To the Editor:
Hyperammonemia is a lethal syndrome in lung recipients characterized by progressive elevation in serum ammonia leading to mental status changes, cerebral edema, and death (1–3). It was previously speculated to result from an inborn error of urea metabolism, unmasked by calcineurin inhibition (3, 4). We recently demonstrated a strong association between Ureaplasma infection and hyperammonemia syndrome and reported that antimicrobial therapy targeting this organism reverses the clinical syndrome (5). Nevertheless, it remains unclear whether the pathogenic form of this organism is of donor or recipient origin. It also remains unknown whether Ureaplasma can lead to complications other than fatal hyperammonemia.
We prospectively evaluated consecutive lung recipients (n = 29) and donors (n = 28) at Northwestern Memorial Hospital from the inauguration of the lung transplant program in July 2014 until May 2016. The study was approved by the Institutional Review Board. Pretransplant urine and bronchoalveolar lavage fluid (BALF) from recipients and BALF from donors were tested for Mollicutes, as previously described using culture and polymerase chain reaction (5). The testing laboratory was blinded to the clinical status of the patients. One (3%) recipient was positive for Ureaplasma urealyticum in the urine before transplantation and was treated using levofloxacin and azithromycin for 2 weeks. Native lung BALF from all recipients was negative, whereas BALF from four donors (14%) was positive for Mollicutes (Ureaplasma alone = 3; Ureaplasma and Mycoplasma hominis = 1). Donors with Mollicutes were younger, at 23.3 versus 38.3 years (P <0.001); were predominantly male (P = 0.07); all were sexually active with multiple sexual partners (P = 0.1); and all had a documented aspiration event before declaration of brain death (P = 0.001; Table 1).
Table 1.
Donor Variables and Associated Recipient Complications
| Ureaplasma Positive (N = 4) | Ureaplasma Negative (N = 24) | All*(N = 28) | P Value | |
|---|---|---|---|---|
| Donor variables | ||||
| Age, yr, mean ± SD | 23.3 ± 3.2 | 38.3 ± 10.7 | 36.2 ± 8.2 | <0.001 |
| Sex, n (%) | 0.07 | |||
| Male | 3 (75) | 14 (58) | 17 (61) | |
| Female | 1 (25) | 10 (42) | 11 (39) | |
| Race, n (%) | 0.8 | |||
| White | 2 (50) | 12 (50) | 14 (50) | |
| Black | 1 (25) | 8 (33) | 9 (32) | |
| Hispanic | 1 (25) | 3 (13) | 4 (14) | |
| Asian | — | 1 (4) | 1 (4) | |
| Sexually active, n (%) | 4 (100) | 12 (50) | 16 (57) | 0.1 |
| Cause of death, n (%) | 0.4 | |||
| Traumatic brain injury | 3 (75) | 14 (58) | 17 (61) | |
| Stroke/intracranial hemorrhage | 1 (25) | 10 (42) | 11 (39) | |
| Aspiration, n (%)† | 4 (100) | 3 (13) | 7 (25) | 0.001 |
| Recipient complications, n (%) | ||||
| Hyperammonemia‡ | 4 (100) | 0 | 4 (14) | <0.001 |
| Primary graft dysfunction, grade 3 | 1 (25) | 1 (4) | 2 (7) | 0.3 |
| Acute renal failure | 3 (75) | 5 (20) | 9 (31) | 0.5 |
| Bronchial dehiscence | 2 (50) | 0 (0) | 2 (7) | 0.02 |
| Acute rejection | 1 (25) | 1 (4) | 2 (7) | 0.2 |
| Mental status changes | 4 (100) | 1 (4) | 2 (7) | 0.2 |
| 60-d mortality | 1 (25) | 1 (4) | 2 (7) | 0.3 |
One donor was used for two recipients of single-lung transplant; therefore, the number of donors is one less than the total recipients.
Aspiration was diagnosed during bronchoscopy by the Organ Procurement Organization at the time of initial donor evaluation and documented into the donor records before acceptance of the organs by the recipient centers.
Hyperammonemia was diagnosed if two consecutive levels of serum ammonia were elevated.
Recipients received induction immunosuppression (methylprednisolone [500 mg], basiliximab [20 mg]), after which they were maintained on tacrolimus (target trough, 8–12 ng/ml), mycophenolate mofetil (1,000 mg twice daily), and prednisone (0.5 mg/kg daily). In addition, patients received empiric vancomycin and cefepime until intraoperative bronchial cultures were finalized. All recipients of Ureaplasma-positive donor lungs developed lung infiltrates and demonstrated systemic inflammatory response syndrome requiring vasopressors to maintain mean arterial pressures equal to or greater than 60 mm Hg on postoperative Day 1. In addition, Ureaplasma-positive, but not Ureaplasma-negative, donor lungs were associated with ammonia elevation in the first 3 days after transplantation, despite vancomycin and cefepime therapy, neither of which are active against Mollicutes because of their lack of cell walls. Recipients of allografts from Ureaplasma-positive donors demonstrated a trend toward greater incidence of grade 3 primary graft dysfunction, acute renal failure, acute rejection, and 60-day mortality (Table 1). In addition, two (50%) of these recipients developed bronchial dehiscence (P = 0.02).
The median time to obtaining Mollicutes test results was 5 days since it was sent out to the Diagnostic Mycoplasma Laboratory at the University of Alabama at Birmingham. Antimicrobial therapy was subsequently initiated in all recipients of Ureaplasma-positive donor lungs. The previously reported index patient developed recurrence of hyperammonemia during azithromycin monotherapy associated with a Ureaplasma strain harboring a A2058G mutation in the 23S ribosomal RNA gene operon 2, causing macrolide resistance (5). Therefore, subsequent patients were treated with a combination of macrolide and fluoroquinolone (Figure 1A). All recipients of Ureaplasma-positive lungs demonstrated improvement in serum ammonia and systemic inflammatory response syndrome within 24 hours of initiation of targeted antimicrobial therapy with complete recovery within 7 days. After 14 days of antimicrobial therapy the Ureaplasma cultures became negative, although polymerase chain reaction remained positive in two patients for up to 4 weeks. None of the treated patients developed relapse of hyperammonemia at a median follow-up of 8 months.
Figure 1.
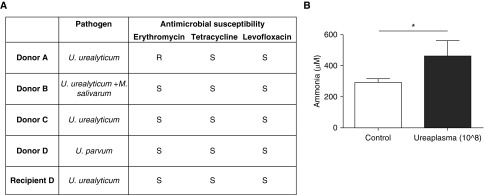
Ureaplasma infection and hyperammonemia. (A) Antimicrobial susceptibilities of Mollicutes-positive donor lung bronchoalveolar lavage fluid cultures and recipient D pretransplant urine. (B) The Ureaplasma isolates were inoculated (count 108) into immunocompetent mice, which resulted in hyperammonemia. n = 5; *P < 0.05. M. salivarum = Mycoplasma salivarum; R = resistant; S = susceptible; U. parvum = Ureaplasma parvum; U. urealyticum = Ureaplasma urealyticum.
Coincidentally, the sole recipient with Ureaplasma in the urine pretransplantation received Ureaplasma-positive donor lungs. Her native lung BALF and urine at the time of transplant were free of Mollicutes, and the organism identified in her BALF post-transplant was Ureaplasma parvum, the same as detected in the donor BALF. Further, pretransplant urine had U. urealyticum. These findings suggest that the hyperammonemia, and possibly systemic inflammatory response syndrome, were attributable to donor-derived organisms. To further establish the causality, we injected donor Ureaplasma isolates into immunocompetent C57Bl/6 mice intravenously, which resulted in hyperammonemia at 8 hours (Figure 1B). Hyperammonemia was prevented by treatment with antimicrobial therapy. These results fulfill Koch’s third postulate, causally linking Ureaplasma with the defining feature of hyperammonemia syndrome.
U. urealyticum and U. parvum are underrecognized human pathogens that lack cell walls and do not grow in routine culture media (6, 7). Ureaplasma species are commensals of the urogenital tract and are found in up to 40–80% of sexually active men and women (8). Pneumonias are common in patients with end-stage lung disease and are usually treated with fluoroquinolones and macrolides. Therefore, the low incidence in pretransplant urine was likely related to antimicrobial therapy in this cohort. Intriguingly, Ureaplasma species are not part of the lung microbiome (9). As all four of the Ureaplasma-positive donors in our series had a documented aspiration event, we speculate that Ureaplasma species colonizing the oral cavity, perhaps from sexual contact with their partners, were introduced into the airway during aspiration. Urinary testing for Ureaplasma was negative in two kidney recipients of the Ureaplasma-positive donors immediately after transplantation. It therefore seems unlikely that Ureaplasma from the donor’s urogenital tract gained access to the lung. However, this needs to be validated in further prospective studies.
Since the identification of Ureaplasma species as pathogens for hyperammonemia, we have initiated a protocol to screen all donors and recipients and treat microbiologically proven infections. Given that macrolides are bacteriostatic for Ureaplasma species and that monotherapy can lead to resistance, we use a combination of a macrolide with a quinolone or doxycycline. Despite that, we noted an increase in morbidity and mortality in recipients of Ureaplasma-positive donor lungs. Because hyperammonemia develops rapidly, the prevalence of Ureaplasma was high in donors (14%), and the historical mortality of hyperammonemia approaches 100%, we suggest that trials of universal prophylaxis pending negative cultures and pathogen-directed therapy against Ureaplasma infection are warranted. Azithromycin was previously shown to reduce the incidence of chronic lung allograft rejection (10). After this, some centers have administered azithromycin routinely immediately after transplantation, and this has indirectly resulted in prevention of hyperammonemia (personal communications with Marie Budev, Cleveland Clinic Foundation, Cleveland, OH, and Rajat Walia, Norton Thoracic Institute, Phoenix, AZ).
In summary, we found that Ureaplasma infection in donor lungs was associated with increased morbidity after lung transplantation. We isolated Ureaplasma species from humans in pure culture and showed that these organisms are sufficient to increase serum ammonia levels when injected into normal mice but not in those treated with antimicrobials. The relatively high prevalence of these organisms in donor lungs and associated mortality warrant the need for future studies to investigate the optimal strategies for Ureaplasma treatment and prophylaxis.
Acknowledgments
Acknowledgment
The authors thank Elena Susan for administrative assistance in formatting and submitting the manuscript.
Footnotes
A.B. is supported by National Institutes of Health grant NIH-HL125940, with matching funds from the Thoracic Surgery Foundation, and the American Lung Association. G.R.S.B. is supported by National Institutes of Health grants NIA-AG049665 and NHLBI-HL071643 and the Veterans Administration.
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Chen C, Bain KB, Iuppa JA, Yusen RD, Byers DE, Patterson GA, Trulock EP, Hachem RR, Witt CA. Hyperammonemia syndrome after lung transplantation: a single center experience. Transplantation. 2016;100:678–684. doi: 10.1097/TP.0000000000000868. [DOI] [PubMed] [Google Scholar]
- 2.Lichtenstein GR, Yang YX, Nunes FA, Lewis JD, Tuchman M, Tino G, Kaiser LR, Palevsky HI, Kotloff RM, Furth EE, et al. Fatal hyperammonemia after orthotopic lung transplantation. Ann Intern Med. 2000;132:283–287. doi: 10.7326/0003-4819-132-4-200002150-00006. [DOI] [PubMed] [Google Scholar]
- 3.Lichtenstein GR, Kaiser LR, Tuchman M, Palevsky HI, Kotloff RM, O’Brien CB, Furth EE, Raps EC, Berry GT. Fatal hyperammonemia following orthotopic lung transplantation. Gastroenterology. 1997;112:236–240. doi: 10.1016/s0016-5085(97)70240-3. [DOI] [PubMed] [Google Scholar]
- 4.Moffatt-Bruce SD, Pesavento T, Von Viger J, Nunley D, Pope-Harman A, Martin S, Ross P. Successful management of immunosuppression in a patient with severe hyperammonemia after lung transplantation. J Heart Lung Transplant. 2008;27:801–803. doi: 10.1016/j.healun.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 5.Bharat A, Cunningham SA, Scott Budinger GR, Kreisel D, DeWet CJ, Gelman AE, Waites K, Crabb D, Xiao L, Bhorade S, et al. Disseminated Ureaplasma infection as a cause of fatal hyperammonemia in humans. Sci Transl Med. 2015;7:284re3. doi: 10.1126/scitranslmed.aaa8419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shepard MC, Lunceford CD. Occurrence of urease in T strains of Mycoplasma. J Bacteriol. 1967;93:1513–1520. doi: 10.1128/jb.93.5.1513-1520.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glass JI, Lefkowitz EJ, Glass JS, Heiner CR, Chen EY, Cassell GH. The complete sequence of the mucosal pathogen Ureaplasma urealyticum. Nature. 2000;407:757–762. doi: 10.1038/35037619. [DOI] [PubMed] [Google Scholar]
- 8.Waites KB, Taylor-Robinson D. Manual of Clinical Microbiology. Washington, DC: ASM Press; 2015. Mycoplasma and Ureaplasma; pp. 1088–1105. [Google Scholar]
- 9.Cui L, Morris A, Huang L, Beck JM, Twigg HL, III, von Mutius E, Ghedin E. The microbiome and the lung. Ann Am Thorac Soc. 2014;11:S227–S232. doi: 10.1513/AnnalsATS.201402-052PL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerhardt SG, McDyer JF, Girgis RE, Conte JV, Yang SC, Orens JB. Maintenance azithromycin therapy for bronchiolitis obliterans syndrome: results of a pilot study. Am J Respir Crit Care Med. 2003;168:121–125. doi: 10.1164/rccm.200212-1424BC. [DOI] [PubMed] [Google Scholar]