Abstract
Threat sensitivity (THT) and weak inhibitory control (or disinhibition; DIS) are trait constructs that relate to multiple types of psychopathology and can be assessed psychoneurometrically (i.e., using self-report and physiological indicators combined). However, to establish that psychoneurometric assessments of THT and DIS index biologically-based liabilities, it is first important to clarify the etiologic bases of these variables and their associations with clinical problems. The current work addressed this important issue using data from a sample of identical and fraternal adult twins (N = 454). THT was quantified using a scale measure and three physiological indicators of emotional reactivity to visual aversive stimuli. DIS was operationalized using scores on two scale measures combined with two brain indicators from cognitive processing tasks. THT and DIS operationalized in these ways both showed appreciable heritability (.45, .68), and genetic variance in these traits accounted for most of their phenotypic associations with fear, distress, and substance use disorder symptoms. Our findings suggest that, as indices of basic dispositional liabilities for multiple forms of psychopathology with direct links to neurophysiology, psychoneurometric assessments of THT and DIS represent novel and important targets for biologically-oriented research on psychopathology.
Keywords: psychopathology, mental disorders, internalizing, externalizing, inhibitory control, disinhibition, threat sensitivity, fear
Introduction
The National Institute of Mental Health’s (NIMH) Research Domain Criteria (RDoC) initiative seeks to reorient psychopathology research toward the study of core biobehavioral constructs such as threat or reward sensitivity and cognitive control, in order to advance neurobiological understanding of psychiatric conditions and improve methods for preventing and treating them (Kozak & Cuthbert, 2016). To facilitate this endeavor, new approaches for assessing mental health problems are needed (Lilienfeld, 2014; Patrick & Hajcak, 2016). One approach, termed psychoneurometrics (Nelson, Patrick, & Bernat, 2011; Patrick et al., 2013; Patrick, Durbin, & Moser, 2012; Yancey, Venables, & Patrick, 2016), involves combining indicators from different assessment domains (e.g., neural, behavioral, psychological-scale) to quantify individual characteristics that relate to mental disorders. Two such characteristics are threat sensitivity (THT) and weak inhibitory control (or disinhibition; DIS). Prior work has shown that joint psychological-scale/neurophysiological (psychoneurometric) assessments of these dispositions show robust relations with patient-reported clinical problems of various types and outperform scale measures in predicting neurophysiological criterion measures (Patrick et al., 2013; Yancey et al., 2016). As a next step in evaluating their substantive nature and scientific utility, the current study used data from an adult twin sample to examine the contributions of genetic and environmental influences to variance in psychoneurometric THT and DIS variables and clarify the etiologic bases of their relations with differing forms of psychopathology.
Dispositional fear/fearlessness, corresponding to “acute threat” in the Negative Valence Systems domain of the RDoC framework, and inhibitory control (inhibition/disinhibition), corresponding to “response inhibition” in the Cognitive Systems domain, are biobehavioral dispositions with potential relevance to many common forms of psychopathology. Dispositional fear (or threat sensitivity; THT), reflecting heightened negative emotional reactivity to threatening situations and stimuli, appears most relevant to focal fear disorders such as specific phobia, social phobia, and panic disorder. Weak inhibitory control (or disinhibition; DIS), reflecting impaired capacity for behavioral restraint, appears most relevant to externalizing conditions such as alcohol and drug dependence and antisocial behavior problems. Both dispositions may play a role in distress (Watson, 2005; or “anxious misery” [Krueger, 1999]) conditions such as major depression, dysthymia, and generalized anxiety disorder—which are characterized by pervasive, dysregulated negative affect.
Nelson et al. (2016) reported on relationships of THT and DIS assessed using self-report scales alone with symptoms of multiple DSM-IV clinical disorders in a large community adult sample. Robust associations with internalizing disorder symptoms were evident for both trait variables, with THT more predictive of fear disorder symptoms and DIS more predictive of distress disorder symptoms. For substance use disorders, prediction was evident only for DIS. Additionally, interactive effects of THT and DIS were found for distress disorders, and to a lesser extent, fear disorders—with participants scoring high on both trait variables exhibiting markedly elevated levels of symptomatology relative to those scoring high on one or the other. The implication is that the presence of both traits is associated with the pervasive, dysregulated negative affect that characterizes conditions such as recurrent depression, generalized anxiety disorder, and posttraumatic stress disorder. Of note, work with community and clinical samples has shown that THT and DIS also predict separately and interactively to suicidal behavior (Venables et al., 2015).
Other research has shown that these trait dispositions remain predictive of disorder symptoms when assessed using self-report scale and neurophysiological indicators combined (i.e., psychoneurometrically), at levels comparable to prediction using scale measures alone. Importantly, psychoneurometric assessments of these traits show appreciably higher associations with neurophysiological criterion measures. Specifically, Patrick et al. (2013) reported that DIS quantified as a composite of two trait-relevant scale measures and two variants of the P300 brain response (known to correlate reliably with disinhibitory tendencies; Patrick et al., 2006; Yancey Yancey, Venables, Hicks, & Patrick, 2013) outperformed self-report DIS substantially in predicting cognitive-brain criterion measures, while predicting externalizing disorder symptoms to an equivalent degree. In parallel with this, Yancey, Venables, and Patrick (2016) reported that THT quantified as a composite of scores on a fear/fearlessness scale (Kramer et al., 2012) along three lab physiological measures of reactivity to discrete aversive stimuli (in a picture-viewing task) outperformed self-report THT by over 30% in predicting separate criterion measures of fear-cue reactivity, with no reduction in prediction of fear disorder symptoms. These results illustrate the potential utility of a cross-domain (‘multi-unit’) approach to assessing psychopathology-related constructs, as advocated by RDoC: Individuals who score high on dispositional dimensions quantified partly by lab neurophysiological indicators can be expected to differ more reliably in other neurobiological characteristics of interest (e.g., brain activations measured using neuroimaging; responsiveness to pharmacological interventions) than those scoring high on dimensions indexed by self-report alone.
Given findings indicating that THT and DIS assessed in this manner show robust associations with clinical problems of various types, an important question is whether and to what extent these observed associations reflect common genetic influences, as opposed to common environmental influences. A prominent genetic basis to observed relations between psychoneurometric measures of these traits and clinical outcomes would support the notion that these cross-domain trait measures index constitutionally-based liability factors for psychopathology. A more appreciable environmental basis to overlap between the two, on the other hand, would suggest that traits quantified this way reflect shaping influences of experiential factors on self-perceptions and reactivity patterns in common with experiential factors that contribute to the occurrence of clinical problems.
The current study addressed key questions regarding the etiological bases of observed relations between psychoneurometric measures of THT and DIS (Patrick et al., 2013; Yancey et al., 2016) and common forms of psychopathology (cf. Krueger, 1999) by undertaking biometric analyses of multi-domain data (self-report, clinical-diagnostic, psychophysiological) from a mixed-gender sample of adult twins. In line with the focus of the RDoC initiative on problem dimensions rather than discrete disorders (Kozak & Cuthbert, 2016), and following prior published work utilizing DSM-based symptom dimensions as clinical criterion measures (e.g., Lang, McTeague, & Bradley, 2016), our analyses focused on broad symptom factors (i.e., fear, distress, substance; Nelson et al., 2016) rather than binary diagnoses or symptom counts for individual disorders. Major study hypotheses were that: (1) psychoneurometric trait variables and clinical symptom variables would each show appreciable heritabilities, and (2) the observed (phenotypic) covariation between psychoneurometric and symptom variables would be accounted for largely by common genetic influences. In addition to examining the etiological bases of observed relations for THT and DIS with broad symptom dimensions, we also assessed contributions of genetic and environmental influences to the relationship for the interaction of the two traits (quantified as a product term) with distress and fear disorder symptoms. Though we did not have specific hypotheses for this interaction term, we expected that knowledge regarding the etiological basis of its association with affective symptomology would help to clarify the construct represented by the product of the two traits.
Method
Participants
The base sample for the study consisted of 508 adult twins (133 female monozygotic [MZ], 124 female dizygotic [DZ], 127 male MZ, and 124 male DZ) recruited from the greater Twin Cities metro area. Most participants were tested concurrently with their same gender co-twin on the same day, but by different experimenters in separate laboratory testing rooms. Participants were selected for participation in lab testing based on levels of THT as indexed by scores on a 55-item Trait Fear scale as described below (see also: Yancey, Vaidyanathan, & Patrick, 2015; Yancey et al., 2016), and as being free from visual or hearing impairments as assessed by a screening questionnaire. (Further information regarding the sampling strategy for the study is provided in Nelson et al. [2006]). Twenty-two members of the base sample were excluded from analyses due to missing individual difference data; 32 others were excluded due to missing or artifact-ridden data for two or all three of the main physiological indicators of THT or DIS. These exclusions resulted in an N of 454 for data analyses involving psychoneurometric variables (51.3% female; M age = 29.5 years, SD = 4.84). Data for the 471 participants reported on by Nelson et al. (2016) were utilized in biometric analyses focusing on diagnostic variables per se. All participants provided informed written consent and were compensated $100 for participation. Study procedures were approved by the University of Minnesota’s Institutional Review Board.
Experimental Paradigms and Physiological Recording Procedures
The data for the current analyses were collected as part of a larger physiological assessment protocol that included affective picture-viewing task and visual oddball task procedures. Participants were seated in a padded recliner, and completed a series of questionnaires while an elastic cap fitted with electroencephalographic (EEG) sensors was attached along with peripheral electrodes to record brain and other physiological reactivity. During testing, participants viewed the task stimuli on a 21” computer monitor, situated 1 m away at eye level. Stimuli were presented using a PC computer running E-Prime software (Psychology Software Tools), and physiological data were collected using a second PC computer running Scan 4 software (Neuroscan, Inc.). Experimental task paradigms as described just below were used to derive physiological indices of THT and DIS.
Affective picture viewing paradigm
The picture-viewing task included 90 picture stimuli from the International Affective Picture System (IAPS; Center for the Study of Emotion and Attention, 1999) depicting pleasant, neutral, and aversive scenes (30 of each). Each picture was presented for 6 s, followed by a 12 s intertrial interval, during which a fixation cross was displayed. Pleasant pictures included erotic, nurturant (babies and small animals), and adventure scenes (10 each). Neutral pictures included household objects, buildings, and neutral faces (also 10 each). Aversive scenes included 20 threatening images (aimed guns and attacking animals) and 10 mutilation scenes (injured bodies, limbs, faces). During 81 of the 90 picture stimuli, abrupt noise probes (50 ms, 105 dB, 10 μs rise time) were presented at 3, 4, or 5 s into the 6 s presentation interval to elicit startle blink responses. Within and between orders, picture stimuli and noise probes were counterbalanced such that all picture valence categories (pleasant, neutral, and aversive) were represented equally across orders at each serial position, with the following constraints: no more than two pictures of the same valence occurred consecutively within any stimulus order; pictures of the same content category never appeared consecutively or across orders; and pictures were rotated so as to serve in both probed and unprobed conditions.
Visual oddball task
The visual oddball task for the study consisted of a modified version of the two-stimulus ‘rotated-heads’ paradigm developed by Begleiter, Porjesz, Bihari, and Kissin (1984), with neutral and affective IAPS picture stimuli included as a third (novel) stimulus category. On 70% of trials (i.e., 168 of 240), frequent non-target stimuli consisting of simple ovals were presented. Target stimuli consisting of schematic heads (simple oval shapes accompanied by a stylized nose and ear) were presented on 15% of (i.e., 72) trials. For each target stimulus, participants were instructed to press the left or right button on a button-box, with either the left or right hand respectively, to indicate whether the ear was on the left or right side of the head. On 50% of target trials, the nose was pointed up; on the remaining trials, the nose was pointed down, requiring a ‘mental rotation’ to correctly identify the ear’s position on the head. The remaining 15% of task stimuli consisted of novel non-targets (i.e., pleasant, neutral, and unpleasant pictures from the IAPS set; 24 of each) interspersed randomly through the stimulus sequence and requiring no response on the part of the participant. Before initiating the test procedure, participants practiced to a level of 85% accuracy using a version of the task that included only target and standard (oval) stimuli.
Physiological data acquisition
EEG activity was recorded from 54 scalp sites using Neuroscan ‘Synamps 2’ amplifiers and sintered Ag-AgCl scalp electrodes, positioned within a head-cap in accordance with the 10–20 system (Jasper, 1958). Separate electrodes were placed above and below the left eye to monitor vertical electrooculogram (VEOG) activity, and adjacent to the outer canthi of the left and right eyes to monitor horizontal electrooculogram (HEOG) activity. Facial electromyographic (EMG) activity was measured using sintered Ag-AgCl electrodes filled with electrolyte paste and positioned above and below the left eye—over the corrugator supercilii muscle and the orbicularis oculi muscle, respectively. Heart rate (HR) was recorded from Ag-AgCl electrodes placed on the forearms. All electrode impedances were kept below 10 KOhms. EEG/EMG signals were digitized on-line at 1000 Hz during data collection with an analog band pass filter of .05–200 Hz.
Scale and Physiological measures of Threat-Sensitivity
Psychometric-scale assessment of threat sensitivity
The psychometric index of THT consisted of a Trait Fear scale developed to index the broad fear/fearlessness dimension from a structural model of various questionnaire measures of this individual-difference domain (Kramer et al., 2012; see also Vizueta, Patrick, Jiang, Thomas, & He, 2012; Yancey et al., 2016). This Trait scale consists of 55 items drawn from several questionnaire measures designed to index dispositional tendencies towards fear and fearlessness, including the Fear Survey Schedule-III (Arrindell et al., 1984), the Fearfulness subscale of the EAS Temperament Survey (Buss and Plomin, 1984), the Harm Avoidance subscale of the Temperament and Personality Questionnaire (Cloninger, 1987), subscales comprising Factor 1 of the Psychopathic Personality Inventory (Lilienfeld and Andrews, 1996), and the Thrill/Adventure Seeking subscale of the Sensation Seeking Scale (Zuckerman, 1979). Internal consistency reliability for items comprising this 55-item Trait Fear (TF-55) scale is very high (Cronbach’s a = .96 within the current analysis sample) and the scale as a whole correlated very highly (r > .9) with scores on the general fear/fearlessness factor from the structural model of the fear/fearlessness domain (Kramer et al., 2012; see also: Patrick, Durbin, & Moser, 2012). For the current work, an aggregate score was computed for each participant as the average item-response value (coded from 0–3, in the direction of high fear) across the scale’s 55 individual items; descriptive statistics for this TF-55 variable in the current sample were: M = 1.13, SD = .47, range = .04 to 2.51.
Physiological indices of threat sensitivity
As described in Yancey et al. (2016), physiological indices of threat sensitivity consisted of the following measures of reactivity to aversive stimuli during the affective picture-viewing paradigm: aversive startle potentiation, corrugator “frown” EMG reactivity, and mid-latency HR acceleration. Corrugator EMG response was quantified as the average change in activity over the initial 3 s following picture onset, relative to a 1s pre-picture baseline—and mean response scores across trials were computed for each picture category (pleasant, neutral, aversive). A difference score between corrugator response for aversive as compared to neutral pictures was then computed and used as a facial reactivity index of threat sensitivity. Startle blink reactivity was quantified as peak magnitude of orbicularis EMG occurring 30 – 120 ms after noise-probe presentation. Blink magnitude values were then standardized across picture trials within subject, with the mean across all trials for each participant scaled to equal 50 (cf. Yancey et al., 2015). HR data were processed using an automated Matlab protocol (Mathworks, Inc.) in which cardiac R-spikes were detected and interbeat intervals were used to compute HR in beats per minute during each picture trial. Based on prior work (Bradley et al., 2001), HR-change values were computed for 500-ms bins spanning the 6-s picture viewing interval, with change for each bin expressed relative to a 1-s pre-picture baseline. Consistent with prior work (Bradley et al., 2001), the morphology of the average HR waveform differed markedly across pictures valence categories, and therefore an index of threat sensitivity was derived in this case from the data for aversive pictures specifically. Inspection of the aggregate HR waveform for pictures of this type revealed an initial deceleratory component followed by a subsequent acceleratory component. For purposes of analysis, the acceleratory component was computed as the peak HR change from baseline across a window of 3 – 6 s after picture onset, and an average score across trials was computed as the index of threat sensitivity. A detailed conceptual-empirical rationale for the choice of these particular physiological indicators of THT is provided by Yancey et al. (2016).
Scale and Physiological Measures of Weak Response Inhibition
Psychometric-scale assessment of weak response inhibition
Two scale measures of DIS were used. The first consisted of a subset of 30 items from the Externalizing Spectrum Inventory (ESI; Krueger, et al., 2007) that has been shown to index general proneness to disinhibitory problems (Yancey et al., 2013). An aggregate score on this 30-item Disinhibition scale (DIS-30) was computed for each participant as the average item-response value (coded from 0–3, in the direction of high disinhibition) across all individual items. The second scale measure of DIS was the 12-item Aggression scale of the brief-form Multidimensional Personality Questionnaire (MPQ-BF; Patrick, Curtin, & Tellegen, 2002), known to correlate both with externalizing problems (Krueger et al., 1998) and brain-response indicators of externalizing proneness (Venables et al., 2011). Internal consistency reliabilities (Cronbach’s a) for the DIS-30 and MPQ Aggression scales in the current sample were .88. and .81, respectively.
Physiological indicators of weak response inhibition
As reported in Patrick et al. (2013), novel-P3 response to incidental emotional pictures during the oddball task and probe-P3 to noise probes occurring during neutral scenes in the affective picture viewing paradigm were used as brain-ERP indicators of DIS. For both ERP components, data epochs from –1000 ms to 2000 ms were extracted from the continuous EEG recordings and corrected for eye movements using the Neuroscan EDIT software package (version 4.3; Neuroscan Inc.). The segmented, blink-corrected EEG data were imported to Matlab (Mathworks, Inc.) for subsequent processing, including low pass anti-aliasing filtering, downsampling to 128 Hz, and artifact checking. Trials in which activity exceeded ±75 μV either within pre- or post-stimulus intervals of interest (−1000 to 0 ms, and 0 to 2000 ms, respectively) were excluded from further processing. Visual inspection of each participant’s average waveform data was undertaken to evaluate the effectiveness of the aforementioned criteria. Data for electrodes identified as containing excessive artifact were replaced by estimates based on data for near-neighboring sites. Novel-P3 response was quantified for electrode site PZ as the maximum positive peak occurring between 273 and 550 ms after novel stimulus onset relative to a 150-ms pre-stimulus baseline. Probe-P3 response was quantified as the maximum positive peak occurring between 250 and 350 ms after onset of the noise-probe stimulus relative to a 300-ms pre-probe baseline.
Psychoneurometric Measures of Threat Sensitivity and Weak Response Inhibition
Analyses utilized psychoneurometric scores for THT and DIS (THTPsyNeuro, DISPsyNeuro) as computed in prior work with the current participant sample (Patrick et al., 2013; Yancey et al., 2016) – consisting of composites of scale and physiological indicators for each trait, weighted according to their loadings on the single common factor emerging from a factor analysis of the four indicators of each. Weightings for the four THTPsyNeuro indicators (cf. Yancey et al., 2016) were as follows: TF-55 = .48, corrugator EMG differentiation = .35; HR acceleration = .33; startle potentiation = .26. Weighting for the four DISPsyNeuro indicators were: DIS-30 = .54, Aggression = .56; novel-stimulus P3 = −.39, and noise-probe P3 = −.36 (Patrick et al., 2013). When operationalized this way (i.e., as psychoneurometric composites), THTPsyNeuro and DISPsyNeuro were uncorrelated (r = −.07, p > .14).
Diagnostic Assessment of Clinical Problems Criterion Measures
All participants were assessed for anxiety, mood, and substance use disorders using the Structured Clinical Interview for DSM-IV-TR Axis I Disorders (SCID-I; First et al., 2002). Interviews were conducted by a PhD-level clinical psychologist and advanced clinical psychology graduate students trained in administration and scoring of the SCID-I diagnostic interview. Interviewers had no knowledge of other assessment data collected from interviewees. Symptom ratings were assigned through a consensus process involving meetings of the study interviewers (cf. Iacono, Carlson, Taylor, Elkins, & McGue, 1999), attended by the project PI (Christopher Patrick) and a licensed clinical psychologist who provided expert consultation on ratings and diagnostic decisions. In addition to symptom counts for individual disorders, symptom composites were computed, consisting of average symptom proportion-scores (cf. Yancey et al., 2016) for clinical conditions within three distinct groups identified by structural-equation and latent-class modeling analyses of common mental disorders (Krueger, 1999; Vaidyanathan, Patrick, & Iacono, 2011): Fear Disorders (average symptoms across panic disorder, agoraphobia, social phobia, and specific phobia), Distress Disorders (average symptoms across major depression, dysthymia, generalized anxiety disorder, and posttraumatic stress disorder), and Substance Use Disorders (average of abuse and dependence symptoms, across alcohol specifically and illegal drugs generally). Mean symptom composite scores (ranging from 0 to 1) for these three problem domains were as follows: Fear = .19 (SD = .45), Distress = .27 (SD = .58), and Substance Use = .43 (SD = .50). The percent of individuals meeting full criteria for at least one disorder within each domain was as follows: Fear = 17.2%, Distress = 22%, and Substance Use = 43%.
Data Analyses
Simple correlations were used to quantify phenotypic associations for THTPsyNeuro and DISPsyNeuro with symptoms of DSM-IV clinical problems, both at the level of individual disorders and substance use, distress, and fear disorder composites (cf. Nelson et al., 2016). In addition, hierarchical regression analyses were performed in which standardized THTPsyNeuro and DISPsyNeuro scores were entered as individual predictors of each symptom variable (individual disorder counts, disorder-composite counts) at step 1, with a term consisting of the product of mean-centered THTPsyNeuro and DISPsyNeuro scores entered at step 2 to test for an incremental contribution of the interaction of the two traits to prediction of clinical problems. The interaction effect was probed for regions of significance using the Johnson-Neyman procedure (Johnson & Neyman, 1936; Preacher, Rucker, & Hayes, 2007), a continuous-score method for evaluating moderating effects of one predictor variable on the relationship between a second predictor and a criterion measure.
Next, we used twin-modeling (biometric) analyses to evaluate the contributions of genetic and environmental influences to major variables of interest (i.e., traits, clinical problems) and their observed covariation. To streamline the presentation of findings, these analyses focused on the disorder composite scores rather than on symptoms of individual disorders. Specifically, we used standard biometric models (Neale & Cardon, 1992) to delineate sources of etiological influence contributing to scores on the two psychoneurometric traits (along their interaction) and symptoms of fear, distress, and substance use disorders, and to evaluate the etiologic basis of observed relations between traits and symptoms. These models conceptualize the variance of a phenotype or trait to be attributable to the following potential sources: additive genetic (A), non-additive (i.e., dominant) genetic (D), shared environmental (C), and nonshared environmental (E) influences. Estimating the relative contributions of these genetic and environmental was accomplished by comparing the similarity of monozygotic (MZ) twins (who share all of their genetic material) on a phenotype relative to dizygotic (DZ) twins (who share on average 50% of their segregating genes) on the phenotype. More specifically, genetic influences on a trait are inferred if the correlation between scores for MZ twins is greater than the correlation for DZ twin pairs (rMZ > r DZ). Heritability is then computed as the ratio of genetic variance to total phenotypic variance (genetic plus environmental) variance. Shared environmental influences refer to environmental factors that contribute to similarity among family members on a trait, and are inferred if 2rDZ > rMZ. Nonshared environmental influences refer to environmental factors that contribute to difference among family members, and are inferred is rMZ < 1, that is, if MZ twins are not identical on scores on a given phenotype. This component of variance also includes measurement error in addition to systematic but nonshared sources of environmental influences.
Based on the observed twin correlation patterns, we fit biometric models using the computer program Mx (Neale, Booker, Xie, and Maes, 2002) using full information maximum likelihood estimation, which can accommodate missing data. For univariate models, we first fit an ACE model. Then, to determine whether the A or C paths contributed significantly, we compared the goodness of fit for alternative AE, CE, and E models with that of the ACE models using the -2 times log-likelihood (-2LL) statistic. The difference between -2LL values for nested models approximates the χ2 distribution, which allows for computation of a likelihood ratio test to compare the relative fit of competing models. We also used Akaike’s Information Criterion (AIC) to evaluate model fit. The AIC fit statistic balances overall fit with model parsimony and penalizes fit for unnecessary parameters (χ2 -2df), with lower values indicative of better fit. Lastly, the sample-size adjusted Bayesian Information Criterion (BIC n adj.), for which lower values indicate better fit, was included as an additional index of model fit. For each psychoneurometric variable, their interaction, and each diagnostic symptom composite variable, the most parsimonious model (i.e., model with the fewest parameters) was selected, provided that dropping a path did not did not significantly reduce fit, and parameters were estimated for this model.
Biometric models can be readily extended to the multivariate case using a Cholesky decomposition to distinguish genetic and environmental influences that are unique to a given phenotype and those that are shared with other phenotypes. Estimates of the genetic covariance can then be standardized to quantify the genetic correlation between two phenotypes, which provides an index of the amount of heritable variance that is shared between two phenotypes (i.e., the magnitude of shared genetic covariance). Similar correlations can also be calculated to index the amount of overlapping shared and nonshared environmental influences across traits. Notably, genetic and environmental correlations are independent of the heritability of a trait or magnitude of the phenotypic association. For example, the heritability estimates for two traits could be high and they could be strongly correlated at the phenotypic level, but the genetic correlation could be low (and vice versa). Finally, the Cholesky decomposition can also be used to partition the extent to which the phenotypic association (however large or small) is attributable to genetic and environmental influences. We fit multivariate biometric models for associations of the psychoneurometric THT and DIS scores with substance use, distress, and fear disorder symptom composites.
Results
Psychoneurometric Indices of THT and DIS: Phenotypic Associations with Clinical Problems
Zero-order correlations and regression models for psychoneurometric traits as predictors of individual disorder symptoms and composite symptom scores are presented in Table 1. Consistent with expectation, THTPsyNeuro showed robust associations with symptoms of specific fear disorders (mean r for individual disorder symptom counts = .21) and with the symptom composite for fear disorders as a whole (r = .41, p < .001). THTPsyNeuro was also associated to more modest degree with distress disorders (mean r for individual disorder symptom counts = .16; r for distress symptom composite = .25, p < .001). By contrast, THTPsyNeuro was not significantly associated with substance use symptomatology. DISPsyNeuro showed weak associations with symptoms of certain individual fear disorders, namely panic disorder and social phobia (rs = .10 and .12, ps<.05, and with fear symptomatology as a whole (r for fear disorder composite = .12, p<.05). DISPsyNeuro predicted more strongly to distress psychopathology (mean r for individual symptom counts = .15; r for distress symptom composite = .23, p < .001), at levels comparable to those for THTPsyNeuro. Additionally, DISPsyNeuro showed robust associations as expected with substance-related problems (mean r for individual disorder symptom counts = .37; r for symptoms composite = .45, p <.001).
Table 1.
Bivariate Correlations (r) and Regression Coefficients (β) for Prediction of DSM-IV Axis-I clinical problems from Psychoneurometric Trait Measures.
| Clinical Symptom Variable | zero-order associations
|
regression model results
|
|||||
|---|---|---|---|---|---|---|---|
| THT PsyNeuro r | DIS PsyNeuro r |
Step 1
|
Step 2
|
Model Summary
|
|||
| THT PsyNeuro β | DIS PsyNeuro β | THT PsyNeuro x DIS PsyNeuro β | Change R2 | Model R | |||
| Fear Disorders | |||||||
| Specific phobia | .33** | .02 | .34** | .05 | .08 | .006 | .34** |
| Social phobia | .35** | .12* | .37** | .15* | .07 | .005 | .38** |
| Panic disorder | .15** | .10* | .16** | .11* | .07 | .005 | .20** |
| Agoraphobia | .07 | .07 | .08 | .08 | .12* | .014* | .15* |
| OCD | .13* | .03 | .14* | .05 | .07 | .005 | .16* |
| Fear composite | .41** | .12* | .44** | .15* | .13* | .017** | .46** |
| Distress Disorders | |||||||
| Major depression | .22** | .21** | .24** | .23** | .07 | .004 | .32** |
| Dysthymia | .16** | .18** | .18* | .20** | .11* | .012* | .27** |
| GAD | .17** | .13* | .20** | .16** | .16** | .026** | .28** |
| PTSD | .10* | .08 | .11* | .09 | .03 | .001 | .14* |
| Distress composite | .25** | .23** | .28** | .26** | .13** | .017* | .38** |
| Substance Use Disorders | |||||||
| Alcohol abuse | −.08 | .40** | −.06 | .39** | −.03 | .001 | .40** |
| Alcohol dependence | −.05 | .42** | −.02 | .42** | −.06 | .001 | .42** |
| Drug abuse | .03 | .33** | .05 | .33** | − .02 | .001 | .33** |
| Drug dependence | .01 | .33** | .03 | .33** | −.02 | .001 | .33** |
| Substance use composite | −.06 | .45** | −.04 | .45** | −.03 | .001 | .45** |
Note: THTPsyNeuro = threat sensitivity assessed using neurophysiological and self-report indicators; DISPsyNeuro = weak response inhibition assessed using neurophysiological and self-report indicators; THTPsyNeuro x DISPsyNeuro Product = interaction term, computed as the product of mean-centered scores for THTPsyNeuro and DISPsyNeuro; r = Pearson correlation coefficient (indexing manifest-score associations); β = standardized regression coefficient; R = multiple regression coefficient; Change R2 = incremental variance accounted for by including the THTPsyNeuro x DISPsyNeuro interaction term. OCD = Obsessive Compulsive Disorder; GAD = Generalized Anxiety Disorder; PTSD = Posttraumatic Stress Disorder.
p < .05;
p <.001
Results from regression analyses, also displayed in Table 1, indicated that THTPsyNeuro and DISPsyNeuro each contributed distinctively to prediction of symptoms of most distress disorders and selected fear disorders, and to symptom composites for disorders of both these types. As predicted, a significant THTPsyNeuro by DISPsyNeuro interaction was observed for both fear and distress disorder composites such that individuals who scored simultaneously higher on THTPsyNeuro and DISPsyNeuro reported more fear and distress symptoms.
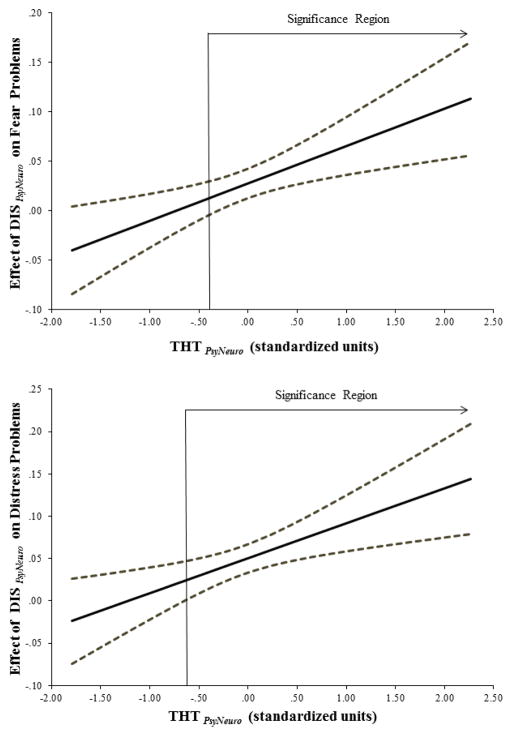
The interaction between psychoneurometric indices of THT and DIS was probed using the Johnson-Neyman technique (Johnson & Neyman, 1936; Preacher et al., 2007). This method identifies values of a moderator variable (designated as THT PsyNeuro in the current analysis) at which the interaction effect is statistically reliable (i.e., <.05) – reflecting the region of significance for the interaction, along with confidence intervals for the effect of a predictor of interest (DIS PsyNeuro, in this case) on the outcome variable (fear and distress problems) across levels of the moderator (THTPsyNeuro). Results from this analysis are depicted in Figure 1, which shows that a synergistic effect of DIS on was evident for participants with THT scores ≥ -.31 SDs below the mean for fear problems and ≥ -.65 SDs below the mean for distress problems (i.e., above which confidence intervals for effect of DIS do not cross zero). Results from this analysis indicate that the effect of DIS PsyNeuro on both fear and distress problems was systematically amplified as a function of increasing THT PsyNeuro scores, but not at particularly low levels of the former trait. This finding is important because it points to a synergistic (i.e., mutually amplifying) impact of these two biobehavioral dispositions on fear and distress problems.
Figure 1.
Depiction of interaction between psychoneurometric measures of threat sensitivity (THTPsyNeuro) and weak response inhibition (DIS PsyNeuro) in predicting fear disorder (upper plot) and distress disorder (lower plot) symptom composites. Values along the y-axis reflect variations (in relative, standard-score units) in the predictive relationship between DIS PsyNeuro and clinical problems as a function of increasing levels of THT PsyNeuro, reflected by values (also in standard-score units) along the x-axis. The solid line reflects point estimates of the association for DIS PsyNeuro with fear (upper plot) and distress problems (lower plot) at differing levels of THT PsyNeuro; the dashed lines reflect upper and lower confidence intervals for these estimates. The point of intersection of the angled arrow labeled “Significance Region” with the x-axis in each plot denotes the level of THT PsyNeuro at which DIS PsyNeuro begins to interact significantly with THT PsyNeuro in predicting problems of each type.
THTPsyNeuro, DISPsyNeuro, and Clinical Problems: The Role of Genetic Contributions
Table 2 displays twin correlation coefficients (computed using Mx) for MZ and DZ twins. In all cases, the coefficient for identicial twins (rMZ) exceeded that for fraternal twins (rDZ), indicating a contribution of genetic influences to twin similarity. Given the observation of rMZ < 1 for all variables, a contribution of nonshared environmental influences was also inferred for each phenotype. For the substance use disorder symptom composite, rMZ < 2rDZ, indicating a possible contribution of nonshared environmental influences. For all six variables tested, comparative model fit indices (see Table S1) indicated that the C path could be dropped without a significant reduction in model fit. As such, the more parsimonious AE model was retained in all cases and the AE parameter estimates are presented in Table 2. Next, to test for the contribution of genetic influences, we dropped the A path from models. In all cases, this resulted in a significant reduction in model fit, indicating a significant influence of genetic factors. Table 2 provides estimates of genetic (A) and nonshared environmental influences for each psychoneurometric and clinical problem phenotype.
Table 2.
Twin correlations and estimates of additive genetic (A), shared environmental (C), and nonshared environmental (E) variance components (and 95% confidence intervals) for trait variables (DISPsyNeuro, THTPsyNeuro) and DSM-IV symptom composites.
| Variable | rMZ | rDZ | A | C | E |
|---|---|---|---|---|---|
| THT PsyNeuro | .45 | .20 | .45 (.30 , .57) | - | .55 (.43 , .70) |
| DIS PsyNeuro | .70 | .35 | .68 (.58 , .76) | - | .32 (.23 , .42) |
| DIS PsyNeuro x THT PsyNeuro | .25 | .02 | .23 (.05, .39) | - | .77 (.61 , .95) |
| Fear Symptoms | .47 | .12 | .45 (.29 , .58) | - | .55 (.42 , .71) |
| Distress Symptoms | .39 | .17 | .38 (.23 , .52) | - | .62 (.49 , .77) |
| Substance Use Symptoms | .56 | .40 | .66 (.55 , .74) | - | .34 (.26 , .45) |
Note. rMZ and rDZ = correlations for MZ and DZ twin pairs, respectively, generated using Mx (Neale et al., 2002).THTPsyNeuro = threat sensitivity assessed using neurophysiological and self-report indicators; DISPsyNeuro = weak response inhibition assessed using neurophysiological and self-report indicators. DSM-IV symptom variables are computed as composites reflecting aggregate symptom counts for Fear, Distress, and Substance Use disorders.
Given the very low rDZ (.02) for the THTPsyNeuro by DISPsyNeuro interaction effect, we also tested for a potential contribution of non-additive genetic (D) influences on this product-term variable. We first fit an ADE model (df = 447, -2LL = 543.724, AIC = −351.342, BIC n adj. = −250.627) along with more parsimonious DE (df = 448, −2LL = 542.658, AIC = −353.342, BIC n adj. = −251.795) and E (df = 449, -2LL = 549.830, AIC = −348.170, BIC n adj. = −249.377) models. Comparison of these models indicated a non-significant difference in model fit between the ADE and DE models (Δχ2 = 1.07, p > .3), suggesting the A path could be dropped without a significant reduction in model fit. Further, comparison of the DE and E models yielded a significant reduction in model fit (Δχ2 = 6.11, p < .05), indicating a significant influence of non-additive genetic influences. Standardized parameter estimates and 95% confidence intervals from the DE model for the THTPsyNeuro by DISPsyNeuro interaction are as follows: D = .27 (.07, .43); E = .73 (.56, .93). However, given a comparable value of BIC n adj. for the counterpart AE model omitting the D path from the ADE model (−251.262, compared to −251.795 for the DE model), it was not possible to establish with confidence whether the genetic influences underlying the interaction term were additive or non-additive in nature.
We then fit a series of bivariate Cholesky models to derive estimates of the genetic and environmental influences on the covariance of the psychoneurometric traits with the disorder symptom composites. Table 3 presents estimated phenotypic, genetic, and nonshared environmental correlations, along with 95% confidence intervals, for the associations of psychoneurometric indices of THT and DIS with substance use, distress, and fear disorder symptom composites. As predicted, THTPsyNeuro exhibited a robust genetic correlation with the fear disorder composite and a more modest genetic correlation with the distress disorder composite. The observed phenotypic associations for THTPsyNeuro with both fear and distress problems were largely attributable to shared genetic influences (90% and 80%, respectively). As predicted for DISPsyNeuro, a robust genetic association with the substance disorder composite and a more modest genetic association with the distress disorder composite were observed. In each case, a large proportion of the phenotypic association was found to be attributable to genetic factors (89% and 100%, respectively). In all bivariate analyses, nonshared environmental correlations emerged as non-significant (i.e., p > .05).
Table 3.
Phenotypic, genetic, and nonshared environmental correlations (and 95% confidence intervals) for each trait variable (DISPsyNeuro, THTPsyNeuro) with DSM-IV symptom composites.
| Variables | r phenotypic | r genetic | r nonshared envir | Percent of Covariance
|
|
|---|---|---|---|---|---|
| A | E | ||||
| THT PsyNeuro - Fear | .40 (.31, .48) | .80 (.58, 1) | .07 (−.09, .24) | 90% | 10% |
| THT PsyNeuro - Distress | .23 (.14, .32) | .46 (.18, .75) | .08 (−.08, .24) | 80% | 20% |
| THT PsyNeuro − Substance Use | −.03 (−.13, .07) | −.13 (−.35, .08) | .07 (−.09, .24) | - | - |
| DIS PsyNeuro - Fear | .11 (.02, .21) | .15 (−.06, .35) | .07 (−.10, .25) | 73% | 27% |
| DIS PsyNeuro − Distress | .20 (.10, .29) | .40 (.19, .62) | −.01 (−.17, .17) | 100% | 0% |
| DIS PsyNeuro - Substance Use | .44 (.37, .52) | .59 (.45, .72) | .14 (−.03, .31) | 89% | 11% |
Note. r values reflect correlations (estimated using MPlus) between total observed variance (r phenotypic) in trait and symptom measures, and between portions of variance in each attributable to genetic influences (r genetic) and to nonshared environmental influences (r nonshared envir). Percent covariance estimates reflect the proportion of phenotypic associations due to additive genetic (A) and nonshared environmental (E) influences. THT PsyNeuro = threat sensitivity assessed using neurophysiological and self-report indicators; DIS PsyNeuro = weak response inhibition assessed using neurophysiological and self-report indicators. DSM-IV symptom variables are computed as composites reflecting aggregate symptom counts for Fear, Distress, and Substance Use disorders.
Discussion
The current work extends findings from prior research (Nelson et al., 2016) by demonstrating robust prediction to common forms of psychopathology for RDoC trait constructs of THT and DIS when operationalized conjointly through neurophysiological and self-report indicators. In the phenotypic correlational analyses, THT PsyNeuro was most strongly associated with symptoms of fear disorders, followed by distress disorder symptoms, and was largely unassociated with substance use problems. On the other hand, DIS PsyNeuro exhibited a pattern of associations reciprocal to that for THT PsyNeuro, showing robust associations with substance use problems, followed by distress disorder symptoms, and weak or negligible associations with fear psychopathology. Further, the observed relationships for psychoneurometric THT and DIS with fear and distress symptom composites became stronger as a function of increasing levels of the other, such that participants scoring high both trait dimensions showed markedly amplified fear and distress problems relative to other participants in the sample. It is notable that the pattern and magnitude of associations between psychoneurometric indices of THT and DIS was comparable to those found using only self-report measures in predicting to clinical problems (Nelson et al., 2016), indicating no loss of clinical predictive power for trait operationalizations that incorporate neurophysiology. Also of note, the finding of a unique contribution of the interaction of psychoneurometric measures of THT and DIS to severity of distress and fear symptomatology extends findings from prior work using self-report-only measures of these traits (Nelson et al., 2016; see Venables et al., 2015, for evidence of a parallel effect in relation to suicidal behavior). The implication is that the interactive effect of these traits on symptomatology may reflect a synergy of two distinct biobehavioral processes—e.g., the propensity to react intensely to threatening stimuli in the environment, and the capacity (or lack thereof) to constrain or regulate emotional and behavioral responses.
The current study is also innovative in that it is the first to decompose etiological sources of variance in psychoneurometric measures of THT and DIS, and to assess for genetic overlap between these traits and distinct subdomains of clinical symptomatology (cf. Krueger, 1999). We found both THT PsyNeuro and DIS PsyNeuro to be appreciably heritable (h2s = .45 and .68, respectively), and an analysis of etiological influences for the THT x DIS product term reflecting the interaction of THT and DIS revealed evidence of modest heritability (h2 =.23). Given the configural nature of this interaction-effect variable and its near-zero concordance for DZ twins, we evaluated the fit of alternative models for this variable specifying additive and non-additive (dominant) genetic influences. Model fit statistics were equivocal as to the more optimal model (i.e., AE vs. DE), leaving open the possibility that genetic contributions to scores on this interaction product-term may be epistatic in nature. In any case, the finding of some genetic component to this interaction term appear consistent with the above-noted possibility that it reflects interplay between distinct biologically-based processes. Findings from the current study and previous work examining the interaction of these traits in relation to clinical problems such as distress-related psychopathology and suicidal behaviors (Nelson et al., 2016; Venables et al., 2015) suggest that the THT x DIS interaction term may reflect emotion dysregulation. However, we offer this hypothesis speculatively and encourage future work aimed at clarifying the psychometric properties of this interaction term and further delineating its nomological network.
The current study was the first to evaluate the etiological overlap between DSM-IV defined symptoms of these common forms of psychopathology and psychoneurometric measures of THT and DIS. Consistent with previous published work (e.g., Kendler et al., 2003), fear, distress, and substance use problems were appreciably heritable (.38 to .66), and evaluation of etiological sources of their relations with the two trait variables revealed evidence for strong genetic overlap. As predicted, we found a very high genetic correlation between THT PsyNeuro and fear disorder symptoms, with 90% of their phenotypic association explained by common genetic influences. Similarly, we found a strong genetic association between DIS PsyNeuro and substance disorder symptoms, with 89% of the phenotypic covariance between the two explained by common genetic influences. Interestingly, both THT and DIS showed moderate genetic associations with pervasive distress symptomatology, and again most of the observed, phenotypic relationship of each trait with problems of this type (90% and 100%, respectively) was explainable by common genetic influences. The observed associations for DIS with distress problems were comparable in magnitude to those reported by Kotov et al. (2010) between self-report scale measures of DIS and disorders such as GAD. As such, the current findings provide further evidence that disinhibitory tendencies may contribute to problems entailing pervasive dysregulated emotion.
Our finding that dispositions quantified using neurophysiological along with psychological-scale indicators showed robust associations with common forms of psychopathology, and these associations were attributable mainly to common genetic influences, is important for both conceptual and practical reasons. Conceptually, the use of neurophysiological indicators along with scale measures to index target dispositions results in a shift in the quantified dimension of variation – away from the domain of self-report and toward the domain of neurobiology (Patrick et al., 2012; Patrick et al., 2013; Yancey et al., 2016). Psychoneurometric assessment of these two RDoC constructs results in constructs that reside in between the two domains, reflecting self-perceived attributes as they intersect with on-line physiological reactivity in trait-relevant contexts (cf. Tellegen, 1991). This is a crucial feature of the psychoneurometric approach to assessment – i.e., it provides a concrete strategy for reframing dispositions in terms that relate more to biological systems, consistent with an RDoC-based approach.
Practically speaking, traits operationalized in this way can be expected to predict effectively to criterion variables in both self-report and physiological domains, and in related domains of measurement (e.g., clinician-ratings, other brain or bodily responses). As evidence for this, we have demonstrated in other work (Nelson et al., 2011; Patrick et al., 2012; Patrick et al., 2013; Yancey et al., 2016) that psychoneurometric assessments of THT and DIS show comparable robust relationships with clinical outcomes as assessed either by self-report measures, or interview-based clinician ratings, and also with criterion measures of physiological response from affective and cognitive processing tasks. In the current work, DSM-IV defined psychopathology symptoms were coded from interview-based assessments, yet were predicted effectively by dispositional variables quantified in part using physiological response measures. While the ability to predict clinical problems using psychoneurometric measures at levels and in patterns comparable to scale-only measures is not advantageous in itself, the crucial added value becomes evident when one seeks to predict outcomes in the domain of physiology: Psychoneurometric assessments of traits greatly outperform scale-only assessments in predicting relevant criterion measures of brain and bodily response (e.g., indices of defensive reactivity to aversive stimuli in the case of THT PsyNeuro [Yancey et al., 2016], and indices of impaired cortical-elaborative processing in the case of DIS PsyNeuro [Patrick et al., 2013]).
The current study results highlight potential advantages to using psychoneurometric assessments in biologically-oriented research on mental disorders over traditional self-report or purely neurophysiological assessments. Besides relating more strongly to electrocortical and visceral-somatic indices of psychopathology-relevant processes, psychoneurometric assessments can be expected to predict more robustly to brain activations occurring during cognitive and emotional tasks in neuroimaging contexts (e.g., Foell et al., in press; Vizueta et al., 2012), and potentially to affiliated neuroanatomical and neurochemical variables. At the same time, use of scale-report measures together with physiological measures as indicators ensures that these trait assessments remain tied to clinical problems that are most commonly assessed in the psychological domain (Patrick et al., 2013; Yancey et al., 2016). As such, psychoneurometric assessments of trait constructs like THT and DIS can serve as bridges between the domains of neurobiological and psychological problems. Consistent with the aims of the NIMH-RDoC research initiative, the psychoneurometric approach seeks to avoid the problem of mind/body dualism by focusing on constructs of the type represented in the RDoC matrix that transcend psychological/biological distinctions, and encouraging a biobehavioral approach to assessment that combines indicators from different domains of measurement.
Some limitations of the current study should be acknowledged. One is the study’s cross-sectional design. It will be important in future work to evaluate the effectiveness of psychoneurometric indices of these trait constructs for predicting mental health outcomes in longitudinal studies. Work of this type will be needed to evaluate whether THT PsyNeuro and DISPsyNeuro represent early-identifiable premorbid processes, or concomitants of emergent psychopathology. While the current results are consistent with the notion that THT PsyNeuro and DISPsyNeuro represent constitutionally-based liability factors, this idea will need to be confirmed using longitudinal designs. Another limitation concerns the neurophysiological variables utilized in the psychoneurometric composites, which were selected based on empirical relations with self-report operationalizations of THT and DIS in prior research. It will be important in future work to incorporate other indicators that are more informative about trait-related variations in neural circuits and processes – including brain-activation scores from relevant neuroimaging paradigms, and performance scores from behavioral tasks known to index distinct neural processes. Through work of this kind, psychoneurometric operationalizations of THT and DIS can be further refined in ways that maximize their value in research directed at identifying neural circuits and biological liabilities for mental health problems. A further limitation of the current work is its exclusive focus on DSM-IV Axis-I clinical problems. It will be important in future work to evaluate phenotypic and etiological relationships of THT and DIS with clinical criterion variables of other types, including personality pathology, suicidal behavior, and other indices of psychological disturbance including emotional distress and psychosocial impairment —operationalized continuously using behavioral and physiological as well as report-based measures.
Notwithstanding these limitations, results from the current study highlight the importance of considering threat sensitivity and inhibitory control capacity as biologically-oriented processes implicated in common forms of psychopathology. Findings from the current study, demonstrating phenotypic associations between combined scale/neurophysiology measures of traits in predicting broad domains of psychopathology, extend results from prior research using scale-only measures (Nelson et al., 2016)—and establish a prominent genetic basis to these trait/psychopathology associations. As such, the current work highlights the importance of assessing core dispositional processes through multiple domains (‘units’) of measurement, as advocated by the RDoC initiative. Combining psychological scales with neurophysiological variables to index key dispositions provides a concrete means for incorporating neuroscience findings/methods into conceptions and assessments of mental health problems. Dispositions assessed in this manner can serve as valuable referents for linking clinical outcomes to neural systems, for clarifying how heritable liabilities contribute to distinct psychological processes associated with specific clinical conditions, and potentially for guiding biologically-oriented approaches to treatment and prevention.
Supplementary Material
Highlights.
Focuses on RDoC traits of threat sensitivity and weak response inhibition
Quantifies these traits as psychoneurometric composite variables
Shows strong genetic basis to relations of these traits with clinical problems
Acknowledgments
This work was supported by grants P50 MH072850 and RC1 MH089727 from the National Institute of Mental Health, and grant W911NF-14-1-0027 from the US Army. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the U.S. Government, Department of Defense, Department of the Army, Department of Veterans Affairs, or U.S. Recruiting Command. Funding sources had no role in the study design in the collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the article for publication.
Footnotes
The authors have no financial disclosures or competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Buss AH, Plomin R. Temperament: Early developing personality traits. Hillsdale: Lawrence Erlbaum Associates; 1984. [Google Scholar]
- Center for the Study of Emotion and Attention. The international affective picture system: Digitized photographs. Gainesville, FL: Center for Research in Psychophysiology, University of Florida; 1999. [Google Scholar]
- Cloninger CR. A systematic method for clinical description and classification of personality variants: A proposal. Archives of General Psychiatry. 1987;44(6):573–588. doi: 10.1001/archpsyc.1987.01800180093014. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzter RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV-TR axis I disorders, non-patient edition. (SCID-I/NP) New York, NY: Biometrics; 2002. [Google Scholar]
- Foell J, Brislin SJ, Strickland CM, Seo D, Sabatinelli D, Patrick CJ. Externalizing proneness and brain response during pre-cuing and viewing of emotional pictures. Social Cognitive and Affective Neuroscience. doi: 10.1093/scan/nsv080. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Taylor J, Elkins IJ, McGue M. Behavioral disinhibition and the development of substance-use disorders: Findings from the Minnesota Twin Family Study. Development and Psychopathology. 1999;11(4):869–900. doi: 10.1017/s0954579499002369. [DOI] [PubMed] [Google Scholar]
- Jasper HH. The ten twenty electrode system of the international federation. Electroencephalography and Clinical Neurophysiology. 1958;10:371–375. [PubMed] [Google Scholar]
- Johnson PO, Neyman J. Tests of certain linear hypotheses and their applications to some educational problems. Statistical Research Memoirs. 1936;1:57–93. [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Archives of general psychiatry. 2003;60(9):929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Kotov R, Gamez W, Schmidt F, Watson D. Linking “big” personality traits to anxiety, depressive, and substance use disorders: A meta-analysis. Psychological Bulletin. 2010;136(5):768. doi: 10.1037/a0020327. [DOI] [PubMed] [Google Scholar]
- Kozak MJ, Cuthbert BN. The NIMH Research Domain Criteria initiative: Background, issues, and pragmatics. Psychophysiology. 2016;53:286–297. doi: 10.1111/psyp.12518. [DOI] [PubMed] [Google Scholar]
- Kramer MD, Patrick CJ, Krueger RF, Gasperi M. Delineating physiologic defensive reactivity in the domain of self-report: phenotypic and etiologic structure of dispositional fear. Psychological Medicine. 2012;42(6):1305–1320. doi: 10.1017/S0033291711002194. [DOI] [PubMed] [Google Scholar]
- Krueger RF. The structure of common mental disorders. Archives of General Psychiatry. 1999;56(10):921–926. doi: 10.1001/archpsyc.56.10.921. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Caspi A, Moffitt TE, Silva PA, McGee R. Personality traits are differently linked to mental disorders: A multitrait-multidiagnosis study of an adolescent birth cohort. Journal of Abnormal Psychology. 1996;105:299–312. doi: 10.1037//0021-843x.105.3.299. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Markon KE, Patrick CJ, Benning SD, Kramer MD. Linking antisocial behavior, substance use, and personality: An integrative quantitative model of the adult externalizing spectrum. Journal of Abnormal Psychology. 2007;116:645–666. doi: 10.1037/0021-843X.116.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, McTeague LM, Bradley MM. RDoC, DSM, and the reflex physiology of fear: A biodimensional analysis of the anxiety disorders spectrum. Psychophysiology. 2016;53(3):336–347. doi: 10.1111/psyp.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilienfeld SO. The Research Domain Criteria (RDoC): An analysis of methodological and conceptual challenges. Behaviour Research and Therapy. 2014;62:129–139. doi: 10.1016/j.brat.2014.07.019. [DOI] [PubMed] [Google Scholar]
- Lilienfeld SO, Andrews B. Development and preliminary validation of a self-report measure of psychopathic personality traits in noncriminal populations. Journal of Personality Assessment. 1996;66(3):488–524. doi: 10.1207/s15327752jpa6603_3. [DOI] [PubMed] [Google Scholar]
- Neale MC, Boker SE, Xie G, Maes HH. Mx: Statistical modeling (Version 6) Dept. of Psychiatry, Virginia Commonwealth University; Richmond, VA: 2002. [Google Scholar]
- Neale MC, Cardon LR. Methodology for genetic studies of twins and families. Dordrecht, The Netherlands: Kluwer; 1992. [Google Scholar]
- Nelson LD, Patrick CJ, Bernat EM. Operationalizing proneness to externalizing psychopathology as a multivariate psychophysiological phenotype. Psychophysiology. 2011;48:64–72. doi: 10.1111/j.1469-8986.2010.01047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson LD, Strickland C, Krueger RF, Arbisi PA, Patrick CJ. Neurobehavioral traits as transdiagnostic predictors of clinical problems. Assessment. 2016;23:75–85. doi: 10.1177/1073191115570110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick CJ, Bernat EM, Malone SM, Iacono WG, Krueger RF, McGue M. P300 amplitude as an indicator of externalizing in adolescent males. Psychophysiology. 2006;43(1):84–92. doi: 10.1111/j.1469-8986.2006.00376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick CJ, Curtin JJ, Tellegen A. Development and validation of a brief form of the Multidimensional Personality Questionnaire. Psychological assessment. 2002;14(2):150. doi: 10.1037//1040-3590.14.2.150. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Durbin CE, Moser JS. Reconceptualizing antisocial deviance in neurobehavioral terms. Development and Psychopathology. 2012;21:912–938. doi: 10.1017/S0954579412000533. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Hajcak G. RDoC: Translating promise into progress. Psychophysiology. 2016;53:415–424. doi: 10.1111/psyp.12612. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Venables NC, Yancey JR, Hicks BM, Nelson LD, Kramer MD. A construct-network approach to bridging diagnostic and physiological domains: Application to assessment of externalizing psychopathology. Journal of Abnormal Psychology. 2013;122:902–916. doi: 10.1037/a0032807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Rucker DD, Hayes AF. Addressing moderated mediation hypotheses: Theory, methods, and prescriptions. Multivariate Behavioral Research. 2007;42:185–227. doi: 10.1080/00273170701341316. [DOI] [PubMed] [Google Scholar]
- Tellegen A. Personality traits: Issues of definition, evidence, and assessment. In: Grove DCWM, editor. Thinking clearly about psychology: Essays in honor of Paul E. Meehl, vol. 2: Personality and psychopathology. Minneapolis: University of Minnesota Press; 1991. [Google Scholar]
- Vaidyanathan U, Patrick CJ, Bernat E. Startle reflex potentiation during aversive picture viewing as an index of trait fear. Psychophysiology. 2009;46:75–85. doi: 10.1111/j.1469-8986.2008.00751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidyanathan U, Patrick CJ, Iacono WG. Patterns of comordibity among common mental disorders: A person-centered approach. Comprehensive Psychiatry. 2011;52:527–535. doi: 10.1016/j.comppsych.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venables NC, Patrick CJ, Hall JR, Bernat EM. Clarifying relations between dispositional aggression and brain potential response: Overlapping and distinct contributions of impulsivity and stress reactivity. Biological Psychology. 2011;86:279–288. doi: 10.1016/j.biopsycho.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venables NC, Sellbom M, Sourander A, Kendler KS, Joiner TE, Drislane LE, … Patrick CJ. Separate and interactive contributions of weak inhibitory control and threat sensitivity to prediction of suicide risk. Psychiatry Research. 2015;226:461–466. doi: 10.1016/j.psychres.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizueta N, Patrick CJ, Jiang Y, Thomas KM, He S. Trait fear and negative affectivity as predictors of neuroimaging response to invisible fear faces. NeuroImage. 2012;59(1):761–771. doi: 10.1016/j.neuroimage.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D. Rethinking the mood and anxiety disorders: a quantitative hierarchical model for DSM-V. Journal of Abnormal Psychology. 2005;114(4):522. doi: 10.1037/0021-843X.114.4.522. [DOI] [PubMed] [Google Scholar]
- Yancey JR, Venables NC, Patrick CJ. Psychoneurometric operationalization of threat sensitivity: Relations with clinical symptom and physiological response criteria. Psychophysiology. 2016;53:393–405. doi: 10.1111/psyp.12512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey JR, Vaidyanathan U, Patrick CJ. Aversive startle potentiation and fear pathology: Mediating role of threat sensitivity and moderating impact of depression. International Journal of Psychophysiology. 2015;98(2):262–269. doi: 10.1016/j.ijpsycho.2014.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey JR, Venables NC, Hicks BM, Patrick CJ. Evidence for a heritable brain basis to deviance-promoting deficits in self-control. Journal of Criminal Justice. 2013;41:309–317. doi: 10.1016/j.jcrimjus.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman M. Sensation seeking: Beyond the optimal level of arousal. Hillsdale, NJ: Lawrence Erlbaum Association; 1979. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.