepitant was applied to the serosal bath. (F) 5-HT3 receptor antagonist ondansetron, 5-HT4 antagonist GR113808, or 5-HT was applied to the serosal bath. (G) Adenosine receptor A1 antagonist (CPX), A2A antagonist (8CC), A2B antagonist PSB603 (PSB), or A3 antagonist MRS3777 (MRS) was applied to the serosal bath. Each datum represents the mean ± S.E.M. (n > 5). *P < 0.05 versus vehicle by paired t test (C) or by ANOVA followed by Dunnett’s test (A, B, D–G).
Effects of Neuronal Channel Inhibitors on Xenin-8-Induced Anion Secretion in the Duodenum.
To identify the neural pathways mediating the secretory response to xenin, the neural cation channel blockers TTX, A803467 [5-(4-chlorophenyl)-N-(3,5-dimethoxyphenyl)-2-furancarboxamide], lidocaine, and/or capsaicin were used. Serosal pretreatment with TTX decreased basal Isc, indicating that TTX-sensitive submucosal neural activity is involved in basal ion transport under stabilized conditions. Xenin-8-induced anion secretion was inhibited 50% in the presence of TTX (Fig. 3B). Serosal capsaicin (10 µM) transiently increased Isc only with the first application (14.0 ± 2.8 µA/cm2, N = 6), suggesting that capsaicin-sensitive afferent nerves innervate duodenal mucosa and contribute to ion secretion. The response to xenin-8 was significantly reduced 15 minutes after capsaicin pretreatment (Cap-t) due to desensitization. The combination of TTX and capsaicin did not additionally inhibit the secretory response to xenin, suggesting that capsaicin-sensitive afferent nerves interact with submucosal neurons to stimulate secretion. The TTX-resistant sodium channel Nav1.8 is selectively inhibited by A803467 (Jarvis et al., 2007), which was tested for its effect on xenin-8-induced anion secretion. Pretreatment with A803467 (1 µM) alone had no effect, but the combination of TTX and A803467 abolished xenin-8-induced anion secretion (Fig. 3B). The local anesthetic lidocaine inhibits sodium channels on TTX-resistant nerves and suppresses colonic secretion and inflammation (Yajima, 1988; McCafferty et al., 1994). Serosal, but not mucosal, treatment with lidocaine (0.5 mM) abolished the secretory response to xenin-8. These results suggest that serosal xenin activates TTX-sensitive and -resistant afferent nerve fibers localized in the submucosal plexus. The L-type Ca2+ channel blocker nifedipine (0.1 mM) significantly inhibited the response to xenin-8 (Fig. 3C), but did not affect forskolin- or bethanechol-induced secretion (data not shown), supporting our hypothesis that xenin-8 activates nerves in the submucosal plexus, rather than directly activates epithelial cells.
Neurotransmitters Involved in Xenin-8-Induced Anion Secretion.
Acetylcholine (ACh) and vasoactive intestinal peptide (VIP) are major and potent neurotransmitters present in submucosal secretomotor neurons, activating muscarinic ACh receptors and VPAC1 on enterocytes, respectively (Xue et al., 2007). Serosal xenin-8-induced secretion was, however, not altered by the muscarinic antagonist atropine (10 µM) or the selective VPAC1 antagonist [Ac-His1, d-Phe2, Lys15, Arg16, Leu27]-VIP(1–7)-GRF(8–27) (1 µM) (Table 2). Calcitonin-gene related peptide (CGRP) is one of neurotransmitters released from capsaicin-sensitive afferent nerves (Maggi et al., 1986). A selective CGRP receptor 1 antagonist SB268262 [10 µM; N-methyl-N-(2-methylphenyl)-3-nitro-4-(2-thiazolylsulfinyl)-benzamide] had no effect on xenin-8-induced Isc increase (Table 2). These results indicated that NTS1-mediated anion secretion was not mediated by ACh, VIP, or CGRP.
TABLE 2.
Xenin-8-evoked Isc increases in the presence or absence of neurotransmitter receptor antagonists or nitric oxide synthase inhibitor
| Vehicle | Treatment | |
|---|---|---|
| µA/cm2 | µA/cm2 | |
| Atropine | 69.5 ± 7.1 | 58.7 ± 7.3 |
| SB268262, 1 µM | 88.1 ± 6.1 | 111.9 ± 26.3 |
| SB268262, 10 µM | 95.7 ± 26.6 | |
| VPAC1 antagonist | 71.4 ± 15.1 | 92.1 ± 22.1 |
| l-NAME | 85.0 ± 20.6 | 111.2 ± 24.1 |
Substance P (SP), 5-hydroxytryptamine (5-HT), and nitric oxide (NO) are peptide, monoamine, and gaseous neurotransmitters, respectively, involved in noncholinergic, nonadrenergic autonomic neurotransmission. To determine the contribution of these transmitters to xenin-induced anion secretion, we tested selective antagonists for tachykinin receptors (NK1, NK2, and NK3), or for 5-HT receptors (5-HT3 and 5-HT4), or the NO synthase inhibitor l-NAME for their effects on xenin-8-induced Isc. The NK1-selective antagonists CP96345 [10 µM; (2S,3S)-N-(2-methoxyphenyl)methyl-2-diphenylmethyl-1-azabicyclo[2.2.2]octan-3-amine] or CP99994 [10 µM; (2S,3S)-N-[(2-methoxyphenyl)methyl]-2-phenyl-3-piperidinamine dihydrochloride] significantly inhibited xenin-induced Isc, whereas the NK2-selective antagonist MEN10376 (1 µM; H-Asp-Tyr-d-Trp-Val-d-Trp-d-Trp-Lys-NH2) or the NK3-preferred antagonist osanetant (1 µM) had no effect (Fig. 3D). A high concentration of osanetant (10 µM) or cotreatment with CP99994 and osanetant inhibited xenin-8-induced Isc as same inhibitory extent as CP99994 alone, suggesting that NK1 is mainly involved in xenin-8-induced anion secretion. Another selective NK1 antagonist, aprepitant [5-[[(2R,3S)-2-[(1R)-1-[3,5-bis(trifluoromethyl)phenyl]ethoxy]-3-(4-fluorophenyl)-4-morpholinyl]methyl]-1,2-dihydro-3H-1,2,4-triazol-3-one], also abolished the response to xenin-8 at 10 µM (Fig. 3E). Ondansetron (10 µM), a 5-HT3-selective antagonist, but not the 5-HT4 selective antagonist GR113808 [10 µM; 1-methyl-1H-indole-3-carboxylic acid, [1-[2-[(methylsulfonyl)amino]ethyl]-4-piperidinyl]methyl ester], significantly reduced the response to xenin by 65% (Fig. 3F). The response to serosal xenin-8 was not altered by pretreatment with 5-HT (0.1 mM) in the serosal bath, suggesting that 5-HT3 receptors are not desensitized under these experimental conditions. Although 5-HT mediates neural NO release in guinea pig colon (Kuwahara et al., 1998), serosal pretreatment with l-NAME (0.1 mM) had no effect on the basal and xenin-8-stimulated Isc change (Table 2).
NT-induced Cl− secretion is mediated by adenosine A1 and A2 receptors in human colonic mucosa (Riegler et al., 2000), whereas rat duodenal HCO3− secretion is stimulated by luminal adenosine through A2B receptors (Ham et al., 2010). We thus tested the effect of the purinergic receptor antagonists CPX (50 µM) for A1 receptors, 8CC (10 µM) for A2A receptors, PSB603 (10 µM) for A2B receptors, and MRS3777 (1 µM) for A3 receptors on xenin-8-induced Cl− secretion in the Ussing chamber. Using the same concentration of antagonists as in our previous study (Ham et al., 2010), none of these selective antagonists affected the xenin-evoked Isc increase (Fig. 3G). Serosal application of CPX alone increased basal Isc (ΔIsc: 23.6 ± 4.0 µA/cm2), suggesting that A1 receptors may be activated during stabilization, and an A1 antagonist unmasks the antisecretory effect of A1 activation. From these data, we concluded that the adenosine pathway is not involved in xenin-NTS1-mediated Cl− secretion in rat duodenum.
Duodenal HCO3− Secretion in Response to Intravenous Infusion of Xenin-8.
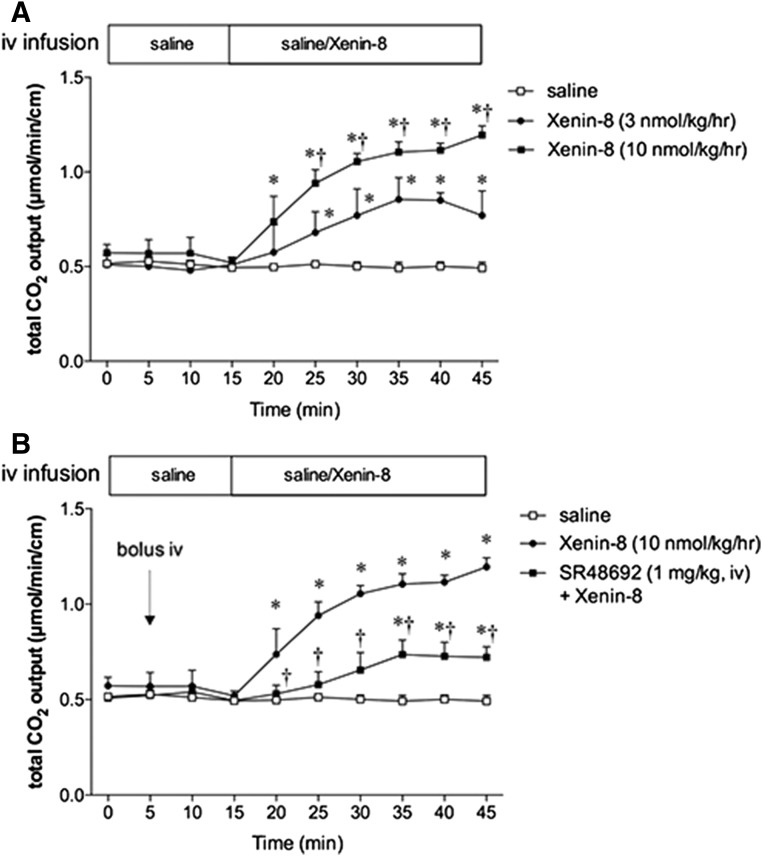
Intravenous infusion of xenin-8 gradually and dose dependently increased the rate of duodenal HCO3− output (Fig. 4A). Intravenous injection of the NTS1 antagonist SR48692 had no effect on basal HCO3− output, but inhibited xenin-8-induced HCO3− secretion (Fig. 4B), suggesting that NTS1 does not contribute to basal HCO3− secretion in fasted rats, whereas xenin enhances protective duodenal HCO3− secretion via NTS1 activation in vivo.
Fig. 4.
Effect of intravenous (iv) infusion of xenin-8 and SR48692 on luminal HCO3− secretion in the duodenum. A duodenal loop was perfused with pH 7 saline and total CO2 output was measured in the perfusate with flow-through pH and CO2 electrodes. (A) Saline with or without xenin-8 was infused into the femoral vein from time = 15 minutes. Intravenous infusion of xenin-8 dose dependently increased total CO2 output. (B) SR48692 was injected at time = 5 minutes. Bolus injection of SR48692 had no effect on basal HCO3− secretion, but significantly inhibited the response to xenin-8. Each data point represents the mean ± S.E.M. (n = 6 rats). *P < 0.05 versus saline, †P < 0.05 versus another group by two-way ANOVA followed by Tukey’s test.
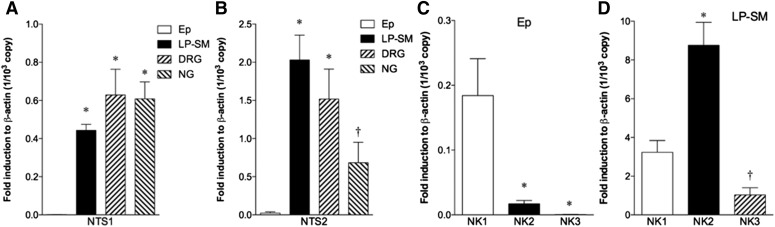
Expression of NTS and NK in the Duodenum and Extrinsic Afferent Neurons.
Real-time polymerase chain reaction was performed for NTS1, NTS2, NK1, NK2, and NK3 in the isolated duodenal epithelial cells (Ep), in the lamina propria + submucosa including the submucosal plexus (LP-SM), and extrinsic afferent neurons, DRG and NG. NTS1 and NTS2 were detected at very low levels in the epithelial cells, whereas both receptors were expressed in LP-SM containing enteric neural plexuses and in extrinsic afferent neurons (Fig. 5, A and B). In contrast to predominant NTS1-mediated secretion in the Ussing chamber studies, NTS2 expression was abundant in duodenal LP-SM, suggesting that NTS2 may be involved in functions other than anion secretion. All NK receptor subtypes were detected in Ep and LP-SM (Fig. 5, C and D). NK1 receptor expression was significantly higher than NK2 or NK3 in Ep, whereas lower than NK2 in LP-SM (Fig. 5D).
Fig. 5.
Expression of mRNA for NTS1, NTS2, and NK receptors in rat tissues determined by real-time polymerase chain reaction. NTS1 (A) and NTS2 (B) expression were compared in isolated duodenal epithelial cells (Ep), lamina propria + submucosa with submucosal plexus from the duodenum (LP-SM), dorsal root ganglia (DRG) and nodose ganglia (NG). *P < 0.05 versus Ep, †P < 0.05 versus LP-SM by ANOVA followed by Tukey’s test. NK1, NK2, and NK3 expressions were compared in Ep (C) and LP-SM (D). *P < 0.05 versus NK1, †P < 0.05 versus NK2 by ANOVA followed by Tukey’s test. Each data represents the mean ± S.E.M. (n = 4).
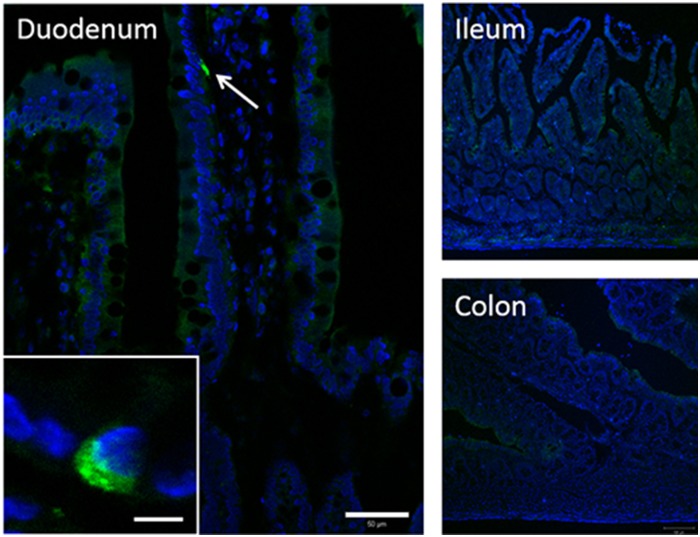
Localization of Xenin in Duodenal Mucosa.
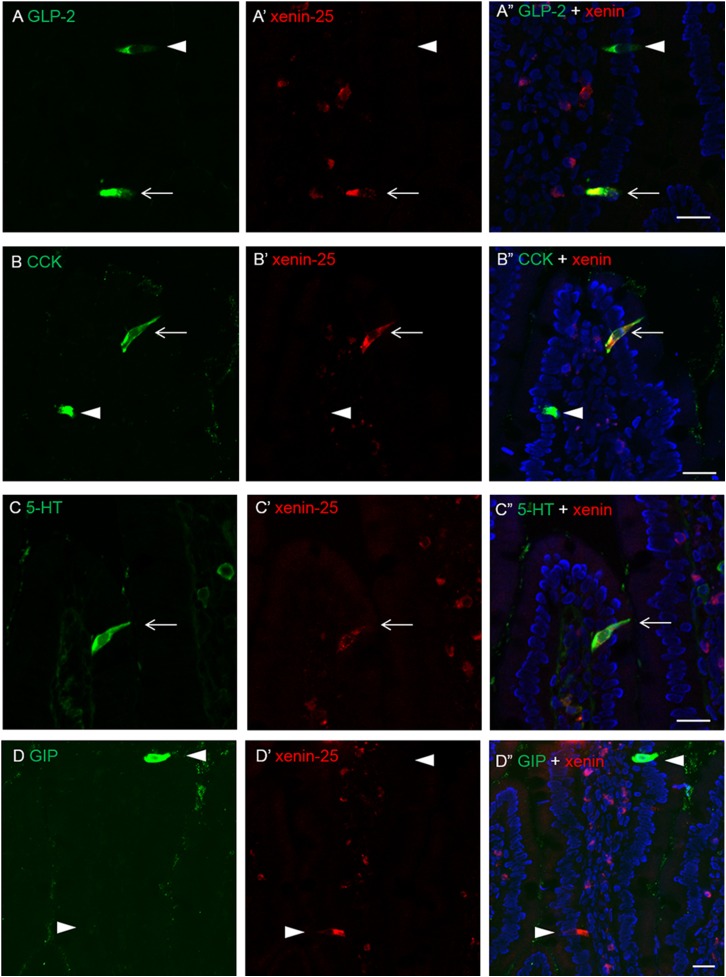
Immunohistochemical study of cryostat intestinal sections revealed that a small subset of epithelial cells expressed xenin-25 in the duodenum, but not in the ileum or colon (Fig. 6). Preabsorption, the antibody with xenin-25 abolished the immunoreactivity (data not shown). Xenin-positive cells in the duodenum possessed long narrow apical processes with storage in the basolateral cytosol, suggesting that xenin-25 is expressed in EECs. Double immunostaining demonstrated that xenin-25-immunoreactive cells co-expressed 5-HT, GLP-2, or CCK (Fig. 7, A–C), suggesting that xenin-25 is released from enterochromaffin, L, and/or I cells. Inconsistent with the study in human and canine duodenum (Anlauf et al., 2000), most GIP-containing K cells had no xenin-25 immunoreactivity and vice versa in rat duodenum (Fig. 7D).
Fig. 6.
Immunoreactivity for xenin-25 in frozen sections of rat duodenum, ileum, and proximal colon. Xenin-25 positive cells in duodenal mucosa (arrow and inset, higher magnification: bar, 5 µm) had enteroendocrine-like morphology. Nuclei were counterstained with DAPI (blue). Bar, 50 µm (duodenum) or 100 µm (ileum and colon).
Fig. 7.
Double immunostaining for xenin-25 and enteroendocrine cell (EEC) markers in frozen sections of rat duodenum. A part of glucagon-like peptide 2 (GLP-2)-, cholecystokinin (CCK)-, or serotonin (5-HT)-containing cells coexpressed xenin-25 (arrows). Most of gastric inhibitory peptide (GIP)-containing cells were xenin-25-negative. Arrowheads indicate EECs, which are stained with a single marker. Nuclei were counterstained with DAPI (blue). Bar, 20 µm.
Discussion
We demonstrated that the gut peptide hormone xenin induced neurogenic HCO3− and Cl− secretion through activation of afferent neural reflexes via NTS1, 5-HT3, and NK1 receptors expressed in rat duodenum. As endogenous xenin was present in a subset of duodenal EECs, postprandial xenin release from the duodenal mucosa may contribute not only to mucosal defense mechanisms but also to satiety signaling via vagal and spinal afferent nerves.
Although ACh and VIP are the major secretagogues for intestinal epithelia in electrically activated submucosal plexus (Krueger et al., 2016), our results showed that serosal xenin failed to activate cholinergic or VIP-ergic neurons. Because the secretory response to xenin was blocked by selective antagonists for NTS1, 5-HT3, and NK1 receptors and Nav1.8 channels that are generally expressed on the afferent nerves, NTS1 receptors may be specifically expressed on the intrinsic and/or extrinsic primary afferent neurons that release SP. 5-HT-induced duodenal anion secretion is predominantly mediated by epithelial 5-HT4 in mice (Tuo et al., 2004). Luminal 5-HT also stimulates HCO3− secretion via 5-HT4 activation in rat proximal colon (Kaji et al., 2015a). Nevertheless, we here showed that xenin-induced Cl− secretion was partially mediated by 5-HT3, but not 5-HT4 activation. In the xenin-evoked neurogenic secretory pathway, 5-HT may not be released from enterochromaffin cells into the lumen as a component of 5-HT4-mediated secretion. Because 5-HT3 is expressed in extrinsic afferent neurons (Glatzle et al., 2002; Raybould et al., 2003) and because the serosally applied selective 5-HT3 agonist SR57227 [1-(6-chloro-2-pyridinyl)-4-piperidinamine hydrochloride] or NK1 agonist Sar-Met-SP increased Isc in a 5-HT3-dependent manner in the duodenum (unpublished observation), endogenous 5-HT may activate afferent axons via 5-HT3 receptors, potentiating SP release during NTS1 activation by xenin, implying that SP is an important stimulant for duodenocytes through activation of NK1 on epithelial cells. Although CGRP may be coreleased with SP from extrinsic afferent nerves, xenin-induced neurogenic secretion was not mediated by CGRP receptors in vitro. It is possible that the secretory effect of released CGRP was minimal, compared with the effect of released SP in our experimental preparation.
The NK1 selective antagonists CP96345 and CP99994 may directly interact with L-type Ca2+ channels (McLean et al., 1993). In addition to these compounds, the L-type Ca2+ channel blocker nifedipine also reduced the response to xenin-8, suggesting that L-type Ca2+ channel activation in neural tissues mediates xenin-8-evoked anion secretion. Because CP99994 has a considerably lower affinity for Ca2+ channels than does CP96345 (McLean et al., 1993), whereas the effects of these two antagonists on the response to xenin were identical, it is likely that the NK1 receptor contributes to xenin-8-induced secretion in the duodenum. Indeed, another type of NK1 antagonist, aprepitant, dose dependently inhibited xenin-8-induced secretion, suggesting that SP-NK1 is downstream of NTS1 activation in xenin-evoked duodenal anion secretion. Although SP is a well-characterized intracellular Ca2+-mediated secretagogue present in intrinsic afferent neurons (Mitsui, 2010), the identity of the physiologic stimulus that releases SP has not yet been identified. Our study revealed that a physiologic concentration of xenin (<1 nM) strongly activated NK1-mediated anion secretion through TTX-sensitive and -resistant pathways, suggesting that NTS1 activation by xenin releases SP from the afferent nerves. In the myenteric plexus, SP is involved in the regulation of motility mediated by cholinergic excitatory motor neurons (Furness et al., 2015). Nonetheless, xenin-induced secretion was independent of the cholinergic secretomotor pathway. The neural circuits of secretomotor and muscle motor functions may differ, although the same neurotransmitter is involved. NK1, as well as NK2 and NK3, are expressed in enteric neurons (Grady et al., 1996), although isolated duodenocytes predominantly expressed NK1, consistent with the functional expression of NK1 in isolated colonocytes (Southwell and Furness, 2001; Hosoda et al., 2002). Although xenin-positive EECs are a tiny subset of all EECs, local SP release may be stimulated by SP itself via NK1 receptors present on the afferent nerves (Mitsui, 2010) and may potentiate the secretory response.
Our immunohistochemical data demonstrated that xenin-25 was present in a part of 5-HT/CCK- or GLP-2-expressing EECs, respectively termed I cells and L cells. Xenin-25 and GIP immunoreactivities were mostly detected in distinct cells; only few GIP-containing K cells coexpressed xenin-25. The concept of EEC classification was recently reconsidered. Egerod et al. (2012) reported that CCK, secretin, GIP, GLP-1, PYY, and NT but not somatostatin were coexpressed in a novel group of EECs, according to the endocrine cell lineage. Cho et al. (2014) reported that 5-HT and CCK were often coexpressed in EECs of mouse duodenum. Therefore, our observation suggests that EECs expressing multiple gut hormones also express xenin-25, particularly in the duodenum. Further studies are required to fully categorize and identify the particular nutrient receptors expressed in these multihormone cells. As the flow rate of luminal contents is quite rapid in the duodenum (Quon et al., 1989), it is reasonable to hypothesize that multiple gut hormones are released at once in response to luminal nutrients to promptly activate postprandial physiologic responses, including mucosally protective ion secretion, metabolic responses, and satiety signals via hormonal and neural effectors.
Nav1.8 is functionally expressed in extrinsic afferent neurons of the NG and DRG, whereas it is rarely identified in enteric neurons (Miranda-Morales et al., 2010; Gautron et al., 2011). Interestingly, although Nav1.8 inhibition alone had no effect, in combination with TTX, Nav1.8 inhibition abolished xenin-8-evoked anion secretion. Furthermore, TTX or capsaicin pretreatment alone similarly inhibited xenin-8-induced secretion, whereas cotreatment with TTX and capsaicin had no additional effect. These results suggest that xenin-8-induced anion secretion is primarily neurogenic, possibly involving the synergistic activation of TTX-sensitive and -resistant afferent nerves. Our study did not distinguish intrinsic afferent activation from extrinsic afferent activation. Because the capsaicin receptor transient receptor potential vanilloid 1 is expressed on extrinsic afferent nerves and because the secretomotor cholinergic pathway was not involved, our results further suggest that xenin-NTS1-SP pathway mainly involves extrinsic afferent nerve activation. These results suggest that intact extrinsic nerve innervation is required to exert full the effect of xenin.
Luminal adenosine induces duodenal HCO3− secretion through the activation of A2B receptors expressed on the apical membrane of duodenocytes, whereas A1 receptors are only expressed in enteric neurons (Ham et al., 2010). Serosal xenin-8-induced Cl− secretion was not altered by any serosal antagonist for A1, A2A, A2B, or A3 receptors, indicating that adenosine receptors expressed on submucosal neurons were not involved in NTS1-mediated Cl− secretion. Consistent with the previous reports in the colon (Hancock and Coupar, 1995; Cooke et al., 1999), A1 receptor inhibition increased Isc, suggesting that adenosine or its precursor ATP/ADP/AMP is released in the submucosal plexus and suppresses basal electrogenic secretion in the duodenum. Therefore, luminal and basolateral stimuli may separately and locally regulate epithelial secretory function, by the same mediator via different receptor activation.
Although the precise t1/2 of xenin-25 in the circulation is unknown, we speculate that xenin activity as a gut hormone may last longer than other gut peptides, because xenin-derived peptide fragments of varying length have similar affinities to its receptor (Martin et al., 2014). Therefore, xenin is not rapidly deactivated by proteolytic cleavage, implying a much-extended physiologic t1/2, which in turn may influence its function in other tissues, such as the central nervous system and pancreas, in addition to the duodenum. Despite the presence of NTS1 expression and NT-containing N cells, the secretory response to xenin was significantly lower in the lower intestine compared with the duodenum or jejunum, suggesting that NTS1 activation is less involved in the secretory response in the lower intestine. NTS1 expression is upregulated in colonocytes obtained from inflamed mucosa (Bossard et al., 2007); furthermore, the expression of NK1 and 5-HT3 is increased in inflamed mucosal afferent nerves (Utsumi et al., 2016). Segmental differences in NTS1-mediated secretory responses suggest varying function of xenin and NT in the upper and lower intestine, respectively.
Consistent with xenin localization in the upper intestine, the presence of xenin signaling in the duodenum for stimulating mucosal protective HCO3− secretion further suggests that the xenin-NTS1 pathway, independent of the cholinergic or VIP-ergic pathways, is the alternative pathway that enhances duodenal mucosal defenses. NTS1 activation is implicated in the suppression of tonic pain, consistent with the abundance of NTS1 expression in DRG neurons (Roussy et al., 2008). Long-lasting xenin fragments may be useful for duodenal mucosal protection, appetite control, and antinociceptive therapeutics as a potent, selective agonist for NTS1 receptors on intrinsic/extrinsic afferent neurons.
Acknowledgments
The authors thank Stacey Jung for manuscript preparation and Drs. Paul H Guth and Eli Engel for their consultancy.
Abbreviations
- ACh
acetylcholine
- CCK
cholecystokinin
- CGRP
calcitonin-gene related peptide
- CPX
8-cyclopentyl-1,3-dipropylxanthine
- DRG
dorsal root ganglia
- EEC
enteroendocrine cell
- Ep
epithelial cells
- GIP
gastric inhibitory peptide
- GLP
glucagon-like peptide
- Gt
tissue conductance
- HPLC
high-performance liquid chromatography
- Isc
short-circuit current
- l-NAME
Nω-nitro-l-arginine methyl ester hydrochloride
- LP-SM
lamina propria + submucosa including the submucosal plexus
- NG
nodose ganglia
- NK1
SP/neurokinin receptor 1
- NO
nitric oxide
- NT
neurotensin
- PBS
phosphate-buffered saline
- SP
substance P
- TTX
tetrodotoxin
- VIP
vasoactive intestinal peptide
- VPAC1
VIP/PACAP receptor 1
Authorship Contributions
Participated in research design: Kaji, Akiba, and Kaunitz.
Conducted experiments and analyzed data: Kaji, Akiba, and Maruta.
Contributed new reagents: Kato and Kuwahara.
Wrote or contributed to the writing of the manuscript: Kaji, Akiba, Kato, Kuwahara, Maruta, and Kaunitz.
Footnotes
This study was supported by VA Merit Review (J.D.K.); the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant R01-DK54221] (J.D.K.); and American Gastroenterology Association-Rome Foundation Functional Gastroenterology and Motility Disorders Pilot Research Award (I.K.).
No conflicts of interest are declared.
References
- Akiba Y, Inoue T, Kaji I, Higashiyama M, Narimatsu K, Iwamoto K, Watanabe M, Guth PH, Engel E, Kuwahara A, et al. (2015) Short-chain fatty acid sensing in rat duodenum. J Physiol 593:585–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiba Y, Kaunitz JD. (2011) Luminal chemosensing in the duodenal mucosa. Acta Physiol (Oxf) 201:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexiou C, Zimmermann JP, Schick RR, Schusdziarra V. (1998) Xenin--a novel suppressor of food intake in rats. Brain Res 800:294–299. [DOI] [PubMed] [Google Scholar]
- Anlauf M, Weihe E, Hartschuh W, Hamscher G, Feurle GE. (2000) Localization of xenin-immunoreactive cells in the duodenal mucosa of humans and various mammals. J Histochem Cytochem 48:1617–1626. [DOI] [PubMed] [Google Scholar]
- Arslan N, Sayin O, Tokgoz Y. (2014) Evaluation of serum xenin and ghrelin levels and their relationship with nonalcoholic fatty liver disease and insulin resistance in obese adolescents. J Endocrinol Invest 37:1091–1097. [DOI] [PubMed] [Google Scholar]
- Bossard C, Souazé F, Jarry A, Bezieau S, Mosnier JF, Forgez P, Laboisse CL. (2007) Over-expression of neurotensin high-affinity receptor 1 (NTS1) in relation with its ligand neurotensin (NT) and nuclear beta-catenin in inflammatory bowel disease-related oncogenesis. Peptides 28:2030–2035. [DOI] [PubMed] [Google Scholar]
- Botto JM, Chabry J, Sarret P, Vincent JP, Mazella J. (1998) Stable expression of the mouse levocabastine-sensitive neurotensin receptor in HEK 293 cell line: binding properties, photoaffinity labeling, and internalization mechanism. Biochem Biophys Res Commun 243:585–590. [DOI] [PubMed] [Google Scholar]
- Cho HJ, Callaghan B, Bron R, Bravo DM, Furness JB. (2014) Identification of enteroendocrine cells that express TRPA1 channels in the mouse intestine. Cell Tissue Res 356:77–82. [DOI] [PubMed] [Google Scholar]
- Chowdhury S, Reeds DN, Crimmins DL, Patterson BW, Laciny E, Wang S, Tran HD, Griest TA, Rometo DA, Dunai J, et al. (2014) Xenin-25 delays gastric emptying and reduces postprandial glucose levels in humans with and without type 2 diabetes. Am J Physiol Gastrointest Liver Physiol 306:G301–G309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke HJ, Wang Y, Liu CY, Zhang H, Christofi FL. (1999) Activation of neuronal adenosine A1 receptors suppresses secretory reflexes in the guinea pig colon. Am J Physiol 276:G451–G462. [DOI] [PubMed] [Google Scholar]
- Cooke JH, Patterson M, Patel SR, Smith KL, Ghatei MA, Bloom SR, Murphy KG. (2009) Peripheral and central administration of xenin and neurotensin suppress food intake in rodents. Obesity (Silver Spring) 17:1135–1143. [DOI] [PubMed] [Google Scholar]
- Dumoulin V, Moro F, Barcelo A, Dakka T, Cuber JC. (1998) Peptide YY, glucagon-like peptide-1, and neurotensin responses to luminal factors in the isolated vascularly perfused rat ileum. Endocrinology 139:3780–3786. [DOI] [PubMed] [Google Scholar]
- Egerod KL, Engelstoft MS, Grunddal KV, Nøhr MK, Secher A, Sakata I, Pedersen J, Windeløv JA, Füchtbauer EM, Olsen J, et al. (2012) A major lineage of enteroendocrine cells coexpress CCK, secretin, GIP, GLP-1, PYY, and neurotensin but not somatostatin. Endocrinology 153:5782–5795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feurle GE. (1998) Xenin--a review. Peptides 19:609–615. [DOI] [PubMed] [Google Scholar]
- Feurle GE, Hamscher G, Kusiek R, Meyer HE, Metzger JW. (1992) Identification of xenin, a xenopsin-related peptide, in the human gastric mucosa and its effect on exocrine pancreatic secretion. J Biol Chem 267:22305–22309. [PubMed] [Google Scholar]
- Feurle GE, Ikonomu S, Partoulas G, Stoschus B, Hamscher G. (2003) Xenin plasma concentrations during modified sham feeding and during meals of different composition demonstrated by radioimmunoassay and chromatography. Regul Pept 111:153–159. [DOI] [PubMed] [Google Scholar]
- Furness JB, Poole DP, Cho HJ, Callaghan BP, Rivera LR. (2015) The innervation of the gastrointestinal tract, in Yamada’s Textbook of Gastroenterology, pp 239–258, John Wiley & Sons, Ltd Oxford, UK. [Google Scholar]
- Gault VA, Martin CM, Flatt PR, Parthsarathy V, Irwin N. (2015) Xenin-25[Lys13PAL]: a novel long-acting acylated analogue of xenin-25 with promising antidiabetic potential. Acta Diabetol 52:461–471. [DOI] [PubMed] [Google Scholar]
- Gautron L, Sakata I, Udit S, Zigman JM, Wood JN, Elmquist JK. (2011) Genetic tracing of Nav1.8-expressing vagal afferents in the mouse. J Comp Neurol 519:3085–3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatzle J, Sternini C, Robin C, Zittel TT, Wong H, Reeve JR, Jr, Raybould HE. (2002) Expression of 5-HT3 receptors in the rat gastrointestinal tract. Gastroenterology 123:217–226. [DOI] [PubMed] [Google Scholar]
- Grady EF, Baluk P, Böhm S, Gamp PD, Wong H, Payan DG, Ansel J, Portbury AL, Furness JB, McDonald DM, et al. (1996) Characterization of antisera specific to NK1, NK2, and NK3 neurokinin receptors and their utilization to localize receptors in the rat gastrointestinal tract. J Neurosci 16:6975–6986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gully D, Canton M, Boigegrain R, Jeanjean F, Molimard JC, Poncelet M, Gueudet C, Heaulme M, Leyris R, Brouard A, et al. (1993) Biochemical and pharmacological profile of a potent and selective nonpeptide antagonist of the neurotensin receptor. Proc Natl Acad Sci USA 90:65–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham M, Mizumori M, Watanabe C, Wang JH, Inoue T, Nakano T, Guth PH, Engel E, Kaunitz JD, Akiba Y. (2010) Endogenous luminal surface adenosine signaling regulates duodenal bicarbonate secretion in rats. J Pharmacol Exp Ther 335:607–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamscher G, Meyer HE, Metzger JW, Feurle GE. (1995) Distribution, formation, and molecular forms of the peptide xenin in various mammals. Peptides 16:791–797. [DOI] [PubMed] [Google Scholar]
- Hancock DL, Coupar IM. (1995) Functional characterization of the adenosine receptor mediating inhibition of intestinal secretion. Br J Pharmacol 114:152–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoda Y, Karaki S, Shimoda Y, Kuwahara A. (2002) Substance P-evoked Cl(-) secretion in guinea pig distal colonic epithelia: interaction with PGE(2). Am J Physiol Gastrointest Liver Physiol 283:G347–G356. [DOI] [PubMed] [Google Scholar]
- Jarvis MF, Honore P, Shieh CC, Chapman M, Joshi S, Zhang XF, Kort M, Carroll W, Marron B, Atkinson R, et al. (2007) A-803467, a potent and selective Nav1.8 sodium channel blocker, attenuates neuropathic and inflammatory pain in the rat. Proc Natl Acad Sci USA 104:8520–8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaji I, Akiba Y, Kaunitz JD. (2013) Digestive physiology of the pig symposium: involvement of gut chemosensing in the regulation of mucosal barrier function and defense mechanisms. J Anim Sci 91:1957–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaji I, Akiba Y, Said H, Narimatsu K, Kaunitz JD. (2015a) Luminal 5-HT stimulates colonic bicarbonate secretion in rats. Br J Pharmacol 172:4655–4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaji I, Iwanaga T, Watanabe M, Guth PH, Engel E, Kaunitz JD, Akiba Y. (2015b) SCFA transport in rat duodenum. Am J Physiol Gastrointest Liver Physiol 308:G188–G197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konturek SJ, Bilski J, Tasler J, Laskiewicz J. (1985) Gut hormones in stimulation of gastroduodenal alkaline secretion in conscious dogs. Am J Physiol 248:G687–G691. [DOI] [PubMed] [Google Scholar]
- Krueger D, Michel K, Zeller F, Demir IE, Ceyhan GO, Slotta-Huspenina J, Schemann M. (2016) Neural influences on human intestinal epithelium in vitro. J Physiol 594:357–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwahara A, Kuramoto H, Kadowaki M. (1998) 5-HT activates nitric oxide-generating neurons to stimulate chloride secretion in guinea pig distal colon. Am J Physiol 275:G829–G834. [DOI] [PubMed] [Google Scholar]
- Maggi CA, Manzini S, Giuliani S, Santicioli P, Meli A. (1986) Extrinsic origin of the capsaicin-sensitive innervation of rat duodenum: possible involvement of calcitonin gene-related peptide (CGRP) in the capsaicin-induced activation of intramural non-adrenergic non-cholinergic neurons. Naunyn Schmiedebergs Arch Pharmacol 334:172–180. [DOI] [PubMed] [Google Scholar]
- Martin CM, Parthsarathy V, Pathak V, Gault VA, Flatt PR, Irwin N. (2014) Characterisation of the biological activity of xenin-25 degradation fragment peptides. J Endocrinol 221:193–200. [DOI] [PubMed] [Google Scholar]
- McCafferty DM, Sharkey KA, Wallace JL. (1994) Beneficial effects of local or systemic lidocaine in experimental colitis. Am J Physiol 266:G560–G567. [DOI] [PubMed] [Google Scholar]
- McLean S, Ganong A, Seymour PA, Snider RM, Desai MC, Rosen T, Bryce DK, Longo KP, Reynolds LS, Robinson G, et al. (1993) Pharmacology of CP-99,994; a nonpeptide antagonist of the tachykinin neurokinin-1 receptor. J Pharmacol Exp Ther 267:472–479. [PubMed] [Google Scholar]
- Miranda-Morales M, Ochoa-Cortes F, Stern E, Lomax AE, Vanner S. (2010) Axon reflexes evoked by transient receptor potential vanilloid 1 activation are mediated by tetrodotoxin-resistant voltage-gated Na+ channels in intestinal afferent nerves. J Pharmacol Exp Ther 334:566–575. [DOI] [PubMed] [Google Scholar]
- Mitsui R. (2010) Immunohistochemical characteristics of submucosal Dogiel type II neurons in rat colon. Cell Tissue Res 340:257–265. [DOI] [PubMed] [Google Scholar]
- Mizumori M, Meyerowitz J, Takeuchi T, Lim S, Lee P, Supuran CT, Guth PH, Engel E, Kaunitz JD, Akiba Y. (2006) Epithelial carbonic anhydrases facilitate PCO2 and pH regulation in rat duodenal mucosa. J Physiol 573:827–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quon MG, Mena I, Valenzuela JE. (1989) Abnormalities in the duodenal transit and motility in duodenal ulcer patients: studies with a new isotopic technique. Gut 30:579–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybould HE, Glatzle J, Robin C, Meyer JH, Phan T, Wong H, Sternini C. (2003) Expression of 5-HT3 receptors by extrinsic duodenal afferents contribute to intestinal inhibition of gastric emptying. Am J Physiol Gastrointest Liver Physiol 284:G367–G372. [DOI] [PubMed] [Google Scholar]
- Riegler M, Castagliuolo I, Wang C, Wlk M, Sogukoglu T, Wenzl E, Matthews JB, Pothoulakis C. (2000) Neurotensin stimulates Cl(-) secretion in human colonic mucosa In vitro: role of adenosine. Gastroenterology 119:348–357. [DOI] [PubMed] [Google Scholar]
- Roussy G, Dansereau MA, Doré-Savard L, Belleville K, Beaudet N, Richelson E, Sarret P. (2008) Spinal NTS1 receptors regulate nociceptive signaling in a rat formalin tonic pain model. J Neurochem 105:1100–1114. [DOI] [PubMed] [Google Scholar]
- Said H, Kaji I, Kaunitz JD. (2015) Gastroduodenal mucosal defense mechanisms. Curr Opin Gastroenterol 31:486–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavo-Cardozo D, Lima MM, Pareja JC, Geloneze B. (2013) Appetite-regulating hormones from the upper gut: disrupted control of xenin and ghrelin in night workers. Clin Endocrinol (Oxf) 79:807–811. [DOI] [PubMed] [Google Scholar]
- Southwell BR, Furness JB. (2001) Immunohistochemical demonstration of the NK(1) tachykinin receptor on muscle and epithelia in guinea pig intestine. Gastroenterology 120:1140–1151. [DOI] [PubMed] [Google Scholar]
- Sterl K, Wang S, Oestricker L, Wallendorf MJ, Patterson BW, Reeds DN, Wice BM. (2016) Metabolic responses to xenin-25 are altered in humans with Roux-en-Y gastric bypass surgery. Peptides 82:76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Masu M, Nakanishi S. (1990) Structure and functional expression of the cloned rat neurotensin receptor. Neuron 4:847–854. [DOI] [PubMed] [Google Scholar]
- Tuo BG, Sellers Z, Paulus P, Barrett KE, Isenberg JI. (2004) 5-HT induces duodenal mucosal bicarbonate secretion via cAMP- and Ca2+-dependent signaling pathways and 5-HT4 receptors in mice. Am J Physiol Gastrointest Liver Physiol 286:G444–G451. [DOI] [PubMed] [Google Scholar]
- Utsumi D, Matsumoto K, Amagase K, Horie S, Kato S. (2016) 5-HT3 receptors promote colonic inflammation via activation of substance P/neurokinin-1 receptors in dextran sulphate sodium-induced murine colitis. Br J Pharmacol 173:1835–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wice BM, Wang S, Crimmins DL, Diggs-Andrews KA, Althage MC, Ford EL, Tran H, Ohlendorf M, Griest TA, Wang Q, et al. (2010) Xenin-25 potentiates glucose-dependent insulinotropic polypeptide action via a novel cholinergic relay mechanism. J Biol Chem 285:19842–19853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue J, Askwith C, Javed NH, Cooke HJ. (2007) Autonomic nervous system and secretion across the intestinal mucosal surface. Auton Neurosci 133:55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajima T. (1988) Luminal propionate-induced secretory response in the rat distal colon in vitro. J Physiol 403:559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]