Abstract
Advances in prenatal diagnosis, perioperative management, and postoperative care have dramatically increased the population of survivors of neonatal and infant heart surgery. The high survival rate of these patients into adulthood has exposed the alarming prevalence of long-term neuropsychological and psychiatric morbidities. Dextro-transposition of the great arteries (d-TGA) is one of the most extensively studied cyanotic congenital heart defect (CHD) with regard to neurodevelopmental outcomes. Landmark studies have described a common neurodevelopmental and behavioral phenotype associated with d-TGA. Children with d-TGA display impairments in key neurocognitive areas, including visual–spatial and fine motor abilities, executive functioning, processing speed, and social cognition. As they grow older, they may face additional challenges with a worsening of deficits in higher order cognitive skills, problems in psychosocial adjustment and a higher-than-expected rate of psychiatric disorders, such as attention-deficit hyperactivity disorder, depression, and anxiety. The aim of this review is to summarize the available recent data on neuropsychological and psychiatric outcomes in individuals with d-TGA after the arterial switch operation. We present findings within a life-span perspective, with a particular emphasis on the emerging literature on adolescent and young adult outcomes. Finally, we propose avenues for future research in the CHD adult neuropsychology field. Among these avenues, we explore the potential mechanisms by which pediatric neurodevelopmental impairments may have lifelong adverse effects as well as alternative interventions that could optimize outcomes.
Keywords: dextro-transposition of the great arteries, neuropsychological outcomes, psychiatric disorders, cognitive, executive function, open-heart surgery
Introduction
Dextro-transposition of the great arteries (d-TGA) accounts for 5–7% of congenital heart defects (CHD), with a prevalence of 0.2 per 1,000 live births (1, 2). Individuals with d-TGA represent a unique and relatively homogeneous study cohort with the Arterial Switch Operation (ASO) being now the standard-of-care. Moreover, d-TGA is infrequently associated with extra-cardiac anomalies, including genetic abnormalities, reducing potential confounding variables in follow-up studies. Since the first successful ASO in 1975 (3), survival rates have significantly increased, resulting in a demographic shift: adults now outnumber children living with d-TGA (4, 5). Long-term outcomes in d-TGA, and in CHD population as a whole, pose a public health challenge for screening, diagnosis, and treatment. The aim of this state-of-the-art review is to integrate recent data on neuropsychological and psychiatric outcomes in d-TGA after the ASO within a life-span perspective. Finally, we propose avenues for future research, including discussion on the potential mechanisms of long-term impairments and interventions to optimize outcomes.
Neuropsychological Outcomes in Children with d-TGA
Much of our knowledge on the neuropsychological profile of children with CHD comes from decades of study of survivors of d-TGA. Although the prevalence of severe neurological disorders is very low in this population (6), neurodevelopmental impairments are consistently reported (7). Early development is characterized by mild to moderate delays in cognitive, motor, and language function, with scores on the Bayley Scales of Infant Development (BSID) 0.5–1 SD below the expected mean values (8–11). The Boston Circulatory Arrest Study (BCAS) offers one of the most comprehensive analyses of the neuropsychological phenotype of these patients (9, 12–14). This longitudinal prospective study followed-up children from infancy to adolescence. At 12 months, 20% of infants achieved a psychomotor score ≤80 on the BSID and were less vocal than expected, suggesting delays in expressive language development (9). Recent findings reported an improvement on early outcomes for infants with d-TGA. Andropoulos et al. (15) reported mean Cognitive scores on the BSID within the normal population range, although Language and Motor scores were 7–10 points lower than the expected means. Although evaluations in infancy are important for early interventions, they are not strongly predictive of long-term scores (16), which may lead to false negatives.
Several studies have characterized the cognitive outcomes of children with d-TGA. It has become clear that intelligence abilities, as measured by Full-scale IQ scores, are generally within the normal range (6, 17). Nevertheless, deficits are often apparent in specific neurocognitive areas. Speech and motor impairments were reported in both European (17, 18) and North-American studies (13, 19). Bellinger et al. (12, 13) reported below age-expected scores for 4- and 8-year-old children in visual–spatial skills, academic achievement, working memory, hypothesis generation, sustained attention, and higher order language skills. In general, lower-level skills were relatively intact, but children had difficulty integrating or coordinating these skills to achieve higher order goals (13).
There is growing awareness that executive functioning is particularly vulnerable in d-TGA (20–22). In the BCAS, 8-year children had substantial difficulties in metacognitive aspects of behavior, such as planning, organizational skills, and cognitive flexibility (13). Impairments were evident on both verbal and non-verbal tasks, with children tending to focus on isolated details at the expense of a coherent organization of elements (13, 23). Calderon et al. (24, 25) corroborated these findings in two cohorts of 5- and 7-year olds with d-TGA. In these studies, children had difficulties elaborating a strategy to achieve a goal, i.e., anticipating the right number of actions to reproduce a visual model. They also had deficits in attentional control and inhibition of automatic tendencies, as well as lower working memory. Executive functioning issues start early in preschool years. Calderon et al. (25) demonstrated that executive function impairments were common at the age of 5, in tasks measuring behavioral control, attention, working memory, and cognitive flexibility. Executive function deficits were also reported in children with other types of complex CHD (26, 27), suggesting that they are part of the “developmental signature” of critical CHD.
Recently, deficits in social cognition were described in children with d-TGA (24, 28, 29) manifested by difficulties to interpret social stimuli and situations, e.g., facial emotional expressions, self- and other’s intentions. A significant proportion had difficulties in identifying the emotional and cognitive states of others (Theory of Mind deficits).
In sum, the cognitive and behavioral challenges faced by children with d-TGA place them at high risk of long-term learning disabilities and academic under-achievement (22). Indeed, nearly 50% requires early remedial services (e.g., psychotherapy, speech therapy, or educational support) (30).
Adolescents with d-TGA: Cognitive and Psychiatric Outcomes
Cognitive Outcomes
To our knowledge, only two studies focused on the cognitive outcomes of adolescents with d-TGA corrected by ASO (14, 31). In the BCAS, 139 adolescents with d-TGA (16.1 ± 0.5 years old) were evaluated (14). Patients’ mean scores were lower than the expected population means on tests assessing academic skills, visuo-spatial skills, long-term memory, executive functions, and social cognition. Frequencies of scores ≥1 SD or ≥2 SD below the normative mean were, respectively, 26 and 7% for academic skills composite, 35 and 17% for memory index, and 54 and 20% for visuo-spatial index (compared to the expected frequencies of 16 and 2%, respectively). By parent report, about one in five patients had attention or executive impairments in daily life. In the Aachen Study (17, 31, 32), 60 patients who underwent the ASO were followed-up from preschool to adolescence (16.9 ± 1.7 years old). Adolescents’ IQ scores were not reduced compared to the population norms, but the frequency of IQ scores ≥2 SD below the expected mean was increased, especially for Performance IQ (11%) (31).
Some study cohorts have included adolescents with d-TGA as well as other types of CHD (33–37), but investigations directly comparing the d-TGA and other CHD groups are scarce. In the study of Matos et al. (34), adolescents with cyanotic CHD scored lower than adolescents with acyanotic CHD on all cognitive domains assessed, although the difference was significant only for visuo-constructive abilities. Cassidy et al. (33) evaluated executive function in 352 adolescents with cyanotic CHD (d-TGA, TOF or single-ventricle anatomy requiring Fontan procedure) and 111 controls. d-TGA, TOF, and Fontan groups had lower performances than controls on cognitive flexibility and verbally mediated executive function skills. Only visuo-spatially mediated skills were higher in the d-TGA group compared to the other groups with CHD. Despite the relative preservation of these abilities in the d-TGA group, impairment (score ≥ 1.5 SD below the expected mean) was twice as frequent when compared to controls.
In sum, we observe a continuum between cognitive impairments observed in children and those detected in adolescents with d-TGA. These issues, which may increase with age (13, 14, 25), involve diverse domains but mostly attention, visuo-spatial abilities, and executive functions.
Psychosocial and Psychiatric Outcomes
Adolescence is a vulnerable time, when a variety of psychosocial and psychiatric problems emerge (38). Successful transition from childhood to adolescence depends on the development of effective self-management skills and a sense of autonomy (7). For individuals with CHD, it is also an opportunity for intervention before transitioning to adult health care (39). This is important, as mental health problems can predict future adjustment difficulties, such as unemployment, risk-taking behaviors, substance abuse, and suicidality (40). Although many adolescents with d-TGA do not have residual cardiac morbidities or health chronic conditions, they may still be at risk of poor psychosocial outcomes and mental health problems. Several studies identified increased incidence of internalizing problems (i.e., anxiety, somatic complaints, depressive symptoms) in adolescents with complex CHD when compared to general population (40–43). Externalizing problems were also consistently reported, on both parent and self-report measures (41, 44). However, because these studies typically include mixed types of CHD, it is difficult to draw inferences about the risk among individuals with d-TGA.
Few studies reported specifically on adolescents with d-TGA. Hövels-Gürich et al. (42) and Karl et al. (19) showed an increased risk of parent-reported psychosocial maladjustment in children and adolescents with d-TGA. In the BCAS, 16-year-olds with d-TGA were more likely than controls (35 versus 20%) to meet criteria for a lifetime psychiatric diagnosis, as evaluated by the Schedule for Affective Disorders and Schizophrenia for School-aged Children (K-SADS) (45). They also had a greater proportion with Attention Deficit Hyperactivity Disorder (ADHD), both lifetime (19 versus 7% for referents) and current (16 versus 3% for referents). However, the frequencies of mood or anxiety disorders did not differ between groups. On the Children’s Global Assessment Scale, which assesses psychosocial functioning in different life-settings, adolescents with d-TGA obtained poorer scores, and 15% fell within the range of pathological functioning. Parent- and self-reports of psychiatric symptoms in the d-TGA group also identified more depressive, anxiety, and post-traumatic stress symptoms. Impaired cognitive functioning and greater parental stress at 8 years were the strongest predictors of poor psychosocial and psychiatric outcomes at 16 years. Of note, the prevalence of psychiatric disorders in d-TGA is lower compared to other forms of critical CHD such as single-ventricle physiology (46). However, the rates of mental health disorders in d-TGA are much higher than those reported in the national US population (47).
Finally, neuropsychological and mental health morbidities can impede successful transition from pediatric to adult health care (7). Studies showed that adolescents with CHD struggle to successfully transit to adult care and assume control of their health-care management (48–50). Focused psychosocial care, including strategies for managing health and coping with medical decision-making, should be a key facet of the transition process (51). According to the American Heart Association’s transition guidelines (39), a standard core educational curriculum should include topics related to lifestyle issues including learning disabilities, anxiety, depression, and high-risk behaviors. The transition process must also include facilitated access to mental health services (50).
Adults with d-TGA: Emerging Evidence
Despite the increased number of adults with d-TGA after the ASO (52), studies on their cognitive or psychological outcomes are infrequent. Thus, to our knowledge, no studies have focused specifically on the cognitive outcomes of adults who underwent the ASO. Two recent studies (53, 54) investigated the neuropsychological outcomes of adults with CHD, including d-TGA. However, in these cohorts, most of the adults with d-TGA had not undergone the ASO but the atrial switch procedure (operation often conducted in this population before the development of the ASO). In the study of Klouda et al. (53), adults with critical CHD (i.e., d-TGA or Fontan, n = 24, 32.8 ± 7.6 years old) had lower scores than expected in multiple domains: psychomotor speed, processing speed, sustained and executive attention, and on the overall, neurocognitive index. Tyagi et al. (54) observed that d-TGA adults (n = 80, 19–50 years old) scored worse than those with mild CHD (n = 84) on an overall neuropsychological index. Furthermore, proportions with cognitive impairments on at least three tests were higher in the d-TGA group (49%) compared to the mild CHD group (26%). Most of the impairments observed in CHD groups involved divided attention, executive functions, and fine motor function.
To our knowledge, no studies have investigated psychiatric risks specifically in adults who underwent the ASO. Two studies evaluated the psychological outcomes of adults who underwent the atrial switch procedure (55, 56), finding that approximately 20% met criteria for a psychiatric disorder. Studies comparing results between cardiac diagnoses showed that psychological outcomes were worse among adults with cyanotic or complex CHD than among adults with acyanotic or mild CHD (41, 57, 58). Findings from mixed cohorts of adults with CHD show high rates of psychiatric disorders (59–62), with about 30% meeting diagnostic criteria for at least one lifetime mood disorder (i.e., major depressive disorder, bipolar disorder, etc.), and 26–28% for at least one anxiety disorder (i.e., generalized anxiety, social phobia, etc.) (61, 62). Findings on the frequency and severity of current anxiety-depressive symptoms assessed by self-report are mixed. Some studies found high symptomatology in adults with CHD (59, 61, 63–65), whereas others did not (66–72). According to several authors (60, 71–74), denial mechanisms or coping strategies are frequently used by CHD patients and could contribute to the favorable psychological outcomes sometimes suggested by self-assessments.
Many CHD patients with psychiatric disorders appear not to receive adequate treatment. Kovacs et al. (61) found that 69% of patients with a mood or anxiety disorder did not receive psychotherapy or psychotropic drugs. Other studies report that only 11–12% of patients receive psychological counseling, despite high rates of anxiety-depressive syndromes (62, 64). In adults with CHD, presence of a high depressive or anxiety symptomatology is associated with higher rates of unemployment (64), lower quality of life (QoL) (62, 64, 68), greater consumption of tobacco and alcohol (75), poorer adherence to treatment (76), and worse cardiac prognosis (65).
In summary, to date, few data are available on the cognitive and psychiatric outcomes of d-TGA adults after the ASO. Elevated risk of attentional and executive impairments and of mood and anxiety disorders may be prevalent. More research is needed to better understand the long-term cognitive and psychological trajectory of these adults and to investigate how cognitive and psychiatric disorders influence social interactions, employability, and achievement.
Futures Avenues
Potential Mechanisms for Long-term Impairment
The mechanisms for lifelong cognitive and psychosocial difficulties in CHD are multifactorial. Early cognitive deficits play a decisive role (7). In children with d-TGA, lower full-scale IQ is associated with lower parent-reported psychosocial QoL (77). Moreover, as reported in other pediatric populations, such as children born preterm (78), a cascade of effects may be observed, where early deficits mediate the expression of new symptoms and/or the worsening of pre-existing impairments. In children with d-TGA, Calderon et al. (25) showed that deficits on some aspects of executive functioning (i.e., cognitive flexibility) became worse between the ages of 5 and 7, whereas more basic processes (e.g., motor control) tended to catch-up. Interestingly, Cassidy et al. (22) reported that reading and math difficulties of adolescents with d-TGA at age 16 were predicted by deficits in processing speed and executive function at age 8. Indeed, executive function deficits and difficulties in other key areas of neurodevelopment are often comorbid. In d-TGA, poor working memory adversely impacted children’s language comprehension and mathematic skills (13) and poor inhibitory control was associated with deficits in social cognition (24). These weaknesses might result in more severe dysfunction as expectations increase with age. Patients with these cognitive challenges may find establishing and maintaining social relationships difficult, especially in adolescence and adulthood (20). Peer interactions and social cues (e.g., body language, irony, sarcasm) become more complex, making decoding them difficult to individuals with d-TGA. These and other cognitive deficits may “derail” the developmental trajectory in the mental health domain. Of note, executive function deficits increase the risk of psychosocial and psychiatric morbidities in adolescents with d-TGA. In BCAS, worse psychosocial outcomes and poorer QoL of 16-year olds were strongly predicted by executive dysfunction, suggesting a robust association between these higher order processes and self-perception and psychosocial adaptation (45).
Finally, it is possible that accumulating cognitive deficits, particularly in organization, flexibility, and self-control, along with poor social interactions trigger a lower threshold for stress, predisposing individuals to anxiety and depression. This hypothesis is, however, speculative and more research is needed.
Interventions to Improve Outcomes
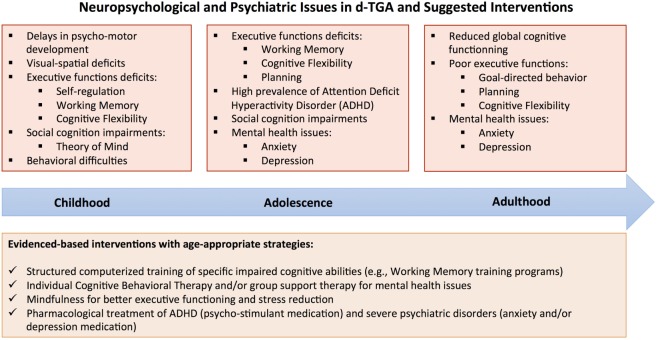
As a result of progress in understanding the challenges patients with CHD face, interventions to prevent, mitigate, or palliate morbidities are emerging (Figure 1). Many hospital-based Cardiac Neurodevelopmental Programs are providing early screening and treatment recommendations. Nevertheless, there is urgent need for well-designed randomized controlled trials evaluating the efficacy of neurocognitive and psychological interventions in this population. McCusker et al. (79) conducted a study evaluating the efficacy of interventions focused on maternal and family functioning, individualized psycho-education, and outreach to community health-care providers. Although maternal mental health and family functioning improved, few benefits were seen on children’s behavioral outcomes and school achievement.
Figure 1.
Neuropsychological and psychiatric issues in dextro-transposition of the great arteries (d-TGA) by age group and suggested interventions.
One major concern in the d-TGA population relates to executive dysfunction, and thus, a proof-of-concept for evidence-based interventions is needed (20). Many behavioral interventions may be implemented from early childhood and throughout the lifespan. Intensive computerized training targeting attention and working memory has improved executive skills and behavioral outcomes in children with ADHD (80) and children born with low birth weight (81). If these programs prove effective, reduction of executive function morbidities could reduce the likelihood that psychiatric disorders emerge (82, 83). Research is ongoing to determine the short- and long-term effects of such structured interventions in youth with CHD.
As recommended by several Associations (39, 84), many transitional and educational programs for patients with CHD are being developed. It may be useful to integrate psychologists into these medico-social programs. Their intervention, within the framework of individual or group support, could prevent subclinical disorders from evolving into depression or anxiety disorders. These interventions could focus on the emotional expression and management, and the development of effective coping strategies. Interventions aiming to optimize the “sense of coherence” (85), that is, the understanding of events, the confidence in one’s power and resources, and the ability to give sense to events, could also improve the psychosocial outcomes of patients with CHD.
Finally, several mental health treatments, including pharmacotherapy and psychotherapy, are available and could be tested in this population. Cognitive behavioral therapy, targeting the modification of maladaptive thought patterns and behaviors, has proven to be effective in treating anxiety and depression disorders (86). Mindfulness and relaxation techniques may also be beneficial. Mindfulness training is associated with substantial reduction in stress-related (82), depressive (83) and ADHD symptoms (87).
Conclusion
Over the last two decades, dramatic progress was made in the understanding of neuropsychological and psychiatric outcomes of individuals with d-TGA (for a summary of main studies, please refer to Table 1). The cardiac neurodevelopmental and mental health field is moving from a descriptive approach of challenges to an in-depth understanding of neurobiological and psychological mechanisms of impairment. Novel hypotheses will be critical to improve outcomes and QoL for individuals with d-TGA.
Table 1.
Overview of selected studies on neuropsychological and psychiatric outcomes for patients with d-TGA.
| Reference | n, age (years), diagnosis | Neurocognitive or psychiatric assessment | Main results |
|---|---|---|---|
| Children | |||
| Bellinger et al. (12) | n = 158, 4 years, d-TGA |
|
|
| Bellinger et al. (13) | n = 155, 8 years, d-TGA |
|
|
| Calderon et al. (24) | n = 21, 7 years, d-TGA |
|
|
| Calderon et al. (25) | n = 45, 5/7 years, d-TGA |
|
|
| Freed et al. (8) | n = 82, 1.5–2 years, d-TGA | BSID II |
|
| Hicks et al. (11) | n = 91, 2 years, d-TGA | BSID III |
|
| Hövels-Gürich et al. (32) | n = 77, 3–9 years, d-TGA |
|
|
| Hövels-Gürich et al. (17) | n = 60, 7–14 years, d-TGA |
|
|
| Hövels-Gürich et al. (42) | n = 60, 7–14 years, d-TGA | Achenbach child behavior checklist |
|
| Karl et al. (19) | n = 74, 4–14 years, d-TGA |
|
|
| McGrath et al. (16) | n = 135, 1/8 years, d-TGA |
Evaluation at 1 year
Evaluation at 8 years
|
|
| Adolescents | |||
| Bellinger et al. (14) | n = 139, 16 years, d-TGA |
|
|
| Cassidy et al. (22) | n = 139, 8/16 years, d-TGA |
|
|
| DeMaso et al. (45) | n = 139, 16 years, d-TGA |
|
|
| Heinrichs et al. (31) | n = 60, 14–21 years, d-TGA |
|
|
| Adults | |||
| Klouda et al. (53) | Total mixed cohort, n = 48, 18–49 years
|
CNS vital signs |
|
| Tyagi et al. (54) | Total mixed cohort, n = 310, 18–76 years
|
|
|
| van Rijen et al. (56) | Total mixed cohort, n = 349, 20–46 years,
|
|
|
BSID, Bayley Scales of Infant Development; CHD, congenital heart defects; d-TGA, dextro-transposition of the great arteries; IQ, intelligence quotient; K-ABC, Kaufman assessment battery for children; SV, single ventricle; TOF, tetralogy of Fallot; WIAT, Wechsler individual achievement test; WISC, Wechsler intelligence scale for children; WPPSI, Wechsler preschool and primary scale of intelligence.
Author Contributions
LK and JC conducted the literature search, drafted this manuscript, and revised it critically for important intellectual content. DB, MM, DK, NG and DCB made substantial contributions to the conception of this review and revised it critically for important intellectual content. All the authors (LK, DB, MM, DK, NG, DCB, and JC) approved the final version of the manuscript and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer MN declared a shared affiliation, though no other collaboration, with the authors DB & JC to the handling Editor, who ensured that the process nevertheless met the standards of a fair and objective review.
Funding
This work was supported by the Association pour la Recherche en Cardiologie du Foetus à l’Adulte (ARCFA).
References
- 1.Samánek M. Congenital heart malformations: prevalence, severity, survival, and quality of life. Cardiol Young (2000) 10(3):179–85. 10.1017/S1047951100009082 [DOI] [PubMed] [Google Scholar]
- 2.Long J, Ramadhani T, Mitchell LE. Epidemiology of nonsyndromic conotruncal heart defects in Texas, 1999-2004. Birth Defects Res A Clin Mol Teratol (2010) 88(11):971–9. 10.1002/bdra.20724 [DOI] [PubMed] [Google Scholar]
- 3.Jatene AD, Fontes VF, Paulista PP, Souza LC, Neger F, Galantier M, et al. Anatomic correction of transposition of the great vessels. J Thorac Cardiovasc Surg (1976) 72(3):364–70. [PubMed] [Google Scholar]
- 4.Warnes CA, Liberthson R, Danielson J, Gordon K, Dore A, Harris L, et al. Task Force 1: the changing profile of congenital heart disease in adult life. J Am Coll Cardiol (2001) 37(5):1170–5. 10.1016/S0735-1097(01)01272-4 [DOI] [PubMed] [Google Scholar]
- 5.Marelli AJ, Mackie AS, Ionescu-Ittu R, Rahme E, Pilote L. Congenital heart disease in the general population changing prevalence and age distribution. Circulation (2007) 115(2):163–72. 10.1161/CIRCULATIONAHA.106.627224 [DOI] [PubMed] [Google Scholar]
- 6.Neufeld RE, Clark BG, Robertson CMT, Moddemann DM, Dinu IA, Joffe AR, et al. Five-year neurocognitive and health outcomes after the neonatal arterial switch operation. J Thorac Cardiovasc Surg (2008) 136(6):1413–21. 10.1016/j.jtcvs.2008.05.011 [DOI] [PubMed] [Google Scholar]
- 7.Marino BS, Lipkin PH, Newburger JW, Peacock G, Gerdes M, Gaynor JW, et al. Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management: a scientific statement from the American Heart Association. Circulation (2012) 126(9):1143–72. 10.1161/CIR.0b013e318265ee8a [DOI] [PubMed] [Google Scholar]
- 8.Freed DH, Robertson CMT, Sauve RS, Joffe AR, Rebeyka IM, Ross DB, et al. Intermediate-term outcomes of the arterial switch operation for transposition of great arteries in neonates: alive but well? J Thorac Cardiovasc Surg (2006) 132(4):845.e–52.e. 10.1016/j.jtcvs.2006.05.046 [DOI] [PubMed] [Google Scholar]
- 9.Bellinger DC, Jonas RA, Rappaport LA, Wypij D, Wernovsky G, Kuban KC, et al. Developmental and neurologic status of children after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. N Engl J Med (1995) 332(9):549–55. 10.1056/NEJM199503023320901 [DOI] [PubMed] [Google Scholar]
- 10.Park IS, Yoon SY, Min JY, Kim YH, Ko JK, Kim KS, et al. Metabolic alterations and neurodevelopmental outcome of infants with transposition of the great arteries. Pediatr Cardiol (2006) 27(5):569–76. 10.1007/s00246-004-0730-5 [DOI] [PubMed] [Google Scholar]
- 11.Hicks MS, Sauve RS, Robertson CMT, Joffe AR, Alton G, Creighton D, et al. Early childhood language outcomes after arterial switch operation: a prospective cohort study. Springerplus (2016) 5(1):1681. 10.1186/s40064-016-3344-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bellinger DC, Wypij D, Kuban KCK, Rappaport LA, Hickey PR, Wernovsky G, et al. Developmental and neurological status of children at 4 years of age after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. Circulation (1999) 100(5):526–32. 10.1161/01.CIR.100.5.526 [DOI] [PubMed] [Google Scholar]
- 13.Bellinger DC, Wypij D, duPlessis AJ, Rappaport LA, Jonas RA, Wernovsky G, et al. Neurodevelopmental status at eight years in children with dextro-transposition of the great arteries: The Boston Circulatory Arrest Trial. J Thorac Cardiovasc Surg (2003) 126(5):1385–96. 10.1016/S0022-5223(03)00711-6 [DOI] [PubMed] [Google Scholar]
- 14.Bellinger DC, Wypij D, Rivkin MJ, DeMaso DR, Robertson RL, Jr, Dunbar-Masterson C, et al. Adolescents with d-transposition of the great arteries corrected with the arterial switch procedure: neuropsychological assessment and structural brain imaging. Circulation (2011) 124(12):1361–9. 10.1161/CIRCULATIONAHA.111.026963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andropoulos DB, Easley RB, Brady K, McKenzie ED, Heinle JS, Dickerson HA, et al. Changing expectations for neurological outcomes after the neonatal arterial switch operation. Ann Thorac Surg (2012) 94(4):1250–6. 10.1016/j.athoracsur.2012.04.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGrath E, Wypij D, Rappaport LA, Newburger JW, Bellinger DC. Prediction of IQ and achievement at age 8 years from neurodevelopmental status at age 1 year in children with D-transposition of the great arteries. Pediatrics (2004) 114(5):e572–6. 10.1542/peds.2003-0983-L [DOI] [PubMed] [Google Scholar]
- 17.Hövels-Gürich HH, Seghaye M-C, Schnitker R, Wiesner M, Huber W, Minkenberg R, et al. Long-term neurodevelopmental outcomes in school-aged children after neonatal arterial switch operation. J Thorac Cardiovasc Surg (2002) 124(3):448–58. 10.1067/mtc.2002.122307 [DOI] [PubMed] [Google Scholar]
- 18.Hövels-Gürich HH, Seghaye M-C, Sigler M, Kotlarek F, Bartl A, Neuser J, et al. Neurodevelopmental outcome related to cerebral risk factors in children after neonatal arterial switch operation. Ann Thorac Surg (2001) 71(3):881–8. 10.1016/S0003-4975(00)02515-7 [DOI] [PubMed] [Google Scholar]
- 19.Karl TR, Hall S, Ford G, Kelly EA, Brizard CP, Mee RB, et al. Arterial switch with full-flow cardiopulmonary bypass and limited circulatory arrest: neurodevelopmental outcome. J Thorac Cardiovasc Surg (2004) 127(1):213–22. 10.1016/j.jtcvs.2003.06.001 [DOI] [PubMed] [Google Scholar]
- 20.Calderon J, Bellinger DC. Executive function deficits in congenital heart disease: why is intervention important? Cardiol Young (2015) 25(7):1238–46. 10.1017/S1047951115001134 [DOI] [PubMed] [Google Scholar]
- 21.Calderon J. Executive function in patients with congenital heart disease: only the tip of the iceberg? J Pediatr (2016) 173:7–9. 10.1016/j.jpeds.2016.02.066 [DOI] [PubMed] [Google Scholar]
- 22.Cassidy AR, White MT, DeMaso DR, Newburger JW, Bellinger DC. Processing speed, executive function, and academic achievement in children with dextro-transposition of the great arteries: testing a longitudinal developmental cascade model. Neuropsychology (2016) 30(7):874–85. 10.1037/neu0000289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bellinger DC, Bernstein JH, Kirkwood MW, Rappaport LA, Newburger JW. Visual-spatial skills in children after open-heart surgery. J Dev Behav Pediatr (2003) 24(3):169–79. 10.1097/00004703-200306000-00007 [DOI] [PubMed] [Google Scholar]
- 24.Calderon J, Bonnet D, Courtin C, Concordet S, Plumet M-H, Angeard N. Executive function and theory of mind in school-aged children after neonatal corrective cardiac surgery for transposition of the great arteries. Dev Med Child Neurol (2010) 52(12):1139–44. 10.1111/j.1469-8749.2010.03735.x [DOI] [PubMed] [Google Scholar]
- 25.Calderon J, Jambaqué I, Bonnet D, Angeard N. Executive functions development in 5- to 7-year-old children with transposition of the great arteries: a longitudinal study. Dev Neuropsychol (2014) 39(5):365–84. 10.1080/87565641.2014.916709 [DOI] [PubMed] [Google Scholar]
- 26.Hövels-Gürich HH, Konrad K, Skorzenski D, Herpertz-Dahlmann B, Messmer BJ, Seghaye M-C. Attentional dysfunction in children after corrective cardiac surgery in infancy. Ann Thorac Surg (2007) 83(4):1425–30. 10.1016/j.athoracsur.2006.10.069 [DOI] [PubMed] [Google Scholar]
- 27.Gaynor JW, Gerdes M, Nord AS, Bernbaum J, Zackai E, Wernovsky G, et al. Is cardiac diagnosis a predictor of neurodevelopmental outcome after cardiac surgery in infancy? J Thorac Cardiovasc Surg (2010) 140(6):1230–7. 10.1016/j.jtcvs.2010.07.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bellinger DC. Are children with congenital cardiac malformations at increased risk of deficits in social cognition? Cardiol Young (2008) 18(1):3–9. 10.1017/S104795110700176X [DOI] [PubMed] [Google Scholar]
- 29.Calderon J, Angeard N, Moutier S, Plumet M-H, Jambaqué I, Bonnet D. Impact of prenatal diagnosis on neurocognitive outcomes in children with transposition of the great arteries. J Pediatr (2012) 161(1):94.e–8.e. 10.1016/j.jpeds.2011.12.036 [DOI] [PubMed] [Google Scholar]
- 30.Calderon J, Bonnet D, Pinabiaux C, Jambaqué I, Angeard N. Use of early remedial services in children with transposition of the great arteries. J Pediatr (2013) 163(4):1105.e–10.e. 10.1016/j.jpeds.2013.04.065 [DOI] [PubMed] [Google Scholar]
- 31.Heinrichs AKM, Holschen A, Krings T, Messmer BJ, Schnitker R, Minkenberg R, et al. Neurologic and psycho-intellectual outcome related to structural brain imaging in adolescents and young adults after neonatal arterial switch operation for transposition of the great arteries. J Thorac Cardiovasc Surg (2014) 148(5):2190–9. 10.1016/j.jtcvs.2013.10.087 [DOI] [PubMed] [Google Scholar]
- 32.Hövels-Gürich HH, Seghaye M-C, Däbritz S, Messmer BJ, von Bernuth G. Cognitive and motor development in preschool and school-aged children after neonatal arterial switch operation. J Thorac Cardiovasc Surg (1997) 114(4):578–85. 10.1016/S0022-5223(97)70047-3 [DOI] [PubMed] [Google Scholar]
- 33.Cassidy AR, White MT, DeMaso DR, Newburger JW, Bellinger DC. Executive function in children and adolescents with critical cyanotic congenital heart disease. J Int Neuropsychol Soc (2015) 21(1):34–49. 10.1017/S1355617714001027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matos SM, Sarmento S, Moreira S, Pereira MM, Quintas J, Peixoto B, et al. Impact of fetal development on neurocognitive performance of adolescents with cyanotic and acyanotic congenital heart disease. Congenit Heart Dis (2014) 9(5):373–81. 10.1111/chd.12152 [DOI] [PubMed] [Google Scholar]
- 35.Murphy LK, Compas BE, Reeslund KL, Gindville MC, Mah ML, Markham LW, et al. Cognitive and attentional functioning in adolescents and young adults with Tetralogy of Fallot and d-transposition of the great arteries. Child Neuropsychol (2017) 23(1):99–110. 10.1080/09297049.2015.1087488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pike NA, Woo MA, Poulsen MK, Evangelista W, Faire D, Halnon NJ, et al. Predictors of memory deficits in adolescents and young adults with congenital heart disease compared to healthy controls. Front Pediatr (2016) 4:117. 10.3389/fped.2016.00117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schaefer C, von Rhein M, Knirsch W, Huber R, Natalucci G, Caflisch J, et al. Neurodevelopmental outcome, psychological adjustment, and quality of life in adolescents with congenital heart disease. Dev Med Child Neurol (2013) 55(12):1143–9. 10.1111/dmcn.12242 [DOI] [PubMed] [Google Scholar]
- 38.Keshavan MS, Giedd J, Lau JYF, Lewis DA, Paus T. Changes in the adolescent brain and the pathophysiology of psychotic disorders. Lancet Psychiatry (2014) 1(7):549–58. 10.1016/S2215-0366(14)00081-9 [DOI] [PubMed] [Google Scholar]
- 39.Sable C, Foster E, Uzark K, Bjornsen K, Canobbio MM, Connolly HM, et al. Best practices in managing transition to adulthood for adolescents with congenital heart disease: the transition process and medical and psychosocial issues a scientific statement from the American Heart Association. Circulation (2011) 123(13):1454–85. 10.1161/CIR.0b013e3182107c56 [DOI] [PubMed] [Google Scholar]
- 40.Luyckx K, Rassart J, Goossens E, Apers S, Oris L, Moons P. Development and persistence of depressive symptoms in adolescents with CHD. Cardiol Young (2016) 26(6):1115–22. 10.1017/S1047951115001882 [DOI] [PubMed] [Google Scholar]
- 41.Freitas IR, Castro M, Sarmento SL, Moura C, Viana V, Areias JC, et al. A cohort study on psychosocial adjustment and psychopathology in adolescents and young adults with congenital heart disease. BMJ Open (2013) 3(1):e001138. 10.1136/bmjopen-2012-001138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hövels-Gürich HH, Konrad K, Wiesner M, Minkenberg R, Herpertz-Dahlmann B, Messmer BJ, et al. Long term behavioural outcome after neonatal arterial switch operation for transposition of the great arteries. Arch Dis Child (2002) 87(6):506–10. 10.1136/adc.87.6.506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karsdorp PA, Everaerd W, Kindt M, Mulder BJM. Psychological and cognitive functioning in children and adolescents with congenital heart disease: a meta-analysis. J Pediatr Psychol (2007) 32(5):527–41. 10.1093/jpepsy/jsl047 [DOI] [PubMed] [Google Scholar]
- 44.Spijkerboer A, Utens E, Bogers A, Verhulst F, Helbing W. Long-term behavioural and emotional problems in four cardiac diagnostic groups of children and adolescents after invasive treatment for congenital heart disease. Int J Cardiol (2008) 125(1):66–73. 10.1016/j.ijcard.2007.02.025 [DOI] [PubMed] [Google Scholar]
- 45.DeMaso DR, Labella M, Taylor GA, Forbes PW, Stopp C, Bellinger DC, et al. Psychiatric disorders and function in adolescents with d-transposition of the great arteries. J Pediatr (2014) 165(4):760–6. 10.1016/j.jpeds.2014.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DeMaso D, Calderon J, Taylor G, Holland J, Stopp C, White M, et al. Psychiatric disorders in adolescents with single ventricle congenital heart disease. Pediatrics (2017) 139(3):e20162241. 10.1542/peds.2016-2241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perou R, Bitsko R, Blumberg S, Pastor P, Ghandour R, Gfroerer J, et al. Mental health surveillance among children – United States, 2005-2011. MMWR Suppl (2013) 62(2):1–35. [PubMed] [Google Scholar]
- 48.Heery E, Sheehan AM, While AE, Coyne I. Experiences and outcomes of transition from pediatric to adult health care services for young people with congenital heart disease: a systematic review. Congenit Heart Dis (2015) 10(5):413–27. 10.1111/chd.12251 [DOI] [PubMed] [Google Scholar]
- 49.Hilderson D, Saidi AS, Van Deyk K, Verstappen A, Kovacs AH, Fernandes SM, et al. Attitude toward and current practice of transfer and transition of adolescents with congenital heart disease in the United States of America and Europe. Pediatr Cardiol (2009) 30(6):786–93. 10.1007/s00246-009-9442-1 [DOI] [PubMed] [Google Scholar]
- 50.Kovacs AH, Utens EM. More than just the heart: transition and psychosocial issues in adult congenital heart disease. Cardiol Clin (2015) 33(4):625–34. 10.1016/j.ccl.2015.07.005 [DOI] [PubMed] [Google Scholar]
- 51.Gurvitz M, Saidi A. Transition in congenital heart disease: it takes a village. Heart (2014) 100(14):1075–6. 10.1136/heartjnl-2014-306030 [DOI] [PubMed] [Google Scholar]
- 52.Villafañe J, Lantin-Hermoso MR, Bhatt AB, Tweddell JS, Geva T, Nathan M, et al. D-transposition of the great arteries: the current era of the arterial switch operation. J Am Coll Cardiol (2014) 64(5):498–511. 10.1016/j.jacc.2014.06.1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Klouda L, Franklin WJ, Saraf A, Parekh DR, Schwartz DD. Neurocognitive and executive functioning in adult survivors of congenital heart disease. Congenit Heart Dis (2017) 12(1):91–8. 10.1111/chd.12409 [DOI] [PubMed] [Google Scholar]
- 54.Tyagi M, Fteropoulli T, Hurt CS, Hirani SP, Rixon L, Davies A, et al. Cognitive dysfunction in adult CHD with different structural complexity. Cardiol Young (2016) 18:1–9. 10.1017/S1047951116001396 [DOI] [PubMed] [Google Scholar]
- 55.Aldén B, Gilljam T, Gillberg C. Long-term psychological outcome of children after surgery for transposition of the great arteries. Acta Paediatr (1998) 87(4):405–10. 10.1111/j.1651-2227.1998.tb01468.x [DOI] [PubMed] [Google Scholar]
- 56.van Rijen EHM, Utens EMWJ, Roos-Hesselink JW, Meijboom FJ, van Domburg RT, Roelandt JRTC, et al. Medical predictors for psychopathology in adults with operated congenital heart disease. Eur Heart J (2004) 25(18):1605–13. 10.1016/j.ehj.2004.06.025 [DOI] [PubMed] [Google Scholar]
- 57.Coelho R, Teixeira F, Silva AM, Vaz C, Vieira D, Proença C, et al. [Psychosocial adjustment, psychiatric morbidity and quality of life in adolescents and young adults with congenital heart disease]. Rev Port Cardiol (2013) 32(9):657–64. 10.1016/j.repc.2013.07.001 [DOI] [PubMed] [Google Scholar]
- 58.Norozi K, Zoege M, Buchhorn R, Wessel A, Geyer S. The influence of congenital heart disease on psychological conditions in adolescents and adults after corrective surgery. Congenit Heart Dis (2006) 1(6):282–8. 10.1111/j.1747-0803.2006.00048.x [DOI] [PubMed] [Google Scholar]
- 59.Bromberg JI, Beasley PJ, D’Angelo EJ, Landzberg M, DeMaso DR. Depression and anxiety in adults with congenital heart disease: a pilot study. Heart Lung (2003) 32(2):105–10. 10.1067/mhl.2003.26 [DOI] [PubMed] [Google Scholar]
- 60.Horner T, Liberthson R, Jellinek MS. Psychosocial profile of adults with complex congenital heart disease. Mayo Clin Proc (2000) 75(1):31–6. 10.4065/75.1.31 [DOI] [PubMed] [Google Scholar]
- 61.Kovacs AH, Saidi AS, Kuhl EA, Sears SF, Silversides C, Harrison JL, et al. Depression and anxiety in adult congenital heart disease: predictors and prevalence. Int J Cardiol (2009) 137(2):158–64. 10.1016/j.ijcard.2008.06.042 [DOI] [PubMed] [Google Scholar]
- 62.Westhoff-Bleck M, Briest J, Fraccarollo D, Hilfiker-Kleiner D, Winter L, Maske U, et al. Mental disorders in adults with congenital heart disease: unmet needs and impact on quality of life. J Affect Disord (2016) 204:180–6. 10.1016/j.jad.2016.06.047 [DOI] [PubMed] [Google Scholar]
- 63.Bang JS, Jo S, Kim GB, Kwon BS, Bae EJ, Noh CI, et al. The mental health and quality of life of adult patients with congenital heart disease. Int J Cardiol (2013) 170(1):49–53. 10.1016/j.ijcard.2013.10.003 [DOI] [PubMed] [Google Scholar]
- 64.Deng L, Khan AM, Drajpuch D, Fuller S, Gleason LP, Ludmir J, et al. Anxiety is more common than depression in adults with congenital heart disease. J Am Coll Cardiol (2015) 65(10). 10.1016/S0735-1097(15)60549-6 [DOI] [Google Scholar]
- 65.Kourkoveli P, Rammos S, Parissis J, Maillis A, Kremastinos D, Paraskevaidis I. Depressive symptoms in patients with congenital heart disease: incidence and prognostic value of self-rating depression scales. Congenit Heart Dis (2015) 10(3):240–7. 10.1111/chd.12200 [DOI] [PubMed] [Google Scholar]
- 66.Cox D, Lewis G, Stuart G, Murphy K. A cross-sectional study of the prevalence of psychopathology in adults with congenital heart disease. J Psychosom Res (2002) 52(2):65–8. 10.1016/S0022-3999(01)00294-X [DOI] [PubMed] [Google Scholar]
- 67.Loup O, von Weissenfluh C, Gahl B, Schwerzmann M, Carrel T, Kadner A. Quality of life of grown-up congenital heart disease patients after congenital cardiac surgery. Eur J Cardiothorac Surg (2009) 36(1):105–11. 10.1016/j.ejcts.2009.03.023 [DOI] [PubMed] [Google Scholar]
- 68.Müller J, Hess J, Hager A. Minor symptoms of depression in patients with congenital heart disease have a larger impact on quality of life than limited exercise capacity. Int J Cardiol (2012) 154(3):265–9. 10.1016/j.ijcard.2010.09.029 [DOI] [PubMed] [Google Scholar]
- 69.Müller J, Hess J, Hager A. General anxiety of adolescents and adults with congenital heart disease is comparable with that in healthy controls. Int J Cardiol (2013) 165(1):142–5. 10.1016/j.ijcard.2011.08.005 [DOI] [PubMed] [Google Scholar]
- 70.Opić P, Roos-Hesselink JW, Cuypers JAA, Witsenburg M, van den Bosch A, van Domburg RT, et al. Psychosocial functioning of adults with congenital heart disease: outcomes of a 30-43 year longitudinal follow-up. Clin Res Cardiol (2015) 104(5):388–400. 10.1007/s00392-014-0792-1 [DOI] [PubMed] [Google Scholar]
- 71.Utens EM, Verhulst FC, Erdman RAM, Meijboom FJ, Duivenvoorden HJ, Bos E, et al. Psychosocial functioning of young adults after surgical correction for congenital heart disease in childhood: a follow-up study. J Psychosom Res (1994) 38(7):745–58. 10.1016/0022-3999(94)90027-2 [DOI] [PubMed] [Google Scholar]
- 72.Utens EM, Bieman HJ, Verhulst FC, Meijboom FJ, Erdman RA, Hess J. Psychopathology in young adults with congenital heart disease. Follow-up results. Eur Heart J (1998) 19(4):647–51. 10.1053/euhj.1997.0824 [DOI] [PubMed] [Google Scholar]
- 73.Apers S, Moons P, Goossens E, Luyckx K, Gewillig M, Bogaerts K, et al. Sense of coherence and perceived physical health explain the better quality of life in adolescents with congenital heart disease. Eur J Cardiovasc Nurs (2013) 12(5):475–83. 10.1177/1474515113477955 [DOI] [PubMed] [Google Scholar]
- 74.Moons P, Norekvål TM. Is sense of coherence a pathway for improving the quality of life of patients who grow up with chronic diseases? A hypothesis. Eur J Cardiovasc Nurs (2006) 5(1):16–20. 10.1016/j.ejcnurse.2005.10.009 [DOI] [PubMed] [Google Scholar]
- 75.Khan M, Monaghan M, Klein N, Ruiz G, John AS. Associations among depression symptoms with alcohol and smoking tobacco use in adult patients with congenital heart disease. Congenit Heart Dis (2015) 10(5):E243–9. 10.1111/chd.12282 [DOI] [PubMed] [Google Scholar]
- 76.White KS, Pardue C, Ludbrook P, Sodhi S, Esmaeeli A, Cedars A. Cardiac denial and psychological predictors of cardiac care adherence in adults with congenital heart disease. Behav Modif (2016) 40(1–2):29–50. 10.1177/0145445515613329 [DOI] [PubMed] [Google Scholar]
- 77.Dunbar-Masterson C, Wypij D, Bellinger DC, Rappaport LA, Baker AL, Jonas RA, et al. General health status of children with D-transposition of the great arteries after the arterial switch operation. Circulation (2001) 104(12 Suppl 1):I138–42. 10.1161/hc37t1.094782 [DOI] [PubMed] [Google Scholar]
- 78.Rose SA, Feldman JF, Jankowski JJ. Modeling a cascade of effects: the role of speed and executive functioning in preterm/full-term differences in academic achievement. Dev Sci (2011) 14(5):1161–75. 10.1111/j.1467-7687.2011.01068.x [DOI] [PubMed] [Google Scholar]
- 79.McCusker CG, Doherty NN, Molloy B, Rooney N, Mulholland C, Sands A, et al. A randomized controlled trial of interventions to promote adjustment in children with congenital heart disease entering school and their families. J Pediatr Psychol (2012) 37(10):1089–103. 10.1093/jpepsy/jss092 [DOI] [PubMed] [Google Scholar]
- 80.Klingberg T, Fernell E, Olesen PJ, Johnson M, Gustafsson P, Dahlström K, et al. Computerized training of working memory in children with ADHD – a randomized, controlled trial. J Am Acad Child Adolesc Psychiatry (2005) 44(2):177–86. 10.1097/00004583-200502000-00010 [DOI] [PubMed] [Google Scholar]
- 81.Løhaugen GCC, Antonsen I, Håberg A, Gramstad A, Vik T, Brubakk A-M, et al. Computerized working memory training improves function in adolescents born at extremely low birth weight. J Pediatr (2011) 158(4):555.e–61.e. 10.1016/j.jpeds.2010.09.060 [DOI] [PubMed] [Google Scholar]
- 82.Spijkerman MPJ, Pots WTM, Bohlmeijer ET. Effectiveness of online mindfulness-based interventions in improving mental health: a review and meta-analysis of randomised controlled trials. Clin Psychol Rev (2016) 45:102–14. 10.1016/j.cpr.2016.03.009 [DOI] [PubMed] [Google Scholar]
- 83.Strauss C, Cavanagh K, Oliver A, Pettman D. Mindfulness-based interventions for people diagnosed with a current episode of an anxiety or depressive disorder: a meta-analysis of randomised controlled trials. PLoS One (2014) 9(4):e96110. 10.1371/journal.pone.0096110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Warnes CA, Williams RG, Bashore TM, Child JS, Connolly HM, Dearani JA, et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (writing committee to develop guidelines on the management of adults with congenital heart disease) developed in collaboration with the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol (2008) 52(23):e143–263. 10.1016/j.jacc.2008.10.001 [DOI] [PubMed] [Google Scholar]
- 85.Antonovsky A. Unraveling the Mystery of Health: How People Manage Stress and Stay Well. San Francisco, CA: Jossey-Bass; (1987). [Google Scholar]
- 86.Butler AC, Chapman JE, Forman EM, Beck AT. The empirical status of cognitive-behavioral therapy: a review of meta-analyses. Clin Psychol Rev (2006) 26(1):17–31. 10.1016/j.cpr.2005.07.003 [DOI] [PubMed] [Google Scholar]
- 87.Cairncross M, Miller CJ. The effectiveness of mindfulness-based therapies for ADHD: a meta-analytic review. J Atten Disord (2016). 10.1177/1087054715625301 [DOI] [PubMed] [Google Scholar]