Abstract
The aim of the present study was to evaluate in patients with acute lymphoblastic leukemia (ALL) the oxidative status and antioxidant defense and its involvement in the relapse of ALL. The plasmatic levels of malondialdehyde (MDA), advanced oxidation of protein products (AOPP) and reduced glutathione (GSH), and the plasmatic activities of catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GPx) were determined in 34 patients newly-diagnosed with ALL and compared with 92 healthy subjects. The plasmatic concentrations of MDA and AOPP were higher in ALL patients than in controls and increased during chemotherapy. A decrease in GPx activity and an increase in CAT and SOD activities and GSH plasma levels were observed in ALL patients, as compared to sex-matched controls. Moreover, SOD activity and GSH levels were significantly correlated with the relapse of ALL patients. These data suggest the involvement of oxidative stress in acute lymphoid leukaemias and leukaemic relapse.
Keywords: Acute lymphoblastic leukemia (ALL), Oxidative stress, Antioxidant defences
Introduction
Acute lymphoblastic leukemia (ALL) is a malignant neoplasm of lymphocytes characterized by uncontrolled proliferation and maturation arrest of lymphoid progenitor cells in bone marrow resulting in an excess of malignant cells [1]. This disease is more common in children which represent 80% of all leukaemias. It is a curable disease with an expected long term survival rate of at least 70%, when treated with modern therapeutic regimens. A common chemotherapy treatment for ALL begins with induction chemotherapy, in which a combination of drugs is used to destroy as many leukemia cells as possible and bring blood counts to normal [2]. This is followed by consolidation chemotherapy, to destroy any remaining leukemia cells that cannot be seen in the blood or bone marrow. Patients with ALL may also receive maintenance chemotherapy.
Nevertheless, in spite of this intensive chemotherapy relapse represents the main cause for treatment failure [3]. Several approaches have been proposed to help predict the poor disease-free and poor overall survival rates for children with ALL [4, 5].
Extensive evidence has shown that disturbances of oxidative stress metabolism are a common feature of transformed tumor cells [6]. Oxidative stress has been characterized by an imbalance between the production of reactive oxygen species (ROS) and a biological system’s ability to repair oxidative damage or to neutralize the reactive intermediates including peroxides and free radicals [7]. Enhanced level of ROS can cause damage to biomolecules such as lipids, proteins and DNA, leading to cellular dysfunction and cell death. The effect of reactive species is balanced by the antioxidant action of non-enzymatic antioxidants, as well as by antioxidant enzymes [8].
Conflicting data have been reported on oxidative status in ALL patients. Moreover, information about the levels of oxidative damage and antioxidant defenses in patient just diagnosed with ALL as compared to those in the different stages of treatment are rare [9].
This study was performed in patients with ALL to evaluate: (i) the oxidative status, through the measurement of lipid peroxidation and the levels of advanced oxidation of protein products (AOPP), (ii) the antioxidant defense through the verification of main enzymatic antioxidant defenses (superoxide dismutase (SOD), catalase (CAT) and Glutathione peroxidase (GPx)) and non-enzymatic antioxidants (thiol antioxidants), and (iii) the involvement of oxidative status in the relapse of ALL.
Methods
Subjects
The sample consisted of 34 patients (22 males and 12 females) newly-diagnosed with ALL, who were prospectively recruited from the hematology department of Hedi Chaker-Hospital University in Sfax (south of Tunisia) from January 2013 to December 2014.
The control group consisted of 92 sex-matched healthy control subjects taken from the general population. Exclusion criteria of this group were: (i) age > 30 years old, (ii) history of alcoholism, smoking, and diseases which induce oxidative stress such as diabetes mellitus, respiratory diseases, cardiovascular diseases etc, (iii) taking any medication or antioxidant supplements.
Patients were diagnosed and treated according to the EORTC-CLG 58951 protocol, a Berlin-Frankfurt-Munster–like trial. The treatment involves a sequential phase of induction, consolidation, interval, intensification and maintenance therapy [10].
Patient data were collected from the hospital records and extracted information like age, gender, subtype of ALL (B-cell ALL or T-cell ALL), biologic disorder in blood (complete blood count, Fibrinogen levels, acid uric levels, liver and renal function tests), blast count in marrow. Information about the risk of relapse of ALL within 2 years from initial diagnosis was also collected for each patient.
This study was conducted in accordance with the Helsinki Declaration and informed consent was taken from all the persons participating in this study.
Plasma samples
Blood was collected in tubes containing ethylene diaminetetraacetic acid (EDTA). Plasma was separated from red cells by centrifugation at 3000 rpm for fifteen minutes. Plasma was immediately aliquoted and stored at −80 °C until analysis.
For each ALL patient included in this study, blood samples were collected at diagnostic just before chemotherapy, then during successive treatment phases: induction, consolidation, interval and maintenance therapy.
Protein rate determination
Total protein concentration was performed by the Bradford method [11], calibrated with bovine serum albumin.
Measurement of malondialdehyde (MDA)
Lipid peroxidation was assessed by thiobarbituric acid reactive substances (TBARS) by measuring plasma levels of MDA, using the method described by Buege and Aust [12]. Briefly, the samples were mixed with thiobarbituric acid (TBA) solution (15% trichloroacetic acid (TCA), 0.8% TBA, 0.25 N HCl). This suspension was incubated at 95°C for 15 min to form pink colored adduct, TBA-MDA adduct. The mixture was then centrifuged at 3000 rpm for 10 min and cooled in ice for 5 minutes. Absorption of supernatants was read at a wavelength of 532 nm. Concentrations were estimated from a standard curve of malondialdehyde bis-(dimethyl acetal) and reported as nmol MDA/mg protein.
Measurement of catalase (CAT) activity
Catalase activity was determined by the method of Aebi based on the decomposition of H2O2 [13]. The plasma was added to a reaction mixture containing 0.1 M potassium phosphate buffer (pH 7) and 0.05 mM H2O2. The conversion of H2O2 into H2O and O2 in 1 min under standard condition was considered to be the enzyme reaction velocity. Catalase activity was calculated based on an extinction coefficient 43.1 M-1 cm-1 for H2O2 at 240nm and expressed as nmols of H2O2 consumed/min/mg of protein.
Measurement of superoxide dismutase (SOD) activity
SOD activity was assayed in terms of its ability to inhibit the photochemical reduction of nitroblue tetrazolium (NBT) according to Beauchamp and Fridovich [14]. The reaction mixture contained, 0.1 M potassium phosphate buffer (pH 7.4), 0.26 mM riboflavin, 2.69 mM methionine and 2.64 mM NBT, with suitably diluted plasma in a total volume of 1.5 ml. The assay mixture was illuminated for 20 min by a 20 W fluorescent lamp, in aluminium foil-lined container. Illumination of riboflavin in the presence of O2 and electron donor like methionine generates superoxide anions. The reduction of NBT by superoxide radicals to blue coloured formazan was followed at 580 nm. Control without the enzyme source was always included. One unit of SOD activity is defined as that amount of enzyme that inhibits the rate of NBT reduction by 50% under the specified conditions. SOD activity was expressed as mUI/mg of protein.
Measurement of reduced Glutathione (GSH)
Plasma GSH levels were measured as described by Weckbecker and cory [15]. The method involved oxidation of GSH by the sulfhydryl reagent 5,5′-dithio-bis(2-nitrobenzoic acid) (DTNB) to form the yellow derivative 5′-thio-2-nitrobenzoic acid (TNB), measurable at 412 nm. Briefly, plasma was diluted (1:1) in 4% sulfosalicylic acid and centrifuged at 3500 rpm for 10 min. The supernatant was mixed with 0.1 M potassium phosphate buffer (pH 7.4), then with 10mM DTNB. Le mixture was incubated for 15 minutes in the dark at room temperature. The amount of GSH in plasma was expressed as nmols/mg of protein, determined from a standard curve constructed with known concentrations of GSH.
Measurement of Glutathione peroxidase (GPx) activity
The activity of GPx was quantified by the procedure of Flohé and Günzler [16]. Briefly, the plasma was added to a reaction mixture containing 0.1 M potassium phosphate buffer (pH 7.4) and 4 mM GSH. After 10 min incubation at 37 °C, 5 mM H2O2 was added in the mixture. The reaction was then stopped by the addition of 5 % TCA. After centrifugation at 3000 rpm for 10 min at 4°C, the supernatant was combined with phosphate buffer and 10 mM DTNB and absorbance was read at 412 nm. The activity of GPx was expressed as nmol of GSH consumed/min/mg protein.
Measurement of advanced oxidation of protein products (AOPP) levels
Levels of advanced oxidation of protein products (AOPP) were determined according to the method of Kayali et al. [17]. Briefly, plasma was treated with phosphate buffer (0.1 M; pH 7.4). After 2 min incubation, 1.16 M potassium iodide and 10% TCA were added to the mixture. The concentration of AOPP for each sample was calculated based on an extinction coefficient of 261 cm-1mM-1 at 340 nm and expressed as nmol/mg of protein.
Statistical analysis
Statistical analysis was carried out by analysis of variance (one-way ANOVA) followed by a Tukey test or Kruskal–Wallis/Dunn’s Multiple comparison test for comparison of the means, using SPSS version 20. Values for means ± SD were used. Kaplan Meier survival curves were also performed. For all comparisons, differences were considered statistically significant at p < 0.05.
Results
Patient’s characteristics
The mean age for ALL patients was 13.7 ± 8.7 years (age range, 3 months–29 years) and for healthy subjects was 18.3 ± 6.1 years (age range, 15–29 years) (p=0.09). B-cell ALL was diagnosed for 23 patients (65.7%) and T-cell ALL for 12 patients (34.3%).
Biological characteristics of the 34 ALL patients at diagnosis and undergoing intensive chemotherapy were summarized in Tables I. We observe the presence of blast cells in the patients just diagnosed. The lower White blood cell count was observed in induction phase. Hemoglobin, mean corpuscular volume and platelets count were decreased at diagnosis and in induction phase and were then increased in the later phases.
Table I.
Levels of different bio-chemicals markers in 34 ALL patients
| Before treatment | Induction phase | Consolidation phase | Interval phase | Maintenance phase | p | |
|---|---|---|---|---|---|---|
| WBC (103 mm3) | 5.6 ± 9.7b | 1.3 ± 3.1a | 5.4 ± 4b | 6 ± 2.5b | 4.8 ± 2b | 10−3 |
| Lymphocytes (103 mm3) | 1.8 ± 5.8a | 3.5 ± 4.6a | 1.8 ± 1.1a | 1.5 ± 0.7a | 1.9 ± 0.9a | 0.16 |
| Neutrophils (103 mm3) | 2.3 ± 4.7a | 1.1 ± 1.7a | 3 ± 3.7a | 3.8 ± 4.4a | 2.1 ± 1.1a | 0.07 |
| Platelets (103 mm3) | 78.2 ± 104.7a | 79.5 ± 84a | 213.8 ± 113.2b | 230 ± 113.4b | 253 ± 104b | 10−3 |
| Hb (g/dL) | 8.2 ± 2.5a | 8.8 ± 2a | 10.3 ± 1.3b | 10.6 ± 1.6 b | 11.7 ± 1.6 b | 10−3 |
| MCV (fL) | 84 ± 9a | 84.7 ± 5.3a | 88.4 ± 6 b | 87 ± 7.6b | 92 ±5.1b | 0.008 |
| ALT (IU/L) | 210.1±32a | 98.9 ± 33a | 38.3 ± 32a | 40.8 ±30a | 109.3 ± 25a | 0.47 |
| AST (IU/L) | 66.2 ± 32a | 36.4 ± 23a | 30.7 ± 16 a | 34.2 ± 24.6a | 36.1 ± 34a | 0.06 |
| ALP (IU/L) | 208 ± 21a | 15405 ± 124a | 162.3 ± 38.3a | 147.1 ±79a | 184 ± 20a | 0.76 |
| Creatinine (mmol/L) | 42.4 ± 19.3a | 39.7± 17a | 34.9 ± 13a | 34.9 ± 16.3a | 35.6 ± 19a | 0.33 |
| blood glucose (mmol/l) | 5.6 ± 1.2a | 5.6 ± 1.2a | 5.4 ± 1.9a | 4.7 ± 1a | 5.4± 0.9a | 0.66 |
| K (mEq/L) | 4.2 ± 0.5a | 3.9 ± 0.4a | 4.1 ± 1.3a | 3.9 ±0.6 a | 3.9 ± 1.1a | 0.55 |
| Na (mEq/L) | 138.7 ± 2.7a | 136.6 ± 2.7a | 139.4 ± 2.5a | 139.4 ±2.5a | 139 ± 4a | 0.32 |
| Fibrinogen (g/L) | 4 ± 1.4a | 3.2 ± 1.5a | 4.3 ± 2a | 4 ± 1.7a | 5.3 ± 1.2a | 0.09 |
| Uric acid (mg/dL) | 3.1 ± 1.6 | 2.1 ± 1.4 | 5.6 ± 6 | - | - | - |
| Protidemia (g/L) | 53.5 ± 9.3a | 53.3 ± 7a | 47 ± 3.3a | 52.6 ± 9.5a | 55.3 ± 5.5a | 0.11 |
| Blasts in marrow (%) | 88.6 ± 14.1 | 50.3 ± 37 | 42 ± 27.1 | - | - | - |
WBC: White blood cell, MCV: Mean corpuscular volume, ALT: alanine transaminase, AST: aspartate aminotransferase, ALP: alkaline phosphatase, K: potassium, Na: sodium. Each result represents mean ± S.D. Tukey’s multiple range test: groups that show different letters are statistically different (p <0.05).
Antioxidant defences levels
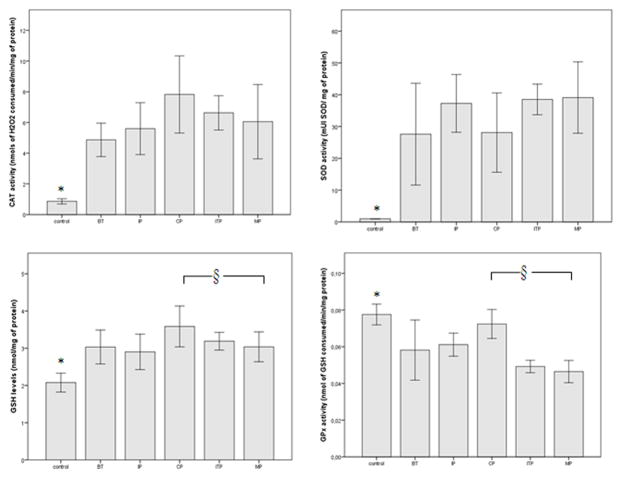
Figure 1 shows the CAT and SOD activities and GSH levels were increased significantly in ALL patients, as compared to sex-matched controls (p < 0.05). We can also observe in this figure, GPx activity was decreased significantly in ALL patients, as compared to age-matched controls (p < 0.05).
Figure 1.
Antioxidant enzymatic and non-enzymatic defences in ALL patients compared with sex-matched controls. Each column represents mean±S.D.
BT: Before treatment; IP: Induction phase; CP: Consolidation phase; ITP: Interval phase; MP: Maintenance phase. *, § p < 0.05, statistically significant differences, control versus ALL patients (*) or patients under therapy versus patients without therapy (§).
Post-hoc comparisons by Tukey’s test revealed that GPx activity and GSH levels were significantly higher in the consolidation phase when compared to the maintenance phase (7.92 ± 1.25 versus 6.05 ± 1.21 nmols of H2O2 consumed/min/mg of protein, and 0.07 ± 0.01 versus 0.04 ± 0.01 nmol of GSH consumed/min/mg of protein, respectively).
Plasma oxidant levels
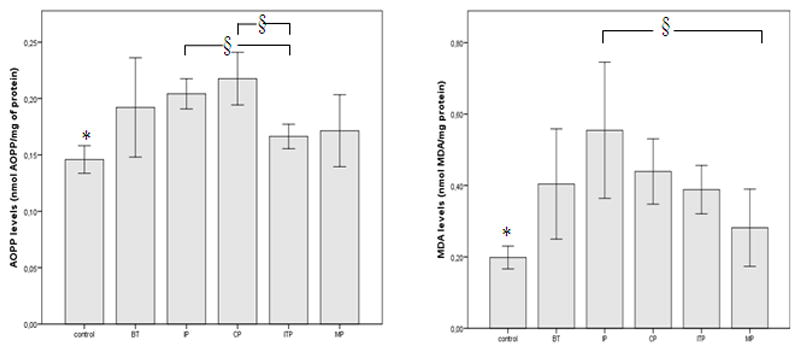
Figure 2 shows the plasma MDA and AOPP levels were increased significantly in ALL patients, as compared to sex-matched controls (p < 0.05).
Figure 2.
Oxidant plasma levels in ALL patients compared with sex-matched controls. Each column represents mean ±S.D.
BT: Before treatment; IP: Induction phase; CP: Consolidation phase; ITP: Interval phase; MP: Maintenance phase. *, § p < 0.05, statistically significant differences, control versus ALL patients (*) or patients under therapy versus patients without therapy (§).
Post-hoc comparisons by Tukey’s test revealed that MDA levels were significantly higher in the induction phase when compared to the maintenance phase (0.55 ± 0.30 nmol MDA/mg of protein versus 0.28 ± 0.12 nmol MDA/mg of protein, respectively). AOPP levels were significantly lower in the interval therapy than in the induction and consolidation phases (0.16 ± 0.05 nmol/mg of protein versus 0.20 ± 0.03 nmol/mg of protein and 0.22 ± 0.04 nmol OPP/mg of protein, respectively).
Nine patients (26.4%) relapsed after a median duration of 380 days. We observed, using Kaplan Meier Analysis, that the highest GSH values and SOD activity were correlated with relapse occurring in ALL patients (Figure 3).
Figure 3.
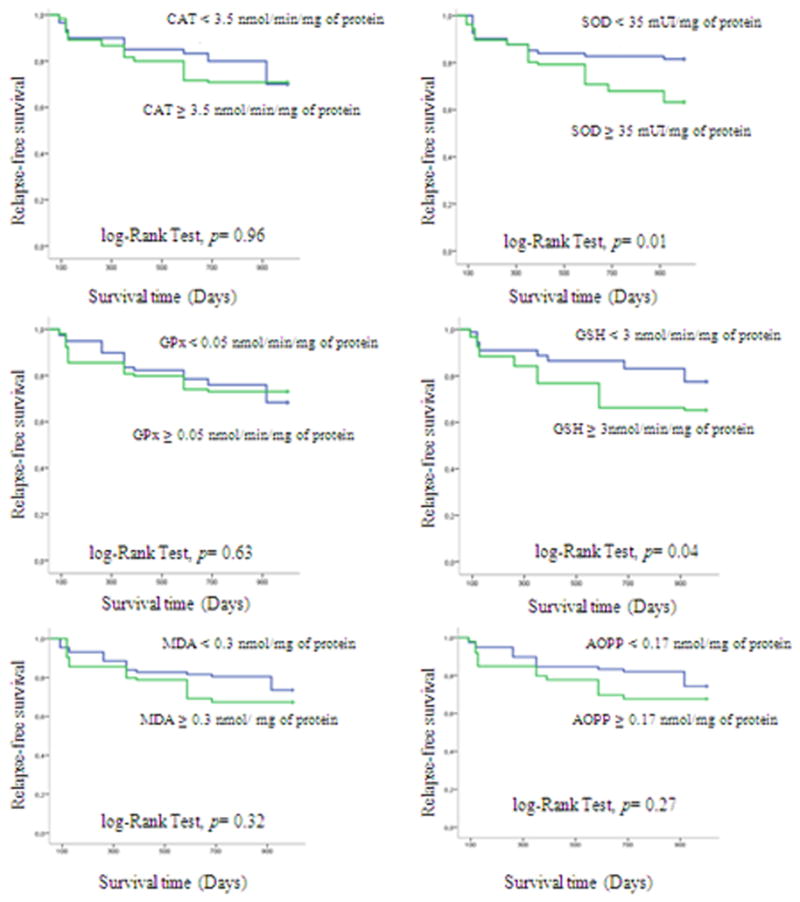
Relapse-free survival curves for acute leukaemic patients using Kaplan–Meier analysis.
Discussion
This study was performed to evaluate the oxidative status and antioxidant defense in patients with ALL and its involvement in leukaemic relapse.
We showed significant alterations of the antioxidant defense and increases in the production of oxygen reactive species in patients with ALL as compared with healthy subjects. This funding support the hypothesis that the cancer or malignant cells produce large numbers of ROS and that there exists a relationship between ROS activity and malignancy [18, 19]. Present observations are in agreement with other reports on various human cancers including hematological malignancies [20–23]. However, there is a controversial between the role of oxidative stress in the development of leukemia cancer and chemotherapeutic drugs which exert their biologic activity through induction of oxidative stress in affected cells [7].
In this context, the activities of antioxidant enzymes (SOD, CAT, GPx) as well as thiol (GSH), MDA and AOPP levels were measured before chemotherapy and during the different stages of treatment.
In the present study, MDA levels which have been applied as biomarkers of lipid peroxidation were shown to be increased significantly in ALL patients before or under chemotherapy when compared with the control group. These results are in accordance with the increase of TBARS levels in the serum of patients with chronic leukemia [24] and acute lymphoblastic leukemia [9, 25].
In addition, AOPP levels which reflected an excess of free radical generation and protein oxidative damages has been found to be higher in ALL patients compared with the control group.
On the other hand, the levels of MDA were more increased in induction and consolidation phases than those in the interval therapy. Similarly, AOPP levels increased in induction phase then decreased progressively to achieve the lowest values in the maintenance phase. We can suggest that the increase of oxidative lesions may involve both the pathogenesis of leukemia and the toxicity of chemotherapeutic agents. The current cytotoxic drugs used in standard leukemia therapy are designed to attack DNA replication process within malignant cells, and it does not discriminate between malignant and nonmalignant cells since it targets cell proliferation [26].
The data reported in the literature concerning antioxidant enzymes in acute leukemia are controversial. In this study, it was demonstrated an increase in SOD in ALL patients when compared with the control group. Moreover, SOD activity was significantly correlated with the relapse of ALL patients. These findings are in accordance with the study of Nishiura et al who reported elevated serum SOD activity in acute leukemia and indicated that regression of the leukemia was accompanied by a decrease in the serum level of SOD [27]. SOD can protect cells from low levels of oxidative stress, catalysing the dismutation of superoxide anion produced in the cell, whereas at high levels this enzyme acts as a peroxidase having a harmful action in the cell through the increment in hydrogen peroxide production [28]. Hydrogen peroxide, in the presence of iron or copper, has been demonstrated to be more toxic than superoxide anion due to the formation of hydroxyl radical, through the Fenton-Haber Weiss reaction, one of the most cytotoxic reactive oxygen species in vivo [29].
In this study, it was demonstrated an increase in CAT activity in ALL patients when compared with the control group. This results is in disagreement with those reported by Battisti V et al, which show a reduced of CAT activity in ALL patients just diagnosed when compared to controls [9]. These findings suggest that there are alterations in the enzymatic antioxidant defenses, which can interfere in the direct removal of free radicals (pro-oxidants) and in the protection for biological sites.
Lower GPx activity was observed in ALL patients as compared to control subjects, similarly to what has been reported to occur in acute lymphoblastic leukaemia by others [30, 31].
Glutathione peroxidase, an enzyme involved in the inactivation of peroxides, catalyzes the oxidation of GSH to its disulphide, GSSG, under oxidative stress conditions. The decrease in the activity of this antioxidant enzyme observed in ALL patients, can lead to the increase in the H2O2, plasma levels and other peroxides and, consequently, to oxidative stress [31].
We also observed higher levels of GSH in ALL patients as compared to control subjects. This non enzymatic antioxidantwas correlated with the relapse of ALL patients, like SOD activity. This result is in agreement with those reported by Sarmento-Ribeiro AB et al., in 2012, which show a correlation of GSH with the relapse and survival of ALL patients [31]. It has been reported the increase in GSH levels was associated with drug resistance in tumor cells [29] [32]. The increase in GSH, leads to increased cell survival, even in the presence of the chemotherapeutic agents and, consequently, to chemotherapeutic drugs resistance and disease relapse.
Another important aspect to be discussed is that, no significant correlation was observed between levels of oxidative damage (MDA or AOPP) and the risk relapse of ALL. We suggest that these findings may be a consequence of small number of ALL patients included in this study. More studies are necessary to confirm the correlation between oxidative status and the relapse and survival of ALL patients.
In conclusion, this research provides additional contribution in the study of the oxidative profile in children just diagnosed with ALL as compared to those in the different stages of treatment. We suggest that GSH level with SOD activity may be a significant relapse predictor in ALL patients. These make very imperative to develop new therapeutic strategies to selectively kill leukaemic cells and circumventing chemoresistance by modulating oxidative stress. Application of antioxidant principles may illicit same effect, for example inhibition of intracellular antioxidants such as GSH proposed by Townsend DM et al [33].
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
Contributor Information
Lobna Ben Mahmoud, University of Sfax, Faculty of Medicine, pharmacology department. Sfax, Tunisia.
Moez Mdhaffar, University of Sfax, Hedi Chaker Hospital, Department of Hematology, Sfax, Tunisia.
Hanene Ghozzi, University of Sfax, Faculty of Medicine, pharmacology department. Sfax, Tunisia.
Mariam Ammar, University of Sfax, Faculty of sciences of Sfax, Sfax, Tunisia.
Ahmed Hakim, University of Sfax, Faculty of Medicine, pharmacology department. Sfax, Tunisia.
Rim Atheymen, University of Sfax, Faculty of Medicine, pharmacology department. Sfax, Tunisia.
Zouheir Sahnoun, University of Sfax, Faculty of Medicine, pharmacology department. Sfax, Tunisia.
Moez Elloumi, University of Sfax, Hedi Chaker Hospital, Department of Hematology, Sfax, Tunisia.
Khaled Zeghal, University of Sfax, Faculty of Medicine, pharmacology department. Sfax, Tunisia.
References
- 1.Plasschaert SLA, Kamps WA, Vellenga A, Vries EGE, De Bont ESJM. Prognosis in childhood and adult acute lymphoblastic leukemia: a question of maturation? Cancer Treat Rev. 2004;30:37–51. doi: 10.1016/S0305-7372(03)00140-3. [DOI] [PubMed] [Google Scholar]
- 2.Bassan R, Rossi G, Pogliani EM, Di Bona E, Angelucci E, et al. Chemotherapy-phased imatinib pulses improve long-term outcome of adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: Northern Italy Leukemia Group protocol 09/00. J Clin Oncol. 2010;28:3644–52. doi: 10.1200/JCO.2010.28.1287. [DOI] [PubMed] [Google Scholar]
- 3.Pui C-H, Evans WE. Acute lymphoblastic leukemia. N Engl J Med. 2006;354:166–178. doi: 10.1056/NEJMra052603. [DOI] [PubMed] [Google Scholar]
- 4.Raetz EA, Bhatla T. Where do we stand in the treatment of relapsed acutelymphoblastic leukemia? Hematology Am Soc Hematol Educ Program. 2012;2012:129–36. doi: 10.1182/asheducation-2012.1.129. [DOI] [PubMed] [Google Scholar]
- 5.Basso G, Veltroni M, Valsecchi MG, Dworzak MN, Ratei R, Silvestri D, et al. Risk of relapse of childhood acute lymphoblastic leukemia is predicted by flow cytometric measurement of residual disease on day 15 bone marrow. J Clin Oncol. 2009;27:5168–74. doi: 10.1200/JCO.2008.20.8934. [DOI] [PubMed] [Google Scholar]
- 6.Skarstein J, Aass N, Fosså SD, Skovlund E, Dahl AA. Anxiety and depression in cancer patients: relation between the Hospital Anxiety and Depression Scale and the European Organization for Research and Treatment of Cancer Core Quality of Life Questionnaire. J Psychosom Res. 2000;49:27–34. doi: 10.1016/s0022-3999(00)00080-5. [DOI] [PubMed] [Google Scholar]
- 7.Udensi UK, Tchounwou PB. Dual effect of oxidative stress on leukemia cancer induction and treatment. J Exp Clin Cancer Res. 2014;33:106. doi: 10.1186/s13046-014-0106-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valko M, Rhodes CJ, Moncola J, Izakovic M, Mazura M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 9.Battisti V, Maders LD, Bagatini MD, Santos KF, Spanevello RM, Maldonado PA, et al. Measurement of oxidative stress and antioxidant status in acute lymphoblastic leukemia patients. Clin Biochem. 2008;41:511–18. doi: 10.1016/j.clinbiochem.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 10.De Moerloose B, Suciu S, Bertrand Y, Mazingue F, Robert A, Uyttebroeck A, et al. Improved outcome with pulses of vincristine and corticosteroids in continuation therapy of children with average risk acute lymphoblastic leukemia (ALL) and lymphoblastic non-Hodgkin lymphoma (NHL): report of the EORTC randomized phase 3 trial 58951. Blood. 2010;116:36–44. doi: 10.1182/blood-2009-10-247965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradford M. A rapid and sensitive method for the quantities of microgram quantities of protein utilizing the principle of proteinbinding. Analytical Biochemistry. 1976;72:248–54. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 12.Buege JA, Aust SD. Microsomal lipid peroxidation. Methods in Enzymology. 1972;51:302–10. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 13.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–6. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 14.Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–87. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- 15.Weckbercker G, Cory JG. Ribonucleotide reductase activity and growth of glutathionedepended mouse leukaemia L 1210 cells in vitro. Cancer Lett. 1988;40:257–264. doi: 10.1016/0304-3835(88)90084-5. [DOI] [PubMed] [Google Scholar]
- 16.Flohe L, Gunzler WA. Analysis of glutathione peroxidase. Methods Enzymol. 1984;105:114–21. doi: 10.1016/s0076-6879(84)05015-1. [DOI] [PubMed] [Google Scholar]
- 17.Kayali R, Cakatay U, Akcay T, Altug T. Effect of alpha-lipoic acid supplementation on markers of protein oxidation in post-mitotic tissues of ageing rat. Cell Biochem Funct. 2006;24:79–85. doi: 10.1002/cbf.1190. [DOI] [PubMed] [Google Scholar]
- 18.Cerutti PA. Oxy-radicals and cancer. Lancet. 1994;344:862–3. doi: 10.1016/s0140-6736(94)92832-0. [DOI] [PubMed] [Google Scholar]
- 19.Dormandy TI. An approach to free radicals. Lancet. 1983;1:1010–4. doi: 10.1016/s0140-6736(83)90989-3. [DOI] [PubMed] [Google Scholar]
- 20.Ray G, Batra S, Shukla NK, Deo S, Raina V, Ashok S, Husain SA. Lipid peroxidation, free radical production and antioxidant status in breast cancer. Breast Cancer Research and Treatment. 2000;59:163–70. doi: 10.1023/a:1006357330486. [DOI] [PubMed] [Google Scholar]
- 21.Singh V, Ghalaut PS, Kharb S, Singh GP. Plasma concentrations of lipid peroxidation products in children with acute leukemia. Ind J Med Sci. 2001;55:215–17. [PubMed] [Google Scholar]
- 22.Ghalaut VS, Ghalaut PS, Singh S. Lipid peroxidation in leukemia. J Asso Phys Ind. 1999;47:403–5. [PubMed] [Google Scholar]
- 23.Hammouda AE, Soliman SF, Tolba KA, el-Kabbany ZA, Makhlouf MS. Plasma concentrations of lipid peroxidation products in children with acute lymphoblastic leukemia. Clin Chem. 1992;38:594–5. [PubMed] [Google Scholar]
- 24.Al-Gayyar MM, Eissa LA, Rabie AM, El-Gayar AM. Measurements of oxidative stress status and antioxidant activity in chronic leukaemia patients. J Pharm Pharmacol. 2007;59:409–17. doi: 10.1211/jpp.59.3.0011. [DOI] [PubMed] [Google Scholar]
- 25.Drabko K, Bojarska-Junak A, Kowalczyk J. Activity of superoxide dismutase and glutathione peroxidase and concentrations of malonyldialdehyde, vitamin E, total antioxidant status and extracellular cytokines concentrations in children with acute lymphoblastic leukaemia (ALL) Med Wieku Rozwoj. 2006;10:861–8. [PubMed] [Google Scholar]
- 26.Estey E, Dohner H. Acute myeloid leukaemia. Lancet. 2006;368:1894–1907. doi: 10.1016/S0140-6736(06)69780-8. [DOI] [PubMed] [Google Scholar]
- 27.Nishiura T, Suzuki K, Kawaguchi T, et al. Elevated serum manganese superoxide dismutase in acute leukemias. Cancer Lett. 1992;62:211–5. doi: 10.1016/0304-3835(92)90098-g. [DOI] [PubMed] [Google Scholar]
- 28.Sun J, Chen Y, Li M, Ge Z. Role of antioxidant enzymes on ionizing recliation resistance. Free Radic Biol Med. 1998;24:586–93. doi: 10.1016/s0891-5849(97)00291-8. [DOI] [PubMed] [Google Scholar]
- 29.Toyokuni S, Okamoto K, Yodoi J, Hiai H. Persistent oxidative stress in cancer. FEBS Lett. 1995;358:1–3. doi: 10.1016/0014-5793(94)01368-b. [DOI] [PubMed] [Google Scholar]
- 30.Nørgaard Jan Maxwell, Hokland Peter. Biology of multiple drug resistance in acute leukemia. Int J Hematol. 2000;72:290–7. [PubMed] [Google Scholar]
- 31.Sarmento-Ribeiro AB, Proença MT, Sousa I, Pereira A, Guedes F, Teixeira A, Oliveira CR. A possible role for oxidation stress in lymphoid leukaemias and therapeutic failure. Leuk Res. 2012;36:1041–8. doi: 10.1016/j.leukres.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 32.Godwin AK, Meister A, O’Dwyer PJ, Huang CS, Hamilton TC, Anderson ME. High resistance to cisplatin in human ovarian cancer cell lines is associated with marked increase of glutathione synthesis. Proc Natl Acad Sci USA. 1992;89:3070–4. doi: 10.1073/pnas.89.7.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Townsend DM, Findlay VL, Tew KD. Glutathione S-transferases as regulators of kinase pathways and anticancer drug targets. Methods Enzymol. 2005;401:287–307. doi: 10.1016/S0076-6879(05)01019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]