Abstract
Background
Few studies have evaluated dietary antioxidant vitamins intake in relation to risk of mortality in Asia.
Methods
We examined the associations between total carotene, vitamin C, and vitamin E from diet and risk of mortality from all causes, cancer, and cardiovascular disease in 134,358 participants (59,739 men and 74,619 women) from the Shanghai Men's Health Study and Shanghai Women's Health Study, two prospective cohort studies of middle-aged and elderly Chinese adults in urban Shanghai. Participants were followed up for a median period of 8.3 and 14.2 years for men and women, respectively. Hazard ratios (HRs) and 95% confidence interval (CIs) were estimated using Cox proportional hazards regression models.
Results
During the 495,332 and 1,029,198 person-years of follow-up for men and women, respectively, there were 10,079 deaths (4170 men and 5909 women). For men, compared with the lowest quintiles, the multivariable-adjusted risk reductions in the highest categories were 17% (HR 0.83; 95% CI, 0.76–0.92) for dietary total carotene and 17% (HR 0.83; 95% CI, 0.75–0.91) for dietary vitamin C. Associations were weaker in women than in men, though they were still statistically significant (highest versus lowest quintiles of dietary total carotene, HR 0.87; 95% CI, 0.80–0.95; dietary vitamin C: HR 0.83; 95% CI, 0.77–0.91). Significant inverse associations were observed between dietary total carotene, vitamin C, and risk of cardiovascular disease mortality but not cancer mortality.
Conclusion
This study suggests that total carotene and vitamin C intake from diet were inversely associated with deaths from all causes and cardiovascular disease in middle-aged or elderly people in China.
Keywords: Antioxidants, Vitamins, Mortality, Cohort studies
Highlights
-
•
Dietary vitamin C and carotene were inversely associated with total deaths in China.
-
•
These associations were more pronounced for cardiovascular disease mortality.
-
•
Dietary antioxidant vitamins might not be associated with cancer mortality risk.
1. Introduction
Accumulating evidence suggests that oxidative stress plays an important role in the pathogenesis of human chronic disease, such as cancer1 and cardiovascular disease.2 Emerging from in vitro studies, evidence suggests that antioxidant vitamins can directly scavenge reactive oxygen.3 Therefore, the intake of antioxidant vitamins has been hypothesized to improve human survival and longevity.4
There has been considerable interest in the role of antioxidant vitamins in relation to risk of mortality. Previous studies have showed an inverse association of vegetable and fruit intakes and risk of all-cause mortality.5, 6 However, whether this protective effect is caused by antioxidant vitamins remains unclear.7 A series of studies have been conducted in Western populations with good nutritional status, which showed conflict results.8, 9, 10, 11, 12, 13 Based on the evidence from epidemiologic research over the past several decades, the effectiveness of antioxidant vitamin intake on mortality should depend on individual nutritional status.14 However, few studies have specifically examined the relation between dietary carotene, vitamin C, and vitamin E and risk of all-cause, cancer, and cardiovascular disease (CVD) mortality in the Asian population.
We aimed to access the dose-response relationship between dietary carotene, vitamin C, and vitamin E intake and risk of all-cause, cancer, and CVD mortality using data from the Shanghai Men's Health Study (SMHS) and Shanghai Women's Health Study (SWHS).
2. Methods
2.1. Study population
The SMHS and SWHS are two ongoing, population-based, prospective cohort studies. Details of the study designs, scientific rationale, and baseline characteristics of the participants have been published previously.15, 16 In brief, the SMHS, established in 2002–2006, recruited 61,491 men aged 40–74 years, with an overall response rate of 74.1% (61,491/83,033). For SWHS, 74,941 women aged 40–70 years were recruited from 1997 to 2000, with a response rate of 92.3% (74,941/81,170). All participants were interviewed using a structured questionnaire to obtain information on demographic characteristics, lifestyle and dietary habits, medical history, family history of cancers, and other exposures. Anthropometric measurements, such as current weight, height, and circumferences of the waist and hip, were also taken at baseline. The two cohorts were approved by the Institutional Review Boards of the Shanghai Cancer Institute, Vanderbilt University, and the National Cancer Institute. All participants in the cohorts were asked to provide informed consent.
In the current analyses, we excluded participants with (i) loss to follow-up immediately after enrollment (n = 30 for men, n = 5 for women); (ii) extreme values for total energy intake (for men: <800 or >4000 kcal/day, n = 256; for women: <500 or >3500 kcal/day, n = 238); (iii) or missing data for any of the covariates of interests (n = 1466 for men, n = 79 for women). After these exclusions, a total of 134,358 participants (59,739 men and 74,619 women) remained in the present study.
2.2. Assessment of total carotene, vitamins C, and vitamin E intake
Dietary intakes of total carotene, vitamins C, and vitamin E for each participant were estimated using food frequency questionnaires (FFQ) that were developed and validated separately for men17 and women.18 In brief, we calculated vitamin intakes based on the Chinese Food Composition Tables.19 Total dietary intake of each nutrient was calculated by summing the nutrients from all food items reported in the FFQs. The Spearman correlation coefficients for total carotene, vitamins C, and vitamin E between the FFQ and the means of multiple 24-h dietary recalls (conducted twice per month during a 12-month period) ranged from 0.38 to 0.49.17, 18
Although we did not collect detailed information for specific types of antioxidant vitamins supplements taken at the baseline survey, the questionnaire included questions asking whether the participant had taken vitamins A, B, C, D, or E, or a multivitamin supplement at least three times per week continuously for more than 2 months. We carried out sensitivity analyses excluding participants who used vitamin supplements to control for the potential influence of vitamin supplements.
2.3. Cohort follow-up and outcome ascertainment
Participant databases were linked to the Shanghai Vital Statistics Registry annually, and in-person visits to participants' homes were made every 2 to 3 years. All possible matches identified through the linkage were verified through in-home visits. For the SMHS, the response rates for the first (2004–2008) and second (2008–2011) in-person follow-up surveys were 97.6% and 91.9%, respectively. For the SWHS, the response rates for the first (2000–2002), second (2002–2004), third (2004–2007), and fourth (2008–2011) in-person follow-up surveys were 99.8%, 98.7%, 96.7%, and 92.0%, respectively.
Causes of death reported in death certificates were recorded according to the International Classification of Diseases, Ninth Revision (ICD-9), with cancer death defined as 140–208 and CVD death as 390–459.20
2.4. Statistical analysis
Dietary antioxidant vitamin intakes were adjusted for total energy using the residual method proposed by Willett21 and standardized to approximate mean daily energy intake calculated from the sex-specific cohort. When density methods were also performed in sensitivity analysis, similar results were observed (eTable 5). These energy-adjusted intakes were categorized by quintile based on their distribution among the entire study population at baseline. Person-years for each participant were counted from the year of enrollment to the date of death or December 31, 2012, whichever came first.
Hazard ratios (HRs) and 95% confidence intervals (CIs) for mortality risk associated with dietary antioxidant vitamin intakes were estimated separately for men and women using Cox proportional hazards regression models, with person-years as the underlying time scale. Results calculated with age as the underlying time scale were similar. We checked proportional hazards assumptions using the Schoenfeld residual method, finding no evidence of violation of these assumptions. Tests for linear trends of HRs across vitamin intake quintiles were conducted by modeling the median values as continuous variables. We also evaluated nonlinear associations between dietary antioxidant vitamins intake and mortality risk using restricted cubic splines, with 3 knots placed at the 5th, 50th, and 95th percentiles of antioxidant vitamin intake.
We applied three models in the above analyses. We adjusted age (per 5-y intervals) and energy (kcal/day, quartiles) in model 1. In model 2, we chose potential confounders a priori based on the previous literature as follows: birth cohort (per 10-y intervals), education (four categories: illiteracy or elementary school, middle school, high school, or graduate school), income (four categories: low, lower middle, upper middle, or high), occupation (three categories for men: professional or administrator, clerical or service worker, or manual laborer; four categories for women: housewife, professional or administrator, clerical or service worker, or manual laborer), smoking status (for men: never, pack-years <20, or pack-years ≥20; for women, never or ever smokers), alcohol intake (never, <2 drinks/day, or ≥2 drinks/day), body mass index (BMI; four categories:<18.5, 18.5–23.9, 24.0–27.9, or ≥28 kg/m2), waist: hip ratio (three categories: <0.90, 0.90–0.99, or ≥1.0 for men; <0.85, 0.85–0.94, or ≥0.95 for women), physical activity (metabolic equivalent-hours per week, quartiles), history of hypertension (yes/no), diabetes (yes/no), coronary heart disease (yes/no), stroke (yes/no), vitamin supplements use (yes/no), menopause status (yes/no, women only), and hormone replacement therapy (yes/no, women only). In model 3, we mutually adjusted total carotene, vitamin C, and vitamin E. However, the risk estimates changed little compared with model 2. To consider possible effect modification by baseline stratifying variable, we reanalyzed theses association between dietary carotene, vitamin C, or vitamin E and total mortality stratified by smoking status, alcohol intake, BMI, physical activity, dietary iron intake, and menopause status (women only).
In secondary analyses, we performed several sensitivity analyses to assess the robustness of our findings. First, we conducted analyses restricted to participants who reported no use of any vitamin supplements at the baseline survey. Second, we repeated the analyses after excluding deaths that occurred during the first 3 years of follow-up to rule out possible reverse causality. Additionally, we analyzed the data in participants free of prevalent cancers, diabetes, coronary heart disease, and stroke at baseline because these comorbidities may have led to a change in dietary habits. Finally, we additionally adjusted for dietary constituents (such as dietary fiber, monounsaturated fatty acid, polyunsaturated fatty acid) that may confound the association between dietary antioxidant vitamins and mortality.
All statistical analyses were performed using SAS software, version 9.3 (SAS Institute, Inc, Cary, NC, USA). Statistical tests were two-sided, and P values of less than 0.05 were considered to indicate statistical significance.
3. Results
During an average follow-up of 8.3 years (5–95% percentile, 6.6–10.4 years) in the SMHS and 14.2 years (5–95% percentile, 10.5–15.6 years) in the SWHS, 10,079 deaths were documented (4170 men and 5909 women). Baseline characteristics of study populations are presented in Table 1 and eTable 1, 2, and 3. As expected, cases were older, more likely to be ever smokers, current drinkers, and had lower education, lower income, and a higher prevalence of hypertension, diabetes, stroke, and coronary heart disease than surviving participants.
Table 1.
Baseline characteristics of cases and study population by sex in SMHS (2002–2012) and SWHS (1997–2012).
| Men, mean (SD) or N (%) |
Women, mean (SD) or N (%) |
|||
|---|---|---|---|---|
| Deaths (n = 4170) | Cohort (n = 59,739) | Deaths (n = 5909) | Cohort (n = 74,619) | |
| Education | ||||
| Elementary school or less | 864 (20.72) | 3982 (6.67) | 3201 (54.17) | 16,076 (21.54) |
| Middle school | 1416 (33.96) | 19,996 (33.47) | 1324 (22.41) | 27,573 (36.95) |
| High school | 1053 (25.25) | 21,592 (36.14) | 926 (15.67) | 20,819 (27.9) |
| Professional education/college or higher | 837 (20.07) | 14,169 (23.72) | 458 (7.75) | 10,151 (13.6) |
| Incomea | ||||
| Low | 486 (11.65) | 7474 (12.51) | 1600 (27.08) | 12,072 (16.18) |
| Lower middle | 2253 (54.03) | 25,489 (42.67) | 2378 (40.24) | 28,540 (38.25) |
| Upper middle | 1205 (28.9) | 20,976 (35.11) | 1267 (21.44) | 20,959 (28.09) |
| High | 226 (5.42) | 5800 (9.71) | 664 (11.24) | 13,048 (17.49) |
| Occupation | ||||
| House wife | – | – | 83 (1.4) | 274 (0.37) |
| Professional, administrator | 1180 (28.3) | 15,712 (26.3) | 1166 (19.73) | 21,329 (28.58) |
| Clerical or service worker | 852 (20.43) | 13,119 (21.96) | 1120 (18.95) | 15,408 (20.65) |
| Manual laborer | 2138 (51.27) | 30,908 (51.74) | 3540 (59.91) | 37,608 (50.4) |
| History of hypertension | 1978 (47.43) | 17,779 (29.76) | 2496 (42.24) | 17,745 (23.78) |
| History of diabetes | 626 (15.01) | 3731 (6.25) | 886 (14.99) | 3272 (4.38) |
| History of coronary heart disease | 578 (13.86) | 3030 (5.07) | 961 (16.26) | 5497 (7.37) |
| History of stroke | 625 (14.99) | 2233 (3.74) | 347 (5.87) | 872 (1.17) |
| Smoke statusb | ||||
| Never smoker | 1247 (29.9) | 18,151 (30.38) | 5486 (92.84) | 72,545 (97.22) |
| Pack-years <20 | 920 (22.06) | 18,262 (30.57) | 423 (7.16) | 2074 (2.78) |
| Pack-years ≥20 | 2003 (48.03) | 23,326 (39.05) | – | – |
| Alcohol intake | ||||
| Never drinker | 2675 (64.15) | 39,810 (66.64) | 5790 (97.99) | 73,179 (98.07) |
| <2 drinks/day | 782 (18.75) | 11,977 (20.05) | 107 (1.81) | 1352 (1.81) |
| ≥2 drinks/day | 713 (17.1) | 7952 (13.31) | 12 (0.2) | 88 (0.12) |
| Supplemental vitamins usersc | 787 (18.87) | 9003 (15.07) | 1207 (20.43) | 14,707 (19.71) |
| Menopause status | – | – | 5021 (84.97) | 36,979 (49.56) |
| Hormone replacement therapy | – | – | 66 (1.12) | 1564 (2.1) |
| Age at baseline, years | 64.32 (9.37) | 55.34 (9.74) | 61.39 (7.99) | 52.62 (9.08) |
| Body mass index, kg/m2 | 23.63 (3.52) | 23.72 (3.08) | 24.84 (4.06) | 24.02 (3.43) |
| Waist-hip ratio | 0.90 (0.06) | 0.90 (0.06) | 0.83 (0.06) | 0.81 (0.05) |
| Physical activity, MET-h/week | 61.77 (37.38) | 59.65 (34.15) | 100.33 (45.99) | 106.45 (45.14) |
| Dietary intaked | ||||
| Total energy, kcal/d | 1803.57 (479.29) | 1908.91 (472.38) | 1598.66 (403.53) | 1674.79 (392.31) |
| Carbohydrate, g/d | 313.29 (36.67) | 313.06 (36.23) | 283.36 (29.91) | 279.08 (29.14) |
| Protein, g/d | 75.15 (13.28) | 76.3 (13.34) | 63.46 (11.2) | 65.33 (11.27) |
| Saturated fat, g/d | 10.04 (4.05) | 9.97 (4.05) | 7.83 (3.26) | 8.3 (3.23) |
| Monounsaturated fat, g/d | 14.85 (6.39) | 14.77 (6.37) | 11.83 (5.03) | 12.47 (4.97) |
| Polyunsaturated fat, g/d | 8.51 (3.08) | 8.36 (3.01) | 7.51 (2.93) | 7.45 (2.66) |
| Fiber, g/d | 11.16 (3.46) | 11.21 (3.46) | 10.5 (3.33) | 10.72 (3.31) |
| Vegetables, g/d | 321.92 (174.74) | 336.15 (177.5) | 276.6 (149.69) | 289.17 (154.32) |
| Fruits, g/d | 131.96 (113.2) | 147.45 (119.94) | 210.99 (154.35) | 258.59 (169.4) |
| Red meat, g/d | 58.52 (38.03) | 60.98 (38.22) | 44.89 (29.34) | 48.97 (31.35) |
| Poultry, g/d | 14.94 (17.51) | 15.48 (18.35) | 12.59 (15.72) | 14.73 (16.98) |
| Fish, g/d | 44.94 (42.27) | 50.32 (44.12) | 40.52 (35.91) | 49.16 (42.07) |
| Total carotene, μg/d | 2947.82 (1719.66) | 3097.4 (1635.89) | 2706.21 (1470.55) | 2856.66 (1441.64) |
| Vitamin C, mg/d | 87.81 (44.53) | 94.04 (47.48) | 80.62 (40.5) | 88.68 (43.91) |
| Vitamin E, mg/d | 14.53 (5.46) | 14.56 (5.4) | 12.98 (5.2) | 13.12 (4.79) |
MET, metabolic equivalent; SD, standard deviation.
Income level, four categories: Low: less than ¥10,000 CNY per family per year for women and less than ¥500 CNY per person per month for men; lower middle: ¥10,000–19,999 CNY per family per year for women and ¥500–999 CNY per person per month for men; Upper middle: ¥20,000–29,999 CNY per family per year for women and ¥1000–1999 CNY per person per month for men; high: greater than ¥30,000 CNY per family per year for women and more than ¥2000 CNY per person per month for men.
Smoking status was collapsed into two groups in SWHS.
Use of any vitamin supplement.
Dietary nutrients intake (except for energy) was energy-adjusted by residual methods.
The associations between use of dietary vitamins and all-cause mortality by quintiles of dietary antioxidant vitamin intakes are presented in Table 2. For men, after adjustment for potential confounders, we observed inverse associations of total carotene and vitamin C with all-cause mortality. Compared with the lowest quintiles, the corresponding risk reductions in the highest quintile were 17% (HR 0.83; 95% CI, 0.76–0.92, P for trend<0.001) for carotene and 17% (HR 0.83; 95% CI, 0.75–0.91, P for trend<0.001) for vitamin C. Mutual adjustment with all three vitamins did not substantially change the results. Tests for nonlinear associations of total intakes of carotene and vitamin C with all-cause mortality were statistically significant among men. For total carotene, compared with those in the lowest quintile (Q1), risk estimates decreased markedly from Q2 to Q4 but tended to be higher in Q5 (P for nonlinearity<0.001). This U-shaped curve was also observed for vitamin C (P for nonlinearity<0.001). The association between low intake of vitamin E and total mortality was attenuated and no longer significant after adjustment for potential confounders.
Table 2.
Associations of energy-adjusted dietary antioxidant vitamins intake with all-cause mortality in SMHS (2002–2012) and SWHS (1997–2012).
| Variable | Intake of energy-adjusted dietary antioxidant vitaminsa |
P for trend | |||||
|---|---|---|---|---|---|---|---|
| Q1 (lowest) | Q2 | Q3 | Q4 | Q5 (highest) | |||
| Men | |||||||
| Total carotene | Median intake, μg/d | 1392.62 | 2175.57 | 2831.91 | 3615.24 | 5099.66 | |
| Person-years | 98,660 | 98,573 | 98,147 | 99,022 | 100,928 | ||
| Number of deaths | 1040 | 850 | 782 | 727 | 771 | ||
| Model 1b | 1.00 | 0.77 (0.70–0.84) | 0.71 (0.65–0.78) | 0.66 (0.60–0.72) | 0.71 (0.64–0.78) | <0.001 | |
| Model 2c | 1.00 | 0.84 (0.76–0.92) | 0.83 (0.75–0.91) | 0.77 (0.70–0.85) | 0.83 (0.76–0.92) | <0.001 | |
| Model 3d | 1.00 | 0.84 (0.77–0.92) | 0.85 (0.77–0.94) | 0.82 (0.72–0.92) | 0.89 (0.78–1.01) | 0.190 | |
| Vitamin C | Median intake, mg/d | 44.55 | 67.95 | 86.73 | 108.70 | 151.69 | |
| Person-years | 98,527 | 98,135 | 98,315 | 99,027 | 101,326 | ||
| Number of deaths | 1044 | 905 | 791 | 725 | 705 | ||
| Model 1b | 1.00 | 0.80 (0.73–0.88) | 0.69 (0.63–0.76) | 0.65 (0.59–0.71) | 0.69 (0.63–0.76) | <0.001 | |
| Model 2c | 1.00 | 0.88 (0.80–0.96) | 0.80 (0.73–0.88) | 0.76 (0.69–0.84) | 0.83 (0.75–0.91) | <0.001 | |
| Model 3d | 1.00 | 0.88 (0.80–0.96) | 0.81 (0.72–0.90) | 0.77 (0.68–0.88) | 0.84 (0.73–0.96) | 0.042 | |
| Vitamin E | Median intake, mg/d | 8.85 | 11.72 | 13.86 | 16.28 | 20.91 | |
| Person-years | 98,326 | 97,923 | 98,521 | 99,135 | 101,425 | ||
| Number of deaths | 842 | 861 | 802 | 858 | 807 | ||
| Model 1b | 1.00 | 0.90 (0.82–0.99) | 0.83 (0.75–0.91) | 0.86 (0.78–0.94) | 0.88 (0.80–0.97) | 0.014 | |
| Model 2c | 1.00 | 0.95 (0.87–1.05) | 0.91 (0.83–1.01) | 0.95 (0.86–1.04) | 0.95 (0.86–1.05) | 0.424 | |
| Model 3d | 1.00 | 0.98 (0.89–1.08) | 0.96 (0.86–1.06) | 1.01 (0.91–1.11) | 1.03 (0.93–1.15) | 0.408 | |
| Women | |||||||
| Total carotene | Median intake, μg/d | 1333.63 | 2042.42 | 2632.21 | 3330.21 | 4602.66 | |
| Person-years | 205,673 | 206,161 | 205,881 | 205,682 | 205,798 | ||
| Number of deaths | 1465 | 1235 | 1128 | 1087 | 994 | ||
| Model 1b | 1.00 | 0.88 (0.82–0.95) | 0.87 (0.81–0.94) | 0.86 (0.80–0.93) | 0.82 (0.76–0.89) | <0.001 | |
| Model 2c | 1.00 | 0.92 (0.86–1.00) | 0.94 (0.87–1.01) | 0.90 (0.83–0.98) | 0.87 (0.80–0.95) | 0.001 | |
| Model 3d | 1.00 | 0.93 (0.86–1.00) | 0.95 (0.87–1.03) | 0.92 (0.83–1.02) | 0.89 (0.80–1.00) | 0.057 | |
| Vitamin C | Median intake, mg/d | 42.51 | 64.40 | 81.95 | 102.86 | 142.72 | |
| Person-years | 204,701 | 205,846 | 205,635 | 205,975 | 207,038 | ||
| Number of deaths | 1575 | 1257 | 1172 | 1040 | 865 | ||
| Model 1b | 1.00 | 0.83 (0.77–0.89) | 0.84 (0.78–0.91) | 0.81 (0.75–0.88) | 0.76 (0.70–0.83) | <0.001 | |
| Model 2c | 1.00 | 0.86 (0.80–0.93) | 0.9 (0.84–0.98) | 0.90 (0.83–0.97) | 0.83 (0.77–0.91) | <0.001 | |
| Model 3d | 1.00 | 0.86 (0.80–0.93) | 0.91 (0.83–0.99) | 0.90 (0.81–1.00) | 0.84 (0.75–0.94) | 0.008 | |
| Vitamin E | Median intake, mg/d | 8.19 | 10.70 | 12.54 | 14.59 | 18.48 | |
| Person-years | 206,584 | 206,198 | 205,534 | 205,662 | 205,216 | ||
| Number of deaths | 1302 | 1210 | 1190 | 1099 | 1108 | ||
| Model 1b | 1.00 | 0.96 (0.89–1.04) | 0.96 (0.89–1.04) | 0.90 (0.83–0.98) | 0.93 (0.86–1.01) | 0.036 | |
| Model 2c | 1.00 | 1.00 (0.92–1.08) | 1.01 (0.93–1.09) | 0.95 (0.87–1.03) | 0.91 (0.84–0.99) | 0.013 | |
| Model 3d | 1.00 | 1.01 (0.93–1.09) | 1.03 (0.94–1.11) | 0.97 (0.89–1.06) | 0.94 (0.86–1.03) | 0.112 | |
Q, quintile.
Dietary antioxidant vitamins and the intakes of all nutrients and foods were adjusted for total energy using the residual method.
Model 1 adjusted for age (per 5-y) and energy (quartiles).
Model 2 adjusted for age (per 5-y intervals), energy (quartiles), birth cohort (per 10-y intervals), education (4 categories), income (4 categories), occupation (3 categories for men, 4 for women), smoking status (3 categories for men, 2 for women), alcohol intake (3 categories), body mass index (4 categories), waist-hip ratio (3 categories), physical activity (quartiles), history of hypertension (yes/no), diabetes (yes/no), coronary heart disease (yes/no), stroke (yes/no), vitamin supplements use (yes/no), menopause status (yes/no, women only), hormone replacement therapy (yes/no, women only).
Model 3 additionally mutually adjusted for other two vitamins.
For women, associations were weaker than in men, although they were still statistically significant. Compared with the lowest quintiles, the corresponding risk reductions in the highest category were 13% (HR 0.87; 95% CI, 0.80–0.95, P for trend<0.001) for carotene and 17% (HR 0.83; 95% CI, 0.77–0.91, P for trend = 0.004) for vitamin C. Total carotene was associated with all-cause mortality in a nonlinear manner (P for nonlinearity = 0.004), while vitamin C was inversely associated with total death linearly (P for nonlinearity = 0.377). When antioxidant vitamins were mutually adjusted, no substantial change was observed. Dietary vitamin E was not associated with all-cause mortality in women when vitamin C and total carotene were adjusted.
Multivariable analyses of cancer and CVD mortality are presented in Table 3 and Table 4. Significant inverse associations of dietary total carotene and vitamin C with CVD mortality were also observed in both men and women. In contrast, there were no significant associations of dietary total carotene and vitamin C with cancer mortality. As with all-cause mortality, no significant associations were observed between dietary vitamin E intake and mortality from cancer or CVD.
Table 3.
Associations of energy-adjusted dietary antioxidant vitamins intake with cancer mortality in SMHS (2002–2012) and SWHS (1997–2012).
| Variable | Intake of energy-adjusted dietary antioxidant vitaminsa |
P for trend | |||||
|---|---|---|---|---|---|---|---|
| Q1 (lowest) | Q2 | Q3 | Q4 | Q5 (highest) | |||
| Men | |||||||
| Total carotene | Number of deaths | 434 | 351 | 332 | 326 | 346 | |
| Model 1b | 1.00 | 0.78 (0.68–0.90) | 0.74 (0.64–0.86) | 0.72 (0.62–0.83) | 0.76 (0.66–0.87) | <0.001 | |
| Model 2c | 1.00 | 0.85 (0.74–0.98) | 0.85 (0.74–0.98) | 0.84 (0.73–0.97) | 0.91 (0.79–1.05) | 0.289 | |
| Model 3d | 1.00 | 0.85 (0.73–0.98) | 0.84 (0.72–0.99) | 0.83 (0.69–1.00) | 0.89 (0.72–1.08) | 0.413 | |
| Vitamin C | Number of deaths | 430 | 360 | 361 | 315 | 323 | |
| Model 1b | 1.00 | 0.81 (0.70–0.93) | 0.80 (0.69–0.92) | 0.70 (0.61–0.82) | 0.76 (0.66–0.88) | <0.001 | |
| Model 2c | 1.00 | 0.88 (0.76–1.01) | 0.91 (0.79–1.05) | 0.83 (0.71–0.96) | 0.91 (0.79–1.06) | 0.205 | |
| Model 3d | 1.00 | 0.87 (0.76–1.01) | 0.90 (0.76–1.06) | 0.81 (0.67–0.98) | 0.89 (0.72–1.09) | 0.382 | |
| Vitamin E | Number of deaths | 368 | 353 | 313 | 373 | 382 | |
| Model 1b | 1.00 | 0.88 (0.76–1.02) | 0.78 (0.67–0.90) | 0.89 (0.77–1.03) | 0.96 (0.83–1.10) | 0.899 | |
| Model 2c | 1.00 | 0.92 (0.79–1.07) | 0.84 (0.72–0.99) | 0.97 (0.84–1.12) | 1.04 (0.90–1.20) | 0.316 | |
| Model 3d | 1.00 | 0.93 (0.80–1.08) | 0.86 (0.74–1.01) | 1.00 (0.86–1.16) | 1.08 (0.92–1.26) | 0.144 | |
| Women | |||||||
| Total carotene | Number of deaths | 596 | 517 | 522 | 503 | 457 | |
| Model 1b | 1.00 | 0.92 (0.82–1.04) | 0.98 (0.87–1.10) | 0.96 (0.85–1.08) | 0.89 (0.79–1.01) | 0.135 | |
| Model 2c | 1.00 | 0.95 (0.84–1.07) | 1.02 (0.91–1.15) | 0.99 (0.88–1.12) | 0.92 (0.82–1.05) | 0.358 | |
| Model 3d | 1.00 | 0.95 (0.84–1.07) | 1.03 (0.91–1.18) | 1.01 (0.87–1.17) | 0.95 (0.80–1.12) | 0.566 | |
| Vitamin C | Number of deaths | 630 | 520 | 550 | 476 | 419 | |
| Model 1b | 1.00 | 0.88 (0.78–0.99) | 0.99 (0.88–1.11) | 0.91 (0.80–1.02) | 0.85 (0.75–0.97) | 0.034 | |
| Model 2c | 1.00 | 0.90 (0.80–1.01) | 1.03 (0.92–1.16) | 0.95 (0.84–1.08) | 0.90 (0.79–1.02) | 0.230 | |
| Model 3d | 1.00 | 0.89 (0.79–1.01) | 1.01 (0.88–1.15) | 0.92 (0.78–1.08) | 0.87 (0.73–1.03) | 0.104 | |
| Vitamin E | Number of deaths | 565 | 528 | 514 | 497 | 491 | |
| Model 1b | 1.00 | 0.99 (0.88–1.12) | 0.98 (0.87–1.10) | 0.95 (0.84–1.08) | 0.93 (0.83–1.05) | 0.208 | |
| Model 2c | 1.00 | 1.02 (0.90–1.15) | 1.01 (0.90–1.15) | 0.99 (0.87–1.12) | 0.95 (0.84–1.07) | 0.307 | |
| Model 3d | 1.00 | 1.02 (0.90–1.15) | 1.02 (0.89–1.15) | 0.99 (0.87–1.13) | 0.95 (0.83–1.08) | 0.342 | |
Q, quintile.
Dietary antioxidant vitamins and the intakes of all nutrients and foods were adjusted for total energy using the residual method.
Model 1 adjusted for age (per 5-y) and energy (quartiles).
Model 2 adjusted for age (per 5-y intervals), energy (quartiles), birth cohort (per 10-y intervals), education (4 categories), income (4 categories), occupation (3 categories for men, 4 for women), smoking status (3 categories for men, 2 for women), alcohol intake (3 categories), body mass index (4 categories), waist-hip ratio (3 categories), physical activity (quartiles), history of hypertension (yes/no), diabetes (yes/no), coronary heart disease (yes/no), stroke (yes/no), vitamin supplements use (yes/no), menopause status (yes/no, women only), hormone replacement therapy (yes/no, women only).
Model 3 additionally mutually adjusted for other two vitamins.
Table 4.
Associations of energy-adjusted dietary antioxidant vitamins intake with CVD mortality in SMHS (2002–2012) and SWHS (1997–2012).
| Variable | Intake of energy-adjusted dietary antioxidant vitaminsa |
P for trend | |||||
|---|---|---|---|---|---|---|---|
| Q1 (lowest) | Q2 | Q3 | Q4 | Q5 (highest) | |||
| Men | |||||||
| Total carotene | Number of deaths | 373 | 301 | 242 | 232 | 231 | |
| Model 1b | 1.00 | 0.75 (0.64–0.87) | 0.61 (0.52–0.71) | 0.58 (0.49–0.68) | 0.59 (0.50–0.70) | <0.001 | |
| Model 2c | 1.00 | 0.81 (0.69–0.94) | 0.70 (0.59–0.82) | 0.67 (0.57–0.80) | 0.67 (0.56–0.79) | <0.001 | |
| Model 3d | 1.00 | 0.82 (0.71–0.96) | 0.76 (0.64–0.91) | 0.80 (0.64–0.99) | 0.82 (0.65–1.03) | 0.115 | |
| Vitamin C | Number of deaths | 362 | 339 | 240 | 226 | 212 | |
| Model 1b | 1.00 | 0.86 (0.74–0.99) | 0.59 (0.50–0.70) | 0.58 (0.49–0.68) | 0.61 (0.51–0.72) | <0.001 | |
| Model 2c | 1.00 | 0.93 (0.80–1.08) | 0.69 (0.58–0.82) | 0.66 (0.56–0.78) | 0.71 (0.59–0.84) | <0.001 | |
| Model 3d | 1.00 | 0.94 (0.80–1.09) | 0.72 (0.59–0.87) | 0.70 (0.56–0.88) | 0.76 (0.59–0.97) | 0.047 | |
| Vitamin E | Number of deaths | 287 | 296 | 281 | 267 | 248 | |
| Model 1b | 1.00 | 0.89 (0.75–1.04) | 0.83 (0.70–0.98) | 0.76 (0.64–0.90) | 0.79 (0.67–0.94) | 0.002 | |
| Model 2c | 1.00 | 0.96 (0.82–1.14) | 0.94 (0.79–1.11) | 0.86 (0.73–1.02) | 0.87 (0.73–1.04) | 0.065 | |
| Model 3d | 1.00 | 1.02 (0.86–1.20) | 1.03 (0.86–1.22) | 0.99 (0.83–1.18) | 1.03 (0.86–1.24) | 0.839 | |
| Women | |||||||
| Total carotene | Number of deaths | 476 | 390 | 337 | 312 | 304 | |
| Model 1b | 1.00 | 0.87 (0.76–0.99) | 0.83 (0.72–0.95) | 0.79 (0.68–0.91) | 0.82 (0.71–0.94) | 0.003 | |
| Model 2c | 1.00 | 0.91 (0.80–1.04) | 0.89 (0.78–1.03) | 0.82 (0.71–0.94) | 0.85 (0.74–0.99) | 0.014 | |
| Model 3d | 1.00 | 0.92 (0.80–1.05) | 0.92 (0.79–1.07) | 0.86 (0.72–1.03) | 0.91 (0.74–1.12) | 0.353 | |
| Vitamin C | Number of deaths | 532 | 393 | 348 | 293 | 253 | |
| Model 1b | 1.00 | 0.77 (0.67–0.88) | 0.76 (0.66–0.87) | 0.71 (0.62–0.82) | 0.72 (0.62–0.84) | <0.001 | |
| Model 2c | 1.00 | 0.80 (0.70–0.91) | 0.80 (0.70–0.92) | 0.79 (0.68–0.92) | 0.78 (0.67–0.91) | 0.002 | |
| Model 3d | 1.00 | 0.80 (0.70–0.91) | 0.80 (0.68–0.94) | 0.79 (0.65–0.95) | 0.77 (0.62–0.95) | 0.033 | |
| Vitamin E | Number of deaths | 426 | 366 | 369 | 315 | 343 | |
| Model 1b | 1.00 | 0.88 (0.77–1.02) | 0.92 (0.80–1.06) | 0.80 (0.69–0.92) | 0.90 (0.78–1.04) | 0.097 | |
| Model 2c | 1.00 | 0.92 (0.80–1.06) | 0.97 (0.84–1.12) | 0.84 (0.72–0.97) | 0.89 (0.77–1.03) | 0.067 | |
| Model 3d | 1.00 | 0.94 (0.81–1.08) | 1.00 (0.87–1.16) | 0.88 (0.75–1.03) | 0.95 (0.81–1.11) | 0.414 | |
CVD, cardiovascular disease; Q, quintile.
Dietary antioxidant vitamins and the intakes of all nutrients and foods were adjusted for total energy using the residual method.
Model 1 adjusted for age (per 5-y) and energy (quartiles).
Model 2 adjusted for age (per 5-y intervals), energy (quartiles), birth cohort (per 10-y intervals), education (4 categories), income (4 categories), occupation (3 categories for men, 4 for women), smoking status (3 categories for men, 2 for women), alcohol intake (3 categories), body mass index (4 categories), waist-hip ratio (3 categories), physical activity (quartiles), history of hypertension (yes/no), diabetes (yes/no), coronary heart disease (yes/no), stroke (yes/no), vitamin supplements use (yes/no), menopause status (yes/no, women only), hormone replacement therapy (yes/no, women only).
Model 3 additionally mutually adjusted for other two vitamins.
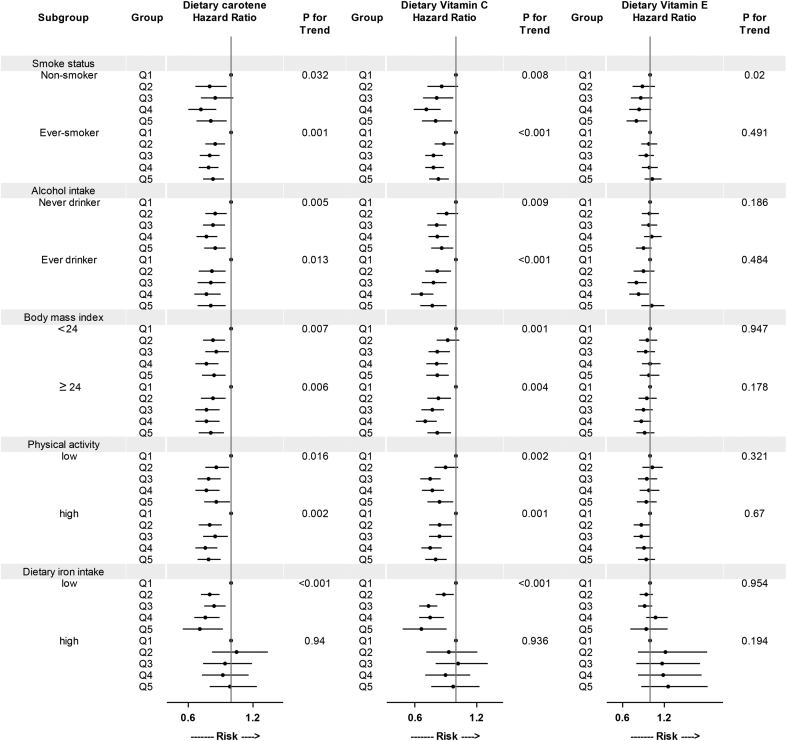
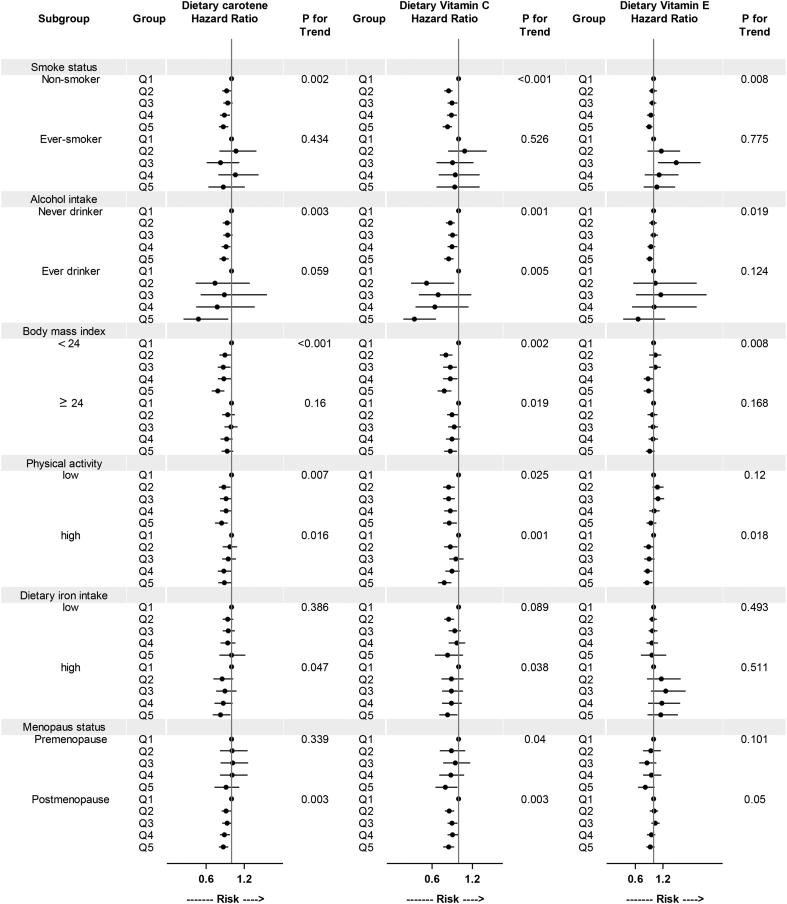
Subgroup analysis of the associations of total carotene, vitamin C, and vitamin E with all-cause mortality stratified by baseline subgroups are presented in Fig. 1 and Fig. 2. The associations for total carotene and vitamin C varied little across smoking status, alcohol intake, BMI, intake of dietary iron, or physical activity in both men and women. Similar results were also observed between premenopausal and postmenopausal women. We observed an inverse association between dietary vitamin E intake and all-cause mortality among never smokers (both in men and in women).
Fig. 1.
Subgroup analysis of associations of energy-adjusted dietary antioxidant vitamins with all-cause mortality in men (2002–2012). HRs for all-cause mortality are presented for dietary antioxidant vitamins intake. The multivariate model was adjusted for covariates as in Table 2. High and low levels of physical activity and dietary iron intake were split at median.
Fig. 2.
Subgroup analysis of associations of energy-adjusted dietary antioxidant vitamins with all-cause mortality in women (1997–2012). HRs for all-cause mortality are presented for dietary antioxidant vitamins intake. The multivariate model was adjusted for covariates as in Table 2. High and low levels of physical activity and dietary iron intake were split at median.
When we reanalyzed the data restricted to participants who reported no use of any vitamin supplements at the baseline survey (excluded 793 deaths in men and 1207 deaths in women), results were similar to those of the main analyses. Exclusion of deaths that occurred within the first 3 years of follow-up (including 1046 deaths in men and 731 deaths in women) did not substantially change the findings. Moreover, we observed similar patterns after excluding participants with a prior history of diabetes, stroke, or coronary heart disease (including 1418 deaths in men and 2094 deaths in women). We additionally adjusted for other dietary constituents (such as dietary fiber, monounsaturated fatty acid, and polyunsaturated fatty acid), with little effect on our main findings. Results of sensitivity analyses are provided in eTable 4.
4. Discussion
In the present study, using data from two population-based prospective cohorts of 134,358 middle-aged and elderly Chinese adults in urban Shanghai, we assessed the associations of dietary antioxidant vitamin intakes and risk of mortality from all causes, cancer, and CVD. We found that total carotene and vitamin C intake were inversely associated with all-cause and CVD mortality in both men and women, but no significant association was seen for cancer mortality. Dietary vitamin E intake was not associated with risk of mortality. Similar association patterns were observed in subgroup and sensitivity analyses.
The results for dietary vitamin C intake in the present study were consistent in direction and magnitude with those from other cohort studies conducted in Europe12, 22, 23 or the United States.24, 25, 26 However, some studies have reported null associations between vitamin C and total mortality.7, 8, 9, 13, 27 The inverse association between vitamin C and CVD mortality was in accordance with a previous study performed in Japan.10 For cancer mortality, we confirmed the null association between dietary vitamin C and cancer mortality reported in previous studies.7, 13, 26, 28
For dietary total carotene, three prospective studies found that total carotene was associated with all-cause mortality, with a reduction of 10–30%.12, 22, 28 The protective effect was mainly driven by reductions in CVD mortality11 rather than cancer mortality.7, 13
We observed a favorable effect of dietary vitamin E on all-cause mortality in women (model 2). However, when we additionally adjusted dietary vitamin C and carotene intake, the benefits disappeared. Dietary vitamin C or carotene intake may confound the mortality-vitamin E association. The null association was consistent with most of the previous studies,7, 9, 12, 22, 27, 28 with the exception of one study that reported a slight inverse association.23, 27
Inconsistencies between the results of our studies and those of previous studies may be partly explained by baseline nutritional status. Of all the studies exploring the associations of dietary antioxidant vitamins with risk of mortality, only one study was conducted in Asia.10 Some studies conducted in the United States or Europe contended that the lack of association between vitamin intake and mortality was due to the fact that the population was well-fed.8, 27 In our cohort, less than five percent of participants were below recommended nutritional intakes. Therefore, additional vitamin E intake may have no beneficial health effects.
Other studies demonstrated that East Asian and Western diets were significantly different in composition of micronutrients.29 Compared with Japan and the United States, the mean intake of beta-carotene and vitamin C were lower both among men and women in China. However, for vitamin E, the mean intake was higher than in the United States and the United Kingdom. These results were consistent with those of another study conducted in 2015.30 Moreover, in the studies conducted in Western countries7, 13, 22, 27 or Japan,10 the median intake of vitamin C was much higher than in our cohort. Additionally, different food sources of antioxidant vitamins may be another explanation. In China, vegetables, especially light colored vegetables, remained the top contributor of dietary vitamin C, providing more than 80% of the total intake, rather than fruits.31 In the United States, the major contributors of vitamin C intake were orange juice, fruit drinks and tomatoes.32 We also included tea drinking in our multiple regression models, but this had little effect on our results (data not shown). The main food source of vitamin E may also be different between Eastern and Western diet.33, 34
The ability of antioxidant vitamins, such as carotene and vitamin C, to prevent oxidative damage is thought to be involved in the pathological process of many chronic diseases.29 In previous studies, the lack of association between vitamin intake and mortality may be due to the study of well-nourished cohort members.30 For poorly nourished populations, previous studies have suggested beneficial effects of antioxidant vitamins in relation to mortality.31 We found a non-linear relationship of total carotene and vitamin C intake with mortality in men. Antioxidant vitamins in the human body may need to be maintained within a narrow concentration range for normal physiological functioning.35 Therefore, additional intake may produce plasma saturation without any biological effect.32 There would be no additional benefits in participants in the top quintiles of carotene or vitamin C intake. For cause-specific mortality, dietary carotene and vitamin C intake may be a protective factor for CVD. Some studies indicated some beneficial impact of dietary carotene and vitamin C on specific cancers, such as prostate cancer36 and head-neck cancer,37 but not for all cancers.38, 39 Combining all cancer sites may dilute the observed associations for specific cancers. The conflicting results regarding the association of antioxidant vitamin intake and cancer mortality need to be further investigated.
Strengths of the present study included the population-based, prospective study design, the relatively large sample size, and the high response rates for both baseline recruitment and follow-up. We had nearly complete ascertainment of deaths, and the FFQs used in our study have relatively good validity and reliability.17, 18 Additionally, few participants (9003 men and 14,707 women) in our population used vitamin supplements. Thus, our study population provided a unique opportunity to evaluate associations of dietary vitamin intakes with mortality, with no confounding from the use of dietary supplements. Furthermore, we adjusted for a wide range of potential confounders in the analyses, including important lifestyle factors and other dietary factors associated with mortality.
Some limitations of our research must also be considered. First, although antioxidant vitamin intakes were estimated using a validated cohort-specific FFQ, misclassification in the estimate of vitamin intakes cannot be ruled out, which may attenuate the real associations of total carotene and vitamin C intakes with the risk of mortality. Second, we tested multiple hypotheses in our present study, so observed associations could have arisen by chance. Therefore, results must be interpreted with caution. Additionally, we cannot exclude residual confounding of unadjusted nutrients (such as polyphenol), even though we had included the important confounders in our model. Finally, dietary intake assessed at baseline may have been affected by preclinical conditions. However, excluding death during the first 3 years of observation and excluding participants with comorbidity at baseline did not alter the results of the overall analyses.
5. Conclusions
In summary, in these two population-based cohort studies of 134,358 men and women, we found that dietary carotene and vitamin C intakes were inversely associated with death from all causes and CVD in middle-aged or older people from China. Based on existing evidence, higher intake of dietary total carotene and vitamin C may increase longevity in China.
Funding
This work was supported by the funds of State Key Laboratory of Oncogene and Related Genes (No. 91-15-10) and the Shanghai Health Bureau Key Disciplines and Specialties Foundation, and grants from US National Institutes of Health (R37 CA070867 and UM1 CA182910, R01 CA082729 and UM1 CA173640).
Conflicts of interest
None declared.
Acknowledgements
We would like to thank the participants and the staffs from the Shanghai Women's and Men's Health Studies for their contribution to this research.
Footnotes
Peer review under responsibility of the Japan Epidemiological Association.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.je.2016.10.002.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Sabharwal S.S., Schumacker P.T. Mitochondrial ROS in cancer: initiators, amplifiers or an Achilles' heel? Nat Rev Cancer. 2014;14:709–721. doi: 10.1038/nrc3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mangge H. Antioxidants, inflammation and cardiovascular disease. World J Cardiol. 2014;6:462–477. doi: 10.4330/wjc.v6.i6.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sies H. Oxidative stress: oxidants and antioxidants. Exp Physiol. 1997;82:291–295. doi: 10.1113/expphysiol.1997.sp004024. [DOI] [PubMed] [Google Scholar]
- 4.Peng C., Wang X., Chen J. Biology of ageing and role of dietary antioxidants. Biomed Res Int. 2014;2014:831–841. doi: 10.1155/2014/831841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okuda N., Miura K., Okayama A. Fruit and vegetable intake and mortality from cardiovascular disease in Japan: a 24-year follow-up of the NIPPON DATA80 Study. Eur J Clin Nutr. 2015;69:482–488. doi: 10.1038/ejcn.2014.276. [DOI] [PubMed] [Google Scholar]
- 6.Wang X., Ouyang Y., Liu J. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: systematic review and dose-response meta-analysis of prospective cohort studies. BMJ. 2014;349:g4490. doi: 10.1136/bmj.g4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Genkinger J.M., Platz E.A., Hoffman S.C., Comstock G.W., Helzlsouer K.J. Fruit, vegetable, and antioxidant intake and all-cause, cancer, and cardiovascular disease mortality in a community-dwelling population in Washington County, Maryland. Am J Epidemiol. 2004;160:1223–1233. doi: 10.1093/aje/kwh339. [DOI] [PubMed] [Google Scholar]
- 8.Paganini-Hill A., Kawas C.H., Corrada M.M. Antioxidant vitamin intake and mortality: the leisure world cohort study. Am J Epidemiol. 2015;181:120–126. doi: 10.1093/aje/kwu294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henriquez-Sanchez P., Sanchez-Villegas A., Ruano-Rodriguez C. Dietary total antioxidant capacity and mortality in the PREDIMED study. Eur J Nutr. 2016;55:227–236. doi: 10.1007/s00394-015-0840-2. [DOI] [PubMed] [Google Scholar]
- 10.Kubota Y., Iso H., Date C. Dietary intakes of antioxidant vitamins and mortality from cardiovascular disease: the Japan collaborative cohort study (JACC) study. Stroke. 2011;42:1665–1672. doi: 10.1161/STROKEAHA.110.601526. [DOI] [PubMed] [Google Scholar]
- 11.Buijsse B., Feskens E.J.M., Kwape L., Kok F.J., Kromhout D. Both alpha- and beta-carotene, but not tocopherols and vitamin C, are inversely related to 15-year cardiovascular mortality in Dutch elderly men. J Nutr. 2008;138:344–350. doi: 10.1093/jn/138.2.344. [DOI] [PubMed] [Google Scholar]
- 12.Bates C.J., Hamer M., Mishra G.D. Redox-modulatory vitamins and minerals that prospectively predict mortality in older British people: the national diet and nutrition survey of people aged 65 years and over. Br J Nutr. 2011;105:123–132. doi: 10.1017/S0007114510003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stepaniak U., Micek A., Grosso G. Antioxidant vitamin intake and mortality in three central and eastern European urban populations: the HAPIEE study. Eur J Nutr. 2016;55:547–560. doi: 10.1007/s00394-015-0871-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goyal A., Terry M.B., Siegel A.B. Serum antioxidant nutrients, vitamin A, and mortality in U.S. adults. Cancer Epidemiol Biomarkers Prev. 2013;22:2202–2211. doi: 10.1158/1055-9965.EPI-13-0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shu X.O., Li H., Yang G. Cohort profile: the Shanghai men's health study. Int J Epidemiol. 2015;44:810–818. doi: 10.1093/ije/dyv013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng W., Chow W.H., Yang G. The Shanghai Women's health study: rationale, study design, and baseline characteristics. Am J Epidemiol. 2005;162:1123–1131. doi: 10.1093/aje/kwi322. [DOI] [PubMed] [Google Scholar]
- 17.Villegas R., Yang G., Liu D. Validity and reproducibility of the food-frequency questionnaire used in the Shanghai men's health study. Br J Nutr. 2007;97:993–1000. doi: 10.1017/S0007114507669189. [DOI] [PubMed] [Google Scholar]
- 18.Shu X.O., Yang G., Jin F. Validity and reproducibility of the food frequency questionnaire used in the Shanghai women's health study. Eur J Clin Nutr. 2004;58:17–23. doi: 10.1038/sj.ejcn.1601738. [DOI] [PubMed] [Google Scholar]
- 19.Yang Y.X., Wang G.Y., Pan X.C. Peking University Medical Press; Beijing, China: 2002. Chinese Food Composition. [Google Scholar]
- 20.International Classification of Diseases: Manual of the International Statistical Classification of Diseases, Injuries, and Causes of Death. World Health Organization; Geneva, Switzerland: 1997. Ninth Revision. [Google Scholar]
- 21.Willett W.C., Howe G.R., Kushi L.H. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65:1220S–1228S. doi: 10.1093/ajcn/65.4.1220S. 1229S-1231S. [DOI] [PubMed] [Google Scholar]
- 22.Agudo A., Cabrera L., Amiano P. Fruit and vegetable intakes, dietary antioxidant nutrients, and total mortality in Spanish adults: findings from the Spanish cohort of the European prospective investigation into cancer and nutrition (EPIC-Spain) Am J Clin Nutr. 2007;85:1634–1642. doi: 10.1093/ajcn/85.6.1634. [DOI] [PubMed] [Google Scholar]
- 23.Todd S., Woodward M., Tunstall-Pedoe H., Bolton-Smith C. Dietary antioxidant vitamins and fiber in the etiology of cardiovascular disease and all-causes mortality: results from the Scottish Heart Health Study. Am J Epidemiol. 1999;150:1073–1080. doi: 10.1093/oxfordjournals.aje.a009931. [DOI] [PubMed] [Google Scholar]
- 24.Rexrode K.M., Manson Antioxidants and coronary heart disease: observational studies. J Cardiovasc Risk. 1996;3:363–367. doi: 10.1177/174182679600300405. [DOI] [PubMed] [Google Scholar]
- 25.Pandey D.K., Shekelle R., Selwyn B.J., Tangney C., Stamler J. Dietary vitamin C and beta-carotene and risk of death in middle-aged men. The Western Electric Study. Am J Epidemiol. 1995;142:1269–1278. doi: 10.1093/oxfordjournals.aje.a117594. [DOI] [PubMed] [Google Scholar]
- 26.Enstrom J.E., Kanim L.E., Klein M.A. Vitamin C intake and mortality among a sample of the United States population. Epidemiology. 1992;3:194–202. doi: 10.1097/00001648-199205000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Roswall N., Olsen A., Christensen J. Micronutrient intake in relation to all-cause mortality in a prospective danish cohort. Food Nutr Res. 2012;56:5466. doi: 10.3402/fnr.v56i0.5466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sahyoun N.R., Jacques P.F., Russell R.M. Carotenoids, vitamins C and E, and mortality in an elderly population. Am J Epidemiol. 1996;144:501–511. doi: 10.1093/oxfordjournals.aje.a008957. [DOI] [PubMed] [Google Scholar]
- 29.Zhou B.F., Stamler J., Dennis B. Nutrient intakes of middle-aged men and women in China, Japan, United Kingdom, and United States in the late 1990s: the INTERMAP study. J Hum Hypertens. 2003;17:623–630. doi: 10.1038/sj.jhh.1001605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang R., Wang Z., Fei Y. The difference in nutrient intakes between Chinese and mediterranean, Japanese and American diets. Nutrients. 2015;7:4661–4688. doi: 10.3390/nu7064661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma Y.X., Zhang B., Wang H.J. Trend on vitamin C intake among Chinese population aged 50-79 years in 9 provinces, from 1991 to 2009. Chin J Epidemiol. 2012;33:496–500. [PubMed] [Google Scholar]
- 32.Cotton P.A., Subar A.F., Friday J.E., Cook A. Dietary sources of nutrients among US adults, 1994 to 1996. J Am Diet Assoc. 2004;104:921–930. doi: 10.1016/j.jada.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 33.Subar A.F., Krebs-Smith S.M., Cook A., Kahle L.L. Dietary sources of nutrients among US adults, 1989 to 1991. J Am Diet Assoc. 1998;98:537–547. doi: 10.1016/S0002-8223(98)00122-9. [DOI] [PubMed] [Google Scholar]
- 34.Zhai F.Y., Yang X.G. People's Medical Publishing House; Beijing: 2006. Dietary and Nutrient Intake Status in 2002: The China National Nutrition and Health Survey (Report Two) [Google Scholar]
- 35.Bjelakovic G., Gluud C. Vitamin and mineral supplement use in relation to all-cause mortality in the Iowa Women's Health Study. Arch Intern Med. 2011;171:1633–1634. doi: 10.1001/archinternmed.2011.459. [DOI] [PubMed] [Google Scholar]
- 36.Bai X.Y., Qu X., Jiang X. Association between dietary vitamin C intake and risk of prostate Cancer: a meta-analysis involving 103,658 subjects. J Cancer. 2015;6:913–921. doi: 10.7150/jca.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Munter L., Maasland D.H., van den Brandt P.A., Kremer B., Schouten L.J. Vitamin and carotenoid intake and risk of head-neck cancer subtypes in the Netherlands cohort study. Am J Clin Nutr. 2015;102:420–432. doi: 10.3945/ajcn.114.106096. [DOI] [PubMed] [Google Scholar]
- 38.Hua Y., Wang G., Jiang W., Huang J., Chen G., Lu C. Vitamin C intake and pancreatic cancer risk: a meta-analysis of published case-control and cohort studies. Plos one. 2016;11:e148816. doi: 10.1371/journal.pone.0148816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeegers M.P., Goldbohm R.A., van den Brandt P.A. Are retinol, vitamin C, vitamin E, folate and carotenoids intake associated with bladder cancer risk? Results from the Netherlands cohort study. Br J Cancer. 2001;85:977–983. doi: 10.1054/bjoc.2001.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.