Abstract
N,N-dimethyl-p-toluidine (DMPT), an accelerant for methyl methacrylate monomers in medical devices, was a liver carcinogen in male and female F344/N rats and B6C3F1 mice in a 2-year oral exposure study. p-Toluidine, a structurally-related chemical, was a liver carcinogen in mice but not in rats in an 18-month feed exposure study. In this current study, liver transcriptomic data was used to characterize mechanisms in DMPT and p-toluidine liver toxicity and for conducting benchmark dose (BMD) analysis. Male F344/N rats were exposed orally to DMPT or p-toluidine (0, 1, 6, 20, 60 or 120 mg/kg/day) for 5-days. The liver was examined for lesions and transcriptomic alterations. Both chemicals caused mild hepatic toxicity at 60 and 120 mg/kg and dose-related transcriptomic alterations in the liver. There were 511 liver transcripts differentially expressed for DMPT and 354 for p-toluidine at 120 mg/kg/day (false discovery rate threshold of 5%). The liver transcriptomic alterations were characteristic of an anti-oxidative damage response (activation of the Nrf2 pathway) and hepatic toxicity. The top cellular processes in gene ontology (GO) categories altered in livers exposed to DMPT or p-toluidine were used for BMD calculations. The lower confidence bound benchmark doses (BMDL) for these chemicals were 2 mg/kg/day for DMPT and 7 mg/kg/day for p-toluidine. These studies show the promise of using 5-day target organ transcriptomic data to identify chemical-induced molecular changes that can serve as markers for preliminary toxicity risk assessment.
Keywords: N,N-Dimethyl-p-toluidine; p-toluidine; liver toxicity; molecular markers
Introduction
In this study we profiled the liver toxicity of N,N-dimethyl-p-toluidine (DMPT), and a related chemical, p-toluidine, after a 5-day exposure. We used the liver transcriptome to provide mechanistic information for understanding DMPT liver toxicity and for benchmark dose (BMD) determinations.
DMPT is a high production volume chemical and is used as an accelerant in methyl methacrylate monomers in medical devices (U. S. Environmental Protection Program 2011). DMPT exposure is of concern because the chemical can leach from medical devices and dental materials (Wang et al. 2013). The State of California has listed DMPT as a chemical known to cause cancer (Office of Environmental Health Hazard Assessment 2014) based on the results of a 2-year cancer study where liver tumors occurred in both rats and mice and nasal tumors in rats (National Toxicology Program 2012). p-Toluidine, a chemical used as intermediate in the manufacture of dyes, pesticides, and pharmaceutical intermediates, was carcinogenic in mice but not in rats after an 18 month diet exposure study (Weisburger et al. 1978).
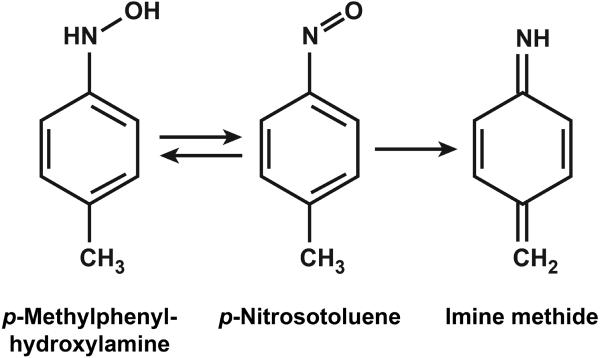
Mechanisms for DMPT and p-toluidine (Fig. 1) liver toxicity may involve oxidative damage and metabolic activation. Both chemicals are known to oxidize hemoglobin to methemoglobin (European Commission 2013; Hazardous Substance Database 2015; National Toxicology Program 2012; Potter et al. 1988). The DMPT-induced methemoglobinemia in animals and humans putatively correlates to the formation and redox cycling of p-methylphenylhydroxylamine (Fig. 2). Phenylhydroxylamine, a similar metabolite of aniline, is a potent inducer of methemoglobinemia in rats (Harrison and Jollow 1987). The formation of p-methylphenylhydroxylamine in DMPT-treated rats is inferred by the presence of N-methyl-p-toluidine and p-(N-acetylhydroxyamino)hippuric acid in the urine (Kim et al. 2007). N-hydroxylated arylamines are also capable of covalently binding to hemoglobin and/or DNA (Marques et al. 1997; Pathak et al. 2016). DNA adduct formation may result in mutations leading to a carcinogenic response. Further, formation of a reactive imine methide via N-hydroxylation (Fig. 2) has been postulated (Dunnick et al. 2014). Imine methides may react with glutathione, other proteins, or nucleic acids (Grillo et al. 2008). The genotoxic potential of DMPT and p-toluidine appears to be low; both compounds were negative in bacteria mutagenicity tests (European Commission 2013; National Toxicology Program 2012).
Figure 1.
DMPT and p-toluidine structures
Figure 2.
Postulated intermediate metabolites of DMPT and p-toluidine
One reported metabolite of p-toluidine in rats is conjugated 2-amino-5-methylphenol excreted in the urine (Cheever et al. 1980). However, N-hydroxylation of p-toluidine has been described in rat liver microsomal preparations and for o-toluidine in rats (Son et al. 1980; Tyrakowska et al. 1993). Therefore, it is speculated that saturation of the detoxication mechanism (ring hydroxylation and conjugation) could result in N-hydroxylation of p-toluidine in vivo in the rat, resulting in formation of active/reactive intermediates such as those postulated for DMPT blood and liver toxicity.
In this study, DMPT and p-toluidine liver transcriptomic changes were grouped by cellular process (gene ontology analysis (GO)) and used to calculate a benchmark dose (using BMDExpress) where the risk for disease could increase by 10% (Thomas et al. 2011; Yang et al. 2007).
Methods
Experimental design
N,N-dimethyl-p-toluidine (Cas. No. 99-97-8) Alfa Aesar, Ward Hill, MA, lot #C15Y012) and p-toluidine (Cas. No. 106-49; Sigma-Aldrich, Allentown, PA, lot STBB8127V) were prepared for oral gavage administration in corn oil (Welch, Holme & Clark Co., Inc. Newark, NJ; Lot# 12-542) to deliver the chemical at doses of 0, 1, 6, 20, 60, or 120 mg/kg/day/chemical for five consecutive days to male F344/N rats in a volume of 2.5-mL/kg body weight. Male F344/N rats (5 animals/dose level/chemical) were obtained from Taconic Laboratory, Germantown, NY. At the start of the study the F344/N rats were 5–6 weeks of age and were housed two per cage. Municipal tap water and NTP-2000 diet (Zeigler Brothers, Inc. Gardners, PA) were made available ad libitum. The care of animals in this study was in accordance with the NIH procedures (available online at http://grants.nih.gov/grants/olaw/olaw.htm#pol).
Clinical chemistry
Blood was collected the retroorbital plexus from animals anesthesized with CO2 and methemoglobin concentration determined by the method of Evelyn and Malloy (1938). Disodium hydrogen phosphate, potassium dihydrogen phosphate, acetic acid aqueous sodium cyanide and aqueous potassium ferricyanide were purchased from Sigma Chemical Company, St. Louis, MO.
Liver Tissue and RNA Collection
Rats were euthanized by CO2 gas. The liver was harvested and weighed, and then a representative section from the midline of the left lateral hepatic lobe was collected and fixed in 10% neutral buffered formalin for histology. Hematoxylin and eosin (H&E)-stained sections (5 μm) were examined by a board certified pathologist. Three samples (approximately 3–4 mm cubes) were dissected from the remaining left lateral hepatic lobe, and flash frozen in liquid nitrogen and stored at −80°C until RNA extraction. RNA was extracted from frozen samples using the Invitrogen PureLink Mini kit (Invitrogen cat# 12183-018A, Carlsbad, CA) according to the manufacturer's protocol. RNA concentration and quality were measured on a Bioanalyzer (Agilent Technologies, Santa Clara, CA). Samples were aliquoted and stored at −80°C until they were analyzed for gene expression studies.
Quantitative Real-time PCR
Quantitative gene expression levels were detected using real-time PCR with the ABI PRISM 7900HT Sequence Detection System (Applied Biosystems) and TaqMan 3′–Minor groove binder-DNA (MGB) probes (FAM [Flourescein amidite] dye labeled). Primers and probes for all genes analyzed were purchased from Applied Biosystems Assays-on-Demand Gene Expression products and the amplification reactions were done according to the manufacturer's protocols. The Taqman assay IDs for the genes validated by RT-PCR include Rn00755544_m1 (Oat), Rn00682418_m1 (Akr7a3), Rn00566528_m1 (Nqo1), Rn00822100_gH (Gpx2) and Rn02770492_gH (Gstp1). The 18S RNA gene was used as the endogenous control for normalization of initial RNA levels. To determine this normalized value, 2–(▵▵Ct) values were compared between treated livers and control livers, where ΔCt (value at which the threshold is crossed) = CtTarget gene − Ct18S RNA, and ΔΔCt = ΔCtTreated − ΔCtControl.
Microarray analysis
Gene expression analysis was conducted using Affymetrix Rat Genome 230 2.0 GeneChip® arrays (Affymetrix, Santa Clara, CA). One hundred ng of total RNA was amplified as directed in the Affymetrix 3' IVT Plus kit protocol. 15 μg of amplified biotin-aRNAs were fragmented and 12.5 μg were hybridized to each array for 16 hours at 45°C in a rotating hybridization oven using the Affymetrix Eukaryotic Target Hybridization Controls and protocol. Array slides were stained with streptavidin/phycoerythrin utilizing a double-antibody staining procedure and then washed for antibody amplification according to the GeneChip Hybridization, Wash and Stain Kit and user manual. Arrays were scanned in an Affymetrix Scanner 3000 and data was obtained using the GeneChip® Command Console and Expression Console Software (AGCC; Version 3.2 and Expression Console; Version 1.2).
Data normalization
Probe intensity data from all Rat Genome 230 version 2 Affymetrix GeneChip® arrays was read into the R software environment (http://www.R-project.org) directly from. CEL files using the R/affy package (Gautier et al. 2004). Probe-level data quality was assessed using image reconstruction, histograms of raw signal intensities and hierarchical clustering of samples. Normalization was carried out using the robust multi-array average (RMA) method using all probe intensity data sets together (Irizarry et al. 2003). Briefly, the RMA method adjusts the background of perfect match (PM) probes, applies a quantile normalization of the corrected PM values, and calculates final expression measures using the Tukey median polish algorithm. RMA scatterplots were used as an additional quality control measure.
Statistical assessment of differential gene expression
Gene expression between control and treated liver tissues was evaluated for each probe set using a bootstrap t-test approach. Pairwise tests were conducted while controlling the false discovery rate (FDR) at the 5% level (Guo et al. 2010). All statistical calculations were performed using the ORIOGEN software package with 10,000 bootstrap samples (Peddada et al. 2005). NextBio analysis was used to identify the most closely related chemicals causing similar gene transcript changes in a large gene expression signature database (Kupershmidt et al. 2010). Over-represented gene sets were determined from the gene list obtained above by testing for association with gene pathway relationships (www.ingenuity.com). Enrichment of pathway members among differentially expressed probe sets were assessed using the one-tailed Fisher exact test for 2 × 2 contingency tables.
Benchmark Dose Analysis
The DMPT and p-toluidine transcriptomic data were used to calculate the benchmark dose (BMD), and a statistically lower confidence bound BMD (BMDL) where little response from chemical exposure would be expected (Crump 1995). BMD and BMDL were calculated for DMPT and p-toluidine using the transcriptomic data and the BMDExpress program (Yang et al. 2007). Briefly, BMDExpress (Thomas et al. 2012; Yang et al. 2007) was used to calculate reference doses for which GO Biological Process Category pathways (http://geneontology.org/page/go-enrichment-analysis) were altered based on liver microarray data from DMPT and p-toluidine exposed animals. All BMD calculations were performed within the BMDExpress framework. First, a classical one-way ANOVA was used to filter the probe list to find transcripts that were altered across dose groups with a false discovery rate threshold of 5% for statistical significance. Next, BMD statistics were calculated for each probe set. The BMD values were averaged across probe sets to obtain a single value for each Entrez ID when more than one probe set mapped to the same Entrez ID and the GO analyses were performed on a gene-specific basis; the program returned a range of summary DMPT or p-toluidine levels (mg/kg/day) representing the central tendencies and variability of BMD (chemical exposure level (mg/kg/day) estimated to result in a 10% extra risk of disease) and BMDL (95% lower bound on BMD) values.
Results
Survival and Body Weights
All animals survived the 5-day exposure period. At the end of the 5-day DMPT exposure, body weight gain for the 0, 1, 6, 20, 60 and 120 mg/kg/day was 131%, 130%, 130%, 130%, 123%*, 119%* respectively, and mean liver weight 6.2 ± 0.29, 6.3 ± 0.40, 6.2 ± 0.23, 6.2 ± 0.32, 6.7 ± 0.30, 7.9* ± 0.37 grams (*p<0.05), respectively. At the end of the 5-day p-toluidine exposure, body weight gain for the 0, 1, 6, 20, 60 and 120 mg/kg/day p-toluidine groups was 127%, 133%, 138%, 133%, 125%*, 118%*, respectively, and mean liver weight 6.4 ± 0.42, 5.6 ± 0.42, 5.8 ± 0.28, 5.9 ± 0.31, 6.0 ± 0.37, 6.8 ± 0.36 grams, respectively. Methemoglobin levels were not increased in treated rats (data not shown).
Liver pathology – DMPT
In the liver of DMPT treated rats, there were increased incidences of individual cell death in the 20, 60 and 120 mg/kg dose groups. Individual cell death was typically observed in the centrilobular to midzonal area, as shrunken, eosinophilic round bodies and cells with brightly eosinophilic cytoplasm and pyknotic, karyorrhectic nuclear debris. Eosinophilic globules were occasionally observed within the cytoplasm of adjacent hepatocytes. Taken together, these changes were suggestive of apoptosis. Grading was based on the number of dead cells observed within each lobule and the number of lobules involved; minimal lesions typically had 3-5 dead cells in about half of the hepatic lobules; mild lesions typically had 5–8 cells with the involvement of most lobules. Two animals in the 60 mg/kg dose group and two animals in the 120 mg/kg dose group had an increase in the number of mitotic figures in the liver. Mitotic figures were common in the control animals due to their young age, and some 20X fields contained 1-3 mitotic figures. In comparison, animals that had minimally increased mitotic figures contained between 3 and 10 mitotic figures per 20X field (Table 1; Fig. 3). All lesions observed were considered to be of minimal to mild severity.
Table 1.
Liver lesions in male F344/NTac Rats after 5 days of DMPT or p-Toluidine exposure
| Dose | 0 | 1 | 6 | 20 | 60 | 120 mg/kg |
|---|---|---|---|---|---|---|
| DMPT | ||||||
| Number examined | 5 | 5 | 5 | 5 | 5 | 5 |
| Individual cell death | 0 | 0 | 0 | 4 [1.0]a | 5 [1.4] | 4 [1.5] |
| Increased mitoses | 0 | 0 | 0 | 0 | 2 [1.0] | 2 [1.0] |
| p-Toluidine | ||||||
| Number examined | 5 | 5 | 5 | 5 | 5 | 5 |
| Individual cell death | 0 | 0 | 0 | 0 | 3 [1.3] | 4 [1.0] |
| Increased mitoses | 0 | 0 | 0 | 0 | 1 [1.0] | 1 [1.0] |
Severity grade
Figure 3.
Control and DMPT liver lesions
A. Centrilobular region of liver from a young, male F344/NTac rat exposed to vehicle control corn oil by gavage for 5 days. There is one mitotic figure (arrow) and no evidence of individual cell death. CV = central vein. H&E.
B. Centrilobular region of liver from a young, male F344/NTac rat exposed to 120 mg/kg DMPT by gavage for 5 days. There are numerous shrunken, eosinophilic bodies, several of which appear to be in clear vacuoles in the cytoplasm of hepatocytes (arrows). A larger hepatocyte with brightly eosinophilic cytoplasm and karyorhectic nuclear debris is also present (arrowhead). CV = central vein. H&E. Same magnification as Fig. 3a.
C. Centrilobular region of liver from a young, male F344/NTac rat exposed to 60 mg/kg DMPT by gavage for 5 days. In the centrilobular region, there are numerous mitotic figures present (arrows). CV = central vein. H&E. Same magnification as Fig. 3a
Liver Pathology - p-Toluidine
In the liver of p-toluidine treated rats, there were increased incidences of individual cell death in the 60 and 120 mg/kg dose groups. Individual cell death was similar to that observed in animals exposed to DMPT. One animal in the 60 mg/kg dose group and one animal in the 120 mg/kg dose group had a minimal increase in the number of mitotic figures in the liver. Mitotic figures were fairly common in the control animals due to their young age, but the two animals that had minimal increased mitotic figures recorded contained more mitotic figures per 20X field (typically 3-5 or more compared to 1-3 in control animals) (Table 1). All lesions observed were considered to be of minimal to mild severity.
Liver transcript analysis
There were treatment-related increases in the number of significant liver transcripts after DMPT or p-toluidine exposure (Table 2 & 3; Supplement 1 and 2; Fig. 4). Because of variability in the data, the gene list sizes presented in Table 2 summarize the number of changes occurring within a dose group, but cannot be used to quantitatively compare gene expression activity across dose groups. Selected transcript changes were confirmed by RT-PCR analysis for select DMPT transcripts (Table 4).
Table 2.
Number of significant liver transcripts significantly altered after 5-day DMPT or p-toluidine exposure
| Dose | 1 | 6 | 20 | 60 | 120 mg/kg |
|---|---|---|---|---|---|
| DMPT transcripts | 6 | 36 | 209 | 136 | 511 |
| DMPT transcripts Corresponding to genes* | 2 | 28 | 176 | 125 | 454 |
| p-Toluidine transcripts | 3 | 18 | 53 | 95 | 354 |
| p-Toluidine transcripts corresponding to genes* | 2 | 11 | 41 | 81 | 305 |
Ingenuity.com (data taken from supplement 1 and 2)
Table 3.
Selected Liver Gene Transcript alterations after 5-day DMPT or p-Toluidine exposure [identified at FDR <0.05 (120 mg/kg)]
| Gene Transcript | Chemical | 1 | 6 | 20 | 60 | 120 mg/kg |
|---|---|---|---|---|---|---|
| Fold Change vs. Control | ||||||
| NRF2 Pathway | ||||||
|
Gstp1 glutathione S-transferase pi 1 1388122_at |
DMPT | 1.3 | 2.9 | 17.9 | 44.7 | 60.1 |
| p-Toluidine | 1.0 | 1.1 | 5.1 | 40.4 | 84.4 | |
|
Gpx2 glutathione peroxidase 2 1374070_at |
DMPT | 1.2 | 1.5 | 1.9 | 2.9 | 6.1 |
| p-Toluidine | 1.4 | 1.4 | 1.7 | 3.7 | 6.8 | |
|
Gsta1 glutathione S-transferase cluster 1368180_s_at |
DMPT | 1.1 | 1.3 | 1.6 | 2.0 | 2.6 |
| p-Toluidine | 1.2 | 1.4 | 1.8 | 2.6 | 3.2 | |
|
Akr7a3 aldo-keto reductase family 7 1368121_at |
DMPT | 1.3 | 2.0 | 1.7 | 5.1 | 12.9 |
| p-Toluidine | 1.4 | 1.9 | 3.1 | 7.8 | 18.7 | |
|
Nqo1 NAD(P)H dehydrogenase, quinone 1 1387599_a_at |
DMPT | 1.0 | 1.1 | 1.3 | 2.6 | 6.6 |
| p-Toluidine | 1.1 | 1.1 | 1.4 | 2.9 | 7.1 | |
|
Aox1 aldehyde oxidase 1 1387376_at |
DMPT | 1.1 | 1.3 | 1.6 | 1.9 | 2.0 |
| p-Toluidine | 1.0 | 1.3 | 1.5 | 2.0 | 2.3 | |
|
Ephx1 epoxide hydrolase1 1387669_a_at |
DMPT | 1.1 | 1.1 | 1.1 | 1.6 | 2.3 |
| p-Toluidine | 1.0 | 1.2 | 1.2 | 1.8 | 2.9 | |
|
Gsr glutathione reductase 1369061_at |
DMPT | −1.2 | 1.1 | 1.3 | 1.5 | 2.0 |
| p-Toluidine | 1.2 | 1.2 | 1.5 | 1.9 | 2.6 | |
| Lipid Metabolism/Xenobiotic Metabolism | ||||||
|
Acaa1 Synthesis of terpenoids 1387783_a_at |
DMPT | −1.0 | 1.3 | 1.5 | 1.8 | 2.1 |
| pToluidine | −1.0 | 1.1 | 1.3 | 1.8 | 1.8 | |
|
Aldh1a1 aldehyde dehydrogenase 1 family, member A1 1387022_at |
DMPT | 1.2 | 1.5 | 1.6 | 1.8 | 2.4 |
| P-Toluidine | 1.2 | 1.4 | 1.7 | 2.3 | 2.6 | |
|
Atf3 activating transcription factor 1369268_at |
DMPT | 1.0 | −1.0 | 2.7 | 5.5 | 2.9 |
| p-Toluidine | −1.1 | −1.1 | −1.1 | 2.4 | 3.8 | |
|
Cyp3a7 Cytochrome enzyme 1370387_at |
DMPT | −1.3 | −1.8 | −2.7 | −2.8 | −2.9 |
| p-Toluidine | −1.4 | −1.3 | −1.6 | −2.3 | −2.2 | |
|
Oat ornithine aminotransferase 1367729_at |
DMPT | −1.0 | −1.8 | −2.2 | −3.1 | −4.3 |
| p-Toluidine | −1.3 | −1.3 | −1.7 | −2.8 | −5.6 | |
|
Inmt indolethylamine N-methyltransferase 1373975_at |
DMPT | 1.4 | 1.3 | −1.4 | −3.0 | −3.0 |
| p-Toluidine | 1.1 | 1.2 | 1.4 | −1.5 | −7.3 | |
|
Sult1c3 sulfotransferase family, cytosolic, 1C, member 3 1369296_at |
DMPT | 1.1 | −1.1 | −1.1 | −1.4 | −2.1 |
| p-Toluidine | −1.1 | −1.1 | −1.2 | −2.2 | −4.0 | |
| Liver Hyperplasia/Liver Cancer | ||||||
|
Adh1c alcohol dehydrogenase 1C 1378260_at |
DMPT | −1.0 | 1.3 | 1.9 | 2.7 | 4.1 |
| p-Toluidine | 1.1 | 1.3 | 1.7 | 2.9 | 3.6 | |
|
Crot carnitine O-octanoyltransferase 1387183_at |
DMPT | −1.3 | −1.3 | −1.7 | −2.0 | −2.9 |
| P-Toluidine | −1.1 | −1.2 | −1.5 | −2.4 | −3.1 | |
|
Epcam epithelial cell adhesion molecule 1388199_at |
DMPT | 1.4 | 1.3 | 2.0 | 2.1 | 2.8 |
| p-Toluidine | 1.3 | 1.5 | 1.7 | 2.4 | 3.6 | |
|
Ptgr1 prostaglandin reductase 1 1388102_at |
DMPT | 1.1 | 1.3 | 1.6 | 2.4 | 4.5 |
| p-Toluidine | 1.0 | 1.1 | 1.5 | 3.1 | 6.0 | |
|
Ca2 carbonic anhydrase II 1367733_at |
DMPT | 1.0 | −1.3 | 1.0 | 1.8 | 5.1 |
| p-Toluidine | 1.1 | −1.1 | −1.3 | 1.3 | 6.5 | |
|
Avpr1a arginine vasopressin receptor 1A 1369664_at |
DMPT | −1.3 | −1.3 | −1.8 | −2.5 | −3.6 |
| p-Toluidine | −1.1 | −1.1 | −1.3 | −2.2 | −3.8 | |
|
Fhit fragile histidine triad 1369318_at |
DMPT | −1.1 | 1.0 | −1.0 | −1.2 | −1.9 |
| p-Toluidine | −1.1 | −1.1 | −1.1 | −1.5 | −2.0 | |
Bolded numbers are significant (FDR<0.05)
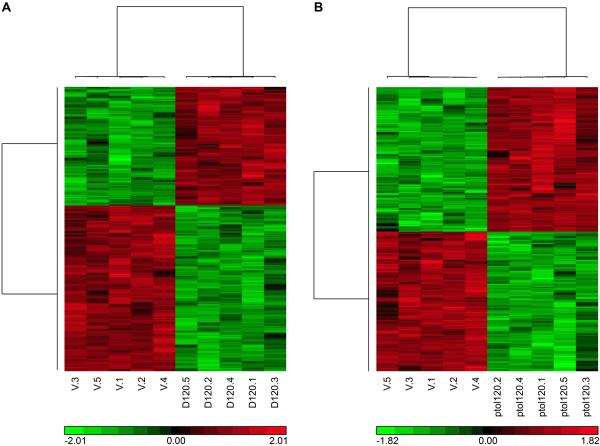
Figure 4.
Heat map showing the unsupervised hierarchical clustering analysis illustrating prominent gene expression patterns at the high dose (120 mg/kg) in (A) significant DMPT liver transcripts and (B) significant p-Toluidine liver transcripts determined at a false discovery rate threshold of 0.05.
Table 4.
Validation of the DMPT liver transcript changes by real time-PCR (fold change compared to controls)
| DMPT genes | 1 mg/kg | 6 mg/kg | 20 mg/kg | 60 mg/kg | 120 mg/kg |
|---|---|---|---|---|---|
| OAT | −1.20 | −2.72 | −2.58 | −2.28 | −2.82 |
| Akr7a3 | 1.07 | 1.54 | 1.71 | 8.09 | 27.79 |
| NQ01 | −1.12 | −1.24 | 1.28 | 3.92 | 10.50 |
| GPX2 | 1.15 | 1.27 | 1.75 | 5.09 | 9.64 |
| Gstp1 | 1.40 | 3.41 | 34.05 | 140.62 | 243.66 |
There were 2, 28, 176, 125, and 454 altered transcripts corresponding to known genes after DMPT exposure, and 2, 11, 41, 81, 305 altered transcripts corresponding to known genes after p-toluidine exposure, for the 1, 6, 20, 60, and 120 mg/kg groups, respectively (Table 2). The small number of differentially expressed transcripts found at 1 mg/kg could reflect random noise. 132 of these liver transcripts were common to both DMPT and p-toluidine at 120 mg/kg. The NrF2 antioxidant pathway was common to both chemicals.
The top canonical pathway for hepatic transcript alteration after DMPT or p-toluidine exposure was the Nrf2 pathway (p<0.001) (Table 3; Supplement 3). While there were no treatment-related liver lesions at 6 mg/kg (with either DMPT or p-toluidine exposure) there were increases in some of these Nrf2 pathway hepatic transcripts (Table 3).
Transcripts characteristic of xenobiotic and lipid metabolism (the second or third most significant pathway after DMPT or p-toluidine exposure (p<0.001)) were upregulated after exposure to both chemicals (Table 3). In addition, hepatic transcripts related to liver injury and cancer (the top disease pathway (p<0.001)) were significantly altered after DMPT and p-toluidine exposures. Transcripts for several solute carriers were increased after DMPT or p-toluidine exposure. The increased mitoses in the liver after DMPT and p-toluidine exposures further supports the significance of these transcriptomic alterations.
Benchmark dose analysis
There were 2882 significant GO categories with more than 5 significant transcripts for DMPT and 2559 for p-toluidine (Supplement 4 and 5). A GO category with more than 5 significant transcripts after both DMPT or p-toluidine exposure was regulation of fatty acid transport (Table 5). Using this GO category, the BMDL (benchmark dose corresponding lower limit of a one-sided 95% confidence interval) was 2 mg/kg/day for DMPT and 7 mg/kg/day for p-toluidine. Other GO categories (e.g. prostanoid or prostaglandin metabolic process) were significant only for DMPT and gave a DMPT BMDL of 0.5 mg/kg.
Table 5.
Selected DMPT and p-Toluidine Benchmark Dose Analysis Based on GO Pathway Analysis of liver transcriptomic changes*
| GO Accession Number | GO name | Chemical | Number of genes in category | Number of significant genes** | Mean BMDL (mg/kg) | BMDL SD |
|---|---|---|---|---|---|---|
| 2000191 | Regulation of fatty acid transport | DMPT | 23 | 5 | 2.0 | 3.3 |
| p-Toluidine | 5 | 7.0 | 11.2 | |||
| 0015986 | ATP synthesis coupled protein transport | DMPT | 16 | 7 | 4.5 | 4.4 |
| p-Toluidine | 6 | 9.7 | 13.4 | |||
| 0052695 | Cellular glucuronidation | DMPT | 8 | 7 | 3.6 | 6.8 |
| p-Toluidine | 7 | 10.8 | 17.8 | |||
| 0000038 | Very long chain fatty acid metabolic process | DMPT | 23 | 11 | 5.7 | 6.5 |
| p-Toluidine | 12 | 13.3 | 15.3 | |||
| 0006750 | Glutathionine biosynthetic process | DMPT | 11 | 5 | 5.2 | 7.8 |
| p-Toluidine | 5 | 55.7 | 42.5 | |||
| 0017144 | Drug Metabolic processes | DMPT | 32 | 16 | 5.8 | 10.1 |
| p-Toluidine | 15 | 24.7 | 21.7 | |||
| 0006749 | Glutathione metabolic processes | DMPT | 41 | 21 | 8.7 | 15.2 |
| p-Toluidine | 24 | 23.9 | 31.0 | |||
| 0006979 | Response to Oxidative stress | DMPT | 314 | 74 | 28,2 | 32.3 |
| p-Toluidine | 70 | 29.9 | 27.2 |
see supplement 4 and 5.
False discovery rate of 0.05%
Data for DMPT taken from supplement 4; data for p-toluidine taken from supplement 5.
Information from supplements 4 and 5: GO Accession number (column A); GO term name (column B); All genes in category (column D); Significant chemical transcripts (column E); BMDL mean (column O); BMDL standard deviations (column R); upregulated genes (column AC).
Discussion
The 5-day DMPT male rat liver transcriptome is similar to that of the structurally-related chemical, p-toluidine, as both show alterations in the Nrf2 antioxidant pathway. These liver transcriptomic patterns provide information for hazard identification and risk assessment using fewer animals and shorter exposure times than traditional toxicity studies, a 21st century goal of the NIH environmental health science programs (Collins et al. 2008).
After DMPT or p-toluidine exposure there was a minimal to mild hepatotoxicity as demonstrated by increased mitosis and individual cell death (60 and 120 mg/kg). Liver hypertrophy, which occurred after longer term DMPT exposure (National Toxicology Program 2012), was not present.
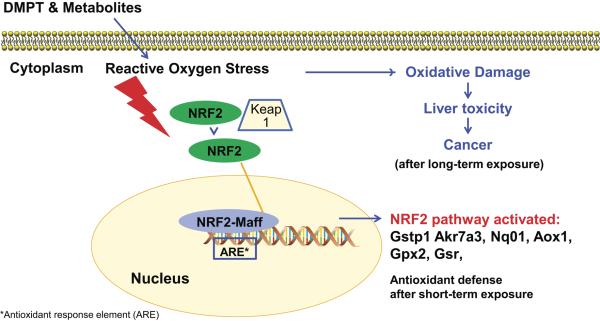
DMPT and p-toluidine exposures caused oxidative stress and subsequently methemoglobinemia in rodents after longer term exposures (European Commission 2013; National Toxicology Program 2012). While methemoglobin formation did not occur in these 5-day DMPT and p-toludine studies, there was evidence for treatment-related redox imbalance in the liver as both DMPT and p-toluidine activated the Nrf2 antioxidant response pathway. Nrf2 is a transcription factor in the cytoplasm and under unstressed conditions is ubiquitinated and degraded by Keap1 and Cullin3. Oxidative stress disrupts the Keap1-Cul3 ubiquitination system and allows cytoplasmic buildup and subsequent nuclear translocation of Nrf2 (Baird et al. 2013). In the nucleus, Nrf2 binds to an antioxidant response element (ARE) and activates gene transcription resulting in upregulation of antioxidant genes (Baird et al. 2013). The Nrf2 pathway is activated in liver disease in humans and in rodents.
The DMPT and p-toluidine activation of the Nrf2 pathway (Fig. 5) occurred both at exposure levels causing liver lesions (60 and 120 mg/kg) and at exposure levels (6 and 20 mg/kg) with no treatment-related liver lesions. For both DMPT and p-toluidine, Nrf2 liver transcripts upregulated included Gpx2, Aox1, Nqo1, Gsr, Akr7a3, and Gstp1. Gpx2 protein, a marker in cancer, can protect against ROS damage (Naiki et al. 2014). The Aox1 protein is involved in oxidizing aldehydes to the corresponding carboxylic acid (Maeda et al. 2012); Nqo1 (NAD(P)H dehydrogenase, quinone 1) plays a role in protection against oxidative stress (Fan et al. 2014); Gsr codes for an oxidative defense enzyme (Djordjevic et al. 2015); and Akr7a3 protects against antioxidant damage and liver injury from chemical-induced hepatotoxicity (Ahmed et al. 2011; Bodreddigari et al. 2008) as does Gstp1 (Casalino et al. 2007). These Nrf2 pathway transcripts are candidate biomarkers for use in toxicity studies.
Figure 5.
Activation of Nrf2 pathway by DMPT and p-toluidine
The upregulation of the Nrf2 antioxidant defense system did not completely prevent DMPT or p-toluidine liver damage (in the 60 and 120 mg/kg groups), and with longer-term chemical exposure, oxidative damage may become more pronounced contributing to the chemical-induced tumors observed in the DMPT 2-year rat and mouse studies (National Toxicology Program 2012).
Nrf2 antioxidant responses occur after exposure to other liver toxins including aflatoxin (Kensler et al. 2014; Merrick et al. 2012; Singh et al. 2015) and carbon tetrachloride (Chen et al. 2014), and are found in other organ toxicities including in the lung (Cho et al. 2015) and nasal cavity (Dunnick et al. 2015).
DMPT and p-toluidine exposures caused increased metabolic transcript expression including Ephx1 whose protein (epoxide hydrolase) detoxifies reactive metabolites (epoxides) by metabolizing them to more soluble and easily excretable metabolites (El-Sherbeni and El-Kadi 2014); Crot (carnityl acetyl transferases) whose protein is involved in lipid metabolism and fatty acid-beta oxidation and facilitates the transport of fatty acids into the cell (van der Leij et al. 2000); and Adhc1 (alcohol dehydrogenase) which can promote oxidation of alcohols to aldehydes (Xue et al. 2012). These aldehydes may have toxic, mutagenic and/or carcinogenic properties (Seitz and Meier 2007).
DMPT and p-toluidine exposure resulted in alterations of several transcripts that play a role in cancer (e.g. ↑Epcam and Ptgr1 transcripts). Epcam is a transmembrane cell adhesion glycoprotein that is over expressed in cancer (Fong et al. 2014), is a biomarker for stem cells (Sulpice et al. 2014), and when increased can lead to more invasive properties. Genetic polymorphisms of Epcam influence susceptibility to cancer (Yu et al. 2014) and blockage of Epcam may provide a possible treatment for cancer (Sulpice et al. 2014). The Ptgr1 (prostaglandin reductase 1) protein metabolizes and inactivates leukotriene B4 (a metabolite of arachidonic acid) (Zhao et al. 2010), and has antioxidant properties (Sanchez-Rodriguez et al. 2014). Ptgr1 has been used as a biomarker for rodent and human liver cancer pathways (Sanchez-Rodriguez et al. 2014). DMPT and p-toluidine decreased Avpr1a (Fernandez-Varo et al. 2016) and Oat expression, and decrease in these transcripts may be a cancer protective response (Zigmond et al. 2015).
There were more transcript changes characteristic of liver toxicity after DMPT exposure than after p-toluidine exposure. For example, DMPT (but not p-toluidine) downregulated Fhit and Inmt; these transcripts are also downregulated after exposure to other liver carcinogens (Auerbach et al. 2010; Boutros et al. 2011; Merrick et al. 2012; Watson et al. 2014).
Using the liver transcriptomic response, the BMDL was calculated as 2 mg/kg/day for DMPT and 7 mg/kg/day for p-toluidine based on the gene ontology category of regulation of fatty acid transport. Disruption of fatty acid metabolism can lead to liver disease (Tanaka et al. 2012). The calculated DMPT BMDL (2 mg/kg) was lower than the lowest dose for carcinogenic activity in rats (20 mg/kg/day) or in mice (6 mg/kg/day) (National Toxicology Program 2012). Using uncertainty factors of 10 for species differences, and 10 for intraspecies differences, a minimal risk level is ~ 0.02 mg/kg/day for DMPT and 0.07 mg/kg/day for p-toluidine.
Conclusion
`The liver transcriptomic changes were suitable for use in quantitating liver toxic dose response, providing information to support a hypothesis for oxidative damage as a mechanism for liver toxicity (Dunnick et al. 2014), and for providing data for benchmark dose analysis. These studies show the promise of using 5-day rodent toxicity and toxicogenomic studies to calculate disease risk using fewer resources than conventional longer-term toxicity studies. Molecular changes associated with disease are usually precursors to histologic alterations and can occur at lower exposure levels and, thus, aid in providing a conservative estimate of the putative disease risk.
Supplementary Material
Acknowledgments
We thank Dr. G. Knudsen, NCI at NIEHS, and Dr. W. Casey, NIEHS, for their review of the manuscript. All persons gave their informed consent prior to their inclusion in the study. This article does not contain clinical studies or patient data.
Funding The intramural program of the National Institute of Environmental Health Sciences (NIEHS), Research Triangle Park, NC, supported this work. However, the statements, opinions or conclusions contained therein do not necessarily represent the statements, opinions or conclusions of NIEHS or NIH. This work was supported by contract ES-75561 with Alion Science & Technology, Inc. and contract ES 55547.
Footnotes
Conflict of interest None of the authors have any conflict of interest to declare.
REFERENCES
- Ahmed MM, Wang T, Luo Y, et al. Aldo-keto reductase-7A protects liver cells and tissues from acetaminophen-induced oxidative stress and hepatotoxicity. Hepatology. 2011;54(4):1322–1332. doi: 10.1002/hep.24493. [DOI] [PubMed] [Google Scholar]
- Auerbach SS, Shah RR, Mav D, et al. Predicting the hepatocarcinogenic potential of alkenylbenzene flavoring agents using toxicogenomics and machine learning. Toxicol Appl Pharmacol. 2010;243(3):300–314. doi: 10.1016/j.taap.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Baird L, Lleres D, Swift S, Dinkova-Kostova AT. Regulatory flexibility in the Nrf2-mediated stress response is conferred by conformational cycling of the Keap1-Nrf2 protein complex. Proc Nat Acad Sci U S A. 2013;110(38):15259–15264. doi: 10.1073/pnas.1305687110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodreddigari S, Jones LK, Egner PA, et al. Protection against aflatoxin B1-induced cytotoxicity by expression of the cloned aflatoxin B1-aldehyde reductases rat AKR7A1 and human AKR7A3. Chem Res Toxicol. 2008;21(5):1134–1142. doi: 10.1021/tx7004458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutros PC, Yao CQ, Watson JD, et al. Hepatic transcriptomic responses to TCDD in dioxin-sensitive and dioxin-resistant rats during the onset of toxicity. Toxicol Appl Pharmacol. 2011;251(2):119–129. doi: 10.1016/j.taap.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Casalino E, Calzaretti G, Landriscina M, Sblano C, Fabiano A, Landriscina C. The Nrf2 transcription factor contributes to the induction of alpha-class GST isoenzymes in liver of acute cadmium or manganese intoxicated rats: comparison with the toxic effect on NAD(P)H:quinone reductase. Toxicology. 2007;237(1–3):24–34. doi: 10.1016/j.tox.2007.04.020. [DOI] [PubMed] [Google Scholar]
- Cheever KL, Richards DE, Plotnick HB. Metabolism of ortho-, meta-, and para-toluidine in the adult male rat. Toxicol Appl Pharmacol. 1980;56(3):361–369. doi: 10.1016/0041-008x(80)90069-1. [DOI] [PubMed] [Google Scholar]
- Chen HW, Huang CS, Li CC, et al. Bioavailability of andrographolide and protection against carbon tetrachloride-induced oxidative damage in rats. Toxicol Appl Pharmacol. 2014;280(1):1–9. doi: 10.1016/j.taap.2014.07.024. [DOI] [PubMed] [Google Scholar]
- Cho HY, Jedlicka AE, Gladwell W, et al. Association of Nrf2 polymorphism haplotypes with acute lung injury phenotypes in inbred strains of mice. Antioxidants & redox signaling. 2015;22(4):325–338. doi: 10.1089/ars.2014.5942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins FS, Gray GM, Bucher JR. Toxicology. Transforming environmental health protection. Science (New York, NY) 2008;319(5865):906–907. doi: 10.1126/science.1154619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump KS. Calculation of benchmark doses from continuous data. Risk Anal. 1995;15:79–89. [Google Scholar]
- Djordjevic J, Djordjevic A, Adzic M, Mitic M, Lukic I, Radojcic MB. Alterations in the Nrf2-Keap1 signaling pathway and its downstream target genes in rat brain under stress. Brain research. 2015;1602:20–31. doi: 10.1016/j.brainres.2015.01.010. [DOI] [PubMed] [Google Scholar]
- Dunnick JK, Brix A, Sanders JM, Travlos GS. N,N-dimethyl-p-toluidine, a component in dental materials, causes hematologic toxic and carcinogenic responses in rodent model systems. Toxicol Pathol. 2014;42(3):603–615. doi: 10.1177/0192623313489604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunnick JK, Merrick BA, Brix A, et al. Toxicol Pathol published online. 2015. Molecular Changes in the Nasal Cavity after N,N-Dimethyl-p-toluidine Exposure. doi:10.1177/0192623316637708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sherbeni AA, El-Kadi AO. The role of epoxide hydrolases in health and disease. Arch Toxicol. 2014;88(11):2013–2032. doi: 10.1007/s00204-014-1371-y. [DOI] [PubMed] [Google Scholar]
- European Commission Recommendation from the Scientific Committee on occupational exposure limits for 4-aminotoluene (p-toluidine) 2013 eceuropaeu/social/BlobServlet?docId=7308&langId=en.
- Evelyn KA, Malloy HT. Microdetermination of oxyhemoglobin, methemoglobin and sulfhemoglobin in a single sample of blood. J Biol Chem. 1938;126:655–662. [Google Scholar]
- Fan Y, Hu D, Feng B, Wang W. The NQO1 C609T polymorphism and hepatocellular carcinoma risk. Tumour Biol. 2014;35(8):7343–7350. doi: 10.1007/s13277-014-1712-8. [DOI] [PubMed] [Google Scholar]
- Fernandez-Varo G, Oro D, Cable EE, et al. Vasopressin 1a receptor partial agonism increases sodium excretion and reduces portal hypertension and ascites in cirrhotic rats. Hepatology. 2016;63(1):207–216. doi: 10.1002/hep.28250. [DOI] [PubMed] [Google Scholar]
- Fong D, Seeber A, Terracciano L, et al. Expression of EpCAM(MF) and EpCAM(MT) variants in human carcinomas. J Clin Pathol. 2014;67(5):408–414. doi: 10.1136/jclinpath-2013-201932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier L, Cope L, Bolstad BM, Irizarry RA. affy--analysis of Affymetrix GeneChip data at the probe level. Bioinformatics. 2004;20(3):307–315. doi: 10.1093/bioinformatics/btg405. [DOI] [PubMed] [Google Scholar]
- Grillo MP, Ma J, Teffera Y, Waldon DJ. A novel bioactivation pathway for 2-[2-(2,6-dichlorophenyl)aminophenyl]ethanoic acid (diclofenac) initiated by cytochrome P450-mediated oxidative decarboxylation. Drug Metab Dispos. 2008;36(9):1740–1744. doi: 10.1124/dmd.108.021287. [DOI] [PubMed] [Google Scholar]
- Guo W, Sarkar SK, Peddada SD. Controlling false discoveries in multidimensional directional decisions, with applications to gene expression data on ordered categories. Biometrics. 2010;66(2):485–92. doi: 10.1111/j.1541-0420.2009.01292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison JH, Jr., Jollow DJ. Contribution of aniline metabolites to aniline-induced methemoglobinemia. Mol Pharmacol. 1987;32(3):423–431. [PubMed] [Google Scholar]
- Hazardous Substance Database [accesssed September 2015];4-Aminotoluene. 2015 https://toxnet.nlm.nih.gov/newtoxnet/hsdb.htm.
- Irizarry RA, Hobbs B, Collin F, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4(2):249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- Kensler KH, Slocum SL, Chartoumpekis DV, et al. Genetic or pharmacologic activation of Nrf2 signaling fails to protect against aflatoxin genotoxicity in hypersensitive GSTA3 knockout mice. Toxicol Sci. 2014;139(2):293–300. doi: 10.1093/toxsci/kfu056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim NC, Ghanbari K, Kracko DA, Weber WM, McDonald JD, Dix KJ. Identification of urinary metabolites of orally administered N,N-dimethyl-p-toluidine in male F344 rats. J Toxicol Environ Health A. 2007;70(10):781–788. doi: 10.1080/15287390701206176. [DOI] [PubMed] [Google Scholar]
- Kupershmidt I, Su QJ, Grewal A, et al. Ontology-based meta-analysis of global collections of high-throughput public data. PLoS One. 2010;5(9) doi: 10.1371/journal.pone.0013066. doi: 10.1007/s12640-015-9574-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda K, Ohno T, Igarashi S, Yoshimura T, Yamashiro K, Sakai M. Aldehyde oxidase 1 gene is regulated by Nrf2 pathway. Gene. 2012;505(2):374–378. doi: 10.1016/j.gene.2012.06.010. [DOI] [PubMed] [Google Scholar]
- Marques MM, Mourato LL, Amorim MT, Santos MA, Melchior WB, Jr., Beland FA. Effect of substitution site upon the oxidation potentials of alkylanilines, the mutagenicities of N-hydroxyalkylanilines, and the conformations of alkylaniline-DNA adducts. Chem Res Toxicol. 1997;10(11):1266–1274. doi: 10.1021/tx970104w. [DOI] [PubMed] [Google Scholar]
- Merrick BA, Auerbach SS, Stockton PS, et al. Testing an aflatoxin B1 gene signature in rat archival tissues. Chem Res Toxicol. 2012;25(5):1132–114. doi: 10.1021/tx3000945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naiki T, Naiki-Ito A, Asamoto M, et al. GPX2 overexpression is involved in cell proliferation and prognosis of castration-resistant prostate cancer. Carcinogenesis. 2014;35(9):1962–1967. doi: 10.1093/carcin/bgu048. [DOI] [PubMed] [Google Scholar]
- National Toxicology Program . NTP TR 579. 2012. NTP Technical Report on the toxicology and carcinogenesis studies of N,N-Dimethyl-p-toluidine (CAS NO. 99-97-8) in F344/N rats and B6C3F1/N mice. [PubMed] [Google Scholar]
- Office of Environmental Health Hazard Assessment C Chemical Listed Effective MAY 2, 2014 as known to the State of California to cause cancer : N,N -Dimethyl-p-toluidine. 2014 http://oehha.ca.gov/Prop65/prop65_list/050214list.html.
- Pathak KV, Chiu TL, Amin EA, Turesky RJ. Methemoglobin Formation and Characterization of Hemoglobin Adducts of Carcinogenic Aromatic Amines and Heterocyclic Aromatic Amines. Chem Res Toxicol. 2016;29(3):255–269. doi: 10.1021/acs.chemrestox.5b00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peddada S, Harris S, Zajd J, Harvey E. ORIOGEN: order restricted inference for ordered gene expression data. Bioinformatics. 2005;21(20):3933–3934. doi: 10.1093/bioinformatics/bti637. [DOI] [PubMed] [Google Scholar]
- Potter JL, Krill CE, Jr., Neal D, Kofron WG. Methemoglobinemia due to ingestion of N,N-dimethyl-p-toluidine, a component used in the fabrication of artificial fingernails. Ann Emerg Med. 1988;17(10):1098–1100. doi: 10.1016/s0196-0644(88)80455-4. [DOI] [PubMed] [Google Scholar]
- Sanchez-Rodriguez R, Torres-Mena JE, De-la-Luz-Cruz M, et al. Increased expression of prostaglandin reductase 1 in hepatocellular carcinomas from clinical cases and experimental tumors in rats. Int J Biochem Cell Biol. 2014;53:186–194. doi: 10.1016/j.biocel.2014.05.017. [DOI] [PubMed] [Google Scholar]
- Seitz HK, Meier P. The role of acetaldehyde in upper digestive tract cancer in alcoholics. Translational Res. 2007;49(6):293–297. doi: 10.1016/j.trsl.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Singh KB, Maurya BK, Trigun SK. Activation of oxidative stress and inflammatory factors could account for histopathological progression of aflatoxin-B1 induced hepatocarcinogenesis in rat. Mol Cell Biochem. 2015;401(1–2):185–196. doi: 10.1007/s11010-014-2306-x. [DOI] [PubMed] [Google Scholar]
- Son OS, Everett DW, Fiala ES. Metabolism of o-[methyl-14C]toluidine in the F344 rat. Xenobiotica. 1980;10(7–8):457–468. doi: 10.3109/00498258009033781. [DOI] [PubMed] [Google Scholar]
- Sulpice L, Rayar M, Turlin B, et al. Epithelial cell adhesion molecule is a prognosis marker for intrahepatic cholangiocarcinoma. J Surg Res. 2014;192(1):117–123. doi: 10.1016/j.jss.2014.05.017. [DOI] [PubMed] [Google Scholar]
- Tanaka N, Matsubara T, Krausz KW, Patterson AD, Gonzalez FJ. Disruption of phospholipid and bile acid homeostasis in mice with nonalcoholic steatohepatitis. Hepatology. 2012;56(1):118–129. doi: 10.1002/hep.25630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas RS, Clewell HJ, 3rd, Allen BC, et al. Application of transcriptional benchmark dose values in quantitative cancer and noncancer risk assessment. Toxicol Sci. 2011;120(1):194–205. doi: 10.1093/toxsci/kfq355. [DOI] [PubMed] [Google Scholar]
- Thomas RS, Clewell HJ, 3rd, Allen BC, Yang L, Healy E, Andersen ME. Integrating pathway-based transcriptomic data into quantitative chemical risk assessment: a five chemical case study. Mutat Res. 2012;746(2):135–143. doi: 10.1016/j.mrgentox.2012.01.007. [DOI] [PubMed] [Google Scholar]
- Tyrakowska B, Boeren S, Geurtsen B, Rietjens IM. Qualitative and quantitative influences of ortho chlorine substituents on the microsomal metabolism of 4-toluidines. Drug Metab Dispos. 1993;21(3):508–59. [PubMed] [Google Scholar]
- U. S. Environmental Protection Program High Production Volume Challenge Program. 2011 http://wwwepagov/HPV/http://iaspubepagov/oppthpv/quicksearchdisplay?pChem=100729http://wwwepagov/hpv/pubs/sumresphtm.
- van der Leij FR, Huijkman NC, Boomsma C, Kuipers JR, Bartelds B. Genomics of the human carnitine acyltransferase genes. Mol Genet Metab Molecular genetics and metabolism. 2000;71(1–2):139–153. doi: 10.1006/mgme.2000.3055. [DOI] [PubMed] [Google Scholar]
- Wang L, Yoon DM, Spicer PP, et al. Characterization of porous polymethylmethacrylate space maintainers for craniofacial reconstruction. J Biomed Mater Res B Appl Biomater. 2013;101(5):813–825. doi: 10.1002/jbm.b.32885. [DOI] [PubMed] [Google Scholar]
- Watson JD, Prokopec SD, Smith AB, Okey AB, Pohjanvirta R, Boutros PC. TCDD dysregulation of 13 AHR-target genes in rat liver. Toxicol Appl Pharmacol. 2014;274(3):445–454. doi: 10.1016/j.taap.2013.12.004. [DOI] [PubMed] [Google Scholar]
- Weisburger EK, Russfield AB, Homburger F, et al. Testing of twenty-one environmental aromatic amines or derivatives for long-term toxicity or carcinogenicity. J Environ Pathol Toxicol. 1978;2(2):325–356. [PubMed] [Google Scholar]
- Xue Y, Wang M, Zhong D, et al. ADH1C Ile350Val polymorphism and cancer risk: evidence from 35 case-control studies. PLoS One. 2012;7(5):e37227. doi: 10.1371/journal.pone.0037227. doi:10.1371/journal.pone.0037227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Allen BC, Thomas RS. BMDExpress: a software tool for the benchmark dose analyses of genomic data. BMC Genomics. 2007;8:387. doi: 10.1186/1471-2164-8-387. doi:10.1186/1471-2164-8-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Ge N, Guo X, et al. Genetic variants in the EPCAM gene is associated with the prognosis of transarterial chemoembolization treated hepatocellular carcinoma with portal vein tumor thrombus. PLoS One. 2014;9(4):e93416. doi: 10.1371/journal.pone.0093416. doi:10.1371/journal.pone.0093416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Weng CC, Tong M, Wei J, Tai HH. Restoration of leukotriene B(4)-12-hydroxydehydrogenase/15- oxo-prostaglandin 13-reductase (LTBDH/PGR) expression inhibits lung cancer growth in vitro and in vivo. Lung Cancer (Amsterdam, Netherlands) 2010;68(2):161–169. doi: 10.1016/j.lungcan.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond E, Ben Ya'acov A, Lee H, et al. Suppression of Hepatocellular Carcinoma by Inhibition of Overexpressed Ornithine Aminotransferase. ACS Med Chem. 2015;6(8):840–844. doi: 10.1021/acsmedchemlett.5b00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.