Abstract
The receptor for advanced glycation endproducts (RAGE) interacts with a unique repertoire of ligands that form and collect in the tissues and circulation in diabetes, aging, inflammation, renal failure and obesity. RAGE is expressed on multiple cell types linked to tissue perturbation in these settings. This Brief Review focuses on the role of RAGE in monocytes/macrophages and how RAGE ligand engagement on these cells mediates seminal changes in monocyte/macrophage migration, oxidative stress, cholesterol efflux and pro- vs. anti-inflammatory cues that signal to tissue damage. Studies using mice devoid of Ager (gene encoding RAGE) or pharmacological antagonists of RAGE are protective in animal models of diabetes, atherosclerosis, and high fat diet-induced obesity, in least in part through key roles in monocytes/macrophages. RAGE signal transduction requires the interaction of RAGE cytoplasmic domain with the formin, DIAPH1 and novel antagonists of this interaction show significant promise in attenuation of the maladaptive effects of RAGE ligands in cellular and in vivo models. Finally, this Brief Review discusses evidence for RAGE axis perturbation in human monocytes/macrophages and how tracing RAGE activity in these cells may identify target engagement biomarkers of RAGE antagonism for future clinical trials.
RAGE: a multi-ligand receptor of the immunoglobulin superfamily
RAGE, a member of the immunoglobulin superfamily of cell surface molecules, was originally discovered for its ability to bind to and transduce the biological effects of advanced glycation endproducts (AGEs), which accumulate in settings such as hyperglycemia, aging, inflammation, renal failure and oxidative stress1, 2. AGEs are an heterogeneous group of modified molecules, which form particularly on lysine and arginine amino acid residues. Among the AGEs, one of the most prevalent species found in human subjects with diabetes, the carboxymethyl lysine (CML) AGEs, are signal transduction ligands of RAGE3. The key to the biology of RAGE lies in its diverse ligand families. The discovery that RAGE bound non-AGE ligands linked the receptor indelibly to mechanisms important in the inflammatory response. Beyond AGEs, RAGE is also a signal transduction receptor for molecules such as members of the S100/calgranulin family, high mobility group box-1 (HMGB1), Mac-1 and lysophosphatidic acid (LPA)4–7. Hence, RAGE and its ligands accumulate in the non-diabetic state and to enhanced degrees in diabetes, at least in part on account of the potent effects of hyperglycemia on stimulating increased formation and accumulation of RAGE ligand AGEs. AGEs, and other ligands, through RAGE, comprise a unique family that imbues chronic inflammatory stress as their chief biological action, for example, through sustained activation of the pro-inflammatory transcription factor NF-κB8, 9. Furthermore, RAGE action downregulates mRNA transcript, protein and activity levels of Glo1 (or glyoxalase1)10. GLO1, which detoxifies the pre-AGE intermediate, methylglyoxal, is a defense against increased AGE production. The finding that RAGE downregulates Glo1 underscores the possibility that RAGE is at the center of a feed forward loop regulating both ligand activity as well as ligand levels. In this context, the finding that RAGE sustains chronic inflammatory cues stimulated by its ligands provides further fuel for sustaining production and accumulation of pro-inflammatory RAGE ligands, which may be escorted into inflammatory sites through S100/calgranulin- or HMGB1-bearing immune cells.
Adding to the complexity of this axis is the consideration that some of the ligands of RAGE, such as HMGB1, also bind to toll like receptors (TLRs). Experiments testing the effects of massive hepatic injury induced by 85% hepatectomy suggest that whereas mice devoid of the TLR signaling effector, Myd88, displayed accelerated mortality after this injury compared to wild-type controls, mice devoid of Ager (gene encoding RAGE) displayed improved survival compared to either wild-type or Myd88 null mice11. Strikingly, mice doubly devoid of Myd88 and Ager displayed comparable mortality to the mice devoid of Myd88, thereby suggesting that RAGE expression, unlike TLR signaling through MYD88, was not essential for the innate response to massive liver injury.
RAGE is expressed on multiple cell types implicated in cardiovascular disease (CVD), such as vascular cells (endothelial cells (ECs) and smooth muscle cells (SMCs)) and immune cells such as monocytes/macrophages, T and B lymphocytes and neutrophils1, 2, 12. This Brief Review, focusing on RAGE and monocytes/macrophages in cardiometabolic disorders, will detail the evidence that points to roles for RAGE not only in recruitment of these key cells in atherosclerosis and obesity but also to their expression of pro-inflammatory mediators and reduced expression of molecules linked to cholesterol efflux. Novel insights into RAGE signal transduction through the formin DIAPH1, and how such signaling may be pharmacologically antagonized, will be discussed. Finally, the evidence in human monocytes/macrophages linking the RAGE axis to cellular perturbations that may contribute to both the development and biomarking of cardiometabolic pathobiology will be presented.
RAGE and Atherosclerosis
RAGE and atherosclerosis in human subjects
Evidence from human subjects places RAGE in atherosclerotic plaques, particularly, but not limited to the diabetic state. Cipollone and colleagues evaluated atherosclerotic plaques from 60 patients undergoing carotid endarterectomy and reported that the diabetic plaques had significantly more macrophages, CD3+ lymphocytes, SMCs and HLA-DR+ cells compared to the non-diabetic plaques13. In parallel, the diabetic plaques demonstrated more RAGE expression (particularly in macrophages and SMCs), activated NF-κB, higher cyclooxygenase 1 (COX2)/microsomal prostaglandin E-synthase1 expression and higher levels of matrix metalloproteinases (MMPs) and gelatinolytic activity. Lower collagen content and higher lipid and oxidized LDL content was also noted in the diabetic plaques13.
In other studies, the expression of RAGE and plaque characteristics in diabetic vs. non-diabetic subjects were examined in the coronary vasculature of subjects who experienced sudden cardiac death. Atherosclerotic plaques from type 2 diabetic subjects revealed larger mean necrotic cores and greater total and distal plaque load compared to the non-diabetic lesions14. The intimal staining for macrophages, T lymphocytes and HLA-DR status was significantly greater in the diabetic vs. the non-diabetic plaques. Expression of RAGE and RAGE ligand S100A12, especially in macrophages and in apoptotic SMCs, was significantly greater in the diabetic lesions14.
Prompted by these observations in human subjects, mechanistic studies in animal models were performed to test the role of RAGE in atherosclerosis, particularly in the context of its role in immune cell recruitment and activity.
RAGE, murine models and atherosclerosis
Studies in atherosclerosis-prone mice have provided definitive evidence for roles for RAGE in the pathogenesis of non-diabetic and diabetic accelerated atherosclerosis. In both mice devoid of Apoe or the Ldlr, deletion of Ager resulted in lower mean atherosclerotic lesion areas12, 15, 16. In these studies, a prominent underlying mechanism was the reduction in lesion levels of Mcp1 and reduced macrophages or T lymphocyte content per lesion area in the atherosclerosis-prone mice globally devoid of Ager. Evidence of generalized reductions in inflammatory and oxidative stress was noted upon deletion of Ager in these settings. In other studies, mice expressing cytoplasmic domain-deleted RAGE in ECs (driven by the pre-pro-endothelin 1 promoter) displayed reduced atherosclerosis in the non-diabetic Apoe null mouse background, in parallel with significant reductions in Vcam1 in the vasculature17.
In distinct studies, mice were treated with soluble (s) RAGE; sRAGE is composed of the extracellular ligand-binding domains of RAGE. Administration of sRAGE to diabetic mice resulted in suppression of early initiation18 and progression of diabetic atherosclerosis19. Prominent effects on down-regulation of inflammatory and oxidative stress responses were observed in sRAGE- vs. vehicle-treated animals18, 19. Beneficial effects of sRAGE were also noted in non-diabetic Apoe null mice, but the extent of benefit was lower, consistent with the premise that although the hyperlipidemic environment of the non-diabetic state stimulates the generation of RAGE ligands, the hyperglycemia of diabetes greatly accelerates formation of RAGE ligands compared to those generated in the non-diabetic state.
The role of myeloid RAGE was definitively tested using bone marrow transplantation strategies. First, these concepts were tested in non-diabetic mice devoid of Apoe. Seven and 23-week old Apoe null mice were lethally-irradiated and reconstituted with bone marrow from Apoe null mice expressing or devoid of Ager. After 16 weeks standard chow diet in the mice irradiated at age 7 weeks, there were no differences in atherosclerotic lesion area vis-à-vis RAGE. However, in the older mice, lesions in the brachiocephalic arteries were significantly smaller in the mice receiving Ager null bone marrow vs. control bone marrow20. In parallel, plaques were more stable and expressed significantly lower levels of Vcam1, Icam1 and Mcp1 vs. the plaques of mice reconstituted with Ager-expressing bone marrow.
In diabetes, streptozotocin-induced Apoe null diabetic mice were lethally irradiated and reconstituted with ApoE null/Ager null or control Ager-expressing Apoe null bone marrow and mice were sacrificed at either 10 or 20 weeks. In both groups of mice, deletion of Ager resulted in reduced atherosclerotic lesion areas and lower levels of Vcam121. Of note, the authors determined in distinct bone marrow transplantation studies that non-bone marrow-derived cells also contributed to diabetic atherosclerosis via RAGE21. In these model systems, deletion of Ager or administration of soluble (s) RAGE had no effect on levels of glucose or on total levels of cholesterol or triglyceride, thereby suggesting that unique RAGE-dependent mechanisms distinct from typical risk factors affected atherosclerosis. Hence, these data suggested that vascular cell RAGE contributes to upregulation of adhesion molecules and chemokines, processes by which RAGE may contribute to the development of atherosclerosis in non-diabetic and diabetic mice. Definitive studies using bone marrow transplantation strategies implicated both myeloid- and non-myeloid cell RAGE directly in atherosclerosis in murine models, both in non-diabetes and diabetes. Evidence of overall reduced macrophage content per lesion area was demonstrated, in parallel with reduced inflammation and oxidative stress. The possible contributions of RAGE in macrophage retention/stasis, proliferation and/or fate (death) in the atherosclerotic lesion remains to be determined.
In addition to testing how RAGE affects diabetic atherosclerosis, the role of RAGE in uremia-associated atherosclerosis has also been addressed. Uremic conditions are linked to highly increased accumulation of RAGE ligand AGEs22. In animal models, performance of the “5/6 nephrectomy” in Apoe null mice resulted in accelerated atherosclerosis in mice expressing Ager but not in mice devoid of Ager23. RAGE ligands serum amyloid A (SAA) and S100B increased significantly in the uremic environment and upon stimulation of SMCs with these ligands, prominent increases in production of reactive oxygen species (ROS) were observed in a RAGE-dependent manner23. In other studies, a neutralizing anti-RAGE IgG was employed in Apoe null mice subjected to the 5/6 nephrectomy. Compared to treatment with isotype control, anti-RAGE antibodies resulted in lower atherosclerotic lesion areas24. Treatment with anti-RAGE antibodies had no effect on a panel of inflammatory markers in the lesions but had a dramatic effect on reduction of oxidative stress. In the section to follow, recent data implicating RAGE in the mechanisms regulating cholesterol metabolism will be discussed.
RAGE, macrophages, cholesterol efflux and reverse cholesterol transport
Macrophage cholesterol efflux in human subjects with diabetes is impaired and such impairment is observed in animal models of diabetes as well25–27. Links to RAGE were uncovered by the finding that in murine bone marrow-derived macrophages (BMDMs) from diabetic mice devoid of Ager, cholesterol efflux to ApoA1 and HDL was higher than that observed in diabetic BMDMs from wild-type mice28. In vivo, macrophage reverse cholesterol transport to plasma, liver, and feces was reduced in diabetic macrophages through RAGE. The underlying mechanism was traced to RAGE ligand-mediated downregulation of the key cholesterol transporters, Abca1 and Abcg1.
In vitro, using RAGE-expressing THP1 cells, and RAGE-overexpressing HEK cells, the RAGE ligand CML-AGE suppressed ABCG1 and ABCA1 promoter luciferase activity and transcription of ABCG1 and ABCA1 through peroxisome proliferator-activated receptor-γ (PPARG)-responsive promoter elements. Interestingly, the effects of RAGE ligands/RAGE were not dependent on the liver X receptor (LXR) elements28. Plasma levels of HDL were lower in diabetic C57BL/6 mice devoid of Ager compared to wild-type mice of the same genetic background28. Laser capture microdissected CD68(+) macrophages from atherosclerotic plaques of Ldlr null mice devoid of Ager vs. the RAGE-expressing Ldlr null mice displayed higher levels of Abca1, Abcg1, and Pparg mRNA transcripts versus Ager-expressing Ldlr null mice independently of glycemia or plasma levels of total cholesterol and triglycerides. Given that PPARG exerts beneficial effects on macrophage inflammation29, these considerations highlighted possible roles for RAGE in downregulation of PPARG-dependent mechanisms in macrophages. Although not the focus of this review, it is important to note that extensive evidence links RAGE and its ligands to microvascular or “small vessel” disease in settings such as diabetes30, aging31 and Alzheimer’s disease32, as examples.
In addition to important roles for macrophages in inflammation, oxidative stress and cholesterol metabolism in atherosclerosis, macrophages are also tightly linked to metabolic regulation of insulin sensitivity, particularly in high fat feeding. The section to follow explores how RAGE contributes to metabolic dysfunction in obesity and the possible links to macrophage functions.
RAGE and High Fat Diet-Induced Obesity
Macrophages exert complex effects in adipose tissue in obesity, not only depending on the adipose tissue depot, but also on the species. Whereas correlations between numbers of adipose tissue macrophages (ATMs) with obesity and insulin resistance have been noted in mice33, 34, the findings in the human obesity are less clear35. Further, markers of “polarization” do not appear to reliably reflect the effects of macrophages on systemic glucose and insulin sensitivity. For example, “M2”-type markers such as CD206 and CD163 have been associated with insulin resistance36, 37. More recently, to link macrophage properties to systemic immunometabolism, an emerging theme relates to how macrophage fatty acid oxidation and handling may regulate inflammatory signatures38.
RAGE, adipose tissue, obesity and human subjects
The study of macrophage RAGE in obesity and systemic metabolism is a work in progress. Recently, Gaens and colleagues examined subcutaneous and visceral adipose tissue from lean vs. obese male human subjects. Compared to the lean subjects, the obese subjects displayed evidence of glucose and insulin-related metabolic dysfunction, as the obese subjects demonstrated significantly higher levels of fasting glucose and fasting insulin and lower glucose infusion rates39. These authors performed immunohistochemical staining to localize RAGE ligand CML-AGE and RAGE in adipose tissue. Whereas only scant immunoreactivity for CML-AGE epitopes was noted in human lean subcutaneous adipose tissue, much more readily detectable CML-AGE was noted in obese tissue. RAGE expression in adipose tissue was also significantly higher in obese vs. lean subjects, particularly in visceral vs. subcutaneous adipose tissue. Both CML-AGE and RAGE epitopes in adipose tissue localized to adipocytes, CD68+ macrophages and CD31+ ECs39. Experiments were performed in mice to determine the mechanistic inferences from these findings.
RAGE, obesity and murine models
Wild-type and homozygous Ager null male mice were fed a high fat diet (60%) vs. standard low fat chow. In the wild-type mice, even before the appearance of insulin resistance or obesity, metabolic tissues displayed significantly higher levels of RAGE ligands, CML-AGE and HMGB140. Surprisingly, despite equal food consumption in mice fed the high fat diet, compared to the wild-type mice, Ager null mice displayed significantly lower body mass; by DEXA scanning, this was accompanied by a significantly lower lean and fat mass in the high fat-fed Ager null mice40. Metabolic phenotyping identified that the Ager null mice fed the high fat diet were more glucose- and insulin sensitive vs. the wild-type control animals; this was confirmed by hyperinsulinemic euglycemic clamp studies. In addition, the Ager null mice displayed significantly higher energy expenditure vs. the wild-type mice, as indicated by higher VO2 consumption and higher VCO2 production40. Interestingly, in the hyperinsulinemic euglycemic clamp studies, even on the low fat diet, mice devoid of Ager were more insulin sensitive than the corresponding wild-type mice.
A key question to address was whether there were correlations between the expression of RAGE, macrophage content and profile, and insulin sensitivity in the visceral (perigonadal) adipose tissue in low fat or high fat feeding. In mice fed the low fat diet, there were no significant differences in Emr1 (F4/80) mRNA transcripts between wild-type and Ager null mice; by immunohistochemistry, significantly lower numbers of F4/80+ and CD11c+ cells populated this tissue in Ager null vs. the wild-type mice on the low fat diet. Although Emr1 mRNA transcripts were significantly higher in wild-type high fat fed- vs. low fat fed-mice, they were significantly lower in Ager null visceral adipose tissue. By immunohistochemistry, on high fat diet, there were significantly lower F4/80+ and CD11c+ cells in the Ager null vs. wild-type visceral adipose tissue. By real time quantitative PCR, levels of Tnfa, Ccl2 and Nos2, normalized to Emr1 transcripts, were either unchanged or higher in Ager null vs. wild-type visceral adipose tissue in high fat feeding; in contrast, levels of Cd163, Il10, Cd209d, Arg1 and Cd209e were all significantly higher in the Ager null vs. wild-type visceral adipose tissue40.
Lethal irradiation of wild-type mice and reconstitution with Ager null vs. wild-type bone marrow resulted in partial protection from high fat diet induced obesity, in parallel with improved glucose tolerance. Interestingly, macrophage content and inflammatory gene expression profiles in mice reconstituted with Ager null bone marrow closely paralleled those in the global Ager null mice40. Taken together, these considerations led us to propose that myeloid/macrophage RAGE appears to play important roles in the response to high fat feeding – both in terms of regulation of body mass, adipose tissue macrophage content and systemic metabolism. The precise mechanisms remain to be identified and are under active investigation. Whereas earlier studies focused on visceral adipose tissue, ongoing work is probing how RAGE expression, in macrophages as well as adipocytes, affects obesity and the metabolic response to high fat feeding in distinct adipose tissue depots of subcutaneous and brown adipose tissue.
RAGE and Signal Transduction
RAGE and DIAPH1: biology
RAGE activates diverse signaling cascades in a range of cell types; until recently, the proximate mechanisms governing RAGE signaling have been elusive. Experiments in a yeast-two-hybrid assay revealed that the cytoplasmic domain of RAGE binds to the formin DIAPH1 and that DIAPH1 is required for RAGE ligands to signal through RAGE41. Formins play key roles in actin cytoskeleton dynamics, cellular migration, cytokinesis, signaling (particularly through the RHO GTPases) and regulation of serum response factor (SRF) activities42–44. In transformed cells, RNAi-knockdown of DIAPH1 suppressed RAGE ligand-mediated activation of Rac1 or Cdc42 and cellular migration41. In SMCs, RNAi knockdown of Diaph1 or global deletion of Diaph1 blocked RAGE ligand-mediated signaling through AKT and suppressed SMC migration45.
In vascular cells and macrophages, it was shown that deletion of Ager protected these cells from hypoxia-stimulated upregulation of Egr1 and its sequelae on inflammatory and pro-thrombotic gene expression46, 47. The mechanistic link to RAGE was illustrated by experiments revealing that exposure of cells to hypoxia resulted in rapid generation of RAGE ligand AGEs, and that blockade of AGEs (using anti-AGE IgG) or aminoguanidine prevented hypoxia-stimulated upregulation of EGR1 in human THP1 macrophage-like cells. In THP1 cells, treatment with AGEs directly upregulated EGR1 in a manner blocked by RNAi-knockdown of DIAPH1. In peritoneal macrophages obtained from Diaph1 null mice, a highly significant reduction in hypoxia-stimulated upregulation of Egr1 was observed when compared to the wild-type cells47. These considerations indicated that RAGE ligand AGEs and hypoxia require DIAPH1 in macrophages in order to upregulate EGR1. Given the profound tissue-damaging effects of EGR1 in hypoxic stress48, identification of roles for RAGE/DIAPH1 in these processes in macrophages and likely other cell types may highlight novel strategies to mitigate tissue damage in hypoxia and ischemia. Hence, it was essential to discern the precise mechanisms accounting for the interaction between the cytoplasmic tail of RAGE and DIAPH1.
RAGE and DIAPH1: physical interaction
Shekhtman and colleagues used a range of techniques, particularly NMR spectroscopy, to discover that the first half of the cytoplasmic domain of RAGE was ordered and that this was the region of the molecule that bound the FH1 (formin homology 1) domain of DIAPH149. They identified that the RAGE cytoplasmic domain possesses an unusual α-turn, which was required for the interaction with DIAPH1. Mutation of amino acid residues R5/Q6 in human RAGE cytoplasmic domain to alanine caused the disruption of the interaction with DIAPH1.
Critically, when these amino acids in the RAGE cytoplasmic domain were mutated to alanine residues in SMCs, activation of AKT and cellular migration and proliferation triggered by the incubation of these cells with RAGE ligand S100B were blocked compared with the control constructs. Importantly, the mutated R5/Q6 (to alanine residues) construct in SMCs displayed no reduction in cellular migration or proliferation triggered by a non-RAGE ligand, PDGF49. Hence, these experiments provided further support for the RAGE-DIAPH1 interaction in cellular signaling.
Recent experiments by Shekhtman and colleagues illustrated that RAGE forms constitutive homo-dimers through its extracellular VC1 and C2 domains and that upon ligand engagement, the molecular dimension of RAGE increases, thereby allowing its cytoplasmic domain to recruit DIAPH1 and to initiate signaling50. In that work, mutations of DIAPH1 were prepared, which indicated that full-length DIAPH1 is required for RAGE signal transduction stimulated by RAGE ligands such as S100B.
RAGE and DIAPH1: Discovering Antagonists of RAGE Signaling
A high throughput binding assay was developed to screen a >58,000 small molecule library for inhibitors of the RAGE-DIAPH1 interaction. Through a series of experiments including binding assays and NMR spectroscopy, 13 small molecules were identified that blocked RAGE-DIAPH1 binding and suppressed the effects of RAGE ligands on signal transduction, that is, phosphorylation of AKT and ERK1/2 in cultured cells. In SMCs and in THP1 macrophage-like cells, these compounds suppressed RAGE ligand-mediated functions such as migration and production of inflammatory mediators. Critically, in SMCs, these compounds had no effect to suppress migration to non-RAGE ligand PDGF. Ex vivo, these compounds were tested for their effects on blocking ischemia/reperfusion injury in the isolated diabetic reperfused heart and a number of them prevented loss of left ventricular developed pressure (LVDP), a marker of heart function. Finally, when RAGE ligand CML-AGE was infused into wild-type mice, upregulation of inflammatory mediators was observed in liver and kidney and prevented, at least in part, by certain of the compounds under study51.
To date, two lead series of molecules have been illuminated from the original 13. Structure activity relationship (SAR) and small molecule refinement studies are underway to optimize these lead series for further development with an eye towards therapeutics. Although ultimate clinical trials testing in diabetes and its complications is the long-term goal for these compounds in human subjects, it is acknowledged that clinical trials in diabetic complications are long in duration and often complicated by factors such as placebo effect or the beneficial effects of rigorously adhered to “standard of care” efforts to lower blood glucose and attenuate other risk factors (such as hyperlipidemia, hypertension or reduce/eliminate smoking). For this reason, it is plausible that given the strong evidence linking RAGE to non-diabetic immune/inflammatory disorders52, 53, a step-wise approach to clinical trial testing of the RAGE hypothesis may be more practical, especially given that exacerbations of such immune/inflammatory disorders may be shorter in duration and more limited in scope, thereby accelerating the timeline for establishing efficacy, or not, of RAGE antagonism in the human subject.
Irrespective of the strategy for further development of these agents, an important challenge is to identify target engagement biomarkers to track RAGE activity in readily accessible tissues in the human subject, such that interim analyses may inform successful blockade of RAGE activity – or not – in RAGE signaling target engagement. Toward this end, two readily accessible sources of trackable material include plasma/serum and peripheral blood monocytes or monocyte-derived macrophages. What is the evidence supporting the use of these tissue sources to biomark the effectiveness of RAGE signaling inhibitors?
RAGE, peripheral blood monocytes and human subjects
Accruing evidence suggests that tracking RAGE expression in peripheral blood monocytes or monocyte-derived macrophages may reflect activity of this axis in disorders in which RAGE ligands accumulate. Su and colleagues assessed AGER mRNA levels in monocytes from the peripheral blood of type 2 diabetic vs. non-diabetic human subjects (50 subjects per group). They reported a statistically-significant increase in levels of AGER in the diabetic vs. non-diabetic monocytes, which paralleled an increase in serum AGE levels and was inversely correlated with levels of soluble RAGE54. Tam and co-workers studied RAGE protein levels by Western blot in 53 diabetic and 52 control subjects and reported that monocyte full-length RAGE expression was significantly higher in the diabetic group but that a splice form of RAGE, called RAGEv1 or “endogenous secretory (es) RAGE” was lower. They reported an inverse association between monocyte RAGE expression and serum sRAGE but not serum esRAGE levels55.
Others asked if RAGE expression in myeloid cells tracked with tissue activities in disease. Sunahori and colleagues examined synovial tissue from rheumatoid arthritis subjects and reported that AGER mRNA expression was higher in this tissue from rheumatoid arthritis vs. osteoarthritis subjects and that CD68+ lining macrophages in the synovial tissue expressed high levels of RAGE expression. Further, they showed that RAGE expression could be augmented on normal monocytes by incubation with rheumatoid arthritis synovial tissue cell culture supernatants or by treatment by cytokines, especially IL1B56. Uhle and colleagues reported that severe trauma or surgical intervention resulted in a downregulation of monocyte RAGE expression by flow cytometry compared to the admission values and a trend toward lower levels of monocyte RAGE when compared to healthy controls. These authors showed that post-trauma, two phases of release of RAGE ligands occurred and they speculated that their interaction with RAGE contributed to modulation of immune responses after severe injuries57. Evidence that the changes in RAGE expression in such settings is not spurious but, rather, that specific cues in distinct environments may result in alterations in monocyte RAGE expression was evident from studies by Stew and colleagues who showed that there no differences in monocyte cell surface RAGE expression between tuberculosis patients with or without type 2 diabetes58.
In other studies, researchers treated monocytes or monocyte-derived macrophages from diabetic or non-diabetic human subjects with RAGE ligands and showed that (1) treatment of monocytes with AGE-bovine serum albumin increased expression of CD36 and production of ROS, which was blocked by RNAi-knockdown of AGER59; (2) treatment of monocytes/macrophages with RAGE ligands AGEs or S100B or incubation with high glucose increased generation of ROS, which was blocked by anti-RAGE, but not control IgGs60; and (3) treatment of THP1 macrophage-like cells with RAGE ligand S100B increased phosphorylation of pleckstrin and consequent phosphorylation of protein kinase C (PKC) substrates, which was suppressed by RNAi-knockdown of PLEK61. These authors showed that phosphorylation of pleckstrin was increased in diabetic mononuclear phagocytes compared to control, thereby linking RAGE ligands to mechanisms that regulate activity of PKC.
RAGE and soluble RAGEs
There are two forms of soluble RAGEs detectable in human subjects; the first is total soluble RAGE (sRAGE), which reflects cell surface cleaved material by the actions of matrix metalloproteinases (MMPs) or ADAM10; and the second is esRAGE, which results from a splice variant of RAGE62. Although the precise source(s) of sRAGEs is not fully clarified, it is plausible that vascular cells and circulating immune cells, such as monocytes, account for detectable sRAGEs in circulation. A recent literature search (PubMed) revealed that >500 papers have been published in which levels of sRAGE and/or esRAGE have been tested in a variety of disorders known to be linked to the accumulation of RAGE ligands. Whether or not monitoring levels of sRAGEs may be of value in biomarking target engagement is yet to be determined. However, it is notable that therapeutic interventions such as dexamethasone63; statins, telmisartan, curcurmin, Vitamin D and rosiglitazone64; physical exercise65; and bariatric surgery66, 67 may modulate levels of sRAGEs.
Summary and Perspectives: Heading into the Future
Much of the past and present work on RAGE in monocytes/macrophages and other key cell types linked to cardiometabolic disorders has focused on probing roles for the receptor and its signaling axis in initiation and progression of diabetic (and non-diabetic) atherosclerosis and in diet induced obesity, insulin resistance and type 2 diabetes. Key future directions include the testing of RAGE/DIAPH1 in regression of diabetic atherosclerosis and in established obesity or weight loss paradigms, as these are equally and highly relevant clinical milieus that need to considered in clinical studies.
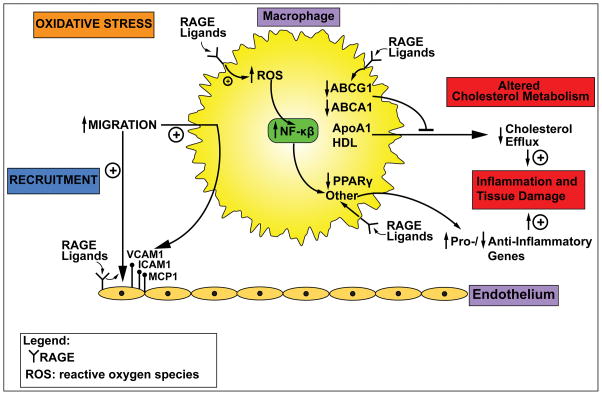
The Figure indicates the known effects of RAGE ligands on monocyte/macrophage properties. Specifically, evidence for important roles for RAGE in monocyte/macrophage recruitment, cholesterol efflux and reverse cholesterol transport, oxidative stress and inflammation stimulate us to keep digging to discover what are the precise biochemical and molecular pathways that underlie experimental findings. Lead pathways for investigation include those that regulate glucose and lipid (fatty acid) metabolism and addressing if energy preferences mediate key RAGE actions in these cells in immunometabolic milieus. Finally, the unexpected, but intriguing finding that mice devoid of Ager are protected from high fat diet induced obesity and its metabolic consequences, at least in part through upregulation of energy expenditure, spurs us to address the unresolved question on RAGE’s natural functions. No doubt that RAGE actions impart considerable maladaptive consequences, leading to chronic disease, especially in cardiometabolic disorders. Perhaps, however, the silver lining is the ability of the RAGE to combat starvation stress by conservation of energy. Answers to these questions will help to unravel the complex biology of RAGE, understand its value through evolution and to establish its utility or not as a target for therapeutic intervention in chronic cardiometabolic and immune/inflammatory diseases.
Figure. RAGE and monocyte/macrophage perturbation.
The unique cadre of RAGE ligands, via RAGE, stimulates key perturbations in monocytes/macrophages, such as induction of oxidative stress and consequent activation of NF-κB; migration (at least in response to RAGE ligand-mediated upregulation of adhesion molecules and chemokines on endothelial cells); downregulation of cholesterol transporters ABCG1 and ABCA1, leading to suppression of cholesterol efflux; and downregulation of PPARG and other mediators that tip the balance to increased expression of PRO-inflammatory vs. reduced expression of ANTI-inflammatory mediators, thereby contributing to sustained inflammation and tissue damage. These facets of RAGE pathobiology in macrophages have implications for atherosclerosis and diet-induced obesity, as well as for chronic inflammatory disorders. More work needs to be done to determine if RAGE modulates glucose/fatty acid metabolism in these cells and if such effects might affect macrophage properties in responses to stress. Findings to date suggest that targeting RAGE may be beneficial in chronic immunometabolic disorders and that biomarking RAGE activity in circulating monocytes/monocyte-derived macrophages may be a key strategy to track target engagement for future clinical trials. Abbreviation: ROS, reactive oxygen species.
Acknowledgments
The author gratefully acknowledges the assistance of Ms. Latoya Woods in the preparation of this manuscript. This work was funded by grants from the U.S. Public Health Service, JDRF, Harrington Discovery Institute and the American Diabetes Association. The author declares no conflicts of interest.
References
- 1.Lopez-Diez R, Shekhtman A, Ramasamy R, Schmidt AM. Cellular mechanisms and consequences of glycation in atherosclerosis and obesity. Biochim Biophys Acta. 2016;1862:2244–52. doi: 10.1016/j.bbadis.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shekhtman A, Ramasamy R, Schmidt AM. Glycation & the RAGE axis: targeting signal transduction through DIAPH1. Expert Rev Proteomics. 2016:1–10. doi: 10.1080/14789450.2017.1271719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kislinger T, Fu C, Huber B, Qu W, Taguchi A, Du Yan S, Hofmann M, Yan SF, Pischetsrieder M, Stern D, Schmidt AM. N(epsilon)-(carboxymethyl)lysine adducts of proteins are ligands for receptor for advanced glycation end products that activate cell signaling pathways and modulate gene expression. J Biol Chem. 1999;274:31740–9. doi: 10.1074/jbc.274.44.31740. [DOI] [PubMed] [Google Scholar]
- 4.Hofmann MA, Drury S, Fu C, et al. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901. doi: 10.1016/s0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- 5.Taguchi A, Blood DC, del Toro G, et al. Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature. 2000;405:354–60. doi: 10.1038/35012626. [DOI] [PubMed] [Google Scholar]
- 6.Chavakis T, Bierhaus A, Al-Fakhri N, Schneider D, Witte S, Linn T, Nagashima M, Morser J, Arnold B, Preissner KT, Nawroth PP. The pattern recognition receptor (RAGE) is a counterreceptor for leukocyte integrins: a novel pathway for inflammatory cell recruitment. J Exp Med. 2003;198:1507–15. doi: 10.1084/jem.20030800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rai V, Toure F, Chitayat S, Pei R, Song F, Li Q, Zhang J, Rosario R, Ramasamy R, Chazin WJ, Schmidt AM. Lysophosphatidic acid targets vascular and oncogenic pathways via RAGE signaling. J Exp Med. 2012;209:2339–50. doi: 10.1084/jem.20120873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andrassy M, Igwe J, Autschbach F, et al. Posttranslationally modified proteins as mediators of sustained intestinal inflammation. Am J Pathol. 2006;169:1223–37. doi: 10.2353/ajpath.2006.050713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bierhaus A, Schiekofer S, Schwaninger M, et al. Diabetes-associated sustained activation of the transcription factor nuclear factor-kappaB. Diabetes. 2001;50:2792–808. doi: 10.2337/diabetes.50.12.2792. [DOI] [PubMed] [Google Scholar]
- 10.Reiniger N, Lau K, McCalla D, et al. Deletion of the receptor for advanced glycation end products reduces glomerulosclerosis and preserves renal function in the diabetic OVE26 mouse. Diabetes. 2010;59:2043–54. doi: 10.2337/db09-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeng S, Zhang QY, Huang J, Vedantham S, Rosario R, Ananthakrishnan R, Yan SF, Ramasamy R, DeMatteo RP, Emond JC, Friedman RA, Schmidt AM. Opposing roles of RAGE and Myd88 signaling in extensive liver resection. FASEB J. 2012;26:882–93. doi: 10.1096/fj.11-192997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bu DX, Rai V, Shen X, Rosario R, Lu Y, D’Agati V, Yan SF, Friedman RA, Nuglozeh E, Schmidt AM. Activation of the ROCK1 branch of the transforming growth factor-beta pathway contributes to RAGE-dependent acceleration of atherosclerosis in diabetic ApoE-null mice. Circ Res. 2010;106:1040–51. doi: 10.1161/CIRCRESAHA.109.201103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cipollone F, Iezzi A, Fazia M, Zucchelli M, Pini B, Cuccurullo C, De Cesare D, De Blasis G, Muraro R, Bei R, Chiarelli F, Schmidt AM, Cuccurullo F, Mezzetti A. The receptor RAGE as a progression factor amplifying arachidonate-dependent inflammatory and proteolytic response in human atherosclerotic plaques: role of glycemic control. Circulation. 2003;108:1070–7. doi: 10.1161/01.CIR.0000086014.80477.0D. [DOI] [PubMed] [Google Scholar]
- 14.Burke AP, Kolodgie FD, Zieske A, Fowler DR, Weber DK, Varghese PJ, Farb A, Virmani R. Morphologic findings of coronary atherosclerotic plaques in diabetics: a postmortem study. Arterioscler Thromb Vasc Biol. 2004;24:1266–71. doi: 10.1161/01.ATV.0000131783.74034.97. [DOI] [PubMed] [Google Scholar]
- 15.Soro-Paavonen A, Watson AM, Li J, et al. Receptor for advanced glycation end products (RAGE) deficiency attenuates the development of atherosclerosis in diabetes. Diabetes. 2008;57:2461–9. doi: 10.2337/db07-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun L, Ishida T, Yasuda T, Kojima Y, Honjo T, Yamamoto Y, Yamamoto H, Ishibashi S, Hirata K, Hayashi Y. RAGE mediates oxidized LDL-induced pro-inflammatory effects and atherosclerosis in non-diabetic LDL receptor-deficient mice. Cardiovasc Res. 2009;82:371–81. doi: 10.1093/cvr/cvp036. [DOI] [PubMed] [Google Scholar]
- 17.Harja E, Bu DX, Hudson BI, et al. Vascular and inflammatory stresses mediate atherosclerosis via RAGE and its ligands in apoE−/− mice. J Clin Invest. 2008;118:183–94. doi: 10.1172/JCI32703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park L, Raman KG, Lee KJ, Lu Y, Ferran LJ, Jr, Chow WS, Stern D, Schmidt AM. Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat Med. 1998;4:1025–31. doi: 10.1038/2012. [DOI] [PubMed] [Google Scholar]
- 19.Bucciarelli LG, Wendt T, Qu W, Lu Y, Lalla E, Rong LL, Goova MT, Moser B, Kislinger T, Lee DC, Kashyap Y, Stern DM, Schmidt AM. RAGE blockade stabilizes established atherosclerosis in diabetic apolipoprotein E-null mice. Circulation. 2002;106:2827–35. doi: 10.1161/01.cir.0000039325.03698.36. [DOI] [PubMed] [Google Scholar]
- 20.Morris-Rosenfeld S, Blessing E, Preusch MR, Albrecht C, Bierhaus A, Andrassy M, Nawroth PP, Rosenfeld ME, Katus HA, Bea F. Deletion of bone marrow-derived receptor for advanced glycation end products inhibits atherosclerotic plaque progression. Eur J Clin Invest. 2011;41:1164–71. doi: 10.1111/j.1365-2362.2011.02514.x. [DOI] [PubMed] [Google Scholar]
- 21.Koulis C, Kanellakis P, Pickering RJ, Tsorotes D, Murphy AJ, Gray SP, Thomas MC, Jandeleit-Dahm KA, Cooper ME, Allen TJ. Role of bone-marrow- and non-bone-marrow-derived receptor for advanced glycation end-products (RAGE) in a mouse model of diabetes-associated atherosclerosis. Clin Sci (Lond) 2014;127:485–97. doi: 10.1042/CS20140045. [DOI] [PubMed] [Google Scholar]
- 22.Odetti P, Cosso L, Pronzato MA, Dapino D, Gurreri G. Plasma advanced glycosylation end-products in maintenance haemodialysis patients. Nephrol Dial Transplant. 1995;10:2110–3. [PubMed] [Google Scholar]
- 23.Belmokhtar K, Robert T, Ortillon J, Braconnier A, Vuiblet V, Boulagnon-Rombi C, Diebold MD, Pietrement C, Schmidt AM, Rieu P, Toure F. Signaling of Serum Amyloid A Through Receptor for Advanced Glycation End Products as a Possible Mechanism for Uremia-Related Atherosclerosis. Arterioscler Thromb Vasc Biol. 2016;36:800–9. doi: 10.1161/ATVBAHA.115.306349. [DOI] [PubMed] [Google Scholar]
- 24.Bro S, Flyvbjerg A, Binder CJ, Bang CA, Denner L, Olgaard K, Nielsen LB. A neutralizing antibody against receptor for advanced glycation end products (RAGE) reduces atherosclerosis in uremic mice. Atherosclerosis. 2008;201:274–80. doi: 10.1016/j.atherosclerosis.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 25.Shiu SW, Zhou H, Wong Y, Tan KC. Endothelial lipase and reverse cholesterol transport in type 2 diabetes mellitus. J Diabetes Investig. 2010;1:111–6. doi: 10.1111/j.2040-1124.2010.00016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou H, Shiu SW, Wong Y, Tan KC. Impaired serum capacity to induce cholesterol efflux is associated with endothelial dysfunction in type 2 diabetes mellitus. Diab Vasc Dis Res. 2009;6:238–43. doi: 10.1177/1479164109344934. [DOI] [PubMed] [Google Scholar]
- 27.de Boer JF, Annema W, Schreurs M, van der Veen JN, van der Giet M, Nijstad N, Kuipers F, Tietge UJ. Type I diabetes mellitus decreases in vivo macrophage-to-feces reverse cholesterol transport despite increased biliary sterol secretion in mice. J Lipid Res. 2012;53:348–57. doi: 10.1194/jlr.M018671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daffu G, Shen X, Senatus L, Thiagarajan D, Abedini A, Hurtado Del Pozo C, Rosario R, Song F, Friedman RA, Ramasamy R, Schmidt AM. RAGE Suppresses ABCG1-Mediated Macrophage Cholesterol Efflux in Diabetes. Diabetes. 2015;64:4046–60. doi: 10.2337/db15-0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li AC, Binder CJ, Gutierrez A, Brown KK, Plotkin CR, Pattison JW, Valledor AF, Davis RA, Willson TM, Witztum JL, Palinski W, Glass CK. Differential inhibition of macrophage foam-cell formation and atherosclerosis in mice by PPARalpha, beta/delta, and gamma. J Clin Invest. 2004;114:1564–76. doi: 10.1172/JCI18730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wautier JL, Zoukourian C, Chappey O, Wautier MP, Guillausseau PJ, Cao R, Hori O, Stern D, Schmidt AM. Receptor-mediated endothelial cell dysfunction in diabetic vasculopathy. Soluble receptor for advanced glycation end products blocks hyperpermeability in diabetic rats. J Clin Invest. 1996;97:238–43. doi: 10.1172/JCI118397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hallam KM, Li Q, Ananthakrishnan R, Kalea A, Zou YS, Vedantham S, Schmidt AM, Yan SF, Ramasamy R. Aldose reductase and AGE-RAGE pathways: central roles in the pathogenesis of vascular dysfunction in aging rats. Aging Cell. 2010;9:776–84. doi: 10.1111/j.1474-9726.2010.00606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mackic JB, Stins M, McComb JG, Calero M, Ghiso J, Kim KS, Yan SD, Stern D, Schmidt AM, Frangione B, Zlokovic BV. Human blood-brain barrier receptors for Alzheimer’s amyloid-beta 1-40. Asymmetrical binding, endocytosis, and transcytosis at the apical side of brain microvascular endothelial cell monolayer. J Clin Invest. 1998;102:734–43. doi: 10.1172/JCI2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW., Jr CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116:115–24. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boutens L, Stienstra R. Adipose tissue macrophages: going off track during obesity. Diabetologia. 2016;59:879–94. doi: 10.1007/s00125-016-3904-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wentworth JM, Naselli G, Brown WA, Doyle L, Phipson B, Smyth GK, Wabitsch M, O’Brien PE, Harrison LC. Pro-inflammatory CD11c+CD206+ adipose tissue macrophages are associated with insulin resistance in human obesity. Diabetes. 2010;59:1648–56. doi: 10.2337/db09-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fjeldborg K, Pedersen SB, Moller HJ, Christiansen T, Bennetzen M, Richelsen B. Human adipose tissue macrophages are enhanced but changed to an anti-inflammatory profile in obesity. J Immunol Res. 2014;2014:309548. doi: 10.1155/2014/309548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Namgaladze D, Brune B. Macrophage fatty acid oxidation and its roles in macrophage polarization and fatty acid-induced inflammation. Biochim Biophys Acta. 2016;1861:1796–807. doi: 10.1016/j.bbalip.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 39.Gaens KH, Goossens GH, Niessen PM, van Greevenbroek MM, van der Kallen CJ, Niessen HW, Rensen SS, Buurman WA, Greve JW, Blaak EE, van Zandvoort MA, Bierhaus A, Stehouwer CD, Schalkwijk CG. Nepsilon-(carboxymethyl)lysine-receptor for advanced glycation end product axis is a key modulator of obesity-induced dysregulation of adipokine expression and insulin resistance. Arterioscler Thromb Vasc Biol. 2014;34:1199–208. doi: 10.1161/ATVBAHA.113.302281. [DOI] [PubMed] [Google Scholar]
- 40.Song F, Hurtado del Pozo C, Rosario R, et al. RAGE regulates the metabolic and inflammatory response to high-fat feeding in mice. Diabetes. 2014;63:1948–65. doi: 10.2337/db13-1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hudson BI, Kalea AZ, Del Mar Arriero M, Harja E, Boulanger E, D’Agati V, Schmidt AM. Interaction of the RAGE cytoplasmic domain with diaphanous-1 is required for ligand-stimulated cellular migration through activation of Rac1 and Cdc42. J Biol Chem. 2008;283:34457–68. doi: 10.1074/jbc.M801465200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kühn S, Geyer M. Formins as effector proteins of Rho GTPases. Small GTPases. 2014;5:e29513. doi: 10.4161/sgtp.29513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bogdan S, Schultz J, Grosshans J. Formin’ cellular structures: Physiological roles of Diaphanous (Dia) in actin dynamics. Commun Integr Biol. 2013;6:e27634. doi: 10.4161/cib.27634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Young KG, Copeland JW. Formins in cell signaling. Biochim Biophys Acta. 2010;1803:183–90. doi: 10.1016/j.bbamcr.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 45.Toure F, Fritz G, Li Q, Rai V, Daffu G, Zou YS, Rosario R, Ramasamy R, Alberts AS, Yan SF, Schmidt AM. Formin mDia1 mediates vascular remodeling via integration of oxidative and signal transduction pathways. Circ Res. 2012;110:1279–93. doi: 10.1161/CIRCRESAHA.111.262519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chang JS, Wendt T, Qu W, Kong L, Zou YS, Schmidt AM, Yan SF. Oxygen deprivation triggers upregulation of early growth response-1 by the receptor for advanced glycation end products. Circ Res. 2008;102:905–13. doi: 10.1161/CIRCRESAHA.107.165308. [DOI] [PubMed] [Google Scholar]
- 47.Xu Y, Toure F, Qu W, Lin L, Song F, Shen X, Rosario R, Garcia J, Schmidt AM, Yan SF. Advanced glycation end product (AGE)-receptor for AGE (RAGE) signaling and up-regulation of Egr-1 in hypoxic macrophages. J Biol Chem. 2010;285:23233–40. doi: 10.1074/jbc.M110.117457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ten VS, Pinsky DJ. Endothelial response to hypoxia: physiologic adaptation and pathologic dysfunction. Curr Opin Crit Care. 2002;8:242–50. doi: 10.1097/00075198-200206000-00008. [DOI] [PubMed] [Google Scholar]
- 49.Rai V, Maldonado AY, Burz DS, Reverdatto S, Yan SF, Schmidt AM, Shekhtman A. Signal transduction in receptor for advanced glycation end products (RAGE): solution structure of C-terminal rage (ctRAGE) and its binding to mDia1. J Biol Chem. 2012;287:5133–44. doi: 10.1074/jbc.M111.277731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xue J, Manigrasso M, Scalabrin M, Rai V, Reverdatto S, Burz DS, Fabris D, Schmidt AM, Shekhtman A. Change in the Molecular Dimension of a RAGE-Ligand Complex Triggers RAGE Signaling. Structure. 2016;24:1509–22. doi: 10.1016/j.str.2016.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Manigrasso MB, Pan J, Rai V, Zhang J, Reverdatto S, Quadri N, DeVita RJ, Ramasamy R, Shekhtman A, Schmidt AM. Small Molecule Inhibition of Ligand-Stimulated RAGE-DIAPH1 Signal Transduction. Sci Rep. 2016;6:22450. doi: 10.1038/srep22450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chuah YK, Basir R, Talib H, Tie TH, Nordin N. Receptor for advanced glycation end products and its involvement in inflammatory diseases. Int J Inflam. 2013;2013:403460. doi: 10.1155/2013/403460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lanati N, Emanuele E, Brondino N, Geroldi D. Soluble RAGE-modulating drugs: state-of-the-art and future perspectives for targeting vascular inflammation. Curr Vasc Pharmacol. 2010;8:86–92. doi: 10.2174/157016110790226642. [DOI] [PubMed] [Google Scholar]
- 54.Su XD, Li SS, Tian YQ, Zhang ZY, Zhang GZ, Wang LX. Elevated serum levels of advanced glycation end products and their monocyte receptors in patients with type 2 diabetes. Arch Med Res. 2011;42:596–601. doi: 10.1016/j.arcmed.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 55.Tam XH, Shiu SW, Leng L, Bucala R, Betteridge DJ, Tan KC. Enhanced expression of receptor for advanced glycation end-products is associated with low circulating soluble isoforms of the receptor in Type 2 diabetes. Clin Sci (Lond) 2011;120:81–9. doi: 10.1042/CS20100256. [DOI] [PubMed] [Google Scholar]
- 56.Sunahori K, Yamamura M, Yamana J, Takasugi K, Kawashima M, Makino H. Increased expression of receptor for advanced glycation end products by synovial tissue macrophages in rheumatoid arthritis. Arthritis Rheum. 2006;54:97–104. doi: 10.1002/art.21524. [DOI] [PubMed] [Google Scholar]
- 57.Uhle F, Lichtenstern C, Brenner T, Fleming T, Koch C, Hecker A, Heiss C, Nawroth PP, Hofer S, Weigand MA, Weismuller K. Role of the RAGE Axis during the Immune Response after Severe Trauma: A Prospective Pilot Study. Mediators Inflamm. 2015;2015:691491. doi: 10.1155/2015/691491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stew SS, Martinez PJ, Schlesinger LS, Restrepo BI. Differential expression of monocyte surface markers among TB patients with diabetes co-morbidity. Tuberculosis (Edinb) 2013;93(Suppl):S78–82. doi: 10.1016/S1472-9792(13)70015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xanthis A, Hatzitolios A, Fidani S, Befani C, Giannakoulas G, Koliakos G. Receptor of advanced glycation end products (RAGE) positively regulates CD36 expression and reactive oxygen species production in human monocytes in diabetes. Angiology. 2009;60:772–9. doi: 10.1177/0003319708328569. [DOI] [PubMed] [Google Scholar]
- 60.Ding Y, Kantarci A, Hasturk H, Trackman PC, Malabanan A, Van Dyke TE. Activation of RAGE induces elevated O2- generation by mononuclear phagocytes in diabetes. J Leukoc Biol. 2007;81:520–7. doi: 10.1189/jlb.0406262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ding Y, Kantarci A, Badwey JA, Hasturk H, Malabanan A, Van Dyke TE. Phosphorylation of pleckstrin increases proinflammatory cytokine secretion by mononuclear phagocytes in diabetes mellitus. J Immunol. 2007;179:647–54. doi: 10.4049/jimmunol.179.1.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schmidt AM. Soluble RAGEs - Prospects for treating & tracking metabolic and inflammatory disease. Vascul Pharmacol. 2015;72:1–8. doi: 10.1016/j.vph.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kosutova P, Mikolka P, Balentova S, Adamkov M, Kolomaznik M, Calkovska A, Mokra D. Intravenous dexamethasone attenuated inflammation and influenced apoptosis of lung cells in an experimental model of acute lung injury. Physiol Res. 2016;65:S663–S72. doi: 10.33549/physiolres.933531. [DOI] [PubMed] [Google Scholar]
- 64.Prasad K, Tiwari S. Therapeutic interventions for Advanced Glycation-End Products and its Receptor-Mediated Cardiovascular Disease. Curr Pharm Des. 2016 doi: 10.2174/1381612822666161006143032. [DOI] [PubMed] [Google Scholar]
- 65.Santilli F, Vazzana N, Iodice P, Lattanzio S, Liani R, Bellomo RG, Lessiani G, Perego F, Saggini R, Davi G. Effects of high-amount-high-intensity exercise on in vivo platelet activation: modulation by lipid peroxidation and AGE/RAGE axis. Thromb Haemost. 2013;110:1232–40. doi: 10.1160/TH13-04-0295. [DOI] [PubMed] [Google Scholar]
- 66.Horwitz D, Saunders JK, Ude-Welcome A, Schmidt AM, Dunn V, Leon Pachter H, Parikh M. Three-year follow-up comparing metabolic surgery versus medical weight management in patients with type 2 diabetes and BMI 30–35. The role of sRAGE biomarker as predictor of satisfactory outcomes. Surg Obes Relat Dis. 2016;12:1337–41. doi: 10.1016/j.soard.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 67.Parikh M, Chung M, Sheth S, McMacken M, Zahra T, Saunders JK, Ude-Welcome A, Dunn V, Ogedegbe G, Schmidt AM, Pachter HL. Randomized pilot trial of bariatric surgery versus intensive medical weight management on diabetes remission in type 2 diabetic patients who do NOT meet NIH criteria for surgery and the role of soluble RAGE as a novel biomarker of success. Ann Surg. 2014;260:617–22. doi: 10.1097/SLA.0000000000000919. discussion 22-4. [DOI] [PMC free article] [PubMed] [Google Scholar]