Abstract
Background
Diabetes mellitus (DM) is associated with higher incidence and poorer prognosis of hepatocellular carcinoma (HCC). The influence of DM on patient survival in different HCC stages is not known.
Methods
A prospective dataset of 3,182 HCC patients was collected between 2002 and 2014. Patients were divided into three groups according to BCLC stages (BCLC stage 0 and stage A, BCLC stage B, BCLC stage C and stage D). We compared the cumulative survival rate of diabetic and non-diabetic patients in different BCLC groups. The correlation between DM and overall survival was also analyzed by multivariate Cox regression model within each group.
Results
DM is present in 25.2% of all patients. Diabetic patients had lower cumulative survival in BCLC stage 0 plus BCLC stage A group (log rank p<0.001), and BCLC stage B group (log rank p = 0.012), but not in BCLC stage C plus BCLC stage D group (log rank p = 0.132). Statistically significant differences in overall survival are found between diabetic and non-diabetic patients in BCLC stage 0 plus stage A group (adjusted hazard ratio [HR] = 1.45, 95% confidence interval [CI] 1.08–1.93, p = 0.013), and BCLC stage B (adjusted HR = 1.77, 95% CI 1.24–2.51, p = 0.002). In contrast, the survival difference is not seen in BCLC stage C plus stage D group (adjusted HR = 1.09, 95% CI 0.90–1.30, p = 0.387).
Conclusions
DM is prevalent in HCC, and is associated with lower survival rate in HCC patients with BCLC stage 0 plus stage A and B, but not in those with BCLC stage C plus stage D.
Introduction
Hepatocellular carcinoma (HCC) is the fifth common neoplasm in men and the seventh in women. It contributed to 745,000 deaths in year 2012 and was the second leading cause of cancer-related mortality worldwide.[1] Well-established risk factors for HCC include chronic hepatitis B virus (HBV) infection, chronic hepatitis C virus (HCV) infection, aflatoxin B1, and alcohol consumption.[2, 3] The pathogenic and prognostic roles of metabolic factors, such as diabetes mellitus (DM), metabolic syndrome, or obesity, had also been studied.[4–6] Epidemiologic studies have disclosed association between presence of DM and higher HCC incidence, suggesting that DM is an independent risk factor for development of HCC.[7–10]
In addition to its role in pathogenesis, DM may also be an important predictor of prognosis.[11, 12] Previous studies analyzing the relation between DM and HCC outcomes focused mainly on resectable or potentially curable diseases. However, the results were inconsistent.[13–18] DM seems to worsen HCC prognosis in some subgroups to a greater extent. Toyoda et al found that the presence of DM led to poorer prognosis only in patients with treatable diseases, and those with tumor size ≤ 3 cm in greatest dimension.[19] Wang et al reported lower overall and disease-free survival in DM patients with cirrhosis and HCC, but not in their non-cirrhotic counterparts.[20]
The Barcelona Clínic Liver Cancer (BCLC) classification is one of the most widely adapted classification systems for HCC. The BCLC system incorporates multiple factors, including tumor burden, liver functional reserve and performance status. With its ability to predict prognosis and guide treatment algorithm, the BCLC staging system is endorsed by the European Association for the Study of Liver (EASL) and American Association for the Study of Liver Diseases (AASLD) HCC management guidelines. BCLC system stratifies patients into several distinct prognostic groups. The association and prognostic impact of DM on HCC patients with different cancer stages remain unclear. In this study, we aim to explore the prognostic role of DM in different BCLC stages.
Methods
Patients
We have prospectively enrolled and retrospectively analyzed newly diagnosed HCC patients admitted to Taipei Veterans General Hospital during 2002 to 2014. Baseline characteristics, including underlying etiologies for HCC, biochemistry profile, tumor extent, vascular invasion, severity of cirrhosis, performance status, and diagnosis of DM were recorded. Patient follow-up was arranged every 3–6 months until death or dropout from the program. Survival was defined from the date of diagnosis to death or last follow-up. Those receiving liver transplantation were censored at the date of transplantation. The study complies with the standards of the Declaration of Helsinki and current ethical guidelines and was approved by the Institutional Review Board of the Taipei Veterans General Hospital (IRB protocol number 2014-03-007AC). Waiver of consent was obtained, and patient records/information was anonymized and de-identified prior to analysis.
Diagnosis and definitions
The diagnosis of HCC was established in accordance with EASL and AASLD HCC management guidelines.[21, 22] BCLC staging information was obtained at the time of diagnosis. We defined vascular invasion as radiological evidence of tumor invasion to intrahepatic vasculatures, portal trunk or abdominal great vessels. DM was defined as a fasting plasma glucose of 126 mg/dl or greater on at least two separate occasions, plasma glucose of 200 mg/dl or greater 2 hours after a 75 g oral glucose tolerance test, a glycated hemoglobin (HbA1C) level > 6.5% for once, or any prescription of hypoglycemic agents.[23]
Treatments
Once diagnosis was confirmed, patient data were reviewed at multi-disciplinary HCC board of Taipei Veterans General Hospital for treatment planning. We provided comprehensive information regarding risks and benefits of each treatment to patients. The final treatment modality taken was decided by shared-decision making between physicians and patients. Written informed consent was obtained prior to all management. Invasive therapies, including radiofrequency ablation, surgical resection, and transarterial chemoembolization were performed through standard procedures as previously reported.[24–26]
Statistics
The cumulative survival rates of diabetic and non-diabetic patients among different BCLC stages were examined by the Kaplan-Meier methods with log-rank tests. Cox proportional hazards regression model was performed for hazard ratio evaluation. Prognostic factors that are probably associated with overall survival, including age, sex, severity and etiology of chronic liver diseases, biochemical laboratory parameters and tumoral status were included in the univariate survival analysis. Factors significant in the univariate analysis (P < 0.1) were introduced into the multivariate Cox model to determine independent predictors of prognosis. The proportional hazard assumption was assessed graphically before being analyzed with Cox model. We used two-tailed χ2 test to compare categorical data, and Mann-Whitney U test to evaluate continuous variables. Interaction between DM and other predictors were assessed using likelihood ratio tests comparing the final model and the final model with the interaction terms. Statistical analyses were conducted with IBM SPSS version 21 (IBM, NY) and SAS version 9.4 (SAS Institute, NC). Statistical significance was set as P value less than 0.05 in a two-tailed test.
Results
Patient characteristics
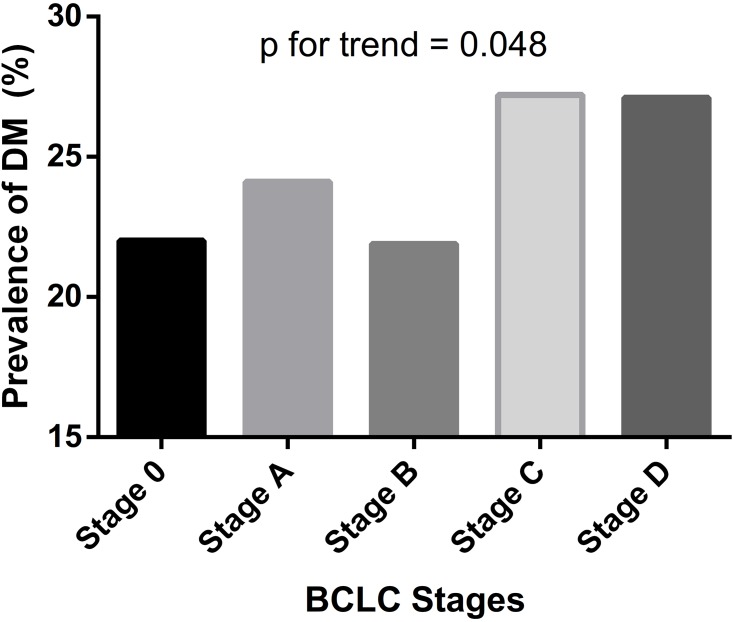
The median age of the study patients is 65 years old. The median follow-up duration is 17 months for the entire cohort, being 19 months for non-diabetic and 15 months for diabetic patients. Among the 3,182 participants, 1001 (31.5%) were early HCC, 503 (15.8%) were intermediate HCC, 1282 (40.3%) were advanced HCC, while 396 patients (12.4%) had terminal HCC at the time of diagnosis. Prevalence of DM was 25.2% as a whole. The respective DM prevalence in BCLC very early, early, intermediate, advanced, and terminal stages were 22.0%, 24.1%, 21.9%, 27.2%, and 27.1%, showing no statistically significant difference (Table 1; p = 0.081). However, a significant trend toward increasing prevalence of DM in more advanced BCLC stages were noted (p for trend = 0.048, Fig 1).
Table 1. Demographic, clinical, and staging information of the entire hepatocellular carcinoma cohort.
| n = 3,182 | BCLC Stage 0 (n = 265) |
BCLC Stage A (n = 736) |
BCLC Stage B (n = 503) |
BCLC Stage C (n = 1282) |
BCLC Stage D (n = 396) |
P value |
|---|---|---|---|---|---|---|
| Age (years, mean ± SD) | 63.3 ± 11.8 | 64.9 ± 11.6 | 64.2 ± 13.6 | 63.8 ± 13.6 | 66.5 ± 14.8 | 0.004 |
| Male gender, n (%) | 185 (69.8) | 528 (71.9) | 414 (82.3) | 1002 (78.3) | 310 (78.3) | <0.001 |
| Diabetes mellitus, n (%) | 58 (22) | 177 (24.1) | 110 (21.9) | 346 (27.2) | 107 (27.1) | 0.081 |
| ECOG, n (%) | <0.001 | |||||
| Performance status = 0 | 265 (100) | 736 (100) | 503 (100) | 296 (23.1) | 6 (1.5) | |
| Performance status = 1 | 0 (0) | 0 (0) | 0 (0) | 680 (53.0) | 24 (6.1) | |
| Performance status = 2 | 0 (0) | 0 (0) | 0 (0) | 306 (23.9) | 30 (7.6) | |
| Performance status = 3 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 225 (56.8) | |
| Performance status = 4 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 111 (28.0) | |
| Vascular invasion, n (%) | 0 (0) | 0 (0) | 0 (0) | 591 (46.1) | 215 (54.3) | <0.001 |
| Hepatitis B, n (%) | 141 (53.2) | 377 (51.2) | 299 (59.4) | 705 (55.0) | 197 (49.7) | 0.019 |
| Hepatitis C, n (%) | 105 (39.6) | 290 (39.4) | 124 (24.7) | 341 (26.6) | 114 (28.8) | 0.001 |
| Alcoholism, n (%) | 28 (10.6) | 98 (13.3) | 67 (13.3) | 298 (23.2) | 91 (23.0) | <0.001 |
| Laboratory values (mean ± SD) | ||||||
| Albumin (g/dl) | 4.0 ± 0.5 | 3.9 ± 0.5 | 3.9 ± 0.5 | 3.6 ± 0.6 | 3.0 ± 0.6 | <0.001 |
| Bilirubin (mg/dl) | 0.86 ± 0.39 | 0.94 ± 0.62 | 0.94 ± 0.83 | 1.42 ± 2.16 | 4.15 ± 5.85 | <0.001 |
| INR (ratio) | 1.04 ± 0.09 | 1.06 ± 0.12 | 1.04 ± 0.12 | 1.10 ± 0.14 | 1.27 ± 0.33 | <0.001 |
| AFP (ng/ml) | 136 ± 411 | 733 ± 9959 | 6281 ± 31499 | 32184 ± 165949 | 58138 ± 135642 | <0.001 |
| Platelet (1000/mm3) | 133.8 ± 64.3 | 137.5 ± 62.3 | 174.5 ± 85.6 | 186.5 ± 106.2 | 196.6 ± 119.6 | <0.001 |
| Na (mEq/l) | 139.4 ± 3.2 | 139.9 ± 3.0 | 139.6 ± 2.8 | 137.8 ± 3.8 | 134.9 ± 5.2 | <0.001 |
| eGFR* (ml/min/1.73 m2) | 76.0 ± 22.0 | 74.4 ± 24.1 | 75.4 ± 24.8 | 78.2 ± 36.5 | 68.2 ± 40.0 | <0.001 |
| Ascites, n (%) | 8 (3) | 46 (6.3) | 22 (4.4) | 388 (30.3) | 279 (70.5) | <0.001 |
| Variceal bleeding, n (%) | 2 (0.8) | 9 (1.2) | 5 (1.0) | 53 (4.1) | 43 (10.9) | <0.001 |
| Total tumor volume (ml) | 2.3 ± 1.3 | 17.1 ± 15.4 | 337.9 ± 541.1 | 570.0 ± 864.9 | 670.8 ± 1051.0 | <0.001 |
| Median follow-up duration (month)+ | 45 (25–78) | 37 (17–68) | 27 (12–59) | 9 (3–26) | 2 (1–9) | <0.001 |
| Child-Turcotte-Pugh class (A/B/C), n (%) | 265/0/0 (100/0/0) | 651/85/0 (88.5/11.5/0) | 459/44/0 (91.3/8.7/0) | 873/409/0 (68.1/31.9/0) | 76/164/156 (19.2/41.4/39.4) | <0.001 |
| MELD score (mean ± SD) | 8.2 ± 2.3 | 8.8 ± 2.9 | 8.7 ± 2.7 | 9.9 ± 3.7 | 14.8 ± 6.6 | <0.001 |
| Treatment modalities (resection/ablation/TACE/systemic therapy/others), % | 38/50/11/0/1 | 42/35/21/0/2 | 42/7/45/2/4 | 22/11/32/17/18 | 3/9/14/14/60 | <0.001 |
* eGFR: estimated glomerular filtration rate, calculated by the four-variable modification of diet in renal disease (MDRD) equation.
+ Data were demonstrated as medians (interquartile range)
Abbreviations: BCLC, Barcelona Clínic Liver Cancer classification; ECOG, Eastern Cooperative Oncology Group scale; INR, international normalized ratio for prothrombin time; AFP, Alpha-fetoprotein; Na, plasma sodium level; MELD, Model for End-Stage Liver Disease.
Fig 1. Prevalence of Diabetes Mellitus (DM) in different BCLC stages.
A trend toward increasing DM prevalence in more advanced BCLC stages is seen at a marginal significance (p for trend = 0.048).
Age, sex, performance status, existence of vascular invasion, HBV or HCV infection, alcoholism, presence of ascites, history of variceal bleeding showed significant differences between different BCLC stages at baseline. Parameters with gradual increment from BCLC stage 0 to stage D were serum bilirubin (p< 0.001), international normalized ratio (INR) (p< 0.001), serum alpha-fetoprotein (AFP) level (p< 0.001), platelet count (p< 0.001), and total tumor volume (p< 0.001). On the contrary, some parameters decreased in graded manner, including serum albumin (p< 0.001), serum sodium (p< 0.001), and estimated glomerular filtration rate (eGFR, p< 0.001).
Survival analysis
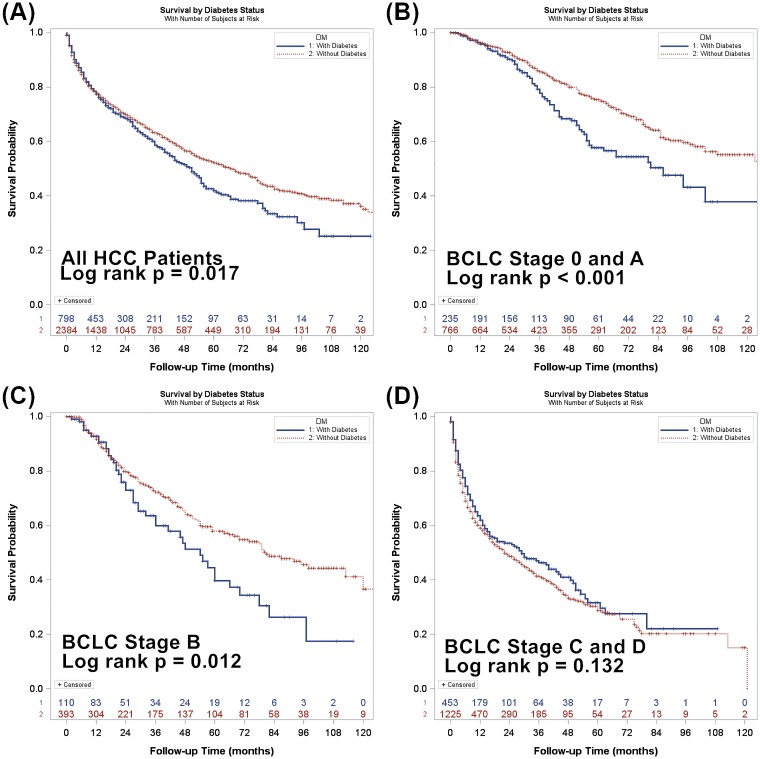
As a whole, diabetic HCC patients had significantly lower overall survival compared with non-diabetic patients (p = 0.017, Fig 2A). In subgroup analysis, diabetic patients also had decreased overall survival in very early/early and intermediate HCC (p<0.001 and 0.012, respectively, Fig 2B & 2C). The survival was similar between diabetic and non-diabetic patients in advanced and terminal HCC (p = 0.132, Fig 2D).
Fig 2. Cumulative survival rates of diabetic and non-diabetic patients among different BCLC staging groups.
Differences in survival between diabetic and non-diabetic patients are significant in the whole cohort (p = 0.017, panel A), BCLC stage 0 and stage A (p< 0.001, panel B), and BCLC stage B (p = 0.012, panel C), but not in BCLC stage C and stage D (p = 0.132, panel D).
Risk factor analysis
All patients
Univariate survival analysis show that DM, age, gender, HBV, alcoholism, albumin, bilirubin, INR, Na, AFP, eGFR, variceal bleeding, total tumor volume, vascular invasion, presence of ascites, and platelet count were significant predictors for survival in HCC patients (Table 2, all p< 0.05). Factors significant in the univariate analysis were introduced into the multivariate Cox model. MELD score and CTP class were not included in the final model because of they contain potentially confounding predictors, such as albumin and bilirubin. After adjustment, DM showed no effect on overall survival (adjusted hazard ratio [HR] 1.09; 95% confidence interval [CI] 0.95–1.25; p = 0.199).
Table 2. Univariate and multivariate Cox survival analysis in the entire hepatocellular carcinoma cohort.
| n = 3,182 | Crude Hazard Ratio | Adjusted Hazard Ratio | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Diabetes mellitus (no/yes) | 1.17 (1.03–1.33) | 0.019 | 1.09 (0.95–1.25) | 0.199 |
| Age (per 10 years increment) | 1.05 (1.00–1.10) | 0.030 | 1.14 (1.09–1.20) | <0.001 |
| Male gender (female/male) | 1.16 (1.01–1.33) | 0.039 | 1.07 (0.92–1.24) | 0.370 |
| Hepatitis B (negative/positive) | 0.93 (0.83–1.04) | 0.222 | - | - |
| Hepatitis C (negative/positive) | 0.84 (0.74–0.95) | 0.007 | 0.97 (0.85–1.12) | 0.702 |
| Alcoholism (no/yes) | 1.37 (1.19–1.59) | <0.001 | 1.16 (0.99–1.35) | 0.064 |
| Albumin (per 1 g/dl increment) | 0.38 (0.35–0.42) | <0.001 | 0.55 (0.50–0.61) | <0.001 |
| Bilirubin (per 1 mg/dl increment) | 1.14 (1.13–1.15) | <0.001 | 1.07 (1.05–1.09) | <0.001 |
| INR (per 1.0 increment) | 4.80 (3.92–5.87) | <0.001 | 1.59 (1.18–2.13) | 0.002 |
| Sodium (per 1 mEq/l increment) | 0.90 (0.89–0.92) | <0.001 | 0.98 (0.97–1.00) | 0.029 |
| AFP (per 10,000 ng/ml increment) | 1.01 (1.01–1.01) | <0.001 | 1.01 (1.01–1.01) | <0.001 |
| eGFR (per 10 mL/min/1.73 m2 increment) | 0.96 (0.94–0.98) | <0.001 | 0.98 (0.96–0.99) | 0.010 |
| Variceal bleeding (no/yes) | 2.17 (1.66–2.83) | <0.001 | 1.13 (0.85–1.49) | 0.404 |
| Total tumor volume (per 1,000 ml increment) | 1.54 (1.48–1.61) | <0.001 | 1.22 (1.15–1.30) | <0.001 |
| Vascular invasion (no/yes) | 5.01 (4.42–5.68) | <0.001 | 3.20 (2.77–3.70) | <0.001 |
| Ascites (no/yes) | 3.52 (3.11–3.99) | <0.001 | 1.72 (1.48–1.99) | <0.001 |
| Platelet count (per 10,000 mm3 increment) | 1.03 (1.02–1.03) | <0.001 | 1.01 (1.01–1.02) | <0.001 |
Factors with P value < 0.1 in the univariate analysis were introduced into the Cox multivariate survival analysis.
The forepart of the parentheses was set as the reference group in univariate and multivariate analysis.
AFP, alpha-fetoprotein; CI, confidence interval; eGFR, estimated glomerular filtration rate; HR, hazard ratio; INR, international normalized ratio
BCLC stage 0 and stage A
DM, age, HBV, HCV, albumin, bilirubin, INR, AFP, eGFR, and platelet count showed significant impact on patient survival (Table 3, p< 0.05). After adjusting in the multivariate model, presence of DM (HR 1.45, 95% CI 1.08–1.93, p = 0.013), higher serum bilirubin (HR 1.27, 95% CI 1.07–1.52, p = 0.008), higher serum AFP (HR 1.34, 95% CI 1.20–1.48, p< 0.001), lower serum albumin (HR 1.82, 95% CI 1.37–2.38, p<0.001), and lower eGFR (HR 1.11, 95% CI 1.04–1.19, p = 0.001), lower platelet count (HR 1.03, 95% CI 1.0–1.05, p = 0.029) were confirmed as independent predictors of a decreased survival rate. We found no interaction between DM and these independent predictors (all p > 0.05).
Table 3. Univariate and multivariate Cox survival analysis in patients with BCLC stage 0 and stage A hepatocellular carcinoma.
| n = 1,001 | Crude Hazard Ratio | Adjusted Hazard Ratio | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Diabetes mellitus (no/yes) | 1.61 (1.23–2.12) | 0.001 | 1.45 (1.08–1.93) | 0.013 |
| Age (per 10 years increment) | 1.24 (1.11–1.38) | <0.001 | 1.11 (0.98–1.26) | 0.089 |
| Male gender (female/male) | 1.02 (0.77–1.43) | 0.902 | - | - |
| Hepatitis B (negative/positive) | 0.66 (0.52–0.85) | 0.001 | 0.73 (0.52–1.01) | 0.054 |
| Hepatitis C (negative/positive) | 1.37 (1.07–1.76) | 0.012 | 0.87 (0.63–1.22) | 0.424 |
| Alcoholism (no/yes) | 1.21 (0.84–1.74) | 0.309 | - | - |
| Albumin (per 1 g/dl decrement) | 2.33 (1.85–2.94) | <0.001 | 1.82 (1.37–2.38) | <0.001 |
| Bilirubin (per 1 mg/dl increment) | 1.51 (1.29–1.76) | <0.001 | 1.27 (1.07–1.52) | 0.008 |
| INR (per 1.0 increment) | 3.58 (1.61–7.97) | 0.002 | 1.37 (0.43–4.30) | 0.593 |
| Sodium (per 1 mEq/l increment) | 1.03 (0.99–1.07) | 0.210 | - | - |
| AFP (per 10,000 ng/ml increment) | 1.30 (1.17–1.44) | <0.001 | 1.34 (1.20–1.48) | <0.001 |
| eGFR (per 10 mL/min/1.73 m2 decrement) | 1.12 (1.06–1.19) | <0.001 | 1.11 (1.04–1.19) | 0.001 |
| Variceal bleeding (no/yes) | 1.39 (0.44–4.33) | 0.574 | - | - |
| Total tumor volume (per 1,000 ml increment) | 1.05 (0.96–1.14) | 0.274 | - | - |
| Vascular invasion (no/yes) | - | - | - | - |
| Ascites (no/yes) | 1.55 (0.90–2.65) | 0.114 | - | - |
| Platelet count (per 10,000 mm3 decrement) | 1.05 (1.03–1.08) | <0.001 | 1.03 (1.0–1.05) | 0.029 |
Factors with P value < 0.1 in the univariate analysis were introduced into the Cox multivariate survival analysis.
The forepart of the parentheses was set as the reference group in univariate and multivariate analysis.
AFP, alpha-fetoprotein; CI, confidence interval; eGFR, estimated glomerular filtration rate; HR, hazard ratio; INR, international normalized ratio
BCLC stage B
In univariate survival analysis for BCLC stage B subgroup, DM, albumin, total tumor volume were associated with survival (Table 4, p< 0.05). The Cox multivariate analysis revealed DM (HR 1.77, 95% CI 1.24–2.51, p = 0.002), serum albumin (HR 1.67, 95% CI 1.27–2.22, p< 0.001), and total tumor volume (HR 1.51, 95% CI 1.19–1.93, p = 0.001) as independent predictors of a poor outcome. There were no interaction between DM and these independent predictors of poor outcome (all p > 0.05).
Table 4. Univariate and multivariate Cox survival analysis in patients with BCLC stage B hepatocellular carcinoma.
| n = 503 | Crude Hazard Ratio | Adjusted Hazard Ratio | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Diabetes mellitus (no/yes) | 1.54 (1.09–2.17) | 0.014 | 1.77 (1.24–2.51) | 0.002 |
| Age (per 10 years increment) | 1.09 (0.97–1.22) | 0.157 | - | - |
| Male gender (female/male) | 0.86 (0.59–1.28) | 0.463 | - | - |
| Hepatitis B (negative/positive) | 1.00 (0.74–1.35) | 0.992 | - | - |
| Hepatitis C (negative/positive) | 0.98 (0.69–1.39) | 0.914 | - | - |
| Alcoholism (no/yes) | 1.29 (0.83–2.00) | 0.260 | - | - |
| Albumin (per 1 g/dl increment) | 1.72 (1.30–1.22) | <0.001 | 1.67 (1.27–2.22) | <0.001 |
| Bilirubin (per 1 mg/dl increment) | 1.11 (0.99–1.24) | 0.082 | 1.10 (0.97–1.25) | 0.144 |
| INR (per 1.0 increment) | 2.24 (1.00–5.02) | 0.051 | 2.36 (0.89–6.27) | 0.085 |
| Sodium (per 1 mEq/l increment) | 0.97 (0.92–1.02) | 0.207 | - | - |
| AFP (per 10,000 ng/ml increment) | 1.02 (0.97–1.07) | 0.395 | - | - |
| eGFR (per 10 mL/min/1.73 m2 increment) | 0.98 (0.92–1.05) | 0.595 | - | - |
| Variceal bleeding (no/yes) | 2.49 (0.62–10.08) | 0.200 | - | - |
| Total tumor volume (per 1,000 ml increment) | 1.29(1.01–1.64) | 0.041 | 1.51 (1.19–1.93) | 0.001 |
| Vascular invasion (no/yes) | - | - | - | - |
| Ascites (no/yes) | 1.34 (0.68–2.62) | 0.396 | - | - |
| Platelet count (per 10,000 mm3 increment) | 0.98 (0.96–1.00) | 0.099 | 0.99 (0.97–1.01) | 0.182 |
Factors with P value < 0.1 in the univariate analysis were introduced into the Cox multivariate survival analysis.
The forepart of the parentheses was set as the reference group in univariate and multivariate analysis.
AFP, alpha-fetoprotein; CI, confidence interval; eGFR, estimated glomerular filtration rate; HR, hazard ratio; INR, international normalized ratio
BCLC stage C and stage D
Variables showing hazardous effects for HCC survival were bilirubin (HR 1.11; 95% CI 1.09–1.12; p<0.001), INR (HR 3.13; 95% CI 2.46–3.97; p< 0.001), serum AFP (HR 1.01; 95% CI 1.01–1.01 p< 0.001), total tumor volume (HR per 1,000ml increment 1.33; 95% CI 1.26–1.40; p< 0.001), variceal bleeding (HR 1.38; 95% CI 1.04–1.83; p = 0.024), vascular invasion (HR 2.59; 95% CI 2.24–3.00; p< 0.001), ascites (HR 2.26; 95% CI 1.95–2.61; p< 0.001), and platelet count (HR per 10,000/m3 increment: 1.02; 95% CI 1.02–1.03; p< 0.001). DM does not appear to be a predictive variable for survival in this group of patients (crude HR 0.88; 95% CI 0.75–1.04; p = 0.136). Significant predictors for decreased survival rate in multivariate Cox model were serum albumin (HR 1.67; 95% CI 1.47–1.89; p< 0.001) and bilirubin (HR 1.06; 95% CI 1.04–1.08; p< 0.001), plasma sodium (HR 1.02; 95% CI 1.01–1.04; p = 0.006), serum AFP (HR per 10,000 ng/ml increment 1.01; 95% CI 1.00–1.01; p< 0.001), eGFR (HR 1.03, 95% CI 1.01–1.05; p = 0.003), total tumor volume (HR per 1,000 ml increment 1.12; 95% CI 1.04–1.20; p = 0.002), vascular invasion (HR 2.34; 95% CI 1.99–2.74; p< 0.001), ascites (HR 1.63; 95% CI 1.39–1.92; p< 0.001), and platelet count (HR per 10,000/mm3 increment 1.01; 95% CI 1.01–1.02; p< 0.001; Table 5).
Table 5. Univariate and multivariate Cox survival analysis in patients with BCLC stage C and stage D hepatocellular carcinoma.
| n = 1,678 | Crude Hazard Ratio | Adjusted Hazard Ratio | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Diabetes mellitus (no/yes) | 0.88 (0.75–1.04) | 0.136 | - | - |
| Age (per 10 years increment) | 0.99 (0.94–1.04) | 0.589 | - | - |
| Male gender (female/male) | 1.19 (0.99–1.42) | 0.059 | 1.09 (0.90–1.30) | 0.387 |
| Hepatitis B (negative/positive) | 1.04 (0.90–1.20) | 0.582 | - | - |
| Hepatitis C (negative/positive) | 0.81 (0.68–0.96) | 0.013 | 1.00 (0.83–1.19) | 0.954 |
| Alcoholism (no/yes) | 1.08 (0.91–1.28) | 0.371 | - | - |
| Albumin (per 1 g/dl decrement) | 2.08 (1.85–2.27) | <0.001 | 1.67 (1.47–1.89) | <0.001 |
| Bilirubin (per 1 mg/dl increment) | 1.11 (1.09–1.12) | <0.001 | 1.06 (1.04–1.08) | <0.001 |
| INR (per 1.0 increment) | 3.13 (2.46–3.97) | <0.001 | 1.22 (0.86–1.72) | 0.260 |
| Sodium (per 1 mEq/l decrement) | 1.09 (1.06–1.10) | <0.001 | 1.02 (1.01–1.04) | 0.006 |
| AFP (per 10,000 ng/ml increment) | 1.01 (1.01–1.01) | <0.001 | 1.01 (1.00–1.01) | <0.001 |
| eGFR (per 10 mL/min/1.73 m2 decrement) | 1.03 (1.01–1.05) | 0.010 | 1.03 (1.01–1.05) | 0.003 |
| Variceal bleeding (no/yes) | 1.38 (1.04–1.83) | 0.024 | 1.00 (0.74–1.34) | 0.983 |
| Total tumor volume (per 1,000 ml increment) | 1.33 (1.26–1.40) | <0.001 | 1.12 (1.04–1.20) | 0.002 |
| Vascular invasion (no/yes) | 2.59 (2.24–3.00) | <0.001 | 2.34 (1.99–2.74) | <0.001 |
| Ascites (no/yes) | 2.26 (1.95–2.61) | <0.001 | 1.63 (1.39–1.92) | <0.001 |
| Platelet count (per 10,000 mm3 increment) | 1.02 (1.02–1.03) | <0.001 | 1.01 (1.01–1.02) | <0.001 |
Factors with P value < 0.1 in the univariate analysis were introduced into the Cox multivariate survival analysis.
The forepart of the parentheses was set as the reference group in univariate and multivariate analysis.
AFP, alpha-fetoprotein; CI, confidence interval; eGFR, estimated glomerular filtration rate; HR, hazard ratio; INR, international normalized ratio
Discussion
In this longitudinally followed-up study from a large patient cohort, notably, a quarter of HCC patients were diabetic. We demonstrate a trend toward increasing prevalence of DM in HCC patients with higher BCLC stages. In addition, DM may differentially affect overall survival in HCC patients with BCLC stage 0, stage A, and stage B subgroups, but not in those with BCLC stage C and D groups.
Relations between DM and HCC prognosis have been studied extensively but show discrepant results. It has been noticed in several epidemiologic studies that the predictive value of DM on HCC prognosis was limited to specific patient subgroups. Wang et al. reported a meta-analysis including 21 studies with total 9,767 HCC patients, and showed that DM is an independent predictor for decreased overall survival (HR 1.55; 95% CI 1.27–1.91; p = 0.001) and disease-free survival (HR 2.15; 95% CI 1.75–2.63; p = 0.001).[12] However, subgroup analyses in that study further disclosed that the effect was only seen in patients receiving hepatic resection (HR 1.91; 95% CI 1.21–3.00; p = 0.005), but not in subjects treated with other modalities.
Whether differences exist in tumors with diverse baseline characteristics is less well established. Our group previously conducted an analysis comparing how DM influenced cumulative survival in patients with small (defined as ≤ 5 cm) and large (> 5 cm) hepatic tumors. We found that non-diabetic patients had significantly better survival after surgical resection compared to diabetic patients when the tumors are small. In contrast, there was no significant difference in survival between DM and non-DM individuals when tumor sizes were large at presentation.[27]
HCC is a highly heterogeneous disease entity, and the size of tumor size does not necessarily correlate well with disease severity, tumor stage, or prognosis. The BCLC classification system involves not only tumor size, but also performance status and liver functional reserve. Thus it may serve as a better disease indicator. In the current study, we divided patients into a total of three subgroups according to their BCLC staging. Our aim is to more precisely evaluate how the presence of DM changes the prognosis of HCC under different disease entities. We found a poor overall survival in diabetic patients with early BCLC stages but not in those with advanced diseases. This phenomenon could be explained by the hepatocarcinogenesis effect of insulin. In type 2 diabetic patients, increased insulin resistance and resulting hyperinsulinemia might upregulate the production of insulin-like growth factor-1 (IGF-1) and insulin receptor subtrate-1 (IRS-1). Elevating IGF-1 stimulates cell proliferation and inhibits apoptosis, thereby inducing carcinogenesis.[28] Consequently, patients with DM may suffer from accelerating tumor growth and poorer survival. However, in chronic and advanced liver diseases, especially in fibrotic liver, insulin resistance increased.[29–32] We consider that in BCLC stage C and stage D populations, the underlying liver disease alone causes insulin resistance and hyperinsulinemia that resemble diabetes status. Therefore, whether or not patients have true clinical diabetes may not significantly influence the prognosis. This assumption was further supported by our demographic data showing that the CTP class was more advanced in higher BCLC stages, representing more severe cirrhosis. In the meanwhile, the same hypothesis could also explain the gradual increasing trend of DM prevalence from BCLC stage 0 to stage D as observed in our cohort, which stems from progressively worsening insulin resistance.
DM is known to cause multi-system complications. Diabetic patients with early stage HCC usually have a better survival, which allows diabetic complications and diabetes-associated death to develop. Thus the increased mortality in diabetic group might come from diabetic complications instead of cancer burden. Alternatively, in later stages, treatment for HCC is greatly limited. According to the suggestions from the AASLD guideline, patients with BCLC stage C disease can only be treated with sorafenib, and stage D patients are less likely to tolerate any treatment except for palliative care. There is a high possibility that patients die before significant diabetic complications take place.
The study has a few limitations. This is a single-center study and the results may not be generalizable to other geographical areas. With more than half of the patients having evidence of HBV infection, our data require validation from other study groups. Secondly, as a tertiary center, referral bias cannot be avoided completely. Thirdly, our primary outcome was all-cause mortality. We could not evaluate the association between DM and its potential morbidities, such as surgical complications and cardiovascular death. Lastly, our study lacks of the information about DM treatment. Recently, the protective effect of several classes of anti-diabetic agents in cancer development and progression, especially metformin and thiazolidinedione, has been emphasized.[33, 34] On the contrary, insulin analogue seems to have mitogenic effects.[35] Therefore, choice of glucose lowering drugs may have its own role in affecting the outcome of HCC patients.
In conclusion, DM is highly prevalent among patients with HCC across different cancer stages. DM worsens overall survival in these patients with early BCLC stages from stage 0 to B. However. For patients with stage C and stage D, the long-term survival is not significantly influenced by the presence of DM.
Abbreviations
- AASLD
American Association for the Study of Liver Diseases
- ADA
American Diabetes Association
- AFP
alpha-fetoprotein
- BCLC
Barcelona Clínic Liver Cancer
- CI
confidence interval
- CTP
Child-Turcotte-Pugh classification
- DM
diabetes mellitus
- EASL
European Association for the Study of the Liver
- ECOG
Eastern Cooperative Oncology Group
- eGFR
estimated glomerular filtration rate
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HR
hazard ratio
- IGF-1
insulin-like growth factor-1
- INR
international normalized ratio
- IRS-1
insulin receptor subtrate-1
- MELD
model for end-stage liver disease
Data Availability
The restrictions set by the Institutional Review Board of Taipei Veterans General Hospital prohibit the authors from making the minimal data set publicly available. For data access, please contact the Director of the IRB of Taipei Veterans General Hospital at d-mre@vghtpe.gov.tw.
Funding Statement
This study was supported by the grants from Taipei Veterans General Hospital (V105C-009, VN106-11), Taipei, Taiwan. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–86. 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 2.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379(9822):1245–55. 10.1016/S0140-6736(11)61347-0 [DOI] [PubMed] [Google Scholar]
- 3.Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol. 2013;47 Suppl:S2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raffetti E, Portolani N, Molfino S, Baiocchi GL, Limina RM, Caccamo G, et al. Role of aetiology, diabetes, tobacco smoking and hypertension in hepatocellular carcinoma survival. Dig Liver Dis. 2015;47(11):950–6. 10.1016/j.dld.2015.07.010 [DOI] [PubMed] [Google Scholar]
- 5.Jinjuvadia R, Patel S, Liangpunsakul S. The association between metabolic syndrome and hepatocellular carcinoma: systemic review and meta-analysis. J Clin Gastroenterol. 2014;48(2):172–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loria P, Lonardo A, Anania F. Liver and diabetes. A vicious circle. Hepatology research: the official journal of the Japan Society of Hepatology. 2013;43(1):51–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126(2):460–8. [DOI] [PubMed] [Google Scholar]
- 8.Davila JA, Morgan RO, Shaib Y, McGlynn KA, El-Serag HB. Diabetes increases the risk of hepatocellular carcinoma in the United States: a population based case control study. Gut. 2005;54(4):533–9. 10.1136/gut.2004.052167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ali Kamkar MM, Ahmad R, Alsmadi O, Behbehani K. Insight into the impact of diabetes mellitus on the increased risk of hepatocellular carcinoma: mini-review. J Diabetes Metab Disord. 2014;13:57 10.1186/2251-6581-13-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ko WH, Chiu SY, Yang KC, Chen HH. Diabetes, hepatitis virus infection and hepatocellular carcinoma: A case-control study in hepatitis endemic area. Hepatology research: the official journal of the Japan Society of Hepatology. 2012;42(8):774–81. [DOI] [PubMed] [Google Scholar]
- 11.Huo TI, Hsu CY, Huang YH, Hsia CY, Lin HC, Lee PC, et al. Diabetes mellitus as an independent prognostic predictor and its association with renal dysfunction in patients with hepatocellular carcinoma. Liver Int. 2010;30(2):198–207. 10.1111/j.1478-3231.2009.02143.x [DOI] [PubMed] [Google Scholar]
- 12.Wang YY, Huang S, Zhong JH, Ke Y, Guo Z, Liu JQ, et al. Impact of diabetes mellitus on the prognosis of patients with hepatocellular carcinoma after curative hepatectomy. PloS one. 2014;9(12):e113858 10.1371/journal.pone.0113858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huo TI, Wu JC, Lui WY, Lee PC, Huang YH, Chau GY, et al. Diabetes mellitus is a recurrence-independent risk factor in patients with hepatitis B virus-related hepatocellular carcinoma undergoing resection. Eur J Gastroenterol Hepatol. 2003;15(11):1203–8. [DOI] [PubMed] [Google Scholar]
- 14.Kawamura Y, Ikeda K, Arase Y, Yatsuji H, Sezaki H, Hosaka T, et al. Diabetes mellitus worsens the recurrence rate after potentially curative therapy in patients with hepatocellular carcinoma associated with nonviral hepatitis. J Gastroenterol Hepatol. 2008;23(11):1739–46. 10.1111/j.1440-1746.2008.05436.x [DOI] [PubMed] [Google Scholar]
- 15.Ting CT, Chen RC, Chen CC, Liu MH, Chu D, Kuo NW. Diabetes worsens the surgical outcomes in cirrhotic patients with hepatocellular carcinoma. Tohoku J Exp Med. 2012;227(1):73–81. [DOI] [PubMed] [Google Scholar]
- 16.Ikeda Y, Shimada M, Hasegawa H, Gion T, Kajiyama K, Shirabe K, et al. Prognosis of hepatocellular carcinoma with diabetes mellitus after hepatic resection. Hepatology. 1998;27(6):1567–71. 10.1002/hep.510270615 [DOI] [PubMed] [Google Scholar]
- 17.Feng YH, Lin CY, Huang WT, Wu CL, Fang JL, Tsao CJ. Diabetes mellitus impairs the response to intra-arterial chemotherapy in hepatocellular carcinoma. Med Oncol. 2011;28(4):1080–8. 10.1007/s12032-010-9650-9 [DOI] [PubMed] [Google Scholar]
- 18.Poon RT, Fan ST, Wong J. Does diabetes mellitus influence the perioperative outcome or long term prognosis after resection of hepatocellular carcinoma? Am J Gastroenterol. 2002;97(6):1480–8. 10.1111/j.1572-0241.2002.05792.x [DOI] [PubMed] [Google Scholar]
- 19.Toyoda H, Kumada T, Nakano S, Takeda I, Sugiyama K, Kiriyama S, et al. Impact of diabetes mellitus on the prognosis of patients with hepatocellular carcinoma. Cancer. 2001;91(5):957–63. [PubMed] [Google Scholar]
- 20.Wang YG, Wang P, Wang B, Fu ZJ, Zhao WJ, Yan SL. Diabetes mellitus and poorer prognosis in hepatocellular carcinoma: a systematic review and meta-analysis. PloS one. 2014;9(5):e95485 10.1371/journal.pone.0095485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bruix J, Sherman M, American Association for the Study of Liver D. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–2. 10.1002/hep.24199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.European Association For The Study Of The Liver, European Organisation For Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56(4):908–43. 10.1016/j.jhep.2011.12.001 [DOI] [PubMed] [Google Scholar]
- 23.American Diabetes A. (2) Classification and diagnosis of diabetes. Diabetes Care. 2015;38 Suppl:S8–S16. [DOI] [PubMed] [Google Scholar]
- 24.Chau GY. Resection of hepatitis B virus-related hepatocellular carcinoma: evolving strategies and emerging therapies to improve outcome. World J Gastroenterol. 2014;20(35):12473–84. 10.3748/wjg.v20.i35.12473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang YH, Chen CH, Chang TT, Chen SC, Chiang JH, Lee HS, et al. The role of transcatheter arterial embolization for patients with unresectable hepatocellular carcinoma: a nationwide, multicentre study evaluated by cancer stage. Aliment Pharmacol Ther. 2005;21(6):687–94. 10.1111/j.1365-2036.2005.02404.x [DOI] [PubMed] [Google Scholar]
- 26.Liu PH, Hsu CY, Hsia CY, Lee YH, Huang YH, Chiou YY, et al. Surgical Resection Versus Radiofrequency Ablation for Single Hepatocellular Carcinoma </ = 2 cm in a Propensity Score Model. Ann Surg. 2016;263(3):538–45. [DOI] [PubMed] [Google Scholar]
- 27.Huo TI, Wu JC, Lui WY, Huang YH, Lee PC, Chiang JH, et al. Differential mechanism and prognostic impact of diabetes mellitus on patients with hepatocellular carcinoma undergoing surgical and nonsurgical treatment. Am J Gastroenterol. 2004;99(8):1479–87. 10.1111/j.1572-0241.2004.30024.x [DOI] [PubMed] [Google Scholar]
- 28.Perumpail RB, Liu A, Wong RJ, Ahmed A, Harrison SA. Pathogenesis of hepatocarcinogenesis in non-cirrhotic nonalcoholic fatty liver disease: Potential mechanistic pathways. World J Hepatol. 2015;7(22):2384–8. 10.4254/wjh.v7.i22.2384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petta S, Camma C, Di Marco V, Macaluso FS, Maida M, Pizzolanti G, et al. Hepatic steatosis and insulin resistance are associated with severe fibrosis in patients with chronic hepatitis caused by HBV or HCV infection. Liver Int. 2011;31(4):507–15. 10.1111/j.1478-3231.2011.02453.x [DOI] [PubMed] [Google Scholar]
- 30.Chu CJ, Hung TH, Hwang SJ, Wang YJ, Yang CF, Lin HC, et al. Association of insulin resistance with hepatic steatosis and progression of fibrosis in Chinese patients with chronic hepatitis C. Hepatogastroenterology. 2008;55(88):2157–61. [PubMed] [Google Scholar]
- 31.Kawaguchi T, Yoshida T, Harada M, Hisamoto T, Nagao Y, Ide T, et al. Hepatitis C virus down-regulates insulin receptor substrates 1 and 2 through up-regulation of suppressor of cytokine signaling 3. Am J Patho. 2004;165(5):1499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawaguchi T, Taniguchi E, Itou M, Sakata M, Sumie S, Sata M. Insulin resistance and chronic liver disease. World J Hepatol. 2011;3(5):99–107. 10.4254/wjh.v3.i5.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lai SW, Chen PC, Liao KF, Muo CH, Lin CC, Sung FC. Risk of hepatocellular carcinoma in diabetic patients and risk reduction associated with anti-diabetic therapy: a population-based cohort study. Am J Gastroenterol. 2012;107(1):46–52. 10.1038/ajg.2011.384 [DOI] [PubMed] [Google Scholar]
- 34.Zhang ZJ, Zheng ZJ, Shi R, Su Q, Jiang Q, Kip KE. Metformin for liver cancer prevention in patients with type 2 diabetes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2012;97(7):2347–53. 10.1210/jc.2012-1267 [DOI] [PubMed] [Google Scholar]
- 35.Weinstein D, Simon M, Yehezkel E, Laron Z, Werner H. Insulin analogues display IGF-I-like mitogenic and anti-apoptotic activities in cultured cancer cells. Diabetes Metab Res Rev. 2009;25(1):41–9. 10.1002/dmrr.912 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The restrictions set by the Institutional Review Board of Taipei Veterans General Hospital prohibit the authors from making the minimal data set publicly available. For data access, please contact the Director of the IRB of Taipei Veterans General Hospital at d-mre@vghtpe.gov.tw.