Abstract
Background
Improvement of postural control in persons with multiple sclerosis (PwMS) is an important target for neurorehabilitation. Although PwMS are able to improve postural performance with training, the neural underpinnings of these improvements are poorly understood.
Objective
To understand the neural underpinnings of postural motor learning in PwMS.
Methods
Supraspinal white matter structural connectivity in PwMS was correlated with improvements in postural performance (balancing on an oscillating surface over 25 trials) and retention of improvements (24 hours later).
Results
Improvement in postural performance was directly correlated to microstructural integrity of white matter tracts, measured as radial diffusivity, in the corpus callosum, posterior parieto-sensorimotor fibers and the brainstem in PwMS. Within the corpus callosum, the genu and midbody (fibers connecting the prefrontal and primary motor cortices, respectively) were most strongly correlated to improvements in postural control. Twenty-four hour retention was not correlated to radial diffusivity.
Conclusion
PwMS who exhibited poorer white matter tract integrity connecting the cortical hemispheres via the corpus callosum showed the most difficulty learning to control balance on an unstable surface. Prediction of improvements in postural control through training (i.e. motor learning) via structural imaging of the brain may allow for identification of individuals who are particularly well-suited for postural rehabilitation interventions.
Keywords: multiple sclerosis, motor learning, balance, posture, diffusion tensor imaging, white matter
INTRODUCTION
Postural dyscontrol is a major source of disability in persons with multiple sclerosis (PwMS), leading to reduced walking speed, falls, and reduced quality of life [1-3]. Given the well-established relationship between postural control and falls [2, 4], improving balance is an important target for neurorehabilitation.
Recent studies demonstrate that PwMS can improve upper extremity (UE) motor performance through practice [5-7], although these improvements may be less pronounced compared to controls [8, 9]. Considerably less is known about the ability of PwMS to improve postural motor control. In fact, to our knowledge only two studies have directly investigated postural motor learning in PwMS. Hatzitaki et al. showed that PwMS were able to learn a postural visuo-motor task in which participants voluntarily leaned to the left and right. However, these improvements were less pronounced than in healthy adults [10].
Similarly, we recently showed that PwMS were able to improve postural responses through repeated exposure to continuous support surface translations [11]. PwMS maintained balance while the support surface continually slid forward and backward at a fixed frequency (see [11, 12] for details). We measured temporal performance, i.e. ability to anticipate changes in direction, and spatial performance, i.e. the ability to control the amplitude of sway, with repeated exposures to this moving support surface. Despite poorer performance than control subjects, PwMS exhibited improvements in temporal performance (over one day of practice) and retention (ability to maintain improvements 24 hours later) in a manner similar to the control group. Conversely, spatial performance improved to a lesser degree in PwMS than in control subjects and was not retained on the following day. We hypothesized that given the temporal consistency of platform movements, participants were able to improve temporal performance via a feedforward control strategy. However, given the relative inconsistency in amplitude of the forward and backward movements, participants were forced to rely on non-specific learning, or improvements based on proprioceptive feedback control mechanisms to improve spatial performance [11]. Therefore, PwMS seemed to exhibit a specific deficit in non-specific learning, but not feedforward learning, compared to people without MS.
The ability of PwMS to acquire and retain improvements in postural performance is promising for balance neurorehabilitation. However, the ability to learn varies widely in PwMS and the neural underpinnings of these motor learning processes are unknown. Previous UE research suggests that numerous structures, including the basal ganglia, cerebellum, and cortex play a role in motor learning. Not surprisingly, the white matter tracts connecting these structures also play an important role in this process [8, 13-15]. White matter tracts such as the corpus callosum (CC) are of particular interest as they are often disrupted in PwMS [16-18]. Further, PwMS exhibit considerable heterogeneity in the degree of degeneration of white matter tracts, which may contribute to the variability in learning ability in this population. Indeed, Bonzano et al. [2011] demonstrated that UE sequence learning was less pronounced in PwMS, and the degree of learning was directly related to structural connectivity of the CC, such that people with more pronounced worsening of CC structural integrity exhibited poorer learning [8]. In a follow-up study, Bonzano et al. [2014] demonstrated that active UE motor rehabilitation resulted in retention of CC fibers and function, further underscoring the importance of the CC in motor learning and neurorehabilitation [19].
Based on these previous reports, it is possible that white matter degradation (particularly within the CC) impacts motor learning in PwMS. However, research relating white matter disruption to motor learning in PwMS has been limited to UE learning tasks (e.g.[8]); thus it is unknown whether these findings generalize to postural control. By investigating whether changes in the integrity of white matter predict deficits in postural learning, we may be able to identify PwMS most suitable for postural training [20], thus improving the utilization of therapeutic resources.
To understand the neural underpinnings of postural motor learning in PwMS, we correlated the acquisition and retention of practice-related improvements of postural control to whole-brain structural connectivity using a tract-based spatial statistical (TBSS) approach. Given previous findings in UE literature [8], we hypothesized that white matter connectivity of the CC, an area which is both important for motor learning [13], and altered in PwMS [16], would be related to improvement in postural control performance and retention of these improvements within PwMS.
METHODS
Participants
We present the data for twenty-nine PwMS and fifteen age- and gender-matched healthy adults (HC) used in our previous report [11]. Inclusion criteria for all participants were: ability to walk 500m without assistance, ability to maintain balance independently by standing on toes for 3s, and no known biomechanical conditions affecting balance. Exclusion criteria for PwMS and HC were: co-existing conditions that can mimic MS (e.g. lupus or fibromyalgia), or additional conditions that may affect gait or balance (e.g. arthritis, joint replacement).
Behavioral protocol
Behavioral testing procedures have been described previously [11]. Briefly, participants underwent one day of balance training and one day of balance testing for retention. Participants stood on a hydraulically controlled platform that oscillated at a fixed, sinusoidal frequency (0.5Hz) in the forward and backward directions (Figure 1). Participants were asked to maintain balance while keeping arms crossed across the chest to minimize contribution of the arms and looking straight ahead. Trials were 48s long and the same sequence was repeated for each trial. On day 1, participants were trained with 5 blocks of 5 trials. On day 2 (24 hours later), participants completed 2 blocks of 5 trials to measure retention. As such, we were able to assess improvements in postural performance across day 1, as well as 24-hour retention of these improvements.
Figure 1.
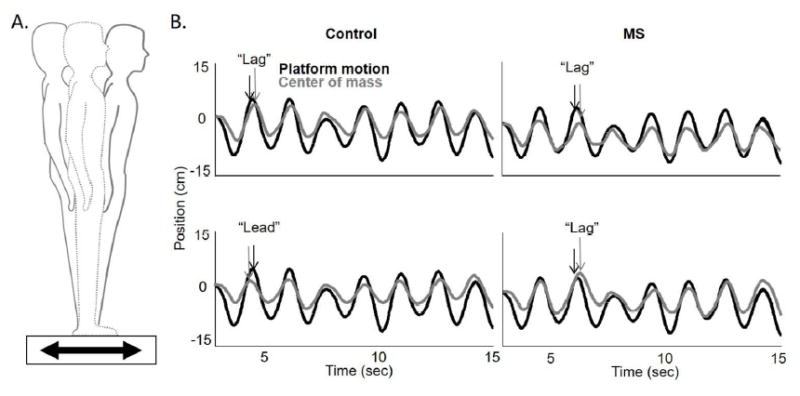
A) Schematic of an individual while on the movable force plate. Forward motion (black) of the force plate results in backward sway, while backward motion of the plate (grey) results in forward sway. B) Illustration of the position of the platform (black) and the center of mass (gray) for a control (left) and multiple sclerosis (MS; right) patient as it moved forward and backward during a trial. Temporal performance is noted as the difference in phase of the platform and center of mass. A “lag” in phase represents poorer performance, and “lead” represents improved performance. The top row shows the mean relative phase in the early training block, and the bottom row shows the late training block. With training, the phase “lag” relationship between the platform motion and center of mass changes to phase “lead” for the control participant but not so for the MS patient. Figure adapted from Gera G, et al. 2015; NNR; Dec. 23; pii:1545968315619700; DOI: 10.1177/1545968315619700.
Behavioral data analysis
Temporal and spatial control of body CoM over the moving surface was used to measure postural performance. Whole body COM was calculated from reflective markers placed on bony landmarks and measurement of body anthrophomorpic data [21]. Motion analysis was sampled at 60Hz and low-pass filtered using a second-order, dual-pass Butterworth filter (5Hz). COM position was tracked in the anteroposterior directions, and temporal and spatial COM outcome measures were calculated. The temporal CoM measure was the mean relative phase of body COM position relative to the platform position at the instant of maximum platform displacement (peaks and valleys of sinusoidal platform movement). Negative values represent the COM “lagging” behind the platform, while positive values reflect the COM “leading” the platform in a predictive manner. Greater negative values represent worse performance. Thus, a phase lag moving toward zero (becoming less negative) represents an improved ability to predict platform perturbations, consistent with feedforward postural control [11]. Spatial CoM performance was calculated as the position (relative to the starting position) of the COM at each peak and valley of the sinusoidal plate movement. COM displacements were normalized to the platform displacement. Thus, a mean gain of 1 would represent equal displacements of the platform and COM and would occur if participants’ COM followed platform motion exactly. Reduction of mean gain (towards zero), represents improved balance control with the body COM displacing less than the support surface and feet. Values at each peak and valley were averaged across each trial.
Improvement in temporal (phase lag) and spatial (mean gain) performance were calculated as the change in performance between block 1 and block 5 on Day 1. Retention of improvement was calculated as the change in performance between block 1 (Day 1) and block 1 (Day 2). Some previous investigations have shown retention as a lack of change between performance at the end of training and retention (i.e. follow-up) periods [22]. We opted to calculate retention as the difference in performance between the beginning of training and the retention period [23-25]. While both methods provide information regarding the retention of improved performance, we chose the latter option because it may provide more information about the degree to which improvements are persist over the baseline value [26]. Improvements in postural motor learning and retention of improvements across subjects were correlated to whole brain structural connectivity via white matter tracks (see below). In addition, average temporal and spatial performance were calculated as the mean performance over all five blocks of practice on Day 1. Group differences in average temporal and spatial performance were compared via independent sample t-tests, and were also related to structural connectivity (see below).
Imaging protocol
On a separate day, less than two weeks following behavioral testing, participants were scanned on a 3.0T Siemens Magentom Tim Trio scanner with a 12-channel head coil at OHSU’s Advanced Imaging Research Center. One high-resolution T1-weighted MPRAGE sequence (orientation = Sagittal, echo time = 3.58 ms, repetition time = 2300 ms, 256 × 256 matrix, resolution 1.0×1.0×1.1 mm. total scan time = 9 min 14 sec) was acquired. A whole-brain echoplanar imaging sequence was used (TR = 9,100 ms, TE = 88 ms, field of view = 240 mm2, b value = 1,000 s/mm2, isotropic voxel dimensions = 2 mm3); images were sensitized for diffusion along 90 different directions with a b-value of 1000 s/mm2. For every 36 diffusion-weighted images, a non-diffusion weighted image (b = 0 s/mm2) was acquired (three total). A static magnetic field map was also acquired using the same parameters as the diffusion weighted sequence. All neuroimaging testing occurred in the morning to maintain homogeneity of testing across participants. T1-weighted structural images were processed using the tools implemented in FMRIB Software Library (FSL; Version 5.0) to quantify metrics of brain volume. Briefly, using the FAST (FMRIB’s Automated Segmentation Tool) toolbox, three-dimensional T1-weighted volumes were segmented to produce grey matter (GM), white matter (WM), and cerebrospinal fluid (CSF) images in standard space [27]. This technique is based on a hidden Markov random field model and an associated Expectation-Maximization algorithm, corrects for spatial intensity variations, and has been shown to be robust and reliable compared to most finite mixture model-based methods [28]. Images were modulated by multiplying each voxel intensity by the Jacobian determinant of the nonlinear transformation used for normalization. The volumes of each tissue were recorded and then used to calculate total brain volume as GM + WM + CSF.”
Diffusion Tensor Imaging (DTI) Data Analysis
Diffusion data were also processed using the tools implemented in FSL. The three raw data sets were first corrected for eddy current distortions and motion artifacts using FMRIB’s diffusion toolbox (FDT 1.0), then averaged to improve signal-to-noise ratio [29] and subsequently skull-stripped (using FSL’s brain extraction tool). The principal diffusion direction was estimated for each voxel as a probability density function, using Bayes’ rules in order to account for noise and uncertainty in the measured data. As described elsewhere [30], the implicit modeling of noise in a probabilistic model enables a fiber tracking procedure without externally added constraints such as fractional anisotropy threshold or fiber angle. Thus, fiber-tracking in or near cortical areas becomes more sensitive. For each individual, the fractional anisotropy images were normalized into Montreal Neurological Institute (MNI) space by using a linear (affine) registration and Fourier interpolation through the FMRIB linear image registration tool. Using the averaged images with b = 0 and b = 1000 s/mm2, the diffusion tensor was calculated. Diagonalization of the diffusion tensor yields the eigenvalues λ1, λ2, and λ3 as well as the eigenvectors that define the predominant diffusion direction.
Radial diffusivity (RD) was chosen as our primary outcome measure to assess white matter microstructural integrity, as this measure is an indirect neural marker of myelination [31]. RD was calculated for each participant by taking the mean of the second and third eigenvalues – (λ2 + λ3) / 2. In addition, we provide complementary analyses of both mean diffusivity (MD) and fractional anisotropy (FA). MD is a measure of the average molecular motion independent of any tissue directionality and is influenced by cellular size and integrity (λ1 + λ2 + λ3) / 3 [32]. For both RD and MD, lower values are interpreted as being indicative of better white matter tract microstructure [33]. FA is a normalized index ranging from 0-1 whereby higher values reflect increased alignment of cellular structures within fiber tracts and better microstructural integrity [34].
Tract Based Spatial Statistics (TBSS)
We performed whole-brain, voxelwise analysis of RD, MD and FA maps using TBSS within the FSL environment. TBSS provides analyses restricted to those white matter voxels that constitute the skeleton (core) of the brain’s connectional architecture and this skeleton can be matched more accurately (compared with whole-brain normalization) across subjects [35]. Each participant’s FA image was used as input for TBSS by registering all subjects’ FA maps to a common space (FMRIB_58 FA MNI template) via a nonlinear transform and then an affine transform to MNI152 space. The two transformations were combined before being applied, to avoid having to resample images twice. The above results in a standard-space version of each subject’s FA image, from which average group FA maps were created and skeletonized. The resulting alignment-invariant representation of the central trajectory of white matter pathways was used for voxelwise statistical analysis (randomize, 10,000 permutations). To identify group differences in white matter fiber tract microstructure, as assessed by RD, MD, and FA, the contrasts MS< HC and HC> MS were examined using threshold-free cluster enhancement [36], with correction for multiple comparisons at α < 0.05 while controlling for age, gender, disease severity (EDSS), total brain volume.
In addition, we utilized whole-brain TBSS skeleton regression analyses to identify relationships between white matter microstructure (RD, MD, and FA) and 1) temporal and spatial postural control for both improvement (over the course of Day 1) and retention and 2) mean temporal and spatial average performance during Day 1. All behavioral data were first demeaned across the entire sample and then regressed against the imaging metrics of interest using randomize within the FSL environment (10,000 permutations). All covariates described in the previous section were also controlled for, in the TBSS regression models.
Post-hoc Region of Interest (ROI) Analyses
White matter regions demonstrating significant correlations with postural motor control underwent post-hoc, ROI analyses to further describe these associations. To provide detailed information regarding interhemispheric callosal localization, we utilized a parcellation technique previously developed by our laboratory to differentiate interhemispheric connections between homologus left and right sensorimotor cortical regions including the pre-supplementary motor area, supplementary motor area, primary motor cortex, and primary somatosensory motor cortex [37]. In addition, we use ROIs of the genu and splenium defined by the Johns Hopkins University white matter labels for both the genu and splenium. These callosal ROIs were identified on the mid-sagittal slice (X = 0) and extend ±4 slices in either direction.
RESULTS
Of the 29 PwMS and 15 HC, data from five PwMS and one HC were excluded due to: inability to complete the protocol (PwMS=4; HC=0), or technical issues during data collection (PwMS=1; HC=1). Of these 5 PwMS, 4 were relapsing remitting, and one secondary progressive. Therefore, data presented below represent 24 PwMS and 14 HC. Of the 24 PwMS included, 19 were relapsing remitting, 2 secondary progressive, 2 primary progressive, and 1 progressive relapsing.
Temporal Performance was worse in PwMS than HC, but improved similarly with training
PwMS exhibited worse temporal performance throughout the Day 1 training period than HC (mean±SD; PwMS: -8.97±6.41; HC: -3.39±3.63; p=0.001). Spatial performance trended toward worse in PwMS than HC, but this difference did not reach statistical significance (PwMS: 0.65±0.09; HC: 0.62±0.05; p=0.208). As described in detail previously [11], PwMS and HC improved similarly on both temporal and spatial performance on Day 1, however, PwMS only retained improvements 24 hours later in temporal performance.
PwMS exhibited worse structural connectivity in the CC and superior cortical white matter tracts
Significantly smaller total brain volume as well as grey and white matter volume were observed within our cohort of PwMS compared to control subjects (Supplemental Figure 1). Furthermore, PwMS showed reduced supraspinal white matter integrity compared to HC. Specifically, we observed significantly increased RD (indicating poorer white matter integrity), principally within white matter tracts of the CC, corona radiata and superior longitudinal fasciculi (SLF; Figure 2). PwMS also exhibited significantly reduced FA (indicating poorer white matter integrity) within similar, although fewer, neural regions as those white matter areas demonstrating increased RD (Supplemental Figure 2A). Finally, PwMS showed significantly increased MD (indicating poorer white matter integrity) compared to their age-matched counterparts within cortical white matter tracts including the SLF and corona radiata. It is worth noting that group differences in MD were lateralized to the right hemisphere (Supplemental Figure 2B).
Figure 2.
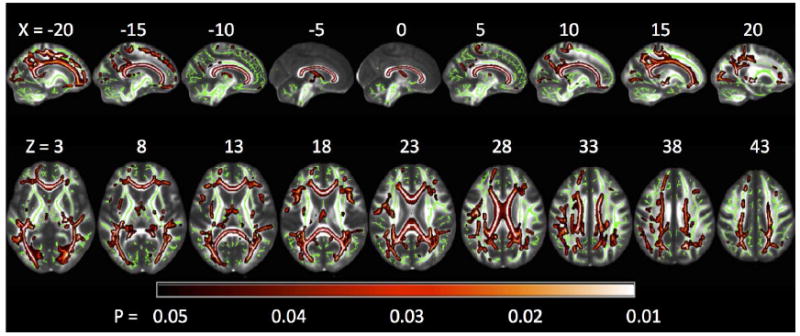
Tract Based Spatial Statistics (TBSS) whole-brain group comparisons of white matter microstructural integrity (assessed via radial diffusivity) showing the contrast of persons with MS (PwMS) > healthy controls (HC), with lighter colors indicating the largest differences between groups. Analysis is restricted to those white matter voxels that constitute the skeleton (green) of the brain’s connectional architecture, whereby this skeleton can be matched across subjects. PwMS had a large network of impaired white matter integrity (reflected by higher radial diffusivity values), most notably within interhemispheric callosal fibers. The reverse contrast of HC > PwMS yielded no significant differences. Results are multiple comparison-corrected and controlled for age, gender, brain volume, and EDSS..
Temporal, but not spatial improvements on Day 1 were correlated to structural connectivity in PwMS
Improved temporal performance over Day 1 was significantly correlated with the primary structural connectivity measure: RD, as well as secondary measures: FA and MD. Improved temporal performance for HC was not related to any of the measures of the white matter integrity. No significant correlations were observed between white matter integrity (RD, MD, or FA) and improvements in spatial performance for either HC or PwMS.
RD and improved temporal performance
Statistically significant correlations were observed for improved temporal performance with RD measures of white matter integrity of the CC, white matter regions connecting the left posterior parietal cortex with the primary sensorimotor cortex of PwMS (Figure 3). Multiple comparison corrected post-hoc analyses showed that correlations between improvement of temporal performance and structural connectivity (RD) were most pronounced in CC regions connecting pre-frontal cortical regions (genu), and the region connecting the primary motor cortex (Table 2; Figure 3). No correlations were observed between temporal performance improvement and any cerebellar structural connectivity (RD) measures.
Figure 3.
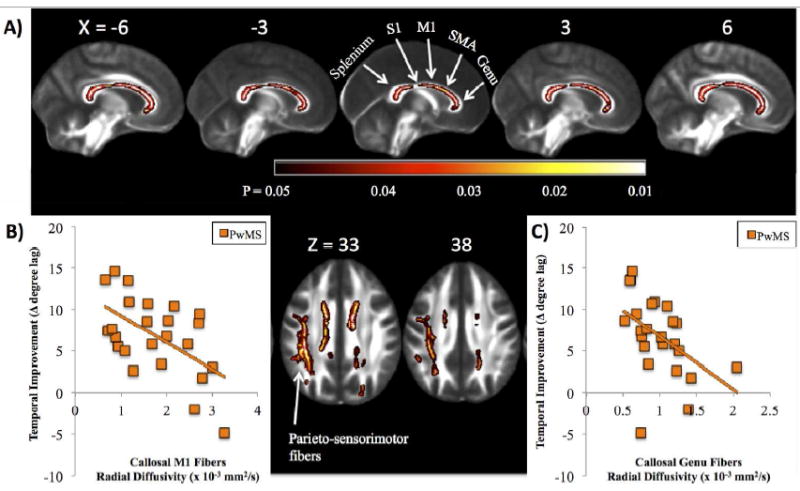
A) Significant associations between temporal improvement and white matter microstructure are shown in people with MS (PwMS), with brighter colors (yellow, white) representing stronger correlation. Significant associations were localized to the corpus callosum (sagittal views: genu, body and splenium; top) and white matter connecting the posterior parietal cortices with the primary sensorimotor cortices within the left hemisphere (axial views; bottom). Results are multiple comparison-corrected and controlled for age, gender, brain volume, and EDSS. Sections of the callosum connecting specific cortical structures (splenium, primary somatosensory cortex [S1]; primary motor cortex [M1]; supplementary motor area [SMA]), and genu are localized. Callosal locations are adapted from Fling et al., 2013 [37]. Scatterplots represent individual values for PwMS displaying the significant association between temporal improvement in postural control and callosal fiber tracts connecting the B) M1: r = -0.56; P = 0.004, as well as the C) genu: r = -0.47; P = 0.01.
Table 2.
Post-hoc analysis correlating fiber tract quality (radial diffusivity) of specific corpus callosum regions with temporal performance and improvement in temporal performance in persons with MS (PwMS). Pearson’s Correlation coefficients are reported.
| Pearson’s Correlation Coefficient(PwMS) | Genu | Pre-supplementary motor area fibers | Supplementary motor area fibers | Primary motor fibers | Primary somatosensory fibers | Splenium | Parieto-sensorimotor fibers |
|---|---|---|---|---|---|---|---|
| Improvement in Temporal Performance | -0.468* | -0.325 | -0.250 | -0.558* | -0.292 | -0.332 | -0.234 |
| Average Temporal Performance | -0.503* | -0.250 | -0.09 | -0.24 | -0.11 | -0.19 | -0.356 |
P<0.01.
FA and improved temporal performance
The relationship between FA and improved temporal performance was similar, albeit less pronounced, to RD (Supplemental Figure 3). Specifically, nonsignificant trends were observed between FA and improved temporal performance in the CC and left SLF.
MD and improved temporal performance
A relationship between MD values and the improved temporal performance was observed in the left hemisphere’s SFL (posterior aspect) and arcuate fasciculus in PwMS (Supplemental Figure 4).
Retention of improvements tested on Day 2 was correlated to MD, but not FA or RD imaging outcomes
No significant correlations were observed between RD or FA and retention of improvements of either temporal or spatial performance. However, MD values within the left hemisphere’s SLF (posterior aspect) and arcuate fasciculus were related to retention in PwMS, such that lower MD values were related to greater retention (Supplemental Figure 5).
Temporal postural performance was correlated to CC and brainstem structural connectivity
Figure 4 shows the correlation between RD and mean temporal postural performance (averaged across Day 1) in PwMS. CC (particularly within the genu of the CC – see Table 2), and brainstem white matter tracts were significantly correlated to temporal performance. Similar to RD, FA of the CC and brainstem was also related to mean temporal performance within PwMS (Supplemental Figure 6). No significant correlation was noted between MD and temporal performance.
Figure 4.
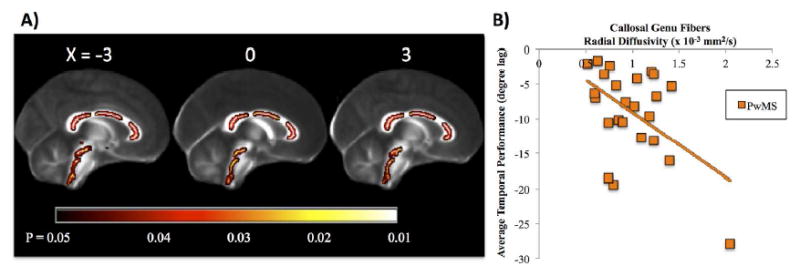
A) In persons with MS (PwMS), significant associations between average temporal performance and white matter microstructure were localized to the corpus callosum (genu, body and splenium) and within the brainstem. Results are multiple comparison-corrected and controlled for age, gender, brain volume, and EDSS. B) Scatterplot of individual participant values displaying the significant association between genu fiber tract integrity and average temporal performance in PwMS: r = -0.50; P = 0.006, but not HC: r = 0.01; P = 0.8 (HC data not shown).
DISCUSSION
Our primary result is that integrity of CC white matter tracts were directly correlated to temporal postural motor performance as well as improvement in performance in PwMS, such that better connectivity was associated with larger improvements in postural control. In contrast, no significant correlations were observed between structural connectivity and spatial measures of postural control. These results are consistent with previous data suggesting the CC is related to improvements in UE motor learning in PwMS [8]. We extend this literature, showing that the CC, and interhemispheric communication, is also likely involved with postural motor learning in this population.
The observed relationship between improved temporal performance and CC integrity (measured via RD and FA) in PwMS is consistent with recent UE literature. Feedforward motor sequence learning is often measured by having participants tap individual fingers in response to repeated sequences of visual stimuli [7, 38] . Feedforward, sequence specific, learning is measured by reduction in reaction time to these repeated sequences. Participants also improve reaction time when exposed to random sequences of visual stimuli. This improvement, characterized as ‘nonspecific’ learning, is related to improved feedback response mechanisms. Bonzano & colleagues [8] showed that the degree of UE feedforward learning, but not nonspecific learning, was strongly associated with the FA of the CC in PwMS. In the current study, CC integrity, measured both by RD and FA, was related to improvements in temporal, but not spatial, postural performance. We have shown previously that the constant sinusoidal frequency of support surface movements throughout trials allows for feedforward learning via prediction of the timing of these movements [11, 12, 39]. However, given the relative inconsistency of movement amplitude forces, participants must rely on nonspecific learning using proprioceptive feedback for spatial improvements [12]. Therefore, our current finding that improvements in temporal performance (i.e. feedforward learning), but not spatial performance (i.e. nonspecific learning) is correlated to CC connectivity is consistent with the findings of Bonzano and colleagues [8]. Together, these findings suggest that feedforward learning, but not nonspecific learning, is related specifically to CC structural connectivity.
Post-hoc analyses showed that the strongest relationship between temporal performance improvement and CC fiber integrity occurred in the genu, connecting the bilateral prefrontal cortices, and the CC midbody, connecting bilateral primary motor cortices. This is consistent with reports showing regions of the CC connecting the prefrontal cortices to be critical for motor learning. For example, Sisti & Swinnen found that upper extremity learning was directly correlated with anterior CC fiber tracts [13]. Ours is the first study to suggest that connectivity between bilateral frontal and prefrontal cortices is also critical for learning to improve postural responses to surface displacements.
Similar to improvements in temporal performance, we also report that mean temporal performance was correlated with both RD and FA of the CC and the brain stem. The relationship between CC integrity and postural performance is consistent with previous research showing that the CC, and the genu specifically, is critical for UE motor performance (for review, see [40]). In fact, the genu of the CC, connecting prefrontal cortices, has been shown to be particularly important for bilateral UE performance in PwMS [41]. Our finding relating postural performance to brainstem structural connectivity is also supported by previous research. Indeed, brainstem structures are critical for postural responses [42, 43], as well as for maintenance of upright posture and tone in normal [44] and neurological [45] populations.
Although alterations in CC structure in PwMS are commonly reported [16-18], the mechanism by which CC structural deficits affect motor learning is unclear. However, recent work suggests reduced interhemispheric inhibition via CC structural deficits may impact learning. Considerable previous work suggests that learning (particularly bimanual learning) is reliant on the CC [8, 13-15], and an important function of the CC is mediating interhemispheric inhibition [46]. Further, intracortical inhibition contributes to motor learning [47-49], and PwMS demonstrate poor contralateral communication and intracortical inhibition [50, 51]. Perhaps unsurprisingly, recent results from our laboratory confirm that PwMS exhibit less specificity of interhemispheric network connectivity, likely related to altered CC structural connectivity [52]. Taken together, this work suggests that reduced interhemispheric inhibition may play a role in learning deficits in PwMS. However, more work is necessary to understand this possible relationship.
In addition to the inter-hemispheric connections between the prefrontal cortices, we also observed a significant correlation between improved postural control and the SLF, a tract that connects intra-hemispheric posterior parietal cortex and motor regions. The posterior parietal cortex plays an integral role in voluntary movements by assessing the context in which movements are being made. Specifically, this region receives somatosensory, proprioceptive, and visual inputs, and uses this feedback to determine such things as the positions of the body and the target [53]. It thereby produces internal models of the movement to be made, prior to the involvement of the premotor and motor cortices. Therefore, alteration to the transmission of these models to the primary motor cortex via disrupted white matter tracts may have contributed to poorer improvement performance in PwMS. It is noteworthy that this association was lateralized to tracts connecting the left, but not right, posterior parietal cortex and sensorimotor regions. Recent work has shown these tracts, namely the dorsal SLF, to be asymmetric with the left hemisphere SLF exhibiting more pronounced connectivity between the dorsal precentral gyrus and the caudal middle frontal gyrus than the right [54]. As noted above, these regions play critical roles for the planning and execution of movement. Therefore, while replication of our finding is necessary, it is possible that left lateralized structural alterations may relate more closely to postural improvements than right-side dysfunction.
Interestingly, we did not observe correlations between white matter tracts to or from the cerebellum. While the cerebellum does play a critical role in learning, we note that no group differences were observed in cerebellar white matter integrity (Figure 2). Therefore, the lack of correlation in the current study may have been the result of relatively intact cerebellar white matter tracts in our group of participants.
Our primary variable of structural connectivity, RD, was not related to 24-hour retention, measured here as the degree to which improvements remained different from baseline. Although numerous cortical and subcortical structures have been shown to be related to retention of skill learning, to our knowledge, no previous studies have investigated the role of white matter integrity in retention. Late stage learning is typically associated with reduced activity of cortical structures (i.e. prefrontal cortex and dorsolateral prefrontal cortex), and increased deep brain activity (i.e. basal ganglia and cerebellum) [55, 56]. Therefore, it is possible that while acquisition of skill (i.e. improvement through practice) relies on transcallosal fibers and cortical structures, later stage learning relies more on deep brain structures. It is therefore notable that white matter tracts emanating from the cerebellum were not related to retention in the current study. However, as discussed above, this may have been related to a lack of structural dysfunction of these tracts or potentially due to the short-term training protocol. Unlike RD, MD within the SLF and arcuate fasciculus of the left hemisphere were related to retention in PwMS. These tracts have previously been related primarily to language comprehension and production [57]. However, given the connectivity to motor regions, they may also play a role in motor function. The disparate findings in the current study regarding RD, FA, and MD are unclear. The lack of consistent coupling with FA is potentially the result of differences in how these measures are calculated, as MD represents the overall tissue diffusivity, whereas FA and RD represent the integrity of the primary and orthogonal directions, or tensors, respectively.
In addition to differences between RD and MD with respect to retention analyses, MS-related changes in MD were found to be somewhat distinct from FA and RD. Specifically, while FA and RD were widespread and consistent [58, 59], with considerable alteration of the CC, MD was lateralized to the right-hemisphere. The reason for these somewhat inconsistent and non-intuitive findings is unclear. However, previous studies have shown changes in MD to be both similar [60] and disparate [61] to FA in PwMS compared to controls. Given the lack of data relating different imaging measures to improvements in postural control with practice, additional work will be necessary to elucidate how each measure predicts postural control and improvement.
Several limitations should be noted. First, our population exhibited mild symptoms of MS. Further, data was collected from a relatively small and somewhat homogeneous (primarily relapsing remitting) population of PwMS. Together, these limitations may reduce the confidence and generalizability of findings. In particular, results may not generalize to patients with more severe symptoms or those with secondary progressive MS. In addition, we were unable to calculate lesion load within this population. It is possible that lesions to grey or white matter also contribute to the heterogeneity of postural control or motor learning in this population. Finally, sensory loss was not accounted for in this analysis. Although sensory loss may have contributed to the reduced performance on the postural task, we find it unlikely to have affected the degree of learning in the MS population.
CONCLUSIONS
This study is the first to demonstrate that CC white matter tracts play a role in practice-related improvements in postural control in PwMS. Retention of these improvements was not related to structural connectivity of the CC white matter tracts. This information highlights the importance of interhemispheric white matter tracts for learning in PwMS. Further; it has the potential to inform patient selection regarding ability to improve balance control over time. However, given the importance of retention of skills for neurorehabilitation, additional research is required to identify predictive factors of retention of skill acquisition in PwMS and how neuroimaging can best be used to predict one’s ability to learn.
Supplementary Material
Table 1.
Participant Characteristics; mean and standard deviation reported unless otherwise noted.
| PwMS | HC | p-value | |
|---|---|---|---|
| N [# female] | 24 (21) | 14 (11) | 0.46 |
| Age [years] | 48 (11) | 47 (13) | 0.73 |
| EDSS (range) | 3.5 (2-4) | -- | -- |
| Years with Disease | 12.9 (7.8) | -- | -- |
| MS Diagnosis (RR/SP/PP) | 19/3/2 | -- | -- |
PwMS- Persons with MS; HC- Healthy Controls; EDSS- Expanded; Disability Status Scale; RR- Relapsing Remitting; PP- Primary Progressive; SP- Secondary Progressive
Acknowledgments
We thank the volunteers for participating in this study. We are also grateful to Heather Schlueter and Jessica Nyugen for assistance in participant recruitment and data collection.
FUNDING
This work was supported by grants from the United States Department of Veteran’s Affairs Rehabilitation Research and Development Service (Career Development Award-1: #I01BX007080; PI: DSP) & VA Merit Award (E1075-R; PI: FBH) and the National Multiple Sclerosis Society (FG 2058-A-1 PI: GG; RG 5273A1/T PI: BWF; MB0011 PI: FBH). Additional support was provided by the Medical Research Foundation of Oregon (BWF, GG), and the NL Tartar Research Fund (BWF). The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Footnotes
CONFLICT OF INTEREST STATEMENT
Dr. Horak and OHSU have an equity/interest in APDM, a company that may have a commercial interest in the results of the study. This potential conflict of interest has been reviewed and managed by the Research & Development Committee at the Portland VA Medical Center and OHSU. No other authors declare any conflict of interest.
LITERATURE CITED
- 1.Hoang PD, Cameron MH, Gandevia SC, Lord SR. Neuropsychological, balance, and mobility risk factors for falls in people with multiple sclerosis: a prospective cohort study. Arch Phys Med Rehabil. 2014 Mar;95(3):480–6. doi: 10.1016/j.apmr.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 2.Kalron A, Achiron A. Postural control, falls and fear of falling in people with multiple sclerosis without mobility aids. J Neurol Sci. 2013 Dec 15;335(1-2):186–90. doi: 10.1016/j.jns.2013.09.029. [DOI] [PubMed] [Google Scholar]
- 3.Nilsagard Y, Lundholm C, Denison E, Gunnarsson LG. Predicting accidental falls in people with multiple sclerosis -- a longitudinal study. Clin Rehabil. 2009 Mar;23(3):259–69. doi: 10.1177/0269215508095087. [DOI] [PubMed] [Google Scholar]
- 4.Cameron MH, Lord S. Postural control in multiple sclerosis: implications for fall prevention. Curr Neurol Neurosci Rep. 2010 Sep;10(5):407–12. doi: 10.1007/s11910-010-0128-0. [DOI] [PubMed] [Google Scholar]
- 5.Casadio M, Sanguineti V, Morasso P, Solaro C. Abnormal sensorimotor control, but intact force field adaptation, in multiple sclerosis subjects with no clinical disability. Multiple sclerosis. 2008 Apr;14(3):330–42. doi: 10.1177/1352458507085068. [DOI] [PubMed] [Google Scholar]
- 6.Vergaro E, Squeri V, Brichetto G, Casadio M, Morasso P, Solaro C, et al. Adaptive robot training for the treatment of incoordination in Multiple Sclerosis. Journal of neuroengineering and rehabilitation. 2010;7:37. doi: 10.1186/1743-0003-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tomassini V, Johansen-Berg H, Leonardi L, Paixao L, Jbabdi S, Palace J, et al. Preservation of motor skill learning in patients with multiple sclerosis. Multiple sclerosis. 2011 Jan;17(1):103–15. doi: 10.1177/1352458510381257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonzano L, Tacchino A, Roccatagliata L, Sormani MP, Mancardi GL, Bove M. Impairment in explicit visuomotor sequence learning is related to loss of microstructural integrity of the corpus callosum in multiple sclerosis patients with minimal disability. Neuroimage. 2011 Jul 15;57(2):495–501. doi: 10.1016/j.neuroimage.2011.04.037. [DOI] [PubMed] [Google Scholar]
- 9.Leocani L, Comi E, Annovazzi P, Rovaris M, Rossi P, Cursi M, et al. Impaired short-term motor learning in multiple sclerosis: evidence from virtual reality. Neurorehabil Neural Repair. 2007 May-Jun;21(3):273–8. doi: 10.1177/1545968306294913. [DOI] [PubMed] [Google Scholar]
- 10.Hatzitaki V, Koudouni A, Orologas A. Learning of a novel visuo-postural co-ordination task in adults with multiple sclerosis. J Rehabil Med. 2006 Sep;38(5):295–301. doi: 10.1080/16501970600680247. [DOI] [PubMed] [Google Scholar]
- 11.Gera G, Fling BW, Van Ooteghem K, Cameron M, Frank JS, Horak FB. Postural Motor Learning Deficits in People With MS in Spatial but Not Temporal Control of Center of Mass. Neurorehabil Neural Repair. 2015 Dec 23; doi: 10.1177/1545968315619700. [DOI] [PubMed] [Google Scholar]
- 12.Van Ooteghem K, Frank JS, Allard F, Buchanan JJ, Oates AR, Horak FB. Compensatory postural adaptations during continuous, variable amplitude perturbations reveal generalized rather than sequence-specific learning. Exp Brain Res. 2008 Jun;187(4):603–11. doi: 10.1007/s00221-008-1329-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sisti HM, Geurts M, Gooijers J, Heitger MH, Caeyenberghs K, Beets IA, et al. Microstructural organization of corpus callosum projections to prefrontal cortex predicts bimanual motor learning. Learn Mem. 2012;19(8):351–7. doi: 10.1101/lm.026534.112. [DOI] [PubMed] [Google Scholar]
- 14.de Guise E, del Pesce M, Foschi N, Quattrini A, Papo I, Lassonde M. Callosal and cortical contribution to procedural learning. Brain. 1999 Jun;122(Pt 6):1049–62. doi: 10.1093/brain/122.6.1049. [DOI] [PubMed] [Google Scholar]
- 15.de Guise E, Lassonde M. Callosal contribution to procedural learning in children. Developmental neuropsychology. 2001;19(3):253–72. doi: 10.1207/S15326942DN1903_2. [DOI] [PubMed] [Google Scholar]
- 16.Fling BW, Dutta GG, Schlueter H, Cameron MH, Horak FB. Associations between Proprioceptive Neural Pathway Structural Connectivity and Balance in People with Multiple Sclerosis. Front Hum Neurosci. 2014;8:814. doi: 10.3389/fnhum.2014.00814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garg N, Reddel SW, Miller DH, Chataway J, Riminton DS, Barnett Y, et al. The corpus callosum in the diagnosis of multiple sclerosis and other CNS demyelinating and inflammatory diseases. J Neurol Neurosurg Psychiatry. 2015 Dec;86(12):1374–82. doi: 10.1136/jnnp-2014-309649. [DOI] [PubMed] [Google Scholar]
- 18.Wahl M, Hubers A, Lauterbach-Soon B, Hattingen E, Jung P, Cohen LG, et al. Motor callosal disconnection in early relapsing-remitting multiple sclerosis. Hum Brain Mapp. 2011 Jun;Jun;32:846–55. doi: 10.1002/hbm.21071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonzano L, Tacchino A, Brichetto G, Roccatagliata L, Dessypris A, Feraco P, et al. Upper limb motor rehabilitation impacts white matter microstructure in multiple sclerosis. Neuroimage. 2014 Apr 15;90:107–16. doi: 10.1016/j.neuroimage.2013.12.025. [DOI] [PubMed] [Google Scholar]
- 20.Stinear CM, Barber PA, Smale PR, Coxon JP, Fleming MK, Byblow WD. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain. 2007 Jan;130(Pt 1):170–80. doi: 10.1093/brain/awl333. [DOI] [PubMed] [Google Scholar]
- 21.Vaughan C, Davis B, O’Connor J. Dynamics of Human Gait. Cape Town, SA: Kiboho Publishers; 1992. [Google Scholar]
- 22.Rochester L, Baker K, Hetherington V, Jones D, Willems AM, Kwakkel G, et al. Evidence for motor learning in Parkinson’s disease: acquisition, automaticity and retention of cued gait performance after training with external rhythmical cues. Brain Res. 2010 Mar 10;1319:103–11. doi: 10.1016/j.brainres.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 23.dos Santos Mendes FA, Pompeu JE, Modenesi Lobo A, Guedes da Silva K, Oliveira Tde P, Peterson Zomignani A, et al. Motor learning, retention and transfer after virtual-reality-based training in Parkinson’s disease--effect of motor and cognitive demands of games: a longitudinal, controlled clinical study. Physiotherapy. 2012 Sep;98(3):217–23. doi: 10.1016/j.physio.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 24.Smania N, Corato E, Tinazzi M, Stanzani C, Fiaschi A, Girardi P, et al. Effect of balance training on postural instability in patients with idiopathic Parkinson’s disease. Neurorehabil Neural Repair. 2010 Nov-Dec;24(9):826–34. doi: 10.1177/1545968310376057. [DOI] [PubMed] [Google Scholar]
- 25.Schlenstedt C, Paschen S, Kruse A, Raethjen J, Weisser B, Deuschl G. Resistance versus Balance Training to Improve Postural Control in Parkinson’s Disease: A Randomized Rater Blinded Controlled Study. PLoS One. 2015;10(10):e0140584. doi: 10.1371/journal.pone.0140584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmidt R, Lee TD. Motor Control and Learning: A Behavioural Emphasis. Champaign, IL: Human Kinetics; 1999. [Google Scholar]
- 27.Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005 Jul 1;26(3):839–51. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE transactions on medical imaging. 2001 Jan;20(1):45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- 29.Eickhoff SB, Jbabdi S, Caspers S, Laird AR, Fox PT, Zilles K, et al. Anatomical and functional connectivity of cytoarchitectonic areas within the human parietal operculum. J Neurosci. 2010 May 5;30(18):6409–21. doi: 10.1523/JNEUROSCI.5664-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, et al. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med. 2003 Nov;50(5):1077–88. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- 31.Gulani V, Webb AG, Duncan ID, Lauterbur PC. Apparent diffusion tensor measurements in myelin-deficient rat spinal cords. Magn Reson Med. 2001 Feb;45(2):191–5. doi: 10.1002/1522-2594(200102)45:2<191::aid-mrm1025>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 32.Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G. Diffusion tensor MR imaging of the human brain. Radiology. 1996 Dec;201(3):637–48. doi: 10.1148/radiology.201.3.8939209. [DOI] [PubMed] [Google Scholar]
- 33.Beaulieu C. What makes diffusion anisotropic in the nervous system? In: Jones DK, editor. Diffusion MRI: theory, methods, and applications. Oxford: Oxford University Press; 2011. pp. 92–109. [Google Scholar]
- 34.Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. Journal of magnetic resonance Series B. 1996 Jun;111(3):209–19. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- 35.Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006 Jul;31(4):1487–505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 36.Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009 Jan;44(1):83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 37.Fling BW, Benson BL, Seidler RD. Transcallosal sensorimotor fiber tract structure-function relationships. Hum Brain Mapp. 2013 Feb;34(2):384–95. doi: 10.1002/hbm.21437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tacchino A, Bove M, Roccatagliata L, Luigi Mancardi G, Uccelli A, Bonzano L. Selective impairments of motor sequence learning in multiple sclerosis patients with minimal disability. Brain Res. 2014 Oct 17;1585:91–8. doi: 10.1016/j.brainres.2014.08.031. [DOI] [PubMed] [Google Scholar]
- 39.Van Ooteghem K, Frank JS, Horak FB. Practice-related improvements in posture control differ between young and older adults exposed to continuous, variable amplitude oscillations of the support surface. Exp Brain Res. 2009 Nov;199(2):185–93. doi: 10.1007/s00221-009-1995-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gooijers J, Swinnen SP. Interactions between brain structure and behavior: the corpus callosum and bimanual coordination. Neurosci Biobehav Rev. 2014 Jun;43:1–19. doi: 10.1016/j.neubiorev.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 41.Bonzano L, Tacchino A, Roccatagliata L, Abbruzzese G, Mancardi GL, Bove M. Callosal contributions to simultaneous bimanual finger movements. J Neurosci. 2008 Mar 19;28(12):3227–33. doi: 10.1523/JNEUROSCI.4076-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Honeycutt CF, Gottschall JS, Nichols TR. Electromyographic responses from the hindlimb muscles of the decerebrate cat to horizontal support surface perturbations. J Neurophysiol. 2009 Jun;101(6):2751–61. doi: 10.1152/jn.91040.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stapley PJ, Drew T. The pontomedullary reticular formation contributes to the compensatory postural responses observed following removal of the support surface in the standing cat. J Neurophysiol. 2009 Mar;101(3):1334–50. doi: 10.1152/jn.91013.2008. [DOI] [PubMed] [Google Scholar]
- 44.Takakusaki K. Neurophysiology of gait: from the spinal cord to the frontal lobe. Mov Disord. 2013 Sep 15;28(11):1483–91. doi: 10.1002/mds.25669. [DOI] [PubMed] [Google Scholar]
- 45.Peterson DS, Horak FB. Neural Control of Walking in People with Parkinsonism. Physiology. 2016 Mar;31(2):95–107. doi: 10.1152/physiol.00034.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takeuchi N, Oouchida Y, Izumi S. Motor control and neural plasticity through interhemispheric interactions. Neural plasticity. 2012;2012 doi: 10.1155/2012/823285. 823285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stagg CJ, Bachtiar V, Johansen-Berg H. The role of GABA in human motor learning. Current biology : CB. 2011 Mar 22;21(6):480–4. doi: 10.1016/j.cub.2011.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Floyer-Lea A, Wylezinska M, Kincses T, Matthews PM. Rapid modulation of GABA concentration in human sensorimotor cortex during motor learning. J Neurophysiol. 2006 Mar;95(3):1639–44. doi: 10.1152/jn.00346.2005. [DOI] [PubMed] [Google Scholar]
- 49.Heba S, Puts NA, Kalisch T, Glaubitz B, Haag LM, Lenz M, et al. Local GABA Concentration Predicts Perceptual Improvements After Repetitive Sensory Stimulation in Humans. Cereb Cortex. 2016 Mar;Mar;26:1295–301. doi: 10.1093/cercor/bhv296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Manson SC, Palace J, Frank JA, Matthews PM. Loss of interhemispheric inhibition in patients with multiple sclerosis is related to corpus callosum atrophy. Exp Brain Res. 2006 Oct;174(4):728–33. doi: 10.1007/s00221-006-0517-4. [DOI] [PubMed] [Google Scholar]
- 51.Warlop NP, Achten E, Debruyne J, Vingerhoets G. Diffusion weighted callosal integrity reflects interhemispheric communication efficiency in multiple sclerosis. Neuropsychologia. 2008;46(8):2258–64. doi: 10.1016/j.neuropsychologia.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 52.Fling BW, Gera Dutta G, Horak FB. Functional connectivity underlying postural motor adaptation in people with multiple sclerosis. NeuroImage Clinical. 2015;8:281–9. doi: 10.1016/j.nicl.2015.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Buneo CA, Andersen RA. The posterior parietal cortex: sensorimotor interface for the planning and online control of visually guided movements. Neuropsychologia. 2006;44(13):2594–606. doi: 10.1016/j.neuropsychologia.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 54.Wang X, Pathak S, Stefaneanu L, Yeh FC, Li S, Fernandez-Miranda JC. Subcomponents and connectivity of the superior longitudinal fasciculus in the human brain. Brain structure & function. 2016 May;221(4):2075–92. doi: 10.1007/s00429-015-1028-5. [DOI] [PubMed] [Google Scholar]
- 55.Steele CJ, Penhune VB. Specific increases within global decreases: a functional magnetic resonance imaging investigation of five days of motor sequence learning. J Neurosci. 2010 Jun;1630(24):8332–41. doi: 10.1523/JNEUROSCI.5569-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Debaere F, Wenderoth N, Sunaert S, Van Hecke P, Swinnen SP. Changes in brain activation during the acquisition of a new bimanual coodination task. Neuropsychologia. 2004;42(7):855–67. doi: 10.1016/j.neuropsychologia.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 57.Dick AS, Tremblay P. Beyond the arcuate fasciculus: consensus and controversy in the connectional anatomy of language. Brain. 2012 Dec;135(Pt 12):3529–50. doi: 10.1093/brain/aws222. [DOI] [PubMed] [Google Scholar]
- 58.Roosendaal SD, Geurts JJ, Vrenken H, Hulst HE, Cover KS, Castelijns JA, et al. Regional DTI differences in multiple sclerosis patients. Neuroimage. 2009 Feb 15;44(4):1397–403. doi: 10.1016/j.neuroimage.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 59.Kern KC, Sarcona J, Montag M, Giesser BS, Sicotte NL. Corpus callosal diffusivity predicts motor impairment in relapsing-remitting multiple sclerosis: a TBSS and tractography study. Neuroimage. 2011 Apr 1;55(3):1169–77. doi: 10.1016/j.neuroimage.2010.10.077. [DOI] [PubMed] [Google Scholar]
- 60.Lin F, Yu C, Jiang T, Li K, Chan P. Diffusion tensor tractography-based group mapping of the pyramidal tract in relapsing-remitting multiple sclerosis patients. AJNR American journal of neuroradiology. 2007 Feb;28(2):278–82. [PMC free article] [PubMed] [Google Scholar]
- 61.Fox RJ, Cronin T, Lin J, Wang X, Sakaie K, Ontaneda D, et al. Measuring myelin repair and axonal loss with diffusion tensor imaging. AJNR American journal of neuroradiology. 2011 Jan;32(1):85–91. doi: 10.3174/ajnr.A2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


