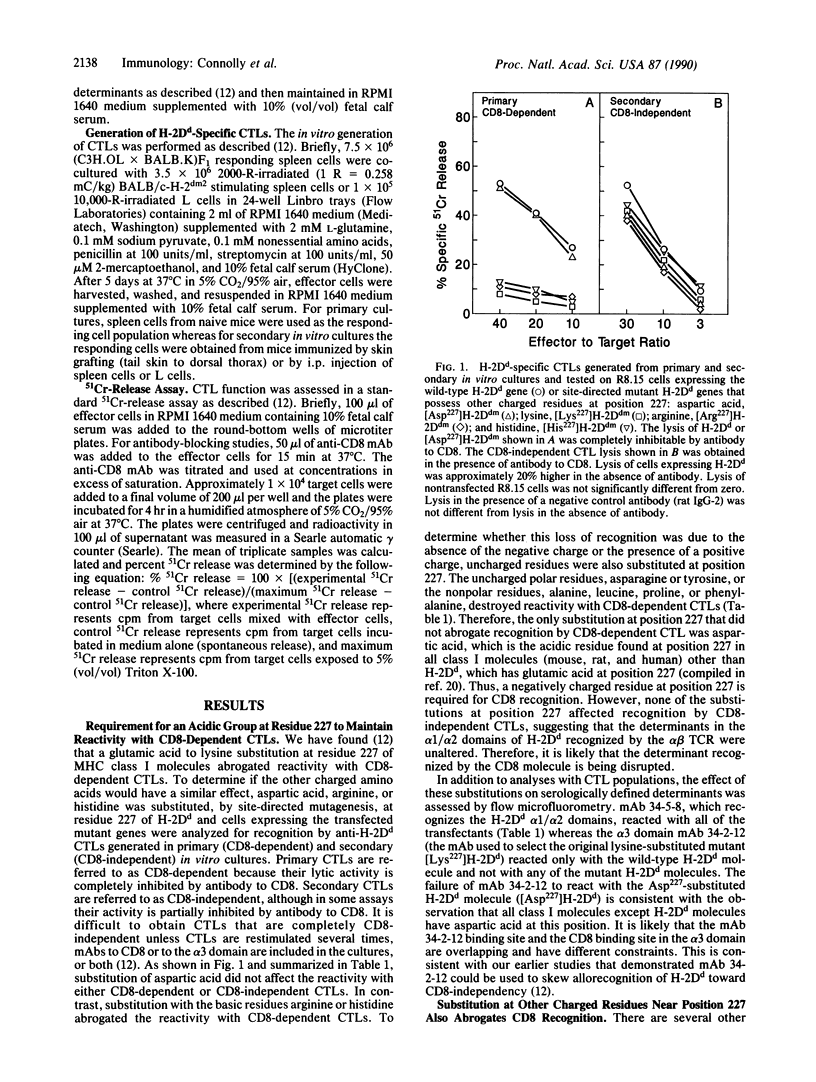
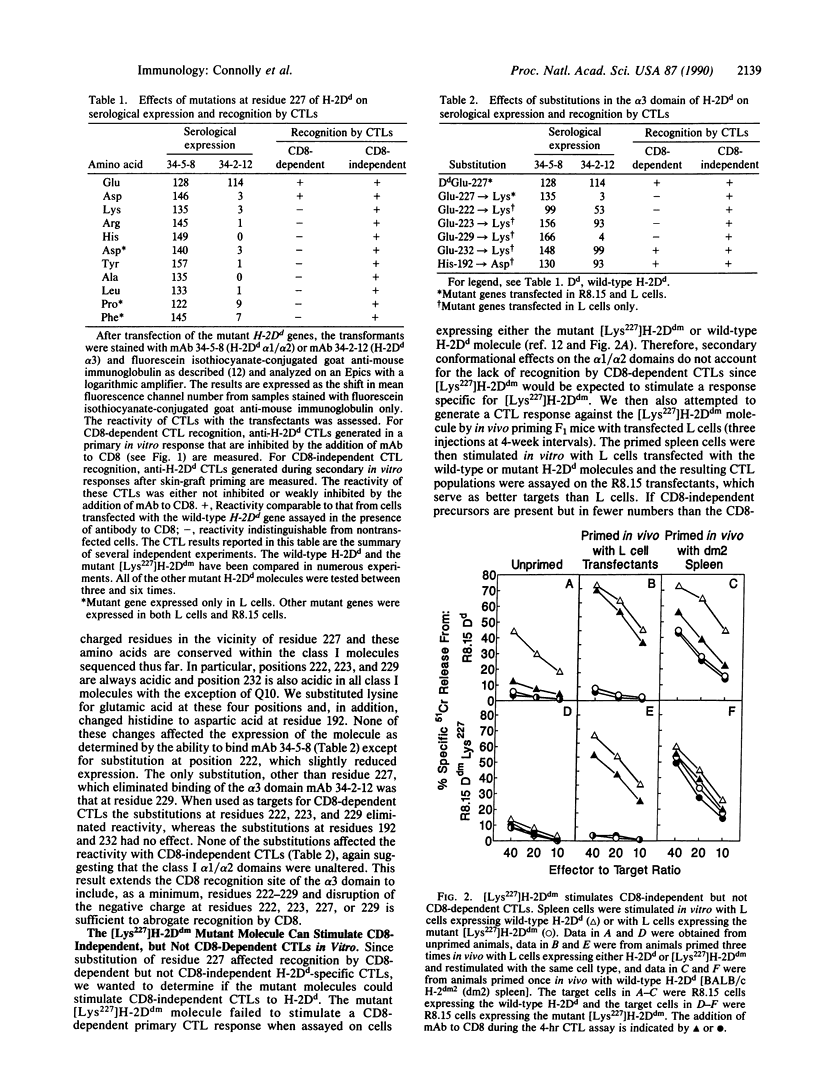
Abstract
The CD8 molecule on class I-reactive cytotoxic T lymphocytes (CTLs) is believed to function as a coreceptor along with the alpha beta T-cell receptor. Whereas the alpha beta T-cell receptor recognizes polymorphic residues in the alpha 1/alpha 2 domains of the class I molecule, the CD8 molecule is believed to recognize monomorphic class I residues. Our previous experiments suggested that residue 227 in the alpha 3 domain of major histocompatibility complex class I molecules contributes to the determinant recognized by CD8. By using a panel of site-directed mutants of H-2Dd, this observation has been extended herein. Our findings indicate that for recognition by CD8-dependent CTLs, residue 227 must be either glutamic acid or aspartic acid and cannot be either basic or uncharged. However, the recognition by CD8-independent CTLs is unaffected by any of the substitutions at position 227 of H-2Dd. Similarly, alterations of other charged residues at positions 222, 223, and 229 have an analogous effect to substitution at residue 227, whereas substitutions at residues 192 and 232 do not affect the reactivity of CD8-dependent or CD8-independent CTLs. In addition, mutant H-2Dd molecules that are not recognized by CD8-dependent CTLs are unable to stimulate a primary CTL response, yet they can stimulate a secondary CD8-independent H-2Dd-specific CTL response. These findings suggest that CD8 recognition is obligatory for the priming of class I-dependent CTL responses. Since endogenous class I molecules were expressed by all of the transfected cell lines, these findings provide direct genetic evidence that CD8 and the alpha beta T-cell receptor must interact with the same class I molecule.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ajitkumar P., Geier S. S., Kesari K. V., Borriello F., Nakagawa M., Bluestone J. A., Saper M. A., Wiley D. C., Nathenson S. G. Evidence that multiple residues on both the alpha-helices of the class I MHC molecule are simultaneously recognized by the T cell receptor. Cell. 1988 Jul 1;54(1):47–56. doi: 10.1016/0092-8674(88)90178-x. [DOI] [PubMed] [Google Scholar]
- Allen H., Wraith D., Pala P., Askonas B., Flavell R. A. Domain interactions of H-2 class I antigens alter cytotoxic T-cell recognition sites. Nature. 1984 May 17;309(5965):279–281. doi: 10.1038/309279a0. [DOI] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. Structure of the human class I histocompatibility antigen, HLA-A2. Nature. 1987 Oct 8;329(6139):506–512. doi: 10.1038/329506a0. [DOI] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. The foreign antigen binding site and T cell recognition regions of class I histocompatibility antigens. Nature. 1987 Oct 8;329(6139):512–518. doi: 10.1038/329512a0. [DOI] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly J. M., Potter T. A., Wormstall E. M., Hansen T. H. The Lyt-2 molecule recognizes residues in the class I alpha 3 domain in allogeneic cytotoxic T cell responses. J Exp Med. 1988 Jul 1;168(1):325–341. doi: 10.1084/jem.168.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dembić Z., Haas W., Zamoyska R., Parnes J., Steinmetz M., von Boehmer H. Transfection of the CD8 gene enhances T-cell recognition. Nature. 1987 Apr 2;326(6112):510–511. doi: 10.1038/326510a0. [DOI] [PubMed] [Google Scholar]
- Doyle C., Strominger J. L. Interaction between CD4 and class II MHC molecules mediates cell adhesion. Nature. 1987 Nov 19;330(6145):256–259. doi: 10.1038/330256a0. [DOI] [PubMed] [Google Scholar]
- Emmrich F., Strittmatter U., Eichmann K. Synergism in the activation of human CD8 T cells by cross-linking the T-cell receptor complex with the CD8 differentiation antigen. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8298–8302. doi: 10.1073/pnas.83.21.8298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans G. A., Margulies D. H., Shykind B., Seidman J. G., Ozato K. Exon shuffling: mapping polymorphic determinants on hybrid mouse transplantation antigens. Nature. 1982 Dec 23;300(5894):755–757. doi: 10.1038/300755a0. [DOI] [PubMed] [Google Scholar]
- Fowlkes B. J., Schwartz R. H., Pardoll D. M. Deletion of self-reactive thymocytes occurs at a CD4+8+ precursor stage. Nature. 1988 Aug 18;334(6183):620–623. doi: 10.1038/334620a0. [DOI] [PubMed] [Google Scholar]
- Gabert J., Langlet C., Zamoyska R., Parnes J. R., Schmitt-Verhulst A. M., Malissen B. Reconstitution of MHC class I specificity by transfer of the T cell receptor and Lyt-2 genes. Cell. 1987 Aug 14;50(4):545–554. doi: 10.1016/0092-8674(87)90027-4. [DOI] [PubMed] [Google Scholar]
- Janeway C. A., Jr, Rojo J., Saizawa K., Dianzani U., Portoles P., Tite J., Haque S., Jones B. The co-receptor function of murine CD4. Immunol Rev. 1989 Jun;109:77–92. doi: 10.1111/j.1600-065x.1989.tb00020.x. [DOI] [PubMed] [Google Scholar]
- Kupfer A., Singer S. J., Janeway C. A., Jr, Swain S. L. Coclustering of CD4 (L3T4) molecule with the T-cell receptor is induced by specific direct interaction of helper T cells and antigen-presenting cells. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5888–5892. doi: 10.1073/pnas.84.16.5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., Rouse R. V., Micklem H. S., Herzenberg L. A. T cell subsets defined by expression of Lyt-1,2,3 and Thy-1 antigens. Two-parameter immunofluorescence and cytotoxicity analysis with monoclonal antibodies modifies current views. J Exp Med. 1980 Aug 1;152(2):280–295. doi: 10.1084/jem.152.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald H. R., Glasebrook A. L., Bron C., Kelso A., Cerottini J. C. Clonal heterogeneity in the functional requirement for Lyt-2/3 molecules on cytolytic T lymphocytes (CTL): possible implications for the affinity of CTL antigen receptors. Immunol Rev. 1982;68:89–115. doi: 10.1111/j.1600-065x.1982.tb01061.x. [DOI] [PubMed] [Google Scholar]
- MacDonald H. R., Hengartner H., Pedrazzini T. Intrathymic deletion of self-reactive cells prevented by neonatal anti-CD4 antibody treatment. Nature. 1988 Sep 8;335(6186):174–176. doi: 10.1038/335174a0. [DOI] [PubMed] [Google Scholar]
- Maziarz R. T., Burakoff S. J., Bluestone J. A. The alpha-3 domain of class I MHC proteins influences cytotoxic T lymphocyte recognition of antigenic determinants located within the alpha-1 and alpha-2 domains. J Immunol. 1988 Jun 15;140(12):4372–4377. [PubMed] [Google Scholar]
- Nakayama E., Shiku H., Stockert E., Oettgen H. F., Old L. J. Cytotoxic T cells: Lyt phenotype and blocking of killing activity by Lyt antisera. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1977–1981. doi: 10.1073/pnas.76.4.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norment A. M., Salter R. D., Parham P., Engelhard V. H., Littman D. R. Cell-cell adhesion mediated by CD8 and MHC class I molecules. Nature. 1988 Nov 3;336(6194):79–81. doi: 10.1038/336079a0. [DOI] [PubMed] [Google Scholar]
- Ozato K., Evans G. A., Shykind B., Margulies D. H., Seidman J. G. Hybrid H-2 histocompatibility gene products assign domains recognized by alloreactive T cells. Proc Natl Acad Sci U S A. 1983 Apr;80(7):2040–2043. doi: 10.1073/pnas.80.7.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozato K., Mayer N. M., Sachs D. H. Monoclonal antibodies to mouse major histocompatibility complex antigens. Transplantation. 1982 Sep;34(3):113–120. doi: 10.1097/00007890-198209000-00001. [DOI] [PubMed] [Google Scholar]
- Potter T. A., Bluestone J. A., Rajan T. V. A single amino acid substitution in the alpha 3 domain of an H-2 class I molecule abrogates reactivity with CTL. J Exp Med. 1987 Oct 1;166(4):956–966. doi: 10.1084/jem.166.4.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter T. A., Rajan T. V., Dick R. F., 2nd, Bluestone J. A. Substitution at residue 227 of H-2 class I molecules abrogates recognition by CD8-dependent, but not CD8-independent, cytotoxic T lymphocytes. Nature. 1989 Jan 5;337(6202):73–75. doi: 10.1038/337073a0. [DOI] [PubMed] [Google Scholar]
- Reiss C. S., Evans G. A., Margulies D. H., Seidman J. G., Burakoff S. J. Allospecific and virus-specific cytolytic T lymphocytes are restricted to the N or C1 domain of H-2 antigens expressed on L cells after DNA-mediated gene transfer. Proc Natl Acad Sci U S A. 1983 May;80(9):2709–2712. doi: 10.1073/pnas.80.9.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstein Y., Ratnofsky S., Burakoff S. J., Herrmann S. H. Direct evidence for binding of CD8 to HLA class I antigens. J Exp Med. 1989 Jan 1;169(1):149–160. doi: 10.1084/jem.169.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd C. E., Trevillyan J. M., Dasgupta J. D., Wong L. L., Schlossman S. F. The CD4 receptor is complexed in detergent lysates to a protein-tyrosine kinase (pp58) from human T lymphocytes. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5190–5194. doi: 10.1073/pnas.85.14.5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter R. D., Norment A. M., Chen B. P., Clayberger C., Krensky A. M., Littman D. R., Parham P. Polymorphism in the alpha 3 domain of HLA-A molecules affects binding to CD8. Nature. 1989 Mar 23;338(6213):345–347. doi: 10.1038/338345a0. [DOI] [PubMed] [Google Scholar]
- Sha W. C., Nelson C. A., Newberry R. D., Kranz D. M., Russell J. H., Loh D. Y. Positive and negative selection of an antigen receptor on T cells in transgenic mice. Nature. 1988 Nov 3;336(6194):73–76. doi: 10.1038/336073a0. [DOI] [PubMed] [Google Scholar]
- Shinohara N., Sachs D. H. Mouse alloantibodies capable of blocking cytotoxic T-cell function. I. Relationship between the antigen reactive with blocking antibodies and the Lyt-2 locus. J Exp Med. 1979 Sep 19;150(3):432–444. doi: 10.1084/jem.150.3.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teh H. S., Kisielow P., Scott B., Kishi H., Uematsu Y., Blüthmann H., von Boehmer H. Thymic major histocompatibility complex antigens and the alpha beta T-cell receptor determine the CD4/CD8 phenotype of T cells. Nature. 1988 Sep 15;335(6187):229–233. doi: 10.1038/335229a0. [DOI] [PubMed] [Google Scholar]
- Veillette A., Bookman M. A., Horak E. M., Samelson L. E., Bolen J. B. Signal transduction through the CD4 receptor involves the activation of the internal membrane tyrosine-protein kinase p56lck. Nature. 1989 Mar 16;338(6212):257–259. doi: 10.1038/338257a0. [DOI] [PubMed] [Google Scholar]



