Abstract
Background
Estimating the current global prevalence of metabolic syndrome (MetS), and its components, among rheumatoid arthritis (RA) patients is necessary in order to formulate preventative strategies and to ensure there are adequate community resources available for these patients. Furthermore, the association between RA and MetS is controversial and has not previously been comprehensively assessed. Therefore, the present study aimed to: 1) determine the prevalence of MetS, and its components, among RA patients across the world 2) update the odds ratio of MetS in RA patients, compared to healthy controls, using a comprehensive systematic review and meta-analysis.
Methods
International databases, including: the Web of Science, PubMed, Scopus, Embase, CINAHL and other relevant databases were searched to identify English language articles which reported the prevalence and risk of MetS in RA patients between January 2000 and August 2016. The meta-analysis only included studies which clearly described the time and location of the study, utilised adequate sampling strategies, and appropriate statistical analyses.
Results
The meta-analyses of prevalence (70 studies [n = 12612]) and risk (43 studies [n = 35220]) of MetS in RA patients were undertaken separately. The overall pooled prevalence of MetS was 30.65% (95% CI: 27.87–33.43), but this varied from 14.32% (95% CI: 10.59–18.05) to 37.83% (95% CI: 31.05–44.61), based upon the diagnostic criteria used. The prevalence of MetS also varied slightly between males (31.94%, 95% CI: 24.37–39.51) and females (33.03%, 95% CI: 28.09–37.97), but this was not statistically significant. The overall pooled odds ratio (OR) of MetS in RA patients, compared to healthy controls, was 1.44 (95% CI: 1.20–1.74), but this ranged from 0.70 (95% CI: 0.27–1.76) to 4.09 (95% CI: 2.03–8.25), depending on the criteria used. The mean age and diagnostic criteria of MetS were identified as sources of heterogeneity in the estimated odds ratios between studies (P<0.05).
Conclusions
According to the high prevalence of MetS in RA patients, and high risk of MetS, measuring metabolic syndrome in RA patients is strongly recommended. Furthermore, as high waist circumference (WC) is the most common metabolic syndrome component, more attention must be paid to nutrition and weight loss among those with RA.
Introduction
Metabolic syndrome (MetS) is comprised of a group of risk factors for type 2 diabetes and cardiovascular diseases, including insulin resistance, abdominal obesity, dyslipidemia, blood pressure, and impaired fasting glucose[1]. The most common clinical manifestations of MetS include: abdominal obesity, hypertriglyceridaemia, reduced high-density lipoprotein cholesterol (HDL-C), hyperglycaemia, and high blood pressure (BP)[2]. MetS is responsible for a three-fold increase in the risk of atherosclerotic cardiovascular diseases (CVDs) and increased mortality from CVD, as well as all-causes, compared to the general population [3]. MetS is also associated with a fourfold increased relative risk of developing diabetes [4, 5]. There are eight commonly used definitions for MetS, but the National Cholesterol Education Programme-Adult Treatment Panel III (NCEP ATP III) and the International Diabetes Federation (IDF) definitions are the most commonly used [6]. These definitions have many similarities, but they differ on several components and on the cut-off points used (Table 1).
Table 1. Summary of the MetS definitions.
| Definitions | WHO | NCEP-ATP III | IDF | EGIR | AACE | AHA/NHLBI | ATP III | JS 2009 |
|---|---|---|---|---|---|---|---|---|
| Number of Criteria | Two or more of: | Three or more of: | Two or more of | Two or more of: | Obesity and two or more of: | Three or more of: | Three or more of: | Three or more of: |
| Obesity | BMI > 30 and/or WHR > 0.9 (men), WHR > 0.85 (women) | WC ≥ 102 cm (men), WC ≥ 88 cm (women | WC ≥ 94 cm men, WC ≥ 80 cm women | WC ≥ 94 cm (men, WC ≥80 cm (women) | WC ≥ 102 cm (men), WC ≥ 88 cm (women | BMI ≥ 30 kg/m2 | WC ≥ 102 cm (men), WC ≥ 88 cm (women | Population- and country-specific definitions |
| Blood pressure mmhg | ≥ 140/90 | ≥ 130/85 or treatment | ≥130/≥85 or treatment | ≥ 140/90 | ≥ 130/85 or treatment | ≥130/85 mmHg or previous hypertension diagnosis | ≥ 130/85 or treatment | ≥ 130/85 or treatment |
| Dyslipidmia: | ||||||||
| HDL-C | ≥ 35 mg/dL (0.9 mmol/L) in men or ≥ 39 mg/dL (≥ 1.0 mmol/L) in women | ≥ 40 mg/dL (1.03 mol/L) in men, ≥ 50 mg/dL (1.29 mmol/L) in women, or treatment | ≥ 40 mg/dL (1.03 mol/L) in men, ≥ 50 mg/dL (1.29 mmol/L) in women, or treatment | ≥ 39 mg/dL (1.0 mmol/L) or treatment | ≥ 40 mg/dL (1.03 mol/L) in men, ≥ 50 mg/dL (1.29 mmol/L) in women, or treatment | ≥ 40 mg/dL (1.03 mol/L) in men, ≥ 50 mg/dL (1.29 mmol/L) in women | ≥ 40 mg/dL (1.03 mol/L) in men, ≥ 50 mg/dL (1.29 mmol/L) in women | ≥ 40 mg/dL (1.03 mol/L) in men, ≥ 50 mg/dL (1.29 mmol/L) in women, or treatment |
| Triglycerides | ≥178 mg/dL(2.0 mmol/L) or treatment | ≥150 mg/dL (1.7 mmol/L) or treatment | ≥150 mg/dL (1.7 mmol/L) or treatment | ≥150 mg/dL (1.7 mmol/L) | ≥150 mg/dL (1.7 mmol/L) or treatment | ≥150 mg/dL (1.7 mmol/L) or treatment | ≥150 mg/dL (1.7 mmol/L) | ≥150 mg/dL (1.7 mmol/L) or treatment |
| Glucose Intolerance or Fasting Plasma Glucose | ≥110 mg/dL (6.1 mmol/l), DM, IGT, IR | ≥100 mg/dL (5.6 mmol/L) or T2D | ≥100 mg/dL (5.6 mmol/L) or T2D | ≥110 mg/dL (6.1 mmol/L) | ≥110 mg/dL (6.1 mmol/l), or treatment | ≥100 mg/dL (5.6 mmol/L) or T2D | ≥110 mg/dL (6.1 mmol/L) | ≥100 mg/dL (5.6 mmol/L) or T2D |
BMI = body mass index; JC = Joint Consensus; DM = diabetes mellitus; EGIR = European Group against Insulin Resistance; HDL-C = high-density lipoprotein cholesterol; IDF = International Diabetes Federation; IGT = impaired glucose tolerance; IR = insulin resistance; NCEP ATPIII = National Cholesterol Education Program Adult Treatment Panel; AACE = American Association of Clinical Endocrinologists; AHA/NHLBI = The American Heart Association / National Heart, Lung, and Blood Institute; JS = Joint Statement; T2 D, type II diabetes mellitus; WC = waist circumference; WHO = World Health Organization; WHR = waist hip ratio.
Therefore, although we could expect slight differences in prevalence rates, according to the criteria used in each study, genetic and geographical differences may also contribute to differences in the rates of MetS. For example, using the ATP III definition, Ford et al. reported the prevalence rate of metabolic syndrome in the USA to be 34.3% [3], while Tillin et al. reported the age-adjusted rates were 18.4% for men and 14.4% for women among Europeans, 28.8% for men and 31.8% for women in South Asians, and 15.5% for men and 23.4% for women in African-Caribbeans. Further, the prevalence rate was reported to be 15.7% in Taiwan, using the same criteria[7, 8].
Rheumatoid arthritis (RA) is a chronic inflammatory disorder of unknown etiology [9] that has a prevalence rate of approximately 0.5 to 1% [10]. Rheumatoid arthritis and metabolic syndrome are considered to be diseases with common traits that can increase the risk of cardiovascular disease[11], with previous research showing an association between the two[12]. Higher frequencies of insulin resistance and MetS have been reported in patients with RA [12, 13], with the frequency of MetS in RA patients ranging from 14 to 56% [14]. This variation can be explained by differences in the definition of MetS, along with differences in ethnicity, geographic area, study design, and study population. However, although many studies have reported a higher prevalence of MetS among RA patients, compared to the general population [15, 16], a number of studies have reported a higher prevalence of MetS in the healthy controls [2].
Research measuring the prevalence of MetS in RA patients has resulted in a wide range of estimates across the world. In addition, research measuring the prevalence of metabolic syndrome using a large sample size is rare. Furthermore, there have been very few meta-analyses on the prevalence of MetS in patients with rheumatoid arthritis [11]. Therefore, the present study aimed to: 1) determine the prevalence of MetS, and its components, in RA patients across the world 2) update the odds ratio of MetS in RA patients, compared to healthy controls, using a comprehensive systematic review and meta-analysis.
Methods
Search strategy and study selection
The current systematic review and meta-analysis was conducted according to PRISMA guidelines [17]. A systematic review was undertaken of English-language medical literature published between January 2000 and August 2016 to identify scientific papers reporting the prevalence and risk of metabolic syndrome and its components (i.e., waist circumference—WC, blood pressure—BP, high-density lipoprotein cholesterol -HDL-C, Triglycerides—TG, fasting blood sugar—FBS) among rheumatoid arthritis patients.
International databases, including: the Web of Science, Medline, Scopus, Embase, CABI, CINAHL, DOAJ, Index Medicus for Eastern Mediterranean Region-IMEMR and Google Scholar were searched using the following medical subject headings (MeSH): “Metabolic Syndrome”, “Dysmetabolic Syndrome”, “Cardiovascular Syndrome”, and “Insulin Resistance Syndrome”, combined with “Rheumatoid Arthritis”, “Prevalence”, “Odds Ratio”, “Comparative Cross-sectional Studies” and “case-control studies”. The search strategy for Medline was developed first and then adapted for the remaining databases. More detailed information regarding the search strategy is presented in Box 1. The grey literature were searched using Google Scholar, as recommended [18], using the abovementioned search strategy. An expert in this field was also consulted to identify additional papers.
Box 1. Search strategy for MEDLINE (MeSH, Medical Subject Headings).
1: Metabolic Syndrome [Text Word] OR Metabolic Syndrome [MeSH Terms]
2: Dysmetabolic Syndrome [Text Word] OR Dysmetabolic Syndrome [MeSH Terms]
3: Cardiovascular Syndrome [Text Word] OR Cardiovascular Syndrome [MeSH Terms]
4: Insulin Resistance Syndrome [Text Word] OR Insulin Resistance Syndrome [MeSH Terms]
5: 1 OR 2 OR 3 OR 4
6: Rheumatoid Arthritis [Text Word] OR Rheumatoid Arthritis [MeSH Terms]
7: 5 AND 6
8: Prevalence [Text Word] OR Prevalence [MeSH Terms]
9: Odds Ratio [Text Word] OR Odds Ratio [MeSH Terms]
10: Risk Ratio [Text Word] OR Risk Ratio [MeSH Terms]
11: Cross-Product Ratio [Text Word] OR Cross-Product Ratio [MeSH Terms]
12: 8 OR 9 OR 10 OR 11
13: Cross-sectional Studies [Text Word] OR Cross-sectional Studies [MeSH Terms]
14: Case-Control Studies [Text Word] OR Case-Control Studies [MeSH Terms]
15: Comparative cross-sectional Studies [Text Word] OR Comparative cross-sectional Studies [MeSH Terms]
16: 13 OR 14 OR 15
17: 7 AND 12 AND 16
All publications were categorized using Endnote X6. The title and abstract of identified publications were systematically screened and full texts were obtained for those which passed the initial screening. All full text publications were then independently evaluated by two reviewers (SS and JH) for inclusion in the review. Disagreements between the reviewers were resolved by consensus using a third expert (MN). In this study, blinding and task separation were also applied to study selection.
All English language observational (cross-sectional and comparative cross-sectional) studies on the prevalence of metabolic syndrome were included in the current study if they clearly described the date of data collection and study location, used appropriate sampling strategies, and conducted appropriate statistical analyses. Case studies and letters to the editor were excluded, along with systematic reviews or meta-analyses. Lastly, studies undertaken on patients with other disorders were also excluded.
Data extraction and quality assessment
Study characteristics (first author’s name, date of publication, and country of origin), participant characteristics (gender, age, and sample size), and MetS prevalence (based on the different criteria) were extracted using the full text reviews. The quality of each included study was also assessed using the STROBE checklist [19].
Statistical analysis
All statistical analyses were undertaken using Review Manager (RevMan) Version 5.3. (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). The prevalence of metabolic syndrome, and its five components, among rheumatoid arthritis patients were pooled using a random-effects model and presented in a forest plot. The odds ratios for metabolic syndrome in rheumatoid arthritis patients, based upon the different diagnostic criteria, in comparative cross-sectional studies were also pooled using a random-effects model and presented in a forest plot. Statistical heterogeneity was assessed using the I2 index and a random-effects model was used when the I2 index was > 0.6. Stata software version 13 (Stata Corp, College Station, TX, USA) was used to determine which factors were responsible for any observed heterogeneity using meta-regression. Publication bias, with regards to the ORs between MetS and RA was assessed using a Funnel plot and Begg's correlation test [20].
Results
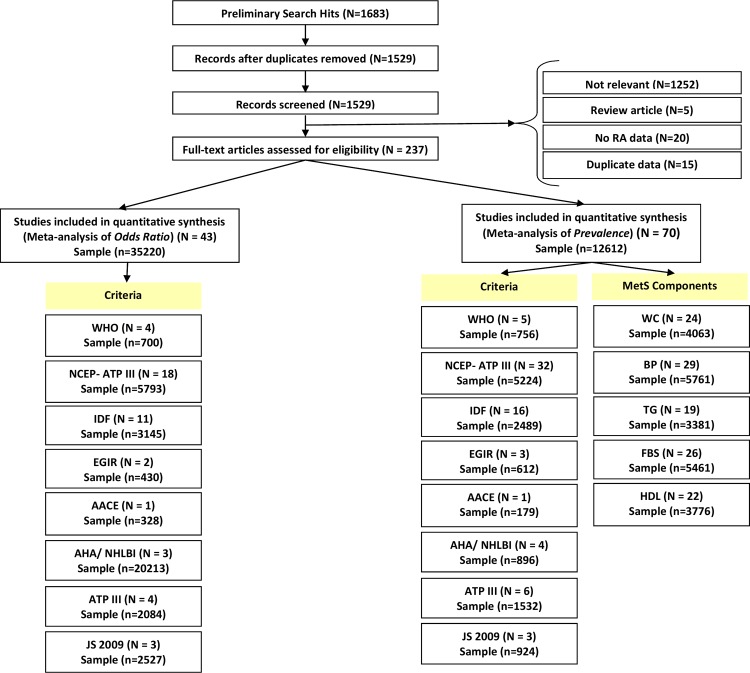
After removing duplicates, our primary search found 237 relevant articles. Following the exclusion of all non-eligible studies a total of 70 cross-sectional studies and 43 comparative cross-sectional studies, from 25 countries, were retained to estimate the prevalence and risk of metabolic syndrome among RA patients. The details of our study selection method are shown in Fig 1. The majority of the studies reporting MetS prevalence (55 studies) included both male and female patients who were aged >18 years. The lowest and highest prevalence of MetS in rheumatoid arthritis patients reported were 10.6% and 55.5%, respectively. More detailed information about each included studies can be found in Table 2.
Fig 1. Flow diagram of the study selection process.
Table 2. Worldwide prevalence (95% CI) of metabolic syndrome in rheumatoid arthritis patients.
| First Author | Country | Criteria | DOP | Age Range | Mean Age | Gender | N. of RA Patients | Prevalence of MetS in RA Patients (%) | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Male | Female | Total | Male | Female | ||||||||
| Lee SH | Korea | AHA/NHLBI | 2016 | ≥12 | 63.6 | Both | 598 | 110 | 488 | 36.4 | 34.5 | 36.9 | [37] |
| Hugo M | France | IDF | 2016 | 18–75 | 57.6 | Both | 57 | 15 | 42 | 24.0 | 25.0 | 24.0 | [38] |
| Zafar ZA | Pakistan | NCEP-ATP III | 2016 | 20–60 | 43.8 | Both | 384 | 97 | 277 | 31.3 | 18.5 | 35.5 | [35] |
| Oliveira BMGB | Brazil | NCEP-ATP III | 2016 | - | 55.5 | Female | 107 | - | 107 | 51.4 | - | 51.4 | [24] |
| Oliveira BMGB | Brazil | IDF | 2016 | - | 55.5 | Female | 107 | - | 107 | 53.4 | - | 53.4 | [24] |
| Muller R | Estonia | NCEP-ATP III | 2016 | - | 51.6 | Both | 91 | 66 | 25 | 35 | [33] | ||
| Dihingia P | India | NCEP-ATP III | 2016 | >12 | 41.5 | Both | 72 | 6 | 66 | 16.7 | [39] | ||
| Ghazaly AHAH | Egypt | ATP III | 2015 | ≥18 | 40.7 | Both | 80 | 13 | 67 | 50.0 | 53.8 | 49.2 | [40] |
| Salamon L | Croatia | ATP III | 2015 | 52–68 | 59 | Both | 583 | 100 | 483 | 43.1 | 40.0 | 43.7 | [41] |
| Tanayakom P | Thailand | NCEP-ATP III | 2015 | - | 59 | Both | 267 | 31 | 236 | 16.1 | 12.9 | 16.5 | [42] |
| Parra-Salcedo F | Mexico | AHA/NHLBI | 2015 | - | 38.1 | Both | 160 | 18 | 142 | 28.0 | [43] | ||
| Parra-Salcedo F | Mexico | IDF | 2015 | - | 38.1 | Both | 160 | 18 | 142 | 18.0 | [43] | ||
| Parra-Salcedo F | Mexico | NCEP-ATP III | 2015 | - | 38.1 | Both | 160 | 18 | 142 | 24.0 | [43] | ||
| Craciun L | Romania | IDF-AHA | 2014 | 32–79 | 55.2 | Both | 51 | 7 | 77 | 19.0 | 10.52 | 82.47 | [23] |
| Craciun L | Romania | NCEP-ATP III | 2014 | 32–79 | 55.2 | Both | 51 | 7 | 77 | 23.0 | [23] | ||
| Craciun L | Romania | IDF | 2014 | 32–79 | 55.2 | Both | 51 | 7 | 77 | 18.0 | [23] | ||
| Craciun L | Romania | AHA | 2014 | 32–79 | 55.2 | Both | 51 | 7 | 77 | 14.0 | [23] | ||
| Bilecik NA | Turkey | IDF | 2014 | 24–65 | 52.0 | Female | 100 | - | 100 | 33.0 | - | 33.0 | [44] |
| Bilecik NA | Turkey | NCEP-ATP III | 2014 | 24–65 | 52.0 | Female | 100 | - | 100 | 27.0 | - | 27.0 | [44] |
| Özmen M | Turkey | NCEP-ATP III | 2014 | - | 51.0 | Both | 52 | 15 | 37 | 17.30 | [45] | ||
| Özmen M | Turkey | WHO | 2014 | - | 51.0 | Both | 52 | 15 | 37 | 28.80 | [45] | ||
| Kumar BS | India | IDF | 2014 | ≥18 | 46.0 | Both | 54 | 6 | 48 | 29.0 | [46] | ||
| Kumar BS | India | NCEP-ATP III | 2014 | ≥18 | 46.0 | Both | 54 | 6 | 48 | 31.0 | [46] | ||
| Abourazzak FE | Morocco | IDF | 2014 | >16 | 49.0 | Both | 179 | 22 | 157 | 30.7 | [26] | ||
| Abourazzak FE | Morocco | NCEP-ATP III | 2014 | >16 | 49.0 | Both | 179 | 22 | 157 | 29.0 | [26] | ||
| Abourazzak FE | Morocco | AACE 2003 | 2014 | >16 | 49.0 | Both | 179 | 22 | 157 | 24.6 | [26] | ||
| Salinas MJH | Argentina | ATP III | 2013 | - | 55.5 | Both | 409 | 69 | 340 | 30.0 | 62.0 | 23.8 | [47] |
| Salinas MJH | Argentina | IDF | 2013 | - | 55.5 | Both | 409 | 69 | 340 | 35.0 | [47] | ||
| Abdul-Qahar | Iraq | NCEP-ATP III | 2013 | - | 46.9 | Both | 203 | 41 | 162 | 51.2 | 12.0 | 92.0 | [48] |
| Rostam S | Morocco | NCEP-ATP III-2004 | 2013 | - | 49.0 | Both | 120 | 10 | 110 | 30.8 | 10.0 | 32.7 | [49] |
| Rostam S | Morocco | NCEP-ATP III-2001 | 2013 | - | 49.0 | Both | 120 | 10 | 110 | 24.6 | [49] | ||
| Rostam S | Morocco | WHO | 2013 | - | 49.0 | Both | 120 | 10 | 110 | 20.0 | [49] | ||
| Rostam S | Morocco | IDF | 2013 | - | 49.0 | Both | 120 | 10 | 110 | 48.6 | [49] | ||
| Rostam S | Morocco | EGIR | 2013 | - | 49.0 | Both | 120 | 10 | 110 | 18.0 | [49] | ||
| Rostam S | Morocco | JC 2009 | 2013 | - | 49.0 | Both | 120 | 10 | 110 | 32.3 | [49] | ||
| Lee SG | Korea | NCEP-ATP III | 2013 | 22–76 | 50.6 | Female | 84 | - | 84 | 19.0 | - | 19.0 | [34] |
| Ormseth MJ | USA | ATP III | 2013 | ≥18 | 54.0 | Both | 162 | 18 | 144 | 36.0 | [50] | ||
| Karakoc | Turkey | IDF | 2012 | - | 49.8 | Both | 54 | 7 | 47 | 42.6 | [51] | ||
| Manka V | Slovakia | IDF | 2012 | ≥18 | 58.8 | Both | 87 | 4 | 83 | 48.3 | [52] | ||
| Manka V | Slovakia | NCEP-ATP III | 2012 | ≥18 | 58.8 | Both | 87 | 4 | 83 | 44.8 | [52] | ||
| Manka V | Slovakia | AHA/NHLBI | 2012 | ≥18 | 58.8 | Both | 87 | 4 | 83 | 47.1 | [52] | ||
| Cunha VR Da | Brazil | NCEP-ATP III | 2012 | ≥18 | 56.8 | Both | 283 | 50 | 233 | 39.2 | [53] | ||
| Goshayeshi L | Iran | NCEP-ATP III | 2012 | - | 45.5 | Both | 120 | 14 | 106 | 45.2 | [21] | ||
| Bkaer JF | USA | IDF | 2012 | 18–85 | 49.5 | Both | 499 | 83 | 416 | 10.6 | [54] | ||
| Crowson CS | USA | NCEP-ATP III | 2011 | ≥18 | 58.8 | Both | 232 | 58 | 174 | 33.0 | 36.0 | 32.0 | [31] |
| Sahaberi M | Iran | IDF | 2011 | - | 45.5 | Both | 120 | 14 | 106 | 30.8 | 28.6 | 41.5 | [55] |
| Sahaberi M | Iran | NCEP-ATP III | 2011 | - | 45.5 | Both | 120 | 14 | 106 | 45.2 | 28.6 | 37.7 | [55] |
| Karimi M | Iran | NCEP | 2011 | ≥18 | 48.3 | Female | 92 | - | 92 | 27.2 | - | 27.2 | [22] |
| Karimi M | Iran | WHO | 2011 | ≥18 | 48.3 | Female | 92 | - | 92 | 19.6 | - | 19.6 | [22] |
| Mok CC | Hong Kong | JS 2009 | 2011 | ≥18 | 53.3 | Both | 699 | 133 | 566 | 20.0 | [56] | ||
| Dao HH | Vietnam | IDF | 2010 | 26–73 | 56.3 | Female | 105 | - | 105 | 40.9 | - | 40.9 | [57] |
| Dao HH | Vietnam | NCEP-ATP III 2004 | 2010 | 26–73 | 56.3 | Female | 105 | - | 105 | 32.4 | - | 32.4 | [57] |
| Dao HH | Vietnam | NCEP-ATP III 2001 | 2010 | 26–73 | 56.3 | Female | 105 | - | 105 | 24.7 | - | 24.7 | [57] |
| Dao HH | Vietnam | JS 2009 | 2010 | 26–73 | 56.3 | Female | 105 | - | 105 | 32.4 | - | 32.4 | [57] |
| Dao HH | Vietnam | WHO | 2010 | 26–73 | 56.3 | Female | 105 | - | 105 | 19.0 | - | 19.0 | [57] |
| Dao HH | Vietnam | EGIR | 2010 | 26–73 | 56.3 | Female | 105 | - | 105 | 16.2 | - | 16.2 | [57] |
| Raterman H G | Netherlands | NCEP | 2010 | 50–75 | 62.1 | Both | 236 | 79 | 157 | 19.9 | [58] | ||
| Solomon A | South Africa | NCEP-ATP III | 2010 | - | 27.2 | Both | 291 | 32 | 259 | 31.3 | [59] | ||
| Solomon B | South Africa | NCEP-ATP III | 2010 | - | 27.2 | Both | 335 | 65 | 270 | 20.3 | [59] | ||
| Giles J | USA | NCEP-ATP III | 2010 | 45–84 | 61 | Both | 131 | 51 | 80 | 36.0 | [60] | ||
| Santos MJ | Portugal | ATP III | 2010 | ≥18 | 49.2 | Female | 98 | 98 | 25.5 | [61] | |||
| Toms TE | UK | IDF | 2009 | 55.5–69.6 | 63.1 | Both | 387 | 105 | 282 | 45.3 | 52.7 | 42.6 | [25] |
| Toms TE | UK | NCEP-ATP III 2004 | 2009 | 55.5–69.6 | 63.1 | Both | 387 | 105 | 282 | 40.1 | 42.5 | 39.2 | [25] |
| Toms TE | UK | NCEP-ATP III 2001 | 2009 | 55.5–69.6 | 63.1 | Both | 387 | 105 | 282 | 38.3 | 40.0 | 37.7 | [25] |
| Toms TE | UK | WHO | 2009 | 55.5–69.6 | 63.1 | Both | 387 | 105 | 282 | 19.4 | 25.5 | 17.2 | [25] |
| Toms TE | UK | EGIR | 2009 | 55.5–69.6 | 63.1 | Both | 387 | 105 | 282 | 12.1 | 22.6 | 8.2 | [25] |
| Chung CP | USA | WHO | 2008 | ≥18 | 59 | Both | 66 | 18 | 48 | 42.0 | [29] | ||
| Zonana-Nacach A | Mexico | NCEP-ATP III | 2008 | - | 42.9 | Both | 107 | 18.7 | [30] | ||||
| Karvounaris SA | Greece | ATP III | 2007 | ≥18 | 63.0 | Both | 200 | 53 | 147 | 44.0 | 39.6 | 45.6 | [32] |
| Montagna G La | Italy | NCEP-ATP III | 2007 | - | 53.8 | Both | 45 | 3 | 42 | 55.5 | [62] |
||
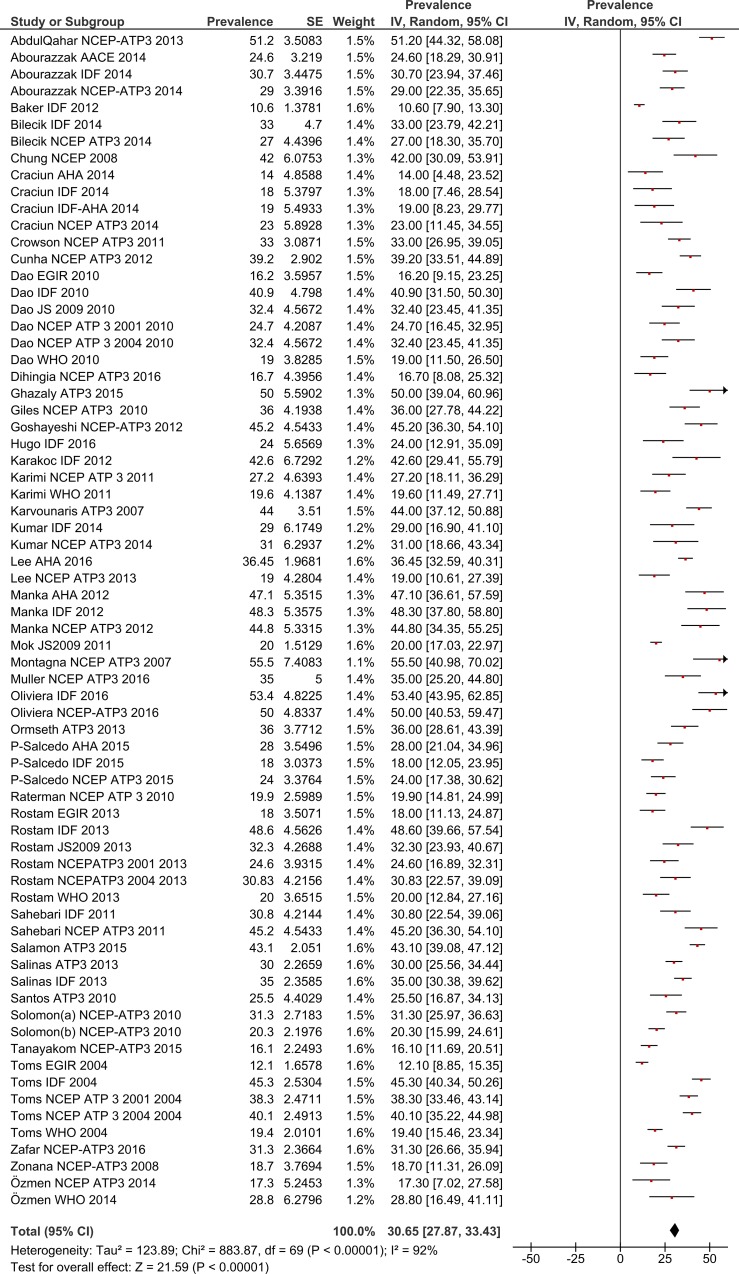
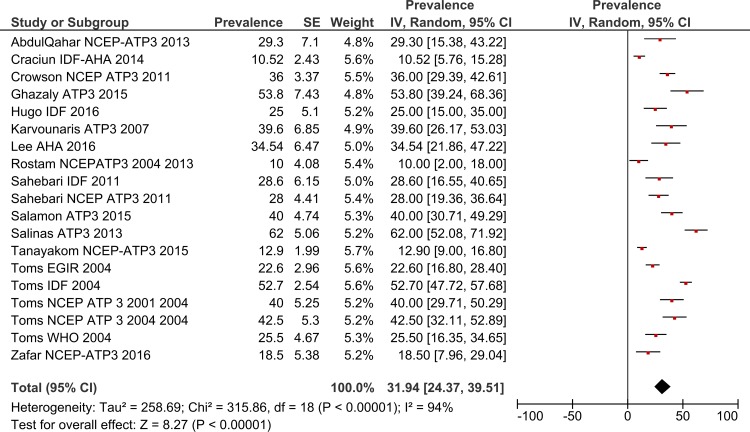
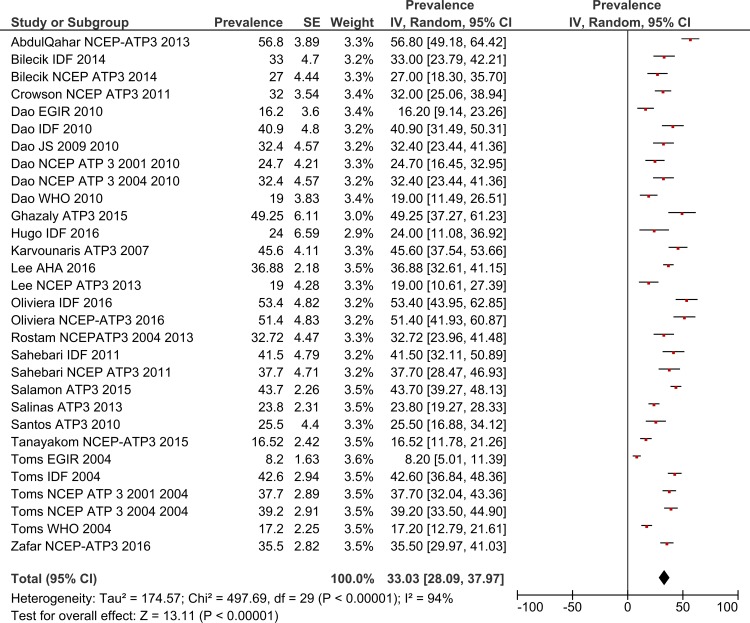
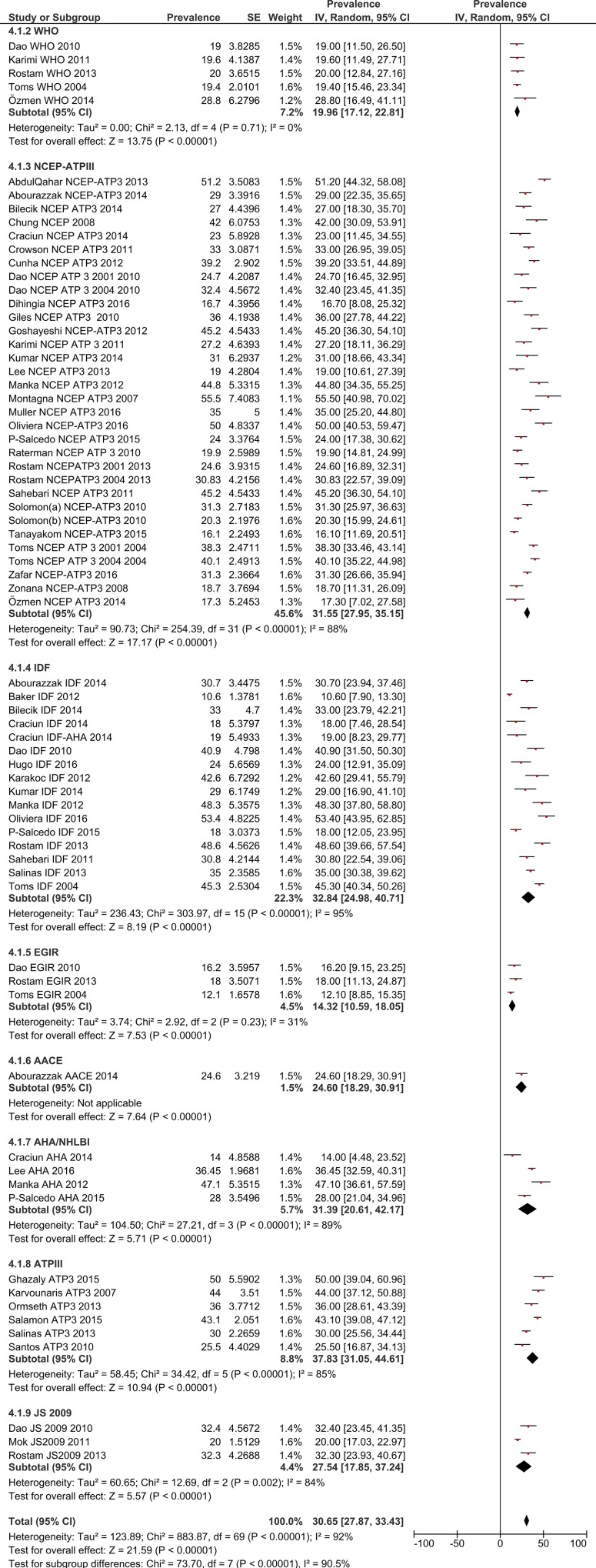
The estimated pooled prevalence, with 95% confidence interval (the diamond below the graph shows the pooled prevalence and the horizontal lines define the reported 95% confidence interval in each study) are presented in graphs by gender and by MetS definition.
Total MetS prevalence in RA patients by gender
Using a random effects model, the estimated worldwide prevalence rate of MetS among RA patients was 30.65% (95% CI: 27.87–33.43) (Fig 2). In addition, information on the prevalence of MetS by gender was available from 19 studies for males and 30 for females. The prevalence rates among males was 31.94% (95% CI: 24.37–39.51) and for females this was 33.03% (95% CI: 28.09–37.97) (Figs 3 and 4).
Fig 2. Forest plot of MetS prevalence in RA Patients.
Fig 3. Forest plot of MetS prevalence among male RA Patients.
Fig 4. Forest plot of MetS prevalence among female RA Patients.
MetS prevalence in RA patients by criteria/definition
The pooled MetS prevalence rates for the eight definitions are: WHO—19.96% (95% CI: 17.12–22.81), NCEP/ATP III—31.55% (95% CI: 27.95–35.15), IDF—32.84% (95% CI: 24.98–40.71), EGIR—14.32% (95% CI: 10.59–18.05), ACCE—24.6% (95% CI: 19.29–30.91), AHA/NHBI—31.39% (95% CI: 20.61–42.17), ATP III—37.83% (95% CI: 31.05–44.61) and JS 2009–27.54 (95% CI: 17.85–37.24) (Fig 5).
Fig 5. Forest plot of MetS prevalence among RA Patients by definition/criteria.
MetS prevalence in rheumatoid arthritis patients by MetS component
The MetS components of FBS, HDL-C, BP, Triglyceride and Waist Circumstance (WC) were reported by 26, 22, 29, 19 and 24 studies, respectively. The pooled MetS prevalence rates, by component, were: FBS—19.47% (95% CI: 15.69–23.25), HDL—41.78% (95% CI: 28.73–54.84), BP—48.65% (95% CI: 41.03–56.26), Triglyceride—28.43% (95% CI: 22.3–34.57) and WC—52.63 (95% CI: 43.76–61.5) (S 1–5 Appendix).
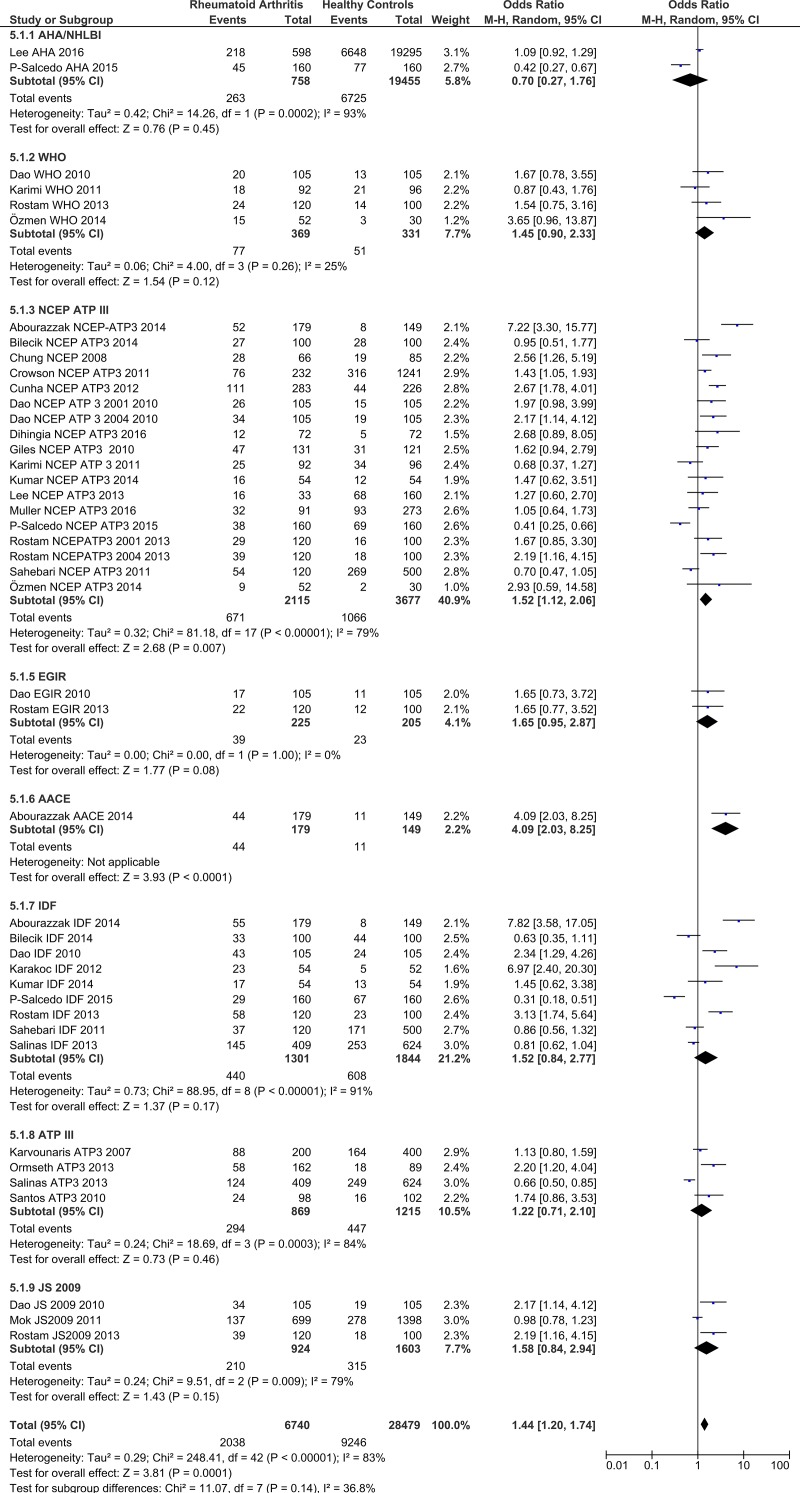
Risk of MetS in rheumatoid arthritis patients by criteria/definition
In this section the prevalence of MetS in RA patients and among healthy controls were compared (Table 3). The pooled estimates identified a significant positive association between rheumatoid arthritis and the risk of MetS (OR = 1.44; 95% CI: 1.20–1.74). The odds ratios for MetS in rheumatoid arthritis patients, according to the definition used, were: WHO—OR = 1.45 (95% CI: 0.9–2.33), NCEP/ATP III—OR = 1.52 (95% CI: 1.12–2.06), IDF—OR = 1.52 (95% CI: 0.84–2.77), EGIR—OR = 1.65 (95% CI: 0.95–2.87), ACCE—OR = 4.09 (95% CI: 2.03–8.25), AHA/NHBI—OR = 0.7 (95% CI: 0.27–1.76), ATP III—OR = 1.22 (95% CI: 0.71–2.1), and JS 2009—OR = 1.58 (95% CI: 0.84–2.94) (Fig 6).
Table 3. Worldwide prevalence (95% CI) of metabolic syndrome in rheumatoid arthritis patients compared to healthy controls.
| First Author | Country | Criteria | DOP | Gender | N. RA Patients | N. Healthy Controls | Reference | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean Age | Age Range | Male | Female | Total | Mean Age | Age Range | Male | Female | Total | ||||||||
| N. | MetS Prev. (%) | N. | MetS Prev. (%) | ||||||||||||||
| Lee SH | Korea | AHA/NHLBI | 2016 | Both | 63.6 | - | 110 | 488 | 598 | 36.45 | 58.4 | - | 8114 | 11181 | 19295 | 34.45 | [37] |
| Muller R | Estonia | NCEP-ATP III | 2016 | Both | 51.6 | - | 66 | 25 | 91 | 35.16 | 51.5 | - | 75 | 198 | 273 | 34.06 | [33] |
| Dihingia P | India | NCEP-ATP III | 2016 | Both | 41.5 | - | 6 | 66 | 72 | 16.66 | - | - | - | - | 72 | 6.94 | [39] |
| Parra-Salcedo F | Mexico | AHA/NHLBI | 2015 | Both | 38.1 | - | 18 | 142 | 160 | 28.12 | 38.0 | - | 18 | 142 | 160 | 4.81 | [43] |
| Parra-Salcedo F | Mexico | IDF | 2015 | Both | 38.1 | - | 18 | 142 | 160 | 18.12 | 38.0 | - | 18 | 142 | 160 | 4.18 | [43] |
| Parra-Salcedo F | Mexico | NCEP-ATP III | 2015 | Both | 38.1 | - | 18 | 142 | 160 | 23.75 | 38.0 | - | 18 | 142 | 160 | 4.31 | [43] |
| Bilecik NA | Turkey | IDF | 2014 | Female | 52.0 | 24–65 | 0 | 100 | 100 | 33.0 | 51.0 | 27–65 | 0 | 100 | 100 | 44.0 | [44] |
| Bilecik NA | Turkey | NCEP-ATP III | 2014 | Female | 52.0 | 24–65 | 0 | 100 | 100 | 27.0 | 51.0 | 27–65 | 0 | 100 | 100 | 28.0 | [44] |
| Özmen M | Turkey | NCEP-ATP III | 2014 | Both | 51.0 | - | 15 | 37 | 52 | 17.30 | 48.0 | - | 9 | 21 | 30 | 6.60 | [45] |
| Özmen M | Turkey | WHO | 2014 | Both | 51.0 | - | 15 | 37 | 52 | 28.84 | 48.0 | - | 9 | 21 | 30 | 10.0 | [45] |
| Kumar BS | India | IDF | 2014 | Both | 46.0 | - | 6 | 48 | 54 | 31.48 | 45.4 | - | 6 | 48 | 54 | 24.07 | [46] |
| Kumar BS | India | NCEP-ATP III | 2014 | Both | 46.0 | - | 6 | 48 | 54 | 29.62 | 45.4 | - | 6 | 48 | 54 | 22.22 | [46] |
| Abourazzak FE | Morocco | IDF | 2014 | Both | 49.0 | - | 22 | 157 | 179 | 30.72 | 51.0 | - | 23 | 126 | 149 | 5.36 | [26] |
| Abourazzak FE | Morocco | NCEP-ATP III | 2014 | Both | 49.0 | - | 22 | 157 | 179 | 29.05 | 51.0 | - | 23 | 126 | 149 | 5.36 | [26] |
| Abourazzak FE | Morocco | AACE 2003 | 2014 | Both | 49.0 | - | 22 | 157 | 179 | 24.58 | 51.0 | - | 23 | 126 | 149 | 7.38 | [26] |
| Salinas MJH | Argentina | ATP III | 2013 | Both | 55.5 | - | 69 | 340 | 409 | 30.31 | 57.3 | - | 103 | 521 | 624 | 39.90 | [47] |
| Salinas MJH | Argentina | IDF | 2013 | Both | 55.5 | - | 69 | 340 | 409 | 35.45 | 57.3 | - | 103 | 521 | 624 | 40.54 | [47] |
| Chung CP | Usa | NCEP-ATP III | 2008 | Both | 59.0 | 43–59 | 18 | 48 | 66 | 42.42 | 52.0 | 44–58 | 30 | 55 | 85 | 22.35 | [29] |
| Dao HH | Vietnam | WHO | 2010 | Female | 56.3 | 26–73 | 0 | 105 | 105 | 19.04 | 55.7 | 25–72 | 56 | 49 | 105 | 12.35 | [57] |
| Dao HH | Vietnam | IDF | 2010 | Female | 56.3 | 26–73 | 0 | 105 | 105 | 40.95 | 55.7 | 25–72 | 56 | 49 | 105 | 22.85 | [57] |
| Dao HH | Vietnam | NCEP-ATP III | 2010 | Female | 56.3 | 26–73 | 0 | 105 | 105 | 24.76 | 55.7 | 25–72 | 56 | 49 | 105 | 14.28 | [57] |
| Dao HH | Vietnam | NCEP-ATP III | 2010 | Female | 56.3 | 26–73 | 0 | 105 | 105 | 32.38 | 55.7 | 25–72 | 56 | 49 | 105 | 18.09 | [57] |
| Dao HH | Vietnam | EGIR | 2010 | Female | 56.3 | 26–73 | 0 | 105 | 105 | 16.19 | 55.7 | 25–72 | 56 | 49 | 105 | 10.47 | [57] |
| Dao HH | Vietnam | JS2009 | 2010 | Female | 56.3 | 26–73 | 0 | 105 | 105 | 32.38 | 55.7 | 25–72 | 56 | 49 | 105 | 18.09 | [57] |
| Karimi M | Iran | NCEP-ATP III | 2011 | Both | 48.3 | - | - | - | 92 | 27.17 | 42.2 | - | - | - | 96 | 35.41 | [22] |
| Rostam S | Morocco | WHO | 2013 | Both | 49.0 | - | 10 | 110 | 120 | 20.00 | 48.5 | - | 10 | 90 | 100 | 14.00 | [49] |
| Rostam S | Morocco | IDF | 2013 | Both | 49.0 | - | 10 | 110 | 120 | 48.60 | 48.5 | - | 10 | 90 | 100 | 23.00 | [49] |
| Rostam S | Morocco | NCEP-ATP III | 2013 | Both | 49.0 | - | 10 | 110 | 120 | 24.16 | 48.5 | - | 10 | 90 | 100 | 16.00 | [49] |
| Rostam S | Morocco | NCEP-ATP III | 2013 | Both | 49.0 | - | 10 | 110 | 120 | 32.50 | 48.5 | - | 10 | 90 | 100 | 18.0 | [49] |
| Rostam S | Morocco | EGIR | 2013 | Both | 49.0 | - | 10 | 110 | 120 | 18.33 | 48.5 | - | 10 | 90 | 100 | 12.00 | [49] |
| Rostam S | Morocco | JS2009 | 2013 | Both | 49.0 | - | 10 | 110 | 120 | 32.50 | 48.5 | - | 10 | 90 | 100 | 18.0 | [49] |
| Crowson CS | Usa | NCEP-ATP III | 2011 | Both | 58.8 | - | 58 | 174 | 232 | 32.75 | 63.9 | - | 560 | 681 | 1241 | 25.46 | [31] |
| Cunha VR da | Brazil | NCEP-ATP III | 2012 | Both | 56.8 | - | 50 | 233 | 283 | 39.22 | 44.5 | - | 34 | 192 | 226 | 19.46 | [53] |
| Giles JT | Usa | NCEP-ATP III | 2010 | Both | 61.0 | - | 51 | 80 | 131 | 35.87 | 63.0 | - | 70 | 51 | 121 | 25.61 | [60] |
| Sahebari M | Iran | NCEP-ATP III | 2011 | Both | 45.5 | - | 14 | 106 | 120 | 45.0 | 45.6 | - | 69 | 431 | 500 | 53.8 | [55] |
| Sahebari M | Iran | IDF | 2011 | Both | 45.5 | - | 14 | 106 | 120 | 30.83 | 45.6 | - | 69 | 431 | 500 | 34.2 | [55] |
| Karakoc M | Turkey | IDF | 2012 | Both | 49.7 | - | 7 | 47 | 54 | 42.59 | 47.0 | - | 43 | 9 | 52 | 9.61 | [51] |
| Santos MJ | Portugal | ATP III | 2010 | Female | 49.2 | - | 0 | 98 | 98 | 24.48 | 47.7 | - | 0 | 102 | 102 | 15.68 | [61] |
| Mok CC | Hong Kong | JS2009 | 2011 | Both | 53.3 | - | 133 | 566 | 699 | 19.59 | 52.9 | - | 266 | 1132 | 1398 | 19.88 | [56] |
Fig 6. Forest plot of MetS risk among RA patients by definition/criteria.
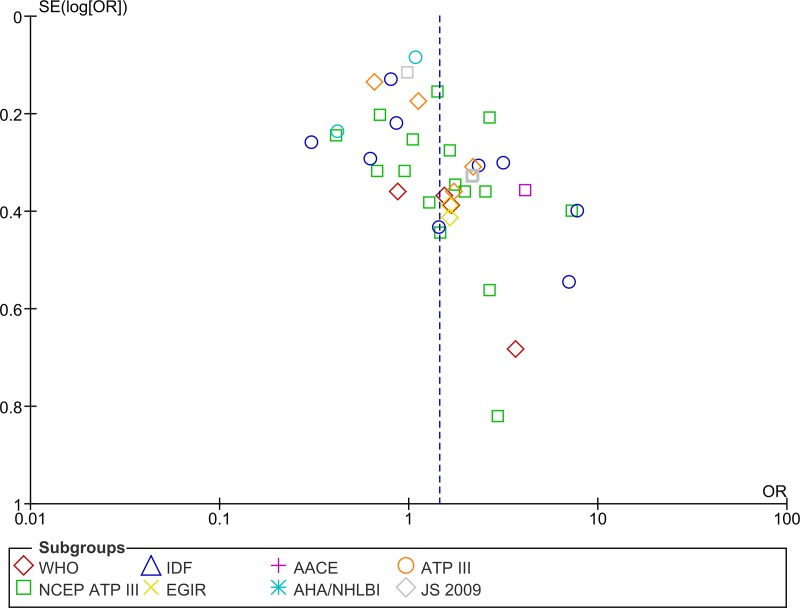
Publication bias
In order to assess publication bias in relation to the OR for MetS and RA, funnel plots and Begg's correlation were used. These found no evidence of any publication bias (Fig 7).
Fig 7. Funnel plot of MetS risk among RA Patients by definition/criteria.
Meta-regression
To assess the sources of heterogeneity, four variables were included in a univariable meta-regression. Our results indicated that the study date (P = 0.60) and country (P = 0.38) were not responsible for the heterogeneity in the ORs for MetS in RA patients, compared to healthy controls, but mean age (P = 0.03) and diagnostic criteria (P = 0.04) could be considered sources of heterogeneity. Hence, subgroup analysis was undertaken based upon the diagnostic criteria.
Discussion
The present study found a MetS prevalence of 30.65% among RA patients, but this rate ranged from 14.32% to 37.83%, depending upon the MetS definition used. The relatively high degree of variability in MetS prevalence, according to the MetS definition used, is clearly a substantial issue that permeates the literature on this topic. For example, research in Asia has reported the prevalence of MetS to be 45.2% among RA patients using the NCEP-ATP III criteria [21] and 19.6% when using the WHO definition[22]. In Europe the prevalence rates reported, according to criteria used were: AHA (27.4%), IDF (35.2%), IDF-AHA (37.2%) and NCEP-ATP III (23.0%)[23]. Furthermore, based on the NCE-P-ATP III criteria, Oliveira et al. found that the prevalence of MetS among RA patients in South American was 51.4%, but using the IDF criteria this proportion was 53.4% [24]. Much larger differences have been reported in research from the UK, with MetS prevalence ranging from 8.2% to 42.6% [25], depending upon the definition used. Moreover, in a cross-sectional study which used three definitions (NCEP-ATP III, IDF and AACE) the prevalence of MetS in RA patients varied from 24.6 to 30.7% [26]. Finally, the results of a case- control study in 2013 showed that the frequency of MetS in RA patients and the control group were 30% versus 39% (respectively) when using the ATP III definition and 35% versus 40% (respectively) when using the IDF [27] definition.
Therefore, it appears that some of the variation in the prevalence reported are to do with i) a lack of definition clarity, with many different criteria in the existing definitions, ii) different and multiple phenotypes included in each definition of MetS, and iii) the lack of consistency in the number of components required by each definition.
However, prevalence rates also vary widely even when comparing studies that have used the same criteria. For example, using the NCE/ATP definition, Dessein et al. reported a MetS prevalence of 19% among 74 RA patients [28], while a separate study using the same definition reported a prevalence rate of 42% in those with long standing RA and 30% in those recently diagnosed with RA[29]. Further, in a study of 107 female RA patients a MetS prevalence of 18.7% [30] was reported, but using the same definition Crowson et al. reported the prevalence to be 33%[31]. Therefore, it is likely that other factors related to the characteristics of the study population, such as: genetic, ethnic, cultural, demographic, socioeconomic and clinical factors, also affect the prevalence. Thus, studies conducted using different populations are critical in order to identify other factors related to MetS.
In this study the risk of MetS in RA patients was 45% higher than that in the healthy control group (OR = 1.45; 95% CI: 1.20–1.75). The OR found in the present study is considerably higher than that reported in a meta-analysis of 12 studies in 2013, which reported an OR of 1.24 (95% CI, 1.03–1.50) [11]. Furthermore, Karvounaris et al. found prevalence of MetS to be similar in RA patients (44%) to their control population (41%), but they also found a relationship between disease activity and the presence of MetS [32]. It is also worth mentioning that several studies have not reported any association between RA and MetS [33, 34].
When we assessed the individual components of MetS (FBS, HDL, BP, Triglyceride, WC), a high WC had the highest prevalence, while the lowest prevalence was high FBS. These findings are consistent with a cross-sectional study by Zafar et al., which found that high FBS (21.9%) was the least prevalent component, while a high WC (46.1%) was the most prevalent component[35]. Furthermore, a study of 200 rheumatoid arthritis outpatients reported that the prevalence of a high WC was 74.8% in female patients and 60.4% in male patients, while the prevalence of high FBS were 30.6% and 26.4% in female and male patients, respectively [32]. In another study, blood pressure, hypoglycemia and HDL had prevalence’s of 35.9%, 22.95 and 68.9%, respectively [36]. Therefore, it seems that in most studies a high WC is the most prevalent MetS component and targeting preventative measures at this may considerably reduce the risk of developing MetS.
Advantages
The present study has a number of advantages over the previous meta-analysis, including: 1) All of the published studies were included in this meta-analysis. 2) The prevalence of metabolic syndrome was investigated in RA patients from across the world. 3) This study reported the prevalence of MetS in RA patients based upon eight separate definitions. 4) This paper included both comparative cross-sectional and cross-sectional studies. 5) The odds ratio for metabolic syndrome was pooled across a large number of studies.
Limitations
1) Several countries have not assessed the prevalence of MetS in RA patients and therefore data from those countries could not be presented in this study. 2) The crude (unadjusted) odds ratio for MetS in RA patients was reported, as different studies used different set(s) of confounders.
Conclusion
The prevalence of MetS in RA patients was relatively high, but did not vary significantly by gender. According to the high prevalence of MetS in RA patients and the high risk of it, monitoring and testing for metabolic syndrome in these patients is clearly recommended. As the most important component of metabolic syndrome was found to be a high WC, it is clearly important to pay more attention to patient nutrition and weight loss. Finally, mean age and the diagnostic criteria used to diagnose MetS were identified as sources of heterogeneity in the estimated risk of MetS.
Supporting information
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
Data Availability
All relevant data are available in the manuscript.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Sidiropoulos P, Karvounaris S, Boumpas D. Metabolic syndrome in rheumatic diseases: epidemiology, pathophysiology, and clinical implications. Arthritis Res Ther. 2008;10:207 10.1186/ar2397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zafar Z, H.Mahmud T, Rasheed A, AhmedWagan A. Frequency of metabolic syndrome in Pakistani cohort of patients with rheumatoid arthritis. J Pak Med Assoc. 2016;66(6):671–6. [PubMed] [Google Scholar]
- 3.Ford E. Risks for All-Cause Mortality, Cardiovascular Disease, and Diabetes Associated With the Metabolic Syndrome A summary of the evidence. Diabetes Care. 2005;28:1769–78. [DOI] [PubMed] [Google Scholar]
- 4.Hanley A, Karter A, Williams K, et a. Prediction of type 2 diabetes mellitus with alternative definitions of the metabolic syndrome: the Insulin Resistance Atherosclerosis Study. Circulation. 2005;112:3713–21. 10.1161/CIRCULATIONAHA.105.559633 [DOI] [PubMed] [Google Scholar]
- 5.Mitchell AJ, Vancampfort D, Sweers K, Winkel RV, Yu W, De Hert M. Prevalence of Metabolic Syndrome and Metabolic Abnormalities in Schizophrenia and Related Disorders—A Systematic Review and Meta-Analysis. Schizophrenia Bulletin. 2013;39(2):306–18. 10.1093/schbul/sbr148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferraz-Amaro I, Gonzalez-Juanatey C, Lopez-Mejias R, Riancho-Zarrabeitia L, Gonzalez-Gay M. Metabolic syndrome in rheumatoid arthritis. Mediators Inflammation. 2013;2013:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tillin T, Forouhi N, DG J, et al. Metabolic syndrome and coronary heart disease in South Asians, African-Caribbeans and white Europeans: a UK population-based cross-sectional study. Diabetologia. 2005;48:649–56. 10.1007/s00125-005-1689-3 [DOI] [PubMed] [Google Scholar]
- 8.Hwang L, Bai C, Chen C. Prevalence of obesity and metabolic syndrome in Taiwan. J Formos Med Assoc. 2006;105:626–35. 10.1016/S0929-6646(09)60161-3 [DOI] [PubMed] [Google Scholar]
- 9.Kojima M, Kojima T, Ishiguro N, Oguchi T, Oba M, Tsuchiya H, et al. Psychosocial factors, disease status, and quality of life in patients with rheumatoid arthritis. J Psychosom Res. 2009;67:425–31. 10.1016/j.jpsychores.2009.01.001 [DOI] [PubMed] [Google Scholar]
- 10.Tierney M, Fraser A, Kennedy N. Physical activity in rheumatoid arthritis: a systematic review. J Phys Act Health. 2012;9:1036–48. [DOI] [PubMed] [Google Scholar]
- 11.Zhang J, Fu L, Shi J, Chen X, Li Y, Ma B, et al. The risk of metabolic syndrome in patients with rheumatoid arthritis: a meta-analysis of observational studies. PLoS One. 2013;8(10):e78151 10.1371/journal.pone.0078151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parra-Salcedo F, Contreras-Yáñez I, Elías-López D, Aguilar-Salinas C, Pascual-Ramos V. Prevalence, incidence and characteristics of the metabolic syndrome (MetS) in a cohort of Mexican Mestizo early rheumatoid arthritis patients treated with conventional disease modifying anti-rheumatic drugs: the complex relationship between MetS and disease activity. Arthritis research & therapy. 2015;17:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dąbrowski P, Majdan M. Insulin resistance and metabolic syndrome—a different image of disorders in rheumatoid arthritis and ankylosing spondylitis. Wiad Lek. 2015;68(3):235–41. [PubMed] [Google Scholar]
- 14.Lee S, Kim J, Lee S, Kim K, Kim J, Yi J, et al. Is the frequency ofmetabolic syndrome higher in South Korean women with rheumatoid arthritis than in healthy subjects? Korean J Intern Med. 2013;28:206–15. 10.3904/kjim.2013.28.2.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dao H, Do Q, Sakamoto J. Increased frequency of metabolic syndrome among Vietnamese women with early rheumatoid arthritis: a cross-sectional study. Arthritis Res Ther. 2010;12:R218 10.1186/ar3203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rostom S, Mengat M, Lahlou R, Hari A, Bahiri R, Hajjaj-Hassouni N. Metabolic syndrome in rheumatoid arthritis: case control study. BMC MusculoskeletDisord. 2013;14:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of internal medicine. 2009;151(4):264–9. [DOI] [PubMed] [Google Scholar]
- 18.Haddaway NR, Collins AM, Coughlin D, Kirk S. The role of Google Scholar in evidence reviews and its applicability to grey literature searching. PLOS ONE. 2015;10(9):e0138237 10.1371/journal.pone.0138237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vandenbroucke JP, Von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Annals of internal medicine. 2007;147(8):W-163-W-94. [DOI] [PubMed] [Google Scholar]
- 20.Sterne JA, Harbord RM. Funnel plots in meta-analysis. Stata Journal. 2004;4:127–41. [Google Scholar]
- 21.Goshayeshi L, Saber H, Sahebari M, Rezaieyazdi Z, Rafatpanah H, Esmaily H, et al. Association between metabolic syndrome, BMI, and serum vitamin D concentrations in rheumatoid arthritis. Clinical rheumatology. 2012;31(8):1197–203. 10.1007/s10067-012-1995-3 [DOI] [PubMed] [Google Scholar]
- 22.Karimi M, Mazloomzadeh S, Kafan S, Amirmoghadami H. The frequency of metabolic syndrome in women with rheumatoid arthritis and in controls. International journal of rheumatic diseases. 2011;14(3):248–54. 10.1111/j.1756-185X.2011.01595.x [DOI] [PubMed] [Google Scholar]
- 23.Crăciun L, Crăciun P, Buicu F. Prevalence of Metabolic Syndrome in Psoriatic Arthritis and Rheumatoid Arthritis. Acta Medica Marisiensis. 2014;60(5):196–9. [Google Scholar]
- 24.Oliveira BMGBd, Medeiros MMdC, Cerqueira JVMd, Quixadá RTdS, Oliveira ÍMAXd. Metabolic syndrome in patients with rheumatoid arthritis followed at a University Hospital in Northeastern Brazil. Revista brasileira de reumatologia. 2016;56(2):117–25. 10.1016/j.rbre.2015.08.016 [DOI] [PubMed] [Google Scholar]
- 25.Toms TE, Panoulas VF, John H, Douglas KM, Kitas GD. Methotrexate therapy associates with reduced prevalence of the metabolic syndrome in rheumatoid arthritis patients over the age of 60-more than just an anti-inflammatory effect? A cross sectional study. Arthritis research & therapy. 2009;11(4):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abourazzak FE, Mansouri S, Najdi A, Tahiri L, Nejjari C, Harzy T. Prevalence of metabolic syndrome in patients with rheumatoid arthritis in Morocco: a cross-sectional study of 179 cases. Clinical rheumatology. 2014;33(11):1549–55. 10.1007/s10067-014-2570-x [DOI] [PubMed] [Google Scholar]
- 27.Kerekes G, Nurmohamed MT, González-Gay MA, Seres I, Paragh G, Kardos Z, et al. Rheumatoid arthritis and metabolic syndrome. Nature Reviews Rheumatology. 2014;10(11):691–6. 10.1038/nrrheum.2014.121 [DOI] [PubMed] [Google Scholar]
- 28.Dessein PH, Tobias M, Veller MG. Metabolic syndrome and subclinical atherosclerosis in rheumatoid arthritis. The Journal of rheumatology. 2006;33(12):2425–32. [PubMed] [Google Scholar]
- 29.Chung CP, Oeser A, Solus JF, Avalos I, Gebretsadik T, Shintani A, et al. Prevalence of the metabolic syndrome is increased in rheumatoid arthritis and is associated with coronary atherosclerosis. Atherosclerosis. 2008;196(2):756–63. 10.1016/j.atherosclerosis.2007.01.004 [DOI] [PubMed] [Google Scholar]
- 30.Zonana-Nacach A, Santana-Sahagún E, Jiménez-Balderas FJ, Camargo-Coronel A. Prevalence and factors associated with metabolic syndrome in patients with rheumatoid arthritis and systemic lupus erythematosus. JCR: Journal of Clinical Rheumatology. 2008;14(2):74–7. 10.1097/RHU.0b013e31816b2faa [DOI] [PubMed] [Google Scholar]
- 31.Crowson CS, Myasoedova E, Davis JM, Matteson EL, Roger VL, Therneau TM, et al. Increased prevalence of metabolic syndrome associated with rheumatoid arthritis in patients without clinical cardiovascular disease. The Journal of rheumatology. 2011;38(1):29–35. 10.3899/jrheum.100346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karvounaris SA, Sidiropoulos PI, Papadakis JA, Spanakis EK, Bertsias GK, Kritikos HD, et al. Metabolic syndrome is common among middle-to-older aged Mediterranean patients with rheumatoid arthritis and correlates with disease activity: a retrospective, cross-sectional, controlled, study. Annals of the rheumatic diseases. 2007;66(1):28–33. 10.1136/ard.2006.053488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Müller R, Kull M, Põlluste K, Aart A, Eglit T, Lember M, et al. The metabolic profile in early rheumatoid arthritis: a high prevalence of metabolic obesity. Rheumatology international. 2016:1–7. [DOI] [PubMed] [Google Scholar]
- 34.Lee S-G, Kim J-M, Lee S-H, Kim K-H, Kim J-H, Yi J-W, et al. Is the frequency of metabolic syndrome higher in South Korean women with rheumatoid arthritis than in healthy subjects? The Korean journal of internal medicine. 2013;28(2):206–15. 10.3904/kjim.2013.28.2.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zafar ZA, Mahmud TH, Rasheed A, Wagan AA. Frequency of metabolic syndrome in Pakistani cohort of patients with rheumatoid arthritis. [PubMed]
- 36.La Montagna G, Cacciapuoti F, Buono R, Manzella D, Mennillo GA, Arciello A, et al. Insulin resistance is an independent risk factor for atherosclerosis in rheumatoid arthritis. Diabetes and Vascular Disease Research. 2007;4(2):130–5. 10.3132/dvdr.2007.031 [DOI] [PubMed] [Google Scholar]
- 37.Lee S-H, Choi H, Cho B-L, An A-R, Seo Y-G, Jin H-S, et al. Relationship between Metabolic Syndrome and Rheumatoid Arthritis. Korean journal of family medicine. 2016;37(1):44–50. 10.4082/kjfm.2016.37.1.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hugo M, Mehsen-Cetre N, Pierreisnard A, Schaeverbeke T, Gin H, Rigalleau V. Energy expenditure and nutritional complications of metabolic syndrome and rheumatoid cachexia in rheumatoid arthritis: an observational study using calorimetry and actimetry. Rheumatology. 2016:kew038. [DOI] [PubMed] [Google Scholar]
- 39.Dihingia P, Das D, Chakraborty A, Debbarma M, Kakati S. INCREASE FREQUENCY OF METABOLIC SYNDROME AMONG THE CASES OF RHEUMATOID ARTHRITIS: A CASE CONTROL STUDY. HYPERTENSION. 41(56.9):56.9.12511530 [Google Scholar]
- 40.Ghazaly AHAH, El-Moez KM, El Shorbagy MS, El-Nahrery EM. Angiopoietin-2 as a biomarker for metabolic syndrome and disease activity in rheumatoid arthritis patients. The Egyptian Rheumatologist. 2016;38(1):9–13. [Google Scholar]
- 41.Šalamon L, Morović-Vergles J, Marasović-Krstulović D, Kehler T, Šakić D, Badovinac O, et al. Differences in the prevalence and characteristics of metabolic syndrome in rheumatoid arthritis and osteoarthritis: a multicentric study. Rheumatology international. 2015;35(12):2047–57. 10.1007/s00296-015-3307-0 [DOI] [PubMed] [Google Scholar]
- 42.Tantayakom P, Koolvisoot A, Arromdee E, Chiowchanwisawakit P, Muangchan C, Katchamart W. Metabolic syndrome is associated with disease activity in patients with rheumatoid arthritis. Joint Bone Spine. 2016. [DOI] [PubMed] [Google Scholar]
- 43.Parra-Salcedo F, Contreras-Yáñez I, Elías-López D, Aguilar-Salinas CA, Pascual-Ramos V. Prevalence, incidence and characteristics of the metabolic syndrome (MetS) in a cohort of Mexican Mestizo early rheumatoid arthritis patients treated with conventional disease modifying anti-rheumatic drugs: the complex relationship between MetS and disease activity. Arthritis research & therapy. 2015;17(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bilecik NA 1, Tuna S, Samancı N, Balcı N, Akbaş H. Prevalence of metabolic syndrome in women with rheumatoid arthritis and effective factors. International journal of clinical and experimental medicine. 2014;7(8):2258 [PMC free article] [PubMed] [Google Scholar]
- 45.Ozmen M, Ozturk S, Soysal D, Koseoglu M. Prevalence of the metabolic syndrome in rheumatoid arthritis. Eur J Rheumatol. 2014;1:1–4. 10.5152/eurjrheum.2014.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kumar BS, Naik GS, Mohan A, Kumar DP, Suresh V, Sarma K, et al. Prevalence of thyroid disorders and metabolic syndrome in adult patients with rheumatoid arthritis.
- 47.Salinas MJH, Bertoli AM, Lema L, Saucedo C, Rosa J, Quintana R, et al. Prevalence and correlates of metabolic syndrome in patients with rheumatoid arthritis in Argentina. JCR: Journal of Clinical Rheumatology. 2013;19(8):439–43. 10.1097/RHU.0000000000000039 [DOI] [PubMed] [Google Scholar]
- 48.Abdul-Qaharr Z, Al-Osami MH. Prevalence of Metabolic Syndrome in Iraqi Patients with Rheumatoid Arthritis. IOSR Journal of Dental and Medical Sciences (IOSR-JDMS). 1(11):69–72. [Google Scholar]
- 49.Rostom S, Mengat M, Lahlou R, Hari A, Bahiri R, Hajjaj-Hassouni N. Metabolic syndrome in rheumatoid arthritis: case control study. BMC musculoskeletal disorders. 2013;14(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ormseth MJ, Lipson A, Alexopoulos N, Hartlage GR, Oeser AM, Bian A, et al. Epicardial adipose tissue is associated with cardiometabolic risk and the metabolic syndrome in patients with rheumatoid arthritis. Arthritis care & research. 2013;65(9):1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Karakoc M, Batmaz I, Sariyildiz MAA, Tahtasiz M, Cevik R, Tekbas E, et al. The relationship of metabolic syndrome with disease activity and the functional status in patients with rheumatoid arthritis. Journal of clinical medicine research. 2012;4(4):279–85. 10.4021/jocmr1001w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Manka V, Galajda P, Sagova I, Klimentova A, Kantarova D, Stancik M, et al. Metabolic Syndrome in Rheumatoid Arthritis. Acta Medica Martiniana. 2012;12(3):19–27. [Google Scholar]
- 53.Da Cunha V, Brenol C, Brenol J, Fuchs S, Arlindo E, Melo I, et al. Metabolic syndrome prevalence is increased in rheumatoid arthritis patients and is associated with disease activity. Scandinavian journal of rheumatology. 2012;41(3):186–91. 10.3109/03009742.2011.626443 [DOI] [PubMed] [Google Scholar]
- 54.Baker JF, Mehta NN, Baker DG, Toedter G, Shults J, Von Feldt JM, et al. Vitamin D, metabolic dyslipidemia, and metabolic syndrome in rheumatoid arthritis. The American journal of medicine. 2012;125(10):1036. e9-. e15. [DOI] [PubMed] [Google Scholar]
- 55.Sahebari M, Goshayeshi L, Mirfeizi Z, Rezaieyazdi Z, Hatef MR, Ghayour-Mobarhan M, et al. Investigation of the association between metabolic syndrome and disease activity in rheumatoid arthritis. The Scientific World Journal. 2011;11:1195–205. 10.1100/tsw.2011.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mok CC, Ko GTC, Ho LY, Yu KL, Chan PT, To CH. Prevalence of atherosclerotic risk factors and the metabolic syndrome in patients with chronic inflammatory arthritis. Arthritis care & research. 2011;63(2):195–202. [DOI] [PubMed] [Google Scholar]
- 57.Dao H-H, Do Q-T, Sakamoto J. Increased frequency of metabolic syndrome among Vietnamese women with early rheumatoid arthritis: a cross-sectional study. Arthritis research & therapy. 2010;12(6):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Raterman H, Van Eijk I, Voskuyl A, Peters M, Dijkmans B, Van Halm V, et al. The metabolic syndrome is amplified in hypothyroid rheumatoid arthritis patients: a cross-sectional study. Annals of the rheumatic diseases. 2010;69(01):39–42. [DOI] [PubMed] [Google Scholar]
- 59.Solomon A, Christian BF, Norton GR, Woodiwiss AJ, Dessein PH. Risk factor profiles for atherosclerotic cardiovascular disease in black and other Africans with established rheumatoid arthritis. The Journal of rheumatology. 2010;37(5):953–60. 10.3899/jrheum.091032 [DOI] [PubMed] [Google Scholar]
- 60.Giles JT, Allison M, Blumenthal RS, Post W, Gelber AC, Petri M, et al. Abdominal adiposity in rheumatoid arthritis: association with cardiometabolic risk factors and disease characteristics. Arthritis & Rheumatism. 2010;62(11):3173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Santos MJ, Vinagre F, Silva J, Gil V, Fonseca J. Cardiovascular risk profile in systemic lupus erythematosus and rheumatoid arthritis: a comparative study of female patients. Acta Reumatológica Portuguesa. 2010:325–32. [PubMed] [Google Scholar]
- 62.La Montagna G, Cacciapuoti F, Buono R, Manzella D, Mennillo G, Arciello A, et al. Insulin resistance is an independent risk factor for atherosclerosis in rheumatoid arthritis. Scand J Rheumatol. 2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
Data Availability Statement
All relevant data are available in the manuscript.