Abstract
Rationale
Recent years have seen an increase in the recreational use of novel, synthetic psychoactive substances. There are little or no data on the abuse liability of many of the newer compounds.
Objectives
The current study investigated the discriminative stimulus and locomotor effects of a series of synthetic analogues of cathinone: alpha-PPP, alpha-PHP, alpha-PVT, 3,4-methylenedioxy-PBP (MDPBP), and ethylone.
Methods
Locomotor activity was assessed in an open-field assay using Swiss-Webster mice. Discriminative stimulus effects were assessed in Sprague-Dawley rats trained to discriminate either cocaine or methamphetamine from vehicle.
Results
Each of the compounds produced an inverted-U dose-effect on locomotor activity. Maximal effects were similar among the test compounds, but potencies varied with relative potencies of MDPBP>α-PPP=α-PHP>ethylone>α-PVT. Each of the test compounds substituted fully for the discriminative stimulus effects of methamphetamine. α-PPP, α-PHP, and ethylone fully substituted for cocaine. α-PVT produced a maximum of 50% cocaine-appropriate responding and MDPBP produced an inverted U-shaped dose-effect curve with maximum effects of 67%.
Conclusions
These data provide initial evidence that these structurally-similar, emerging novel psychoactive substances demonstrate potential for abuse and may be utilized for their stimulant-like effects, given their ability to stimulate locomotor activity and their substitution for the discriminative stimulus effects of the classical psychostimulants cocaine and/or methamphetamine.
Among recreational drug users, there has been a significant increase in the use of novel psychoactive substances in recent years. Novel psychoactive substances have presented a significant health risk with numerous adverse reactions and hospitalizations as they become available for recreational use at a faster rate than they can be appropriately characterized (Baumann, 2016).
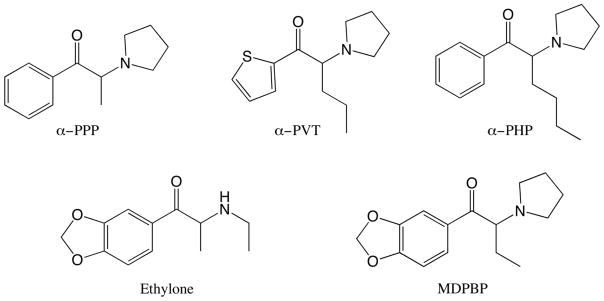
The present study examines a set of five compounds flagged for study by the United States Drug Enforcement Agency because of their increasing prevalence in drug seizures (Brandt et al., 2011; Kikura-Hanajiri et al., 2014; Odoardi et al., 2016; Reitzel et al., 2012; Schneir et al., 2014). The compounds are synthetic analogues of cathinone (Figure 1), the pyrrolidines α-pyrrolidinohexiophenone (α-PHP), α-pyrrolidinopropiophenone (α-PPP), α-pyrrolidinopentiothiophenone (α-PVT), 3,4-methylenedioxybutiophenone (MDPBP), and the methylenedioxy-substituted compound 3,4-methylenedioxyethcathinone (ethylone). α-PVT is a structurally novel cathinone derivative as it has a thiophene ring substituted for the phenyl ring. These compounds add to the many public health risks posed by novel psychoactive substances, as impaired driving, suicide attempts, and overdose deaths have been reported in users of compounds such as ethylone, α-PPP, and α-PHP (Know et al., 2014; Sellors et al., 2014; McIntyre et al., 2015; Klavž et al., 2016).
Figure 1.
Structures of compounds tested
Mechanistic studies report that α-PPP blocked the dopamine and norepinephrine transporters with relative potency DAT>NET and produced increases in locomotor activity (Marusich et al., 2014). α-PVT has been reported to be a dopamine reuptake inhibitor with significant neurotoxic effects (Eshleman et al., 2016; Wojcieszak et al., 2016). Similarly, ethylone inhibited the dopamine, norepinephrine, and serotonin transporters, weakly elicited 5-HT release, and bound to the serotonin 5-HT1A receptor (Eshleman et al., 2016; Simmler et al., 2013). Taken together, these findings indicate that these compounds have mechanisms of action similar to abused psychostimulants such as cocaine and methamphetamine.
Structurally similar compounds have been tested for their discriminative stimulus effects. The pyrrolidines α-pyrrolidinopropiobutiophenone (α-PBP), α-pyrrolidinopentiophenone (α-PVP), 4-methyl-α-pyrrolidinopropiophenone (4-MePPP), 3,4-methylenedioxypyrovalerone (MDPV), and naphyrone all produced full substitution for methamphetamine in rats (Gatch et al., 2013; 2015a; Naylor et al., 2015). All of these compounds except 4-MePPP fully substituted for cocaine in rats (Gatch et al., 2013; 2015a). MDPV fully substituted for cocaine in mice (Gannon et al., 2016), and α-PVP, MDPV and 4-MePVP fully substituted for cocaine in monkeys (Smith et al., 2016). Taken together with the mechanistic studies, these findings suggest that the five related compounds tested in the present study are also likely to have cocaine- and/or methamphetamine-like subjective effects.
The purpose of the present study was to assess the behavioral effects of five compounds: α-PHP, α-PPP, α-PVT, MDPBP, and ethylone in order to contribute to the understanding of their abuse liability. The criteria for abuse liability includes, having chemical structure similar to drugs of abuse, having mechanisms of action similar to drugs of abuse, having discriminative stimuli similar to drugs of abuse, and producing reinforcing effects. As previously mentioned, these compounds have chemical structures and mechanisms of action similar to known drugs of abuse. The locomotor activity assay was used to determine the dose range and time course of the psychoactive effects of the test compounds. Since all five of the test compounds are being used as quasi-legal substitutes for psychostimulants, it was expected that they would produce locomotor stimulant effects. The drug discrimination assay was used to model the subjective effects of the test compounds and is commonly used for predicting the abuse potential of drugs (Carter and Griffiths, 2009; Horton et al., 2013).
Methods
Subjects
Male Swiss–Webster mice were obtained from Envigo (Indianapolis, IN) at approximately 8 weeks of age and tested at approximately 10 weeks of age. Mice were group housed in cages on a 12:12-h light/dark cycle and were allowed free access to food and water. Male Sprague-Dawley rats were obtained from Envigo. All rats were housed individually and were maintained on a 12:12 light/dark cycle (lights on at 7:00 AM). Body weights were maintained at 320–350 g by limiting food to 15 g/day which included the food received during operant sessions. Water was readily available. All housing and procedures were in accordance with Guidelines for the Care and Use of Laboratory Animals (National Research Council, 2011) and were approved by the University of North Texas Health Science Center Animal Care and Use Committee.
Locomotor Activity
The study was conducted using 40 Digiscan (model RXYZCM, Omnitech Electronics, Columbus, OH) locomotor activity testing chambers (40.5 X 40.5 X 30.5 cm) housed within sound-attenuating chambers in sets of two. A panel of infrared beams (16 beams) and corresponding photodetectors were located in the horizontal direction along the sides of each activity chamber. A 7.5-W incandescent light above each chamber provided dim illumination and fans provided an 80-dB ambient noise level within the chamber.
Separate groups of 8 mice were injected with either vehicle (0.9% saline), or a dose of α-PPP (2.5, 5, 10, 25 or 50 mg/kg), α-PHP (1, 2.5, 5, 10, or 25 mg/kg), α-PVT (5, 10, 25, 50 or 100 mg/kg), MDPBP (0.1, 0.25, 0.5, 1 or 2.5 mg/kg), or ethylone (2.5, 5, 10, 25 or 50 mg/kg) immediately prior to locomotor activity testing. Separate vehicle controls were tested for each test compound. In all studies, horizontal activity (interruption of photocell beams) was measured for 8 hours within 10-min periods, in order to establish a time-course of locomotor effects, beginning at 0800 hrs (1 hr after lights on). Studies typically began with 1 mg/kg, after which higher and/or lower doses were tested from no effect, defined as not statistically different from vehicle, to full effect, defined as less than 50% of vehicle control for depressants and less than peak locomotor effect for stimulants.
Discrimination Procedures
Standard behavior-testing chambers (Coulbourn Instruments, Allentown, PA) were connected to IBM-PC compatible computers via LVB interfaces (Med Associates, East Fairfield, VT). The computers were programmed in Med-PC for Windows, version IV (Med Associates) for the operation of the chambers and collection of data.
Using a two-lever choice methodology, a pool of rats was trained to discriminate either methamphetamine (1 mg/kg) or cocaine (10 mg/kg) from saline. Rats received an injection of either saline or drug and were subsequently placed in the behavior-testing chambers, where food (45 mg food pellets; Bio-Serve, Frenchtown, NJ) was available as a reinforcer for every ten responses on a designated injection-appropriate lever. The pretreatment time was 10 min both cocaine and methamphetamine. Each training session lasted a maximum of 10 min, and the rats could earn up to a maximum of 20 food pellets. The rats received approximately 60 of these sessions before they were used in tests for substitution of the experimental compounds. Rats were used in testing once they had achieved 9 of 10 sessions at 85% injection-appropriate responding for both the first reinforcer and total session. The training sessions occurred on separate days in a double alternating fashion (drug-drug-saline-saline-drug; etc.) until the training phase was complete, after which substitution tests were introduced into the training schedule such that at least one saline and one drug session occurred between each test (drug-saline-test-saline-drug-test-drug; etc.). The substitution tests occurred only if the rats had achieved 85% injection-appropriate responding on the two prior training sessions.
α-PPP, α-PHP, α-PVT, MDPBP, and ethylone were tested for substitution in methamphetamine- and cocaine-trained rats. Test sessions lasted for a maximum of 20 min. In contrast with training sessions, both levers were active, such that 10 consecutive responses on either lever led to reinforcement. Data were collected until all 20 reinforcers were obtained, or for a maximum of 20 min. Each compound was tested in groups of six rats. A repeated-measures design was used, such that each rat was tested at all doses of a given drug, including vehicle and training-drug controls. Each group was drawn from a pool of trained rats and some rats may have previously been used to test other compounds. The dose effect of each compound was tested from no effect to full effect or rate suppression (<20% of vehicle control) or adverse effects. Starting doses and pretreatment times were inferred from the locomotor activity testing. Doses were tested in no particular order. For dose-effect experiments, intraperitoneal (i.p.) injections (1 ml/kg) of vehicle or test compound were administered with a pretreatment of 15 min for each compound. Rats that failed to complete the first fixed ratio were excluded from the analysis of drug-appropriate responding, but were used for analysis of response rate.
Drugs
(+)-Methamphetamine hydrochloride, (−)-cocaine hydrochloride, α-pyrrolidinopropiophenone hydrochloride (α-PPP), α-pyrrolidinohexiophenone hydrochloride (α-PHP), α-pyrrolidinopentiothiophenone hydrochloride (α-PVT), 3,4-methylenedioxybutiophenone hydrochloride (MDPBP), and 3,4-methylendioxyethcathinone hydrochloride (bk-MDEA, ethylone) were all supplied by the National Institute on Drug Abuse Drug Supply Program. All test compounds were racemates. All compounds were dissolved in 0.9% saline and administered i.p. in a volume of 1 ml/kg in rats and 10 ml/kg in mice.
Data Analysis
Locomotor activity data were expressed as the mean number of photocell counts in the horizontal plane (ambulation counts) during each 10-min period of testing. A 30-min period, beginning when maximal stimulation of locomotor activity first appeared at the lowest effective dose, was used for analysis of dose-response data. A two-way repeated measures analysis of variance was conducted on horizontal activity counts/10 min interval. A one-way analysis of variance was conducted on horizontal activity counts for the 30-min period of maximal effect (defined as the earliest time period in which a peak effect was observed), and planned comparisons were conducted for each dose against saline control using single degree-of-freedom F tests. A one-way ANOVA was conducted on peak ambulation adjusted for control (peak effects = locomotor activity drug - locomotor activity vehicle) for the five test compounds. The cut-off for statistical significance was set at p<0.05.
Drug discrimination data are expressed as the mean percentage of drug-appropriate responses occurring in each test period. Graphs for percent drug-appropriate responding and response rate were plotted as a function of dose of test compound (log scale). Percent drug-appropriate responding was shown only if at least 3 rats completed the first fixed ratio. Full substitution was defined as ≥80% drug-appropriate responding and not statistically different from the training drug. Rates of responding were expressed as a function of the number of responses made divided by the total session time. Response rate data were analyzed by one-way repeated measures analysis of variance. Effects of individual doses were compared to the vehicle control value using a priori contrasts.
The potencies (ED50 and 95%-confidence intervals) of the test compounds in both assays were calculated by fitting straight lines to the linear portion of the dose-response data for each compound by means of OriginGraph (OriginLab Corporation, Northampton, MA). A one-way ANOVA was conducted on the ED50 values.
Results
Locomotor Activity
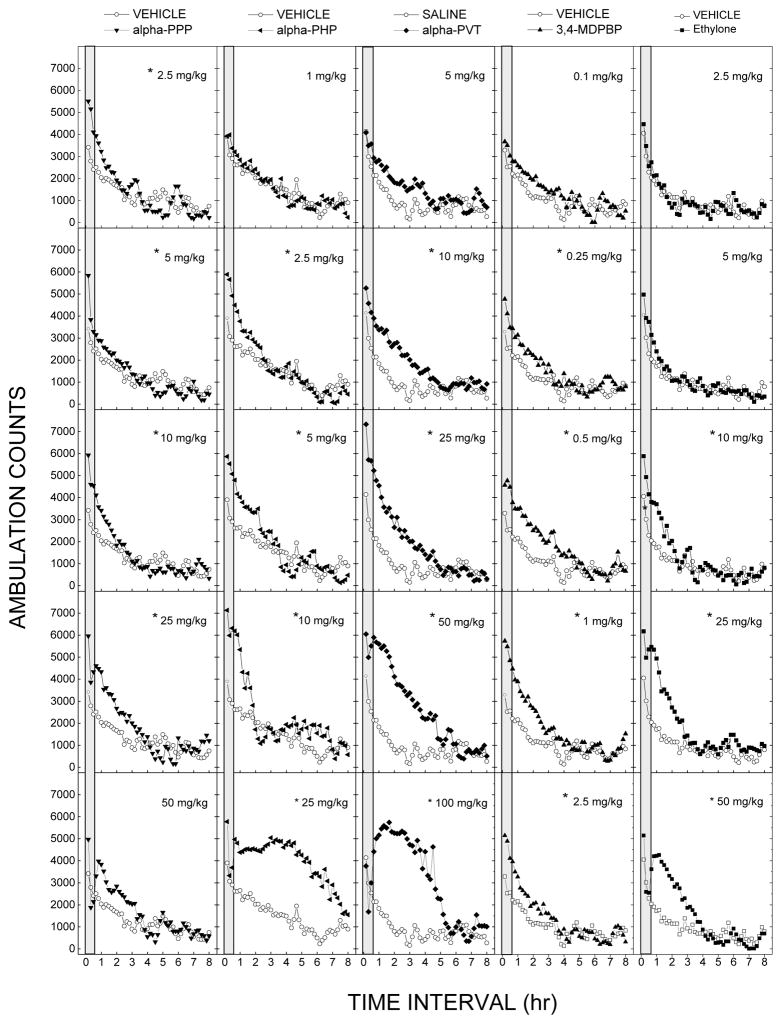
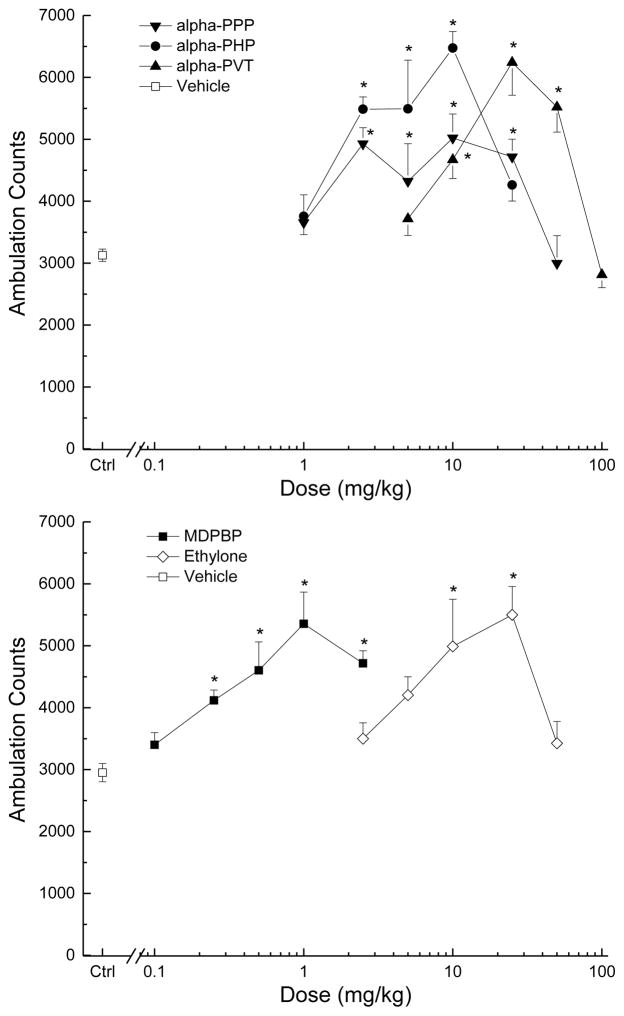
Each of the test compounds produced stimulation of locomotor activity that peaked within the first 30 min at some dose range. Figure 2 shows the average horizontal activity counts/10 min as a function of time (0–8 h) and dose for each of the test compounds. Figure 3 shows the dose-effect curves at the time of peak effect for each compound. There were no differences between the peak effects of the test compounds (adjusted for differing vehicle control levels). There were significant differences in potencies among the test compounds [F(4,35)=61.447, p<.001], with relative potencies 3,4MDPBP>α-PPP=α-PHP>ethylone>α-PVT. ED50 values are shown in Table 1.
Figure 2. Time course of locomotor stimulant effects.
Average horizontal activity counts/10min (ambulation counts) as a function of time and dose for α-PHP, α-PPP, α-PVT, MDPBP, and ethylone. Each panel shows the effects of one dose of compound versus the vehicle; n=8 for each dose. The gray bar shows the time range of peak effect. *indicates stimulant effects (p<0.05) against vehicle control at any time period.
Figure 3. Dose effect of locomotor activity.
Average horizontal activity counts/10 min (±SE) during the 30 min of peak effect as a function of dose for each of the five cathinones. The top panel shows the effects of the three alpha compounds, α-PHP, α-PPP, and α-PVT. The bottom panel shows the effects of the compounds with a methylenedioxy ring, MDPBP and ethylone. n=8 for each dose. Ctrl indicates vehicle control. * indicates stimulant effects (p<0.05) against vehicle control.
Table 1.
ED50 values (mg/kg) for locomotor activity in mice and for discriminative stimulus effects of the test compounds in cocaine- and methamphetamine-trained rats.
| Compound | Locomotor Activity | Methamphetamine Discrimination | Cocaine Discrimination |
|---|---|---|---|
| α-PPP | 1.10±0.19 | 3.01±0.09 | 7.35±0.11 |
| α-PHP | 1.46±0.09 | 1.23±0.10 | 2.86±0.08 |
| α-PVT | 10.47±0.06 | 11.39±0.17 | -- |
| MDPBP | 0.15±0.10 | 1.25±0.12 | -- |
| ethylone | 6.73±0.09 | 5.01±0.08 | 3.37±0.11 |
Treatment with α-PPP resulted in time- and dose-dependent stimulation of locomotor activity following 2.5 to 50 mg/kg (Figure 2). The stimulant effects of 2.5 to 25 mg/kg occurred within 10 minutes following injection and lasted 60 to 190 minutes. The stimulant effects of 50 mg/kg were delayed until 50 min after administration and lasted 140 min. A two-way analysis of variance did not indicate a significant effect of treatment, but yielded a significant effect of time F(47,2961)=101.52, p<.001, and the interaction of time and treatment F(376,2961)=2.04, p<.001. During the time period of maximal stimulant effect (0–30 min), α-PPP produced an inverted U-shaped dose-effect curve with the peak at 10 mg/kg (Figure 3). A one-way analysis of variance indicated a significant effect of treatment F(6,49)=5.95, p<.001. The 1 mg/kg data are omitted from Figure 2 for clarity.
α-PHP produced time- and dose-dependent stimulation of locomotor activity following 2.5, 5 and 10 mg/kg. Stimulant effects occurred within 10 minutes following injection and lasted 100 minutes. Following 25 mg/kg, there was a sharp reduction in stimulant effects at 20–40 min, followed by profound stimulant effects lasting 8 h. There was a significant effect of treatment F(5,42)=18.81, p<.001, of time F(47,1974)=69.82, p<.001, and the interaction of time and treatment F(235,1974)=4.39, p<.001. During the time period of maximal stimulant effect (0–30 min), α-PPP produced an inverted U-shaped dose-effect curve with the peak at 10 mg/kg [F(5,42)=9.495, p<.001].
Treatment with α-PVT resulted in time- and dose-dependent stimulation of locomotor activity following 10, 25 and 50 mg/kg (Figure 2). Stimulant effects of 10 to 50 mg/kg occurred within 10 minutes following injection and lasted 240–250 minutes. After administration of 100 mg/kg, no stimulant effects were observed during the first 30 minutes, followed by strong stimulant effects lasting 250 min. There was a significant effect of treatment F(7,56)=11.97, p<.001, of time F(47,2632)=68.76, p<.001, and the interaction of time and treatment F(329,2632)=4.89, p<.001. During the time period of maximal stimulant effect (0–30 min), α-PVT produced an inverted U-shaped dose-effect curve with the peak at 25 mg/kg [F(5,42)=15.967, p<.001] as shown in Figure 3.
MDPBP produced time- and dose-dependent stimulation of locomotor activity following 0.1 to 2.5 mg/kg (Figure 2). Stimulant effects of 0.25 to 1 mg/kg occurred within 10–20 minutes following injection and lasted 160 to 270 minutes. There was a significant effect of treatment F(5,42)=2.96, p=.022, of time F(47,1974)=79.97, p<.001, and the interaction of time and treatment F(235,1974)=1.82, p<.001. During the time period of maximal stimulant effect (0–30 min), MDPBP produced an inverted U-shaped dose-effect curve with the peak at 1 mg/kg [F(5,42)=8.823, p<.001] as shown in Figure 3.
Treatment with ethylone resulted in time- and dose-dependent stimulation of locomotor activity following 10, 25, and 50 mg/kg (Figure 2). Stimulant effects of 10 and 25 mg/kg occurred within 10 minutes following injection and lasted 120–160 minutes. Stimulant effects of 50 mg/kg were delayed until 40 min after administration, and lasted until 4 h after administration. There was a significant effect of treatment F(7,56)=3.40, p=.004, time F(47,2632)=92.84, p<.001, and the interaction of time and treatment F(329,2632)=3.31, p<.001. During the time period of maximal stimulant effect (0–30 min), ethylone produced an inverted U-shaped dose-effect curve with the peak at 25 mg/kg, as shown in Figure 3. A one-way analysis of variance on the ascending portion of the curve indicated a significant effect of treatment F(5,42)=4.497, p=.002.
Drug Discrimination
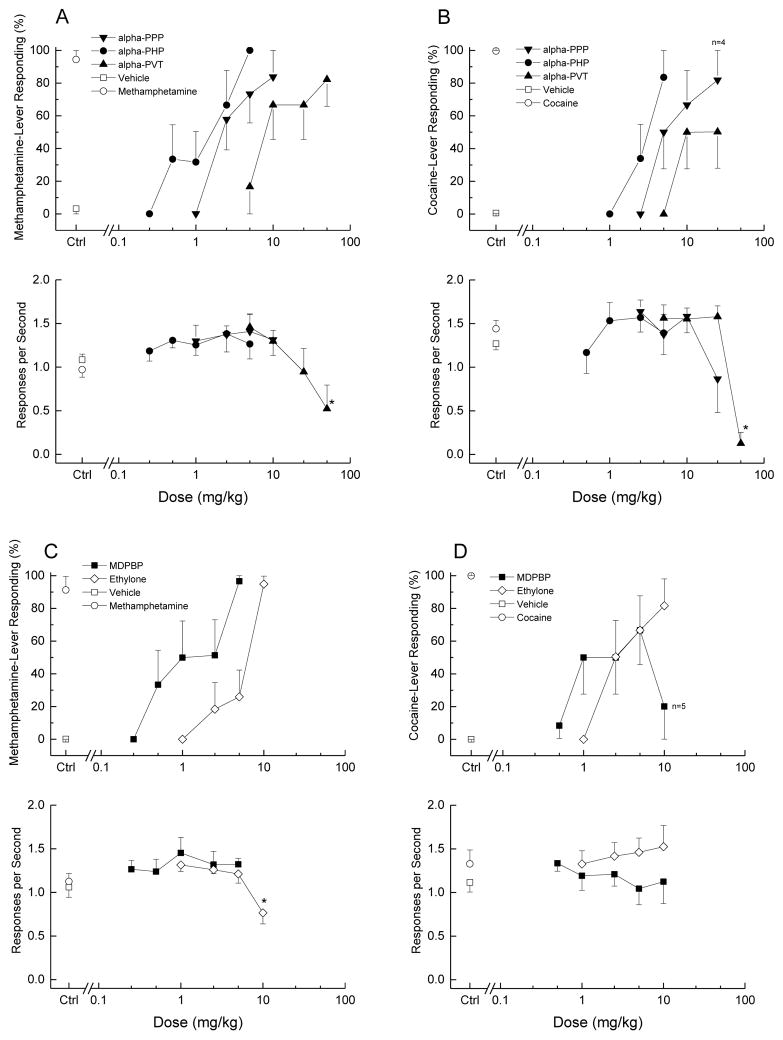
Drug discrimination data are illustrated in figure 4. α-PPP, α-PHP, α-PVT, MDPBP, and ethylone all dose-dependently and fully substituted (≥80% drug-appropriate responding) for the discriminative stimulus effects of methamphetamine. ED50 values are shown in Table 1.α-PHP and MDPBP produced peak discriminative stimulus effects at 5 mg/kg, α-PPP and ethylone at 10 mg/kg, whereas α-PVT required 50 mg/kg to fully substitute for methamphetamine. α-PPP, α-PHP, and MDPBP had no effect on response rate, whereas α-PVT produced an decrease in rate at 50 mg/kg [F(4,20)=3.29, p=.032], and ethylone produced a decrease in rate at 10 mg/kg [F(4,20)=8.29, p<.001].
Figure 4. Substitution for the discriminative stimulus effects of cocaine or methamphetamine.
The top graph in each panel shows the percentage of total responses made on the drug-appropriate lever. The bottom graph shows the rate of responding in responses per second (r/s). Panel A shows the effects of the three alpha compounds, α-PHP, α-PPP and α-PVT in cocaine-trained rats. Panel B shows the effects of α-PHP, α-PPP and α-PVT in methamphetamine-trained rats. Panel C shows the effects of the compounds with a methylenedioxy ring, MDPBP and ethylone, in cocaine-trained rats. Panel D shows the effects of MDPBP and ethylone in methamphetamine-trained rats. n=6 for each compound. Ctrl indicates vehicle and training drug controls. * indicates rate effects (p<0.05) against vehicle control
In contrast, only α-PPP, α-PHP, and ethylone fully substituted for the discriminative stimulus effects of cocaine. ED50 values are shown in Table 1. α-PHP produced full substitution at 5 mg/kg, α-PPP at 25 mg/kg, and ethylone at 10 mg/kg. α-PHP, and ethylone had no effect on response rate. Two out of six rats failed to complete the first fixed ratio following 25 mg/kg α-PPP; however, overall response rate was not affected. α-PVT produced a maximum effect of 50% cocaine-appropriate responding at 10 and 25 mg/kg. Response rate decreased following 50 mg/kg [F(4,20)=23.5, p<.001], such that 5 of 6 rats failed to receive a food pellet. MDPBP produced an inverted U-shaped dose effect with peak effect (67% cocaine-appropriate responding) at 5 mg/kg. A higher dose produced only 20% cocaine-appropriate responding. Despite one of six rats failing to complete the first fixed ratio following 10 mg/kg MDPBP, overall rate of responding was not affected by MDPBP.
Discussion
The present study examines behavioral effects of several cathinone analogs, including the pyrrolidines α-PHP, α-PPP, α-PVT, and MDPBP, as well as the N-ethyl analog ethylone. All five of the compounds produced an inverted u-shaped dose effect curve in the locomotor activity assay, with lower doses producing increasing stimulant effects, whereas the highest doses produced levels of activity below the peak effects. This pattern is consistent with other psychostimulants (e.g., Katz et al., 2001). Two of the compounds, α-PHP and α-PVT, produced very long-acting stimulant effects (5–8 h), even after the immediate effects of the compounds were to depress locomotor activity, which seems to be characteristic of methamphetamine, but not cocaine (Gatch et al., 2013). Similar long-lasting effects were produced by MDPV, naphyrone, α-PVP (Gatch et al., 2015a), methcathinone and pentylone (Gatch et al., 2015b). Ethylone also produced delayed stimulant effects, but the magnitude and duration of the effect was smaller, similar to that produced by methylone and butylone (Gatch et al., 2013).
All five of the test compounds produced full substitution for methamphetamine, although α-PVT did so at a dose that decreased rate of responding. Stimulus control was not affected as the slowing in responding occurred late in the test session after the rats had selected a lever. This is consistent with earlier reports that most other cathinones fully substitute for the discriminative stimulus effects of methamphetamine (Gatch et al., 2013; 2015a; Naylor et al., 2015). The exception thus far is that 4-MEC did not produce significant levels of methamphetamine-appropriate responding at doses up to 8 mg/kg, which significantly decreased rate of responding (Naylor et al., 2015). This is in apparent contrast to a study in which 4-MEC fully substituted for methamphetamine (Gatch et al., 2015b); however, in our study a dose of 50 mg/kg was necessary to produce full substitution. In fact, the dose-effect curve in the Naylor study (1 to 8 mg/kg) was quite similar to the lower portion of the curve in our study (1 to 5 mg/kg). For reasons that are unclear, we were able to test significantly higher doses without the decreases in response rate observed in the Naylor study, although 2 of 6 rats failed to make enough responses to obtain a reinforcer at the 50 mg/kg dose in our study.
In the present study, α-PHP, α-PPP, and ethylone fully substituted for cocaine, whereas α-PVT produced maximum cocaine-appropriate responding of only 50% and MDPBP produced an inverted U-shaped dose effect curve with a maximum of 67% cocaine-appropriate responding. α-PPP fully substituted at a dose that suppressed responding in 2 of 6 rats. Three of the four remaining rats responded robustly on the drug lever, whereas the fourth responded on both levers. In general, most cathinone compounds also fully substitute for the cocaine discriminative stimulus (Gatch et al., 2013; 2015a; 2015b), but there have been other reports of a few compounds failing to substitute for cocaine. 4-MePPP did not fully substitute for cocaine in rats (Gatch et al., 2015a), nor did mephedrone (4-methylmethcathinone) fully substitute in rhesus monkeys (Smith et al., 2016), despite fully substituting for cocaine in rats (Gatch et al., 2013). It should be noted that α-PVT has been reported to produce full substitution in both cocaine- and methamphetamine-trained rats (Cheong et al., 2017), although that study used a lower training dose of cocaine (5.6 mg/kg). There is still a possibility that mephedrone does not have robust cocaine-like discriminative stimulus effects as rats trained to discriminate mephedrone from saline did not fully generalize to cocaine (Varner et al. 2013). 4-MePVP did fully substitute for cocaine (Smith et al., 2016), so the presence of a methyl group at the 4 position does not generally reduce efficacy.
Similarly, there is a possibility that addition of a methylenedioxy ring may reduce efficacy, as 3,4-methylenedioxy analogs of cathinone, amphetamine and methamphetamine had less potency and efficacy in monkeys trained to discriminate cocaine (Smith et al. 2017). This agrees with the finding that α-PBP produced full substitution in cocaine-trained rats (Gatch et al., 2105a), but its 3,4-methylenedioxy analog failed to fully substitute for cocaine in the present study. However, the 3,4-methylenedioxy analogs of α-PVP or methcathinone did not have decreased potency or efficacy (Smith et al., 2016), and several other cathinones with 3,4-methylenedioxy rings, including MDPV, methylone, ethylone, butylone, and pentylone, also fully substitute for cocaine in mice, rats and monkeys (Gannon et al., 2016; Gatch et al., 2013; 2015b; Smith et al., 2016).
The reasons for the differential substitution patterns between methamphetamine- and cocaine-trained rats are not readily apparent from the in vitro literature. With the exception of ethylone, which acts as a hybrid dopamine reuptake inhibitor and serotonin releasing agent, each of the test compounds function as DAT-selective monoamine reuptake inhibitors (Eshleman, 2016). MDPBP and α-PVT, which failed to substitute for cocaine’s discriminative stimulus effects, are not more or less DAT-selective than the other compounds which did substitute (Eshleman, 2016). Given cocaine’s nonselective pharmacodynamic profile, the increased DAT-selectivity of these compounds may explain the preferential substitution for methamphetamine relative to cocaine, but not necessary the differences among the compounds in cocaine-trained rats. Further in vitro studies assessing non-transporter targets and use of selective antagonist in vivo are needed to clarify the substitution profile in cocaine.
In conclusion, all five of the test compounds, α-PHP, α-PPP, α-PVT, MDPBP, and ethylone, fully substituted for the discriminative stimulus effects of methamphetamine, which suggests that all of these compounds may share a similar abuse liability with methamphetamine. α-PVT and MDPBP did not fully substitute for the discriminative stimulus effects of cocaine, which may indicate that they may not support the same range of recreational use as α-PHP, α-PPP, and ethylone. However, α-PVT did produce conditioned place preference in mice and was self-administered in mice, which suggests that it does have substantial abuse liability (Cheong et al., in press). Drug discrimination studies have been very useful for predicting abuse liability, but do not directly test the reinforcing properties of drugs (Horton, et al., 2013). More detailed information on the patterns of recreational use of the compounds, together with further studies of their rewarding and reinforcing effects with conditioned place preference and self-administration will be necessary to make predictions about the extent the degree of substitution for the discriminative stimulus effects of various recreationally used compounds will predict their substitution for recreational use of currently controlled substances.
References
- Baumann MH. The changing face of recreational drug use. Cerebrum. 2016 pii: cer-01-16. [PMC free article] [PubMed] [Google Scholar]
- Brandt SD, Freeman S, Sumnall HR, Measham F, Cole J. Analysis of NRG ‘legal highs’ in the UK: identification and formation of novel cathinones. Drug Test Anal. 2011;3:569–75. doi: 10.1002/dta.204. [DOI] [PubMed] [Google Scholar]
- Carter LP, Griffiths RR. Principles of laboratory assessment of drug abuse liability and implications for clinical development. Drug Alcohol Depend. 2009;105S:S14–S25. doi: 10.1016/j.drugalcdep.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong JH, Choi MJ, Jang CG, Lee YS, Lee S, Kim HJ, Seo JW, Yoon SS. Behavioral evidence for the abuse potential of the novel synthetic cathinone alpha-pyrrolidinopentiothiophenone (PVT) in rodents. Psychopharmacology (Berl) 2017 Jan 9; doi: 10.1007/s00213-017-4526-8. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Eshleman AJ, Wolfrum KM, Reed JF, Kim SO, Swanson TS, Johnson RA, Janowsky A. Structure-activity relationships of substituted cathinones, with transporter binding, uptake and release. J Pharmacol Exp Ther. 2016 Oct; doi: 10.1124/jpet.116.236349. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon BM, Williamson A, Suzuki M, Rice KC, Fantegrossi WE. Stereoselective effects of abused Bath Salt constituent 3,4-methylenedioxypyrovalerone (MDPV) in mice: Drug discrimination, locomotor activity, and thermoregulation. J Pharmacol Ex Ther. 2016;356:615–623. doi: 10.1124/jpet.115.229500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Dolan SB, Forster MJ. Comparative behavioral pharmacology of three pyrrolidine-containing synthetic cathinone derivatives. J Pharmacol Exp Ther. 2015a;354:103–10. doi: 10.1124/jpet.115.223586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Rutledge M, Forster MJ. Discriminative and locomotor effects of five synthetic cathinones in rats and mice. Psychopharmacology. 2015b;232:1197–1205. doi: 10.1007/s00213-014-3755-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Taylor CM, Forster MJ. Locomotor stimulant and discriminative stimulus effects of “Bath Salt” cathinones. Behavioural Pharmacology. 2013;24:437–47. doi: 10.1097/FBP.0b013e328364166d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton DB, Potter DM, Mead AN. A translational pharmacology approach to understanding the predictive value of abuse potential assessments. Behav Pharmacol. 2013;24:10–36. doi: 10.1097/FBP.0b013e3283644d2e. [DOI] [PubMed] [Google Scholar]
- Katz JL, Agoston GE, Alling KL, Kline RH, Forster MJ, Woolverton WL, Kopajtic TA, Newman AH. Dopamine transporter binding without cocaine-like behavioral effects: synthesis and evaluation of benztropine analogs alone and in combination with cocaine in rodents. Psychopharmacology (Berl) 2001;154:362–374. doi: 10.1007/s002130000667. [DOI] [PubMed] [Google Scholar]
- Kikura-Hanajiri R, Kawamura NU, Goda Y. Changes in the prevalence of new psychoactive substances before and after the introduction of the generic scheduling of synthetic cannabinoids in Japan. Drug Test Anal. 2014;6:832–9. doi: 10.1002/dta.1584. [DOI] [PubMed] [Google Scholar]
- Klavž J, Gorenjak M, Marinšek M. Suicide attempt with a mix of synthetic cannabinoids and synthetic cathinones: Case report of non-fatal intoxication with AB-CHMINACA, AB-FUBINACA, alpha-PHP, alpha-PVP and 4-CMC. Forensic Sci Int. 2016;265:121–4. doi: 10.1016/j.forsciint.2016.01.018. [DOI] [PubMed] [Google Scholar]
- Knoy JL, Peterson BL, Couper FJ. Suspected impaired driving case involving α-pyrrolidinovalerophenone, methylone and ethylone. J Anal Toxicol. 2014;38:615–7. doi: 10.1093/jat/bku073. [DOI] [PubMed] [Google Scholar]
- Marusich JA, Antonazzo KR, Wiley JL, Blough BE, Partilla JS, Baumann MH. Pharmacology of novel synthetic stimulants structurally related to the “bath salts” constituent 3,4-methylenedioxypyrovalerone (MDPV) Neuropharmacology. 2014;87:206–13. doi: 10.1016/j.neuropharm.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre IM, Hamm CE, Sherrard JL, Gary RD, Burton CG, Mena O. Acute 3,4-methylenedioxy-N-ethylcathinone (ethylone) intoxication and related fatality: a case report with postmortem concentrations. J Anal Toxicol. 2015;39:225–8. doi: 10.1093/jat/bku146. [DOI] [PubMed] [Google Scholar]
- Naylor JE, Freeman KB, Blough BE, Woolverton WL, Huskinson SL. Discriminative-stimulus effects of second generation synthetic cathinones in methamphetamine-trained rats. Drug Alcohol Depend. 2015;149:280–4. doi: 10.1016/j.drugalcdep.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odoardi S, Romolo FS, Strano-Rossi S. A snapshot on NPS in Italy: Distribution of drugs in seized materials analysed in an Italian forensic laboratory in the period 2013–2015. Forensic Sci Int. 2015;265:116–20. doi: 10.1016/j.forsciint.2016.01.037. [DOI] [PubMed] [Google Scholar]
- Reitzel LA, Dalsgaard PW, Müller IB, Cornett C. Identification of ten new designer drugs by GC-MS, UPLC-QTOF-MS, and NMR as part of a police investigation of a Danish internet company. Drug Test Anal. 2012;4:342–54. doi: 10.1002/dta.358. [DOI] [PubMed] [Google Scholar]
- Schneir A, Ly BT, Casagrande K, Darracq M, Offerman SR, Thornton S, Smollin C, Vohra R, Rangun C, Tomaszewski C, Gerona RR. Comprehensive analysis of “bath salts” purchased from California stores and the internet. Clin Toxicol (Phila) 2014;52:651–8. doi: 10.3109/15563650.2014.933231. [DOI] [PubMed] [Google Scholar]
- Sellors K, Jones A, Chan B. Death due to intravenous use of α-pyrrolidinopentiophenone. Med J Aust. 2014;201:601–3. doi: 10.5694/mja13.00203. [DOI] [PubMed] [Google Scholar]
- Simmler LD, Buser TA, Donzelli M, Schramm Y, Dieu LH, Huwyler J, Chaboz S, Hoener MC, Liechti ME. Pharmacological characterization of designer cathinones in vitro. Br J Pharmacol. 2013;168(2):458–70. doi: 10.1111/j.1476-5381.2012.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DA, Blough BE, Banks ML. Cocaine-like discriminative stimulus effects of amphetamine, cathinone, methamphetamine, and their 3,4-methylenedioxy analogs in male rhesus monkeys. Psychopharmacology (Berl) 2017;234:117–127. doi: 10.1007/s00213-016-4444-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DA, Negus SS, Poklis JL, Blough BE, Banks ML. Cocaine-like discriminative stimulus effects of alpha-pyrrolidinovalerophenone, methcathinone and their 3,4-methylenedioxy or 4-methyl analogs in rhesus monkeys. Addict Biol. 2016 Apr 6; doi: 10.1111/adb.12399. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varner K, Daigle K, Weed P, Lewis P, Mahne S, Sankaranarayanan A, Winsauer P. Comparison of the behavioral and cardiovascular effects of mephedrone with other drugs of abuse in rats. Psychopharmacology (Berl) 2013;225:675–685. doi: 10.1007/s00213-012-2855-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcieszak J, Andrzejczak D, Woldan-Tambor A, Zawilska JB. Cytotoxic activity of pyrovalerone derivatives, an emerging group of psychostimulant designer cathinones. Neurotox Res. 2016;30:239–50. doi: 10.1007/s12640-016-9640-6. [DOI] [PubMed] [Google Scholar]