Abstract
Background
There is a lack of knowledge of those contemporary factors associated with modifying subtherapeutic treatments in hypercholesterolemic patients. The aim of this study was to assess determinants of treatment modification in patients not attaining their low-density lipoprotein cholesterol goals.
Methods
The centralized Pan-Asian survey on the under-treatment of hypercholesterolemia enrolled patients taking stable lipid-lowering medications. The study physicians then determined existing patient treatments, which were to be continued or modified when treatments failed. The patient questionnaire surveying patient attitudes and perceptions toward their hypercholesterolemia management was prospectively collected. The odds ratios (ORs) (95% confidence intervals) were calculated.
Results
Among the 420 patients included for analysis, 35.7% were designated for planned treatment modification. Those patients assigned to treatment modification were more likely to have a family history of premature coronary heart disease (40% vs. 19%), an indication for secondary prevention (76% vs. 61%), elevated triglyceride (60% vs. 48%) and fasting sugar (84% vs. 67%), and were less adherent to their medications (29% vs. 12%) than patients assigned to treatment continuation. Patient recognition of treatment failure [OR, 1.82 (1.13-2.94)], the lower frequency of cholesterol checkup [OR, 2.40 (1.41-4.08)], patient satisfaction with provided cholesterol information [OR, 2.30 (1.21-4.39)], and their feelings toward cholesterol management [OR, 0.25 (0.10-0.62) and 3.80 (2.28-6.32)] for confusion and no strong feeling, respectively were determinants of the treatment modification assignment.
Conclusions
There was a large gap between evidence-based goals and modification of subtherapeutic treatments, particularly among patients with lower treatment satisfaction and better compliance. Our findings have emphasized the need to further reduce inertia in implementing hypercholesterolemia management.
Keywords: Clinical inertia, Epidemiology, Hypercholesterolemia, Low-density lipoprotein cholesterol, Treatment modification
INTRODUCTION
Atherosclerotic disease is a substantial and growing global healthcare burden. The success in managing factors in cardiovascular health metrics, particularly high cholesterol, is associated with the reduction in cardiovascular mortality.1 Despite an overall increase in patient awareness and more aggressive therapeutic strategies, the control of hypercholesterolemia in patients at high cardiovascular risk is still suboptimal, even with contemporary treatments.2,3 Rates of low-density lipoprotein cholesterol (LDL-C) goal attainment has varied across Asian countries.4 Prospective registries and real-world data generally have suggested that the underuse of high-potency treatments is a prevalent phenomenon in Asia.5-7
Statin is a well-tolerated and effective agent to control LDL-C. The practice guidelines for lowering LDL-C recommend intensifying lipid-lowering treatments, either by increasing the therapeutic dose of statin or adding an additional agent to the standard dose statin, in patients who fail to attain LDL-C goals. However, the ineffective treatment in controlling LDL-C to the therapeutic target has rarely been adjusted.5 Patient attitudes and perceptions toward hypercholesterolemia and its management are important elements for physicians in their decisions to allocate effective statin treatments.8 Meanwhile, patient characteristics and educational status are further associated with maximization of lipid-lowering treatments in addition to aggressiveness of the healthcare system.9
Physician behaviors that recognize problems but fail to act accordingly define clinical inertia.10 Though well-recognized, there is not yet any published report on determinants of clinical inertia in response to patient attitudes and perceptions subsequent to patient failure in LDL-C goal attainment. The CEntralized Pan-Asian survey on tHE Under-treatment of hypercholeSterolemia (CEPHEUS-PA) assessed contemporary information on LDL-C goal attainment in patients with ≥ 2 coronary heart disease (CHD) risk factors. Follow-up treatment plans were given to patients who failed to attain their physician-designated LDL-C goals. This study, as part of the CEPHEUS-PA, investigated determinants of the assignment of treatment modification by using data from the Taiwanese cohort.
METHOD
The CEPHEUS-PA (ClinicalTrials.gov Identifier: NCT 00687492) enrolled patients who had been treated pharmacologically for hypercholesterolemia for ≥ 3 months (without treatment adjustment ≥ 6 weeks) across 8 Asian countries. Patient characteristics, the results of physical examinations, cardiovascular risk factors and histories, and indications for lipid-lowering treatments were retrieved by reviewing medical records. An overnight fasting blood sample was obtained from each patient at the beginning of this study to determine blood glucose and lipid concentrations at the local laboratory of each participating hospital.
The LDL-C goal of each patient was prespecified according to the recommendation of the 2004 National Cholesterol Education Program Adult Treatment Panel III guidelines. Before physical and biochemistry assessments began, patients were interviewed and completed the patient questionnaire regarding their attitudes, experiences, and perceptions toward hypercholesterolemia management, including satisfaction, compliance, and involvement in the process. In addition, the questionnaire also addressed their feelings regarding changes of hypercholesterolemia management.
After receiving laboratory results, physicians were asked to determine the follow-up treatment plan for each patient who was not at the designated LDL-C goal. The treatment plans included: 1) continuing existing treatments; 2) adding additional lifestyle modification; 3) intensifying the therapeutic dose; 4) switching to other medications; or 5) switching to other medications after intensifying the initial treatment.
The study protocol was approved by the local Institutional Review Board of each hospital, and written informed consent was obtained from each patient before enrollment. The design and main findings have been previously reported in detail.11 Only patients not attaining LDL-C goals, with valid data were included in this study.
Statistics
Continuous variables were presented in mean ± standard deviation, and no statistical comparison was made because of limited patient numbers in the different treatment plans. Categorical variables were presented in numbers and frequency distributions. Data stratified according to follow-up treatment plans were compared with the Chi-squared test, and factors potentially affecting the assignment of treatment plans were investigated in the univariate analysis. The odds ratio (OR) and associated 95% confidence interval (CI) were calculated. All analyses were based on the per-protocol population and were performed with Statistical Analysis System statistical software (Cary, NC, USA). Two-sided p values were calculated through the study.
RESULTS
Patient characteristics
Between April-December 2008, 1072 patients were enrolled in this study in Taiwan. The contemporaneous Taiwan National Health Insurance reimbursement policy for lipid-lowering treatments is presented in Table 1. Among 504 patients who failed to attain therapeutic LDL-C goals, 420 patients with valid data were included. The follow-up treatment plan to continue existing treatments was assigned to 270 patients, whereas the follow-up treatment plan to modify treatments was assigned to 150 patients (additional lifestyle modification planned for 38 patients, current dose intensification planned for 38 patients, medication switches planned for 63 patients, and medication switches after current dose intensification planned for 11 patients).
Table 1. The Taiwan National Health Insurance reimbursement policy for lipid-lowering treatments in 2008.
| LDL-C goal or TC goal | Pharmacological treatment* | |
| CHD or CHD risk equivalents# | LDL-C ≤ 100 mg/dL | LDL-C ≥ 130 mg/dL‡ |
| TC < 160 mg/dL | TC ≥ 200 mg/dL‡ | |
| ≥ 2 risk factors, 10-year risk > 20%† | LDL-C < 130 mg/dL | LDL-C ≥ 130 mg/dL§ |
| TC < 200 mg/dL | TC ≥ 200 mg/dL§ | |
| ≥ 2 risk factors, 10-year risk 10-20%† | LDL-C < 130 mg/dL | LDL-C ≥ 130 mg/dL§ |
| TC < 200 mg/dL | TC ≥ 200 mg/dL§ | |
| ≥ 2 risk factors, 10-year risk < 10%† | LDL-C < 130 mg/dL | LDL-C ≥ 130 mg/dL§ |
| TC < 200 mg/dL | TC ≥ 200 mg/dL§ | |
| 0-1 risk factor† | LDL-C < 160 mg/dL | LDL-C ≥ 160 mg/dL§ |
| TC < 240 mg/dL | TC ≥ 240 mg/dL§ |
* Down-titration of lipid-lowering drugs once patients are at the therapeutic goals is mandated. # Abdominal aortic aneurysm, > 50% carotid stenosis without previous stroke, and asymptomatic peripheral artery disease are not included; brain hemorrhage is included. † Risk factors do not include low high-density lipoprotein cholesterol. ‡ A lipid-lowering drug is indicated simultaneously with therapeutic lifestyle modification. § A lipid-lowering drug is indicated after therapeutic lifestyle modification fails.
CHD, coronary heart disease; LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol.
The majority of patients had complex cardiovascular risk factors. It appeared that distributions of CHD, very-high risk characteristics, and secondary prevention were greater in patients assigned to either the plan of dose intensification, medication switches, or both (Table 2). With respect to elements of metabolic syndrome, the most prevalent feature was elevated blood pressure, followed by impaired fasting glucose and abdominal obesity.
Table 2. Characteristics of patients who failed to attain LDL-C goals stratified by follow-up treatment plans.
| Continue existing treatment (n = 270) | Add additional lifestyle modification (n = 38) | Intensify therapeutic dose (n = 38) | Switch medication (n = 63) | Switch medication after dose intensification (n = 11) | |
| Age, years | 65 ± 11 | 63 ± 11 | 68 ± 11 | 65 ± 11 | 63 ± 15 |
| Women | 89 (33) | 16 (42) | 11 (29) | 26 (41) | 2 (18) |
| Smoking | 85 (31) | 11 (29) | 10 (26) | 16 (25) | 5 (45) |
| Family history of premature CHD | 52 (19) | 14 (37) | 21 (55) | 21 (33) | 2 (18) |
| Hypertension | 247 (91) | 35 (92) | 36 (95) | 57 (90) | 10 (91) |
| Diabetes | 140 (52) | 25 (66) | 20 (53) | 35 (56) | 6 (55) |
| Multiple risk factors with 10-year risk for CHD > 20% | 75 (28) | 5 (13) | 12 (32) | 11 (17) | 2 (18) |
| CHD | 149 (55) | 11 (29) | 28 (74) | 30 (48) | 9 (82) |
| Carotid artery disease | 0 (0) | 0 (0) | 3 (8) | 5 (8) | 1 (9) |
| LDL-C target < 70 mg/dL | 207 (77) | 23 (61) | 34 (89) | 49 (78) | 10 (91) |
| Very high-risk category | 197 (73) | 23 (61) | 34 (89) | 51 (81) | 10 (91) |
| Secondary prevention | 164 (61) | 30 (79) | 34 (89) | 40 (63) | 10 (91) |
| Statin monotherapy | 228 (84) | 29 (76) | 36 (95) | 54 (86) | 9 (82) |
| Metabolic syndrome element# | |||||
| Abdominal obesity | 192 (71) | 29 (76) | 29 (76) | 47 (75) | 5 (45) |
| Abnormal triglyceride | 127 (48) | 22 (58) | 22 (58) | 36 (57) | 10 (91) |
| Low HDL-C | 119 (45) | 19 (50) | 18 (47) | 28 (44) | 6 (55) |
| Abnormal BP | 236 (87) | 32 (84) | 38 (100) | 57 (90) | 10 (91) |
| Impaired fasting glucose | 179 (67) | 30 (79) | 32 (84) | 57 (90) | 7 (64) |
* Data are in numbers (%) unless noted otherwise. # Abdominal obesity (waist > 90 cm in men or > 80 cm in women); abnormal triglyceride (≥ 150 mg/dL); low HDL-C (< 40 mg/dL in men or < 50 mg/dL in women); abnormal BP (≥ 130/≥ 85 mmHg); impaired fasting glucose (≥ 100 mg/dL).
BP, blood pressure; CHD, coronary heart disease; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
Patient questionnaire
Patient perceptions about their satisfaction level and extent of compliance with hypercholesterolemia management are illustrated in Figure 1. In general, patients were satisfied with the level of information they received related to hypercholesterolemia (76% for patients assigned to treatment continuation vs. 88% for patients assigned to treatment modification; p value = 0.01). With respect to perceptions concerning compliance, the majority of patients agreed to always take their lipid-lowering medications (90% for patients assigned to treatment modification vs. 91% for patients assigned to treatment continuation; p value = 0.81) and disagreed with stopping medications when normal cholesterol level was reached (64% for both; p value = 0.96). However, 51% of patients assigned to treatment continuation and 56% of patients assigned to treatment modification sometimes forgot to take medications (p value = 0.37).
Figure 1.
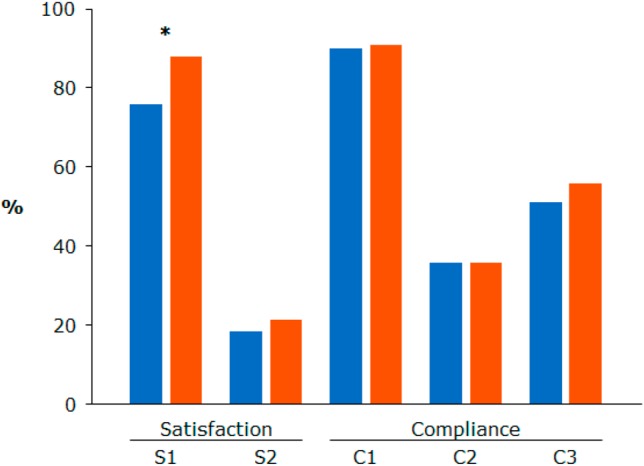
Patient perceptions toward satisfaction and compliance with hypercholesterolemia management. * p value < 0.05. Blue indicates patients assigned to treatment continuation; red indicates patients assigned to treatment modification. S1, feels satisfied with the level of information available to them about high cholesterol; S2, feels frustrated that they are unsure whether tablets are sufficiently effective; C1, always takes tablets daily to lower cholesterol; C2, stops tablets when cholesterol returns to normal; C3, sometimes forgets to take tablets.
Patients assigned to treatment modification had higher awareness, as high proportions of patients recognized hypercholesterolemia information or understood their current situations. Nevertheless, real compliance with their hypercholesterolemia management was suboptimal. Patient attitudes and experiences towards hypercholesterolemia management are presented in Table 3. A greater proportion of patients assigned to treatment modification rather than treatment continuation had their cholesterol checkup every 6-12 months and missed their lipid-lowering medications more than once a week (23% vs. 12% and 29% vs. 12%, respectively). Experiences in relation to initial hypercholesterolemia management were similar across both populations.
Table 3. Patient attitudes and experiences toward hypercholesterolemia management.
| Continue existing treatment (n = 270) | Modify existing treatment (n = 150) | p value | |
| Patient awareness | |||
| Heard or has been told about LDL-C | < 0.01 | ||
| Yes | 88 (33) | 77 (51) | |
| No | 106 (39) | 61 (41) | |
| Cannot remember | 76 (28) | 12 (8) | |
| Heard or has been told about HDL-C | < 0.01 | ||
| Yes | 87 (33) | 79 (53) | |
| No | 106 (40) | 59 (39) | |
| Cannot remember | 75 (28) | 12 (8) | |
| Has been informed about the cholesterol level | 0.07 | ||
| Yes | 154 (57) | 99 (66) | |
| No | 116 (43) | 51 (34) | |
| Has been given a target cholesterol level | < 0.01 | ||
| Yes | 107 (56) | 82 (79) | |
| No | 83 (44) | 22 (21) | |
| Current situation according to the patient | < 0.01 | ||
| Not been given a cholesterol target | 37 (16) | 32 (22) | |
| Not reach the cholesterol target | 14 (6) | 41 (28) | |
| Unsure whether reaching the cholesterol target | 147 (63) | 41 (28) | |
| Reached the cholesterol target | 35 (15) | 32 (22) | |
| Patient compliance | |||
| Frequency the patient was seen for checkup of cholesterol level | 0.01 | ||
| More frequently than once every 3 months | 67 (26) | 34 (25) | |
| Every 3 months | 163 (63) | 71 (52) | |
| Every 6 to 12 months | 31 (12) | 32 (23) | |
| Frequency of lipid-lowering medications missed | < 0.01 | ||
| More than once a week | 18 (12) | 24 (29) | |
| Once a week or less | 126 (88) | 59 (71) | |
| Frequency that patient thought that missing medications would not affect the cholesterol level | 0.22 | ||
| More than once a week | 64 (29) | 25 (23) | |
| Once a week or less | 156 (71) | 85 (77) | |
| Patient experience | |||
| Measures taken by the doctor when the patient was first diagnosed | 0.64 | ||
| Only advised to change lifestyle | 21 (8) | 16 (11) | |
| Only prescribed medications | 43 (16) | 21 (14) | |
| Both advised lifestyle changes and prescribed medications | 201 (76) | 111 (75) | |
| Neither advised lifestyle changes nor prescribed medications | 1 (< 1) | 0 (0) | |
| Changes of lipid-lowering medications since first prescribed | 0.50 | ||
| Still on the same tablet | 163 (70) | 95 (64) | |
| Still on the same tablet but the dose has been increased | 4 (2) | 3 (2) | |
| Have changed tablets | 66 (28) | 50 (34) |
* Data are in numbers (%).
HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
With respect to patient involvement with hypercholesterolemia management, most patients were satisfied and motivated; only a few expressed concerns. A larger proportion of patients assigned to treatment continuation felt confused by cholesterol management relative to patients assigned to treatment modification (16% vs. 5%; p value < 0.01). A greater proportion of patients assigned to treatment modification had no strong feeling compared with patients assigned to treatment continuation (81% vs. 52%; p value < 0.01). The feelings of patients toward treatment changes were only exploratory since less than a quarter of patients completed the questionnaire. The majority of patients were satisfied with their medical management, whether they were assigned to the continuation or modification groups (88% for patients assigned to treatment continuation vs. 92% for patients assigned to treatment modification; p value = 0.47). However, a modest majority of patients still had no strong feeling (58% for patients assigned to treatment continuation and 73% for patients assigned to treatment modification; p value = 0.15) (Figure 2).
Figure 2.
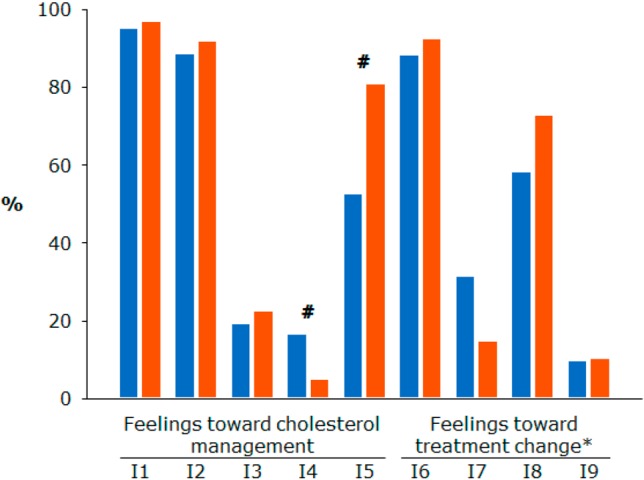
Patient involvement with hypercholesterolemia management and feelings to treatment changes. * Less than 25% of patients answered this part of the questionnaire. # p value < 0.05. Blue indicates patients assigned to treatment continuation; red indicates patients assigned to treatment modification. I1, being satisfied with cholesterol management; I2, being motivated by cholesterol management; I3, being concerned about cholesterol management; I4, being confused by cholesterol management; I5, having no strong feeling about cholesterol management; I6, being satisfied with treatment change; I7, being concerned that condition is now a serious illness; I8, having strong feelings; I9, being less motivated to keep taking tablets.
Determinants of the treatment modification assignment
Treatment modification was more likely to be assigned to patients with a family history of premature CHD (OR, 2.67; 95% CI, 1.71-4.18; p value < 0.01), an indication for secondary prevention (OR, 2.07; 95% CI, 1.32-3.24; p value < 0.01), triglycerides ≥ 150 mg/dL (OR, 1.65; 95% CI, 1.10-2.48; p value = 0.02), and fasting glucose ≥ 100 mg/dL (OR, 2.58; 95% CI, 1.56-4.28; p value < 0.01). Univariate determinants of treatment plan assignment are presented in Table 4. Other determinants included patients who were given a target cholesterol level (OR, 2.89; 95% CI, 1.67-5.02; p value < 0.01), patients who were aware of not achieving targets (OR, 1.82; 95% CI, 1.13-2.94; p value = 0.01), and patients who were seen for cholesterol checkup less frequently than every 3 months (OR, 2.40; 95% CI, 1.41-4.08; p value < 0.01).
Table 4. Determinants of the assignment of treatment modification.
| Odds ratio (95% confidence interval) | p value | |
| Family history of premature CHD, n = 419 | 2.67 (1.71-4.18) | < 0.01 |
| Secondary prevention, n = 417 | 2.07 (1.32-3.24) | < 0.01 |
| Metabolic syndrome element* | ||
| Abnormal triglycerides, n = 417 | 1.65 (1.10-2.48) | 0.02 |
| Impaired fasting glucose, n = 417 | 2.58 (1.56-4.28) | < 0.01 |
| Patients have been given a target cholesterol level, n = 294 | 2.89 (1.67-5.02) | < 0.01 |
| Patients were aware of not achieving targets, n = 412 | 1.82 (1.13-2.94) | 0.01 |
| Patients seen for cholesterol checkup less frequently than every 3 months, n = 402 | 2.40 (1.41-4.08) | < 0.01 |
| Frequency of lipid-lowering medications missed more than once a week, n = 227 | 2.85 (1.44-5.65) | < 0.01 |
| Satisfied with the level of information available to them about high cholesterol, n = 315 | 2.30 (1.21-4.39) | 0.01 |
| Feelings toward cholesterol management | ||
| Confused, n = 334 | 0.25 (0.10-0.62) | < 0.01 |
| No strong feeling, n = 347 | 3.80 (2.28-6.32) | < 0.01 |
* Abnormal triglyceride (≥ 150 mg/dL); impaired fasting glucose (≥ 100 mg/dL).
CHD, coronary heart disease
From the patient perspective, those who were satisfied with the lipid information provided (OR, 2.30; 95% CI, 1.21-4.39; p value = 0.01) or had no strong feeling about cholesterol management (OR, 3.80; 95% CI, 2.28-6.32; p value < 0.01) were more frequently assigned to treatment modification, whereas treatment continuation was more likely to be assigned to patients who were confused with their cholesterol management.
DISCUSSION
Our findings indicated that physicians tended to remain with the same treatments in the majority of patients at high cardiovascular risk, notwithstanding the fact that they failed to attain LDL-C goals. Among patients whose treatments would be modified by their physicians, medications were planned to be switched either promptly or after dose intensification in 49% of the patients. Furthermore, patients assigned to treatment modification had an enhanced awareness about, but lower compliance with hypercholesterolemia management.
The undertreatment of hypercholesterolemia has multiple facets involving both physicians and patients. Although lifestyle modification is the backbone of hypercholesterolemia management, adding additional lifestyle changes to an existing ineffective treatment may still be inadequate.12 Measures available to improve hypercholesterolemia control focus on the intensification of pharmacological treatments in addition to lifestyle modification.13 Since the majority of patients commonly receive low- or medium-potency treatments,14 even those patients at higher cardiovascular risk, up-titration of existing treatments is a useful method for further LDL-C reductions.15 Combining another lipid-lowering agent with statin or switching to another more effective statin has also been proven effective for LDL-C goal attainment.16 Current evidence suggests that combination with ezetimibe reduces cardiovascular risk.17-19 Since the goal attainment rate plateaus after 3 months, even with up-titration of doses or medication switches,5 the most effective contemporary lipid-modifying treatment for LDL-C goal attainment is by use of a potent statin in the treatment initiation before the approval of new agents.
The final decisions of physicians influence the quality and quantity of information they provide to their patients. Well-informed patients might have a greater understanding of their disease, which can encourage their involvement with treatment and further affect their motivation to comply with treatment.20 The positive treatment experience of physicians and good patient compliance was associated with LDL-C goal attainment.8 Our findings also collaterally reflect the physician attitude that patients with positive treatment experiences should be treated more aggressively, as treatment modification was more likely assigned to patients who were better informed or aware of their situations. Public awareness campaigns have been successful in disease management.21,22 Currently, technology solutions by improved patient engagement have been employed to improve heath.23 With media coverage and social networking, awareness can in fact be raised.24,25 As a result, multichannel education programs are warranted to increase the knowledge and awareness of patients to overcome the large treatment gap between the guideline recommendations and real-world LDL-C goal attainment rates.
We found no statistical difference in the proportion of patients who would sometimes miss taking their tablets and those patients assigned to either strategy when assessing patient perceptions toward their compliance with hypercholesterolemia management in general. However, once patients were asked to recall and indicate real compliance, those who forgot medications more than once a week were more likely to be assigned to treatment modification. In all likelihood, physicians were probably aware that poor patient compliance was associated with treatment failure; therefore, they were eager to change therapeutic strategies in those patients.
In the CEPHEUS-PA, physicians were blinded to results of the patient questionnaire and determined follow-up treatment plans of their patients after receiving laboratory results. Our results strongly mirrored the opinions and knowledge of physicians about the clinical guidelines and their applications to their patients. In the real world, patients at high cardiovascular risk were more likely to have stringent LDL-C goals.26 Despite advances that have been made in cardiovascular disease assessment and treatment, with physicians typically confident about their disease management,8,26 such patients were less likely to attain their therapeutic goals.27 Although evidence supports the use of statin drugs in patients with CHD at LDL-C levels lower than the current insurance reimbursement policy,28 recent data have persistently demonstrated the underuse of secondary prevention drugs for cardiovascular disease.29 Therefore, the need to improve preventive measures remains high until healthy lifestyles can be adopted and implemented throughout the human lifespan. Adequate hypercholesterolemia control depends largely on aggressive lifestyle management, both in diet and physical activity, and in proactive pharmacological modification, particularly in patients at high cardiovascular risk.30 Finally, our findings support the continuous need to advocate against inertia that can lull physicians and their patients into maintaining existing ineffective treatments and to reinforce compliance of patients in the implementation of hypercholesterolemia management.
Limitations
The CEPHEUS-PA was cross-sectional and observational, which by its nature had several limitations that need to be addressed. First, patients to whom a certain follow-up treatment plan was assigned might not have precisely received that particular treatment. Second, patient compliance with hypercholesterolemia management was evaluated by patient questionnaire and their recollections instead of ascertainment from medical records or prescription refills. Third, some factors such as LDL-C levels, socio-economic status, and patient perspectives were not included in the analysis; the physician characteristics were not included in this study as well. Fourth, this part of the analyses focused on Taiwanese patients, which may not be representative of a larger population for purposes of estimating treatment failure. Finally, implications of inertia to continue the same treatment in patients who have failed to attain LDL-C goals certainly necessitates a follow-up study.
CONCLUSIONS
The majority of patients who failed to attain their LDL-C goals were allocated to remaining the current treatment and patient involvement had a complex interaction with the allocation of treatment strategies. Our findings advocate to reduce inertia in remaining the ineffective treatment in very-high-risk patients and to reinforce patient compliance to hypercholesterolemia management.
LIST OF ABBREVIATIONS
CEPHEUS-PA: CEntralized Pan-Asian survey on tHE Under-treatment of hypercholeSterolemia.
CHD: coronary heart disease.
LDL-C: low-density lipoprotein cholesterol.
Acknowledgments
This study was supported in part by grants from the Ministry of Health and Welfare (MOHW106-TDU-B-211-113001) and the Ministry of Science and Technology (MOST104-2314-B-075-028) and was financially supported and monitored by AstraZeneca (as part of the CEPHEUS-PA). The responsibility for opinions, conclusions, and interpretation of data was attributable to authors only.
DISCLOSURE
Kang-Ling Wang has received honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, and Daiichi-Sankyo outside the submitted work.
All other authors have no conflict of interest to disclose.
REFERENCES
- 1.Huang WC, Lin TW, Chiou KR, et al. The effect of intensified low density lipoprotein cholesterol reduction on recurrent myocardial infarction and cardiovascular mortality. Acta Cardiol Sin. 2013;29:404–412. [PMC free article] [PubMed] [Google Scholar]
- 2.Waters DD, Brotons C, Chiang CW, et al. Lipid treatment assessment project 2: a multinational survey to evaluate the proportion of patients achieving low-density lipoprotein cholesterol goals. Circulation. 2009;120:28–34. doi: 10.1161/CIRCULATIONAHA.108.838466. [DOI] [PubMed] [Google Scholar]
- 3.Chiang CE, Ferrieres J, Gotcheva NN, et al. Suboptimal control of lipid levels: results from 29 countries participating in the centralized pan-regional surveys on the undertreatment of hypercholesterolaemia (CEPHEUS). J Atheroscler Thromb. 2016;23:567–587. doi: 10.5551/jat.31179. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen HN, Fujiyoshi A, Abbott RD, Miura K. Epidemiology of cardiovascular risk factors in Asian countries. Circ J. 2013;77:2851–2859. doi: 10.1253/circj.cj-13-1292. [DOI] [PubMed] [Google Scholar]
- 5.Kim HS, Wu Y, Lin SJ, et al. Current status of cholesterol goal attainment after statin therapy among patients with hypercholesterolemia in Asian countries and region: the Return on Expenditure Achieved for Lipid Therapy in Asia (REALITY-Asia) study. Curr Med Res Opin. 2008;24:1951–1963. doi: 10.1185/03007990802138731. [DOI] [PubMed] [Google Scholar]
- 6.Wang KL, Liu CJ, Chao TF, et al. Statins, risk of diabetes, and implications on outcomes in the general population. J Am Coll Cardiol. 2012;60:1231–1238. doi: 10.1016/j.jacc.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 7.Munawar M, Hartono B, Rifqi S. LDL cholesterol goal attainment in hypercholesterolemia: CEPHEUS Indonesian Survey. Acta Cardiol Sin. 2013;29:71–81. [PMC free article] [PubMed] [Google Scholar]
- 8.Wang KL, Wu CH, Wang KF, et al. The Association between low-density lipoprotein cholesterol goal attainment, physician and patient attitudes and perceptions, and healthcare policy. J Atheroscler Thromb. 2014;21:1044–1054. doi: 10.5551/jat.24158. [DOI] [PubMed] [Google Scholar]
- 9.Arnold SV, Kosiborod M, Tang F, et al. Patterns of statin initiation, intensification, and maximization among patients hospitalized with an acute myocardial infarction. Circulation. 2014;129:1303–1309. doi: 10.1161/CIRCULATIONAHA.113.003589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phillips LS, Branch WT, Cook CB, et al. Clinical inertia. Ann Intern Med. 2001;135:825–834. doi: 10.7326/0003-4819-135-9-200111060-00012. [DOI] [PubMed] [Google Scholar]
- 11.Park JE, Chiang CE, Munawar M, et al. Lipid-lowering treatment in hypercholesterolaemic patients: the CEPHEUS Pan-Asian survey. Eur J Prev Cardiol. 2012;19:781–794. doi: 10.1177/1741826710397100. [DOI] [PubMed] [Google Scholar]
- 12.Stefanick ML, Mackey S, Sheehan M, et al. Effects of diet and exercise in men and postmenopausal women with low levels of HDL cholesterol and high levels of LDL cholesterol. N Engl J Med. 1998;339:12–20. doi: 10.1056/NEJM199807023390103. [DOI] [PubMed] [Google Scholar]
- 13.Catapano AL. Perspectives on low-density lipoprotein cholesterol goal achievement. Curr Med Res Opin. 2009;25:431–447. doi: 10.1185/03007990802631438. [DOI] [PubMed] [Google Scholar]
- 14.Wang KL, Liu CJ, Chao TF, et al. Risk of new-onset diabetes mellitus versus reduction in cardiovascular events with statin therapy. Am J Cardiol. 2014;113:631–636. doi: 10.1016/j.amjcard.2013.10.043. [DOI] [PubMed] [Google Scholar]
- 15.Chen YP, Chang KC, Tseng WK, et al. Increased rosuvastatin dose versus concomitant fenofibrate and rosuvastatin therapy to achieve lipid goal in patients with diabetes or atherosclerosis with metabolic syndrome. Acta Cardiol Sin. 2013;29:421–428. [PMC free article] [PubMed] [Google Scholar]
- 16.Bays HE, Averna M, Majul C, et al. Efficacy and safety of ezetimibe added to atorvastatin versus atorvastatin uptitration or switching to rosuvastatin in patients with primary hypercholesterolemia. Am J Cardiol. 2013;112:1885–1895. doi: 10.1016/j.amjcard.2013.08.031. [DOI] [PubMed] [Google Scholar]
- 17.Ginsberg HN, Elam MB, Lovato LC, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563–1574. doi: 10.1056/NEJMoa1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 19.Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397. doi: 10.1056/NEJMoa1410489. [DOI] [PubMed] [Google Scholar]
- 20.Kitagawa Y, Teramoto T, Daida H. Adherence to preferable behavior for lipid control by high-risk dyslipidemic Japanese patients under pravastatin treatment: the APPROACH-J study. J Atheroscler Thromb. 2012;19:795–805. doi: 10.5551/jat.12682. [DOI] [PubMed] [Google Scholar]
- 21.Tomkowski WZ, Dybowska M, Kuca P, et al. Effect of a public awareness campaign on the incidence of symptomatic objectively confirmed deep vein thrombosis: a controlled study. J Thromb Haemost. 2012;10:2287–2290. doi: 10.1111/j.1538-7836.2012.04915.x. [DOI] [PubMed] [Google Scholar]
- 22.Nishijima H, Kon T, Ueno T, et al. Effect of educational television commercial on pre-hospital delay in patients with ischemic stroke. Neurol Sci. 2016;37:105–109. doi: 10.1007/s10072-015-2372-1. [DOI] [PubMed] [Google Scholar]
- 23.Lee JM, Hirschfeld E, Wedding J. A patient-designed do-it-yourself mobile technology system for diabetes: promise and challenges for a new era in medicine. JAMA. 2016;315:1447–1448. doi: 10.1001/jama.2016.1903. [DOI] [PubMed] [Google Scholar]
- 24.Brzezinski M, Klikowicz P. Facebook as a medium for promoting statement of intent for organ donation: 5-years of experience. Ann Transplant. 2015;20:141–146. doi: 10.12659/AOT.892494. [DOI] [PubMed] [Google Scholar]
- 25.Advani R, Naess H, Kurz M. Mass media intervention in Western Norway aimed at improving public recognition of stroke, emergency response, and acute treatment. J Stroke Cerebrovasc Dis. 2016;25:1467–1472. doi: 10.1016/j.jstrokecerebrovasdis.2016.02.026. [DOI] [PubMed] [Google Scholar]
- 26.Wang KF, Chang CC, Wang KL, et al. Determinants of low-density lipoprotein cholesterol goal attainment: insights from the CEPHEUS Pan-Asian Survey. J Chin Med Assoc. 2014;77:61–67. doi: 10.1016/j.jcma.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 27.Su MI, Tsai CT, Yeh HI, Chen CY. Factors associated with lipid goal attainment among patients with deployed drug eluting stent. Acta Cardiol Sin. 2014;30:325–332. [PMC free article] [PubMed] [Google Scholar]
- 28.Kuwabara M, Kondo F, Hamada T, et al. Impact of statins therapy for ischemic heart disease patients with low-density lipoprotein cholesterol levels less than 100 mg/dL. Acta Cardiol Sin. 2016;32:565–569. doi: 10.6515/ACS20151013E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin YC, Yang CC, Chen YJ, et al. Utilization of statins and aspirin among patients with diabetes and hyperlipidemia: Taiwan, 1998-2006. J Chin Med Assoc. 2012;75:567–572. doi: 10.1016/j.jcma.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 30.Koba S, Maruyama C, Sasaki J. Comment on the 2013 ACC/AHA Guidelines on lifestyle management to reduce cardiovascular risk by the JAS Guidelines Committee. J Atheroscler Thromb. 2014;21:375–377. doi: 10.5551/jat.ed003. [DOI] [PubMed] [Google Scholar]


