Abstract
Traumatic brain injury (TBI) is a devastating problem for people of all ages, but the nature of the response to such injury is often different in children than in adults. Cerebral vessel damage and dysfunction are common following TBI, but age-dependent, large-deformation vessel response has not been characterized. Our objective was to investigate the mechanical properties of cerebral arteries as a function of development. Sheep middle cerebral arteries from four age groups (fetal, newborn, juvenile, and adult) were subjected to biaxial loading around physiological conditions and then to failure in the axial direction. Results show little difference among age groups under physiological loading conditions, but response varied significantly with age in response to large axial deformation. Vessels from all age groups reached the same ultimate stretch level, but the amount of stress carried at a given level of stretch increased significantly with age through the developmental period (fetal to juvenile). Our results are the first to identify changes in cerebral vessel response to large deformations with age and may lead to new insights regarding differences in response to TBI with age.
Keywords: cerebral artery development, blood vessel mechanical testing, large deformations, traumatic brain injury, vessel microstructure
INTRODUCTION
Traumatic brain injury (TBI) in the pediatric population is responsible for over 400,000 emergency department visits, 29,000 hospitalizations, and 3,000 deaths each year in the United States.1 Many of these injuries result in life-long neurological deficits. While TBI is not unique to children, the disease often manifests itself differently in children than in adults. For example, contusions in infants are seen as tears in the cerebral white matter and in the outer layers of the cortex rather than as hemorrhage and necrosis.2 Cerebral blood flow (CBF) and autoregulation are commonly disturbed following TBI at all ages, but the response in children is more variable than in adults.3-5 Uncontrollable brain swelling due to an increase in cerebral blood volume and CBF is another unique feature of pediatric TBI.6-8 Reasons for these differences are largely unknown.
Cerebral blood vessels are frequently damaged as part of head trauma in both children and adults, leading to hemorrhage and other vascular dysfunction.9 Because TBI is initiated by mechanical loading, determining tissue-level load response is an important component of understanding the development of injury. We previously characterized the large-deformation properties of human adult cerebral arteries,10-12 but similar data have not been obtained for children. Blood vessels generally become less compliant with age, but most studies showing this emphasized aging rather than development (e.g.13-15). A few investigations on carotid and cerebral arteries obtained from both fetal humans and sheep confirmed a general increase in stiffness across development.16-18 However, only physiological levels of loading in the circumferential direction were explored. In contrast, large axial deformations are thought to play an important role in TBI. Further characterization of age-dependent properties of cerebral vessels is important to better define thresholds for pediatric TBI and may also lend insight into the unique response to TBI seen in children.
We hypothesized that the passive mechanical properties of cerebral arteries, both at subfailure and failure levels, are age dependent. Specifically, we anticipated that vessels from early development would be more extensible but carry less stress than those from later life at similar stretch levels. We tested our hypothesis using cerebral arteries in four age groups: fetal, newborn, juvenile, and adult. Due to the limited availability of human tissue in these age groups, tests were conducted on cerebral vessels from sheep.
METHODS AND MATERIALS
Tissue Collection and Preparation
Middle cerebral arteries (MCAs) were collected from sheep ranging in age from fetal to adult. In some cases, both left and right MCAs from the same sheep were used. Forty-five MCAs were tested from 9 fetal lambs (128-135 days gestation; 85-90% full gestation; n = 11), 7 newborn lambs (8 days old after full term or 150 days gestation; n = 12), 6 juvenile lambs (3–7 months; n = 11), and 9 adult ewes (3–7 years; n = 11). All fetal, four juvenile, and all but one adult vessels were obtained from the Lamb Intensive Care Unit (LICU) at the University of Utah; the remaining vessels were collected from local slaughterhouses. Sheep from the lamb LICU were euthanized by injection of beuthanasia solution, while those from slaughterhouses were sacrificed by either electric or percussion stunning. In the latter cases, the impact site was located away from the Circle of Willis to prevent damage to the MCAs prior to testing. Sheep MCAs were removed shortly after death and placed in cold, calcium-free, phosphate-buffered saline (PBS; KH2PO4 1.54, NaCl 155.2, Na2HPO4-7H2O 2.7; concentrations in mM). Vessel testing always occurred within 48 hours of death. Procedures for obtaining MCAs were prospectively approved by the Institutional Animal Care and Use Committee at the University of Utah.
Following death, the head of the sheep was removed and the top of the skull was cut away. The brain was freed from attachments to the inner surface of the skull, and the rostral portion of the spinal cord was transected. The brain was separated from the dura, taking care to not damage the MCAs, and placed in a petri dish. A proximal segment of the MCA, usually about 8 mm long, was removed with microscissors and placed in cold PBS. Under a dissecting microscope, remaining pia-arachnoid tissue was carefully peeled away from the MCA with forceps. Branches were ligated using unwound 6-0 silk sutures. Cross sections were typically obtained from the proximal end of the dissected vessel and photographed for subsequent measurement.
Mechanical Testing
Mechanical tests were performed using procedures previously reported by our group.12,19 In brief, isolated MCAs were mounted by ligating their ends over grooved, 22-gauge needles using 6-0 silk suture and a small amount of cyanoacrylate. Tests used a custom load frame controlled by LabVIEW (National Instruments, Austin, TX). Axial displacement was produced using either a custom linear stage (Parker Automation, Cleveland, OH) or a voice coil (MGV52-25-1.0, Akribis, Singapore), while force was measured with an inline load cell (Model 31 Low, 250 or 1000 g; Honeywell, Golden Valley, MN). Pressure was controlled with a saline-filled syringe attached to a linear actuator (D-A0.25-ABHT17075-4-P, Ultra Motion, Cutchogue, NY), and inline pressure sensors (26PCDFM6G, Honeywell, Golden Valley, MN) were located equidistant both upstream and downstream of the mounted vessel. Pressure and force measurements were recorded at 100 Hz, while images of the vessel were taken at 3 Hz using a high-resolution camera (PL-A641, Pixelink, Ottawa, Canada). Vessels were immersed in calcium-free PBS to eliminate any active contribution from smooth muscle cells. Tests were performed at room temperature.
Mounted MCAs were preconditioned by cycling the pressure (between 6.7 and 20 kPa) while length was held constant at up to 1.2 times the approximate unloaded length, L, defined subsequently by the point where force began to increase during an unpressurized axial stretch test. MCAs were subjected to six biaxial loading scenarios around the physiological loading range, three inflation tests (up to 20 kPa) with vessel length held constant (at 1.05, 1.07, or 1.10 times the in vivo length – defined below) and three axial stretch tests (up to 1.1 times the in vivo length) with constant pressures (6.7, 13.3, and 20 kPa). MCAs were finally stretched axially to failure under a constant internal pressure of 13.3 kPa. All tests were done quasi-statically.
Data Processing and Analysis
MCAs were assumed to be incompressible, homogeneous, circular cylinders with uniform wall thickness. Unloaded outer diameter (De) and cross sectional area (A) were obtained from images of vessel rings using Vision Assistant (National Instruments, Austin, TX). Reference inner diameter (Di) was calculated from these measurements. Current vessel length (l) was determined from actuator displacement, and current outer diameter (de) was measured from video images obtained during testing. The axial (λZ) and circumferential (λθ) stretch ratios were defined as shown in Eqs. 1 and 2.
| Eq. 1 |
| Eq. 2 |
Current vessel inner diameter (di) was calculated using Eq. 3, assuming incompressibility.
| Eq. 3 |
The mean axial and circumferential Cauchy stresses (Tz, Tθ) were calculated as defined in Eqs. 4 and 5.
| Eq. 4 |
| Eq. 5 |
where pi is pressure and F is axial force. Force data were post-filtered with the butterworth, 4-pole, phaseless filter specified in the SAE J211 standard, and diameters were interpolated between test images to match corresponding force and pressure data.
Axial and circumferential stiffness values were obtained from physiological range stress-stretch data for comparison among age groups. In vivo stiffness was defined by fitting an exponential function to a segment of the stress-stretch curve around the in vivo stretch values (λZ_IV and λθ_IV ± 0.01) and by calculating its derivative at those stretch values. In vivo axial stretch (λZ_IV) was found during preconditioning and was defined as the axial stretch at which force did not change during an increase in pressure up to 20 kPa.20,21 In vivo circumferential stretch (λθ_IV) was determined as the circumferential stretch (Eq. 1) at physiological pressures during a circumferential test conducted at in vivo length. Because blood pressure increases with age,22,23 axial in vivo stiffness values were determined from axial tests having pressures of 6.7 and 13.3 kPa for the two younger and two older age groups, respectively.
Points corresponding with maximum stiffness (MMAX, λMAX) and ultimate failure (TULT, λULT) were derived from the failure stress-stretch curves. Maximum stiffness was defined as the maximum slope between the toe region and the ultimate stress, and was found by plotting the slope of the curve between every 20th data point. This spacing reduced noise while still representing the behavior of the curve. The ultimate point was defined where the maximum stress in the failure test was achieved.
The influence of age on vessel response was evaluated using standard statistical methods, with a p-value ≤ 0.05 considered significant (QIMacros for Excel; KnowWare International, Inc. Denver CO). Levene's test was first used to determine homogeneity of variance. Where variances were found to be similar, group differences were evaluated with ANOVA, followed by LSD post hoc tests. Otherwise, Welch's ANOVA and Games Howell post hoc testing were utilized. In some cases, physiological level stress-stretch data were not obtained. As a result, some analyses did not include a sample size with the aforementioned group numbers.
Qualitative Vessel Histology
Three-four untested vessel cross sections from each age group were fixed in 4% paraformaldehyde and processed for qualitative histological analysis (ARUP Laboratories, Integrated Oncology and Genetics Services Division, Research Histology, Salt Lake City, UT). Cross sections were embedded in paraffin wax, cut into 3 μm thick sections, and stained with Hematoxylin and Eosin (H&E) or Elastica van Gieson (EVG) to identify arterial wall structures. Overall, medial, and internal elastic lamina (IEL) thicknesses were measured. We also estimated IEL tortuosity (length of IEL path divided by arc length, both between the same two points) and number of medial smooth muscle layers. No statistics were performed on section parameters due to the small numbers analyzed.
RESULTS
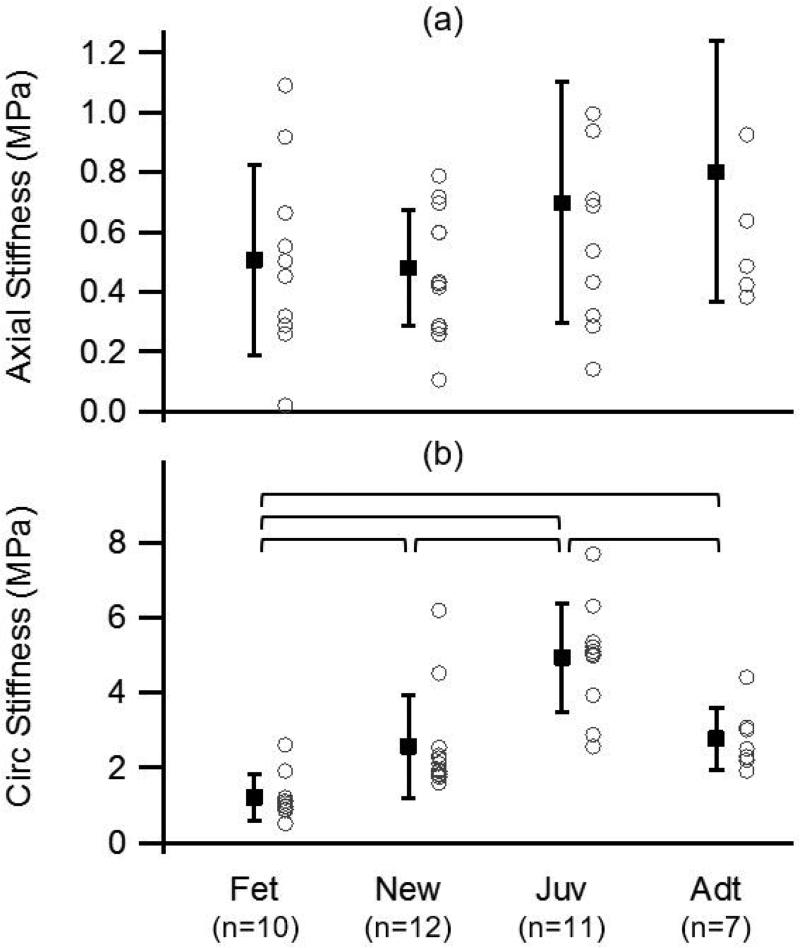
Similar to other soft biological materials, sheep MCA stretch-stress curves demonstrated a typical nonlinear, multiaxial response. Stress-stretch behavior was not obviously different among groups around physiological loads for either direction of loading. Quantitative analysis showed that average in vivo stiffness in the axial direction slightly decreased from fetal to newborn and then increased from newborn to adult, but differences among the means were not statistically significant (ANOVA, p=0.101; Figure 1). In contrast, circumferential stiffness values increased between the fetal and juvenile groups and then surprisingly decreased from juvenile to adult. ANOVA indicated overall differences among groups (p<0.0001), and post-hoc testing revealed statistical significance among all groups except for the comparison between newborn and adult. In vivo stretch and corresponding stress did not change significantly with age. Mean in vivo stretch values were approximately 1.1 and 1.7 for the axial and circumferential directions, respectively, while mean in vivo stress was approximately 0.1 MPa for both directions.
Figure 1.
Mean and standard deviation, along with individual values, of axial (a) and circumferential (b) in vivo stiffness of sheep MCA for the four age groups (fetal, newborn, juvenile, and adult); brackets identify groups statistically different from one another. Stiffness generally increased with age, but changes were not statistically significant in the axial direction.
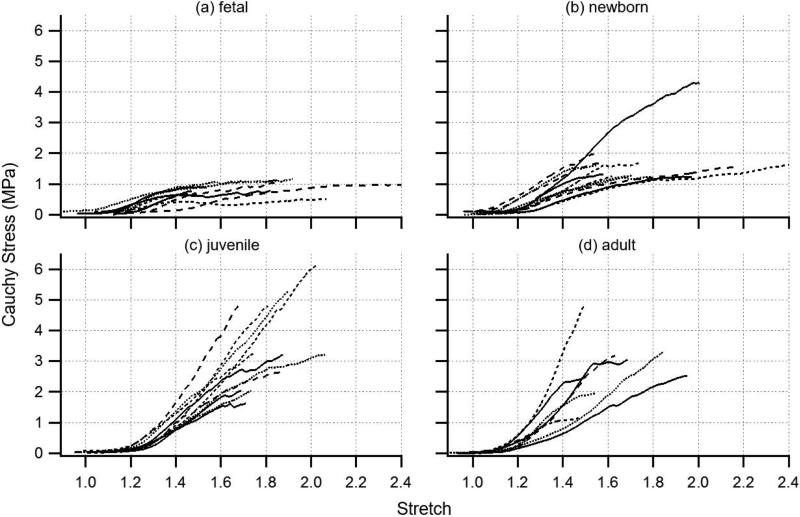
Consistent with the lack of distinction in tests conducted at physiological loads, the toe regions of the large-deformation axial stress-stretch curves were indistinguishable by age group (Figure 2), but a number of other interesting curve shape transitions occurred with age. With axial loading beyond physiological levels in fetal MCAs, stiffness initially increased but then quickly decreased until the stress plateaued with continued stretch. Curves from newborn vessels displayed similar characteristics but typically included a more sustained region of constant stiffness before transitioning relatively abruptly into a stress plateau. The stress level associated with the plateau was typically higher for these vessels than for those from the fetal group. Interestingly, the plateau was uncommon in the juvenile and adult traces; instead, stress typically continued to increase with stretch up to a distinct ultimate failure point, followed by a dramatic drop in stress (not shown in figure). Ultimate stretch did not appear to change significantly among groups. As a result, strain energy, or the area under the stress-stretch curve, also increased with age. In general, there was little difference between juvenile and adult vessels. While some distinct differences were noted among age groups, each collection included traces whose appearance was similar to that of a neighboring group. This may be an indication that the pace of development is somewhat variable from animal to animal.
Figure 2.
Large-deformation axial stress-stretch responses for fetal (a), newborn (b), juvenile (c), and adult (d) ovine MCA reveal a general increase in stiffness and strength with development; various line types serve only to distinguish individual traces within groups.
As shown in Figure 3, overall evaluation of the failure curves revealed significant differences among age groups for the three parameters MMAX, λMAX, and TULT (p<0.0001 in each case); Welch's ANOVA and Games Howell post hoc testing were used with TULT, while ANOVA and LSD post hoc testing were used with the remaining parameters. Between-age differences in maximum stiffness MMAX were found for comparisons between the two young and the two older groups. The stretch at max stiffness, λMAX, was also significantly different among many of the groups. Similar to MMAX, ultimate stress TULT also significantly increased with age, at least up to the juvenile group; ultimate stress tended to be smaller in adults than juveniles, but this difference was not significant. Finally, no statistically significant change was detected in ultimate stretch λULT with age (p=0.151).
Figure 3.
Mean and standard deviation, along with individual values, of large-deformation parameters: stretch at maximum stiffness (a), maximum stiffness (b), ultimate stretch (c), and ultimate stress (d); solid brackets between groups indicate statistical difference by ANOVA and LSD post hoc testing; dashed brackets indicate significance by Welch's ANOVA and Games Howell post hoc testing. All parameter values but those of ultimate stretch generally increased with development.
As an expected part of development, significant differences were indicated among age groups for all three dimensional parameters (p<0.0001 in each case; Figure 4). Post hoc tests showed differences in each case among all age groups except between the fetal and newborn groups and between the newborn and juvenile groups. Each parameter increased consistently with age.
Figure 4.
Mean and standard deviation, along with individual values, of cross sectional area (a), reference outer diameter (b), and wall thickness (c) of sheep MCAs in the unloaded, reference configuration. All dimensions increased with development.
Vessel cross sections stained with H&E and EVG showed some minor structural differences among age groups (Figure 5). Interestingly, the large increase in adult wall thickness seen in fresh sections was not apparent in the histological samples, perhaps a consequence of dehydration. As previously reported for cerebral arteries,24 no external elastic lamina or medial elastic laminae were seen, but the boundary between the adventitia and media was generally better defined in the younger groups. All vessels had a well-developed IEL, though the layer was notably less tortuous in the adult vessels (1.2 compared to 2.1 in the juvenile and 1.6 in the newborn and fetal groups). Within the media, there was a slight increase in the average number of smooth muscle cell layers, increasing from approximately 5 in the fetal and newborn groups to almost 8 in the juvenile and adult groups, though individual layers were difficult to detect without medial elastic laminae.
Figure 5.
Stained sections of ovine MCA at various ages; row (a): H&E (cell nuclei – blue; extracellular matrix – pink), row (b): EVG (elastin – dark purple; collagen – red); scale bar = 100 um
DISCUSSION
The objective of this study was to characterize the development of mechanical properties in cerebral arteries. We tested sheep MCAs at four developmental ages to model development in humans. Results from physiological level data indicate that stiffness is dependent on age in the circumferential, but not axial, direction. In contrast, large-deformation tests in the axial direction revealed significantly stiffer, higher-stress responses with age.
Physiological Level Response
The relatively weak dependence of axial stiffness on age at physiological levels of loading was unexpected, given the large age span studied, but our circumferential results appear to compare reasonably with other studies reporting similar data in developing sheep cerebral arteries.17 Normal mean arterial blood pressure values of lambs and adult sheep are approximately 4-722 and 10-12 kPa,23 respectively, so circumferential stiffness would be expected to increase to accommodate the rise in pressure with age. It should be noted that our tests were generally conducted around a mean pressure of about 13 kPa, slightly higher than normal physiological levels. This difference between test and in vivo pressures, in combination with the nonlinear nature of the stress-stretch curves, may have resulted in higher circumferential stiffness estimates in the younger groups compared to the adult group, potentially explaining the observed drop in circumferential stiffness in adult vessels. Similar to previous research investigating histological changes in cerebral arteries with age,17,25 vessel measurements and the limited histology obtained show changes in vessel dimensions and wall organization with age, but the stress-stretch data suggest that the vessels develop in such a way that they largely maintain their properties through the age range studied, particularly in the axial direction. Pearce et al suggested that the cerebral vessels mature earlier than vessels closer to the heart,17 another possible explanation for the lack of significant change in axial stiffness. However, it is also possible that our apparatus and methods were not sensitive enough to distinguish subtle changes with axial loading. Indeed, the relatively large left-right scatter in the toe regions of the fetal and newborn stress-stretch curves of Figure 2 seem to support the idea that a more precise load cell would have more effectively captured small forces at low deformations. With accompanying tests to failure, however, we also needed to measure relatively high forces, requiring a sensor with less sensitivity at low stretch values.
An unexpected result of our study is that circumferential stiffness values were, on average, multiple times greater than those in the axial direction across all age groups. Our previous work on both human cerebral arteries12 and rat MCAs19 indicate that the opposite is typically true; that is, that the axial direction is stiffer than the circumferential. The relatively high pressures used in this work may have led to higher predictions of circumferential wall stiffness, so the comparison to axial stiffness may not be reliable here.
Large-Deformation Response
In contrast to results at physiological levels of loading, failure tests in the axial direction revealed clear differences with age in response to large deformations. To our knowledge, these are the first data reporting response of pediatric cerebral vessels to large axial stretch. Previous work has shown that the low and high stiffness portions of the circumferential stretch-stretch curve of blood vessels are governed primarily by elastin and collagen, respectively.26 Assuming that similar contributions apply to axial loading, the observed similarity of the toe regions across age groups suggests that the elastin component of the vessel is relatively mature by late gestation in sheep. Using the same reasoning, the considerable differences at larger deformations suggest that collagenous components are still under significant development up to the juvenile period. Our results are also consistent with findings that elastin expression is dramatically reduced at early ages.27,28 However, in the referenced works, the peak of elastin expression occurred after birth. This difference in timing may be species and artery type-dependent, since the prior research was on mouse aorta, but it may also be a manifestation of cerebral arteries maturing earlier than vessels closer to the heart.17
In addition to relative contributions from elastin and collagen, similarities between the evolution of the stress-stretch curve observed here (Figure 2) and that associated with the study of enzymatic cross-links in collagen fibrils (cf. Fig. 5 in 29) are also intriguing. Both experimental 30 and computational 29,31 studies indicate that stress-stretch curves of mature individual fibrils generally demonstrate three phases. The first includes a toe region and linear portion where collagen fibrils are aligning and uncoiling and there is little distinction among fibrils with different cross-link densities and maturities. Immature fibrils with low cross-link density demonstrate a second phase where yielding occurs with intermolecular sliding, resulting in a reduced stiffness that transitions into a plateau until ultimate failure. In contrast, fibrils with a relatively high density of mature cross-links show minimal loss of stiffness in the second phase but progress to a third stage, just prior to catastrophic failure, where stiffness increases to a value higher than previously seen in the loading curve. Age-related changes in the stress-stretch curves observed here display similar patterns, suggesting that they may be associated with increases in both the density and the maturity of enzymatic cross-links in the collagen of the vessel wall. This would also be consistent with the previously identified roles of elastin and collagen, where elastin provides significant support at lower stresses from an early age, and collagen contributes more with age as it is added to the matrix and as cross-links form. However, we have not confirmed that the changes observed here are related to cross-link density, so it should be clear that this is speculation, but others have similarly identified collagen cross-links as having particular influence on arterial stiffening in both development18 and disease.32,33 Additionally, we note that elastin is also enzymatically cross-linked,28 but we are not aware of similar molecular level investigations of elastin cross-linking and mechanics.
Implications for TBI
Although our tests were limited to passive response, results have potentially significant implications for TBI. First, the lack of any difference in ultimate stretch with age indicates that cerebral arteries are similarly vulnerable to hemorrhage throughout all age groups. Other factors surely also contribute to potential differences in injury susceptibility with age, but this study clarifies that vessel ultimate stretch does not. Second, our previous work shows that adult cerebral blood vessels are approximately three orders of magnitude stiffer, in both directions, than the brain parenchyma10,12 and are thus believed to provide much of the supportive structure for brain tissue.34,35 With this in mind, our observations here suggest that pediatric and adult brains would be similarly resistant to deformation at relatively small loads, given similar vessel stiffness values at low strains, but that the pediatric brain would be more susceptible to injury with large applied loads since stiffness and stress are significantly lower at high strains in less developed vessels. While these observations are interesting, it is not clear that they would play a direct role in the unique pediatric physiological response to TBI. However, it has been demonstrated that subfailure mechanical loading can alter extracellular matrix properties,36 as well as smooth muscle cell behavior.37,38 More research is needed to better understand how traumatic loading may impair vessel physiology through development, especially considering our observation of an increasing number of smooth muscle cell layers in the vessel wall during early years.
In considering the reported response to axial loading, it is important to understand that the nature of the loading cerebral vessels experience during TBI is currently unknown, though it likely depends upon both loading conditions and vessel location. Nearly all TBI events include head rotation, leading to relative motion and associated shearing both within the brain parenchyma and between the brain and skull. With this applied shear, it is expected that vessels in these regions rotate until they are able to resist the load axially. As a result, we focused our large deformation tests on axial loading. Despite the provided reasoning, research clarifying TBI-induced vessel strains is needed to better understand what combinations of both axial and circumferential strains may be relevant. Large circumferential strains, not investigated here, may also play an important role in some cases. Due to the thinness of the cerebral vessel wall, shear strains are not expected to be significant at the tissue level.
Limitations
While the presented data are reliable, some limitations in methodology should be noted. Vessels were refrigerated for up to 48 hours before testing. While this methodology has been utilized by many in similar prior studies (see 39), recent work suggests that such storage may lead to changes in properties.40 Unfortunately, storage effects have not been rigorously studied on cerebral arteries, or more broadly, muscular arteries, so it is not clear how this concern may apply to the current study. Regardless, it would have been better for our tests to be conducted within a shorter time window, but our vessel sources did not allow for this. Another potential limitation relates to test pressures. Given the referenced typical physiological pressure ranges of lambs compared to adult sheep, vessels were subjected to relatively high pressures during physiological-level testing. Because our objective was to compare tissue properties between pediatric and adult vessels, it was necessary to subject all age groups to a similar loading range. While it is common to test vessels at pressures slightly above the physiological range,41-43 we found that the imposed pressures resulted in tissue softening in the circumferential direction, typically producing rightward shifts of stretch values between 0.05 and 0.1. However, the observed softening was consistent across age groups. Another limitation is that stretch values were calculated from actuator displacement rather than tissue fiducial markers, which could lead to higher reported stretch values because axial stretch tends to be higher at the ends where circumferential stretch is constrained.44 Comparisons between stretch values calculated from microsphere and actuator motion indicate differences between 2 and 10%. Additionally, it is unknown whether the changes in mechanical characteristics with age presented here are similar in humans. Previous work in our lab with adult human arteries suggests some potential for quantitative differences, but we would at least expect similar observations qualitatively. While this study explored age dependence from the fetal (approximately 86% gestation) to adult periods, exact correspondence with human development is unknown.
Summary
Consistent with previous work,13-18 our findings indicate a stiffening of the cerebral artery with advancing age, including during development. The present findings are the first to consider the influence of development on mechanical properties in the axial direction, as well as on failure level deformations. Variation in the mechanical properties of cerebral vessels during development may contribute to differences in TBI symptoms observed in children relative to adults. The characterization of age-related changes in cerebral vessels is an important step toward better identification of TBI-producing loads and the development of improved diagnostic and treatment strategies for victims.
ACKNOWLEDGEMENTS
Financial support was provided, in part, by the Primary Children's Medical Center Foundation (PCMCF-ISA-KM-01-2012-02 to KLM) and by the National Institutes of Health (HL-110002 to KHA).
Footnotes
The authors report no conflicts of interest.
REFERENCES
- 1.Faul M, Xu L, Wald MM, Coronado VG. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002-2006. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; Atlanta, GA: 2010. [Google Scholar]
- 2.Lindenberg R, Freytag E. Morphology of brain lesions from blunt trauma in early infancy. Arch. Pathol. 1969;87:298–305. [PubMed] [Google Scholar]
- 3.Langfitt TW, Obrist WD, Gennarelli TA, O'Connor MJ, Weeme CA. Correlation of cerebral blood flow with outcome in head injured patients. Ann. Surg. 1977;186:411–414. doi: 10.1097/00000658-197710000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Udomphorn Y, Armstead WM, Vavilala MS. Cerebral blood flow and autoregulation after pediatric traumatic brain injury. Pediatr. Neurol. 2008;38:225–234. doi: 10.1016/j.pediatrneurol.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armstead WM. Cerebral hemodynamics after traumatic brain injury of immature brain. Exp. Toxicol. Pathol. 1999;51:137–142. doi: 10.1016/S0940-2993(99)80087-6. [DOI] [PubMed] [Google Scholar]
- 6.Kochanek PM. Pediatric traumatic brain injury: quo vadis? Dev. Neurosci. 2006;28:244–255. doi: 10.1159/000094151. [DOI] [PubMed] [Google Scholar]
- 7.Snoek JW, Minderhoud JM, Wilmink JT. Delayed deterioration following mild head injury in children. Brain. 1984;107(Pt 1):15–36. doi: 10.1093/brain/107.1.15. [DOI] [PubMed] [Google Scholar]
- 8.Bruce DA. Head injuries in the pediatric population. Curr. Probl. Pediatr. 1990;20:61–107. doi: 10.1016/0045-9380(90)90009-p. [DOI] [PubMed] [Google Scholar]
- 9.DeWitt D, Prough DS. Traumatic cerebral vascular injury: the effects of concussive brain injury on the cerebral vasculature. J. Neurotrauma. 2003;20:795–825. doi: 10.1089/089771503322385755. [DOI] [PubMed] [Google Scholar]
- 10.Monson KL, Goldsmith W, Barbaro NM, Manley GT. Axial mechanical properties of fresh human cerebral blood vessels. J. Biomech. Eng. 2003;125:288–294. doi: 10.1115/1.1554412. [DOI] [PubMed] [Google Scholar]
- 11.Monson KL, Goldsmith W, Barbaro NM, Manley GT. Significance of source and size in the mechanical response of human cerebral blood vessels. J. Biomech. 2005;38:737–744. doi: 10.1016/j.jbiomech.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Monson KL, Barbaro NM, Manley GT. Biaxial response of passive human cerebral arteries. Ann. Biomed. Eng. 2008;36:2028–2041. doi: 10.1007/s10439-008-9578-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Busby D, Burton A. The effect of age on the elasticity of the major brain arteries. Can. J. Physiol. Pharmacol. 1965;43:185–202. doi: 10.1139/y65-018. [DOI] [PubMed] [Google Scholar]
- 14.Cox RH. Effects of age on the mechanical properties of rat carotid artery. Am. J. Physiol. 1977;233:H256–H263. doi: 10.1152/ajpheart.1977.233.2.H256. [DOI] [PubMed] [Google Scholar]
- 15.Nagasawa S, Handa H, Okumura A, Naruo Y, Moritake K, Hayashi K. Mechanical properties of human cerebral arteries. Part 1: Effects of age and vascular smooth muscle activation. Surg. Neurol. 1979;12:297–304. [PubMed] [Google Scholar]
- 16.Bevan RD, Vijayakumaran E, Gentry A, Wellman T, Bevan JA. Intrisic Tone of Cerebral Artery Segments of Human Infants between 23 Weeks of Gestation and Term. Pediatr. Res. 1998;43:20–27. doi: 10.1203/00006450-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Pearce WJ, Hull AD, Long DM, Longo LD. Developmental changes in ovine cerebral artery composition and reactivity. Am. J. Physiol. 1991;261:R458–R465. doi: 10.1152/ajpregu.1991.261.2.R458. [DOI] [PubMed] [Google Scholar]
- 18.Roach MR. The static elastic properties of carotid arteries from fetal sheep. Can. J. Physiol. Pharmacol. 1970;48:695–708. [PubMed] [Google Scholar]
- 19.Bell ED, Kunjir RS, Monson KL. Biaxial and failure properties of passive rat middle cerebral arteries. J. Biomech. 2013;46:91–96. doi: 10.1016/j.jbiomech.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Loon P, Klip W, Bradley EL. Length-force and volume-pressure relationships of arteries. Biorheology. 1977;14:181–201. [PubMed] [Google Scholar]
- 21.Weizsacker HW, Lambert H, Pascale K. Analysis of the passive mechanical properties of rat carotid arteries. J Biomech. 1983;16:703–715. doi: 10.1016/0021-9290(83)90080-5. [DOI] [PubMed] [Google Scholar]
- 22.Unno N, Wong CH, Jenkins SL, Wentworth RA, Ding X-Y, Li C, Robertson SS, Smotherman WP, Nathanielsz PW. Blood pressure and heart rate in the ovine fetus: ontogenic changes and effects of fetal adrenalectomy. Am. J. Physiol. 1999;276:H248–H256. doi: 10.1152/ajpheart.1999.276.1.H248. [DOI] [PubMed] [Google Scholar]
- 23.Dawes G, Johnston BM, Walker D. Relationship of arterial pressure and heart rate in fetal, new-born and adult sheep. J. Physiol. 1980;309:405. doi: 10.1113/jphysiol.1980.sp013516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee RMKW. Morphology of cerebral arteries. Pharmacology & Therapeutics. 1995;66:149–173. doi: 10.1016/0163-7258(94)00071-a. [DOI] [PubMed] [Google Scholar]
- 25.Goyal R, Henderson DA, Chu N, Longo LD. Ovine middle cerebral artery characterization and quantification of ultrastructure and other features: changes with development. Am. J. Physiol. 2012;302:R433–R445. doi: 10.1152/ajpregu.00519.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roach MR, Burton AC. The reason for the shape of the distensibility curves of arteries. Can. J. Biochem. Physiol. 1957;35:681–690. [PubMed] [Google Scholar]
- 27.Kelleher CM, McLean SE, Mecham RP. Vascular Extracellular Matrix and Aortic Development. In: Gerald PS, editor. Current Topics in Developmental Biology. Academic Press; 2004. pp. 153–188. [DOI] [PubMed] [Google Scholar]
- 28.Wagenseil JE, Mecham RP. Vascular extracellular matrix and arterial mechanics. Physiol. Rev. 2009;89:957–989. doi: 10.1152/physrev.00041.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Depalle B, Qin Z, Shefelbine SJ, Buehler MJ. Influence of cross-link structure, density and mechanical properties in the mesoscale deformation mechanisms of collagen fibrils. J. Mech. Behav. Biomed. Mater. 2015;52:1–13. doi: 10.1016/j.jmbbm.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Svensson Rene B., Mulder H, Kovanen V, Magnusson SP. Fracture Mechanics of Collagen Fibrils: Influence of Natural Cross-Links. Biophys. J. 2013;104:2476–2484. doi: 10.1016/j.bpj.2013.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buehler MJ. Nanomechanics of collagen fibrils under varying cross-link densities: Atomistic and continuum studies. J. Mech. Behav. Biomed. Mater. 2008;1:59–67. doi: 10.1016/j.jmbbm.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 32.Wang Z, Lakes RS, Eickhoff JC, Chesler NC. Effects of collagen deposition on passive and active mechanical properties of large pulmonary arteries in hypoxic pulmonary hypertension. Biomech. Model. Mechanobiol. 2013;12:1115–1125. doi: 10.1007/s10237-012-0467-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tian L, Wang Z, Liu Y, Eickhoff JC, Eliceiri KW, Chesler NC. Validation of an arterial constitutive model accounting for collagen content and crosslinking. Acta. Biomater. 2016;31:276–287. doi: 10.1016/j.actbio.2015.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang L, Bae J, Hardy WN, Monson KL, Manley GT, Goldsmith W, Yang KH, King AI. Computational study of the contribution of the vasculature on the dynamic response of the brain. Stapp. Car. Crash. J. 2002;46:145–164. doi: 10.4271/2002-22-0008. [DOI] [PubMed] [Google Scholar]
- 35.Ho J, Kleiven S. Dynamic response of the brain with vasculature: a three-dimensional computational study. J Biomech. 2007;40:3006–3012. doi: 10.1016/j.jbiomech.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 36.Bell ED, Sullivan JW, Monson KL. Subfailure overstretch induces persistent changes in the passive mechanical response of cerebral arteries. Frontiers in Bioengineering and Biotechnology. 3:2015. doi: 10.3389/fbioe.2015.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alford PW, Dabiri BE, Goss JA, Hemphill MA, Brigham MD, Parker KK. Blast-induced phenotypic switching in cerebral vasospasm. Proc. Natl. Acad. Sci. U. S. A. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:12705–12710. doi: 10.1073/pnas.1105860108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boock R. Vascular Response to Mechanical Deformations. University of Pennsylvania; Philadephia: 1991. [Google Scholar]
- 39.Humphrey JD. Cardiovascular Solid Mechanics : Cells, Tissues, and Organs. Springer; New York: 2002. [Google Scholar]
- 40.Stemper BD, Yoganandan N, Stineman MR, Gennarelli TA, Baisden JL, Pintar FA. Mechanics of fresh, refrigerated, and frozen arterial tissue. J Surg Res. 2007;139:236–242. doi: 10.1016/j.jss.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 41.Dobrin P, Rovick A. Influence of vascular smooth muscle on contractile mechanics and elasticity of arteries. Am. J. Physiol. 1969;217:1644–1651. doi: 10.1152/ajplegacy.1969.217.6.1644. [DOI] [PubMed] [Google Scholar]
- 42.Cox RH. Comparison of carotid artery mechanics in the rat, rabbit, and dog. Am. J. Physiol. 1978;234:H280–H288. doi: 10.1152/ajpheart.1978.234.3.H280. [DOI] [PubMed] [Google Scholar]
- 43.Docherty CC, Kalmar-Nagy J, Engelen M, Nathanielsz PW. Development of fetal vascular responses to endothelin-1 and acetylcholine in the sheep. Am. J. Physiol. 2001;280:R554–R562. doi: 10.1152/ajpregu.2001.280.2.R554. [DOI] [PubMed] [Google Scholar]
- 44.Monson KL, Mathur V, Powell DA. Deformations and end effects in isolated blood vessel testing. J. Biomech. Eng. 2011;133:011005. doi: 10.1115/1.4002935. [DOI] [PMC free article] [PubMed] [Google Scholar]