Abstract
Due to Cytochrome P450 3A (CYP3A) metabolism, clinical trials of ibrutinib-treated CLL patients prohibited concurrent medications metabolized by CYP3A. We evaluated concomitant medication use in 118 ibrutinib-treated CLL patients outside the context of clinical trials. Seventy-five (64%) patients were on medications that could increase ibrutinib toxicity and 4 (3%) were on drugs that could decrease ibrutinib efficacy. Nineteen (16%) patients were on concomitant CYP3A inhibitors (11 moderate, 8 strong), and 4 (3%) were on CYP3A inducers (two patients were on both CYP3A inhibitors and inducers). Although the ibrutinib starting dose was changed in 18 patients on CYP3A interacting medications, no difference in 18-month progression-free survival or rate of ibrutinib discontinuation was observed in patients who were not. In routine clinical practice, 2 of 3 CLL patients commencing ibrutinib are on a concomitant medication with potential to influence ibrutinib metabolism. Formal medication review by a pharmacist should be considered in all patients initiating ibrutinib.
INTRODUCTION
Ibrutinib, a first-in-class Bruton’s tyrosine kinase inhibitor, represents a major treatment advance in the management of patients with CLL.1–6 Ibrutinib was FDA approved for relapsed or refractory CLL in February 2014 based on a study of 391 patients with relapsed or refractory CLL who received single-agent ibrutinib versus ofatumumab.1 In July 2014, ibrutinib obtained an expanded indication for CLL patients with deletion 17p13. Recently, untreated CLL patients 65 years or older were randomized to ibrutinib or chlorambucil. Risk of CLL progression and relative risk of death was 84% lower with ibrutinib versus chlorambucil and resulted in an expanded indication as first-line therapy in patients with CLL.4
Ibrutinib is extensively metabolized and eliminated by the Cytochrome P450 CYP3A with minor involvement of CYP2D6. Both CYP3A and CYP2D6 are part of the Cytochrome P450 enzymatic machinery in the liver, and constitute the most significant CYP pathways in the oxidative biotransformation of numerous medications.8,9,10 Therefore, the concomitant use of ibrutinib and medications that alter CYP3A metabolism can potentially result in ibrutinib toxicity or reduced efficacy. In healthy subjects, the co-administration of ibrutinib with ketoconazole (a strong CYP3A inhibitor) increased the maximum serum concentration (Cmax) and area under the curve (AUC) of ibrutinib by 29- and 24-fold, respectively. The co-administration of ibrutinib with rifampin (a strong CYP3A inducer) decreased the Cmax and AUC by more than 13- and 10-fold, respectively.11 Given these significant changes in the concentration of ibrutinib in the presence of medications that inhibit or induce CYP3A pathways, clinical trials of ibrutinib in CLL excluded patients who were on these medications concurrently. In addition to prescription medications, supplements such as garlic, ginkgo biloba, Echinacea, ginseng, St. John’s wort and grape seed.19,20 alter exposure of prescription medications metabolized by CYP3A.
Spontaneous bruising or petechiae during ibrutinib was reported to occur in approximately 50% of CLL patients treated on clinical trials.12 Although the reasons for the increased risk of bleeding are not completely understood, it is thought to be due to defective collagen-mediated platelet aggregation.13,14 CLL patients who were on concomitant anticoagulation therapy with warfarin were excluded from ibrutinib clinical trials, and patients requiring other anticoagulant or antiplatelet medications were enrolled with caution.
CLL is a disease of older adults who frequently have co-existent health problems. Consistent with this fact, patients are frequently on concomitant medications including anti-infectives, anticoagulants and antiplatelet therapy at the time they require treatment for CLL. It is unknown what proportion of CLL patients starting ibrutinib therapy in everyday practice are on medications metabolized through the CYP3A enzymes or other medications that could potentially increase its toxicity. We evaluated these aspects in a large cohort of CLL patients treated with ibrutinib in routine clinical practice.
METHODS & MATERIALS
After approval from the Mayo Clinic Institutional Review Board, all CLL patients who received ibrutinib therapy outside the context of a clinical trial between November 2013 and January 2016 were considered eligible for this study. Baseline clinical and laboratory characteristics were recorded for all patients.
An oncology pharmacist reviewed each patient’s electronic medical record in detail prior to the start of ibrutinib therapy. Concomitant medications at the start of ibrutinib therapy were recorded and categorized as follows: 1) CYP3A inhibitors; 2) CYP3A inducers; 3) anticoagulants; 4) antiplatelet agents; and 5) other medications/supplements (selective serotonin reuptake inhibitors [SSRIs], Vitamin E, fish oil, or other herbals). Determination of the CYP3A inducer and inhibitor status was made according to the FDA’s table of substrates, inhibitors and inducers of the cytochrome P450 family of enzymes and Cytochrome P450 Drug Interaction table from the Indiana University School of Medicine.21,22
Our clinical approach to patients on medications with potential to interfere with ibrutinib metabolism was to either stop the interacting medication or switch to an alternative non-interacting medication if possible. If we were unable to do either of these, we performed the following: 1) for concomitant moderate CYP3A inhibitors, we followed the ibrutinib package insert recommendations12 and reduced the ibrutinib dose to 140 mg once daily; 2) for concomitant strong CYP3A inhibitors (due to no manufacturer recommendations), we decreased the ibrutinib frequency to 140 mg every 48 to 72 hours; 3) for concomitant CYP3A inducers (strong and moderate with no labeling endorsement for ibrutinib dose modifications), we initiated patients on 420 mg once daily with close monitoring for efficacy in the first 8 weeks of therapy, and planned to increase ibrutinib dosing if necessary; 4) for those on anticoagulation or antiplatelet therapy, we evaluated bleeding risk versus benefit of continuing these agents on an individual basis; and 5) discontinued all supplements with potential CYP3A interaction or bleeding risk (including Vitamin E, fish oil, and herbals).
All patients were followed at Mayo Clinic during the course of their ibrutinib therapy. Evaluations included monthly visits for the first three months, then every three months for the duration of therapy. At each visit, patients were evaluated for toxicity with ibrutinib. Clinically significant bleeding events were defined as those requiring hospitalization, transfusion or procedure. All other bleeding events were classified as minor.
Statistical Analysis
We used Chi-square and Fisher’s exact tests to compare discrete variables, and the Kruskal Wallis test to compare continuous variables. Time to ibrutinib discontinuation was defined as the interval between initiation of ibrutinib therapy and discontinuation for any reason (toxicity or progression of disease), death date, or last known alive date. Cumulative incidence curves, accounting for competing risk of death, were generated to depict time to ibrutinib discontinuation; when analyzing time to discontinuation for a specific reason (i.e., toxicity or progression of disease); the other reason was coded as a competing event. Discontinuation rates were calculated by CYP3A interactions as well as by patients who were on CYP3A inhibitors or inducers, and differences between groups were testing using the Gray K-sample test. Cumulative incidence functions were used to estimate risk of bleeding events while on ibrutinib therapy, accounting for risk of death. Progression free survival (PFS) was defined as the interval between start of ibrutinib therapy and death due to any reason or disease progression; patients were censored if they 1) stopped ibrutinib due to toxicity prior to progression of disease and who were not yet deceased (censored on the last date of ibrutinib) or 2) were still alive (censored on last known alive date); PFS was plotted using the Kaplan-Meier method, and differences were tested using the log-rank test. All statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
RESULTS
One hundred eighteen CLL patients started ibrutinib during the study period. Median age was 59 years (range, 29–83 years), and 78 (66%) were male. Indications to initiate ibrutinib therapy were relapsed/refractory CLL in 106 (90%) patients, previously untreated CLL with del17p13 in 7 (6%) patients and CLL with Richter’s transformation in 5 (4%) patients. The median number of prior therapies among 111 previously treated CLL patients (including those with Richter’s transformation) was 3 (range 1–18). The baseline characteristics of all patients are shown in Table 1. Seventy-five patients (64%) were taking concurrent medications with the potential to alter ibrutinib metabolism and/or increase risk of ibrutinib toxicity while 4 (3%) of patients were on drugs potentially reducing ibrutinib efficacy. Potentially interacting medications included strong and moderate inhibitors of CYP3A4 (16%), CYP3A4 inducers (3%), anticoagulants (including warfarin, low molecular weight heparin and novel anticoagulants, 11%), and antiplatelet medications (48%). Individual patients were counted only once if they were on more than one potentially interacting medication.
Table 1.
Baseline Characteristics at diagnosis of all patients
| N (%) or [range] | |
|---|---|
|
| |
| Median age at ibrutinib initiation (range) | 59 [29–83] |
|
| |
| Sex | |
| - Female | 40 (34%) |
| - Male | 78 (66%) |
|
| |
| FISH | |
| - Normal | 15 (20%) |
| - Del 13q- | 20 (26%) |
| - Trisomy 12 | 18 (24%) |
| - Del 11q- | 10 (13%) |
| - Del 17p- | 13 (17%) |
| - Missing | 42 |
|
| |
| IGHV mutation status | |
| - Mutated | 25 (24%) |
| - Unmutated | 77 (76%) |
| - Missing | 16 |
|
| |
| CD38 | |
| - Negative | 55 (70%) |
| - Positive | 24 (30%) |
| - Missing | 39 |
|
| |
| CD49d | |
| - Negative | 30 (51%) |
| - Positive | 29 (49%) |
| - Missing | 59 |
|
| |
| ZAP70 | |
| - Negative | 28 (38%) |
| - Positive | 45 (62%) |
| - Missing | 45 |
|
| |
| Rai stage | |
| - Rai 0 | 45 (45%) |
| - Rai I–II | 44 (44%) |
| - Rai III–IV | 11 (11%) |
| - Missing | 18 |
|
| |
| Diagnosis | |
| - CLL | 105 (89%) |
| - SLL | 13 (11%) |
Concomitant CYP3A Medications
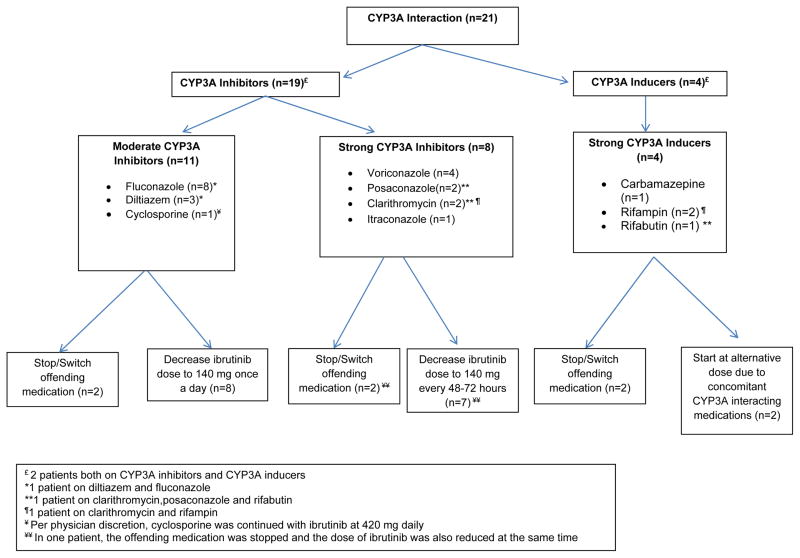
At ibrutinib initiation, 21 (18%) patients were on concomitant medications known to induce or inhibit CYP3A. This included 19 (16%) patients on concomitant CYP3A inhibitors (including 11 [9%] on moderate CYP3A inhibitors and 8 [7%] on strong CYP3A inhibitors), and 4 (3%) on strong CYP3A inducers. Two patients were on both a strong CYP3A inhibitor and a strong CYP3A inducer. No patient was on a moderate CYP3A inducer. Figure 1 lists the names of these interacting medications and the interventions recommended by the pharmacist prior to the start of ibrutinib therapy. Of note, two patients on strong CYP3A4 inducers were also on concurrent strong CYP3A4 inhibitors. The ibrutinib dose was adjusted to 140 mg once every other day to account for the potential increased toxicity with the concomitant use of the strong CYP3A4 inhibitor. Both patients developed lymphocytosis commonly seen after ibrutinib initiation. Overall, medications interfering with CYP3A metabolism were discontinued or replaced with an alternative medication prior to the start of ibrutinib therapy in 5 patients. A modification of the ibrutinib starting dose was recommended in 16 patients who continued on medications altering CYP3A metabolism. During the course of ibrutinib, an additional 8 (7%) patients were started on CYP3A4 inhibitors or inducers which necessitated ibrutinib dose modifications in all 8 patients.
Figure 1.
Pharmacist recommended interventions for CLL patients on Concomitant CYP3A Medications at ibrutinib initiation
Concomitant Anticoagulants/Antiplatelet Agents
At the time of commencing ibrutinib, 13 (11%) patients were on anticoagulants (7 warfarin, 3 enoxaparin, and 3 direct oral anticoagulants), 34 patients were on aspirin (3 also on clopidogrel), and 9 were on NSAIDs (2 were also on aspirin). In an attempt to reduce bleeding risk, warfarin was switched to enoxaparin in two patients, to a direct oral anticoagulant in one patient, to aspirin in one patient, and discontinued in one patient. In two patients, an alternative anticoagulant could not be used, so ibrutinib was begun at 140 mg daily in conjunction with warfarin and titrated upward as tolerated. Aspirin was discontinued in 10 patients, and three patients switched from 325 mg to 81 mg daily. Clopidogrel and NSAIDs were continued at current dose in three and 9 patients, respectively. Overall, 11 patients on concurrent anticoagulant/antiplatelet medications were discontinued or replaced with an alternative medication prior to the start of ibrutinib therapy.
Other Medications and Supplements
Prior to starting ibrutinib, 23 (19%) patients were on SSRI’s. SSRI’s were continued in all ibrutinib patients. Sixteen patients (14%) were on fish oil or Vitamin E, and 27 (23%) were on herbal medications deemed to modify CYP3A metabolism or increase risk of bleeding; all were discontinued.
Bleeding
Thirteen patients had bleeding on ibrutinib including four clinically significant bleeds requiring hospitalization (1 subdural hematoma, 1 intramastoid bleed, 2 hematomas). Of these four patients, one was on enoxaparin, two on a SSRI, and one on a NSAID. Nine patients had minor bleeding resulting in a dose reduction of ibrutinib to 140–280 mg daily. The risk of any bleeding at 12 months was 11% (95% Confidence Interval [CI] 5–17%).
Discontinuation of Ibrutinib
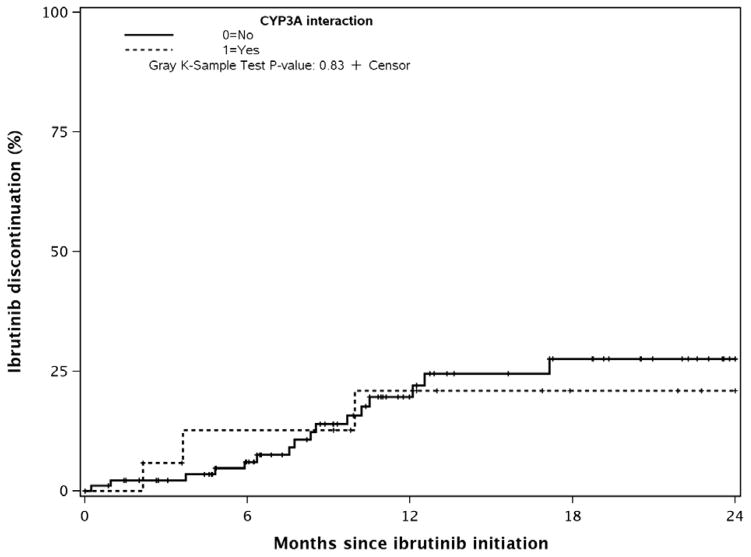
After a median follow-up of 13 months, 94 (73%) patients remain on ibrutinib. Of the 24 patients who discontinued ibrutinib therapy, 14 discontinued due to toxicity/intolerance and 10 due to progression of disease (6 CLL progression and 4 Richter’s transformation). There was no difference in the 12-month rates of discontinuation of ibrutinib between patients who continued on CYP3A interacting medications with initiation of ibrutinib versus those who did not (21% vs 20%, p-value=0.83, Figure 2); there were no differences by CYP3A interactions if each reason for discontinuation was evaluated separately. After accounting for baseline CYP3A medication use, there was no significant difference in time to ibrutinib discontinuation between patients who stopped ibrutinib due to toxicity or due to progression of disease.
Figure 2.
Discontinuation of Ibrutinib (Progression or Toxicity) by CYP3A Interaction
Progression-free Survival
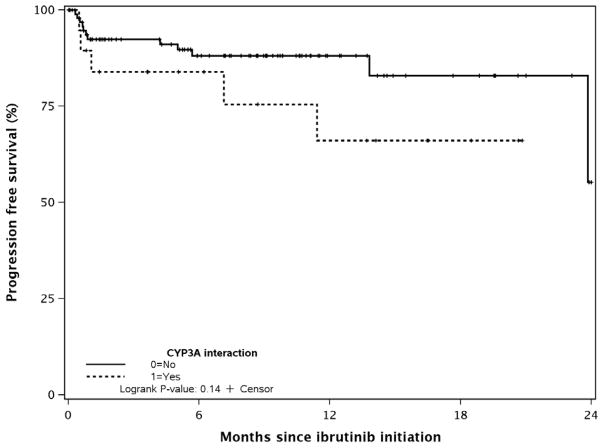
The estimated 18-month PFS for all patients was 79% (95% CI 69–90%,). There was no significant difference in the 18-month PFS between patients who were on a CYP3A interacting medication at the time of ibrutinib initiation vs. not (76% [95% CI 52–100%] vs. 82% [95% CI 71–96%], p-value=0.14, Figure 3).
Figure 3.
Progression-Free Survival by CYP3A Interaction
DISCUSSION
This is the first study that systematically analyzes pharmacovigilance among CLL patients who received ibrutinib therapy in routine clinical practice. Our results indicate that two out of three CLL patients commencing ibrutinib therapy are on a preexisting medication with the potential to alter ibrutinib metabolism and/or toxicity resulting in a potentially clinically significant drug-drug interaction with ibrutinib. With the expertise of a clinical pharmacist, all these patients were able to initiate ibrutinib by either altering or stopping offending medications or adjusting ibrutinib dose. These interventions did not appear to negatively impact the time to discontinuation of ibrutinib or PFS.
It is estimated that CYP3A, the main isozyme of the P450 family, metabolizes approximately 50% of all available medications.23 With the advent of targeted therapies in cancer, particularly tyrosine kinase inhibitors (TKI), which are all metabolized by CYP3A (28 FDA approved kinase inhibitors as of July 2015, including ibrutinib),24 there is an urgent need for recognizing the potential for significant drug-drug interactions. In a study using a de-identified pharmacy claims database who were co-prescribed one of 9 oral TKIs and a drug metabolized by the CYP3A enzyme during a 12-month interval, ~40% patients were co-prescribed medications that could decrease the efficacy of the TKI, and ~50% were co-prescribed medications that could increase its toxicity.25 Although this study predated the approval of ibrutinib, it underscores the problem of significant drug-drug interactions in this era of targeted cancer therapies.
It is well documented that drug-drug interactions occur in approximately one-third of oncology patients.26,27 A recent analysis of 898 cancer patients receiving oral cancer therapies found that 46% had risk of at least one potential drug-drug interaction. Sixteen percent of patients had a documented major drug-drug interaction that potentially could have caused harm to the patient or toxicity requiring intensive monitoring.28 The role of the oncology pharmacist in assessing patient medications for drug-drug interactions has been deemed important by patients and providers, even if associated with added time and cost for the pharmacist visit.29,30 The results from our study emphasize the need for a multidisciplinary approach to the care of CLL patients. We found that one of five CLL patients initiating ibrutinib therapy is on a concurrent medication that is metabolized by the CYP3A family of enzymes. The vast majority of drug classes implicated in this interaction include antifungals, antihypertensives, anticonvulsants and other tyrosine kinase inhibitors. Additionally, ~10% patients are started on a medication metabolized by the CYP3A isozyme during the course of ibrutinib therapy which suggests that an ongoing review of concurrent medications needs to also be included in the plan of care.
Although dose adjustments of ibrutinib were made due to a potential for drug interactions in approximately two-thirds of patients in our study, no significant difference in either the rate of discontinuation of ibrutinib or PFS was observed. In a recent analysis of the ibrutinib arm of the RESONATE study (which compared ibrutinib to ofatumumab in relapsed/refractory CLL), patients who had a higher mean dose intensity of ibrutinib experienced a longer PFS compared to patients who did not (particularly those who skipped more than 7 days of ibrutinib).31 The results from our study suggest that the reduced dose of ibrutinib did not significantly alter the rates of discontinuation of ibrutinib or PFS. Additionally, there was no significant difference in bleeding risks among patients who had a CYP3A medication interaction at the time of ibrutinib initiation.
There are several promising drugs available for relapsed/refractory CLL – including idelalisib,32 acalabrutinib,33 and venetoclax.34 All of these agents are metabolized by the CYP3A isozyme and will also need careful scrutiny for drug-drug interactions when they are used more extensively for the treatment of CLL. Additionally, these novel agents are all being tested in patients with previously untreated CLL where their use will significantly expand in the future, thereby underscoring the need to monitor for potential drug-drug interactions. Given that most of these therapies are administered for an indefinite length of time, a careful drug history must be obtained at each visit, and patients must be educated to discuss any new prescription drug or over-the-counter medication with their hematologist/oncologist.
Our study has several strengths. To the best of our knowledge, it is the first study that comprehensively describes the number of concomitant medications in CLL patient starting ibrutinib in routine practice. Second, an oncology pharmacist reviewed the medication list for each patient and assigned risks for drug-drug interactions, including several medications that have the potential to adversely impact the efficacy and safety of ibrutinib. This approach appears more accurate than use of electronic software to identify potential drug-drug interactions. For example, in a study by Yap and colleagues,35 four major drug databases including Micromedex.com were able to accurately identify an interaction in only 8–56% cases. Our study also has several limitations. It is a single-center study and caution must be exercised prior to generalization to other settings. The vast majority of our patients had received prior therapy for CLL (median number of prior treatments was 3). This may skew the results towards a more heavily pre-treated CLL patient population who are on multiple medications, with a large potential for drug-drug interactions. Finally, we did not perform BTK occupancy studies among patients in whom the dose of ibrutinib was changed to determine if we were achieving adequate blocking of BTK phosphorylation, a valid pharmacodynamics endpoint.2 Of note, no dose limiting toxicity or maximum tolerated dose was identified in a phase I study looking at 420 mg versus 840 mg ibrutinib daily dosing, limiting knowledge of an ibrutinib dose-response relationship with toxicity.2 Because of this, we initiated dosing of ibrutinib at 140 to 280 mg once daily in patients on concomitant anticoagulants and titrated the ibrutinib dose upward as tolerated to 420 mg daily. Given that the outcomes of patients treated with an altered dose of ibrutinib were not significantly different from those patients who received full dose ibrutinib, our results suggest that, even if this difference was present, it was not clinically meaningful.
In summary, approximately two of three CLL patients initiating ibrutinib therapy are on concurrent medications which can potentially alter its efficacy or cause increased toxicity. Our results indicate that dose modifications of ibrutinib with concomitant CYP3A interacting medications do not appear to negatively impact ibrutinib treatment efficacy for CLL. Formal medication review by a clinical pharmacist should be considered in all patients initiating ibrutinib therapy.
Acknowledgments
REASEARCH SUPPORT:
This work was supported by funding from the National Cancer Institute (grants K12 CA090628 to Sameer Parikh, MD, K23CA160345 to Wei Ding, MD, CA95241 to Neil Kay, MD and K12 CA090628 to Saad Kenderian MD) and in part by the Predolin Foundation.
Tait D. Shanafelt, MD is a clinical scholar of the Leukemia and Lymphoma Society.
Footnotes
PREVIOUS PRESENTATION OF THIS RESEARCH:
The results of this study were presented at the 57th Annual American Society of Hematology Meeting in Orlando, FL in December 2015.
DISCLAIMER:
Heidi Finnes, PharmD and Sameer A. Parikh, MD had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Byrd JC, Brown JR, O’Brien S, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. The New England journal of medicine. 2014;371(3):213–223. doi: 10.1056/NEJMoa1400376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. The New England journal of medicine. 2013;369(1):32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chanan-Khan A, Cramer P, Demirkan F, et al. Ibrutinib combined with bendamustine and rituximab compared with placebo, bendamustine, and rituximab for previously treated chronic lymphocytic leukaemia or small lymphocytic lymphoma (HELIOS): a randomised, double-blind, phase 3 study. The Lancet Oncology. doi: 10.1016/S1470-2045(15)00465-9. [DOI] [PubMed] [Google Scholar]
- 4.Burger JA, Tedeschi A, Barr PM, et al. Ibrutinib as Initial Therapy for Patients with Chronic Lymphocytic Leukemia. The New England journal of medicine. 2015;373(25):2425–2437. doi: 10.1056/NEJMoa1509388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farooqui MZ, Valdez J, Martyr S, et al. Ibrutinib for previously untreated and relapsed or refractory chronic lymphocytic leukaemia with TP53 aberrations: a phase 2, single-arm trial. The Lancet Oncology. 2015;16(2):169–176. doi: 10.1016/S1470-2045(14)71182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burger JA, Keating MJ, Wierda WG, et al. Safety and activity of ibrutinib plus rituximab for patients with high-risk chronic lymphocytic leukaemia: a single-arm, phase 2 study. The Lancet Oncology. 2014;15(10):1090–1099. doi: 10.1016/S1470-2045(14)70335-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.FDA NEWS RELEASE. 2014;2016 [Google Scholar]
- 8.Niel N, Rechencq E, Muller A, et al. Synthesis and contractile activity of new pseudopeptido and thioaromatic analogues of leukotriene D4. Prostaglandins. 1992;43(1):45–54. doi: 10.1016/0090-6980(92)90063-y. [DOI] [PubMed] [Google Scholar]
- 9.Zanger UM, Schwab M. Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacology & Therapeutics. 2013;138(1):103–141. doi: 10.1016/j.pharmthera.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 10.Scheers E, Leclercq L, de Jong J, et al. Absorption, metabolism, and excretion of oral (1)(4)C radiolabeled ibrutinib: an open-label, phase I, single-dose study in healthy men. Drug metabolism and disposition: the biological fate of chemicals. 2015;43(2):289–297. doi: 10.1124/dmd.114.060061. [DOI] [PubMed] [Google Scholar]
- 11.de Jong J, Skee D, Murphy J, et al. Effect of CYP3A perpetrators on ibrutinib exposure in healthy participants. Pharmacology research & perspectives. 2015;3(4):e00156. doi: 10.1002/prp2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.IMBRUVICA® [package insert] Vol. 2016 Pharmacyclics. Inc; Sunnyvale, CA: Feb, 2014. [Google Scholar]
- 13.Kamel S, Horton L, Ysebaert L, et al. Ibrutinib inhibits collagen-mediated but not ADP-mediated platelet aggregation. Leukemia. 2015;29(4):783–787. doi: 10.1038/leu.2014.247. [DOI] [PubMed] [Google Scholar]
- 14.Levade M, David E, Garcia C, et al. Ibrutinib treatment affects collagen and von Willebrand factor-dependent platelet functions. Blood. 2014;124(26):3991–3995. doi: 10.1182/blood-2014-06-583294. [DOI] [PubMed] [Google Scholar]
- 15.Jones JA, Hillmen P, Coutre S, et al. Pattern of Use of Anticoagulation and/or Antiplatelet Agents in Patients with Chronic Lymphocytic Leukemia (CLL) Treated with Single-Agent Ibrutinib Therapy. Blood. 2014;124(21):1990–1990. [Google Scholar]
- 16.Turner MS, May DB, Arthur RR, Xiong GL. Clinical impact of selective serotonin reuptake inhibitors therapy with bleeding risks. Journal of internal medicine. 2007;261(3):205–213. doi: 10.1111/j.1365-2796.2006.01720.x. [DOI] [PubMed] [Google Scholar]
- 17.Jeong BO, Kim SW, Kim SY, Kim JM, Shin IS, Yoon JS. Use of serotonergic antidepressants and bleeding risk in patients undergoing surgery. Psychosomatics. 2014;55(3):213–220. doi: 10.1016/j.psym.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 18.Jiang HY, Chen HZ, Hu XJ, et al. Use of selective serotonin reuptake inhibitors and risk of upper gastrointestinal bleeding: a systematic review and meta-analysis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2015;13(1):42–50. e43. doi: 10.1016/j.cgh.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 19.Sparreboom A, Cox MC, Acharya MR, Figg WD. Herbal remedies in the United States: potential adverse interactions with anticancer agents. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22(12):2489–2503. doi: 10.1200/JCO.2004.08.182. [DOI] [PubMed] [Google Scholar]
- 20.Zhou S, Gao Y, Jiang W, Huang M, Xu A, Paxton JW. Interactions of herbs with cytochrome P450. Drug metabolism reviews. 2003;35(1):35–98. doi: 10.1081/dmr-120018248. [DOI] [PubMed] [Google Scholar]
- 21.United States FDA. Drug Development and Drug Interactions: Table of Substrates, Inhibitors and Inducers. 2016. [Google Scholar]
- 22.DAF . Drug Interactions: Cytochrome P450 Drug Interaction Table. Vol. 2016. Indiana University School of Medicine; 2007. [Google Scholar]
- 23.Teo YL, Ho HK, Chan A. Metabolism-related pharmacokinetic drug–drug interactions with tyrosine kinase inhibitors: current understanding, challenges and recommendations. British Journal of Clinical Pharmacology. 2015;79(2):241–253. doi: 10.1111/bcp.12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu P, Nielsen TE, Clausen MH. FDA-approved small-molecule kinase inhibitors. Trends in Pharmacological Sciences. 36(7):422–439. doi: 10.1016/j.tips.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 25.Bowlin SJ, Xia F, Wang W, Robinson KD, Stanek EJ. Twelve-month frequency of drug-metabolizing enzyme and transporter-based drug-drug interaction potential in patients receiving oral enzyme-targeted kinase inhibitor antineoplastic agents. Mayo Clinic proceedings. 2013;88(2):139–148. doi: 10.1016/j.mayocp.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 26.Riechelmann RP, Del Giglio A. Drug interactions in oncology: how common are they? Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2009;20(12):1907–1912. doi: 10.1093/annonc/mdp369. [DOI] [PubMed] [Google Scholar]
- 27.Scripture CD, Figg WD. Drug interactions in cancer therapy. Nature reviews Cancer. 2006;6(7):546–558. doi: 10.1038/nrc1887. [DOI] [PubMed] [Google Scholar]
- 28.van Leeuwen RW, Brundel DH, Neef C, et al. Prevalence of potential drug-drug interactions in cancer patients treated with oral anticancer drugs. British journal of cancer. 2013;108(5):1071–1078. doi: 10.1038/bjc.2013.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McKee M, Frei BL, Garcia A, Fike D, Soefje SA. Impact of clinical pharmacy services on patients in an outpatient chemotherapy academic clinic. Journal of oncology pharmacy practice : official publication of the International Society of Oncology Pharmacy Practitioners. 2011;17(4):387–394. doi: 10.1177/1078155210389217. [DOI] [PubMed] [Google Scholar]
- 30.Ruder AD, Smith DL, Madsen MT, Kass FH., 3rd Is there a benefit to having a clinical oncology pharmacist on staff at a community oncology clinic? Journal of oncology pharmacy practice : official publication of the International Society of Oncology Pharmacy Practitioners. 2011;17(4):425–432. doi: 10.1177/1078155210389216. [DOI] [PubMed] [Google Scholar]
- 31.Barr PMBJ, Hillmen P, O’Brien S, Barrientos JC, Reddy NM, Coutre S, Mulligan SP, Jäger U, Furman RR, Cymbalista F, Montillo M, Dearden C, Robak T, Moreno C, Pagel J, Burger JA, Suzuki S, James DF, Byrd JC. Dose adherence and baseline exposure analysis of the ibrutinib 420 mg dose administered to patients with previously treated chronic lymphocytic leukemia (CLL) Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;(33) [Google Scholar]
- 32.Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and Rituximab in Relapsed Chronic Lymphocytic Leukemia. New England Journal of Medicine. 2014;370(11):997–1007. doi: 10.1056/NEJMoa1315226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Byrd JC, Harrington B, O’Brien S, et al. Acalabrutinib (ACP-196) in Relapsed Chronic Lymphocytic Leukemia. The New England journal of medicine. 2016;374(4):323–332. doi: 10.1056/NEJMoa1509981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roberts AW, Davids MS, Pagel JM, et al. Targeting BCL2 with Venetoclax in Relapsed Chronic Lymphocytic Leukemia. The New England journal of medicine. 2016;374(4):311–322. doi: 10.1056/NEJMoa1513257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yap KY, Raaj S, Chan A. OncoRx-IQ: a tool for quality assessment of online anticancer drug interactions. International journal for quality in health care : journal of the International Society for Quality in Health Care / ISQua. 2010;22(2):93–106. doi: 10.1093/intqhc/mzq004. [DOI] [PubMed] [Google Scholar]