Abstract
miR-21 induces epithelial-mesenchymal transition (EMT) of human cholangiocarcinoma (CCA) cells. However, the mechanism by which this occurs remains unclear. In the present study, high throughput platform was employed to detect the genes that are differential expressed in QBC939 cells transfected with a hsa-miR-21 antagomir or control vectors. The EMT-related Krüppel-like factor 4 (KLF4) gene was down-regulated after miR-21 was knocked down. Overexpression of miR-21 upregulated KLF4, Akt, ERK and mesenchymal cell markers (N-cadherin and vimentin), downregulated the expression of epithelial cell marker E-cadherin and reduced cell migration and invasion. Immunohistochemistry showed that KLF4, pAkt and pERK were upregulated in tumor xenografts transfected with miR-21 mimics. Inhibitors of the PI3K-Akt and ERK1/2 pathways, LY294002 and U0126, significantly suppressed the EMT phenotype. The present data demonstrated that overexpression of miR-21, accompanied with KLF4, augmented the EMT via inactivation of Akt and ERK1/2 pathways. In conclusion, we have identified a novel mechanism that may be targeted in an attempt to relieve the malignant biological behavior of CCA cells.
Keywords: cholangiocarcinoma, KLF4, miR-21, signal pathway, epithelial-mesenchymal transition
Introduction
Cancer metastasis is a highly coordinated and sequential process. An epithelial-mesenchymal transition (EMT) is important for disseminating cancer cells by endowing them with greater motility and invasiveness (1,2). Transformed cells downregulate epithelial cell marker E-cadherin whilst upregulating mesenchymal markers N-cadherin and vimentin (3–6).
MicroRNAs (miRNAs) are a class of small, endogenous non-coding RNAs that regulate target gene expression through pairing to its 3′-UTR (7). Accumulating evidence demonstrates that miRNAs are integral to the progression and metastasis of many human malignancies (8,9), including cholangiocarcinoma (CCA).
miR-21 is a major oncogenic miRNA, which is associated with several biomarkers and therapeutic targets in a variety of tumor types, such as renal (10), bladder (11), colorectal cancer (12) and others. miR-21 is an important critical regulator of the biological behavior of cancer cells and plays a role in apoptosis (10) and proliferation (13). Increasing evidence has demonstrated that miR-21 is implicated with induction and regulation of the EMT program (14–17). In addition, recent studies have suggested that miR-21 increases the proliferation, migration and invasion of cancer cells of various origin through activation of the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) and mitogen-activated protein kinase (MAPK)/extracellular signal regulated kinase1/2 (ERK1/2) pathways (18,19), both of which are dysregulated during the maintenance of EMT (20–22). Notably, Yan et al (23) found that PI3K/AKT inhibition by miR-21 knockdown reversed EMT in breast cancer in vitro.
We previously demonstrated that miR-21 is involved in the EMT of CCA cells (24). Krüppel-like factor 4 (KLF4) has been considered to be critical for EMT (25), however, the interactions between miR-21 and KLF4 that trigger the EMT of CCA cells remain unclear. The present study aimed to identify if miR-21, jointly with KLF4, is involved in the maintenance of EMT of CCA cells via AKT and ERK1/2 pathways.
Materials and methods
Reagents and antibodies
Human hsa-miR-21 (MIMAT 0000076) antagomir, mimics, scramble control and negative control were purchased from Guangzhou Land Unicomed Biotechnology, Co., Ltd. (Guangzhou, China). PCR primers were synthesized by Sangon Biotech, Co., Ltd. (Shanghai, China). Real-time PCR assay kits were purchased from Takara Bio (Dalian, China). Specific siKLF4 duplexes and a scramble control siRNA sequence (SsiKLF4) were purchased from Guangzhou Land Unicomed Biotechnology. LY294002 and U0126 were purchased from Sigma-Aldrich (St. Louis, MO, USA). Lipofectamine 2000 was purchased from Invitrogen (Carlsbad, CA, USA). Rabbit anti-human raised against E-cadherin, N-cadherin, vimentin, KLF4, Akt, phospho-Akt (p-Akt), ERK1/2, phospho-ERK1/2 (p-ERK1/2), GAPDH and an HRP-conjugated anti-rabbit IgG secondary antibody were purchased from Cell Signaling Technology (Danvers, MA, USA).
Cell lines and xenografts
Human QBC939 cholangiocarcinoma cells were purchased from the Cell Bank of the Chinese Academy of Sciences. Cells were maintained in high-glucose Dulbecco's modified Eagle's medium (DMEM; Gibco, Waltham, MA, USA), supplemented with 10% fetal bovine serum (FBS; Zhejiang Tianhang Biotechnology, Co. Ltd., Huzhou, China), 100 units/ml penicillin, 100 mg/ml streptomycin and 1% glutamine (Invitrogen) and were grown at 37°C in a humidified atmosphere of 95% air and 5% CO2. Xenografts, procured from our previous study (24), were embedded within consecutive paraffin sections and sliced prior to immunohistochemical analysis.
Vectors and transfection
Specific siKLF-4 duplexes and a scramble control siRNA sequence (SsiKLF-4) were designed, synthesized and annealed by Sigma-Aldrich. The selected RNA duplex (siKLF-4) corresponded to nucleotide sequences of KLF-4 mRNA sense: 5′-CCC ATC TTT CTC CAC GTT CG-3′ and antisense: 5′-TGC TTG ATC TTG GGG CAC GT-3′. LY294002 (inhibitor of PI3K/Akt) and U0126 (inhibitor of ERK1/2) were purchased from Sigma-Aldrich. Human miR-21 mimics, inhibitor and negative control were purchased from Guangzhou RiboBio, Co., Ltd. (Guangzhou, China). QBC939 cells were transfected with either: i) miR-21 mimics (mimic group); ii) miR-21 negative control (control group); or ii) miR-21 inhibitor (inhibitor group) using Lipofectamine 2000 transfection reagent (Invitrogen) following the manufacturer's guidelines. Briefly, 5×105 cells were seeded into 6-well plates and grown to 60% confluence. Human miR-21 antagomir or its negative control were transfected directly into QBC939 cells at a final concentration of 50 nmol/l. Human miR-21 mimics or its negative control were allowed to form transfection complexes with Lipofectamine™ 2000 in Opti-MEMH I serum-free medium (Invitrogen) at a final concentration of 40 nmol/l. Transfections were performed in triplicate. The transfection efficiency was determined by fluorescent microscopy 24 h post transfection. Finally, the transfected cells were harvested for total RNA and protein extraction.
Real-time RT-PCR analysis
We investigated the expression of miR-21, KLF4 and ERK in transfected QBC939 CCA cells. Initially, total RNA was extracted using TRIzol reagent (Invitrogen) following the manufacturer's guidelines. Next, RNA was reverse transcribed into cDNA using a PrimeScripts RT reagent kit (Takara Bio). For the reverse transcription of miR-21, a miR-21 reverse transcription (RT) primer (5′-GTC GTA TCC AGT GCA GGG TCC GAG GTA TTC GCA CTG GAT ACG ACT CAA CA-3′) and quantitative real-time RT-PCR (qPCR) primers (forward, 5′-GCC CGC TAG CTT ATC AGA CTG ATG-3′ and reverse, 5′-GTG CAG GGT CCG AGG T-3′) were used. qPCR reactions were carried out using SYBRH Premix Ex Taq™ II (Takara Bio) using an Applied Biosystems real-time PCR system (Life Technologies, Carlsbad, CA, USA). U6 small nuclear RNA was used as an internal control for miR-21. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA was used as an internal control for KLF4 and ERK genes. The following primers were used: miR-21: forward, 5′-ACA CTC CAG CTG GGT AGC TTA TCA GAC TGA TG-3′ and reverse, 5′-CTC AAC TGG TGT CGT GGA-3′; U6: forward, 5′-CTC GCT TCG GCA GCA CA-3′ and reverse, 5′-AAC GCT TCA CGA ATT TGC GT-3′. PCR reactions were done in triplicate. All samples were normalized to internal controls and fold changes were calculated using the relative quantification method (2−ΔΔCT).
Western blotting
Western blotting of five proteins was conducted as described in our previous study (24). Briefly, cells and xenografts were washed in phosphate-buffered saline (PBS) and incubated in lysis buffer (Sangon Biotech, Co., Ltd., Shanghai, China). Protein was separated by 12% SDS-PAGE and transferred to a PVDF membrane (Sangon Biotech). Non-specific binding sites were blocked by incubation with TBST containing 5% (w/v) non-fat dried milk. Next, the membrane was incubated with rabbit anti-human Akt, p-Akt, KLF-4, ERK or p-ERK (1:1,000 dilution) primary antibodies at 37°C for 1 h. Subsequently, membranes were incubated overnight with an HRP-conjugated anti-rabbit IgG secondary antibody (1:1,000 dilution) at 4°C. Signals were visualized by an ECL chemiluminescence detection kit and semi-quantitated using ImageJ software. Equal protein loading was assessed by the expression of GAPDH.
Immunohistochemical staining
Tissues were obtained from mouse xenografts as previously described (24), and were fixed in formalin, embedded in paraffin and sectioned (2 μm). Slides were stained with KLF-4, p-Akt and p-ERK antibodies, and developed with a streptavidin-peroxidase (S-P) kit (Fuzhou Maixin Biotechnology Development, Co., Ltd., Fuzhou, China). The proportion of positive cells was determined using five ×200 magnification fields per slide.
Migration and invasion assays
Migration and invasion of cells were determined with the Transwell chamber assay (8-μm pore size; Millipore, Billerica, MA, USA) completed as per the manufacturer's instructions. To determine invasion, the chamber was coated with 80 μl Matrigel (BD Biosciences, San Jose, CA, USA) hydrated with 50 μl serum-free medium. Next, 200 μl transfected cell suspension (1×105 cells/ml) was added to the upper chamber, and media supplemented with 30% fetal bovine serum (FBS) was added to the bottom wells. After culturing for 24 h, cells were fixed for 15 min in 4% formaldehyde and stained with 1% crystal violet. Cell numbers were counted under an optical microscope. Cell migration was also evaluated using the Transwell chamber assay with the exception that the chamber was not coated with Matrigel. Each experiment was repeated at least three times.
Construction and transfection of siKLF4
Based on the human KLF4 mRNA sequence (NM_000314), specific siRNA duplexes were designed, synthesized and annealed by Land Unicomed Biotechnology. The selected RNA duplex (siKLF4) corresponding to nucleotides 1567-1585 of KLF4 mRNA was defined as sense: 5′-GCU ACC UGU UAA AGA AUC AdTdT-3′ and antisense: 5′-UGA UUC UUU AAC AGG UAG CdTdT-3′. The scramble-control siRNA sequence (SsiKLF4) was also designed by Land Unicomed Biotechnology, and has no significant homology to any known human gene sequence. Prior to transfection, 5×105 cells were seeded into 6-well plates and grown to 70% confluence. Human siKLF4 or SsiKLF4 (final concentration of 50 nmol/l) was allowed to form transfection complexes with the Lipofectamine 2000 reagent (Invitrogen) in Opti-MEMH I serum-free medium (Invitrogen) as per the manufacturer's guidelines.
Scratch wound migration assay
For the scratch wound migration assay, 1×106 cells were seeded into 6-well plates and grown to 60% confluence. Next, the cells were transfected with a miR-21 mimic and co-incubated with LY294002 or U0126 inhibitors. A 'wound' was then created by scratching the cell cultures with a plastic filter tip. The cells were rinsed three times with PBS and incubated at 37°C. The average distance that cells migrated after 0, 24 and 48 h was determined using an inverted microscope with ×8 vision. Migration distance was calculated by image processing using Prolog software. From the data, the migration rate = (1-distance at indicated time/distance at 0 h)%. Experiments were performed in triplicate.
Statistical analysis
All data are expressed as the mean ± SD. Data were analyzed using SPSS V20.0 software. The Student's t-test was used to determine statistical differences between treatment groups. A P≤0.05 was considered statistically significant.
Results
Transfected miR-21 and its inhibitor change the expression of miR-21 in QBC939 cells
To evaluate whether miR-21 mimics and inhibitors can modify the expression of miR-21, QBC939 cells were transfected with a miR-21 mimic or inhibitor for 48 h after which the relative expression of miR-21 was measured by RT-PCR. The relative expression of miR-21 was significantly upregulated in QBC939/miR-21 cells relative to NC cells (P<0.01; Fig. 1A). However, the relative expression of miR-21 was significantly downregulated in QBC939/miR-21 inhibitor cells relative to NC cells (P<0.01; Fig. 1A). These results suggested that hsa-miR-21 upregulates expression of miR-21 within QBC939 cells, whereas the hsa-miR-21 inhibitor could downregulate miR-21 in QBC939/miR-21 inhibitor cells.
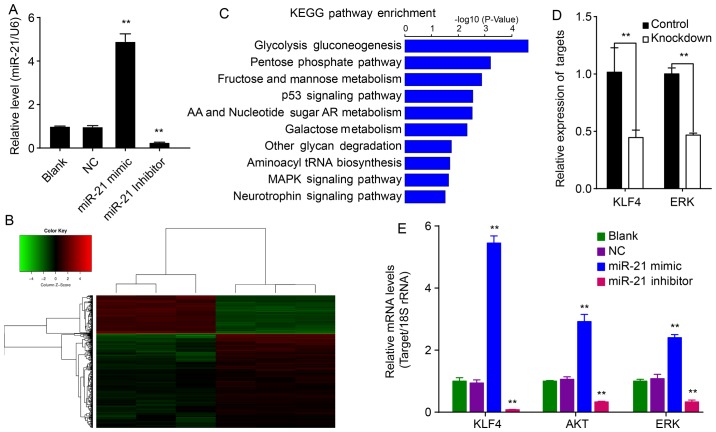
Figure 1.
Altered expression of miR-21, EMT-related genes, KLF4, Akt and ERK after transfection with miR-21 mimic, miR-21 inhibitor, or control. (A) QBC939 cells treated with miR-21 mimic showed increased expression of miR-21, whereas cells treated with the miR-21 inhibitor showed decreased expression of miR-21 compared to the NC group (P<0.01). (B) Clustered and (C) pathway enrichment of differentially expressed genes induced by miR-21 knockdown. (D and E) Altered levels of KLF4 and ERK by qPCR. The qPCR reactions were performed in triplicate. As loading control, 18S rRNA was used. (**P<0.01, compared with NC group).
Inhibition of miR-21 downregulates KLF4 in QBC939 cells
To investigate the role of miR-21 on EMT, QBC939 cells transfected with the hsa-miR-21 inhibitor or control differentially expressed genes and EMT-related genes were analyzed by Human GeneChip PrimeView. The data showed that 400 genes were differentially expressed (115 upregulated and 285 downregulated genes) in miR-21-knockdown cells compared to NC cells (Fig. 1B). These differentially expressed genes were mainly enriched for the glycolysis gluconeogenesis, pentose phosphate, fructose and mannose metabolism and p53 signaling pathways (Fig. 1C). Further analysis of these genes using IPA software showed that genes KLF4 and ERK are related to EMT. KLF4 and ERK expression were verified by qPCR (Fig. 1E).
miR-21 augments levels of KLF4, Akt and ERK in QBC939 cells
To investigate the mechanism of miR-21 on EMT in QBC939 cells, the relative mRNA expression of KLF4, Akt and ERK was determined. miR-21 increased mRNAs levels of KLF4, Akt and ERK relative to corresponding control cells (P<0.01; Fig. 1E). Conversely, the miR-21 inhibitor decreased KLF4, Akt and ERK levels (P<0.01). These results suggest that miR-21 affects mRNA expression of KLF4, Akt and ERK.
miR-21 increases expression of KLF4, and Akt and ERK activation in xenografts
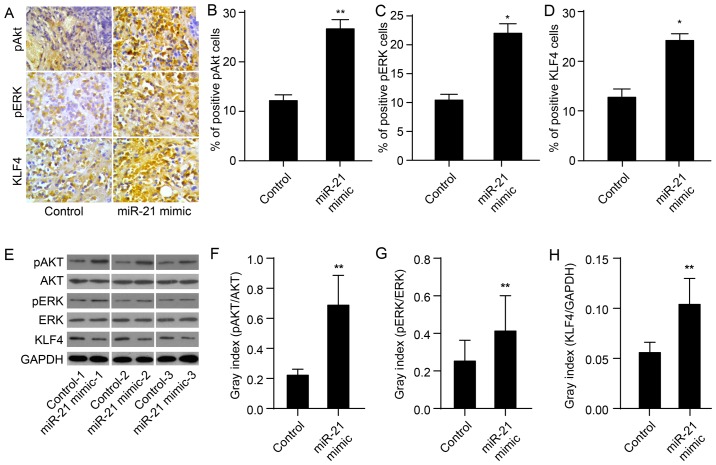
To evaluate whether miR-21 upregulates the expression of KLF4 and of Akt and ERK activation, we used immunohistochemistry and western blotting to determine miR-21 protein levels in tissues obtained from mouse xeno-grafts as previously described (24). As expected, the protein expression of KLF4 in the miR-21 mimic group was markedly upregulated compared to the control group (P<0.01; Fig. 2A, D and I). In the miR-21 mimic group, levels of pAkt and pERK were also significantly upregulated (P<0.01; Fig. 2A–C and E–G). These results demonstrate that miR-21 mimics upregulate the expression of KLF4 and activate Akt and ERK.
Figure 2.
miR-21 mimic facilitates the expression of KLF4, pAkt and pERK in tumor xenografts. (A) Immunohistochemical analysis showing positive staining for p-Akt, p-ERK or KLF4. (B-D) Cell counts for staining with p-Akt, p-ERK or KLF4 are shown. (E) Western blot analysis showing relative protein levels of KLF4, Akt and ERK (F–H). Bands were semi-quantified using Quantity One software. GAPDH was used as loading control. Experiments were performed in triplicate and representative data are shown (**P<0.01).
miR-21 antagonism accompanies KLF4 knockdown inactivated AKT and ERK1/2
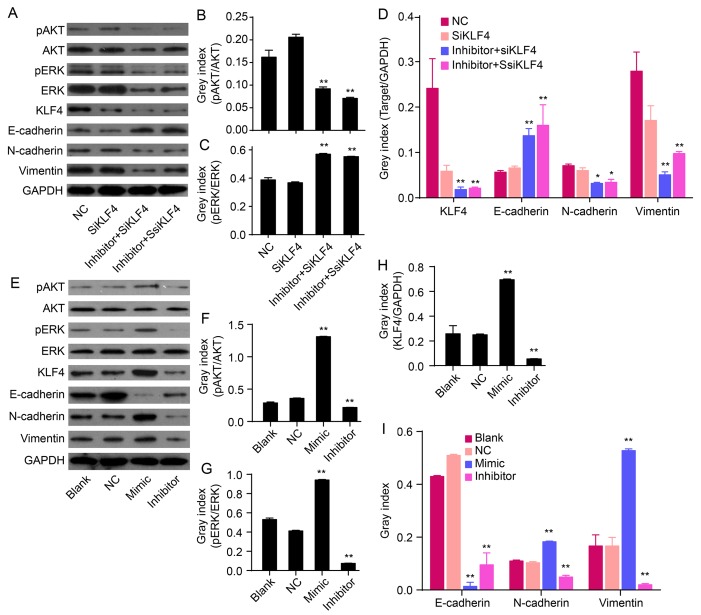
Akt and ERK1/2 are two major components of signaling pathways that are involved in the regulation of cell proliferation, migration and survival. To determine the signaling molecules involved in the antagonism of KLF4 and miR-21-induced EMT phenotype, protein levels of phosphorylated Akt (p-Akt), Akt, phosphorylated ERK1/2 (p-ERK1/2) and ERK1/2 were determined by western blot analysis. The protein levels of p-Akt and p-ERK1/2 in QBC939/anti-KLF4/miR-21 inhibitor cells were markedly lower compared to levels in anti-KLF4 cells (P<0.01; Fig. 3A–C). miR-21 antagonism, in combination with KLF4 knockdown, could reverse EMT via suppressing Akt and ERK1/2 activation.
Figure 3.
Effects of miR-21 on the expression of KLF4, E-cadherin, N-cadherin, vimentin and/or Akt/ERK1/2 pathways. (A) The relative levels of p-Akt, Akt, p-ERK, ERK, KLF4 and EMT marker proteins were measured by western blot analysis. (B-D) Bands were semi-quantified using Quantity One software. (E) Western blot analysis showing protein levels of p-Akt, Akt, p-ERK1/2, ERK1/2, KLF4, mesenchymal markers (N-cadherin and vimentin) and epithelial cell marker (E-cadherin). (F-I) Bands were semi-quantified using Quantity One software. GAPDH was used as loading control (**P<0.01).
Overexpression of miR-21 induces an EMT phenotype accompanied with upregulation of KLF4 and activation of AKT and ERK1/2
To confirm the role of miR-21 in regulating an EMT phenotype, hsa-miR-21 mimic or inhibitor were transfected into QBC939 cells. The protein expression of EMT biomarkers (N-cadherin, vimentin and E-cadherin), KLF4, p-Akt, Akt, p-ERK1/2 and ERK1/2 were measured by western blot analysis. Compared to NC cells, overexpression of miR-21 increased the protein expression of N-cadherin and vimentin, but decreased the expression of E-cadherin (P<0.01; Fig. 3E and I). In addition, low expression of miR-21, induced by the miR-21 inhibitor, decreased the levels of N-cadherin and vimentin and increased the expression of E-cadherin (P<0.01; Fig. 3E and I). This suggests that overexpression of miR-21 induces an EMT phenotype in QBC939 cells. Overexpression of miR-21 increased the expression of KLF4 (P<0.01; Fig. 3E and H). In addition, re-expression of miR-21 increased the expression of p-Akt and p-ERK1/2 in QBC939 cells (P<0.01; Fig. 3F and G), thereby suggesting that an overexpression of miR-21 could activate Akt and ERK1/2 pathways. Together, these data imply that miR-21 regulates EMT and is related to alterations in KLF4 expression and Akt/ERK1/2 pathways.
miR-21 regulates an EMT phenotype and increases invasion and migration through AKT and ERK1/2 pathways
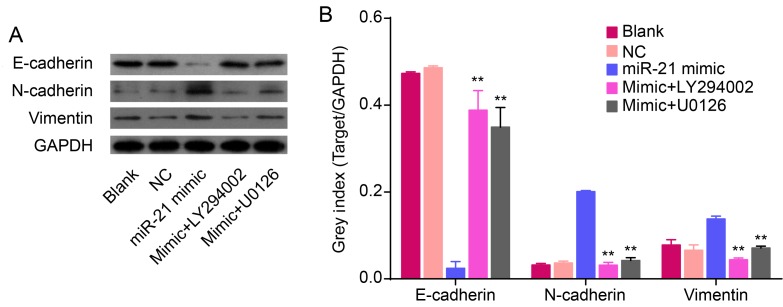
To further investigate the involvement of Akt and ERK1/2 pathways in miR-21 regulating an EMT, QBC939 cells were transfected with miR-21 mimics for 72 h. Cells were treated for 24 h with either the LY294002 inhibitor of Akt or the U0126 inhibitor of ERK1/2. Our data confirm that treatment with LY294002 and U0126 resulted in abolished overexpression of miR-21, induced inhibition of E-cadherin, and activation of N-cadherin and vimentin (P<0.01; Fig. 4). Thus, we have demonstrated that miR-21 overexpression and its ability to induce the EMT phenotype were inhibited by LY294002 or U0126 in QBC939 cells.
Figure 4.
miR-21 regulates EMT through mediating Akt and ERK1/2 activation in QBC939 cells. (A) Relative and (B) semi-quantified protein levels of EMT markers from Blank, NC, miR-21 mimic, LY294002 and U0126 treatment groups. GAPDH was used as loading control (**P<0.01 compared with miR-21 mimic group).
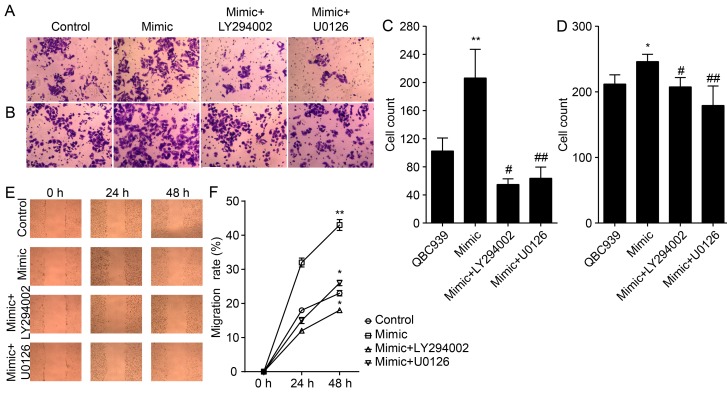
We also studied the effect of antagonizing miR-21 on the migration and invasion of CCA cells (Fig. 5A, B and E). The number of migrated and invaded QBC939/anti-miR-21 cells were significantly lower compared to that of NC cells (P<0.01; Fig. 5C, D and F), thereby suggesting that miR-21 antagonists reduced the migration and invasion of CCA cells.
Figure 5.
miR-21 regulates the invasion and migration of CCA cells through activation of Akt and ERK1/2 pathways. (A) The invasive and (C) migration properties of indicated cells were tested in invasion and migration assays in Transwell inserts. (B and D) Penetrated cells were counted and analyzed. (E) Scratch-wound assay was performed in QBC939 cells and (F) migration rate were quantified from results shown in (E). Experiments were performed in triplicate (*P<0.05; **P<0.01).
Discussion
Increasing evidence suggests that the expression of miR-21 is upregulated in various cancer types, including cholangiocarcinoma (CCA), where it is frequently associated with EMT (15,16,26). Overexpression of miR-21 in CCA biopsies is predictive for a poor outcome (24), and is accompanied with low expression of E-cadherin and high expression of N-cadherin and vimentin (27–29). In our previous study, we showed that miR-21 complicates the EMT of CCA cells. We also showed that that CCA xenografts have high levels of N-cadherin and vimentin and low levels of E-cadherin (24). In the present study, we first identified genes that are differentially expressed in a CCA xenograft model transfected with miR-21 knockdown or control lentivirus, and found that KLF4 and ERK were significantly downregulated. Both KLF4 and ERK genes are associated with EMT. Tiwari et al (25) previously showed that KLF4 was significantly downregulated during EMT of breast cancer cells. However, the role of KLF4 in the EMT of CCA cells has not been elucidated. Accordingly, we studied the effect of miR-21 on the expression of KLF4, its role in the EMT, and investigated the potential underlying mechanisms of action. We used immunohistochemistry and western blot analysis to show that, in QBC939 CCA cells, overexpression of miR-21 promoted upregulation of KLF4 and expression of Akt and ERK proteins. Therefore, our data suggest that miR-21 and KLF4 regulate EMT through AKT/ERK1/2 pathways.
To investigate the roles of miR-21 and KLF4 in regulating the EMT phenotype of CCA cells, miR-21 mimics or inhibitors were transfected into QBC939 cells. In addition, KLF4 was silenced by using siKLF4. The data showed that the EMT phenotype was maintained by miR-21 mimics and reversed by miR-21 inhibitor. The results are consistent with previous studies, in which was shown that miR-21 facilitated EMT of pancreatic cancers (15,30), thyroid (16) and CCA origin and decreased activation of Akt and ERK1/2 pathways (24). We also demonstrated that the downregulation of KLF4 by using RNAi reversed the EMT and suppressed the activation of Akt and ERK1/2 signaling. Studies have demonstrated that Akt and/or ERK1/2 pathways are required for augmenting the EMT of several different cancer cell lines, including MCF-7 breast cancer cells (20), breast epithelial cells (21,22), cervical cancer cells (20), ovarian carcinoma cells (31) and colon cancer cells (32). These results demonstrate that miR-21 and KLF4 play an important role in mediating EMT of CCA cells through the AKT and ERK1/2 pathway.
Finally, we evaluated the invasion and migration of QBC939 cells. Overexpression of miR-21 by miR-21 mimics promoted the activation of Akt and ERK1/2 pathways and resulted in an increase in invasive ability and migration rate. However, these effects were suppressed by treatment of the cells with PI3K-Akt or the ERK1/2 inhibitor LY294002 or U0126.
In summary, our data demonstrate that miR-21 and KLF4 synergistically regulated EMT of QBC939 CCA cells through Akt and ERK1/2 pathways. The present study illustrated a mechanism by which miR-21 and KLF4 maintain an EMT within CCA cells, which may be beneficial for the development of novel therapies to treat cancer.
Acknowledgments
The present study was supported by the National Natural Science Foundation of China (no. 81272397) and the Research Project of Anhui Natural Science for Colleges and Universities (KJ2013Z143).
References
- 1.Tsai JH, Yang J. Epithelial-mesenchymal plasticity in carcinoma metastasis. Genes Dev. 2013;27:2192–2206. doi: 10.1101/gad.225334.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wells A, Chao YL, Grahovac J, Wu Q, Lauffenburger DA. Epithelial and mesenchymal phenotypic switchings modulate cell motility in metastasis. Front Biosci (Landmark Ed) 2011;16:815–837. doi: 10.2741/3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest. 2009;119:1429–1437. doi: 10.1172/JCI36183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 5.Christiansen JJ, Rajasekaran AK. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res. 2006;66:8319–8326. doi: 10.1158/0008-5472.CAN-06-0410. [DOI] [PubMed] [Google Scholar]
- 6.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Wen KC, Sung PL, Yen MS, Chuang CM, Liou WS, Wang PH. MicroRNAs regulate several functions of normal tissues and malignancies. Taiwan J Obstet Gynecol. 2013;52:465–469. doi: 10.1016/j.tjog.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Olson P, Lu J, Zhang H, Shai A, Chun MG, Wang Y, Libutti SK, Nakakura EK, Golub TR, Hanahan D. MicroRNA dynamics in the stages of tumorigenesis correlate with hallmark capabilities of cancer. Genes Dev. 2009;23:2152–2165. doi: 10.1101/gad.1820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farazi TA, Horlings HM, Ten Hoeve JJ, Mihailovic A, Halfwerk H, Morozov P, Brown M, Hafner M, Reyal F, van Kouwenhove M, et al. MicroRNA sequence and expression analysis in breast tumors by deep sequencing. Cancer Res. 2011;71:4443–4453. doi: 10.1158/0008-5472.CAN-11-0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou X, Zhang J, Jia Q, Ren Y, Wang Y, Shi L, Liu N, Wang G, Pu P, You Y, et al. Reduction of miR-21 induces glioma cell apoptosis via activating caspase 9 and 3. Oncol Rep. 2010;24:195–201. doi: 10.3892/or_00000846. [DOI] [PubMed] [Google Scholar]
- 11.Zhang HH, Qi F, Cao YH, Zu XB, Chen MF. Expression and clinical significance of microRNA-21, maspin and vascular endothelial growth factor-C in bladder cancer. Oncol Lett. 2015;10:2610–2616. doi: 10.3892/ol.2015.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukushima Y, Iinuma H, Tsukamoto M, Matsuda K, Hashiguchi Y. Clinical significance of microRNA-21 as a biomarker in each Dukes' stage of colorectal cancer. Oncol Rep. 2015;33:573–582. doi: 10.3892/or.2014.3614. [DOI] [PubMed] [Google Scholar]
- 13.Zhou X, Ren Y, Moore L, Mei M, You Y, Xu P, Wang B, Wang G, Jia Z, Pu P, et al. Downregulation of miR-21 inhibits EGFR pathway and suppresses the growth of human glioblastoma cells independent of PTEN status. Lab Invest. 2010;90:144–155. doi: 10.1038/labinvest.2009.126. [DOI] [PubMed] [Google Scholar]
- 14.Kothari AN, Mi Z, Zapf M, Kuo PC. Novel clinical therapeutics targeting the epithelial to mesenchymal transition. Clin Transl Med. 2014;3:35. doi: 10.1186/s40169-014-0035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bao B, Wang Z, Ali S, Kong D, Li Y, Ahmad A, Banerjee S, Azmi AS, Miele L, Sarkar FH. Notch-1 induces epithelial-mesenchymal transition consistent with cancer stem cell phenotype in pancreatic cancer cells. Cancer Lett. 2011;307:26–36. doi: 10.1016/j.canlet.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Braun J, Hoang-Vu C, Dralle H, Hüttelmaier S. Down-regulation of microRNAs directs the EMT and invasive potential of anaplastic thyroid carcinomas. Oncogene. 2010;29:4237–4244. doi: 10.1038/onc.2010.169. [DOI] [PubMed] [Google Scholar]
- 17.Kumarswamy R, Volkmann I, Jazbutyte V, Dangwal S, Park DH, Thum T. Transforming growth factor-β-induced endothelial-to-mesenchymal transition is partly mediated by microRNA-21. Arterioscler Thromb Vasc Biol. 2012;32:361–369. doi: 10.1161/ATVBAHA.111.234286. [DOI] [PubMed] [Google Scholar]
- 18.Weng LP, Smith WM, Brown JL, Eng C. PTEN inhibits insulin-stimulated MEK/MAPK activation and cell growth by blocking IRS-1 phosphorylation and IRS-1/Grb-2/Sos complex formation in a breast cancer model. Hum Mol Genet. 2001;10:605–616. doi: 10.1093/hmg/10.6.605. [DOI] [PubMed] [Google Scholar]
- 19.Han M, Liu M, Wang Y, Chen X, Xu J, Sun Y, Zhao L, Qu H, Fan Y, Wu C. Antagonism of miR-21 reverses epithelial-mesenchymal transition and cancer stem cell phenotype through AKT/ERK1/2 inactivation by targeting PTEN. PLoS One. 2012;7:e39520. doi: 10.1371/journal.pone.0039520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li J, Zhou BP. Activation of β-catenin and Akt pathways by Twist are critical for the maintenance of EMT associated cancer stem cell-like characters. BMC Cancer. 2011;11:49. doi: 10.1186/1471-2407-11-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iliopoulos D, Polytarchou C, Hatziapostolou M, Kottakis F, Maroulakou IG, Struhl K, Tsichlis PN. MicroRNAs differentially regulated by Akt isoforms control EMT and stem cell renewal in cancer cells. Sci Signal. 2009;2:ra62. doi: 10.1126/scisignal.2000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Irie HY, Pearline RV, Grueneberg D, Hsia M, Ravichandran P, Kothari N, Natesan S, Brugge JS. Distinct roles of Akt1 and Akt2 in regulating cell migration and epithelial-mesenchymal transition. J Cell Biol. 2005;171:1023–1034. doi: 10.1083/jcb.200505087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yan LX, Liu YH, Xiang JW, Wu QN, Xu LB, Luo XL, Zhu XL, Liu C, Xu FP, Luo DL, et al. PIK3R1 targeting by miR-21 suppresses tumor cell migration and invasion by reducing PI3K/AKT signaling and reversing EMT, and predicts clinical outcome of breast cancer. Int J Oncol. 2016;48:471–484. doi: 10.3892/ijo.2015.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Z, Jin ZY, Liu CH, Xie F, Lin XS, Huang Q. MicroRNA-21 regulates biological behavior by inducing EMT in human cholangiocarcinoma. Int J Clin Exp Pathol. 2015;8:4684–4694. [PMC free article] [PubMed] [Google Scholar]
- 25.Tiwari N, Meyer-Schaller N, Arnold P, Antoniadis H, Pachkov M, van Nimwegen E, Christofori G. Klf4 is a transcriptional regulator of genes critical for EMT, including Jnk1 (Mapk8) PLoS One. 2013;8:e57329. doi: 10.1371/journal.pone.0057329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng G, Li N, Jia X, Peng C, Luo L, Deng Y, Yin J, Song Y, Liu H, Lu M, et al. MYCN-mediated miR-21 overexpression enhances chemo-resistance via targeting CADM1 in tongue cancer. J Mol Med (Berl) 2016;94:1129–1141. doi: 10.1007/s00109-016-1417-0. [DOI] [PubMed] [Google Scholar]
- 27.Huang Q, Liu L, Liu CH, You H, Shao F, Xie F, Lin XS, Hu SY, Zhang CH. MicroRNA-21 regulates the invasion and metastasis in cholangiocarcinoma and may be a potential biomarker for cancer prognosis. Asian Pac J Cancer Prev. 2013;14:829–834. doi: 10.7314/APJCP.2013.14.2.829. [DOI] [PubMed] [Google Scholar]
- 28.Liu CZ, Liu W, Zheng Y, Su JM, Li JJ, Yu L, He XD, Chen SS. PTEN and PDCD4 are bona fide targets of microRNA-21 in human cholangiocarcinoma. Chin Med Sci J. 2012;27:65–72. [PubMed] [Google Scholar]
- 29.Selaru FM, Olaru AV, Kan T, David S, Cheng Y, Mori Y, Yang J, Paun B, Jin Z, Agarwal R, et al. MicroRNA-21 is overexpressed in human cholangiocarcinoma and regulates programmed cell death 4 and tissue inhibitor of metalloproteinase 3. Hepatology. 2009;49:1595–1601. doi: 10.1002/hep.22838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bao B, Ali S, Kong D, Sarkar SH, Wang Z, Banerjee S, Aboukameel A, Padhye S, Philip PA, Sarkar FH. Anti-tumor activity of a novel compound-CDF is mediated by regulating miR-21, miR-200, and PTEN in pancreatic cancer. PLoS One. 2011;6:e17850. doi: 10.1371/journal.pone.0017850. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Latifi A, Abubaker K, Castrechini N, Ward AC, Liongue C, Dobill F, Kumar J, Thompson EW, Quinn MA, Findlay JK, et al. Cisplatin treatment of primary and metastatic epithelial ovarian carcinomas generates residual cells with mesenchymal stem cell-like profile. J Cell Biochem. 2011;112:2850–2864. doi: 10.1002/jcb.23199. [DOI] [PubMed] [Google Scholar]
- 32.Wang YK, Zhu YL, Qiu FM, Zhang T, Chen ZG, Zheng S, Huang J. Activation of Akt and MAPK pathways enhances the tumorigenicity of CD133+ primary colon cancer cells. Carcinogenesis. 2010;31:1376–1380. doi: 10.1093/carcin/bgq120. [DOI] [PubMed] [Google Scholar]