Abstract
Background
Circulating adiponectin concentration increases in patients with chronic heart failure (HF). We sought to explore the prognostic value of temporal changes in adiponectin concentration following treatment for chronic HF.
Methods
Serum adiponectin levels were measured at baseline and after a 3-month anti-failure treatment in 124 patients with symptomatic chronic systolic HF. Major adverse cardiac events (MACE) including death, heart transplantation, or hospitalization with worsening HF during a median follow-up period of 752 days were determined.
Results
Univariate and multivariate analysis showed that high levels of adiponectin after a 3-month treatment were associated with a 3.8-fold increased risk of MACE (p = 0.03), independent of amino-terminal pro-B-type natriuretic peptide (NT-proBNP) levels. Moreover, the combining of circulating levels of adiponectin with NT-proBNP provided independent and additional prognostic value in identifying high risk patients with MACE during follow-up.
Conclusions
Changes in adiponectin and NT-proBNP over time provide prognostic information. When adiponectin is used in conjunction with NT-proBNP in chronic HF, the prognostic value may be better than if each biomarker is used separately.
Keywords: Adiponectin, Chronic heart failure, Natriuretic peptide, Prognosis
INTRODUCTION
It is hypothesized that neurohormones and inflammatory cytokines produced in response to heart failure (HF) contribute to progressive deterioration of cardiac performance.1-3 In support of these hypotheses, poor outcomes linked to elevated circulating biomarkers of myocardial wall stress and inflammation were found.3-9
Recent studies suggest that elevated levels of adiponectin, an adipokine, in HF patients may be linked to metabolic and cardiovascular abnormalities, energy wasting, and exercise intolerance, and are related to adverse outcomes.4-9 Increased levels of adiponectin have been reported in patients with both chronic and acute HF, and are associated with the severity of HF, cachexia, and higher mortality.5,10-13 In acute decompensated HF, serum adiponectin levels decreased following treatment, and the treatment-induced decrease in serum adiponectin concentration is also an important determinant of positive outcome.13 However, previous studies showed inconsistent results concerning the effects of anti-HF drugs on circulating adiponectin levels.14-19 Beta-blockers decrease plasma adiponectin concentration,14,15 while renin-angiotensin system blocking agents significantly increase adiponectin levels.16-19 Moreover, an association between circulating adiponectin levels and cardiovascular morbidity or mortality cannot be ascertained in some studies.20,21 Such discrepant findings clearly demand further research into the clinical relevance of adiponectin in HF.
We hypothesize that treatment of chronic HF with guideline-directed medical therapy may, in general, influence neurohormonal and inflammatory function, and help prevent HF exacerbations. The purpose of this study was to ascertain whether the circulating levels of adiponectin can be lowered over time, and the decrease of adiponectin levels following treatment can provide prognostic information concerning patients with chronic HF, compared to the amino-terminal pro-B-type natriuretic peptide (NT-proBNP), a well-established prognostic biomarker of myocardial stress.3
METHODS
Patient population
Between January 2009 and December 2010, we enrolled 124 consecutive patients with mild to moderate chronic stable HF (96 men, 28 women, aged 58 ± 15 years) from the outpatient clinic of the cardiology department at Cheng Hsin General Hospital, presenting with New York Heart Association (NYHA) functional class II-III symptoms of HF, and a left ventricular ejection fraction (LVEF) < 40% by echocardiography.
Patients excluded from this study were those with acute decompensated HF, hemodynamically significant obstructive valvular heart disease, corpulmonale, restrictive or hypertrophic cardiomyopathy, myocarditis, constrictive pericarditis, congenital heart disease, significant liver (defined as liver enzymes > 3 times the normal) or renal diseases (defined as serum creatinine > 2.0 mg/dL), concurrent infection or any documented inflammatory illness, such as arthritis or connective tissue diseases, or any other malignancy.
The etiology of HF was determined to be ischemic when coronary angiography revealed > 50% luminal diameter narrowing in at least two major epicardial coronary arteries, when there was documented myocardial infarction, or when there was HF caused by post-infarction ventricular aneurysm. In those patients with chronic HF without coronary artery disease, where the endomyocardial biopsy revealed findings compatible with dilated cardiomyopathy, the cause of HF was determined to be dilated cardiomyopathy or non-ischemic HF.
This study complied with the Declaration of Helsinki, and the Cheng Hsin General Hospital’s institutional committee on human research approved the research protocol. All patients involved have submitted their informed consents.
Clinical evaluation and blood sampling
Baseline clinical evaluations of all 124 patients with chronic HF were conducted by a physician. Blood sampling and echocardiography were done at baseline and at the 3-month follow-up after continuous anti-HF treatment. Throughout the 3-month period, those patients were in stable condition and their anti-HF medications were not changed. After blood sampling, the serum was separated by centrifugation and then frozen to under -20 °C and then stored at that same temperature until analysis. Additionally, LVEF was measured by 2-dimensional echocardiography.
Measurement of circulating levels of biomarkers
The NT-proBNP was determined with Roche Elecsys ®NT-proBNP (Roche Diagnostics GmbH), a quantitative electrochemiluminescence immunoassay. Measurements were performed with the Elecsys 2010 immunoassay analyzer (Roche Diagnostics GmbH). Serum concentrations of adiponectin were measured with commercial sandwich enzyme-linked immunosorbent assays (R&D Systems, Inc., Minneapolis, MN, USA).
Clinical follow-up
Clinical information regarding major adverse cardiac events (MACE), including cardiac death, requirement for heart transplantation, and hospitalization with worsening HF, was gathered during a median follow-up of 752 days after enrollment. The information was collected by the treating cardiologists unaware of the patients’ biomarker levels.
Data analysis
All values are expressed as mean ± SD. Because plasma concentrations of NT-proBNP and adiponectin were not normally distributed, they were transformed logarithmically before analysis. Linear regression analysis was used to determine the correlation between the circulating levels of biomarkers and LVEF.
For survival analysis, the HF patients were divided into two groups, depending upon whether or not MACE occurred during follow-up. Univariate comparisons of clinical characteristics and laboratory measurements between the two groups were made with appropriate tests. In the Kaplan-Meier analyses, the differences between event-free curves were tested by log-rank test. In the multivariate Cox proportional hazards analyses, the independent predictors of MACE in the study patients was determined.
All variables are two-tailed, and p < 0.05 was considered statistically significant. The statistical software package SPSS version 12.0 (SPSS Inc, Chicago, IL, USA) was used for all analyses.
RESULTS
Patient characteristics
The characteristics of the 124 patients meeting the study criteria were shown in Table 1. There were more men than women in this study population. The causes of chronic HF were of ischemic origin in 39 (32%) of the study subjects. The incidences of comorbidities were demonstrated and a total of 44 (35%) of the study population had type II diabetes mellitus. Because those patients were in stable conditions, their anti-HF medications were basically unchanged throughout the 3 months and were also demonstrated. As to the glucose-lowering agents, the most commonly used were sulfonylureas in 24/44 (55%), followed by biguanides in 22/44 (50%), alpha-glucosidase in 9/44 (20%), insulin in 6/44 (14%), meglitinides in 2/44 (5%), and thiazolidinediones in 2/44 (5%). However, in 2011, the U.S. Food and Drug Administration implemented a stringent "restricted access program" for the prescription of rosiglitazone. Since HF appears to be a class-specific adverse effect with respect to thiazolidinediones, they were no longer used for HF patients in our institution after 2011.
Table 1. Clinical characteristics of the 124 patients with chronic stable heart failure.
| Age, years | 58 ± 15 |
| Male, n (%) | 96 (77%) |
| Ischemic heart failure, n (%) | 39 (32%) |
| Diabetes mellitus, n (%) | 44 (35%) |
| Hypertension, n (%) | 71 (57%) |
| Smoking, n (%) | 40 (32%) |
| Dyslipidemia, n (%) | 113 (91%) |
| Previous myocardial infarction, n (%) | 15 (12%) |
| Previous stroke, n (%) | 9 (7%) |
| Chronic kidney disease, stage 3, n (%) | 64 (52%) |
| Anemia, n (%) | 4 (3%) |
| Medications | |
| Diuretics, n (%) | 102 (82%) |
| Aldosterone antagonist, n (%) | 54 (44%) |
| ACEI/ARB, n (%) | 70 (57%) |
| Beta-blockers, n (%) | 72 (58%) |
| Digitalis, n (%) | 58 (47%) |
| Vasodilators, n (%) | 51 (41%) |
| Statins, n (%) | 33 (27%) |
| Body mass index at baseline, kg/m2 | 26 ± 5 |
| Body mass index at 3-month, kg/m2 | 26 ± 5 |
| LVEF at baseline, % | 29 ± 11 |
| LVEF at 3-month, % | 34 ± 12 |
| NYHA functional class at baseline | |
| II, n (%) | 65 (52%) |
| III, n (%) | 59 (48%) |
| NYHA functional class at 3-month | |
| II, n (%) | 94 (76%) |
| III, n (%) | 30 (24%) |
| Log NT-proBNP at baseline, pg/mL | 2.74 ± 0.76 |
| Log NT-proBNP at 3-month, pg/mL | 2.66 ± 0.77 |
| Log Adiponectin at baseline, ng/mL | 3.88 ± 0.52 |
| Log Adiponectin at 3-month, ng/mL | 3.82 ± 0.52 |
| Total MACE, n (%) | 40 (32%) |
| Mortality, n (%) | 17 (14%) |
ACEI/ARB, angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers; LVEF, left ventricular ejection fraction; MACE, major adverse cardiac events; NT-proBNP, amino-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association.
At baseline, mean body mass index (BMI) was 26 ± 5 kg/m2, mean LVEF was 29 ± 11%, and 65 (52%) patients were in NYHA class II and 59 (48%) were in class III. At the 3-month follow-up, mean BMI was 26 ± 5 kg/m2, mean LVEF was 34 ± 12%, and 94 (76%) patients were in NYHA class II and 30 (24%) were in class III. The mean log NT-proBNP levels and log adiponectin levels at baseline and at 3 months were 2.74 ± 0.76 pg/mL, 2.66 ± 0.77 pg/mL, 3.88 ± 0.52 ng/mL, and 3.82 ± 0.52 ng/mL, respectively.
Prognosis
The median follow-up period was 752 days (475 to 986 days, 25th to 75th percentiles). There was a 32% (40/124) overall event rate in the HF population. Eleven of the 124 patients died of cardiac causes, and all of them were of intractable end-stage HF; whereas, there were two other patients died of pneumonia, during the follow-up period. Four patients underwent heart transplantation and 23 were re-hospitalized for worsening HF.
When the 124 chronic HF patients were divided into two groups depending on whether a patient had MACE or not during follow-up (Table 2), significant differences in the use of diuretics, the mean LVEF at 3 months, and the NYHA functional class at baseline and at 3 months were detected between the two groups. The concentrations of NT-proBNP at baseline and at 3 months and the concentrations of adiponectin at 3 months in the group with MACE were significantly higher than those in the event-free group. In multivariable Cox proportional hazards analyses, high levels of adiponectin at 3 months after treatment was an independent predictor of clinical outcomes and were associated with an about 3.8-fold increase risk of MACE (p = 0.03). Multivariate analysis also identified that elevated NT-proBNP levels at 3 months (HR = 2.24, p = 0.008) and improved LVEF at 3-month (HR = 0.941, p = 0.001) to be independently associated with MACE.
Table 2. Patient characteristics of those who had major adverse cardiac events during the follow-up, and those who experienced event-free follow-up.
| MACE (+) | MACE (-) | Univariate | Multivariate | |
| (N = 40) | (N = 84) | p value | p value | |
| Age, years | 61 ± 16 | 57 ± 14 | 0.15 | |
| Male, n (%) | 29 (73%) | 67 (80%) | 0.37 | |
| Ischemic heart failure, n (%) | 16 (40%) | 23 (27%) | 0.16 | |
| Diabetes mellitus, n (%) | 13 (33%) | 31 (37%) | 0.63 | |
| Hypertension, n (%) | 25 (63%) | 46 (55%) | 0.42 | |
| Smoking, n (%) | 10 (25%) | 30 (36%) | 0.23 | |
| Dyslipidemia, n (%) | 35 (88%) | 78 (93%) | 0.52 | |
| Previous myocardial infarction, n (%) | 6 (15%) | 9 (11%) | 0.70 | |
| Previous stroke, n (%) | 4 (10%) | 5 (6%) | 0.66 | |
| Chronic kidney disease, stage 3, n (%) | 26 (65%) | 38 (45%) | 0.06 | 0.85 |
| Anemia, n (%) | 0 (0%) | 4 (5%) | 0.39 | |
| Medications | ||||
| Diuretics, n (%) | 37 (93%) | 65 (77%) | 0.04 | 0.49 |
| Aldosterone antagonist, n (%) | 21 (53%) | 33 (39%) | 0.23 | |
| ACEI/ARB, n (%) | 20 (50%) | 50 (60%) | 0.32 | |
| Beta-blockers, n (%) | 21 (53%) | 51 (61%) | 0.39 | |
| Digitalis, n (%) | 20 (50%) | 38 (45%) | 0.62 | |
| Vasodilators, n (%) | 19 (48%) | 32 (38%) | 0.32 | |
| Statins, n (%) | 9 (23%) | 24 (29%) | 0.48 | |
| Body mass index at baseline, kg/m2 | 26 ± 4 | 26 ± 5 | 0.52 | |
| Body mass index at 3-month, kg/m2 | 26 ± 4 | 26 ± 5 | 0.54 | |
| LVEF at baseline, % | 27.18 ± 9.97 | 30.44 ± 10.69 | 0.11 | |
| LVEF at 3-month, % | 26.83 ± 10.83 | 37.44 ± 10.80 | < 0.001 | 0.001* |
| NYHA functional class at baseline | ||||
| II, n (%) | 13 (33%) | 52 (62%) | 0.004 | |
| III, n (%) | 27 (67%) | 32 (38%) | 0.004 | |
| NYHA functional class at 3-month | ||||
| II, n (%) | 14 (35%) | 80 (95%) | < 0.001 | |
| III, n (%) | 26 (65%) | 4 (5%) | < 0.001 | |
| Log NT-proBNP at baseline, pg/mL | 2.97 ± 0.68 | 2.63 ± 0.77 | 0.02 | |
| Log NT-proBNP at 3-month, pg/mL | 3.10 ± 0.77 | 2.44 ± 0.68 | < 0.001 | 0.008 |
| Log Adiponectin at baseline, ng/mL | 3.99 ± 0.39 | 3.83 ± 0.56 | 0.11 | |
| Log Adiponectin at 3-month, ng/mL | 4.08 ± 0.42 | 3.70 ± 0.52 | < 0.001 | 0.03 |
| Follow-up, days | 508 ± 275 | 866 ± 296 | < 0.001 |
ACEI/ARB, angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers; LVEF, left ventricular ejection fraction; MACE, major adverse cardiac events; NT-proBNP, amino-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association.
* LVEF and NYHA functional class at 3-month are confounding to each other, so only LVEF at 3-month was included in the final analysis.
When we divided the HF patients into two groups, depending upon whether their circulating adiponectin levels were above or below the median at their 3-month follow-up (Figure 1), those patients with adiponectin levels above the median had a significantly worse outcomes compared to those whose adiponectin levels were below the median (log-rank p = 0.005).
Figure 1.
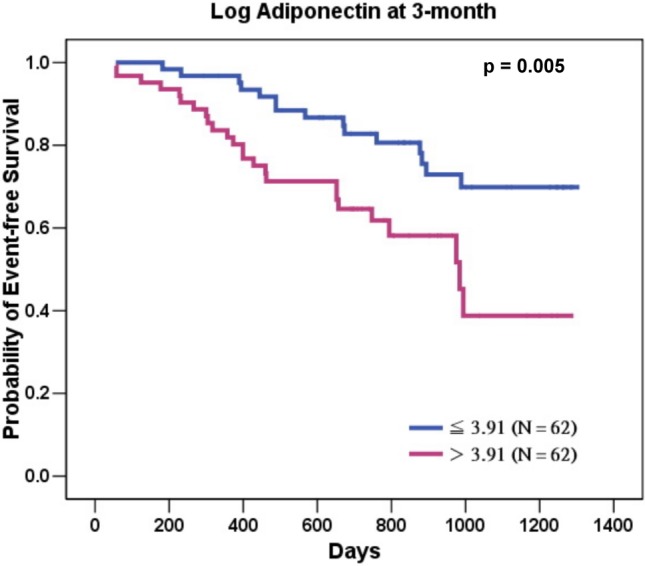
The 124 chronic heart failure patients were divided into two groups, depending upon whether or not their circulating adiponectin levels were above or below the median at 3-month follow-up. Those patients with adiponectin levels above the median had a significant worse outcomes compared to those adiponectin levels were below the median (log-rank p = 0.005).
Relationship between levels of the adiponectin and NT-proBNP, and LVEF at 3-month
At 3 months, significant positive correlation, with the correlation coefficient between adiponectin and NT-proBNP of 0.512 (p < 0.001) was noted (Figure 2A). Significant negative correlation, with the correlation coefficients between adiponectin and LVEF of -0.216 (p = 0.02), was also noted (Figure 2B).
Figure 2.
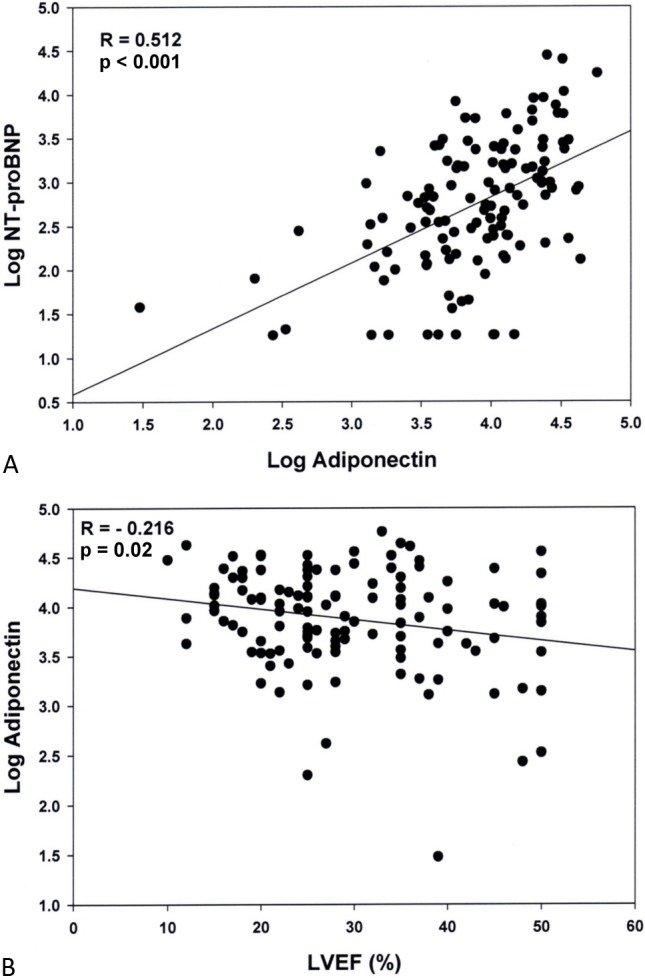
Relationship between levels of the adiponectin and amino-terminal pro-B-type natriuretic peptide (NT-proBNP), and left ventricular ejection fraction (LVEF) at 3-month was demonstrated. Significant positive correlation, with the correlation coefficient between adiponectin and NT-proBNP of 0.512 (p < 0.001) was noted (A). Significant negative correlation, with the correlation coefficients between adiponectin and LVEF of -0.216 (p = 0.02) was also noted (B).
Prognostic stratification according to the coupling between adiponectin and NT-proBNP
To test whether simultaneous assessment of biomarkers enables clinicians to stratify risk more effectively among patients with chronic HF, we have constructed survival curves after dividing the study participants into three groups as showed in Figure 3. The study patients were categorized on the basis of whether they had a log adiponectin at 3 months higher or lower than the median value of 3.91 ng/mL and a log NT-proBNP level at 3 months higher or lower than the median level of 2.64 pg/mL. Forty-eight of the 124 patients (39%) had no elevation in either adiponectin or NT-proBNP (Group 1); 28 (22%) had an elevation in one (Group 2); and 48 (39%) had elevations in two (Group 3). The differences in event-free survival curves between Group 1 and Group 3, and Group 2 and Group 3 were significant (log-rank p < 0.001 and p = 0.01, respectively). These findings suggest the combination of the serum level of adiponectin with NT-proBNP may provide independent and additive prognostic value in identifying high risk patients hospitalized with acute HF.
Figure 3.
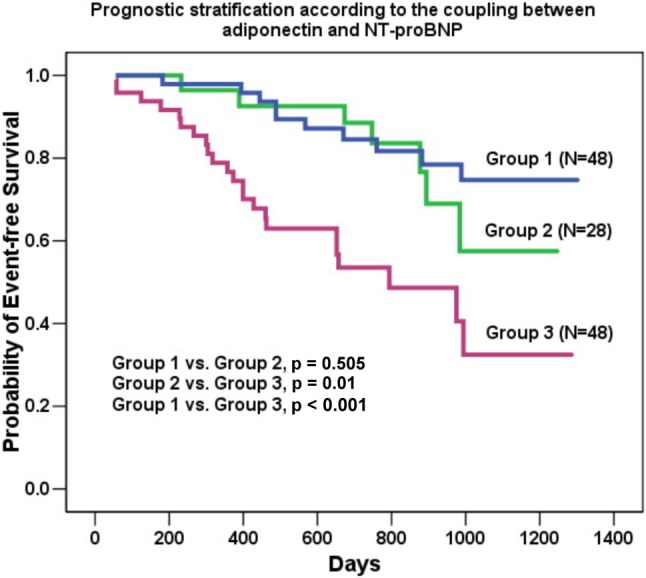
The study patients were categorized on the basis of whether they had a Log adiponectin level at 3 months higher or lower than the median value of 3.91 ng/mL and a Log NT-proBNP level at 3 months higher or lower than the median baseline level of 2.64 pg/mL. Fourty-eight of the 124 patients (39%) had no elevation in either adiponectin or NT-proBNP (Group 1); 28 (22%) had elevation in one (Group 2); and 48 (39%) had elevations in two (Group 3). The difference in event-free survival curves among the 3 groups was significant.
Changes in adiponectin over time following treatment in patients with chronic stable HF and the possible pathophysiologic basis to these changes
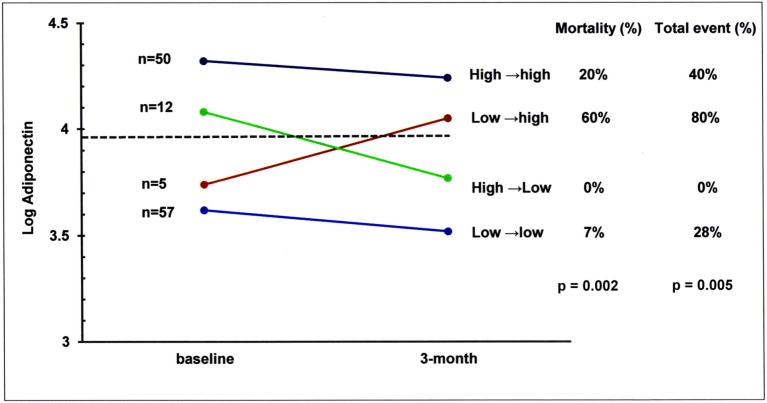
Although the majority of the patients had stable adiponectin levels below or above the threshold of 8138 ng/mL (log adiponectin = 3.91ng/mL) from baseline to 3 months, some moved across the median value as shown in Figure 4. Of the total number of patients, 12 (9.6%) were in the high→low group (adiponectin from above to below the median) and 5 (4%) were in the low→high group (adiponectin from below to above the median).
Figure 4.
Serum levels of adiponectin in the study patients grouped according to changes of adiponectin over time following treatment. The number of patients (left) and subsequent mortality and MACE rates (right) are presented for the four subgroups of adiponectin changes, from baseline to 3 months. MACE, major adverse cardiovascular events.
As shown in Table 3, the incidence of chronic kidney disease were significantly higher in the low→high and high→high groups. It is also worth noting that the use of angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers was significantly lower in the low→ high group, and the prescription rate of beta-blockers was significantly lower in the high→high group.
Table 3. Clinical characteristics of the 124 patients with chronic stable heart failure stratified by changes in adiponectin over time following treatment.
| Low→low (N = 57) | High→low (N = 12) | Low→high (N = 5) | High→high (N = 50) | p value | |
| Age, years | 52 ± 13 | 63 ± 14 | 58 ± 15 | 63 ± 15 | 0.001 |
| Male, n (%) | 47 (83%) | 10 (83%) | 3 (60%) | 36 (72%) | 0.43 |
| Ischemic heart disease, n (%) | 18 (32%) | 4 (33%) | 2 (40%) | 15 (30%) | 0.97 |
| Diabetes mellitus, n (%) | 22 (39%) | 5 (42%) | 1 (20%) | 16 (32%) | 0.75 |
| Hypertension, n (%) | 31 (54%) | 7 (58%) | 3 (60%) | 30 (60%) | 0.95 |
| Smoking, n (%) | 22 (39%) | 3 (25%) | 1 (20%) | 14 (28%) | 0.55 |
| Dyslipidemia, n (%) | 56 (98%) | 12 (100%) | 4 (80%) | 41 (82%) | 0.01 |
| Previous MI, n (%) | 7 (12%) | 0 (0%) | 2 (40%) | 6 (12%) | 0.15 |
| Previous stroke, n (%) | 5 (9%) | 1 (8%) | 1 (20%) | 2 (4%) | 0.53 |
| CKD, stage 3, n (%) | 20 (35%) | 5 (42%) | 4 (80%) | 35 (70%) | 0.002 |
| Anemia, n (%) | 1 (2%) | 1 (8%) | 0 (0%) | 2 (4%) | 0.65 |
| Medication | |||||
| Diuretics, n (%) | 42 (74%) | 12 (100%) | 5 (100%) | 43 (86%) | 0.07 |
| Aldosterone antagonist, n (%) | 26 (46%) | 5 (42%) | 2 (40%) | 21 (42%) | 0.98 |
| ACEI/ARB, n (%) | 30 (53%) | 11 (92%) | 1 (20%) | 28 (56%) | 0.03 |
| Beta-blockers, n (%) | 38 (67%) | 9 (75%) | 4 (80%) | 21 (42%) | 0.02 |
| Digitalis, n (%) | 24 (42%) | 9 (75%) | 2 (40%) | 23 (46%) | 0.22 |
| Vasodilators, n (%) | 24 (42%) | 5 (42%) | 2 (40%) | 20 (40%) | 1.00 |
| Statins, n (%) | 17 (30%) | 4 (33%) | 3 (60%) | 9 (18%) | 0.15 |
| Body mass index at baseline, kg/m2 | 27.11 ± 4.64 | 26.00 ± 4.71 | 24.60 ± 3.97 | 24.97 ± 5.25 | 0.14 |
| Body mass index at 3-month, kg/m2 | 27.43 ± 4.90 | 26.35 ± 4.95 | 25.27 ± 3.78 | 25.09 ± 5.23 | 0.12 |
| LVEF at baseline, % | 30.91 ± 10.44 | 27.25 ± 9.80 | 23.60 ± 7.93 | 28.74 ± 10.94 | 0.34 |
| LVEF at 3-month, % | 37.25 ± 10.38 | 36.83 ± 10.28 | 20.40 ± 11.10 | 31.02 ± 12.42 | 0.002 |
| NYHA functional class at baseline | |||||
| II, n (%) | 37 (65%) | 7 (58%) | 0 (0%) | 21 (42%) | 0.01 |
| III, n (%) | 20 (35%) | 5 (42%) | 5 (100%) | 29 (58%) | 0.01 |
| NYHA functional class at 3-month | |||||
| II, n (%) | 47 (83%) | 11 (92%) | 2 (40%) | 34 (68%) | 0.04 |
| III, n (%) | 10 (17%) | 1 (8%)0 | 3 (60%) | 16 (32%) | 0.04 |
| Log NT-proBNP at baseline, pg/mL | 2.43 ± 0.72 | 2.91 ± 0.73 | 2.70 ± 0.52 | 3.05 ± 0.70 | < 0.001 |
| Log NT-proBNP at 3-month, pg/mL | 2.22 ± 0.63 | 2.58 ± 0.74 | 3.07 ± 0.66 | 3.12 ± 0.65 | < 0.001 |
| Log Adiponectin at baseline, ng/mL | 3.48 ± 0.46 | 4.11 ± 0.12 | 3.72 ± 0.19 | 4.30 ± 0.20 | < 0.001 |
| Log Adiponectin at 3-month, ng/mL | 3.42 ± 0.43 | 3.71 ± 0.24 | 4.06 ± 0.08 | 4.28 ± 0.21 | < 0.001 |
| Total MACE, n (%) | 16 (28%) | 0 (0%) | 4 (80%) | 20 (40%) | 0.005 |
| Mortality, n (%) | 4 (7%) | 0 (0%) | 3 (60%) | 10 (20%) | 0.002 |
ACEI/ARB, angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers; CKD, chronic kidney disease; LVEF, left ventricular ejection fraction; MACE, major adverse cardiac events; MI, myocardial infarction; NYHA, New York Heart Association.
Patients had different outcomes regarding mortality and MACE rates according to the type of change in adiponectin (p = 0.002 and p = 0.005, respectively). Patients whose adiponectin levels improved from above to below the median (high→low), had a similar mortality and MACE rates compared with the low→low patients. Patients whose adiponectin concentration worsened (low→high) had a significantly higher mortality and MACE rates than patients in the low→low and high→ low groups, and indistinguishable from the high→high group.
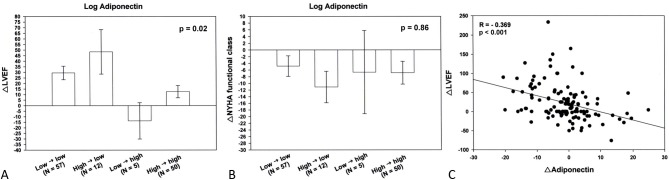
Changes in LVEF and NYHA functional class from baseline to 3 months according to the type of changes in adiponectin are presented in Figure 5A and 5B. Significant improvements in LVEF and NYHA functional class at 3 months were observed both in the patients with adiponectin levels stable below the median (low→low) and in the patients with improved adiponectin from above to below the median (high→low). Changes in LVEF and NYHA functional class were similar in both groups at 3 months. Patients who worsened in terms of adiponectin levels (low→high) were the only ones displaying reduction in LVEF at 3 months, although the 95% confidence intervals do not cross the zero line. Also, the NYHA functional class was unchanged after treatment. Although LVEF was only minimally improved, the NYHA functional class was improved in patients with adiponectin stable above the median (high→high) at 3 months. In other words, worsening of adiponectin (i.e., low→high group) was not associated with improvement in LVEF and NYHA functional class after treatment. Actually, a significant negative correlation, with the correlation coefficients between changes in adiponectin and changes in LVEF of -0.369 (p < 0.001) was noted (Figure 5C).
Figure 5.
Changes in left ventricular ejection fraction (LVEF) (A) and New York Heart Association (NYHA) functional class (B) by the 4 subgroups of changes in adiponection from baseline to 3 months were illustrated. Changes are expressed as least square means (95% confidence limits). A significant negative correlation, with the correlation coefficients between changes in adiponectin and changes in LVEF of -0.369 (p < 0.001) was also noted (C).
DISCUSSION
In patients with chronic stable symptomatic HF, the present study confirmed that changes in adiponectin levels over time with respective to a threshold value of 8138 ng/mL (log adiponectin = 3.91 ng/mL) convey significant additional prognostic information on top of baseline adiponectin values. Moreover, simultaneous assessment of adiponectin and NT-proBNP provided complementary information and enabled powerful prediction of a patient’s risk of MACE in patients with chronic HF.
As far as we know, although experimental studies have suggested that adiponectin has a cardioprotective effect,4,6,8,19,22 administration of adiponectin can nevertheless increase energy expenditure and decrease body weight in lab animals.23,24 Moreover, globular adiponectin may activate nuclear factor – kB and activating protein-1, resulting in expression of proinflammatory genes and enhancement of angiotensin II – induced proliferation in cardiac fibroblasts, which may in turn play a role in HF progression.25,26 The positive correlation between adiponectin and NT-proBNP is in keeping with the identified lipolytic and lipid-mobilization effects of natriuretic peptides.27 It suggests that high levels of BNP might, through their lipolytic effect, exaggerate the wasting process in HF and increase adiponectin levels. Hence, rather than merely being a biomarker reflecting changes in body composition, adiponectin may be involved in the metabolic and endocrine imbalances and inflammation accompanying HF and negatively affect the prognosis in patients with established HF.2,4,7-9,23-25 Therefore, adiponectin may be considered as a cardio-metabolic, and BNP or NT-proBNP a hemodynamic, biomarker in patients with HF; in fact, they are interrelated but can provide complimentary information.28
Because circulating natriuretic peptides are among the most sensitive and specific biomarkers of HF and subsequent outcomes,29,30 whether measurements of adiponectin can provide additional prognostic information to the NT-proBNP levels or not, is a major concern. In our previous report, we demonstrated that a single measurement of adiponectin level at baseline is an independent predictor of prognosis in chronic HF patients, especially in those with an elevated NT-proBNP level.5 The present study further demonstrated that high adiponectin levels after treatment may convey significant additional prognostic information on top of baseline adiponectin values, and the combined evaluation of both parameters as a method of risk detection is superior to the use of either adiponectin or NT-proBNP alone.
The present study has demonstrated a proof of concept that changes in adiponectin and NT-proBNP levels is related to prognosis in chronic HF. The clinical message that can be applied is that once a particular anti-HF treatment has been initiated, serial measurements of both adiponectin and NT-proBNP may help to indicate how patients respond to the treatment. Subsequent adjustments should be made according to the responses indicated by the changes in biomarker levels. If both the adiponectin and NT-proBNP levels either remain or become low, supported by similar clinical features, then the treatment can be considered to be beneficial or satisfactory, and should be continued. If the biomarker levels remain or become high, then alternative therapies should be considered, and instituted as required in an attempt to improve the condition of the patients with HF progression. However, although the prognostic value of repeated determinations of adiponectin has been shown in patients in acute decompensated HF13 and chronic stable HF as in the present study, it was difficult to prove convincingly just how much additional information in terms of outcome serial measurements was conveyed because of the limited sample size and the heterogeneity of the patients being studied. The bottom line is that our study ascertained the course of time allowing adiponectin to reflect the progression of HF and the outcomes by coupling adiponectin and NT-proBNP, which may improve the accuracy of the prognostic stratification of patients treated for chronic HF.
Regarding the possible pathophysiologic basis to those changes in adiponectin over time following treatment in patients with chronic stable HF, our data demonstrated that similar concentrations of adiponectin can have profoundly different prognostic values, depending on the trend over time in these patients. For example, patients with baseline adiponectin values below the median have significant lower mortality hazards of those with baseline adiponectin values above the median, but the subgroup of those whose adiponectin increased to above median (low→high) at 3 months, had a remarkably high risk for mortality and adverse outcomes compared with those whose adiponectin levels remained below median (low→low). Similarly, those with elevated baseline adiponectin levels had really low hazards if their adiponectin levels decreased below the median at 3 months (high→low), compared with the subgroup whose adiponectin remained elevated (high→ high). Simplistically, a trend in adiponectin to decrease seems to reflect an improvement in HF possibly because of effective treatment; while an increase in adiponectin suggests progression of HF. The findings of the present study are consistent with those reported in patients with acute decompensated HF showing that serum adiponectin concentrations was elevated in acute decompensated HF and decreased following the treatment, and how much adiponectin decreases in response to treatment is an important determinant of the prognosis.13 Those observations receive further support from the consistency of our findings in terms of a broader spectrum of chronic stable HF patients being recruited and measurements of adiponectin over a longer period of time after treatment.
The fact that patients whose adiponectin decreases over 3 months from above to below the median (high→low) may have a mortality and MACE rates similar to those who have been constantly below (low→low) suggests that outcome improvement can be detected in some patients with chronic HF in relatively short times. On the other hand, patients whose adiponectin levels worsened from below to above the median (low→high) had a worse outcome suggests that patients whose adiponectin increases over time (HF progression) carry a significant higher risk than those patients with advanced HF, but in stable condition after treatment. Our findings regarding the worsening or improving of adiponectin over time is associated with qualitatively similar changes in LVEF and NYHA functional class provide physiologic and clinical support to the present analysis and extend the previous observations.13,14 In particular, given the overall trend toward improvement over time in LVEF and NYHA functional class, the group low→high shows a poor response in terms of improvement of LVEF and NYHA class after treatment. This finding supports the close relationship not only between LV structural changes and clinical symptoms and circulating adiponectin, but also outcome in HF.5-15
Last but not least, we demonstrated in Table 3 that the prescription rate of angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers was significantly lower in the low→high group, and that of the beta-blockers was significantly lower in the high→high group. Although the higher incidence of chronic kidney disease in the low→high and high→high groups may partially explain the underuse of evidence-based therapies in those patients, the low rates of prescription of drugs based on evidence in the study patients as a whole suggest that searching for a better therapy for HF is urgently necessary. Actually, reliable data from certain recent studies have reported that Taiwanese HF patients have inferior outcomes to those from other countries, with reduced quality of life, more re-hospitalizations, and a greater incidence of cardiovascular death, which might have resulted from underperformance of hospital physicians in HF diagnosis and management, especially the underuse and under-dosing of evidence-based therapies.31-33 Therefore, there is a major unmet need for better therapies for HF in Taiwan. How to overcome the possible underlying obstacles facilitating underperformance of HF treatment in Taiwan remains of paramount importance, such as unfamiliarity with the impact of HF and exaggerated concerns over treatment risks and side-effects, so as to provide timely and appropriate anti-HF treatments not just relieving the symptoms but also improving the overall morbidity and mortality.
Several limitations have to be acknowledged. First, the current study is limited by its modest sample size. The relationship of these biomarkers to prognosis on the basis of the limited numbers of MACE is relatively weak. Therefore, further studies on a larger scale will be needed to confirm and to refine these findings. Second, the cutoff points used for adiponectin and NT-proBNP in the present study population may not be applicable to other HF populations, although these significant cross-sectional associations suggest that our assay methods were reasonably robust. Finally, although potentially clinically relevant, the value of changes of adiponectin over time intervals of longer than 3 months also cannot be assessed. More serial measurements are needed to quantify and correct for possible underestimation or overestimation of associations due to fluctuations of adiponectin values within an individual over time.
CONCLUSIONS
Even if a single adiponectin measurement may provide prognostic information in HF, the present study demonstrated evidence in favor of the use of changes in adiponectin over time in the management of patients with chronic stable HF, and provides a pathophysiologic basis to these changes. When adiopnectin is used in conjunction with NT-proBNP in chronic HF settings, the prognostic value may be better than using each biomarker separately. The decision-making process outlined above should be tested in studies of larger scale and with longer term of follow-up.
Acknowledgments
This study was supported by NSC grant 99-2320-B-350-002-MY3 and Cheng Hsin General Hospital grant 98-21.
CONFLICTS OF INTEREST
All the authors declare no conflict of interest.
REFERENCES
- 1.Sackner-Bernstein JD, Hart D. Neurohormonal antagonism in heart failure:what is the optimal strategy? Mt Sinai J Med. 2004;71:115–126. [PubMed] [Google Scholar]
- 2.Manabe I. Chronic inflammation links cardiovascular, metabolic and renal disease. Circ J. 2011;75:2739–2748. doi: 10.1253/circj.cj-11-1184. [DOI] [PubMed] [Google Scholar]
- 3.Gullestad L, Ueland T, Vinge LE, et al. Inflammatory cytokines in heart failure: mediators and markers. Cardiology. 2012;122:23–35. doi: 10.1159/000338166. [DOI] [PubMed] [Google Scholar]
- 4.Karmazyn M, Purdham DM, Rajapurohitam V, Zeidan A. Signalling mechanisms underlying the metabolic and other effects of adipokines on the heart. Cardiovasc Res. 2008;79:279–286. doi: 10.1093/cvr/cvn115. [DOI] [PubMed] [Google Scholar]
- 5.Yin WH, Wei J, Huang WP, et al. Prognostic value of circulating adipokine levels and expressions of adipokines in the myocardium of patients with chronic heart failure. Circ J. 2012;76:2139–2147. doi: 10.1253/circj.cj-11-1549. [DOI] [PubMed] [Google Scholar]
- 6.Park M, Sweeney G. Direct effects of adipokines on the heart: focus on adiponectin. Heart Fail Rev. 2013;18:631–644. doi: 10.1007/s10741-012-9337-8. [DOI] [PubMed] [Google Scholar]
- 7.Conraads VM, Van Craenenbroeck EM, De Maeyer C, et al. Unraveling new mechanisms of exercise intolerance in chronic heart failure: role of exercise training. Heart Fail Rev. 2013;18:65–77. doi: 10.1007/s10741-012-9324-0. [DOI] [PubMed] [Google Scholar]
- 8.Hatzis G, Deftereos S, Tousoulis D, et al. Adiponectin: merely a bystander or the missing link to cardiovascular disease? Curr Top Med Chem. 2013;13:139–163. doi: 10.2174/1568026611313020005. [DOI] [PubMed] [Google Scholar]
- 9.Van Berendoncks AM, Garnier A, Ventura-Clapier R, Conraads VM. Adiponectin: key role and potential target to reverse energy wasting in chronic heart failure. Heart Fail Rev. 2013;18:557–566. doi: 10.1007/s10741-012-9349-4. [DOI] [PubMed] [Google Scholar]
- 10.Kistorp C, Faber J, Galatius S, et al. Plasma adiponectin, body mass index, and mortality in patients with chronic heart failure. Circulation. 2005;112:1756–1762. doi: 10.1161/CIRCULATIONAHA.104.530972. [DOI] [PubMed] [Google Scholar]
- 11.Tamura T, Furukawa Y, Taniguchi R, et al. Serum adiponectin level as an independent predictor of mortality in patients with congestive heart failure. Circ J. 2007;71:623–630. doi: 10.1253/circj.71.623. [DOI] [PubMed] [Google Scholar]
- 12.McEntegart MB, Awede B, Petrie MC, et al. Increase in serum adiponectin concentration in patients with heart failure and cachexia: relationship with leptin, other cytokines, and B-type natriuretic peptide. Eur Heart J. 2007;28:829–835. doi: 10.1093/eurheartj/ehm033. [DOI] [PubMed] [Google Scholar]
- 13.Matsumoto M, Lee-Kawabata M, Tsujino T, et al. Decrease in serum adiponectin levels in response to treatment predicts good prognosis in acute decompensated heart failure. J Clin Hypertens (Greenwich) 2010;12:900–904. doi: 10.1111/j.1751-7176.2010.00368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamaji M, Tsutamoto T, Tanaka T, et al. Effect of carvedilol on plasma adiponectin concentration in patients with chronic heart failure. Circ J. 2009;73:1067–1073. doi: 10.1253/circj.cj-08-1026. [DOI] [PubMed] [Google Scholar]
- 15.Van Berendoncks AM, Beckers P, Hoymans VY, et al. Beta-blockers modify the prognostic value of adiponectin in chronic heart failure. Int J Cardiol. 2011;150:296–300. doi: 10.1016/j.ijcard.2010.04.039. [DOI] [PubMed] [Google Scholar]
- 16.Han SH, Quon MJ, Kim JA, Koh KK. Adiponectin and cardiovascular disease: response to therapeutic interventions. J Am Coll Cardiol. 2007;49:531–538. doi: 10.1016/j.jacc.2006.08.061. [DOI] [PubMed] [Google Scholar]
- 17.Koh KK, Quon MJ, Han SH, et al. Anti-inflammatory and metabolic effects of candesartan in hypertensive patients. Int J Cardiol. 2006;108:96–100. doi: 10.1016/j.ijcard.2005.07.040. [DOI] [PubMed] [Google Scholar]
- 18.Furuhashi M, Ura N, Higashiura K, et al. Blockade of the renin-angiotensin system increases adiponectin concentrations in patients with essential hypertension. Hypertension. 2003;42:76–81. doi: 10.1161/01.HYP.0000078490.59735.6E. [DOI] [PubMed] [Google Scholar]
- 19.Yu F, Chen R, Takahashi T, et al. Candesartan improves myocardial damage in obese mice with viral myocarditis and induces cardiac adiponectin. Int J Cardiol. 2008;129:414–421. doi: 10.1016/j.ijcard.2007.07.130. [DOI] [PubMed] [Google Scholar]
- 20.Lawlor DA, Davey Smith G, Ebrahim S, et al. Plasma adiponectin levels are associated with insulin resistance, but do not predict future risk of coronary heart disease in women. J Clin Endocrinol Metab. 2005;90:5677–5683. doi: 10.1210/jc.2005-0825. [DOI] [PubMed] [Google Scholar]
- 21.Sattar N, Wannamethee G, Sarwar N, et al. Adiponectin and coronary heart disease: a prospective study and meta-analysis. Circulation. 2006;114:623–629. doi: 10.1161/CIRCULATIONAHA.106.618918. [DOI] [PubMed] [Google Scholar]
- 22.Goldstein BJ, Scalia RG, Ma XL. Protective vascular and myocardial effects of adiponectin. Nat Clin Pract Cardiovasc Med. 2009;6:27–35. doi: 10.1038/ncpcardio1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fruebis J, Tsao TS, Javorschi S, et al. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci USA. 2001;98:2005–2010. doi: 10.1073/pnas.041591798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qi Y, Takahashi N, Hileman SM, et al. Adiponectin acts in the brain to decrease body weight. Nat Med. 2004;10:524–529. doi: 10.1038/nm1029. [DOI] [PubMed] [Google Scholar]
- 25.Hattori Y, Hattori S, Kasai K. Globular adiponectin activates nuclear factor-kappaB in vascular endothelial cells, which in turn induces expression of proinflammatory and adhesion molecule genes. Diabetes Care. 2006;29:139–141. doi: 10.2337/diacare.29.1.139. [DOI] [PubMed] [Google Scholar]
- 26.Hattori Y, Hattori S, Akimoto K, et al. Globular adiponectin activates nuclear factor-kappaB and activating protein-1 and enhances angiotensin II-induced proliferation in cardiac fibroblasts. Diabetes. 2007;56:804–808. doi: 10.2337/db06-1405. [DOI] [PubMed] [Google Scholar]
- 27.Sengenès C, Berlan M, De Glisezinski I, et al. Natriuretic peptides: a new lipolytic pathway in human adipocytes. FASEB J. 2000;14:1345–1351. [PubMed] [Google Scholar]
- 28.Okamoto H. Can adiponectin be a novel metabolic biomarker for heart failure? Circ J. 2009;73:1012–1013. doi: 10.1253/circj.cj-09-0235. [DOI] [PubMed] [Google Scholar]
- 29.Mentz RJ, Felker GM. Natriuretic peptide-guided therapy for heart failure. Circ J. 2011;75:2031–2037. doi: 10.1253/circj.cj-11-0660. [DOI] [PubMed] [Google Scholar]
- 30.Li JJ, Xiang XL, Tian XY, Shi YF. Clinical research on brain natriuretic peptide guiding the application of β1 receptor blocker in patients with moderate to severe heart failure. Acta Cardiol Sin. 2015;31:52–58. doi: 10.6515/ACS20140728A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang CH, Chien KL, Chen WJ, et al. Impact of heart failure and left ventricular function on long-term survival--report of a community-based cohort study in Taiwan. Eur J Heart Fail. 2007;9:587–593. doi: 10.1016/j.ejheart.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 32.Tseng CH. Clinical features of heart failure hospitalization in younger and elderly patients in Taiwan. Eur J Clin Invest. 2011;41:597–604. doi: 10.1111/j.1365-2362.2010.02447.x. [DOI] [PubMed] [Google Scholar]
- 33.Mao CT, Liu MH, Hsu KH, et al. Effect of multidisciplinary disease management for hospitalized heart failure under a national health insurance programme. J Cardiovasc Med. 2015;16:616–624. doi: 10.2459/JCM.0000000000000089. [DOI] [PubMed] [Google Scholar]