Abstract
Objective
Platelets, which are mainly known for their role in hemostasis, are now known to play a crucial role in metastasis. Tamoxifen is a selective estrogen receptor modulator that is widely used for the treatment of breast cancer. Tamoxifen and its metabolites have been shown to directly impact platelet function suggesting that this drug has additional mechanisms of action. The purpose of this study was to determine whether tamoxifen exerts anti-tumor effects through direct platelet inhibition.
Approach and Results
This study found that pretreatment with tamoxifen leads to a significant inhibition of platelet activation. Platelets exposed to tamoxifen released significantly lower amounts of pro-angiogenic regulator VEGF. In vitro angiogenesis assays confirmed that tamoxifen pretreatment led to diminished capillary tube formation and decreased endothelial migration. Tamoxifen and its metabolite, 4-Hydroxytamoxifen, also significantly inhibited the ability of platelets to promote metastasis in vitro. Using a membrane based array, we identified several proteins associated with angiogenesis metastasis that were lower in activated releasate from tamoxifen treated platelets including angiogenin, CXCL1, CCL5, EGF, CXCL5 and PDGF-BB while anti-angiogenic angiopoietin-1 was elevated. Platelets isolated from patients on tamoxifen maintenance therapy were also found to have decreased activation responses, diminished VEGF release and lower angiogenic and metastatic potential.
Conclusions
We demonstrate that tamoxifen and its metabolite 4-Hydroxytamoxifen directly alters platelet function leading to decreased angiogenic and metastatic potential. Furthermore, this study supports the idea of utilizing targeted platelet therapies to inhibit the platelet’s role in angiogenesis and malignancy.
Keywords: platelets, tamoxifen, angiogenesis, metastasis
INTRODUCTION
The role of platelets in malignancy is emerging as an important and attractive area of investigation. It has now been demonstrated that platelets are essential to all stages of primary tumor growth as well as metastatic spread.1 Platelets aid disseminating tumor cells by protecting them from high shear forces and immune surveillance within the circulation, forming tumor cell-platelet aggregates that facilitate embolization, promoting adhesion of tumor cells to the vascular endothelium, and releasing a variety of soluble factors that promote tumor growth, metastasis, and angiogenesis.2 Platelets carry a plethora of angiogenic factors within their alpha granules including the potent pro-angiogenic protein Vascular Endothelial Growth Factor (VEGF).3,4 In fact, platelets appear to be the main storage site for angiogenic proteins; 80% of circulating VEGF is stored in platelet alpha granules.5 Previously, our group and others have demonstrated that platelets differentially release pro- and anti-angiogenic factors such as VEGF and endostatin in an agonist-dependent manner.5–8 Platelets are also activated upon exposure to breast tumor cells, and this leads to the release of potent pro-angiogenic mediators.6 In breast cancer in particular, angiogenesis is key to tumor growth and metastasis including ductal carcinoma in situ.9 Overexpression of angiogenic factors such as VEGF in breast tumor tissue is associated with poor clinical outcome and lower response to chemotherapeutic and hormonal-based treatment regimens.10,11 In addition, VEGF levels in serum have been deemed an independent prognostic factor for survival and there is a correlation between platelet levels of VEGF and disease progression.12,13 Recently, we have demonstrated that intervention with aspirin and anticoagulant drugs can significantly diminish the release of granule components from platelets. As such, the angiogenic potential of aspirin-treated platelets is inhibited in response to breast cancer tumor cells.6,14
Tamoxifen is an estrogen receptor modulator (SERM) that is used widely as anti-estrogen therapy for breast cancer. Tamoxifen treatment is associated with a 50% reduction in the risk of invasive and noninvasive breast cancer in women who utilized the drug for at least 5 years.15 Interestingly, tamoxifen has demonstrated anti-cancer efficacy in estrogen negative cancers suggesting that this drug has additional mechanisms of action.16 Platelets express the receptors for estrogen (ER alpha and ER beta) and a role for estrogen in platelet function has been investigated previously.17,18 Some studies have suggested that tamoxifen may stimulate platelet aggregation in vitro and in vivo 19,20; however more recent data support an inhibitory role in platelet activation.21,22 Mechanistically, Chang and colleagues have recently demonstrated that tamoxifen inhibits platelet activation through the inhibition of PLC-2-PKC-p38 signaling.21 Though the impact of tamoxifen on platelets has been examined in the context of cardiovascular disease, to date no studies have investigated the effect of platelet inhibition by tamoxifen on breast cancer angiogenesis or metastasis.
In this manuscript, we aim to demonstrate that tamoxifen and its metabolites significantly diminish the release of platelet angiogenic factors and metastatic factors leading to decreased tumor cell support. The mechanism is directly linked to tamoxifen’s inhibitory role in platelet activation, causing altered release of key angiogenic and metastatic factors during tumor cell and platelet cross-talk.
MATERIALS AND METHODS
Materials and Methods are available in the online-only Data Supplement.
RESULTS
Patients on tamoxifen therapy release less VEGF and have diminished platelet angiogenic potential
Previous work from our group has shown that drugs that inhibit platelet function, such as aspirin and anticoagulants,6,14 can disrupt pro-angiogenic platelet-tumor cell crosstalk. Recent in vitro studies have shown that tamoxifen can directly alter platelet function; therefore, we hypothesized that tamoxifen therapy may augment the pro-angiogenic response of platelets to tumor cells.21,22 We conducted translational studies using blood samples collected from breast cancer patients on tamoxifen therapy to determine if systemic, therapeutic levels of tamoxifen impacted platelet-mediated angiogenesis. Patients receiving adjuvant tamoxifen therapy for breast cancer for at least one month and who were not taking any additional platelet inhibiting drugs were selected for this study. Platelets were isolated from patients or healthy controls and activated ex vivo by a 10 minute exposure to MCF-7 breast tumor cells to generate an activated platelet releasate (1A, schematic). Previously, we showed that platelets release pro-angiogenic VEGF in response to tumor cells.6 We measured VEGF in releasate collected from activated platelets and found that platelets from patients receiving tamoxifen release significantly less VEGF upon activation by MCF-7 tumor cells (1C). To determine if tamoxifen therapy lead to a net decrease in angiogenic potential, we used these platelet releasates in capillary tube formation assays. Capillary tube formation was significantly lower in patient samples compared to controls (1D-E), demonstrating that tamoxifen therapy inhibits the pro-angiogenic potential of platelets during platelet-tumor cell cross talk.
Tamoxifen inhibits tumor-cell induced platelet activation
To determine if the decrease in VEGF release and angiogenic potential were the result of impaired platelet activation in response to tumor cells, surface expression of the platelet activation marker P-selectin was measured using flow cytometry. We have previously shown that MCF-7 breast tumor cells induce platelet activation.6 We confirmed that P-selectin increased 2.3 fold in MCF-7-activated platelets from healthy donors (1B). However, platelets from patients taking tamoxifen displayed significantly diminished activation response (1B), indicating that therapeutic levels of tamoxifen cause inhibition of platelet activation in response to breast tumor cells.
To further examine the effect of tamoxifen on platelet activation, platelets from healthy human donors were pretreated ex vivo with tamoxifen or vehicle control (2A schematic), washed, and then stimulated with various agonists: ADP, MCF-7 breast tumor cells, or thrombin receptor activating peptide (TRAP). Doses of 10–20 µM tamoxifen were selected based on previously reported studies.21–23 ADP, MCF-7 cells, and TRAP all significantly increased P-selectin surface expression compared to resting platelets (2B-C). However, pretreatment of platelets with tamoxifen caused a dose-dependent decrease in ADP-induced activation (2D). Tamoxifen did not alter activation in response to the strong agonist TRAP at either concentration examined. Importantly, pretreatment of platelets with tamoxifen significantly reduced MCF-7-induced activation. Tamoxifen treatment alone had no effect on P-Selectin expression in resting platelets (2C). Representative histograms showing P-Selectin expression are depicted in Figure 2B. These results confirm that tamoxifen directly and dose-dependently inhibits tumor cell-induced platelet activation, disrupting platelet-tumor cell cross-talk.
Figure 2. Tamoxifen inhibits platelet activation.
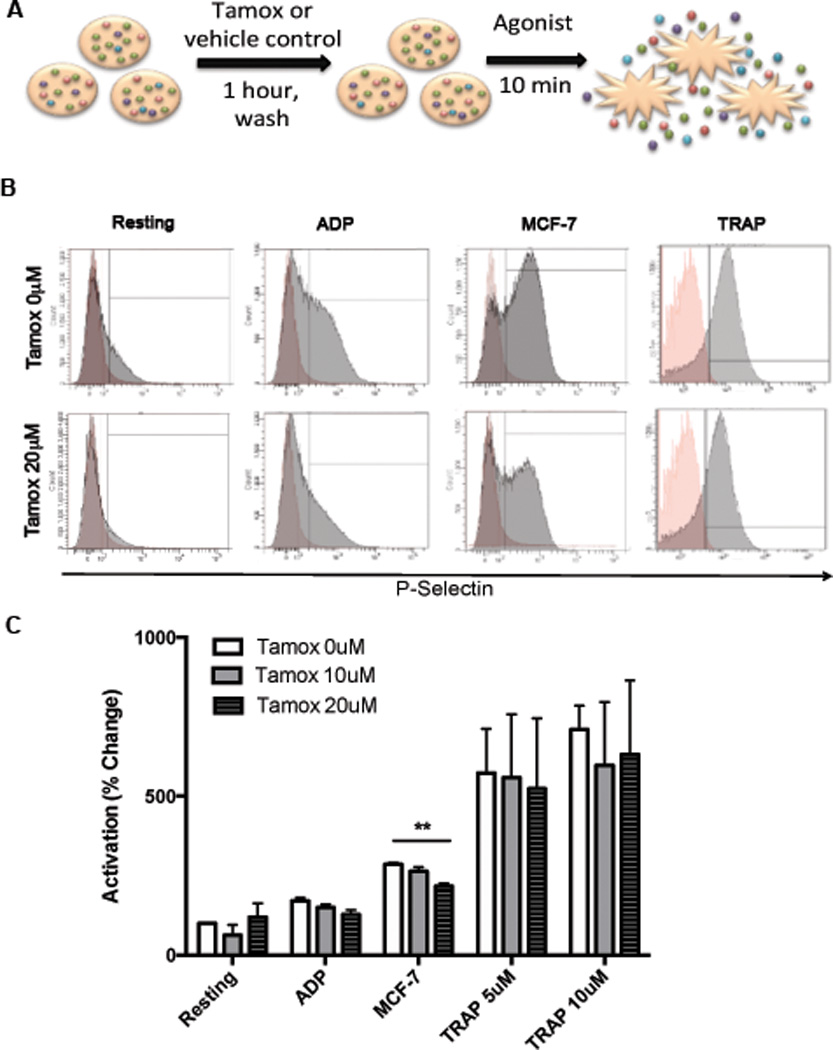
To measure platelet activation, platelets were isolated from healthy donors, pretreated with 10–20 µM of tamoxifen or vehicle control, washed, and exposed to agonists (ADP, MCF-7 tumor cells or TRAP). (A). P-selectin surface expression, a marker of activation, was determined by flow cytometry. Representative histograms are shown, with P-selectin stained platelets (gray) overlaid onto platelets stained with isotype control antibodies (red) (B). Quantified results are shown in C. Bars indicate SEM. P<*0.01 compared to resting control unless otherwise indicated by ANOVA, n=3–6 independent replicates per treatment group.
Tamoxifen directly inhibits angiogenic potential of tumor cell activated platelets
Next we sought to further examine the effect of tamoxifen on the angiogenic potential of tumor cell activated platelets that we observed in our patient cohort. We pretreated platelets with tamoxifen prior to activation by MCF-7 cells and measured the release of VEGF (schematic 3A). Activation with MCF-7 cells increased the release of pro-angiogenic VEGF while tamoxifen pretreatment reduced VEGF release to baseline levels (3B). Next, we sought to determine if tamoxifen could directly alter the net angiogenic effect of platelets. We have previously shown that releasate from platelets activated by MCF-7 cells increases endothelial cell migration and capillary tube formation.6 Here, we pretreated platelets with tamoxifen (10 µM or 20 µM) prior to activation with MCF-7 cells and tested the angiogenic potential of the resulting releasate using functional angiogenesis assays (schematic 3A). We also tested the angiogenic potential of these releasates in capillary tube formation assays. MCF-7-activated platelet releasate induced increased capillary tube formation compared to releasate from resting, unactivated (resting) platelets (3C-D). Releasates from platelets pretreated with tamoxifen prior to activation induced significantly less capillary tube formation after 6 hours compared to controls (3D). Endothelial migration, a critical step in angiogenesis, was sharply and significantly increased in response to releasate from MCF-7-activated platelets (3E,F). However, no significant increase in endothelial cell migration was observed when platelets were treated with tamoxifen prior to MCF-7 activation (3E,F). These results suggest that tamoxifen lowers the net angiogenic potential of platelets and that this may be one of the ways by which tamoxifen works to control tumor growth.
Tamoxifen decreases the metastatic potential of platelets
Because platelets are also known to have pro-metastatic effects in breast cancer,24–26 we next examined the effect of tamoxifen on platelet-mediated metastasis. To test this, we examined the effect of tamoxifen on the metastatic potential of platelets using standard in vitro metastasis assays: tumor cell invasion and transendothelial migration. For invasion assays, human platelets were pretreated with 20 µM tamoxifen or DMSO vehicle control, washed, and activated with MCF-7 tumor cells to generate a releasate. We measured the effect of these releasates on MCF-7 tumor cell invasion through matrigel and found that activated platelet releasate increased MCF-7 invasion compared to resting releasate (4A,B). Pre-treatment with tamoxifen diminished the ability of activated releastes to promote MCF-7 invasion by 76% (4A-B). Transendothelial migration, or the ability of tumor cells to cross an endothelial barrier, is a critical step in the metastatic process. Platelets can aid tumor cells in this process, as demonstrated by the ability of live platelets to increase the migration of MCF-7 tumor cells across an endothelialized membrane by 2.6 fold over platelet-free control (4C). In contrast, tamoxifen pretreatment completely prevented platelets from promoting the transendothelial migration of tumor cells (4C).
Tamoxifen metabolite 4-OH inhibits platelet activation and VEGF release
Tamoxifen is a pro-drug and is metabolized into more active forms in the body.27 Therefore, we hypothesized that a metabolite may contribute to the dramatic results seen in patient samples (Fig 1). 4-Hydroxytamoxifen (4-OH), a tamoxifen metabolite found in the blood,27,28 has been shown to inhibit platelets activation and aggregation,22 Therefore we tested whether 4-OH could also inhibit platelet-mediated angiogenesis and metastasis. Platelets from healthy human donors were pretreated with 25 µM or 50 µM of 4-OH or vehicle control, washed to remove the drug, and then activated by various agonists. Unlike tamoxifen, 4-OH significantly and dose dependently inhibited platelet activation in response to the strong agonist TRAP (5A). 4-OH also lowered activation in response to MCF-7 tumor cells, however this reduction did not reach statistical significance (5A). More significant inhibition was however achieved in response to the aggressive, triple negative breast cancer cell line MDA-MB-231. VEGF release also trended toward a reduction with 4-OH (5B) prompting us to look for other angiogenic factors through angiogenic protein array studies as shown in 5C-E. The robust response, particularly against the strong agonist TRAP and the aggressive MDA-231 cells, suggests that the 4-OH metabolite of tamoxifen has potent effects on platelets. Therefore, we further characterized the effect of 4-OH on platelet-mediated angiogenesis and metastasis.
Figure 1. Patients taking tamoxifen have altered platelet function and diminished platelet angiogenic potential.
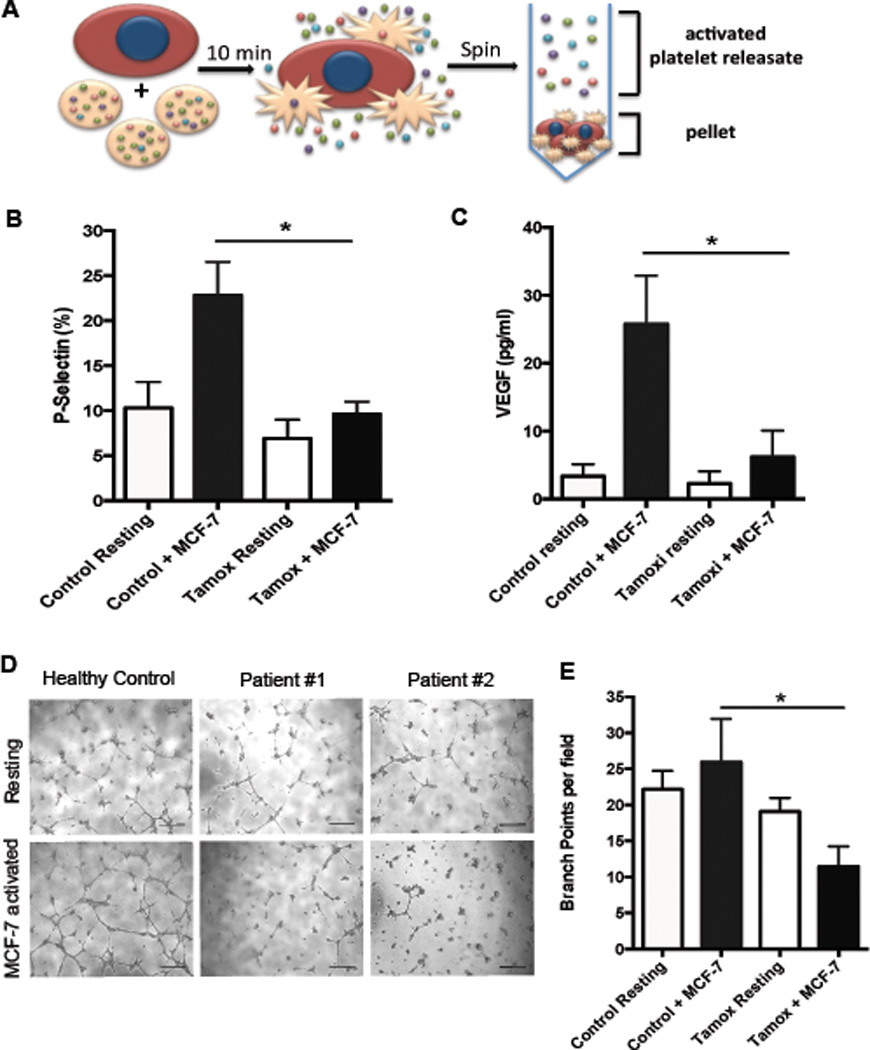
Platelets were isolated from patients on tamoxifen maintenance therapy or healthy controls and activated by exposure to MCF-7 breast tumor cells for 10 minutes to generate an “activated platelet releasate” (A). Activation was determined by P-selectin surface expression using flow cytometry following activation with MCF-7 tumor cells (B). VEGF release from platelets was measured in resulting releastes by ELISA (C). MCF-7 activated or resting platelet releasates from patients were used in capillary tubes assays and compared to releasates from healthy controls. Representative images are shown (D) and results from all replicates are quantified (E). Bars indicate SEM. P<*0.05 by ANOVA, n=3–6 independent replicates per treatment group. Scale bars represent 100 µm.
Tamoxifen metabolite 4-OH dampens release of pro-angiogenic and metastatic factors
To further characterize how 4-OH alters the release of platelet factors, we performed a membrane-based array (C1000, Ray Biotech) to simultaneously detect 43 proteins known to be involved in angiogenesis and cancer. Releasates generated from MCF-7 or TRAP activated platelets following treatment with 50 µM 4-OH or vehicle control were compared by membrane-based array. Proteins that showed a 1.5 fold or greater change in response to tamoxifen or 4-OH are reported (5C-E). We found that 4-OH inhibited the release of key regulators of angiogenesis and metastasis including angiogenin, CCL5 (Rantes), CXCL1, EGF, MMP-1, PDGF, and TGFβ (5C). A complete list of proteins found to be altered by 4-OH is depicted in Figure 5E. For MCF-7 activated platelets, a similar effect was observed (5D). 4-OH also inhibited the release of CXCL1, CCL5, EGF, CXCL5, and PDGF from MCF-7 activated platelets (5C). Interestingly, 4-OH increased the release of Angiopoietin-1, a protein known to inhibit tumor angiogenesis, in response to MCF-7 cells (5C). Results are summarized in Figure 5E. Overall, these array date indicate that 4-OH can alter the release of factors from platelets in ways that could limit the pro-angiogenic and metastatic role of platelets in breast cancer.
Figure 5. Tamoxifen metabolite 4-hydroxytamoxifen inhibits platelet activation and alters release of stored factors.
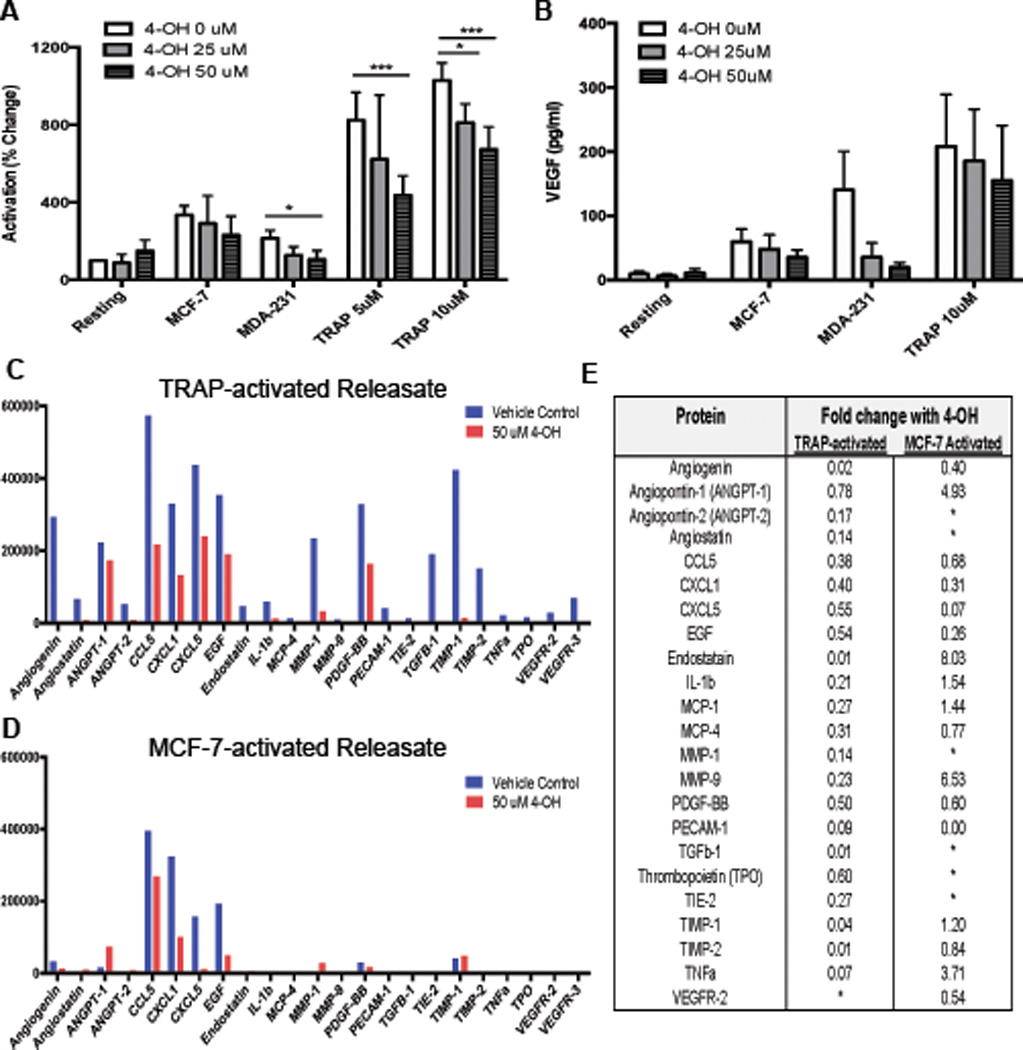
To measure the effect of 4-Hydroxytamoxifen (4-OH) on platelet activation, platelets were isolated from healthy donors, pretreated with 25–50 µM of 4-OH or vehicle control, washed, exposed to agonists, (MCF-7 or MDA-MB-231 tumor cells or TRAP) and P-selectin was measured by flow cytometry (A). VEGF release was measured by ELISA (B). A membrane-based array was performed to further characterize changes in protein release caused by 4-OH (C–E). Platelets from normal, healthy donors were isolated, treated with 50 uM 4-OH or vehicle control, washed, then activated with 5 µM TRAP (C) or MCF-7 tumor cells (D) to generate releasates. Releasate were assayed for 43 proteins by membrane-based array. Proteins showing differences of more than 1.5-fold between vehicle control and 4-OH are shown (C-D). Summary of array results lists all proteins with a 1.5-fold or greater change between drug and vehicle control (E). Bars indicate SEM. P<*0.05, ***0.001 by ANOVA, n=3 independent replicates per treatment group for A–B. Releasates from 3 independent replicates were pooled for use in array.
Tamoxifen metabolite 4-OH decreases platelet angiogenic and metastatic potential
The tamoxifen metabolite 4-OH caused potent inhibition of activation and protein release above and beyond what we observed with tamoxifen. Therefore, we next examined the impact of 4-OH on the net angiogenic and metastatic effects of platelets. Platelets were pretreated with 4-OH, washed, and activated with tumor cells or TRAP to generate releasates. Both TRAP and tumor cell-activated platelet releasates significantly increased capillary tube formation over releasate from resting platelets (6A-B). Similar to what we observed with tamoxifen, 4-OH pretreatment abolished the increase in capillary tube formation caused by activated platelet releasate (6A-B). Metastatic potential was also diminished by 4-OH; activated platelet releasaes promoted the invasion of MCF-7 tumor cells through matrigel while 4-OH pretreatment abrogated this effect, returning invasion to baseline (6C-D).
Overall, our work suggests that tamoxifen and its 4-OH metabolite directly alter platelet function, leading to dampened activation responses and an alteration in angiogenic and metastatic protein release. The net effect leads to platelets that have significantly reduced angiogenic and metastatic effects in response to breast tumor cells (6E, model). Translational studies using platelets isolated from patients undergoing adjuvant tamoxifen therapy confirm that therapeutic, systemic tamoxifen use leads to platelet inhibition and lower net angiogenic potential.
DISCUSSION
Previous studies have shown that tamoxifen can directly alter platelet function and that tamoxifen use may impact the role of platelets in thrombosis and cardiovascular disease.19,21–23 Although platelets have a well-established role in cancer progression and metastasis o date, this study is the first to examine the effect of tamoxifen on platelet function in the context of breast cancer, the disease for which tamoxifen is most widely utilized. Overall, our studies reveal that tamoxifen directly inhibits tumor cell induced platelet activation and substantially dampens the pro-angiogenic and pro-metastatic effects of platelets
The existing body of literature on the effect of tamoxifen on platelets is somewhat contradictory, with a few studies showing platelet activation or aggregation in response to tamoxifen.19 However, the majority of recent studies report inhibition of platelet activation;21,22 the discrepancy between studies could be due to doses, agonists used, or aspect of platelet function analyzed. Serum levels of tamoxifen and its metabolites have been reported to be highly variable, with up to a ten-fold difference detected among patients on comparable doses.29,30 Interestingly, Kisanga et al. reported that breast tumor tissue contains significantly higher concentrations of tamoxifen compared to serum.31 It is well established that platelets interact with tumor cells in both the blood and tumor tissue and they may therefore encounter different doses of tamoxifen based on location. While these findings make it challenging to directly compare in vitro and in vivo studies, we have demonstrated decreased activation and angiogenic potential in platelets treated with tamoxifen ex vivo and more importantly in platelets isolated from patients on tamoxifen therapy.
The exact mechanism by which tamoxifen and its metabolites impact function has not been fully elucidated but some key studies provide insight. Platelets express estrogen receptors alpha and beta but it remains unclear if tamoxifen exerts its effects on platelets through these receptors.23 Of note, use of estrogen receptor blockers such as ICI 182.780 do not reverse the effects of tamoxifen, suggesting that the effects of tamoxifen on platelet inhibition are not mediated directly through the estrogen receptor.21,22 Mechanistically, Chang et al. have demonstrated that tamoxifen inhibits the PKC pathway via PLCγ2 as well as the p38 MAPK pathway in platelets.21 Interestingly, this group and others have shown that tamoxifen also causes a rise in platelet intracellular calcium, which would suggest a pro-activation effect; however abrogation of downstream pathways such as PKC or cAMP production could explain inhibition in the presence of elevated calcium.21,23 Our results are in line with the majority of studies, confirming that tamoxifen and 4-OH inhibit platelet activation. Furthermore, we went on to show that tamoxifen and 4-OH also specifically inhibit platelet activation in response to breast tumor cells.
In addition to inhibiting activation, tamoxifen also altered the release of angiogenic mediators from platelets. We found that tamoxifen inhibited the release of VEGF. This finding is supported by the work of Holmes et al. who reported altered levels of VEGF in patients on tamoxifen therapy.5 Furthermore, our array results show that 4-OH inhibits release of other pro-angiogenic factors including angiogenin and PDGF. Our array results also detected elevated Angiopoietin-1 in releasates from tamoxifen and 4-OH treated platelets. Angiopoietin-1 is primarily involved in vessel stabilization and has a well-known anti-angiogenic role in cancer.32,33 Overall, these changes could lead to a platelet releasate with enhanced anti-angiogenic properties. Indeed, releasates from tamoxifen-treated platelets had a dramatically reduced net angiogenic potential in functional assays. Because angiogenesis is critical for breast cancer progression and platelets are a main source of angiogenic regulators including VEGF, these results strongly suggest that tamoxifen may improve breast cancer outcomes by limiting the pro-angiogenic effects of platelets.
The importance of platelets in cancer progression and metastasis is now widely appreciated.2 In this study, we found that tamoxifen pretreatment potently inhibited the ability of platelets to promote metastasis in vitro. Platelets support metastasis through direct, paracrine effects on tumor cells that have been shown to enhance tumor cell migration, invasion, and epithelial to mesenchymal transition.2 Platelets also support metastasis by exerting effects on other cells in the tumor microenvironment such as endothelial cells and cells of the immune system.6,34–36 Our array results identified decreased levels of CXCL1, CCL5, EGF, and CXCL5 in tumor cell-activated releasate from 4-OH treated platelets. These proteins are all known to play a role in cancer progression and metastasis.37–41 Future studies are needed to examine these factors individually in the context of tamoxifen and look at how tamoxifen reprograms platelets in ways that limit cancer progression.
Recently, interest in anti-platelet agents as cancer therapeutics has grown. GPIIbIIIa blockers and the P2Y12 antagonist Clopidogrel show anti-tumor and anti-metastasis properties in vitro and in murine models of cancer 42,43. Anti-coagulants including low molecular weight heparins and Fondaparinux inhibit tumor cell induced platelet activation and attenuate the angiogenic potential of platelets in vitro.14 Aspirin is perhaps the most intriguing anti-platelet agent that has been studied to date, and several epidemiological studies have suggested that individuals who take aspirin daily are less likely to be diagnosed with cancer and show improved survival if they do develop cancer.44 The randomized phase III Aspirin for Breast Cancer (ABC) Trial will prospectively study whether adjuvant aspirin can reduce the risk of breast cancer recurrence. Tamoxifen, a SERM, is predominantly indicated for the treatment of hormone receptor positive cancers; however, our findings build upon a growing body of evidence demonstrating that tamoxifen also has direct anti-platelet activity. This report highlights the need to examine the efficacy of tamoxifen in estrogen-receptor negative cancers as an anti-platelet agent and supports the exploration of anti-platelet agents as cancer fighting therapeutics.
Supplementary Material
Figure 3. Tamoxifen decreases the angiogenic potential of platelets.
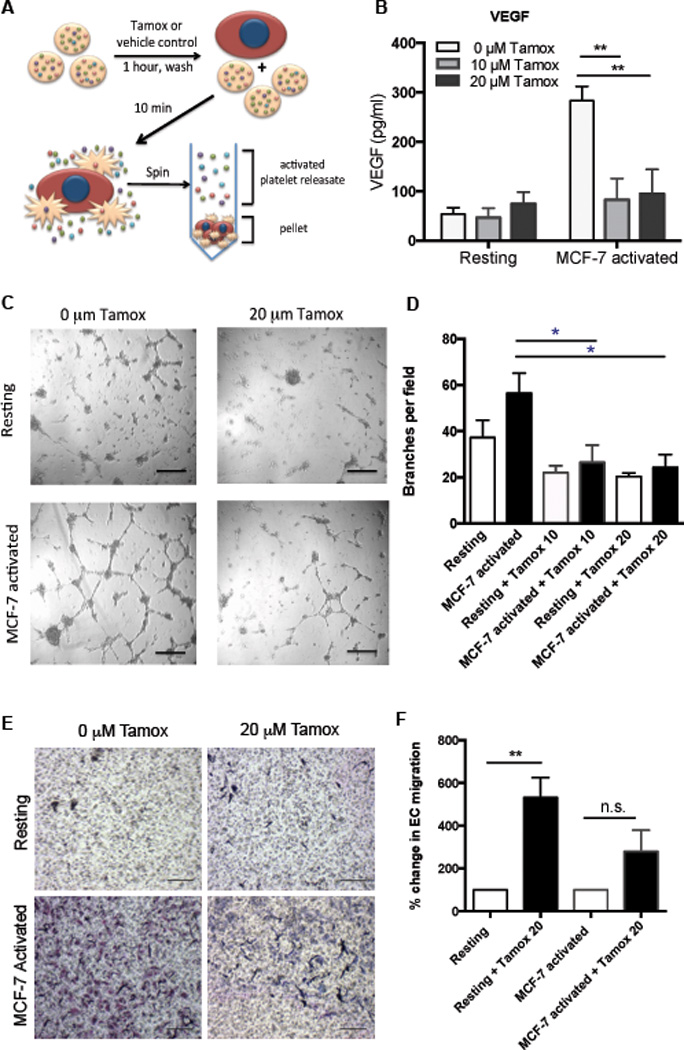
Platelets were pretreated with 0, 10, or 20 µM tamoxifen, washed, and activated with MCF-7 tumor cells or left unactivated (resting) to generate releasates (A). VEGF was quantified in releasates by ELISA (B). Capillary tube formation in HUVECs was assessed following 6 hours of exposure to platelet releasates and quantified as the average number of branch points per field of view (D), with representative images shown (C). Endothelial migration in the presence of resting or MCF-7-activated releasates generated from tamoxifen (20 µM) or control treated platelets was quantified (D-E). Representative images are shown (E) and data from all replicates are quantified as the average number of migrated HUVECs per field (D). Bars indicate SEM. P<*0.05, **0.01 by ANOVA, n=3 independent replicates per treatment group. Scale bars represent 100 µm.
Figure 4. Tamoxifen decreases the metastatic potential of platelets.
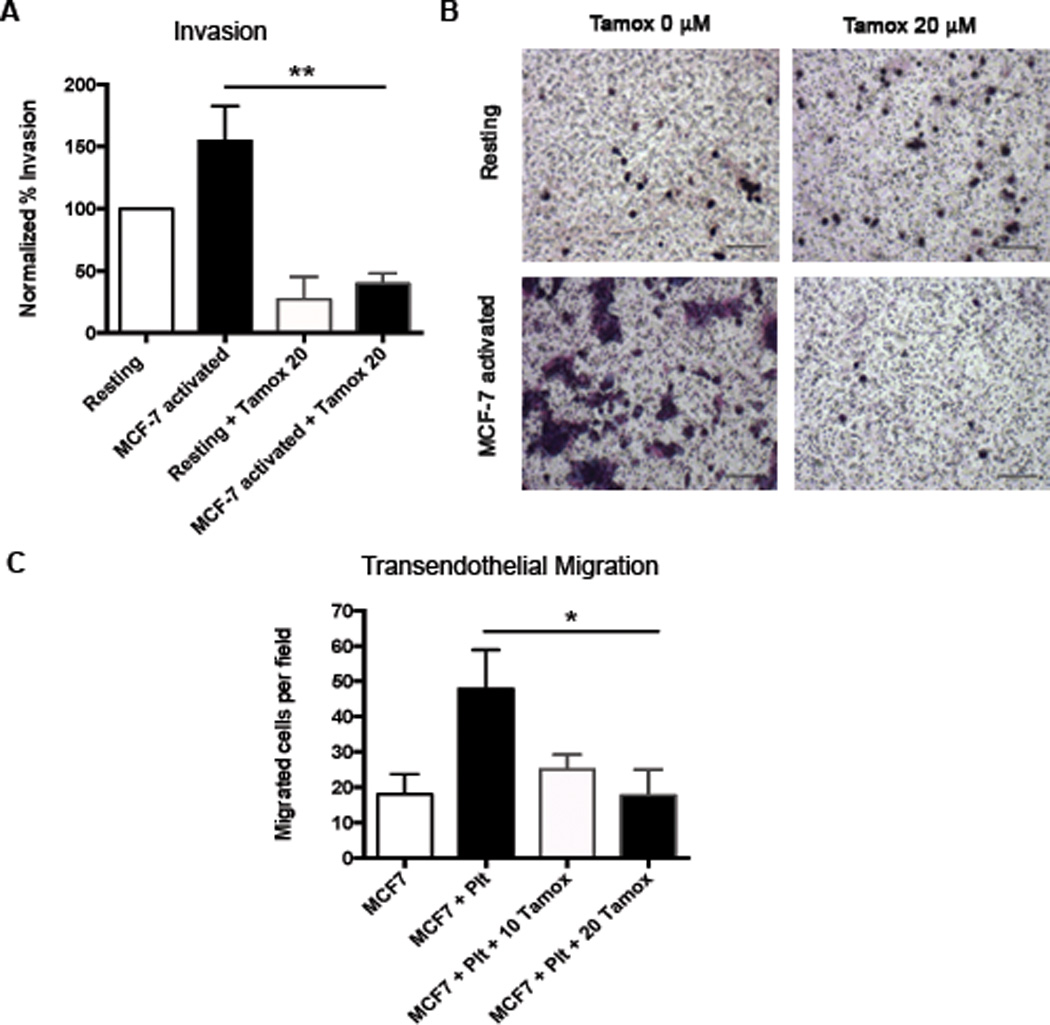
To determine the metastatic potential of tamoxifen-treated platelets, releasates were generated as described in Figure 3A and used in standard transwell invasion assays. The ability of MCF-7 tumor cells to invade through Matrigel in response to platelet releasates was quantified (A) and representative images are shown (B). Transendothelial migration assays were performed in which MCF-7 tumor cells cross an endothelialized transwell membrane in the presence or absence of live platelets pretreated with 0 or 20 µM tamoxifen (C). Bars indicate SEM. P<*0.05, **0.01 by ANOVA, n=3–5 independent replicates per treatment group. Scale bars represent 100 µm.
Figure 6. Tamoxifen metabolite 4-hydroxytamoxifen inhibits platelet angiogenic and metastatic potential.
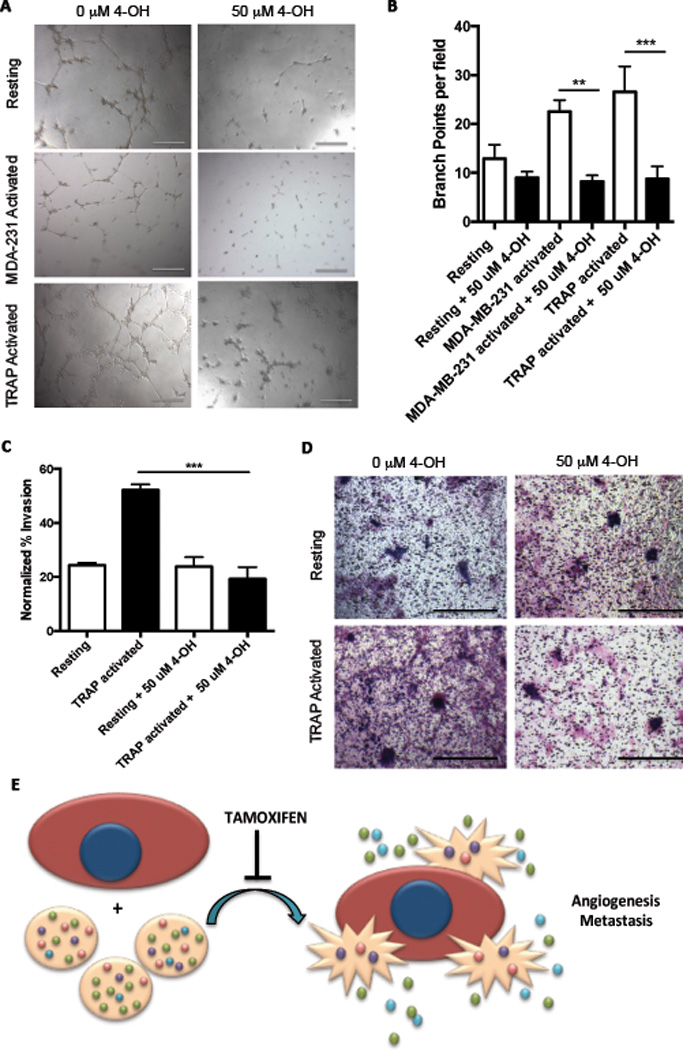
Releasates from platelets treated with 50 µM 4-OH or vehicle control were assayed for angiogenic potential using capillary tube formation assays. Capillary tube formation was quantified as the average number of branch points per field of view (B), with representative images shown (A). To determine the metastatic potential of 4-OH-treated platelets, releasates were generated as previously described and used in standard transwell invasion assays. The ability of MCF-7 tumor cells to invade through Matrigel in response to platelet releasates was quantified (D) and representative images are shown (D). Model: tamoxifen and its metabolites directly inhibit platelet activation in response to breast tumor cells, leading to a decreased release of tumor-supporting pro-angiogenic and pro-metastatic factors from platelets (E). Bars indicate SEM. P<**0.01, ***0.001 by ANOVA, n=3 independent replicates per treatment group.
HIGHLIGHTS.
Breast cancer patients un dergoing adjuvant tamoxifen therapy exhibit platelet inhibition and lower net angiogenic potential.
Tamoxifen and its metabolite directly inhibit platelet activation and alter the release of angiogenic and pro-metastatic factors.
Tamoxifen and its metabolite directly diminish platelet net angiogenic and metastatic potential.
Acknowledgments
The authors would like to thank Nivisha Naik for her assistance with patient recruitment.
Sources of Funding
This work was supported by National Institutes of Health, National Heart, Lung, and Blood Institute grants T32HL116324 (K.E.J.), and R01HL68130 (J.E.I.) and Susan G. Komen for the Cure (E.M.B.)
Nonstandard Abbreviations and Acronyms
- ADP
Adenosine diphosphate
- VEGF
Vascular Endothelial Growth Factor
- HUVEC
Human Umbilical Vein Endothelial Cells
Footnotes
Disclosures: J.E.I. has financial interest in and is a founder of Platelet BioGenesis, a company that aims to produce donor-independent human platelets from human-induced pluripotent stem cells at scale. The interests of J.E.I. were reviewed and are managed by the Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict-of-interest policies. The remaining authors declare no competing financial interests.
REFERENCES
- 1.Sharma D, Brummel-Ziedins KE, Bouchard BA, Holmes CE. Platelets in tumor progression: a host factor that offers multiple potential targets in the treatment of cancer. Journal of cellular physiology. 2014;229:1005–1015. doi: 10.1002/jcp.24539. [DOI] [PubMed] [Google Scholar]
- 2.Bambace NM, Holmes CE. The platelet contribution to cancer progression. Journal of thrombosis and haemostasis : JTH. 2011;9:237–249. doi: 10.1111/j.1538-7836.2010.04131.x. [DOI] [PubMed] [Google Scholar]
- 3.Pinedo HM, Verheul HM, D'Amato RJ, Folkman J. Involvement of platelets in tumour angiogenesis? Lancet. 1998;352:1775–1777. doi: 10.1016/s0140-6736(98)05095-8. [DOI] [PubMed] [Google Scholar]
- 4.Peterson JE, Zurakowski D, Italiano JE, Jr, Michel LV, Fox L, Klement GL, Folkman J. Normal ranges of angiogenesis regulatory proteins in human platelets. American journal of hematology. 2010;85:487–493. doi: 10.1002/ajh.21732. [DOI] [PubMed] [Google Scholar]
- 5.Holmes CE, Huang JC, Pace TR, Howard AB, Muss HB. Tamoxifen and aromatase inhibitors differentially affect vascular endothelial growth factor and endostatin levels in women with breast cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14:3070–3076. doi: 10.1158/1078-0432.CCR-07-4640. [DOI] [PubMed] [Google Scholar]
- 6.Battinelli EM, Markens BA, Italiano JE., Jr Release of angiogenesis regulatory proteins from platelet alpha granules: modulation of physiologic and pathologic angiogenesis. Blood. 2011;118:1359–1369. doi: 10.1182/blood-2011-02-334524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Italiano JE, Jr, Richardson JL, Patel-Hett S, Battinelli E, Zaslavsky A, Short S, Ryeom S, Folkman J, Klement GL. Angiogenesis is regulated by a novel mechanism: pro- and antiangiogenic proteins are organized into separate platelet alpha granules and differentially released. Blood. 2008;111:1227–1233. doi: 10.1182/blood-2007-09-113837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma L, Perini R, McKnight W, Dicay M, Klein A, Hollenberg MD, Wallace JL. Proteinase-activated receptors 1 and 4 counter-regulate endostatin and VEGF release from human platelets. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:216–220. doi: 10.1073/pnas.0406682102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engels K, Fox SB, Whitehouse RM, Gatter KC, Harris AL. Distinct angiogenic patterns are associated with high-grade in situ ductal carcinomas of the breast. J Pathol. 1997;181:207–212. doi: 10.1002/(SICI)1096-9896(199702)181:2<207::AID-PATH758>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 10.Foekens JA, Peters HA, Grebenchtchikov N, Look MP, Meijer-van Gelder ME, Geurts-Moespot A, van der Kwast TH, Sweep CG, Klijn JG. High tumor levels of vascular endothelial growth factor predict poor response to systemic therapy in advanced breast cancer. Cancer research. 2001;61:5407–5414. [PubMed] [Google Scholar]
- 11.Gasparini G, Toi M, Gion M, Verderio P, Dittadi R, Hanatani M, Matsubara I, Vinante O, Bonoldi E, Boracchi P, Gatti C, Suzuki H, Tominaga T. Prognostic significance of vascular endothelial growth factor protein in node-negative breast carcinoma. J Natl Cancer Inst. 1997;89:139–147. doi: 10.1093/jnci/89.2.139. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto Y, Toi M, Kondo S, Matsumoto T, Suzuki H, Kitamura M, Tsuruta K, Taniguchi T, Okamoto A, Mori T, Yoshida M, Ikeda T, Tominaga T. Concentrations of vascular endothelial growth factor in the sera of normal controls and cancer patients. Clinical cancer research : an official journal of the American Association for Cancer Research. 1996;2:821–826. [PubMed] [Google Scholar]
- 13.Weidner N, Folkman J, Pozza F, Bevilacqua P, Allred EN, Moore DH, Meli S, Gasparini G. Tumor angiogenesis: a new significant and independent prognostic indicator in early-stage breast carcinoma. J Natl Cancer Inst. 1992;84:1875–1887. doi: 10.1093/jnci/84.24.1875. [DOI] [PubMed] [Google Scholar]
- 14.Battinelli EM, Markens BA, Kulenthirarajan RA, Machlus KR, Flaumenhaft R, Italiano JE., Jr Anticoagulation inhibits tumor cell-mediated release of platelet angiogenic proteins and diminishes platelet angiogenic response. Blood. 2014;123:101–112. doi: 10.1182/blood-2013-02-485011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fisher B, Costantino JP, Wickerham DL, Cecchini RS, Cronin WM, Robidoux A, Bevers TB, Kavanah MT, Atkins JN, Margolese RG, Runowicz CD, James JM, Ford LG, Wolmark N. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97:1652–1662. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- 16.Yang LH, Tseng HS, Lin C, Chen LS, Chen ST, Kuo SJ, Chen DR. Survival benefit of tamoxifen in estrogen receptor-negative and progesterone receptor-positive low grade breast cancer patients. J Breast Cancer. 2012;15:288–295. doi: 10.4048/jbc.2012.15.3.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jayachandran M, Miller VM. Human platelets contain estrogen receptor alpha, caveolin-1 and estrogen receptor associated proteins. Platelets. 2003;14:75–81. doi: 10.1080/0953710031000080562. [DOI] [PubMed] [Google Scholar]
- 18.Nealen ML, Vijayan KV, Bolton E, Bray PF. Human Platelets Contain a Glycosylated Estrogen Receptor. Circulation Research. 2001;88:438–442. doi: 10.1161/01.res.88.4.438. [DOI] [PubMed] [Google Scholar]
- 19.Vitseva O, Flockhart DA, Jin Y, Varghese S, Freedman JE. The effects of tamoxifen and its metabolites on platelet function and release of reactive oxygen intermediates. The Journal of pharmacology and experimental therapeutics. 2005;312:1144–1150. doi: 10.1124/jpet.104.076315. [DOI] [PubMed] [Google Scholar]
- 20.Dobrydneva Y, Weatherman RV, Trebley JP, Morrell MM, Fitzgerald MC, Fichandler CE, Chatterjie N, Blackmore PF. Tamoxifen stimulates calcium entry into human platelets. J Cardiovasc Pharmacol. 2007;50:380–390. doi: 10.1097/FJC.0b013e31811ec748. [DOI] [PubMed] [Google Scholar]
- 21.Chang Y, Lee JJ, Chen WF, Chou DS, Huang SY, Sheu JR. A novel role for tamoxifen in the inhibition of human platelets. Translational research : the journal of laboratory and clinical medicine. 2011;157:81–91. doi: 10.1016/j.trsl.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Nayak MK, Singh SK, Roy A, Prakash V, Kumar A, Dash D. Anti-thrombotic effects of selective estrogen receptor modulator tamoxifen. Thrombosis and haemostasis. 2011;106:624–635. doi: 10.1160/TH11-03-0178. [DOI] [PubMed] [Google Scholar]
- 23.Shah VP, Chegini HA, Vishneski SR, Weatherman RV, Blackmore PF, Dobrydneva Y. Tamoxifen promotes superoxide production in platelets by activation of PI3-kinase and NADPH oxidase pathways. Thrombosis research. 2012;129:36–42. doi: 10.1016/j.thromres.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 24.Scholz HS, Petru E, Gucer F, Haas J, Tamussino K, Winter R. Preoperative thrombocytosis is an independent prognostic factor in stage III and IV endometrial cancer. Anticancer research. 2000;20:3983–3985. [PubMed] [Google Scholar]
- 25.Suppiah R, Shaheen PE, Elson P, Misbah SA, Wood L, Motzer RJ, Negrier S, Andresen SW, Bukowski RM. Thrombocytosis as a prognostic factor for survival in patients with metastatic renal cell carcinoma. Cancer. 2006;107:1793–1800. doi: 10.1002/cncr.22237. [DOI] [PubMed] [Google Scholar]
- 26.Taucher S, Salat A, Gnant M, et al. Impact of pretreatment thrombocytosis on survival in primary breast cancer. Thrombosis and haemostasis. 2003;89:1098–1106. [PubMed] [Google Scholar]
- 27.Brauch H, Murdter TE, Eichelbaum M, Schwab M. Pharmacogenomics of tamoxifen therapy. Clin Chem. 2009;55:1770–1782. doi: 10.1373/clinchem.2008.121756. [DOI] [PubMed] [Google Scholar]
- 28.Johnson MD, Zuo H, Lee KH, Trebley JP, Rea JM, Weatherman RV, Desta Z, Flockhart DA, Skaar TC. Pharmacological characterization of 4-hydroxy-N-desmethyl tamoxifen, a novel active metabolite of tamoxifen. Breast cancer research and treatment. 2004;65:151–158. doi: 10.1023/B:BREA.0000025406.31193.e8. [DOI] [PubMed] [Google Scholar]
- 29.Madlensky L, Natarajan L, Tchu S, Pu M, Mortimer J, Flatt SW, Nikoloff DM, Hillman G, Fontecha MR, Lawrence HJ, Parker BA, Wu AH, Pierce JP. Tamoxifen metabolite concentrations, CYP2D6 genotype, and breast cancer outcomes. Clin Pharmacol Ther. 2011;89:718–725. doi: 10.1038/clpt.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lien EA, Soiland H, Lundgren S, Aas T, Steen VM, Mellgren G, Gjerde J. Serum concentrations of tamoxifen and its metabolites increase with age during steady-state treatment. Breast cancer research and treatment. 2013;141:243–248. doi: 10.1007/s10549-013-2677-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kisanga ER, Gjerde J, Guerrieri-Gonzaga A, Pigatto F, Pesci-Feltri A, Robertson C, Serrano D, Pelosi G, Decensi A, Lien EA. Tamoxifen and metabolite concentrations in serum and breast cancer tissue during three dose regimens in a randomized preoperative trial. Clinical cancer research : an official journal of the American Association for Cancer Research. 2004;10:2336–2343. doi: 10.1158/1078-0432.ccr-03-0538. [DOI] [PubMed] [Google Scholar]
- 32.Hayes AJ, Huang WQ, Yu J, Maisonpierre PC, Liu A, Kern FG, Lippman ME, McLeskey SW, Li LY. Expression and function of angiopoietin-1 in breast cancer. Br J Cancer. 2000;83:1154–1160. doi: 10.1054/bjoc.2000.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahmad SA, Liu W, Jung YD, Fan F, Wilson M, Reinmuth N, Shaheen RM, Bucana CD, Ellis LM. The effects of angiopoietin-1 and-2 on tumor growth and angiogenesis in human colon cancer. Cancer research. 2001;61:1255–1259. [PubMed] [Google Scholar]
- 34.Pipili-Synetos E, Papadimitriou E, Maragoudakis ME. Evidence that platelets promote tube formation by endothelial cells on matrigel. British journal of pharmacology. 1998;125:1252–1257. doi: 10.1038/sj.bjp.0702191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuznetsov HS, Marsh T, Markens BA, Castano Z, Greene-Colozzi A, Hay SA, Brown VE, Richardson AL, Signoretti S, Battinelli EM, McAllister SS. Identification of luminal breast cancers that establish a tumor-supportive macroenvironment defined by proangiogenic platelets and bone marrow-derived cells. Cancer discovery. 2012;2:1150–1165. doi: 10.1158/2159-8290.CD-12-0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palumbo JS, Talmage KE, Massari JV, La Jeunesse CM, Flick MJ, Kombrinck KW, Jirouskova M, Degen JL. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood. 2005;105:178–185. doi: 10.1182/blood-2004-06-2272. [DOI] [PubMed] [Google Scholar]
- 37.Velasco-Velazquez M, Jiao X, De La Fuente M, Pestell TG, Ertel A, Lisanti MP, Pestell RG. CCR5 antagonist blocks metastasis of basal breast cancer cells. Cancer research. 2012;72:3839–3850. doi: 10.1158/0008-5472.CAN-11-3917. [DOI] [PubMed] [Google Scholar]
- 38.Nunes-Xavier CE, Elson A, Pulido R. Epidermal growth factor receptor (EGFR)-mediated positive feedback of protein-tyrosine phosphatase epsilon (PTPepsilon) on ERK1/2 and AKT protein pathways is required for survival of human breast cancer cells. The Journal of biological chemistry. 2012;287:3433–3444. doi: 10.1074/jbc.M111.293928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niwa Y, Akamatsu H, Niwa H, Sumi H, Ozaki Y, Abe A. Correlation of tissue and plasma RANTES levels with disease course in patients with breast or cervical cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2001;7:285–289. [PubMed] [Google Scholar]
- 40.Acharyya S, Oskarsson T, Vanharanta S, Malladi S, Kim J, Morris PG, Manova-Todarova K, Leversha M, Hogg N, Seshan VE, Norton L, Brogi E, Massague J. A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell. 2012;150:165–178. doi: 10.1016/j.cell.2012.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hsu YL, Hou MF, Kuo PL, Huang YF, Tsai EM. Breast tumor-associated osteoblast-derived CXCL5 increases cancer progression by ERK/MSK1/Elk-1/Snail signaling pathway. Oncogene. 2013;32:4436–4447. doi: 10.1038/onc.2012.444. [DOI] [PubMed] [Google Scholar]
- 42.Amirkhosravi A, Mousa SA, Amaya M, Blaydes S, Desai H, Meyer T, Francis JL. Inhibition of tumor cell-induced platelet aggregation and lung metastasis by the oral GpIIb/IIIa antagonist XV454. Thrombosis and haemostasis. 2003;90:549–554. doi: 10.1160/TH03-02-0102. [DOI] [PubMed] [Google Scholar]
- 43.Su X, Floyd DH, Hughes A, et al. The ADP receptor P2RY12 regulates osteoclast function and pathologic bone remodeling. The Journal of clinical investigation. 2012;122:3579–3592. doi: 10.1172/JCI38576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rothwell PM, Fowkes FG, Belch JF, Ogawa H, Warlow CP, Meade TW. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 2011;377:31–41. doi: 10.1016/S0140-6736(10)62110-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


