Abstract
Background
A systematic literature review was performed to investigate the occurrence of multidrug-resistant tuberculosis (MDR TB) in prisons located in countries formerly part of the Soviet Union.
Methods
A systematic search of published studies reporting MDR TB occurrence in prisons located in former Soviet countries was conducted by probing PubMed and Cumulative Index Nursing and Allied Health Literature for articles that met predetermined inclusion criteria.
Results
Seventeen studies were identified for systematic review. Studies were conducted in six different countries. Overall, prevalence of MDR TB among prisoners varied greatly between studies. Our findings suggest a high prevalence of MDR TB in prisons of Post-Soviet states with percentages as high as 16 times more than the worldwide prevalence estimated by the WHO in 2014.
Conclusion
All studies suggested a high prevalence of MDR TB in prison populations in Post-Soviet states.
Introduction
It is estimated that as of 2014, there were 480,000 cases of MDR TB worldwide [1]. In 2015, of the 27 countries worldwide designated as having a high burden for multidrug-resistant tuberculosis (MDR TB) by the World Health Organization (WHO), 15 of them were in Post-Soviet states [2]. In fact, of all MDR TB cases reported in 26 European countries between 2003 and 2007, 65% were concentrated in three Post-Soviet states: Latvia, Lithuania and Estonia [3]. MDR TB takes longer to diagnose, longer to treat, costs more money and has a greater risk of treatment failure than drug-susceptible tuberculosis [4–6]. While MDR TB can arise when patients do not complete a full course of anti-TB medications, it can also be transmitted from person-to-person via infected droplets in the air, even to those who have never taken anti-TB drugs [7–10]. This makes MDR TB a particular concern in crowded, in-door areas, such as prisons.
Prisoners, on average, have a higher prevalence of tuberculosis (TB) relative to the general population [11,12]. This can be explained in part due to the fact that prisoners often come from populations that are considered high risk for developing TB (substance abuse, mental illness, homelessness). However, prisons themselves can facilitate the spread of TB via poor ventilation systems, overcrowding and poor nutrition [13–17]. Not only does this represent a threat to prisoners, but to the general population as a whole. Prisoners who acquire TB can spread it beyond the confines of their prison cells through daily interaction with prison staff, visitors and prisoners who are about to be released [18].
Post-Soviet states have some of the highest incarceration rates in the world [19], and the TB epidemic within those prisons is well documented [20]. Mistreatment of prisoners in former Soviet states continues to be a major issue and may suggest negative Soviet era attitudes towards prisoners persist [20,21]. In addition to economic and health system collapse following the dissolution of the Soviet Union, these stigmas could also be contributing to inadequate attention to prisoner health.
Despite being more than half of the world’s high burden MDR TB countries, there has never been a direct comparison of the literature on MDR TB in prisons of the Post-Soviet states [2]. While studies exist that report global MDR TB, they fail to elucidate the scope of the problem in the Post-Soviet region. Thus, the aim of this study is to provide the first systematic review of studies that examine the prevalence of MDR TB in Post-Soviet prisons.
Methods
Definition of Post-Soviet states
Also known as the former Soviet Union, Post-Soviet states refers to the 15 independent nation-states that emerged following the collapse of the Union of Soviet Socialist Republics (USSR) in 1991. These include Armenia, Azerbaijan, Belarus, Estonia, Georgia, Kazakhstan, Kyrgyzstan, Latvia, Lithuania, Moldova, Russia, Tajikistan, Turkmenistan, Ukraine, and Uzbekistan [22,23].
Definition of multidrug-resistant tuberculosis
MDR TB is defined by the WHO as being resistant to isoniazid and rifampicin, with or without resistance to any other first-line drugs [24]. All studies included in this review followed that definition when reporting MDR TB cases [25–41].
Literature search strategy and inclusion criteria
This study was not registered with any systematic review protocol registry. Because the Soviet Union dissolved in 1991, we included articles published between 1992 and 2015. PubMed and Cumulative Index Nursing and Allied Health Literature (CINAHL) were searched for relevant studies based on predetermined inclusion criteria. The following MeSH terms were used: tuberculosis, TB, multi drug-resistant, prison, jail, incarcerated, inmate, penitentiary, and the individual names of all the countries that are defined as Post-Soviet. Studies were included if they were original research, published in a peer-reviewed scientific journal, were in the English language, focused on one or more Post-Soviet countries, included prisoners as the study population, reported prevalence of MDR TB among prisoners, and used data that were collected between 1992 and 2015.
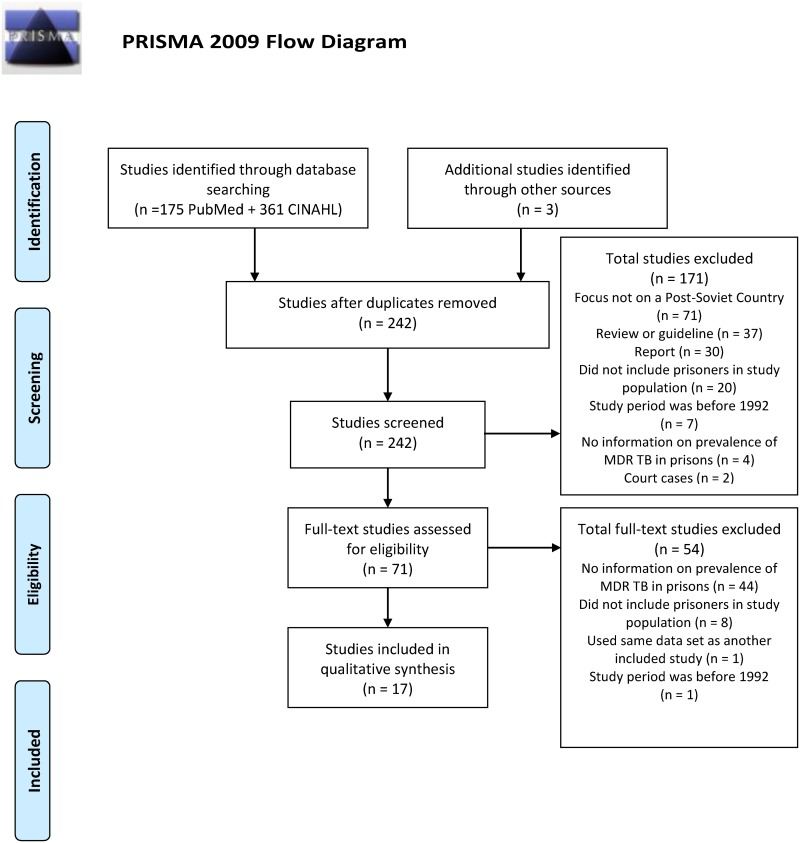
A total of 239 potentially eligible studies were initially identified. We subsequently excluded 225 studies for the following reasons: 71 did not focus on a Post-Soviet country, 48 did not have information on MDR TB prevalence in prisons 37 were reviews or guidelines, 30 were reports, 28 did not include prisoners in the study population, 8 included a study period before 1992, 2 were excluded because they were court cases, and 1 was excluded because it used the same data set as a previously included study. Three additional studies that matched all of the inclusion and none of the exclusion criteria were brought in through looking at sources of other review papers. Seventeen studies were included for review. Two researchers (AJ and AMJ) independently assessed the methodological integrity of each included study. Fig 1 provides a flow diagram of the inclusion and exclusion process.
Fig 1. PRISMA 2009 flow diagram.
From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(7): e1000097. doi: 10.1371/journal.pmed1000097. For more information, visit www.prisma-statement.org.
Results
A total of 17 studies met the inclusion criteria for systematic review [25–41]. Characteristics of included studies are presented in Table 1. Seven of the studies reported MDR TB in both prisoner and civilian populations [26,30–33,35,36]; whereas, 10 of the studies only reported MDR TB among prisoners [25,27–29,34,37–41]. The majority of studies were cross-sectional studies [25–29,32–41] and two were prospective cohorts studies [30,31]. Five of the studies used data from a national database [25,29,32–34], while, 12 used regional data [26–28,30,31,35–41]. Ten studies were conducted in Russia [26–28,30,31,35,37,38,40,41], 3 in Georgia [25,33,34], 1 in Moldova [32],1 in Azerbaijan [29], 1 in Kazakhstan [36], and 1 in Kyrgyzstan [39]. The studies done in Russia, Kazakhstan and Kyrgyzstan used regional data; whereas, studies in Georgia, Moldova and Azerbaijan were national studies [25–41].
Table 1. Characteristics of included studies.
| Author, Year [Citation] | Country | Study Design | Data Sources | Participants |
|---|---|---|---|---|
| Aerts, 2000 [25] | Georgia | Cross-sectional | Prison system of Georgia between 1997 and 1998 | 445 TB positive prisoners |
| Balabnova, 2006 [30] | Russia | Prospective cohort | Patients observed in Samara Oblast, Russia between 2002 and 2008 | 2099 TB positive patients (640 prisoners and 1459 civilians) |
| Balabnova, 2011 [31] | Russia | Prospective cohort | Patients recruited in Samara Oblast, Russia between 2002–2003 | 880 TB positive patients (164 prisoners, 716 civilians) |
| Bonnet, 2005 [37] | Russia | Cross-sectional | Kemerovo prison, Kemerovo Oblast, Russia in 2001 | 459 TB positive prisoners |
| Ibrayeva, 2014 [36] | Kazakhstan | Cross-sectional | Prison systems of Karaganda and Akmola regions in 2012 “Civilian sector” of Kazakhstan | 185 TB positive patients (60 prisoners, 125 civilians) |
| Ignatova, 2006 [28] | Russia | Cross-sectional | Ozerki prison hospital, Tula Oblast, Russia between June, 2001 and June, 2002 | 87 TB positive prisoners |
| Jenkins, 2013 [32] | Moldova | Cross-sectional | Moldovan TB database of all notified TB cases diagnosed nationwide between January 2007 and December 2010 | 23,152 TB positive patients (9,071 testable cases with 606 prisoners and 8,465 civilians) |
| Jenkins, 2014 [33] | Georgia | Cross-sectional | All notified TB cases in Georgia between 2009 and 2011 All patients hospitalized and started on SLD in Georgia between 2009 and 2011 | 6931 TB positive patients (1,582 prisoners, 5,349 civilians) |
| Jugheli, 2008 [34] | Georgia | Cross-sectional | Prison system of Georgia between 2001 and 2003 | 270 TB positive prisoners |
| Kimerling, 1999 [38] | Russia | Cross-sectional | Colony 33, Kemerovo Oblast, Russia between December 1997 and March 1998 | 164 TB positive prisoners |
| Mar'iandyshev, 2005 [41] | Russia | Cross-sectional | Arkhangelsk prison system, Arkhangelsk Oblast, Russia | 343 TB positive prisoners |
| Mokrousov, 2009 [39] | Kyrgyzstan | Cross-sectional | Moldovanovka prison TB hospital, Bishkek, Kyrgyzstan between August and November of 2008 | 56 TB positive prisoners |
| Pfyffer, 2001 [29] | Azerbaijan | Cross-sectional | Central Penitentiary Hospital of Azerbaijan | 65 TB positive prisoners |
| Ruddy, 2005 [26] | Russia | Cross-sectional | Samara Oblast, Russia | 600 TB positive patients (291 prisoners, 309 civilians) |
| Shemyakin, 2004 [40] | Russia | Cross-sectional | Serpukhov prison, Moscow Oblast, Russia between January and December 2001 | 130 TB positive prisoners |
| Spradling, 2002 [35] | Russia | Cross-sectional | DST records of the Central Tuberculosis Dispensary of Oryol Oblast, Russia from 1 July 1999 through 30 June 2000 | 212 TB positive patients (41 prisoners, 171 civilians) |
| Toungoussova, 2003 [27] | Russia | Cross-sectional | Archangel (Arkhangelsk) prison, Arkhangelsk Oblast, Russia | 114 TB positive prisoners |
All studies reported prevalence of MDR TB among prisoners who were culture positive for TB. Not all prisoners who had active TB were culture positive [25–41], with some studies reporting less than half of all sputum samples being successfully cultured in a laboratory [30,34]. As drug susceptibility testing can only be done with culture positive cases, the actual number of MDR TB cases is likely underestimated.
Overall, prevalence of MDR TB among prisoners varied greatly between studies. One study in the Oryol region of Russia found MDR TB prevalence among prisoners with active TB to be 12.0%; whereas, another study in the Tula region of Russia found a prevalence of 71.2% [28,35]. Since no national MDR TB data exists for Russia, the mean average of MDR TB prevalence data from the Russian studies was used to calculate a national average prevalence among prisoners of 43.14% [26–28,30,31,35,37,38,40,41]. Prevalence in Georgia ranged from 13.0% to 18.1% [25,33,34]. Prevalence of MDR TB among prisoners in Azerbaijan was 52.3%; whereas, in Moldova, it was 65.8% [29,32]. In Kazakhstan, the prevalence of MDR TB in prisons was 81.67%; while, in Kyrgyzstan it was found to be 26.8% [36,39]. In all but one study [29], previously treated TB cases had a higher prevalence of MDR TB than new TB cases [25–28,30–34,36,37,41].
Five studies reported adjusted odds ratios (AOR) using multivariate analysis [25,26,30,33,37]. While one study in Russia reported that those with MDR TB had a 4 times greater odds of being a prisoner [30], a study done in Georgia found no association between MDR TB and prisoners [33]. The same study in Georgia found that older age was associated with decreased odds of MDR TB, while another study in Georgia found the odds of having MDR TB increased with age [25,33].
Prisoners with MDR TB had greater odds of being overweight or obese (body mass index [BMI] > 25kg/m2) compared to those with drug-susceptible TB. However, the same study found individuals with drug-susceptible TB were at decreased odds of being overweight or obese. No association between MDR TB and a BMI under 20 kg/m2 was reported, but prisoners with drug-susceptible TB had increased odds of having a BMI under 20 kg/m2 compared to those with MDR TB [25].
The only study that examined recreational drug users found an association between illicit drug use and MDR TB. However, those with MDR TB were at decreased odds of having HIV [26].
Every study that looked at current or previous TB treatment as a risk factor found significant associations between treatment and MDR TB. Two studies found that those with MDR TB had 2.7 times greater odds of having had previous treatment [25,26]. Another 2 studies found that those with MDR TB had over 5 times greater odds of having had treatment failure [30,37]. One study that followed a cohort of 18 prisoners through TB treatment found that 94% (17) developed MDR TB [38]. The major findings for each study are presented in Table 2.
Table 2. Summary of study finding.
| Author, Year [Citation] | Prevalence of MDR TB among Prisoners with TB | Other Findings | Adjusted Odds Ratio (AOR [CIa]) |
|---|---|---|---|
| Aerts, 2000 [25] | 13.0% (36 cases out of 276) |
|
|
| Balabnova, 2006 [30] | 41.6% (84 cases out of 202 cases) |
|
|
| Balabnova, 2011 [31] | 49.3% (33 cases out of 67) |
|
|
| Bonnet, 2005 [37] | 27.9% (128 cases out of 459) |
|
|
| Ibrayeva, 2014 [36] | 81.7% (49 cases out of 60) |
|
|
| Ignatova, 2006 [28] | 71.3% (62 cases out of 87) |
|
|
| Jenkins, 2013 [32] | 65.8% (399 cases out of 606) |
|
|
| Jenkins, 2014 [33] | 18.1% (287 cases out of 1582) |
|
|
| Jugheli, 2008 [34] | 14.4% (39 cases out of 271) |
|
|
| Kimerling, 1999 [38] | 22.6% (37 cases out of 164) |
|
|
| Mar'iandyshev, 2005 [41] | 53.7% (87 cases out of 162) |
|
|
| Mokrousov, 2009 [39] | 26.8% (15 cases out of 56) |
|
|
| Pfyffer, 2001 [29] | 52.3% (34 cases out of 65) |
|
|
| Ruddy, 2005 [26] | 49.8% (145 cases out of 291) |
|
|
| Shemyakin, 2004 [40] | 65.4% (85 cases out of 130) |
|
|
| Spradling, 2002 [35] | 12.2% (5 cases out of 41) |
|
|
| Toungoussova, 2003 [27] | 37.7% (43 cases out of 114) |
|
|
aConfidence Interval of 95%
Discussion
The goal of this study was to investigate the prevalence of MDR TB among prisoners of Post-Soviet states. We used systematic review to analyze studies and extrapolate results. Our findings suggest a high prevalence of multidrug-resistance in prisons of Post-Soviet states with percentages as high as 16 times higher than the worldwide prevalence estimated by the WHO in 2014 [1,36]. While there were no common risk factors among studies reporting adjusted odds ratios [25,26,30,33,37], all studies suggested a high prevalence of MDR TB in prison populations in Post-Soviet states.
Within the Soviet Union, even after the advent of antibiotics for TB, the standard procedures for treatment were diagnosis by X-rays, admittance to a sanitarium, isolation, and occasionally surgery [42]. This treatment was, in fact, effective as TB mortality rates in the Soviet Union decreased from 400 per 100,000 persons in the 1910s to 17.3 per 100,000 men and 1.9 per 100,000 women in 1990 [43,44].
In 1991, the Soviet Union dissolved and the entire interconnected health, economic, and prison system collapsed with it. Suddenly millions of people found themselves living in poverty in newly formed nations [45]. Between 1991 and 1998, TB incidence in most Post-Soviet states more than doubled [46]. Additionally, during this time period, both crime and incarceration rates increased in the region as arrests tripled between 1988 and 1995 [20]. The gulag prison work camps of the former Soviet Union were repurposed to serve as prisons for the newly formed counties in which they were located. These prisons were not only over-crowded but also characterized by inadequate ventilation and malnutrition [20]. Such conditions resulted in high transmission of TB [7–10]. Between 1991 and 1997 the mortality rate in Russian prisons more than doubled with approximately half of these deaths being attributed to TB [47] and by 1999, one-third of all TB cases in Russia were in prisons [48].
Following the collapse in 1991, many of the newly formed nations started to implement the direct observed therapy short course (DOTS) system as recommended by the WHO. However, among physicians of the Post-Soviet states there was strong opposition to this move as they felt it threatened their livelihoods [49]. Conflicting instructions on how to best treat TB during the early 1990s likely did little to help the situation as isoniazid, rifampin and other TB medications started to enter the market. Partly because of stigmas associated with undergoing DOTS, TB medications took on value and were sold in open air markets, without prescription from pharmacists trying to make ends meet, and traded among inmates in prisons [49,50].
By 1998, it was estimated 20% of all TB cases in Russian prisons were multidrug-resistant [51]. Our findings suggest the prevalence of MDR TB in Russian prisons has since increased, and in some cases, more than doubled [26–28,30,31,35,37,38,40,41]. In Azerbaijan, Moldova, and Kazakhstan the proportion of TB among prisoners that was multidrug-resistant was found to be around 52%, 65% and 81% respectively [29,32,36]. Georgia and Kyrgyzstan, although similar in terms of incarceration rates to Azerbaijan, Moldova and Kazakhstan, had the lowest prevalence of multidrug-resistance with a reported prison prevalence of 18.1% and 26.8%, respectively [19,33,39].
Although, still around triple the global rate estimated by the WHO in 2014 [1], the prevalence rate of MDR TB in Georgian prisons seems to be one of the lowest in the Post-Soviet region [25–41]. To explain the differences in prevalence rates among studies, we looked at several factors including incarceration rates, use of fixed-dose combinations (FDC), nutrition, and per capita gross domestic product (GDP).
Among the countries in this study, lower incarceration rates were not associated with lower MDR TB. The studies from Russia, which had one of the highest rates of incarceration in the world at the beginning of each study period, had a more than 20% lower mean prevalence than Moldova which had one of the lowest rates of incarceration among all the countries in this study [19,26–28,30–32,35,37,38,40,41], Georgia, which had one of the highest rates of incarceration and one of the highest percentages of pre-trial detention in the world [19], had the lowest prevalence of MDR TB in prisons of Post-Soviet states [25,33,34].
The use of FDC has been suggested to account for low MDR TB prevalence in Sub-Saharan Africa [52,53]. However, no information on FDC usage was mentioned in any of the studies [25–41]. Only one study suggested the implementation of FDC as a possible solution for decreasing MDR TB prevalence rate in the Samara Oblast of Russia.26
Data on nutrition and daily caloric intake of prisoners in Post-Soviet states has not been found, however several studies have suggested that nutritional status is fairly standard among prisoners in much of the Post-Soviet region [20,21]. There also seems to be conflicting data regarding prison capacity and overcrowding, making it difficult to assess these findings [19,54].
Earlier studies have suggested MDR TB is inversely associated with GDP [55,56]. Our findings, however, did not show such a relationship. Regions with some of the highest GDPs also had the highest rates of MDR TB; whereas, regions with some of the lowest GDPs also had the lowest rates of MDR TB [25–41,57,58]. In fact, among the 3 Georgian studies conducted in 1997, 2001 and 2009, MDR TB seems to be increasing during this period even though during the same period the GDP almost tripled [25,33,34,58]. This may result from better detection and reporting in Post-Soviet states [59,60]. However, Georgia, in comparison to other Post-Soviet states included for review, has a relatively low rate of multidrug-resistance. Further investigation is needed to elucidate possible reasons why.
Since four studies [25,26,30,37] found MDR TB to be associated with TB treatment and the majority of MDR TB cases were from patients who had previously undergone TB treatment [25–28,30–37,41], inadequate or inappropriate treatment and medical noncompliance is likely a mode of spreading MDR TB in Post-Soviet states’ penal systems.
It is important for future research to focus on risk factors so that this epidemic could be better understood and addressed. With conflicting data and small sample sizes of publications, more studies are needed to create a more comprehensive landscape of MDR TB in Post-Soviet prisons. Notably, none of the studies reported a previous history of TB infection among prisoners. Many included previously treated cases, and indeed most of these did have MDR TB [25–28,30–37,41], but studies failed to differentiate between treatment failure cases and those that successfully completed treatment and then were re-infected. Information on the length of prison terms were lacking. Only one study looked at this potential association and found that prisoners who had MDR TB were at decreased odds of having spent a prison term between 2–4 years [25]. This may suggest prison terms less than 2 years and more than 4 years could correlate with MDR TB but further investigation is needed.
Malnutrition has been considered a problem in Post-Soviet prisons [20,21], but only one study included BMI and it found that being overweight was associated with MDR TB [25]. While being overweight is not an indicator of proper nutrition, this link needs to be investigated further. It is not clear if diets varying among countries could have an effect as nutrition has been shown to be a correlated with TB [13–17].
While the link between TB and HIV in prisons has been well established [58–63], the link between MDR TB and HIV is less clear. Studies have found conflicting results and one review focusing on prisons worldwide found that overall there was no significant association, although the relationship was variable based on region [64,65]. The HIV epidemic in the Post-Soviet states has been fueled by injection drug use [62,66,67]. Injection drug use has also been speculated to be associated with increased likelihood of becoming a prisoner as well as acquiring MDR TB [68], but only one of our studies looked at it as a factor [26]. It found that injection drug use was associated with MDR TB but also that those with MDR TB were at decreased odds of having HIV compared to those without MDR TB [26]. The other three studies we included in the review that looked at HIV in prisons found that all MDR TB patients were HIV negative [27,39,40]. As the current sample size of studies is too small to suggest an association, more studies are needed to examine the pathway between injection drug use, MDR TB, and HIV in Post-Soviet prisons.
As suggested by our studies, the rate of MDR TB in prisons of the former Soviet Union is above the world average [1,25–41]. Additionally, consecutive studies done within the same country at different times suggest an increase in MDR TB prevalence overtime [25–27,30,31,33,34,37,38,41]. For effective control, targeted MDR TB control programs tailored to Post-Soviet states, specifically within the penal systems, are warranted.
Limitations
Our review did encounter some limitations. Because our inclusion criteria included studies published from 1992 to 2015, studies published after this timeframe would not have been identified. Additionally, although PubMed and CINAHL are extensive databases for health research, limiting our search to these two databases may have resulted in the exclusion of any potential studies not cataloged in either PubMed or CINAHIL.
Data Availability
All reviewed studies are available from the PubMed and CINAHL databases. The 17 included studies can be found in Table 1 and the References section.
Funding Statement
The authors received no specific funding for this work.
References
- 1.World Health Organization. Multi Drug-Resistant Tuberculosis (MDR-TB) 2015 Update. 2015. http://www.who.int/tb/challenges/mdr/mdr_tb_factsheet.pdf. Accessed 1 May 2016.
- 2.World Health Organization. Global Tuberculosis Report 2015. Geneva: WHO Press; 2015. http://apps.who.int/iris/bitstream/10665/191102/1/9789241565059_eng.pdf. Accessed 1 May 2016.
- 3.Devaux I, Kremer K, Heersma H, Van Soolingen D. Clusters of multidrug-resistant mycobacterium tuberculosis cases, Europe. Emerg Infect Dis. 2009;15:1052–60. 10.3201/eid1507.080994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perkins MD, Roscigno G, and Zumla A. Progress towards improved tuberculosis diagnostics for developing countries. Lancet. 2006;367:942–3. 10.1016/S0140-6736(06)68386-4 [DOI] [PubMed] [Google Scholar]
- 5.Nathanson E, Lambregts-van Weezenbeek C, Rich M, Gupta R, Bayona J, Blönda K, et al. Multidrug-resistant tuberculosis management in resource-limited settings. Emerg Infect Dis. 2006;12:1389–97. 10.3201/eid1209.051618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mukherjee JS, Rich ML, Socci AR, Joseph JK, Virú FA, Shin SS, et al. Programmes and principles in treatment of multidrug-resistant tuberculosis. Lancet. 2004;363:474–81. 10.1016/S0140-6736(04)15496-2 [DOI] [PubMed] [Google Scholar]
- 7.Cox HS, Niemann S, Ismailov G, Doshetov D, Orozco JD, Blok L, et al. Risk of acquired drug resistance during short-course directly observed treatment of tuberculosis in an area with high levels of drug resistance. Clin Infect Dis. 2007;44:1421–7. 10.1086/517536 [DOI] [PubMed] [Google Scholar]
- 8.Rullán JV, Herrera D, Cano R, Moreno V., Godoy P., Peiró E. F., et al. Nosocomial transmission of multidrug-resistant Mycobacterium tuberculosis in Spain. Emerg Infect Dis. 1996;2:125–9. 10.3201/eid0202.960208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Victor TC, Streicher EM, Kewley C, Jordaan AM, van der Spuy GD, Bosman M, et al. Spread of an emerging Mycobacterium tuberculosis drug-resistant strain in the western Cape of South Africa. Int J Tuberc Lung Dis. 2007;11:195–201. [PubMed] [Google Scholar]
- 10.Zhao M, Li X, Xu P, Shen Xin, Gui Xiaohong, Wang Lili, et al. Transmission of MDR and XDR tuberculosis in Shanghai, China. PLoS ONE. 2009;4:e4370 10.1371/journal.pone.0004370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Angie B, Ann A, Malgosia G, Kimerling M, Kluge H, Levy M et al. Tuberculosis control in prisons: A manual for programme managers. Geneva: WHO Press; 2000. [Google Scholar]
- 12.Dara M, Grzemsk M, Kimerling ME, Reyes H, Zagorskiy A. Guidelines for control of tuberculosis in prisons. US Agency for International Development; 2009. [Google Scholar]
- 13.Reyes H, Coninx R. Pitfalls of tuberculosis programmes in prisons. BMJ. 1997;315:1447–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braun MM, Truman BI, Maguire B, DiFerdinando GT Jr, Wormser G, Broaddus R, et al. Increasing incidence of tuberculosis in a prison inmate population. association with HIV infection. JAMA. 1989;261:393–7. [PubMed] [Google Scholar]
- 15.Skolnick AA. Correction facility TB rates soar; some jails bring back chest roentgenograms. JAMA. 1992;268:3175–6. [PubMed] [Google Scholar]
- 16.Coninx R, Eshaya-Chauvin B, Reyes H. Tuberculosis in prisons. Lancet. 1995;346:1238–9. [DOI] [PubMed] [Google Scholar]
- 17.Long R, Njoo H, Hershfield E. Tuberculosis: Epidemiology of the disease in Canada. CMAJ. 1999;160:1185–90. [PMC free article] [PubMed] [Google Scholar]
- 18.Niveau G. Prevention of infectious disease transmission in correctional settings: A review. Public Health. 2006;120:33–41. 10.1016/j.puhe.2005.03.017 [DOI] [PubMed] [Google Scholar]
- 19.Walmsley R. World prison brief. Institute for Criminal Policy Research; 2016. http://www.prisonstudies.org/highest-to-lowest/prison-population-total Accessed 6 May 2016.
- 20.Bobrik A, Danishevski K, Eroshina K, McKee M. Prison health in Russia: The larger picture. J Public Health Policy. 2005;26:30–59. 10.1057/palgrave.jphp.3200002 [DOI] [PubMed] [Google Scholar]
- 21.Mataev SI, Sukhoveĭ IuG, Petrov SA, Popov AV, Unger IG, Vasil'kova TN, et al. The peculiaries of the nutrition and immune status of people in the condition of penitentiary establishment. Vopr Pitan. 2004;73:25–30. [PubMed] [Google Scholar]
- 22.Arbatov A, Chayes A, Handler C, Olson L. Managing conflict in the former Soviet Union: Russian and American perspectives. Cambridge: The MIT Press; 1997. [Google Scholar]
- 23.Bremmer I. The post-Soviet nations after independence In: Barrington L, ed. After independence: Making and protecting the nation in postcolonial and postcommunist states. Ann Arbor: The University of Michigan Press; 2006:141–61. [Google Scholar]
- 24.Barrera L, Cooreman E, de Dieu Iragena J, Drobniewski F, Duda P, Havelkovae M, et al. Policy guidance on drug-susceptibility testing (DST) of second-line antituberculosis drugs. Geneva: WHO Press; 2008. [PubMed] [Google Scholar]
- 25.Aerts A, Habouzit M, Mschiladze L, Malakmadze N, Sadradze N, Menteshashvili O, et al. Pulmonary tuberculosis in prisons of the ex-USSR state Georgia: Results of a nationwide prevalence survey among sentenced inmates. Int J Tuberc Lund Dis. 2000;4:1104–10. [PubMed] [Google Scholar]
- 26.Ruddy M, Balabanova Y, Graham C, Fedorin I, Malomanova N, Elisarova E, et al. Rates of drug resistance and risk factor analysis in civilian and prison patients with tuberculosis in samara region, Russia. Thorax. 2005;60:130–5. 10.1136/thx.2004.026922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toungoussova OS, Mariandyshev A, Bjune G, Sandven P, Caugant DA. Molecular epidemiology and drug resistance of mycobacterium tuberculosis isolates in the archangel prison in russia: Predominance of the W-Beijing clone family. Clin Infect Dis. 2003;37:665–72. 10.1086/377205 [DOI] [PubMed] [Google Scholar]
- 28.Ignatova A, Dubiley S, Stepanshina V, Shemyakin I. Predominance of multi-drug-resistant LAM and Beijing family strains among mycobacterium tuberculosis isolates recovered from prison inmates in Tula region, Russia. J Med Microbiol. 2006;55:1413–8. 10.1099/jmm.0.46575-0 [DOI] [PubMed] [Google Scholar]
- 29.Pfyffer GE, Strassle A, van Gorkum T, Portaels F, Rigouts L, Mathieu C, et al. Multidrug-resistant tuberculosis in prison inmates, Azerbaijan. Emerg Infect Dis. 2001;7:855–61. 10.3201/eid0705.017514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balabanova Y, Drobniewski F, Fedorin I, Zakharova S, Nikolayevskyy V, Atun R, et al. The directly observed therapy short-course (DOTS) strategy in Samara Oblast, Russian Federation. Respir Res. 2006;7:44 10.1186/1465-9921-7-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balabanova Y, Nikolayevskyy V, Ignatyeva O, Kontsevaya I, Rutterford CM, Shakhmistova A, et al. Survival of civilian and prisoner drug sensitive, multi and extensive drug- resistant tuberculosis cohorts prospectively followed in Russia. PLoS ONE. 2011;6:e20531 10.1371/journal.pone.0020531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jenkins HE, Plesca V, Ciobanu A, Crudu V, Galusca I, Soltan V, et al. Assessing spatial heterogeneity of multi drug-resistant tuberculosis in a high-burden country. Eur Respir J. 2013;42:1291–1301. 10.1183/09031936.00111812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jenkins HE, Gegia M, Furin J, Kalandadze I, Nanava U, Chakhaia T, et al. Geographical heterogeneity of multidrug-resistant tuberculosis in Georgia, January 2009 to June 2011. Euro Surveill. 2014;19:20743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jugheli L, Rigouts L, Shamputa IC, Bram de Rijk W, Portaels F. High levels of resistance to second-line anti-tuberculosis drugs among prisoners with pulmonary tuberculosis in Georgia. Int J Tuberc Lung Dis. 2008;12:561–6. [PubMed] [Google Scholar]
- 35.Spradling P, Nemtsova E, Aptekar T, Shulgina M, Rybka L, Wells C, et al. Anti-tuberculosis drug resistance in community and prison patients, Orel Oblast, Russian Federation. Int J Tuberc Lung Dis. 2002;6:757–62. [PubMed] [Google Scholar]
- 36.Ibrayeva A, Kozhamkulov U, Raiymbek D, Alenova A, Igilikova S, Zholdybayeva E, et al. Molecular epidemiology of mycobacterium tuberculosis strains circulating in the penitentiary system of Kazakhstan. Int J Tuberc Lung Dis. 2014;18:298–301. 10.5588/ijtld.13.0558 [DOI] [PubMed] [Google Scholar]
- 37.Bonnet M, Sizaire V, Kebede Y, Janin A, Doshetov D, Mirzoian B, et al. Does one size fit all? drug resistance and standard treatments: Results of six tuberculosis programmes in former Soviet countries. Int J Tuberc Lung Dis. 2005;9:1147–54. [PubMed] [Google Scholar]
- 38.Kimerling ME, Kluge H, Vezhnina N, Iacovazzi T, Demeulenaere T, Portaels F, et al. Inadequacy of the current WHO re-treatment regimen in a central Siberian prison: Treatment failure and MDR-TB. Int J Tuberc Lung Dis. 1999;3:451–3. [PubMed] [Google Scholar]
- 39.Mokrousov I, Valcheva V, Sovhozova N, Aldashev A, Rastogi N, Isakova J. Penitentiary population of mycobacterium tuberculosis in Kyrgyzstan: Exceptionally high prevalence of the Beijing genotype and its Russia-specific subtype. Infect Genet Evol. 2009;9:1400–5. 10.1016/j.meegid.2009.07.007 [DOI] [PubMed] [Google Scholar]
- 40.Shemyakin IG, Stepanshina VN, Ivanov IY, Lipin MY, Anisimova VA, Onasenko AG, et al. Characterization of drug-resistant isolates of mycobacterium tuberculosis derived from Russian inmates. Int J Tuberc Lung Dis. 2004;8:1194–1203. [PubMed] [Google Scholar]
- 41.Mar'iandyshev AO, Nizovtseva NI, Glomozda PG, Panasik VP, Duginova TN, Kuznetsov AA. Spread of multidrug resistance of mycobacterium and its impact on the efficiency of treatment in patients with tuberculosis in the penitentiaries of the arkhangelsk region. Probl Tuberk Bolezn Legk. 2005;5:19–21. [PubMed] [Google Scholar]
- 42.Banatvala N, Peremitin Gg. Tuberculosis, Russia, and the holy grail. Lancet. 1999;353:999–1000. 10.1016/S0140-6736(99)02059-0 [DOI] [PubMed] [Google Scholar]
- 43.Shkolnikov V, Mesle F. The Russian epidemiological crisis as mirrored by mortality trends. RAND conference proceedings. 1996:113–62.
- 44.Stern V. Sentenced to die? The problem of TB in prisons in Eastern Europe and Central Asia. London: International Centre for Prison Studies; 1999. [Google Scholar]
- 45.Kim JY, Millen JV, Irwin A, Gershman J. The Russian health crisis In: Kim JM, ed. Dying for growth. Maine: Common Courage Press; 2000. [Google Scholar]
- 46.World Health Organization. World Health Organization global tuberculosis control. Geneva: WHO Press; 2000. [Google Scholar]
- 47.Ministry of the Interior/Ministry of Justice of the Russian. Official statistics of the penal system, 1989–1999.
- 48.Bone A, Aerts A, Grzemska M, Kimerling M. Tuberculosis control in prisons: A manual for programme managers. Geneva: WHO Press; 2000. [Google Scholar]
- 49.Das V, Das Rk. Global pharmaceuticals: Ethics, markets, practices In: Pharmaceuticals in urban ecologies: The register of the local. Durham: Duke University Press; 2006. [Google Scholar]
- 50.Whyte SR, Sjaak G, Anita H. Social lives of medicines. Cambridge: Cambridge University Press; 2002. [Google Scholar]
- 51.Newsletter of the penal reform project in Eastern Europe and Central Asia. 1998.
- 52.Lukoye D, Ssengooba W, Musisi K, Kasule GW, Cobelens FG, Joloba M, et al. Variation and risk factors of drug resistant tuberculosis in sub-saharan africa: A systematic review and meta-analysis. BMC Public Health. 2015;15:291 10.1186/s12889-015-1614-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fattorini L, Mustazzolu A, Borroni E, Piccaro G, Giannoni F, Cirillo DM, et al. Tuberculosis migrants from 106 countries to Italy, 2008–2014. Eur Respir J. 2016;47:1273–1276 10.1183/13993003.01844-2015 [DOI] [PubMed] [Google Scholar]
- 54.Davitaia A. ISHR georgia report 2010: Prison conditions in the Republic of Georgia. 2010.
- 55.Blondal K, Viiklepp P, Blondal P, Altraja A. Countrywide management of pulmonary tuberculosis reverses increasing incidence. Int J Tuberc Lung Dis. 2011;15:892–8. 10.5588/ijtld.10.0601 [DOI] [PubMed] [Google Scholar]
- 56.Cohen T, Jenkins HE, Lu C, Mclaughlin M, Floyd K, Zignol M. On the spread and control of MDR-TB epidemics: An examination of trends in anti-tuberculosis drug resistance surveillance data. Drug Resist Updat. 2014;17:105–23. 10.1016/j.drup.2014.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gross regional product of the Russian Federation in 1998–2007.
- 58.International comparison program database. The World Bank Group. 2016.
- 59.Atun R, Olynik I. Resistance to impementing policy change: The case of Ukraine. Bull World Health Organ. 2008;86:147–54. 10.2471/BLT.06.034991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dimitrova B, Balabanova D, Atun A, Drobneiwski F, Levicheva V, Coker R. Health service providers' perceptions of barriers to tuberculosis care in Russia. Health Policy Plan. 2006;21:265–74. 10.1093/heapol/czl014 [DOI] [PubMed] [Google Scholar]
- 61.Henostroza G, Topp SM, Hatwiinda S, Maggard KR, Phiri W, Harris JB, et al. The high burden of tuberculosis (TB) and human immunodeficiency virus (HIV) in a large Zambian prison: A public health alert. PLoS ONE. 2013;8:e67338 10.1371/journal.pone.0067338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O'grady J, Hoelscher M, Atun R, Bates M, Mwaba P, Kapata N, et al. Tuberculosis in prisons in sub-Saharan Africa—the need for improved health services, surveillance and control. Tuberculosis. 2011;91:173–8. 10.1016/j.tube.2010.12.002 [DOI] [PubMed] [Google Scholar]
- 63.Stephens TT, Braithwaite R, Cozza S, Robillard A, Arriola KJ. History of prior TB infection and HIV/AIDS risk behaviours among a sample of male inmates in the USA. Int J STD AIDS. 2003;14:514–8. 10.1258/095646203767869101 [DOI] [PubMed] [Google Scholar]
- 64.Mesfin YM, Hailemariam D, Biadgilign S, Biadglign S, Kibret Kt. Association between HIV/AIDS and multi-drug resistance tuberculosis: A systematic review and meta-analysis. PLoS ONE. 2014;9:e82235 10.1371/journal.pone.0082235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Suchindran S, Brouwer ES, Van Rie A. Is HIV infection a risk factor for multi-drug resistant tuberculosis? A systematic review. PLoS ONE. 2009;4:e5561 10.1371/journal.pone.0005561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boltaev AA, El-bassel N, Deryabina AP, Terlikbaeva A, Gilbert L, Hunt T, et al. Scaling up HIV prevention efforts targeting people who inject drugs in Central Asia: A review of key challenges and ways forward. Drug Alcohol Depend. 2013;132:41–7. [DOI] [PubMed] [Google Scholar]
- 67.Mathers BM, Degenhardt L, Phillips B, Wiessing L, Hickman M, Strathdee SA, et al. Global epidemiology of injecting drug use and HIV among people who inject drugs: A systematic review. Lancet. 2008;372:1733–45. 10.1016/S0140-6736(08)61311-2 [DOI] [PubMed] [Google Scholar]
- 68.Schluger NW, El-bassel N, Hermosilla S, Terlikbayeva A, Darisheva M, Aifah A, et al. Tuberculosis, drug use and HIV infection in Central Asia: An urgent need for attention. Drug Alcohol Depend. 2013;132:32–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All reviewed studies are available from the PubMed and CINAHL databases. The 17 included studies can be found in Table 1 and the References section.