Introduction
After partial injury of the central nervous system, neurological function can be restored either by repairing damaged neural circuits or by enabling spared circuits to take over lost function. 4-aminopyridine (4-AP) is an organic compound which is thought to improve function in people with multiple sclerosis (MS) by restoring axon conduction through areas of demyelination. 4-AP blocks voltage-gated potassium channels in the demyelinated internodes of injured axons and thus prolongs action potentials1. This mechanism of action is supported by laboratory studies demonstrating improved conduction in demyelinated axons1 and also in improved physiology of humans with MS2,3. Phase 3 efficacy studies3 led to FDA approval of 4-AP for improving walking in people with MS. Thus, 4-AP is generally considered to improve function in people with MS, by reversing conduction block in areas of demyelination, although it can act on other nervous system targets as well as on muscle4,5.
An alternative and complementary mechanism of action for 4-AP after partial injury is that 4-AP increases spared neural circuit excitability, allowing them to assume functions of injured circuits. This mechanism suggests that 4-AP can be effective in neurological disorders of axonal injuries and is supported by preclinical and clinical studies demonstrating efficacy in stroke6,7. In rats with permanent middle cerebral artery occlusion6, administration of 4-AP improved sensorimotor function, even at drug levels similar to those observed in the MS clinical trials. This positive preclinical study led to clinical trials of the sustained-release formulation of 4-AP (dalfampridine) in people with stroke. Phase 1 and 2 trials indicate that 4-AP is safe and that walking speed, the primary outcome measure, improved in people taking dalfampridine7.
We have previously observed that in rats with an acute transection lesion to the pyramidal tract, an injury model that specifically targets axonal injury, 4-AP is able to restore weak responses to electrical stimulation8. Rats, like humans, have strong connections to the contralateral half of the spinal cord and weak connections to the ipsilateral half of the spinal cord through the corticospinal tract (CST). CST is the primary descending motor pathway that controls voluntary movements in rats and humans. Intracortical micro-stimulation of the motor cortex in intact rats can produce motor evoked potentials (MEPs), albeit weaker, from ipsilateral forelimb muscles. When the pyramidal tract from one hemisphere was cut or the motor cortex was inactivated, ipsilateral MEPs were completely lost from the uninjured hemisphere8. We tested whether the lost innervation could be compensated by pharmacological excitation through systemic administration of 4-AP. Indeed, ipsilateral MEPs were restored after rats were given 2 mg/kg of 4-AP by intraperitoneal injection.
Previous findings from our laboratory are limited by the fact that a relatively high oral dose of 4-AP, 2 mg/kg, was administered to rats with CST injury resulting in an average plasma level of 142.4 ng/ml6. Data from clinical trials for MS indicate that drug plasma levels above 100 ng/ml1,9 or drug doses above 0.8 mg/kg are likely to produce adverse effects10–12. Thus, it is not known if 4-AP can strengthen motor responses in rodents with CST injury at clinically relevant plasma levels.
The current study was designed to fill these gaps by testing whether 4-AP at Running Title: Strengthening spared motor-circuits with 4-AP plasma levels similar to clinical trials would enhance the excitability of intact neural circuits in a transection pyramidal tract injury. Based on our published work, we further hypothesized that 4-AP will be more efficacious in restoring MEPs from intact circuits of rats with partial injury than rats without injury. To test these hypotheses, we first conducted a dose-finding study to produce plasma levels of 4-AP in the range found in clinical studies. We achieved blood plasma levels exceeding 20 ng/ml, the nadir of blood plasma levels in clinical trials, and avoided blood plasma levels of 100 ng/ml to minimize adverse effects3,12. The effects of this lower dose on muscle responses to motor cortex and cervical spinal cord stimulation in intact and pyramidal tract (CST)-lesioned rats were evaluated. In all groups of animals, the left cortex and the dorsal spinal cord were stimulated and the corresponding changes in resultant MEPs from biceps brachii muscles were studied to test where the effects of drug would be most effective13. In uninjured rats and rats with pyramidotomy, 4-AP augmented MEPs from both motor cortex and spinal cord stimulation. Importantly, motor responses were more strongly augmented in the spared connections of rats with pyramidal tract injury compared to uninjured rats. Thus, 4-AP augments intact circuits, an effect that is more robust after partial injury. This warrants further investigation of the potential for 4-AP to support recovery of function in CNS injury or disease.
Methods
Overview
Pharmacokinetic studies were first conducted to determine the best delivery mode (continuous versus bolus) to achieve stable and desired plasma concentration. Then, we measured physiological motor responses to 4-AP by quantifying muscle responses to motor cortex and spinal cord electrical stimulation before and after administration of saline(intact rats only) and 4-AP (both in intact and injured rats receiving a unilateral pyramidal tract injury one week prior to physiology tests). A timeline of the experimental protocol is shown in Fig 1.
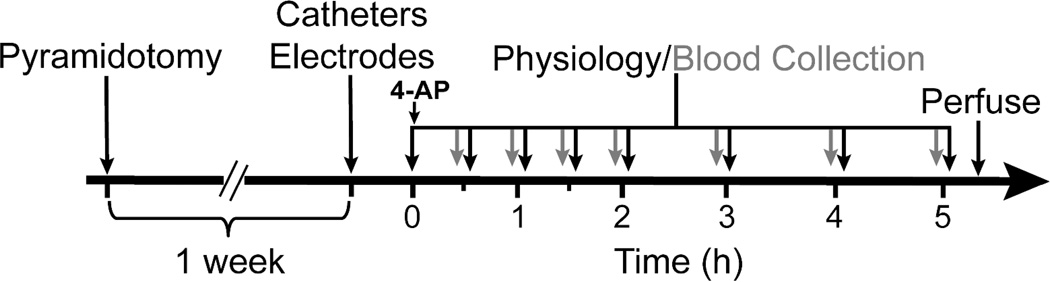
Figure 1. Timeline.
Timeline shows experimental design for bolus infusion of 4-AP along with the physiology and blood plasma collection set-up. Femoral vein was catheterized for plasma collection and an intraperitoneal catheter was also placed for continuous infusion of anesthesia. Bipolar cortical and spinal electrodes were placed over the forelimb area and the cervical region of spinal cord respectively. Also, bicep brachii muscle was implanted with steel EMG electrode. Animals in injury group were pyramidotomized one week prior to physiology experiments. Following i.p. injection of the drug, the cortical and spinal physiology data were collected at different time points (black arrows) as shown and 300 uL of blood plasma was also collected at different time points (grey arrows).
Subjects, randomization and blinding procedures
Female Sprague Dawley rats (250–300g; n=32) were used to limit variability in the pharmacokinetic profile and pharmacologic responses to 4-AP; in addition, female rats perform better on reaching tasks than males in this model14. Seven animals were used for the dose finding studies; four received continuous infusion and three received bolus infusion. The other 25 rats were randomly assigned to one of 3 groups (n=8 each, except injury group n=9) using a random number generator in Excel. 1) Saline controls (to ensure that the physiological measures stayed stable throughout the testing period), 2) naive rats with 4-AP infusion, and 3) injured rats with 4-AP (n=9; 1 rat had an incomplete lesion and was removed from the study). All pharmaco-physiological testings and subsequent analyses were performed by an experimenter who was blinded to the treatment condition. The rats were housed in standard conditions. All procedures were approved by the Institute Animal Care and Use Committee at Weill Cornell Medical College.
Surgical
The surgeries were performed under general anesthesia and aseptic conditions. We also monitored the animal’s body temperature and heart rate (Physiosuite, Kent Scientific) throughout the procedure. General anesthesia was induced with intraperitoneal injection of ketamine (90 mg/kg) and xylazine (10 mg/kg) and maintained by continuous infusion of ketamine (delivered by an i.p. catheter) at 40 mg/kg/hr. This combination was used to preserve motor responses8,15–17.
Corticospinal Injury and Validation
The left pyramidal tract was cut in the rostral medulla as in prior studies8,15,18–20. To confirm the injury was complete, responses from the motor cortex stimulation on the side of the lesion were measured. Only the animals that did not produce any MEP response from the cut circuit within the physiological stimulus intensity range (below 4 mA) were included. In addition, at the end of study following perfusion, the lesions were visually inspected by placing the brain stem ventral side up under a dissecting scope at 20× magnification. One animal out of the nine animals tested was excluded because of an incomplete lesion. This lesion model was chosen due to its ability to cause selective unilateral damage to the CST and the relative ease of quantifying changes in motor function following CST damage21–23.
Muscle responses to dorsal spinal cord stimulation
Bipolar ball electrodes (silver, 2mm) were placed over the center of the spinal cord cervical enlargement at the C5 and C6 segments, a location known to innervate the biceps brachii muscles24. Epidural stimulation engages spinal motor circuits through the recruitment of large diameter afferent fibers25. A single pulse was delivered into the spinal cord (Model 1700, A-M System) as shown in Fig.2 A1. We assessed right bicep MEPs for each of the three groups (Fig.4 A1).
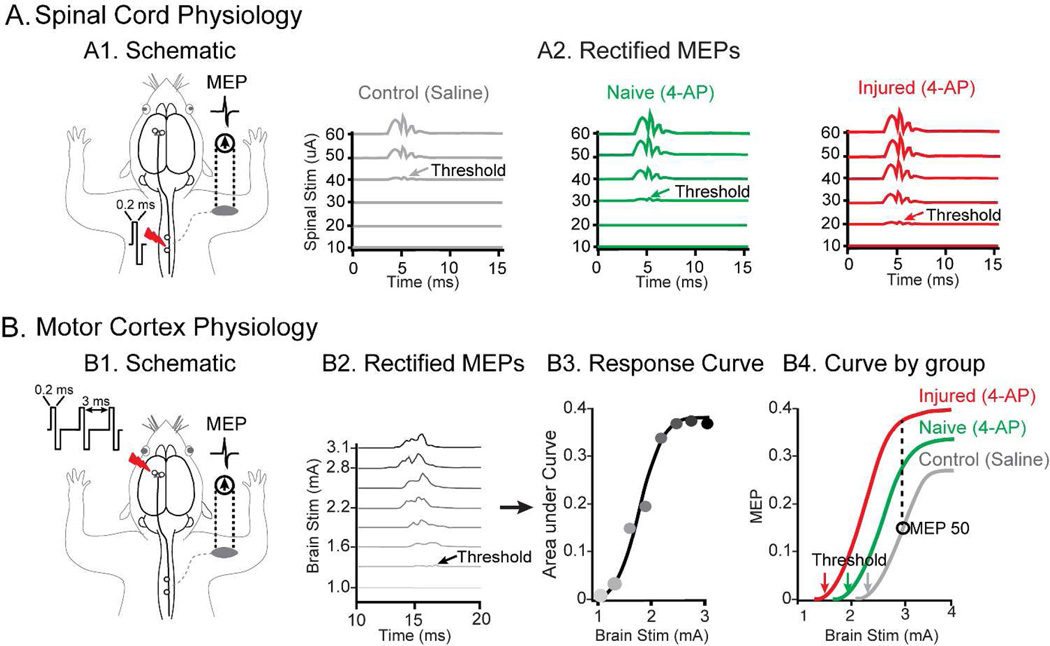
Figure 2. Spinal and cortical electrophysiology methods.
(A) Spinal cord stimulation and recordings. (A1) Schematic for spinal stimulation is shown. (A2) Stimulus strengths of varying magnitude were applied over the cord until the minimum threshold required to generate MEP response was reached (shown with arrows color coded for each group). Threshold detection for each of the three groups tested is shown for control, naïve and injured group. These thresholds were obtained from rectified signals and were used to compute spinal excitability which is reciprocal of the minimum stimulus threshold required to obtain the MEPs. (B) Cortical stimulation and recordings. (B1) Schematic for cortical stimulation. (B2) The signals obtained were rectified and in order to calculate MEP50 (see method for details), brain stimuli of varying strengths were applied to generate corresponding MEPs as shown. (B3) The areas under the curve from these MEPs were used to generate the response curve from which MEP50 values are calculated. (B4) An example of MEP50 calculations for different experimental conditions is shown demonstrating MEP50 for control (grey), increasing MEP50 with 4-AP in uninjured animals (green) and further increment with combination of injury and 4-AP treatment (red). Note, we also calculated cortical thresholds (grey, green and red arrows) and use them to generate cortical excitability data (see methods for details). The data shown in the figures are representative data from a single animal.
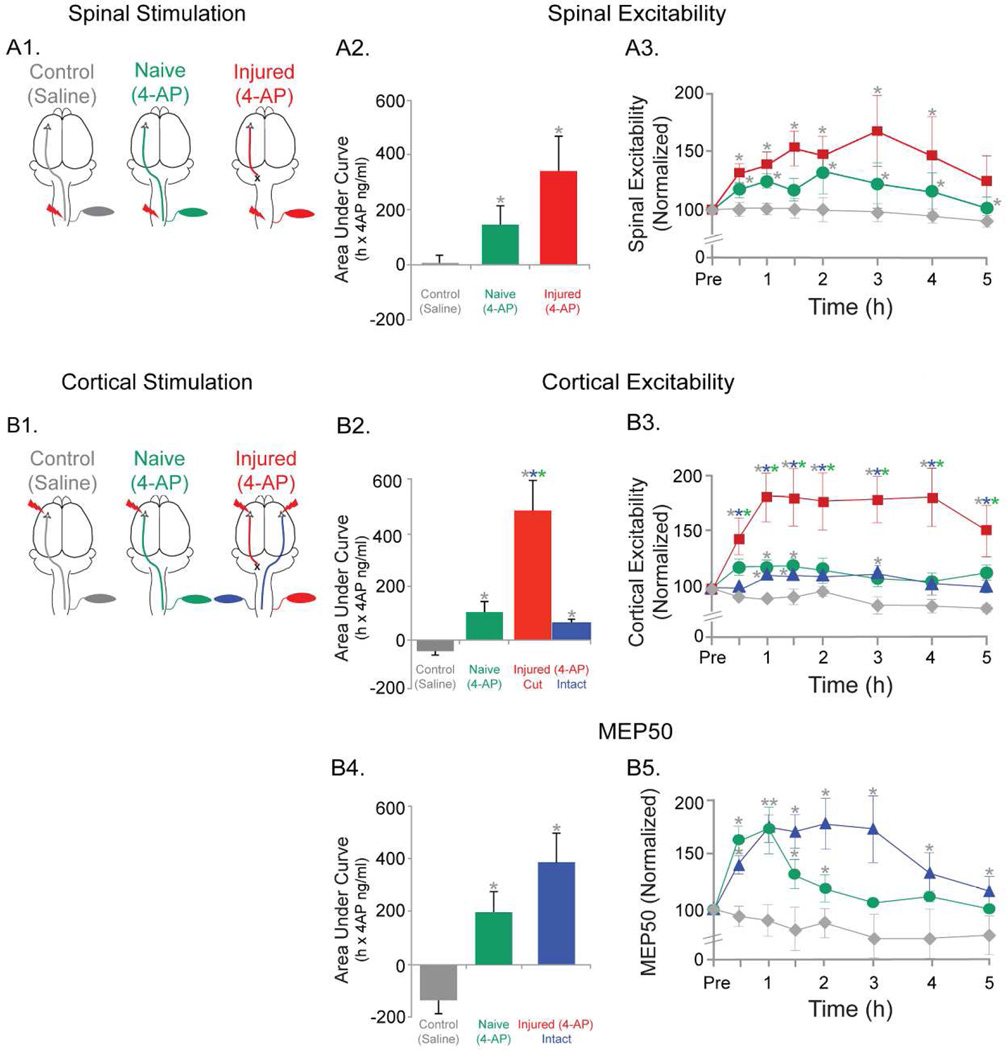
Figure 4. Effects of 4-AP on cortical and spinal electrophysiology parameters.
(A) Spinal excitability. (A1) Schematic of spinal stimulation and the three groups tested are shown. (A2) AUC for spinal excitability changes for the three groups are shown. The AUC changes were significantly higher in the naïve (4-AP) and the injured (4-AP) group compared to saline (control). (A3) Spinal excitability changes for each of the time points recorded are shown for the three groups. Significantly different comparisons for each time points are shown with asterisks next to the symbols.
(B) Cortical excitability and MEP50. (B1) Schematic of cortical stimulation and the three groups tested are shown. Note that in the injured (4-AP) group, cortical stimulations were applied to both hemispheres and recordings from contralateral muscles for each hemisphere are shown. (B2) AUC for cortical excitability changes for the three groups are shown. Note that in injured (4-AP) group, bilateral data are shown. Cut side of Injured (4-AP) group was significantly different from both other groups and from intact side within group as well. Naïve (4-AP) and intact side of injured (4-AP) is significantly different from control (saline) group. (B3) Cortical excitability for each time points recorded are shown for the three groups. Significantly different comparisons for each time points are shown with asterisks next to the symbols. (B4) AUC for MEP50 changes for the three groups are shown. The naïve (4-AP) group and the injured (4-AP) group data were significantly different from the control (saline) group. (B5) The MEP50 changes for each of the time points recorded are shown. Significantly different comparisons for each time points are shown with asterisks next to the symbols.
Note: The asterisks next to each symbol represents significant changes following post-hoc tests. The color of the asterisks represents the group from which the comparisons were significant. P<0.05
We assessed the spinal excitability, which is defined as 1/threshold. The threshold was defined as the stimulation intensity needed to evoke an MEP (Fig. 2A1 and 2A2, arrows). Baseline data were acquired by stepwise increments. Once an MEP was observed, the threshold was confirmed by increasing and decreasing intensity in increments of 10µA. The responses after 4-AP administration (or saline in the case of the control group) was normalized to baseline responses and expressed as a percent change from baseline (Fig. 4A3). The area under the spinal excitability-time curve (AUC, calculated using the trapezoidal rule26) was used as the primary measure of spinal excitability (Fig.4 A2).
Muscle responses to motor cortex stimulation
Cortical MEPs were evoked by epidural stimulation of the caudal forelimb area of the motor cortex using silver ball electrodes (silver, 2 mm) placed at two locations in relation to bregma: 2.5 mm lateral and 1.0 mm anterior; 3.5 mm lateral and 2.0 mm anterior. Ball electrodes were chosen for 3 reasons: 1) These electrodes are stable over long periods of time; 2) they can deliver high intensity stimuli; and 3) they sample a large portion of the forelimb motor cortex.
The cortical stimulations were delivered as a train of 3 biphasic pulses; each pulse was 0.2ms long with 3ms interval in between8 (Fig.2 B1). This paradigm provokes MEPs from cortical stimulation15 as opposed to single pulse stimulation, which largely excites subcortical motor pathways27. MEPs were recorded with the help of invasive bipolar EMG electrodes that were implanted in the biceps brachii muscle. MEPs were amplified (X1000) and filtered (1–1000 Hz) using a differential AC amplifier (model 1700, A–M Systems) and then digitized and recorded at 1 KHz using a CED Micro 1401 amplifier and Signal 5.08 software (Cambridge Electronic Design). Epochs of 258–272 ms were saved and exported into MATLAB (Mathworks, Version 2013a) for further processing and calculations of area under the curve.
Cortical stimulation intensity was initiated below threshold to evoke an MEP response and then increased until the MEPs no longer increased (Fig.2 B2). In the naive(4-AP) and control(saline) groups, only right bicep MEPs were recorded. Both the injured and uninjured hemispheres were stimulated in rats with lesions. The motor cortex on the injured side is connected to the spinal cord through connections to brainstem motor nuclei, including the red nucleus28, but since this is not the dominant descending motor pathway, it requires higher intensity cortical stimulation to evoke responses. Hence, current intensities greater than 4 mA were used to elicit MEPs from the right bicep. We obtained two cortical electrophysiological parameters: Cortical excitability and MEP50, which are described in further detail below. For each parameter, the responses after 4-AP administration (or saline in the case of the control group) was normalized to baseline responses and expressed as a percent change from baseline (Fig. 4B3 and 4B5).
Cortical excitability (primary outcome): This is defined the same way as spinal excitability: 1/threshold, where threshold is the threshold required to generate an MEP (Fig.2 B2 and B4).
MEP50 (secondary outcome): This is defined as the amplitude of MEPs at the 50th percentile of maximum stimulus intensity (Fig.2 B2–B4). When the motor cortex is stimulated with increasing intensity, it generates a characteristic sigmoidal response curve (Fig.2 B3). The response curve shifts left when excitability increases. MEP50 for the baseline (black circle on grey curve in Fig.2 B4) is compared with MEPs for the rest of the groups at the same cortical stimulation intensity (black interrupted line in Fig.2 B4).
4-AP Preparation and Administration
4-AP (Sigma Aldrich) was prepared by dissolving it in sterile saline. Then, it was either delivered intraperitoneally (i.p.) with a catheter for 5 continuous hours (0.36 mg/kg/h-continuous infusion) or injected as bolus (0.32 mg/kg or 0.48 mg/kg) over one minute. We found that bolus infusion was more optimal for maintaining clinically meaningful plasma levels of the drug. Hence, this method was used for pharmaco-physiology experiments.
Blood Collection and 4-AP Levels
300 µL of blood were collected into microfuge tubes containing EDTA from a catheter passing from the femoral vein into the inferior vena cava29. Blood was immediately centrifuged at 10,000 rpm for 5 minutes at 4°C and the plasma was transferred and stored at −20°C. 4-AP plasma concentrations were measured using liquid chromatography and tandem mass spectrometry by Covance, Inc (Madison, WI) using standard controls6.
Statistics
Statistical analyses were performed using SPSS (Version 22, Chicago, IL). All data points are mean ±: standard error. All data are expressed as a percentage of baseline values acquired before 4-AP or saline was administered. The normality of the data was determined using the Shapiro-Wilk test. The plasma 4-AP data from the continuous infusion experiments were normally distributed and a Student’s t-test was used to test the significance between groups. The electrophysiologic data were not normally distributed, so the non-parametric Friedman’s related-samples one-way ANOVA was used in which the significance of the main test statistics is determined using a chi-squared distribution. Post-hoc comparisons were performed using the Mann-Whitney U-test and adjusted for multiple comparisons using the False Discovery Rate30,31. Group analysis for the control group was only employed to test whether changes occurred over the testing period. Significance was set at p<0.05.
Results
Optimizing 4AP delivery and dose to produce target plasma concentrations
The initial goal was to establish a dose and delivery method to maintain plasma drug levels between 20–100 ng/ml (see Introduction). In the first experiment (n=4), a dose of 0.36 mg/kg/h of 4-AP was continuously infused using an i.p. catheter for 5 hours to mimic the pharmacokinetics of dalfampridine, the extended release formulation of 4-AP used in human trials. The plasma drug levels steadily increased during the first 5h during infusion with the drug level peaking at 141.8±15.3 ng/ml on average at 5h (Fig.3A, grey diamond). After the infusion, the average drug level for the next 5h was 71.5±2.3 ng/ml.
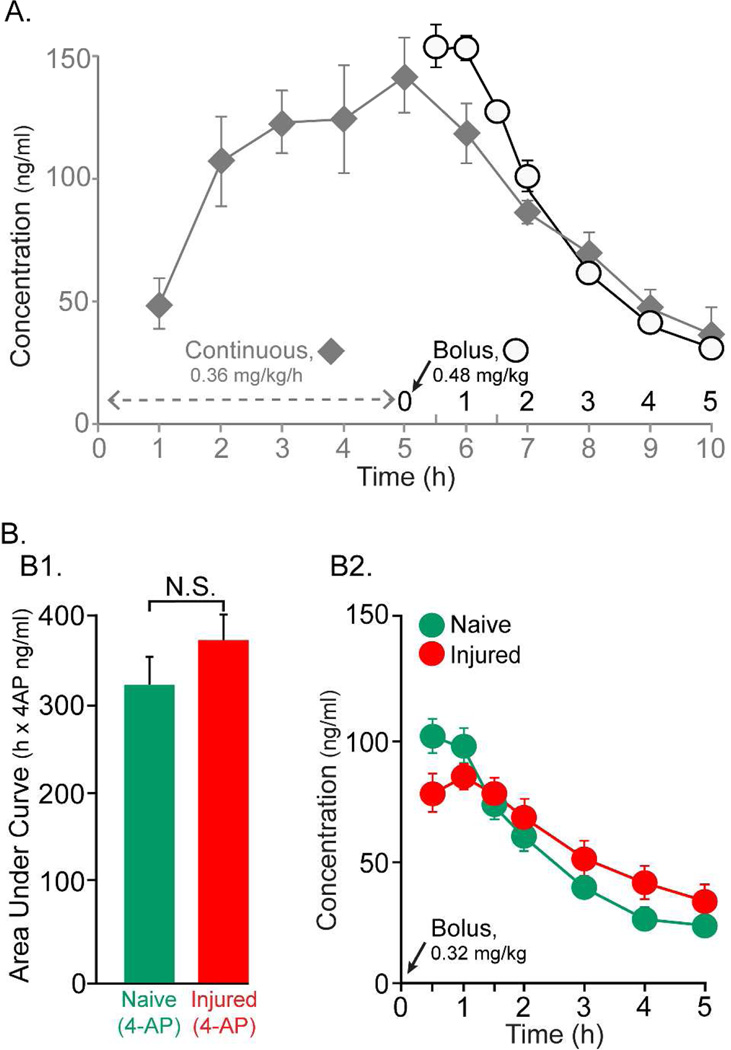
Figure 3. 4-AP Plasma concentrations.
(A) In the first set of experiments (n=4), 4-AP was continuously infused using an i.p. catheter (0.36 mg/kg/h) over 5h and blood plasma was collected during both the first five hours of drug infusion as well as 5h subsequent to infusion (grey diamond symbol). The plasma drug levels steadily increased during the first 5h during infusion with peak levels of approximately 140 ng/ml at the 5h time point. Drug levels steadily declined during the next 5 h. In another experiment (n=3), a single i.p. bolus infusion of 4-AP at 0.48 mg/kg resulted in slightly higher peak drug plasma levels during the first 1.5h and the drug levels then declined steadily over the next 3.5 h (open circle symbols). (B) A slightly lower dose of drug (0.32 mg/kg) was given as a single i.p bolus to a group of uninjured (n=8) and injured (n=8) animals. (B1) The AUC data for the naïve group (green) and injured group (red) are shown. The difference between two groups was not significant. (B2) The changes in plasma drug levels for each time point tested are shown for each group are shown in same color scheme as B1. The drug levels peaked at 100 ng/ml for both of these groups of animals during the first 1.5 hr. Drug levels declined steadily declined in the next 3.5 hr post drug injections. However, note that the drug levels even at end of 5th hour were at 21.5 ng/ml, well above zero.
Drug infusion was then switched to a bolus to shorten the duration of the experiment which is important for maintaining suitable physiological responses. Responses to motor cortex and spinal cord stimulation were tested just before collecting plasma, allowing for a tight correlation of motor responses and plasma drug levels. In the next group of rats (n=3), a single bolus of 4-AP (0.48 mg/kg) was given intraperitoneally. The 4-AP plasma level was, on average, 154.4±6.2 ng/ml at 0.5 h after drug infusion (Fig.3A, open circles symbols). The average drug level during the 5 hr post-infusion was 98.6±18.7 ng/ml. The drug level steadily declined over the next 3.5 hrs at a rate similar to what was observed after ceasing the continuous infusion. The plasma drug level in both of these experiments was above the desired range (20–100 ng/mL), so the dose was lowered to 0.32 mg/kg. This new dose level was administered to two groups of animals (n=8/group): naive(4-AP) and injured(4-AP) (Fig.3B). These two groups were used for electrophysiology experiments. Average AUCs for the two groups were not different (Fig.3 B1; p>0.3). The changes in plasma drug level for each time points after drug infusion are shown in Fig.3 B2. The average plasma drug level in naive animals was 59.7±2.7 ng/ml (Fig.3 B2, green circles) and in injured animals was 63.3±0.9 ng/ml (Fig.3 B2, red diamonds). Rats with saline infusion had 4-AP levels below the limit of quantitation (<1.0 ng/mL; data not shown).
Spinal cord excitability is elevated with 4-AP, especially in rats with pyramidal injury
Based on the dose-finding studies above, a 4-AP dose of 0.32 mg/kg was delivered as bolus to test its effects on the physiology of the motor system (Fig. 4). Spinal excitability was strongly augmented by 4-AP in both naïve(4-AP) and injured(4-AP) animals. The schematics for spinal cord stimulation and recording are shown in Fig. 4A1. The main effects of 4-AP on spinal excitability are shown in Fig.4 A2. The AUC for naïve(4-AP) increased by 133.6±64.5 (%-hr) on average from the baseline and 309.1±116.2 (%-hr) in the injured(4-AP) animals. The AUC did not change significantly for the control(saline) animals. Overall, the mean AUCs between the three groups differed significantly (Χ2(2)=7.7, p=0.02). Significant differences (p<0.05) were found between the naïve(4-AP) vs control(saline) animals as well as injured(4-AP) vs the control(saline) animals.
4-AP produced robust early and late effects on spinal excitability in naïve(4-AP) and injured(4-AP) animals following bolus administration. The spinal excitability on average increased by 19±3.7% in the naïve(4-AP) animals (Fig.4 A3, green). In comparison, the excitability in the injured(4-AP) animals increased by 46.1± 5.3% (Fig.4 A3, red). Spinal excitability did not change significantly over time for the control(saline) animals (within group analysis; Χ2(7)=7.9, p=0.4; Fig.4 A3, grey). Since we did not have a saline treated injured animal group, we compared the spinal excitability changes within the injured group to its own baseline. We found that the changes were significantly different (within group analysis; Χ2(7)=21.5, p=0.003), and post-hoc tests found the changes to be significant for all the time points tested from the baseline up to the 3rd hour of testing. Overall, the 3 groups differed significantly (Χ2(7)=26.1, p<0.01). The differences between the mean excitability in naïve(4-AP) animals vs control(saline) were significant for all but one time point (grey asterisks above green symbols, Fig.4 A3) (p<0.05). Similarly, differences in mean excitability between the injured(4-AP) and control(saline) groups were significant for all but one time point(grey symbols above red symbols, Fig.4 A3) (p<0.05).
Cortical responses are strongly elevated by 4-AP, especially in injured rats
Cortical excitability was strongly enhanced by 0.32 mg/kg of 4-AP (Fig.4 B3 & B2). The AUC in the naïve(4-AP) animals increased by 105.6 ± 41.3 (%-hr) from the baseline. In comparison, AUCs from the injured(4-AP) animals for MEPs from the injured side increased by 484.1±114.7 (%-hr) (Fig.4 B2, red), while the AUC for the uninjured side in the same group was increased by 56.9±16.4 (%-hr) (Fig.4 B2, blue). The AUC did not change significantly for the control(saline) animals (Fig.4 B2, grey). When compared across groups, they were significantly different from each other (Χ2(3)=18.2, p<0.01). Mean AUCs were significantly different between all the comparisons(p<0.05), except between the naive(4AP) (green) and contralesional circuit in the injured(4-AP) groups (blue).
Similar to findings for spinal excitability, cortical excitability increased throughout the testing period. The changes in the cortical excitability for each time point are shown in the Fig.4 B3. In the naïve(4-AP) group, the cortical excitability increased by 14±2.1% (Fig.4 B3, green). On the other hand, in the injured(4-AP) animals, the cortical excitability of the injured side was increased by 70.9±6.3% on average (Fig.4 B3, red). In comparison, the cortical excitability of the contralesional circuit in the same group was not as strongly increased, 8.3±2.2% on average (Fig.4 B3, blue). Within group analysis in the control(saline) group indicated that no significant changes occurred in cortical excitability with time from its baseline (Χ2(7)=5.1, p=0.7) (Fig.4 B3, grey). Here too, since we did not have the saline treated injured animals we compared the changes in cortical excitability in the injured(4-AP) group to its own baseline. We found the differences to be significant (within group analysis; Χ2(7)=25.1, p=0.001), and the post-hoc tests found all the time points tested to be significantly different from the baseline. The differences between the three groups overall were significant (Χ2(7)=32.8, p<0.01). The naïve(4-AP) and saline(control) were different at 2 time points (1h and 1.5h; grey asterisks above green symbols, Fig.4 B3), and all the time points were different between injured(4-AP) animals and rest of the groups (grey, blue and green asterisks above red symbols, Fig.4 B3). Responses from the contralesional circuit of injured(4-AP) rats were significantly different from the control(saline) animals at 3 time points (1h, 1.5h and 3h; grey asterisks above blue symbols, Fig.4 B3).
To understand the effects of 4-AP on MEPs better, MEP50 was measured at same time points as cortical excitability. AUCs for the MEP50 data were also enhanced by 4-AP treatment (Fig.4 B4). As described above, MEPs could not be evoked from the injured side of inured(4-AP) animals (red cut circuit) at physiological stimulus intensities. Instead, the uninjured cortex was stimulated and MEPs were determined for the left biceps (blue circuit). The AUC in the naïve(4-AP) animals increased by 196.3±81.8 (%-hr) from its baseline. And in the case of injured(4-AP) animals, there was a marked increase in AUC by 384.6±112.8 (%-hr). However, in the control(saline) animals the mean AUC decreased by 134.4±52.2 (%-hr) from its baseline. Similar to the other two parameters above, we also compared the changes in MEP50 for the injured group with its own baseline to account for the absence of a saline treated injured group. Here too, we found significant differences (within group analysis; Χ2(7)=19.8, p=0.006), and the post-hoc tests indicated the changes were significantly different between baseline and all the time points tested up to the 4th hour of testing. Furthermore, the three groups differed significantly overall (Χ2(2)=8.2, p=0.017). The differences in mean AUC between saline(control) animals and naïve(4-AP) as well as differences between saline(control) and injured(4-AP) were significant (p<0.05). Although the AUC was greater in the injured(4-AP) group than the naïve(4-AP) group, differences were not significant (p>0.05).
The MEP50 for each time point was also markedly enhanced by 4-AP. In the naïve(4-AP) animals, the MEP50 increased by an average of 26.1±12.0% (Fig.4 B5, green). In the injured(4-AP) animals, recording a recruitment curve was possible only for the contralesional circuit (Fig.4 B5, blue). MEP50 from the intact hemisphere in injured(4-AP) animals increased on average by 56.4±8.3% (Fig.4 B5, blue). MEP50 did not change significantly over time in the control(saline) animals as indicated by within group analysis (Χ2(7)=8.6, p=0.3). The groups’ MEP50 means were different overall (Χ2(7)=39.5, p<0.01). Differences in mean MEP50’s were observed between the naive(4-AP) and saline(control) animals between 0.5–2h time points (grey asterisks above green symbols, Fig.4 B5) (p<0.05). In contrast, the mean MEP50’s in the contralesional circuit in the injured(4-AP) and control(saline) animals were significantly different at all time points tested (grey asterisks above blue symbols, Fig.4 B5) (p<0.05).
Discussion
4-AP enhances motor responses in the intact motor circuits. This is true in the uninjured animals and the intact circuits of injured animals. Past studies have also demonstrated that 4-AP can enhance conduction in normally myelinated neurons32,33. However, the effects in the injured(4-AP) animals, following a cut lesion of pyramidal tract, are of particular interest because it indicates that 4-AP is effective in restoring motor responses following axonal injuries. And given we observed these effects with clinically relevant 4-AP plasma concentrations (i.e. exposure), these results are clinically meaningful as well.
4-AP augmented physiological responses from both the spinal cord and motor cortex more strongly in the injured(4-AP) rats. These results indicate that the weak intact circuits that persist after an acute lesion are more sensitive to 4-AP than the uninjured circuits of naive rats. We postulate that the observed effects of 4-AP on the evoked motor responses from injured and spared circuits are due to its ability to lower the excitation threshold necessary to activate such weakened circuits. These strengthened motor responses in the injured animals could have the advantage of enhancing weak connections preferentially.
Strong electrophysiological effects of 4-AP were observed at plasma drug levels consistent with previously published clinical studies. Our goal was to keep the plasma drug levels between 20–100 ng/ml based on clinical studies3,12,34. In both naïve(4-AP) and injured(4-AP) animals, the drug levels did not peak beyond 100 ng/ml (Fig.3 B2). In fact, a strong concordance exists between the drug exposure (plasma AUC data) in animals from this study (Fig.3 B1) to that from the first five hours of plasma data in clinical trial for MS patients35. The enhancement of spinal excitability in the injured(4-AP) animals peaked at 2 hours after infusion, when 4-AP levels were below 50 ng/ml (Fig.3 B2 & Fig.4 A3). Similarly, cortical excitability enhancement and MEP50 enhancements in the same group also continue to be significantly strong beyond 2 hours after infusion (Fig.4 B3 and B5). Despite these strong effects of 4-AP at clinically meaningful plasma levels, one limitation regarding clinical relevance to the drug infusion paradigm in the present study was the lack of an extended-release (ER) oral formulation of the drug mimicking that seen in humans. Since the ER version of the drug is the most efficacious and safe version for clinical use34, future studies will be necessary to test if such version of the drug will also be similarly effective at raising excitability of the spared neural circuits.
There are three other limitations of our study. Firstly, even though plasma levels were the same in the injured and uninjured animals, we did not measure the CNS levels. CNS injuries, like stroke, can result in disruption of the blood brain barrier (BBB)36. In the injured animals, it is plausible that such BBB disruption could result in more rapid passage into the CNS compartment. But given that 4-AP is lipid soluble and capable of BBB penetration37, it is a less likely that there will be higher CNS levels of 4-AP in injured than uninjured rats. Second, in our study we did not treat the injured animals with saline. This limits our ability to interpret our results regarding how injured animals treated with saline would directly compare to the drug treated injured animals. However, our data from uninjured(control) animals treated with saline showed that there is a visually apparent but statistically non-significant decline in cortical excitability and MEP50 (Fig. 4B3 and 4B5, grey symbols). These results suggest that if the injured animals treated with saline were to respond similar to the uninjured animals, the effect size of the drug would remain similarly large. Future studies with direct comparison between the injured animals treated with saline and drug will be necessary to draw proper conclusions. And finally, with regards to the decline in cortical excitability and MEP50 in the uninjured(control) animals, we suspect that prolonged exposure to anesthesia (5+ hours) and prolonged physiological testing periods may have contributed towards these effects. However, within subject analysis indicated these effects were not significant.
In summary, the current data suggest that 4-AP increases excitability in uninjured neural connections, especially in the presence of injury to other circuits. In most of the injuries to the nervous system, including stroke, some of the neural circuits that have potential to take over the function of the injured ones are preserved. The increased motor cortex and spinal cord excitability observed with 4-AP should be corroborated by studies examining improvements in motor skill at the same dose, in addition to the improvement in reflexive behaviors already tested6. Many other studies demonstrate a strong link between increased excitability of spared motor circuits and motor recovery after CNS injury38,39. Hence, a critical next step to the electrophysiologic evidence presented here is to test the behavioral effect of 4-AP in models of spared circuits. These findings provide an important groundwork for expanding the translational potential of 4-AP.
Acknowledgments
The authors gratefully acknowledge Thelma Bethea’s contributions in blood sample collections for pharmacology.
The authors disclosed following potential conflicts of interest to the research, authorship, and/or publication of this article: JBC and AS received grant from the Acorda Therapeutics for the conduction of the study. JBC owns no shares and has no financial interest in Acorda Therapeutics. JFI and TJP are employees and stockholders for Acorda Therapeutics. JFI and TJP participated in designing the experiment and writing the manuscript but did not contribute to data collection, analysis or the decision to publish.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Acorda Therapeutics grant Dal-MECH-Car-1 awarded to JBC and by NIH grants K08 NS073796 and RO1 NS092875(JBC).
Footnotes
Conflict of Interest Statement
Other authors have no conflict to report.
References
- 1.Hayes KC. The use of 4-aminopyridine (fampridine) in demyelinating disorders. CNS Drug Rev. 2004;10:295–316. doi: 10.1111/j.1527-3458.2004.tb00029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belavic JM. Dalfampridine (Ampyra) for multiple sclerosis. Nurse Pract. 2010;35:7–9. doi: 10.1097/01.NPR.0000389051.00652.80. [DOI] [PubMed] [Google Scholar]
- 3.Goodman AD, et al. A phase 3 trial of extended release oral dalfampridine in multiple sclerosis. Annals of neurology. 2010;68:494–502. doi: 10.1002/ana.22240. [DOI] [PubMed] [Google Scholar]
- 4.Hara Y, Kitamura K, Kuriyama H. Actions of 4-aminopyridine on vascular smooth muscle tissues of the guinea-pig. Br J Pharmacol. 1980;68:99–106. doi: 10.1111/j.1476-5381.1980.tb10704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith KJ, Felts PA, John GR. Effects of 4-aminopyridine on demyelinated axons, synapses and muscle tension. Brain. 2000;123(Pt 1):171–184. doi: 10.1093/brain/123.1.171. [DOI] [PubMed] [Google Scholar]
- 6.Iaci JF, et al. Dalfampridine improves sensorimotor function in rats with chronic deficits after middle cerebral artery occlusion. Stroke; a journal of cerebral circulation. 2013;44:1942–1950. doi: 10.1161/STROKEAHA.111.000147. [DOI] [PubMed] [Google Scholar]
- 7.Simpson DM, et al. Dalfampridine in Chronic Sensorimotor Deficits after Ischemic Stroke: A Proof of Concept Study. J Rehabil Med. 2015;47:924–931. doi: 10.2340/16501977-2033. [DOI] [PubMed] [Google Scholar]
- 8.Brus-Ramer M, Carmel JB, Martin JH. Motor cortex bilateral motor representation depends on subcortical and interhemispheric interactions. J Neurosci. 2009;29:6196–6206. doi: 10.1523/JNEUROSCI.5852-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bever CT, et al. The Pharmacokinetics and Tolerability of a Slow-Release Formulation of 4-Aminopyridine in Multiple-Sclerosis Patients. Neurology. 1995;45:A351–A351. [Google Scholar]
- 10.Ball AP, et al. Human botulism caused by Clostridium botulinum type E: the Birmingham outbreak. Q J Med. 1979;48:473–491. [PubMed] [Google Scholar]
- 11.Murray NM, Newsom-Davis J. Treatment with oral 4-aminopyridine in disorders of neuromuscular transmission. Neurology. 1981;31:265–271. doi: 10.1212/wnl.31.3.265. [DOI] [PubMed] [Google Scholar]
- 12.Van Diemen HA, et al. 4-Aminopyridine in patients with multiple sclerosis: dosage and serum level related to efficacy and safety. Clin Neuropharmacol. 1993;16:195–204. doi: 10.1097/00002826-199306000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Minassian K, Hofstoetter U, Tansey K, Mayr W. Neuromodulation of lower limb motor control in restorative neurology. Clinical neurology and neurosurgery. 2012;114:489–497. doi: 10.1016/j.clineuro.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simpson J, Kelly JP. An investigation of whether there are sex differences in certain behavioural and neurochemical parameters in the rat. Behavioural Brain Research. 2012;229:289–300. doi: 10.1016/j.bbr.2011.12.036. [DOI] [PubMed] [Google Scholar]
- 15.Brus-Ramer M, Carmel JB, Chakrabarty S, Martin JH. Electrical stimulation of spared corticospinal axons augments connections with ipsilateral spinal motor circuits after injury. J Neurosci. 2007;27:13793–13801. doi: 10.1523/JNEUROSCI.3489-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stoicea N, et al. Ketamine-Based Anesthetic Protocols and Evoked Potential Monitoring: A Risk/Benefit Overview. Fronteirs in neurology. 2016;10:37. doi: 10.3389/fnins.2016.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zandieh S, Hopf R, Redl H, Schlag MG. The effect of ketamine/xylazine anesthesia on sensory and motor evoked potentials in the rat. Spinal Cord. 2003;41:16–22. doi: 10.1038/sj.sc.3101400. [DOI] [PubMed] [Google Scholar]
- 18.Carmel JB, Berrol LJ, Brus-Ramer M, Martin JH. Chronic electrical stimulation of the intact corticospinal system after unilateral injury restores skilled locomotor control and promotes spinal axon outgrowth. J Neurosci. 2010;30:10918–10926. doi: 10.1523/JNEUROSCI.1435-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carmel JB, Kim S, Brus-Ramer M, Martin JH. Feed-forward control of preshaping in the rat is mediated by the corticospinal tract. The European journal of neuroscience. 2010;32:1678–1685. doi: 10.1111/j.1460-9568.2010.07440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carmel JB, Kimura H, Martin JH. Electrical stimulation of motor cortex in the uninjured hemisphere after chronic unilateral injury promotes recovery of skilled locomotion through ipsilateral control. J Neurosci. 2014;34:462–466. doi: 10.1523/JNEUROSCI.3315-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Porter R. Corticospinal Control of Movement. Science and Practice in Clinical Neurology. 1993:61–74. [Google Scholar]
- 22.Schlaug G, Zheng X. Structural Changes In The Corticospinal Tract After 10 Days Of Combined Physical Therapy And Transcranial Direct Current Stimulation. Stroke; a journal of cerebral circulation. 2015:46. [Google Scholar]
- 23.Stinear C. Prediction of recovery of motor function after stroke. Lancet Neurol. 2010;9:1228–1232. doi: 10.1016/S1474-4422(10)70247-7. [DOI] [PubMed] [Google Scholar]
- 24.McKenna JE, Prusky GT, Whishaw IQ. Cervical motoneuron topography reflects the proximodistal organization of muscles and movements of the rat forelimb: a retrograde carbocyanine dye analysis. J Comp Neurol. 2000;419:286–296. doi: 10.1002/(sici)1096-9861(20000410)419:3<286::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 25.Capogrosso M, et al. A computational model for epidural electrical stimulation of spinal sensorimotor circuits. J Neurosci. 2013;33:19326–19340. doi: 10.1523/JNEUROSCI.1688-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiou WL. Critical evaluation of the potential error in pharmacokinetic studies of using the linear trapezoidal rule method for the calculation of the area under the plasma level--time curve. J Pharmacokinet Biopharm. 1978;6:539–546. doi: 10.1007/BF01062108. [DOI] [PubMed] [Google Scholar]
- 27.Zappulla RA, et al. Noncortical origins of the spinal motor evoked potential in rats. Neurosurgery. 1988;22:846–852. [PubMed] [Google Scholar]
- 28.Carmel JB, Kimura H, Berrol LJ, Martin JH. Motor cortex electrical stimulation promotes axon outgrowth to brain stem and spinal targets that control the forelimb impaired by unilateral corticospinal injury. The European journal of neuroscience. 2013;37:1090–1102. doi: 10.1111/ejn.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jespersen B, Knupp L, Northcott CA. Femoral Arterial and Venous Catheterization for Blood Sampling, Drug Administration and Conscious Blood Pressure and Heart Rate Measurements. Jove-J Vis Exp. 2012 doi: 10.3791/3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Effron B. Local False Discovery Rates. Technical Report. 2005:29. [Google Scholar]
- 31.Mcdonald JH. Handbook of Biological Statistics. Baltimore, Maryland: Sparky House Publishing; 2014. [Google Scholar]
- 32.Kim YI, Goldner MM, Sanders DB. Facilitatory effects of 4-aminopyridine on neuromuscular transmission in disease states. Muscle Nerve. 1980;3:112–119. doi: 10.1002/mus.880030203. [DOI] [PubMed] [Google Scholar]
- 33.Lundh H. Effects of 4-aminopyridine on neuromuscular transmission. Brain research. 1978;153:307–318. doi: 10.1016/0006-8993(78)90409-2. [DOI] [PubMed] [Google Scholar]
- 34.Weir S, Gao Y, Henney HR., 3rd Population pharmacokinetics and pharmacodynamics of dalfampridine-ER in healthy volunteers and in patients with multiple sclerosis. Curr Med Res Opin. 2013;29:1637–1645. doi: 10.1185/03007995.2012.749222. [DOI] [PubMed] [Google Scholar]
- 35.Vollmer T, Blight AR, Henney HR., 3rd Steady-state pharmacokinetics and tolerability of orally administered fampridine sustained-release 10-mg tablets in patients with multiple sclerosis: a 2-week, open-label, follow-up study. Clin Ther. 2009;31:2215–2223. doi: 10.1016/j.clinthera.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 36.Yang Y, Rosenberg GA. Blood-brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke; a journal of cerebral circulation. 2011;42:3323–3328. doi: 10.1161/STROKEAHA.110.608257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lemeignan M, Millart H, Lamiable D, Molgo J, Lechat P. Evaluation of 4-aminopyridine and 3,4-diaminopyridine penetrability into cerebrospinal fluid in anesthetized rats. Brain research. 1984;304:166–169. doi: 10.1016/0006-8993(84)90875-8. [DOI] [PubMed] [Google Scholar]
- 38.Clarkson AN, Huang BS, Macisaac SE, Mody I, Carmichael ST. Reducing excessive GABA-mediated tonic inhibition promotes functional recovery after stroke. Nature. 2010;468:305–309. doi: 10.1038/nature09511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Di Pino G, et al. Modulation of brain plasticity in stroke: a novel model for neurorehabilitation. Nat Rev Neurol. 2014;10:597–608. doi: 10.1038/nrneurol.2014.162. [DOI] [PubMed] [Google Scholar]