Abstract
Rationale: Lengthy, multidrug, toxic, and low-efficacy regimens limit management of pulmonary nontuberculous mycobacterial disease.
Objectives: In this phase II study, we investigated the efficacy and safety of liposomal amikacin for inhalation (LAI) in treatment-refractory pulmonary nontuberculous mycobacterial (Mycobacterium avium complex [MAC] or Mycobacterium abscessus) disease.
Methods: During the double-blind phase, patients were randomly assigned to LAI (590 mg) or placebo once daily added to their multidrug regimen for 84 days. Both groups could receive open-label LAI for 84 additional days. The primary endpoint was change from baseline to Day 84 on a semiquantitative mycobacterial growth scale. Other endpoints included sputum conversion, 6-minute-walk distance, and adverse events.
Measurements and Main Results: The modified intention-to-treat population included 89 (LAI = 44; placebo = 45) patients. The average age of the sample was 59 years; 88% were female; 92% were white; and 80 and 59 patients completed study drug dosing during the double-blind and open-label phases, respectively. The primary endpoint was not achieved (P = 0.072); however, a greater proportion of the LAI group demonstrated at least one negative sputum culture (14 [32%] of 44 vs. 4 [9%] of 45; P = 0.006) and improvement in 6-minute-walk test (+20.6 m vs. −25.0 m; P = 0.017) at Day 84. A treatment effect was seen predominantly in patients without cystic fibrosis with MAC and was sustained 1 year after LAI. Most adverse events were respiratory, and in some patients it led to drug discontinuation.
Conclusions: Although the primary endpoint was not reached, LAI added to a multidrug regimen produced improvements in sputum conversion and 6-minute-walk distance versus placebo with limited systemic toxicity in patients with refractory MAC lung disease. Further research in this area is needed.
Clinical trial registered with www.clinicaltrials.gov (NCT01315236).
Keywords: culture conversion, efficacy, safety, nontuberculous mycobacteria
At a Glance Commentary
Scientific Knowledge on the Subject
Management of pulmonary nontuberculous mycobacterial (PNTM) disease is limited by lengthy multidrug regimens, drug toxicity and/or intolerance, and poor drug efficacy. Liposomes are taken up by lung macrophages, allowing intracellular delivery of high levels of amikacin into nontuberculous mycobacteria (NTM)-infected cells, and inhalation allows localized drug delivery. In select patients with treatment-refractory PNTM disease, liposomal amikacin for inhalation (LAI) added to guidelines-based therapy may be able to achieve early and sustained negative sputum cultures with limited systemic toxicity.
What This Study Adds to the Field
This multicenter clinical trial assessed the novel drug formulation LAI in patients with persistently positive NTM cultures despite having received guidelines-based treatment for at least 6 months prior to screening. Although the primary endpoint was not achieved, more patients receiving LAI treatment than patients receiving placebo had a negative NTM sputum culture at Day 84 (14 [32%] of 44 vs. 4 [9%] of 45; P = 0.006). Most of these patients (n = 14 of 18) also had a negative culture at 28 days after LAI discontinuation. Culture conversion, used in tuberculosis trials to predict lasting mycobacterial eradication, occurred mainly in patients without cystic fibrosis with Mycobacterium avium complex infection. The results of this study indicate that, in select patients with treatment-refractory PNTM disease, LAI added to guidelines-based therapy can achieve early and sustained negative sputum cultures. Further, culture conversion in response to treatment with LAI may be associated with improvements in functional capacity and, relative to treatment with parenteral amikacin, limited systemic toxicity.
Pulmonary nontuberculous mycobacterial (PNTM) disease is a chronic, frequently progressive infection characterized by necrotizing inflammation, bronchiectasis, and cavitation with associated irreversible lung damage and increased mortality (1–5). Compromised pulmonary function impairs physical activity and negatively impacts patients’ quality of life (6). In North America, the prevalence of PNTM disease roughly doubled in selected populations between 1997 and 2007 (7, 8).
Treatment options for the management of PNTM infection, particularly in patients with treatment-refractory disease, remain limited (9, 10). Contemporary treatment guidelines recommend lengthy multidrug antibiotic regimens, with or without parenteral agents (9). The use of parenteral aminoglycosides for advanced PNTM disease is limited by the risk of ototoxicity, vestibular toxicity, and nephrotoxicity (9, 11).
Because it achieves targeted and localized drug delivery, liposomal amikacin for inhalation (LAI) may yield improved efficacy and reduced systemic toxicity (12). A novel amikacin formulation in development for the treatment of PNTM disease, LAI is composed of small (∼0.3 μm), charge-neutral, highly biocompatible liposomes that encapsulate amikacin (12, 13). The liposomes are taken up by lung macrophages, allowing intracellular delivery of high levels of amikacin into nontuberculous mycobacteria (NTM)–infected cells (12), which can effectively reduce mycobacteria colony counts. Additionally, LAI results in lower serum levels of amikacin than parenteral amikacin and thus may reduce systemic toxicity (14, 15).
We report the first prospective study of the efficacy and tolerability of a novel drug for patients with treatment-refractory PNTM disease (www.clinicaltrials.gov identifier NCT01315236). Some of the results of this study have been reported previously in the form of abstracts (16–25).
Methods
Study Design and Treatment
This phase II, randomized, double-blind, placebo-controlled trial, which was followed by an open-label extension study, was conducted at 19 sites in North America (see Figure E1 in the online supplement). Patients were stratified on the basis of the presence or absence of cystic fibrosis (CF) and the predominance of Mycobacterium avium complex versus Mycobacterium abscessus at screening. Patients were randomly assigned in a 1:1 ratio to receive LAI at a dose of 590 mg or placebo (empty liposomes) once daily via a customized investigational eFlow Technology nebulizer (PARI Pharma GmbH, Starnberg, Germany) added to their ongoing, stable multidrug regimen for the initial 84 days of the study.
Eligible patients could consent to receive open-label treatment with daily LAI and their continuing background regimen for an additional 84 days. The study included a safety follow-up visit 28 days after the last dose of the study drug. Patients who completed the study also had the option to return for a follow-up visit 1 year after the last dose of study treatment. Approximately 90 patients were planned for randomization.
Patient Eligibility
Each site’s institutional review board approved the protocol, and patient informed consent was obtained. Eligible adults met criteria for PNTM disease as defined by the American Thoracic Society/Infectious Disease Society of America (ATS/IDSA) (9), had received ongoing ATS/IDSA guidelines–based multidrug treatment (9) for at least 6 months prior to screening, and had persistently positive cultures for M. avium complex or M. abscessus. Seventy-two (approximately 81%) of 89 patients had been treated with a standard multidrug regimen for NTM for at least 12 months, and almost half (42 [47%] of 89) of the patients had been treated for more than 24 months prior to randomization into the study. Key exclusion criteria included current smoking; FEV1 less than 30% of predicted; clinically significant cardiac, pulmonary, hepatic, or renal disease; systemic immune deficiency; and malignancy. Patients were not excluded on the basis of amikacin resistance.
Pharmacokinetics
Blood samples were drawn predose (from 0 to 4 h before the dose) on Days 1, 2, 28, 56, 84, 112, and 168; 0–1 hour postdose on Days 1 and 2; 3–4 hours postdose on Days 1, 28, 56, and 84; and 1–3 hours postdose on Days 112 and 168.
Efficacy Measurements
Microbiology
At least two predose expectorated or induced sputum specimens were to be collected at study visits; the heaviest growth for a single visit was reported. Patients were stratified on the basis of predominance of M. avium complex or M. abscessus as determined by the quantity of the species present on screening cultures.
Sputum specimens were processed centrally, with standardized decontamination, fluorochrome microscopy, broth culture (26, 27) (VersaTREK; Thermo Fisher Scientific, Inc., Waltham, MA), and solid media culture with a Middlebrook 7H11 agar biplate (Mitchison’s agar) with and without antibiotics (carbenicillin, trimethoprim, amphotericin, and polymyxin B). A positive culture was defined as growth in broth and/or agar. M. avium complex isolates were identified to the complex level using AccuProbe (Gen-Probe; Hologic, Marlborough, MA), and subspecies within the M. abscessus group were identified by polymerase chain reaction restriction fragment length analysis (28). Isolates were banked.
A semiquantitative scale (SQS) was used to assess relative mycobacterial growth (see Table E1). A negative sputum culture was defined at a single time point. Culture conversion was defined in post hoc analysis as three consecutive negative culture results, with the first negative result indicating time of conversion; this could be associated with the background regimen alone (conversion prior to study drug administration) or with the addition of LAI or placebo. Sustainability was defined as continuous negative cultures while on the study drug. Durability was defined as negative sputum culture at the 28-day follow-up visit (off study drug). A microbiologic recurrence was defined as one or more positive cultures after culture conversion. Cultures positive for M. avium complex occurring after culture conversion were molecularly compared with pretreatment isolates using partial 16S ribosomal RNA (rRNA) gene sequencing and variable number tandem repeat (VNTR) to determine if the isolates belonged to the same M. avium complex species and genotype (relapse) (29–31) or if their sequencing or VNTR differed (new infection) (31).
In vitro macrolide and amikacin resistance was assessed by broth microdilution susceptibility testing and by molecular identification of mutations. For amikacin, isolates were sequenced for a mutation at position 1,408 in the rrs (16S rRNA) gene if the amikacin minimum inhibitory concentration (MIC) was greater than or equal to 64 μg/ml (32).
Functional and quality-of-life assessment
The 6-minute-walk test distance walked at baseline was compared with distances achieved at the end of the double-blind and open-label phases as a measure of change in functional capacity (33). At each visit, quality of life was evaluated using patient-reported outcome instruments. (Additional information is provided in the online supplement.)
Safety
At each visit, treatment-emergent adverse events were evaluated on the basis of their severity, seriousness, and relatedness to study treatment. (Additional information is provided in the online supplement.)
Endpoints and Statistical Analyses
Efficacy and safety analyses were performed using the modified intention-to-treat population, defined as all randomized patients who received at least one dose of the study drug. The primary efficacy endpoint was change from baseline to Day 84 in the SQS (see Table E1) for mycobacterial growth for the LAI arm versus the placebo arm. With a two-sided significance level of 0.05, 50 subjects per arm (randomized 1:1) would provide 80% power to detect a difference between the treatment arms of at least 0.94 steps in the SQS in the change from baseline in mycobacterial culture, assuming a pooled SD of 1.6 (based on simulated information), based on a Wilcoxon rank-sum test. The primary efficacy analysis of SQS was performed using a stratified Wilcoxon rank-sum test (i.e., van Elteren test), adjusting for the randomization strata, to compare the treatment arms at a two-sided significance level of 0.05. The SAS statistical software package (version 9.2 or later; SAS Institute, Cary, NC) was used to perform the analysis. The analysis of the SQS data presented in this article was based on missing values equaling failure.
Key secondary microbiologic endpoints included the proportion of patients in each group with sputum cultures negative for NTM at Day 84. Because treatment regimens and response rates for M. avium complex and M. abscessus infections differ considerably (9), culture results were assessed separately in evaluable patients during both the double-blind and open-label periods. Additionally, the proportion of patients with sputum culture conversion was assessed post hoc.
Change from baseline in distance walked in the 6-minute walk test was compared between treatment groups. Changes in quality-of-life measures were summarized at each visit by treatment arm. (Additional information is provided in the online supplement.) Correlations between the change from baseline in SQS and health-related quality of life and 6-minute-walk test at Day 84 were assessed post hoc.
Results
Patient Disposition and Demographics
Of the 136 patients screened, 90 patients who met the eligibility criteria were stratified and randomly assigned to receive treatment with LAI or placebo (see Figure E2). One study participant no longer met the entry criteria at Day 1 and did not receive the study drug. Eighty-nine patients (44 LAI and 45 placebo) were included in the modified intention-to-treat population: 19% had CF, 64% had predominantly M. avium complex infection, and 36% had predominantly M. abscessus infection. Concomitant antibiotic therapies during the double-blind treatment phase are listed in Tables E2–E4.
During the double-blind treatment phase, nine patients from the LAI group discontinued the study drug (seven because of adverse events, two for other reasons); five of these patients completed all visits, and four (including one who died) discontinued the study early. Seventy-eight patients (35 LAI and 43 placebo) enrolled in, and 59 completed, study drug dosing during the open-label phase.
Baseline patient demographics and characteristics were comparable between treatment groups (Table 1). Most patients were white (92%) and female (88%). The mean age of the sample was 58.5 (SD, ±15.8) years. The baseline demographics were generally comparable between treatment groups. At baseline, no notable between-group differences in lung function (mean FEV1 percent predicted, 63.6 ± 21.3% and 62.6 ± 17.2% for LAI and placebo groups, respectively) or in percentage of patients with negative sputum cultures (6.8% and 6.7% for the LAI and placebo groups, respectively) were observed. Because baseline SQS scores were not stratified, imbalances were noted, with higher SQS mycobacterial growth (≥2+) at baseline in a greater proportion of patients in the placebo group (25 [55.6%] of 45) than in the LAI group (19 [43.2%] of 44). However, the imbalance was not statistically significant.
Table 1.
Baseline Demographics and Characteristics (Modified Intention-to-Treat Population)*
| LAI (n = 44) | Placebo (n = 45) | Overall (n = 89) | P Value | |
|---|---|---|---|---|
| Race/ethnicity, n (%) | 0.386 | |||
| White (not of Hispanic origin) | 42 (95.5) | 40 (88.9) | 82 (92.1) | |
| Hispanic | 0 | 2 (4.4) | 2 (2.2) | |
| African | 0 | 1 (2.2) | 1 (1.1) | |
| Asian | 2 (4.5) | 2 (4.4) | 4 (4.5) | |
| Sex, n (%) | 0.717 | |||
| Male | 6 (13.6) | 5 (11.1) | 11 (12.4) | |
| Female | 38 (86.4) | 40 (88.9) | 78 (87.6) | |
| Height, cm, mean (SD) | 165.4 (7.63) | 164.2 (9.12) | 164.8 (8.39) | 0.503 |
| Weight, kg, mean (SD) | 59.71 (10.132) | 60.15 (15.373) | 59.93 (12.976) | 0.874 |
| BMI, kg/m2, mean (SD) | 21.76 (2.705) | 22.10 (3.941) | 21.93 (3.372) | 0.637 |
| Age, yr, mean (SD) | 58.0 (16.61) | 59.1 (15.20) | 58.5 (15.83) | 0.745 |
| FEV1 % predicted, mean (SD) | 63.56 (21.339) | 62.56 (17.168) | 63.06 (19.239) | 0.808 |
| Stratification at screening, n (%) | ||||
| Predominantly Mycobacterium avium complex | 29 (65.9) | 28 (62.2) | 57 (64.0) | 0.717 |
| Predominantly Mycobacterium abscessus | 15 (34.1) | 17 (37.8) | 32 (36.0) | 0.717 |
| CF | 8 (18.2) | 9 (20.0) | 17 (19.1) | 0.827 |
| M. avium complex and CF | 2 (4.5) | 1 (2.2) | 3 (3.4) | 0.544 |
| M. abscessus and CF | 6 (13.6) | 8 (17.8) | 14 (15.7) | 0.592 |
| Mycobacterial load,† n (%) | 0.761 | |||
| Culture negative | 3 (6.8) | 3 (6.7) | 6 (6.7) | |
| Growth in liquid medium only | 3 (6.8) | 3 (6.7) | 6 (6.7) | |
| 1–49 colonies | 17 (38.6) | 10 (22.2) | 27 (30.3) | |
| 1+ (50–100 colonies) | 2 (4.5) | 4 (8.9) | 6 (6.7) | |
| 2+ (>100–200 colonies) | 2 (4.5) | 2 (4.4) | 4 (4.5) | |
| 3+ (>200–500 colonies) | 3 (6.8) | 4 (8.9) | 7 (7.9) | |
| 4+ (>500 colonies) | 14 (31.8) | 19 (42.2) | 33 (37.1) | |
| Cavitary disease‡ | 33 (75.0) | 35 (77.8) | 68 (76.4) | 0.807 |
| Macrolide resistant | 8 (18.0) | 15 (33.3) | 23 (25.8) | 0.146 |
| Amikacin mutation positive | 2 (4.5) | 6 (13.3) | 8 (8.9) | 0.266 |
| Macrolide resistant or amikacin mutation positive | 9 (20.5) | 16 (35.6) | 25 (28.1) |
Definition of abbreviations: BMI = body mass index; CF = cystic fibrosis; LAI = liposomal amikacin for inhalation.
Baseline is defined as the measurement prior and closest to the administration of study drug.
At least two sputum samples (preferably three sputum samples) were obtained.
This designation included patients with primarily nodular bronchiectasis.
Pharmacokinetics
Summary statistics for amikacin serum exposure are listed in Table E5. The mean and median values for maximum serum concentration are well within or below desired trough levels in clinical practice (34).
Efficacy
Change from baseline in semiquantitative culture results at Day 84
The primary endpoint of change from baseline on the SQS in the LAI group versus placebo did not reach statistical significance (P = 0.072), although a positive numerical trend in favor of the LAI arm was seen (Figure 1). Consequently, all P values mentioned below for the nonprimary analyses are presented for completeness and do not imply statistical significance.
Figure 1.
Mean change from baseline on the semiquantitative scale (SQS) for mycobacterial culture growth through the end of the open-label phase (missing value equals failure; modified intent-to-treat population). The SQS is a mycobacterial culture reporting method expressed on a seven-step scale that uses outcomes ranging from no growth at 6 weeks (step 1), growth in liquid media only (step 2), agar positive (1–49 colonies) (step 3), 1+ (step 4), 2+ (step 5), 3+ (step 6), and 4+ (step 7). Change from baseline ranges from +6 (worsening) to −6 (improvement) steps. Death, regardless of cause, was considered a failure and is represented by a +7-step worsening. *All patients in the open-label phase received liposomal amikacin for inhalation (LAI). PBO = placebo.
Negative sputum cultures at Day 84, Day 168, and 28-day follow-up
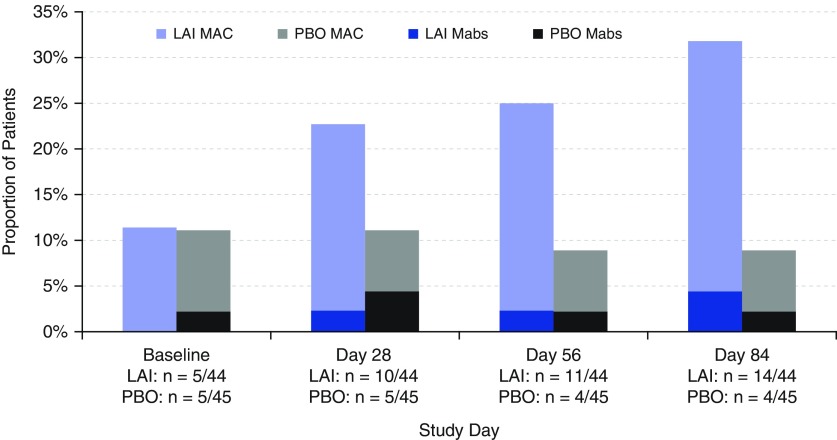
A greater percentage of patients in the LAI group than in the placebo arm had negative sputum cultures at Day 84 (32% [14 of 44] vs. 9% [4 of 45]; P = 0.006) (Figure 2). Most of these patients continued to have persistently negative cultures at Day 168 (11 LAI, 4 placebo) and 28-day follow-up (11 LAI, 3 placebo). A shorter time to first negative sputum culture was also observed with LAI than in the placebo group during the double-blind phase (hazard ratio, 5.68; 95% confidence interval [CI], 1.25–25.79; P = 0.0129).
Figure 2.
Proportion of patients with negative sputum cultures for nontuberculous mycobacteria in the double-blind phase (last observation carried forward; modified intention-to-treat population). A greater proportion of patients achieved negative sputum cultures at Day 84 on liposomal amikacin for inhalation (LAI) (14 [32%] of 44) versus placebo (4 [9%] of 45) (P = 0.006). Mabs = Mycobacterium abscessus; MAC = Mycobacterium avium complex; PBO = placebo.
Sputum culture conversion at 28-day end-of-study follow-up visit
Of the 23 patients who achieved culture conversion by the 28-day end-of-study follow-up visit, 4 (2 patients in the LAI group and 2 in the placebo arm) converted at baseline (Day 1), prior to the administration of the study drug. Nineteen patients achieved culture conversion after baseline (Day 1), ten of whom were randomized to LAI in the double-blind phase and seven after entering the open-label phase (one of these seven was on LAI and the other six were on placebo prior to the open-label phase); the remaining two patients achieved culture conversion while receiving placebo during the double-blind phase.
Patients with M. avium complex infection represented the vast majority (19 [82.6%] of 23) of those who achieved culture conversion; however, 4 of these patients converted at baseline (2 patients in the LAI group and 2 in the placebo arm). Of the remaining 15, 14 converted after receiving LAI: 9 during the double-blind phase and 5 after entering the open-label phase (4 received placebo and 1 received LAI in the double-blind phase). One patient converted while receiving placebo in the double-blind phase. Of the four patients with M. abscessus who achieved culture conversion, three converted after receiving LAI (one during the double-blind phase and two during the open-label phase) and one while receiving placebo in the double-blind phase.
Durability of sputum culture conversion at 12-month safety follow-up
The durability of sputum culture conversion was not a prespecified endpoint; however, the majority of patients who achieved culture conversion during the double-blind phase (12 on LAI, 4 on placebo) continued to have negative cultures through the end of the open-label phase (n = 14) and at the 28-day follow-up visit (n = 13). In addition, seven of these patients continued to have negative cultures at the 12-month follow-up visit.
At the 12-month safety follow-up visit, 9 (64%) of 14 patients with M. avium complex infection who converted after starting LAI had sustained negative cultures after LAI discontinuation (7 had negative sputum cultures; 2 were unable to produce sputum and were off all NTM treatments); 2 patients had positive sputum cultures; and 3 did not consent to participate in the 12-month follow-up phase. Of the four patients with M. abscessus infection who converted, two had negative cultures 12 months after LAI discontinuation, one reverted to positive cultures, and one did not consent to participate in the 12-month follow-up phase.
Microbiologic recurrence
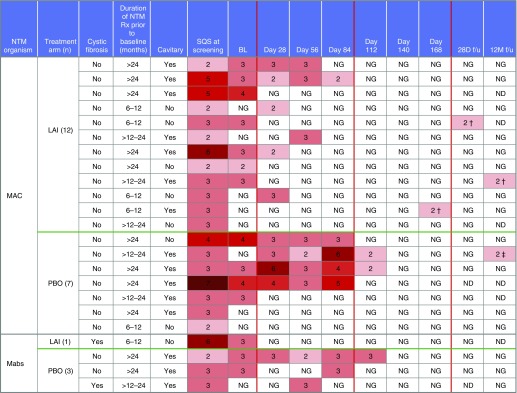
Four patients who achieved culture conversion had a subsequent liquid media–only positive culture; three were different species from the baseline isolate or “new infections,” and one represented a true relapse with the same M. avium complex species and VNTR type on both baseline and late positive cultures. The latter patient had only two negative cultures while on LAI, with the third negative culture occurring 28 days after LAI treatment (Figure 3).
Figure 3.
Patients achieving culture conversion through the end of the open-label phase. Of the 23 patients who achieved culture conversion by the 28-day end-of-study follow-up visit, 4 (LAI = 2 patients; placebo = 2 patients) converted at baseline (Day 1) prior to the administration of study drug. Nineteen patients achieved culture conversion after baseline (Day 1), 10 of whom were randomized to LAI in the double-blind phase and 7 after entering the open-label phase (1 of 7 on prior LAI and 6 of 7 on prior placebo). The remaining two patients achieved culture conversion while receiving add-on placebo. All of the converters with Mycobacterium avium complex were patients without cystic fibrosis. Two of the converters with Mycobacterium abscessus infection (LAI, one patient; PBO, one patient) had cystic fibrosis, and two were patients without cystic fibrosis. Four patients who achieved culture conversion had a subsequent positive liquid media–only culture result: three of these cultures were different species from the baseline isolate, or “new infections” (M. avium at baseline and Mycobacterium timonense 28 d after LAI; M. avium at baseline and Mycobacterium chimaera at 12 mo after LAI; M. avium at baseline and Mycobacterium intracellulare at Day 168), and one was the same or “relapse.” †New infection; ‡relapse. BL = baseline; f/u = follow-up; LAI = liposomal amikacin for inhalation; Mabs = Mycobacterium abscessus; MAC = Mycobacterium avium complex; ND = not done; NG = no growth at 6 weeks; NTM = nontuberculous mycobacteria; PBO = placebo; Rx = medication; SQS = semiquantitative scale.
Amikacin resistance
The protocol included a plan to bank all mycobacterial isolates for future selective susceptibility testing for correlation with microbiologic and clinical response. Macrolide and amikacin resistance MICs (and amikacin resistance mutational status) were processed as soon as the capacity for testing was in place. These data were kept blinded until after data collection was completed and the database was locked. No patient isolate with a molecularly determined amikacin resistance mutation or an MIC greater than 64 μg/ml achieved culture conversion, and no converter developed mutational resistance on treatment. Amikacin mutational resistance was detected in 9 patients before LAI exposure (9 [10.1%] of 89 patients enrolled in the study). Amikacin mutational resistance was detected in five patients after LAI exposure at Day 84 (LAI group) or Day 168 (placebo and LAI group) (nonconverters; four with M. avium complex infection, one with M. abscessus infection: 5 [5.7%] of 87 patients exposed to LAI during this study; 2 of 89 patients received placebo during the double-blind phase and did not enter the open-label phase). Among these five patients, two who were randomized to placebo during the double-blind phase and continued into the open-label phase of the study had isolates (one for M. avium complex, one for M. abscessus) with high MICs (MIC, ≥64 μg/ml) prior to LAI exposure without evidence of mutations subsequently developed mutations (16S) while having persistently high amikacin MIC values at Day 168.
Functional and quality-of-life assessment
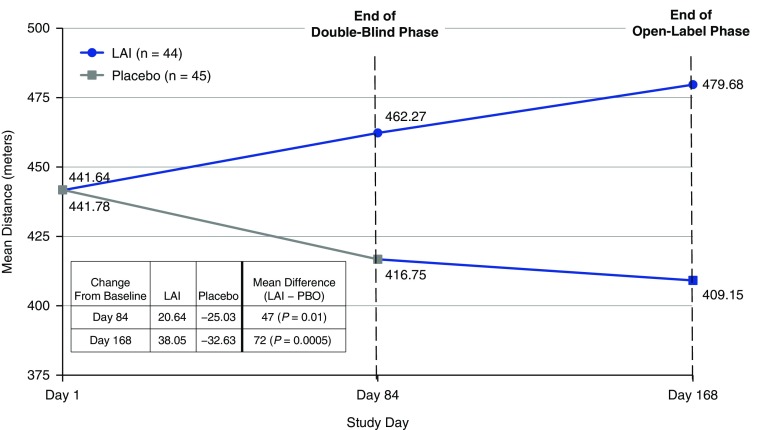
Baseline mean (SD) distance walked in the 6-minute-walk test was similar between groups: 441.6 (133.6) meters and 441.8 (111.6) meters for the LAI and placebo groups, respectively. At the end of the double-blind phase, the LAI group demonstrated improvement compared with the placebo group in mean (SD) distance walked: +20.6 (62.4) meters versus −25.0 (100.2) meters, respectively (P = 0.017) (Figure 4). Further, at the end of the open-label phase, patients in the LAI group (n = 35) continued to demonstrate improvement from baseline in mean distance walked in the 6-minute-walk test (+42.4 [105.9] meters), whereas patients in the prior placebo group who started LAI treatment during the open-label phase (n = 43) demonstrated a reduced rate of decline from baseline: −28.4 (88.1) meters, a treatment difference of 70.8 m (P = 0.012). A greater mean (SD) improvement in distance walked (Day 168) was noted in patients achieving culture conversion (regardless of study drug allocation) by Day 168 compared with those who did not achieve culture conversion (+34.6 [86.1] m vs. −7.1 [101.9] m, respectively; P = 0.073).
Figure 4.
Mean distance walked in the 6-minute-walk test (last observation carried forward; modified intention-to-treat population). LAI = liposomal amikacin for inhalation; PBO = placebo.
No significant difference (P = 0.2039) was observed for subjects without CF in mean change from baseline to Day 84 in quality of life, bronchiectasis, and NTM module scores between the LAI group (−7.935 [14.1998]; n = 36) and the placebo group (−2.829 [13.6733]; n = 36). Changes in the Cystic Fibrosis Questionnaire–Revised respiratory symptoms scale were assessed in patients with CF. The number of patients with CF was too small to determine any meaningful differences between the treatment groups during either double-blind (eight LAI, nine placebo) or open-label (six LAI, eight placebo) treatment.
Safety
Adverse events
The majority (∼90%) of patients in both groups experienced at least one treatment-emergent adverse event (Table 2). Most patients experienced mild or moderate treatment-emergent adverse events. During the double-blind phase, a greater percentage of patients treated with LAI than with placebo experienced dysphonia (43.2% vs. 8.9%), bronchiectasis exacerbation (38.6% vs. 20.0%), cough (31.8% vs.13.3%), oropharyngeal pain (20.5% vs. 2.2%), fatigue (15.9% vs. 8.9%), chest discomfort (11.4% vs. 0%), wheezing (9.1% vs. 2.2%), and infective pulmonary exacerbation of CF (9.1% vs. 2.2%) (see Figure E3). No clinically relevant changes were detected in laboratory values or vital signs.
Table 2.
Overview of Treatment-Emergent Adverse Events by Treatment Phase
| Double-Blind Phase* | Open-Label Phase† | ||||
|---|---|---|---|---|---|
| LAI (n = 44) | Placebo (n = 45) | LAI‡ (n = 35) | Placebo‡ (n = 43) | ||
| Patients with TEAEs, n (%) | 41 (93.2) | 40 (88.9) | 31 (88.6) | 42 (97.7) | |
| Number of TEAEs, n | 240 | 140 | 107 | 160 | |
| Patients with TEAEs by maximum severity, n (%) | |||||
| Grade 1: mild | 12 (27.3) | 25 (55.6) | 16 (45.7) | 10 (23.3) | |
| Grade 2: moderate | 24 (54.5) | 10 (22.2) | 10 (28.6) | 24 (55.8) | |
| Grade 3: severe | 4 (9.1) | 5 (11.1) | 4 (11.4) | 8 (18.6) | |
| Grade 4: life-threatening or disabling | 0 | 0 | 0 | 0 | |
| Grade 5: death§ | 1 (2.3) | 0 | 1 (2.9) | 0 | |
| Patients with TEAEs by seriousness, n (%) | |||||
| Serious | 8 (18.2) | 4 (8.9) | 5 (14.3) | 5 (11.6) | |
| Not serious | 33 (75.0) | 36 (80.0) | 26 (74.3) | 37 (86.0) | |
| Relationship to study drug, n (%) | |||||
| Related | 32 (72.7) | 17 (37.8) | 17 (48.6) | 26 (60.5) | |
| Not related | 9 (20.5) | 23 (51.1) | 14 (40.0) | 16 (37.2) | |
| Patients with audiovestibular TEAEs (e.g., tinnitus and dizziness), n (%) | 5 (11.4) | 5 (11.1) | 2 (5.7) | 2 (4.7) | |
| Patients with renal TEAEs, n (%) | 1 (2.3) | 0 | 1 (2.9) | 0 | |
| Patients with TEAEs leading to study drug discontinuation, n (%) | 7 (15.9) | 0 | 6 (17.1) | 12 (27.9) | |
Definition of abbreviations: LAI = liposomal amikacin for inhalation; TEAEs = treatment-emergent adverse events.
TEAEs during the double-blind phase are those with onset from the date of first dose of double-blind study drug.
TEAEs during the open-label phase are those with onset from the date of first dose of open-label study drug.
All patients in the open-label phase received LAI. “LAI” and “placebo” here refer to treatment assignment in the double-blind phase.
Deaths were caused by acute respiratory distress syndrome and urosepsis, and both TEAEs were unrelated to study drug.
Serious adverse events
In the double-blind phase, the overall incidence of serious adverse events was higher in the LAI group than in the placebo group (18.2% vs. 8.9%). The most frequently reported serious adverse events were bronchiectasis exacerbation (LAI, two patients; placebo, one patient) and pneumonia (LAI, one patient; placebo, two patients).
During the open-label phase, the incidence rates of serious adverse events were 14.3% and 11.6% in patients previously treated with LAI and placebo, respectively. The most frequently reported serious adverse events were CF exacerbation (prior LAI, two patients; prior placebo, three patients) and pneumonitis (one in each group).
One patient in the LAI group died during the double-blind phase as a result of pneumonia and acute respiratory distress syndrome, and one patient in the prior LAI group died during the open-label phase because of urosepsis, intestinal ischemia, and multiorgan failure (postsurgery); investigators considered neither death to be related to the study drug.
Adverse events leading to study drug discontinuation
In the double-blind phase, seven patients (15.9%) in the LAI group and none in the placebo group discontinued the study drug because of a treatment-emergent adverse event; two other patients in the LAI group discontinued the study drug for other reasons. The most common adverse events leading to study drug discontinuation were bronchiectasis exacerbation (three patients [6.8%]) and dyspnea (two patients [4.5%]). A single patient reported each of the other events: cough, oropharyngeal pain, respiratory disorder, and pneumonia. In the open-label phase, drug discontinuation because of a treatment-emergent adverse event occurred in fewer patients who received LAI during the double-blind phase than among those who received placebo (6 [17.1%] vs. 12 [27.9%]). Two patients discontinued the study drug for similar respiratory events while on LAI (respiratory disorder during the double-blind phase, pneumonitis during the open-label phase), ultimately considered to be allergic alveolitis. The mechanism is unclear and may represent a Herxheimer-like reaction (both patients had high SQS scores at baseline) or a direct effect of LAI.
Audiologic observations and audiovestibular events
In the double-blind phase, audiovestibular events occurred at a similar rate in both groups (∼11%), with tinnitus and dizziness being the most common events. Audiovestibular events occurred in two patients in each group (∼5%) in the open-label phase. At Day 168, a grade 1 change from baseline (based on Common Terminology Criteria for Adverse Events criteria) in audiology was observed for five patients (LAI, two patients; placebo, three patients), and a grade 2 change from baseline was recorded for one patient who had received prior placebo.
Renal events
One patient in the LAI group experienced mild (non–clinically meaningful) worsening of a previously abnormal glomerular filtration rate in the double-blind phase that was reversing within 28 days off LAI, and one patient in the LAI group experienced moderate nephrolithiasis for a single day during open-label treatment.
Lung function
Small, clinically insignificant increases in FEV1 percent predicted (0.32 ± 5.5% vs. 0.16 ± 6.0% in the LAI and placebo groups, respectively) were observed in both treatment groups at the end of the double-blind phase.
Discussion
In this multicenter clinical trial, we assessed the novel drug formulation LAI in patients who had persistently positive NTM cultures despite having received ATS/IDSA guidelines–based treatment (9) for at least 6 months before screening. Concern that this treatment-refractory, advanced-disease population, a high proportion of whom had cavitary disease and macrolide resistance (Table 1), would not achieve negative sputum cultures within the 3-month controlled phase led to selection of a reduction in semiquantitative growth as the primary endpoint. Although the primary endpoint did not reach statistical significance, owing in part to adjustments in SQS score for one death in the LAI group (removal of this adjustment yielded a significant difference: P = 0.04), an unanticipated greater proportion of patients treated with LAI relative to placebo achieved negative sputum cultures for NTM, and time to negative sputum culture conversion favored LAI treatment. In the overall analysis, because the primary endpoint was measurement of the change in SQS steps from baseline, an imbalance may have affected the outcome. The impact of such an effect is unknown because a greater improvement may be more achievable mathematically in patients who have higher SQS scores than those with lower SQS scores at baseline, owing to improvement from the lower end of the SQS being inherently more limited.
The significant change in SQS determined a priori was one step (e.g., going from 4+ growth on agar to 3+ growth on agar or from liquid media growth only to no growth or culture negative). In a treatment-naive population of patients with nodular bronchiectasis associated with M. avium complex, Griffith and coworkers (35) showed that a one-step change in SQS scores from baseline to Month 2 was significantly predictive of culture conversion by Month 12. (With each additional 1-point improvement [i.e., a lower value] in culture score from baseline to Month 2, the odds of a patient converting were seven times greater in both univariate and multivariate models [univariate odds ratio, 6.9; 95% CI, 2.9–16.6; P = 0.0001; multivariate odds ratio, 7.2; 95% CI, 2.3–22.0; P = 0.0006]). In the Griffith and colleagues study (35), improvement in SQS significantly correlated with improvement in symptom scores (especially for cough) and radiographic improvement.
Culture conversion, used in tuberculosis trials to predict lasting mycobacterial eradication, occurred in more patients receiving LAI treatment than in patients who received placebo at Day 84, and most had persistently negative cultures at 28 days and 1 year after discontinuation of LAI. Conversion occurred mainly in non-CF patients with M. avium complex infection. Microbiologic effectiveness appeared to correlate with the level of amikacin susceptibility. Culture conversion was seen only at MICs of 64 μg/ml or lower (the upper limit of amikacin concentration tested). Isolates with an MIC greater than this upper limit were more likely to carry amikacin resistance mutations. Patients with isolates having MICs greater than 64 μg/ml or the 16S rRNA gene mutation at position 1,408 do not appear to be candidates for the drug. Emergent mutational resistance occurred in 5.7% of patients exposed to LAI and was not observed with culture conversion on LAI treatment.
No clear correlation was noted between improvement in the microbiologic SQS and results for the St. George’s Respiratory Questionnaire, Quality of Life-Bronchiectasis NTM symptoms, or 6-minute walk distance. There may be several reasons for this. First of all, this primary endpoint as used had significant “penalties” assigned a priori for missing values, as described above. Imbalances in missing values such as the death that occurred in the active drug group may have masked an ability to see a correlation. Second, the worsening of airway-related symptoms associated with inhalation of LAI in some patients, particularly with initiation of LAI, may have masked eventual symptomatic improvement correlating with microbiologic response. Third, many of these patients had advanced underlying bronchiectasis, and many were coinfected with other pathogens. Overlap in symptom changes between microbiologic effect on NTM and the status of the underlying bronchiectasis or effects from other pathogens not in the antimicrobial spectrum of LAI may have affected correlations. In the study by Griffith and colleagues (35), improvement in SQS scores from a treatment-naive population with underlying nodular bronchiectasis receiving standard-of-care treatment did correlate with symptom improvement after 12 months. Last, the phenomenon of the change in scores is such that patients with lower quantities of organisms at baseline would have less change in score when becoming culture negative than would patients with higher quantities of organisms at baseline. Slight imbalances in baseline between the two groups for individuals who converted to negative may explain why a change in SQS score might not correlate as well as absolute culture conversion for significant improvement in functional status represented by the improvement in 6-minute walk test scores.
The 6-minute walk test was used to measure the functional capacity of converters versus nonconverters, regardless of treatment arm. Functional capacity improved with LAI treatment relative to placebo, and patients who had culture conversion experienced greater improvements in distance walked than those who did not convert, suggesting a correlation between culture conversion and improved functional capacity.
No between-group changes were noted in any measures of quality of life during the study. One reason for this may be attributable to the increased respiratory system–related adverse events associated with LAI because these quality-of-life scales are heavily weighted for respiratory symptoms. The majority of adverse events in the LAI group were respiratory in nature, and these respiratory events led to more discontinuations of the study drug. The frequency of respiratory symptoms declined with continued drug use in those patients who remained on the drug.
Aminoglycoside-associated risks of nephrotoxicity, ototoxicity, and vestibular toxicity were relatively low and comparable between groups. These results are consistent with those of prior retrospective reports demonstrating a lower prevalence of these risks with inhaled versus parenteral conventional amikacin (11, 36, 37).
Conclusions
Although the primary endpoint was not achieved, the results of this study suggest that, in patients with treatment-refractory PNTM disease, the addition of LAI to an ATS/IDSA guidelines–based multidrug regimen can achieve early and sustained negative sputum cultures. Further, culture conversion in response to treatment with LAI may be associated with improvements in functional capacity and, relative to treatment with parenteral amikacin, limited systemic toxicity. This study was limited in design, owing to balancing concerns about prolonged use of placebo in a sick population versus a relatively short 3-month period for comparative assessment of LAI efficacy. The findings suggest that addition of LAI may be an effective treatment option for patients with NTM lung disease that is refractory to available multidrug treatment regimens. Additional research is needed to confirm these findings.
Acknowledgments
Acknowledgment
The authors acknowledge the LAI NTM Study Group (i.e., principal investigators, co–principal investigators, and study coordinators who participated in the study); Chiltern International Ltd.; ICON Central Laboratories; PARI Pharma GmbH; Vitalograph Ltd.; Xerimis Inc.; The University of Texas Health Science Center at Tyler (Angela Smith, Jim Post, and Janice Hoeft); and patients who contributed their time and faith to participate in the study. The authors also acknowledge the medical writing assistance in the development of the manuscript provided by Kelly Park, Pharm.D., M.B.A.; Lawrence Nussbaum, M.D.; and Dayton Yuen, Pharm.D. (all from Insmed Incorporated); as well as Duke Duguay, Ph.D. (Connexion Healthcare, Newtown, PA). Editorial and design support was provided by Connexion Healthcare.
Footnotes
This study was supported by Insmed Incorporated, and in part (K.N.O.) by the intramural research programs of the National Institute of Allergy and Infectious Diseases (NIAID) (Cooperative Research and Development Agreement 2011-0473) and the NHLBI and the National Institutes of Health (NIH).
Author Contributions: The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors, were fully responsible for all content and editorial decisions, and were involved at all stages of manuscript development. Study conception and design: K.N.O., D.E.G., R.G., and R.J.W.; data collection: K.N.O., D.E.G., C.L.D., K.L.W., S.R., D.J.A.-H., P.A.F., D.D., M.S., B.A.B.-E., and R.J.W.; and data interpretation: K.N.O., G.E., J.P.M., L.M., K.L., R.G., and R.J.W.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201604-0700OC on October 17, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Winthrop KL, McNelley E, Kendall B, Marshall-Olson A, Morris C, Cassidy M, Saulson A, Hedberg K. Pulmonary nontuberculous mycobacterial disease prevalence and clinical features: an emerging public health disease. Am J Respir Crit Care Med. 2010;182:977–982. doi: 10.1164/rccm.201003-0503OC. [DOI] [PubMed] [Google Scholar]
- 2.O’Connell ML, Birkenkamp KE, Kleiner DE, Folio LR, Holland SM, Olivier KN. Lung manifestations in an autopsy-based series of pulmonary or disseminated nontuberculous mycobacterial disease. Chest. 2012;141:1203–1209. doi: 10.1378/chest.11-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Ingen J, Bendien SA, de Lange WC, Hoefsloot W, Dekhuijzen PN, Boeree MJ, van Soolingen D. Clinical relevance of non-tuberculous mycobacteria isolated in the Nijmegen-Arnhem region, The Netherlands. Thorax. 2009;64:502–506. doi: 10.1136/thx.2008.110957. [DOI] [PubMed] [Google Scholar]
- 4.Mirsaeidi M, Machado RF, Garcia JG, Schraufnagel DE. Nontuberculous mycobacterial disease mortality in the United States, 1999-2010: a population-based comparative study. PLoS One. 2014;9:e91879. doi: 10.1371/journal.pone.0091879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomson RM, Yew WW. When and how to treat pulmonary non-tuberculous mycobacterial diseases. Respirology. 2009;14:12–26. doi: 10.1111/j.1440-1843.2008.01408.x. [DOI] [PubMed] [Google Scholar]
- 6.Mehta M, Marras TK. Impaired health-related quality of life in pulmonary nontuberculous mycobacterial disease. Respir Med. 2011;105:1718–1725. doi: 10.1016/j.rmed.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Adjemian J, Olivier KN, Seitz AE, Holland SM, Prevots DR. Prevalence of nontuberculous mycobacterial lung disease in U.S. Medicare beneficiaries. Am J Respir Crit Care Med. 2012;185:881–886. doi: 10.1164/rccm.201111-2016OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marras TK, Mendelson D, Marchand-Austin A, May K, Jamieson FB. Pulmonary nontuberculous mycobacterial disease, Ontario, Canada, 1998-2010. Emerg Infect Dis. 2013;19:1889–1891. doi: 10.3201/eid1911.130737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, et al. ATS Mycobacterial Diseases Subcommittee American Thoracic Society Infectious Disease Society of America An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases Am J Respir Crit Care Med 2007175367–416.[Published erratum appears in Am J Respir Crit Care Med 2007;175:744–745.] [DOI] [PubMed] [Google Scholar]
- 10.Griffith DE, Aksamit TR. Therapy of refractory nontuberculous mycobacterial lung disease. Curr Opin Infect Dis. 2012;25:218–227. doi: 10.1097/QCO.0b013e3283511a64. [DOI] [PubMed] [Google Scholar]
- 11.Peloquin CA, Berning SE, Nitta AT, Simone PM, Goble M, Huitt GA, Iseman MD, Cook JL, Curran-Everett D. Aminoglycoside toxicity: daily versus thrice-weekly dosing for treatment of mycobacterial diseases. Clin Infect Dis. 2004;38:1538–1544. doi: 10.1086/420742. [DOI] [PubMed] [Google Scholar]
- 12.Rose SJ, Neville ME, Gupta R, Bermudez LE. Delivery of aerosolized liposomal amikacin as a novel approach for the treatment of nontuberculous mycobacteria in an experimental model of pulmonary infection. PLoS One. 2014;9:e108703. doi: 10.1371/journal.pone.0108703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meers P, Neville M, Malinin V, Scotto AW, Sardaryan G, Kurumunda R, Mackinson C, James G, Fisher S, Perkins WR. Biofilm penetration, triggered release and in vivo activity of inhaled liposomal amikacin in chronic Pseudomonas aeruginosa lung infections. J Antimicrob Chemother. 2008;61:859–868. doi: 10.1093/jac/dkn059. [DOI] [PubMed] [Google Scholar]
- 14.Okusanya OO, Bhavnani SM, Hammel J, Minic P, Dupont LJ, Forrest A, Mulder GJ, Mackinson C, Ambrose PG, Gupta R. Pharmacokinetic and pharmacodynamic evaluation of liposomal amikacin for inhalation in cystic fibrosis patients with chronic pseudomonal infection. Antimicrob Agents Chemother. 2009;53:3847–3854. doi: 10.1128/AAC.00872-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Contreras AM, Gamba G, Cortés J, Santiago Y, Nares F, Jimenez-Sanchez G, Bobadilla J, López G, Valadez A, Espinosa A, et al. Serial trough and peak amikacin levels in plasma as predictors of nephrotoxicity. Antimicrob Agents Chemother. 1989;33:973–976. doi: 10.1128/aac.33.6.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daglian D, Lau S, Eagle G, McGinnis J, Micioni L, Addrizzo-Harris D. Case report of a patient with treatment-refractory nontuberculous mycobacteria (NTM) lung infection treated with once daily (QD) liposomal amikacin for inhalation (LAI) [abstract] Chest. 2015;148(4 Suppl):162A. [Google Scholar]
- 17.Olivier KN, Griffith DE, Winthrop KL, Brown-Elliott BA, Eagle G, McGinnis J, Wallace RJ. 12-Month follow-up data from a phase 2 trial of liposomal amikacin for inhalation (LAI) in patients with refractory nontuberculous mycobacterial (NTM) lung infection [abstract] Am J Respir Crit Care Med. 2016;193:A3722. [Google Scholar]
- 18.Olivier KN, Maas-Moreno R, Whatley M, Cheng K, Lee J, Fiorentino C, Shaffer R, Macdonald S, Gupta R, Corcoran TE, et al. Airway deposition and retention of liposomal amikacin for inhalation in patients with pulmonary nontuberculous mycobacterial disease [abstract] Am J Respir Crit Care Med. 2016;193:A3732. [Google Scholar]
- 19.Rubino CM, Olivier KN, Griffith DE, Eagle G, McGinnis JP, II, Micioni L, Winthrop KL. Pharmacokinetic (PK) evaluation of liposomal amikacin for inhalation (LAI) in patients with treatment-refractory nontuberculous mycobacteria (NTM) lung infection. Presented at the 55th Interscience Conference on Antimicrobial Agents and Chemotherapy/28th International Congress of Chemotherapy Meeting (ICAAC/ICC 2015). September 17–21, 2015, San Diego, CA, Poster A-012. [Google Scholar]
- 20.Olivier KN, Eagle G, McGinnis JP, II, Micioni L, Brown-Elliott BA, Wallace RJ., Jr Amikacin (AMK) minimum inhibitory concentrations (MICs) and mutational resistance in patients with treatment-refractory nontuberculous mycobacteria (NTM) lung disease (LD) treated with liposomal amikacin for inhalation (LAI) [abstract] Eur Respir J. 2015;46(Suppl 59):PA371. [Google Scholar]
- 21.Winthrop KL, Eagle G, McGinnis JP, Micioni L, Daley CL, Ruoss SJ, Addrizzo-Harris DJ, Flume PA, Dorgan DJ, Salathe M, et al. Subgroup analyses of baseline demographics and efficacy in patients with refractory nontuberculous mycobacteria (NTM) lung infection treated with liposomal amikacin for inhalation (LAI) [abstract] Am J Respir Crit Care Med. 2015;191:A6294. [Google Scholar]
- 22.Biller JA, Eagle G, McGinnis JP, Micioni L, Daley CL, Winthrop KL, Ruoss SJ, Addrizzo-Harris DJ, Flume PA, Dorgan DJ, et al. Efficacy of liposomal amikacin for inhalation (LAI) in achieving nontuberculous mycobacteria (NTM) culture negativity in patients whose lung infection is refractory to guideline-based therapy [abstract] Am J Respir Crit Care Med. 2015;191:A6295. [Google Scholar]
- 23.Ruoss SJ, Eagle G, McGinnis JP, Micioni L, Daley CL, Winthrop KL, Addrizzo-Harris DJ, Flume PA, Dorgan DJ, Salathe M, et al. Analysis of functional exercise capacity (via the six-minute walk test [6MWT]) and culture negativity in patients with nontuberculous mycobacteria (NTM) lung infection refractory to guideline-based therapy treated with liposomal amikacin for inhalation (LAI) [abstract] Am J Respir Crit Care Med. 2015;191:A6296. [Google Scholar]
- 24.Olivier KN, Eagle G, McGinnis JP, Micioni L, Daley CL, Winthrop KL, Ruoss S, Addrizzo-Harris DJ, Flume P, Dorgan D, et al. Randomized, double-blind (DB), placebo-controlled study and open-label (OL) extension of liposomal amikacin for inhalation (LAI) in patients with refractory nontuberculous mycobacterial (NTM) lung disease (LD) [abstract WS02.3] J Cyst Fibros. 2015;14(Suppl 1):S3. [Google Scholar]
- 25.Olivier KN, Eagle G, McGinnis JP, II, Micioni L, Daley CL, Winthrop KL, Ruoss S, Addrizzo-Harris DJ, Flume P, Dorgan D, et al. Randomized, double-blind, placebo-controlled, open-label study of liposomal amikacin for inhalation (LAI) in patients with recalcitrant nontuberculous mycobacterial lung disease (NTM-LD). Presented at the 28th Annual North American Cystic Fibrosis Conference (NACFC). October 9–11, 2014, Atlanta, GA. [Google Scholar]
- 26.Wallace RJ, Jr, Brown BA, Griffith DE, Girard WM, Murphy DT, Onyi GO, Steingrube VA, Mazurek GH. Initial clarithromycin monotherapy for Mycobacterium avium-intracellulare complex lung disease. Am J Respir Crit Care Med. 1994;149:1335–1341. doi: 10.1164/ajrccm.149.5.8173775. [DOI] [PubMed] [Google Scholar]
- 27.Wallace RJ, Jr, Brown BA, Griffith DE, Girard WM, Murphy DT. Clarithromycin regimens for pulmonary Mycobacterium avium complex: the first 50 patients. Am J Respir Crit Care Med. 1996;153:1766–1772. doi: 10.1164/ajrccm.153.6.8665032. [DOI] [PubMed] [Google Scholar]
- 28.Devallois A, Goh KS, Rastogi N. Rapid identification of mycobacteria to species level by PCR-restriction fragment length polymorphism analysis of the hsp65 gene and proposition of an algorithm to differentiate 34 mycobacterial species. J Clin Microbiol. 1997;35:2969–2973. doi: 10.1128/jcm.35.11.2969-2973.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hall L, Doerr KA, Wohlfiel SL, Roberts GD. Evaluation of the MicroSeq system for identification of mycobacteria by 16S ribosomal DNA sequencing and its integration into a routine clinical mycobacteriology laboratory. J Clin Microbiol. 2003;41:1447–1453. doi: 10.1128/JCM.41.4.1447-1453.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iakhiaeva E, Howard ST, Brown Elliott BA, McNulty S, Newman KL, Falkinham JO, III, Williams M, Kwait R, Lande L, Vasireddy R, et al. Variable-number tandem-repeat analysis of respiratory and household water biofilm isolates of “Mycobacterium avium subsp. hominissuis” with establishment of a PCR database. J Clin Microbiol. 2016;54:891–901. doi: 10.1128/JCM.02409-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iakhiaeva E, McNulty S, Brown Elliott BA, Falkinham JO, III, Williams MD, Vasireddy R, Wilson RW, Turenne C, Wallace RJ., Jr Mycobacterial interspersed repetitive-unit–variable-number tandem-repeat (MIRU-VNTR) genotyping of Mycobacterium intracellulare for strain comparison with establishment of a PCR-based database. J Clin Microbiol. 2013;51:409–416. doi: 10.1128/JCM.02443-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown-Elliott BA, Iakhiaeva E, Griffith DE, Woods GL, Stout JE, Wolfe CR, Turenne CY, Wallace RJ., JrIn vitro activity of amikacin against isolates of Mycobacterium avium complex with proposed MIC breakpoints and finding of a 16S rRNA gene mutation in treated isolates J Clin Microbiol 2013513389–3394.[Published erratum appears in J Clin Microbiol. 2014;52:1311.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute-walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 34.Seshadri P, Climaco AB. Amikacin level. [updated 2014 Feb 7; accessed 2016 Aug 15]. Available from: http://emedicine.medscape.com/article/2089686-overview.
- 35.Griffith DE, Adjemian J, Brown-Elliott BA, Philley JV, Prevots DR, Gaston C, Olivier KN, Wallace RJ., Jr Semiquantitative culture analysis during therapy for Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2015;192:754–760. doi: 10.1164/rccm.201503-0444OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clancy JP, Dupont L, Konstan MW, Billings J, Fustik S, Goss CH, Lymp J, Minic P, Quittner AL, Rubenstein RC, et al. Arikace Study Group. Phase II studies of nebulised Arikace in CF patients with Pseudomonas aeruginosa infection. Thorax. 2013;68:818–825. doi: 10.1136/thoraxjnl-2012-202230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olivier KN, Shaw PA, Glaser TS, Bhattacharyya D, Fleshner M, Brewer CC, Zalewski CK, Folio LR, Siegelman JR, Shallom S, et al. Inhaled amikacin for treatment of refractory pulmonary nontuberculous mycobacterial disease. Ann Am Thorac Soc. 2014;11:30–35. doi: 10.1513/AnnalsATS.201307-231OC. [DOI] [PMC free article] [PubMed] [Google Scholar]