Abstract
Background
Although advancements in the treatment of atrial fibrillation have improved patient prognosis for this persistent condition, interest in atrial fibrillation development is growing. Of note is the fact that additional attention is being focused on the accompanying effect of insomnia. The aim of the study was to investigate the effects of insomnia on the risk of atrial fibrillation development.
Methods
This was a nationwide population-based retrospective cohort study using data from the Taiwan National health Insurance Research Database. We analyzed 64,421 insomnia cases and 128,842 matched controls without insomnia from January 1, 2000, to December 31, 2010. A Cox regression model was used to estimate the adjusted hazard ratios (HRs) and 95% confidence intervals (CI) for atrial fibrillation development.
Results
During the follow-up period, the incidence of atrial fibrillation development was significantly higher in the insomnia cases than in the comparison cohort (2.6% vs. 2.3%, p < 0.001). Insomnia was associated with an increased risk of atrial fibrillation (HR = 1.08, 95% CI: 1.01-1.14). Males, those > 65 years of age, and patients with peripheral artery disease who have insomnia had a higher rate of atrial fibrillation development.
Conclusions
The findings of this nationwide analysis support the hypothesis that insomnia is associated with a significant risk of atrial fibrillation development.
Keywords: Atrial fibrillation, Cohort study, Insomnia
INTRODUCTION
The incidence of insomnia is increasing worldwide, especially in developed and developing countries.1,2 Insomnia is associated with an increased risk of accidents, increased health care utilization, and diminished work and academic performance as well as poor quality of life.3 Moreover, insomnia has detrimental effects on health such as increasing the risk for cardiovascular disease, diabetes mellitus, obesity, cancer, depression, and total mortality.2,4
Atrial fibrillation is the most common sustained cardiac arrhythmia, and occurs in approximately 2% of the general population.5,6 Atrial fibrillation is associated with decreased quality of life, increased thromboembolic events, and increased rates of death.7-9 Most importantly, stroke due to atrial fibrillation is often severe and results in long-term disability or death.5 Although advancements in the diagnosis and treatment of atrial fibrillation have improved its prognosis, interest in atrial fibrillation development is growing.
The association of insomnia and arrhythmias is important and of substantial interest, but has rarely been studied. The definitive mechanism and effects of insomnia on arrhythmias, including atrial fibrillation, are not well-known. We hypothesized that insomnia could be associated with the risk of atrial fibrillation development. To test this hypothesis, we conducted a retrospective cohort study in Taiwan to investigate the effects of insomnia on the risk of atrial fibrillation development.
MATERIALS AND METHODS
Data source
The National Health Insurance (NHI) program, which was started in Taiwan on March 1, 1995, is a national compulsory health insurance program. Under this nationwide health insurance, up to 99% of the nation’s population receives a wide range of health care services including outpatient services, inpatient care, traditional Chinese medicine, dental care, prenatal care/obstetric services, physical therapy, preventive health care, home care, and rehabilitation. The NHI maintains a comprehensive, validated patient database, which contains information on patient diagnoses and drug prescriptions. The quality of its information on prescription use, diagnoses, and hospitalisations has been shown to be excellent.10 The NHI sample files, which are constructed and managed by the National Health Research Institute, consist of comprehensive use and enrolment information for a randomly selected sample of 1,000,000 NHI beneficiaries, representing approximately 5% of all the enrolled persons in Taiwan in 2000. A multistage stratified systematic sampling design was used to create the sample, and there are no statistically significant differences in sex or age between the sample group and all enrollees.
All information allowing a specific patient to be identified has been encrypted. The confidentiality of the data abides by the data regulations of the Bureau of National Health Insurance. The Institutional Review Board (IRB) of Taipei City Hospital approved this study (IRB No.: TCHIRB-1020715-W).
Insomnia cases and control subjects
A retrospective cohort study was conducted from January 1, 2000 to December 31, 2010 based on ambulatory care and inpatient discharge records. In order to improve the accuracy of insomnia diagnosis,11 patients with a diagnosis of insomnia 3 times [based on International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) code 780.52] within one year in the claims database between January 1, 2000 and December 31, 2010 were retrieved as case subjects from the NHI database (Figure 1). Patients younger than age 18 were excluded because the number of patients within that age group was relatively low. Patients with a diagnosis of hyperthyroidism (ICD-9-CM codes 242.9) were also excluded. After age, gender, and the same observational period matching of cases and insomnia-free controls at a ratio of 1:2, we reached a final total of 64,421 insomnia cases and 128,842 matched controls without insomnia. Matched controls without insomnia by using propensity score (as described by Rosenbaum and Rubin)12 were also calculated.
Figure 1.
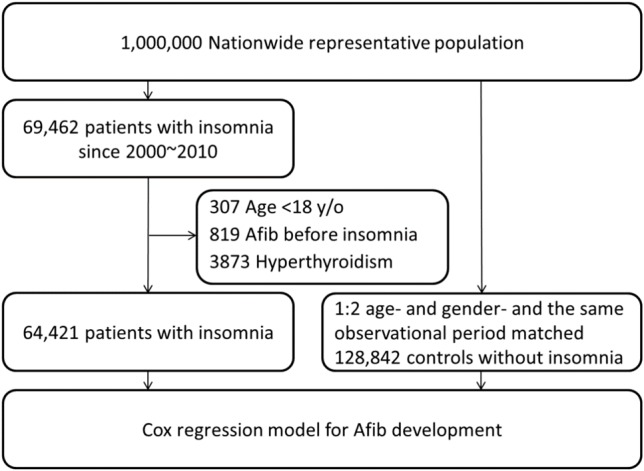
Flow diagram illustrating the selection of patients and controls. Afib, atrial fibrillation.
Outcome measures
Both cohorts were followed-up from the index date to the onset of atrial fibrillation (ICD-9-CM codes 427.31), withdrawal from the insurance system, death, or until December 31, 2010. Atrial fibrillation development is defined as the diagnosis of atrial fibrillation in an out-patient department and admission. Comorbidities were recorded for patients who were identified either in an inpatient setting or from 3 or more ambulatory care claims with the diagnosis hypertension (ICD-9-CM codes 401-405), diabetes mellitus (ICD-9-CM code 250), hyperlipidemia (ICD-9-CM code 272), and chronic obstructive pulmonary disease (COPD) (ICD-9-CM code 496).
Statistical analysis
The risk of atrial fibrillation development among insomnia patients and insomnia-free controls between January 1, 2000 and December 31, 2010 was analyzed. The atrial fibrillation-free survival rates of the 2 cohorts were estimated using the Kaplan-Meier method, utilizing the log-rank test. A Cox regression model was used to estimate the adjusted hazard ratio (HR) and 95% confidence interval (CI) for atrial fibrillation risk. Potential risk factors for the development of atrial fibrillation6,13,14 including diabetes mellitus, hypertension, dyslipidemia, COPD, congestive heart failure, coronary artery disease, chronic kidney disease, stroke, peripheral artery disease, sleep apnea, valvular heart diseases, depression and bipolar disorder were incorporated. Furthermore, the instances of insomnia diagnosis were also calculated. Statistical analysis was performed using the SAS statistical package version 9.2 (SAS Institute, Inc.).
RESULTS
From January 1, 2000 to December 31, 2010, 64,421 patients with insomnia were identified as the study cohort, and 128,842 matched subjects without insomnia were identified as the comparison cohort. Between 2000-2010, the median follow-up time in cases and controls was 5.91 years (25th-75th 3.30-8.40 years). Among cases and matched controls, approximately 60% were female and 51% were 41 to 65 years of age. The characteristics and comorbidities of patients included in this study are shown in Table 1. There was a higher rate of diabetes mellitus, hypertension, dyslipidemia, COPD, congestive heart failure, coronary artery disease, chronic kidney disease, stroke, peripheral artery disease, sleep apnea, valvular heart diseases, depression and bipolar disorder in the case group compared with the controls (all, p < 0.001). During the follow-up period of 5.91 years, the incidence of atrial fibrillation development was significantly higher in the cases than in the comparison cohort (2.6% vs. 2.3%, p < 0.001). The characteristics and comorbidities of patients included in this study by using propensity score are shown in Table 2.
Table 1. Baseline characteristics of the study subjects.
| Insomnia | Non-insomnia | p-value | |||
| n = 64421 | % | n = 128842 | % | ||
| Gender | 1.00 | ||||
| Female | 38860 | 60.3 | 77719 | 60.3 | |
| Male | 25561 | 39.7 | 51123 | 39.7 | |
| Age at baseline, y | 1.00 | ||||
| 18-40 | 14402 | 22.4 | 28804 | 22.4 | |
| 41-65 | 32621 | 50.6 | 65242 | 50.6 | |
| ≥ 65 | 17398 | 27 | 34796 | 27 | |
| Co-morbidity | |||||
| DM | 14041 | 21.8 | 26113 | 20.3 | < 0.001 |
| Hypertension | 29390 | 45.6 | 49272 | 38.2 | < 0.001 |
| Dyslipidemia | 20546 | 31.9 | 34812 | 27 | < 0.001 |
| COPD | 4679 | 7.3 | 6848 | 5.3 | < 0.001 |
| Congestive heart failure | 5287 | 8.2 | 8675 | 6.7 | < 0.001 |
| Coronary artery disease | 14954 | 23.2 | 22396 | 17.4 | < 0.001 |
| Chronic kidney disease | 2856 | 4.4 | 5106 | 4 | < 0.001 |
| Stroke | 4073 | 6.3 | 6984 | 5.4 | < 0.001 |
| Peripheral artery disease | 640 | 1 | 100 | 0.1 | < 0.001 |
| Sleep apnea | 216 | 0.3 | 282 | 0.2 | < 0.001 |
| Valvular heart diseases | 819 | 1.3 | 1160 | 0.9 | < 0.001 |
| Depression | 6291 | 9.8 | 3459 | 2.7 | < 0.001 |
| Bipolar disorder | 8274 | 12.8 | 4972 | 3.9 | < 0.001 |
| Afib | Median follow-up years = 5.91 (3.30-8.40) | < 0.001 | |||
| No | 62747 | 97.4 | 125917 | 97.7 | |
| Yes | 1674 | 2.6 | 2925 | 2.3 |
Afib, atrial fibrillation; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus.
Table 2. Baseline characteristics of the study subjects (propensity score matching).
| Variables | Insomnia | Non-insomnia | p-value | ||
| n = 59674 | % | n = 119348 | % | ||
| Gender | 0.65 | ||||
| Female | 35385 | 59.3 | 70902 | 59.4 | |
| Male | 24289 | 40.7 | 48446 | 40.6 | |
| Age at baseline, y | 0.31 | ||||
| 18-40 | 14322 | 24 | 28557 | 23.9 | |
| 41-65 | 30098 | 50.4 | 59887 | 50.2 | |
| ≥ 65 | 15254 | 25.6 | 30904 | 25.9 | |
| Co-morbidity | |||||
| DM | 12402 | 20.8 | 25317 | 21.2 | 0.04 |
| Hypertension | 25722 | 43.1 | 52361 | 43.9 | 0.002 |
| Dyslipidemia | 17839 | 29.9 | 36187 | 30.3 | 0.06 |
| COPD | 3895 | 6.5 | 8029 | 6.7 | 0.11 |
| Congestive heart failure | 4526 | 7.6 | 9236 | 7.7 | 0.25 |
| Coronary artery disease | 12692 | 21.3 | 25728 | 21.6 | 0.16 |
| Chronic kidney disease | 2516 | 4.2 | 5158 | 4.3 | 0.30 |
| Stroke | 3507 | 5.9 | 7291 | 6.1 | 0.05 |
| Peripheral artery disease | 548 | 0.9 | 1058 | 0.9 | 0.50 |
| Sleep apnea | 173 | 0.3 | 375 | 0.3 | 0.38 |
| Valvular heart diseases | 660 | 1.1 | 1377 | 1.2 | 0.37 |
| Depression | 3460 | 5.8 | 6373 | 5.3 | < 0.001 |
| Bipolar disorder | 4931 | 8.3 | 9506 | 8 | 0.03 |
| Afib | < 0.001 | ||||
| No | 57735 | 96.8 | 116353 | 97.5 | |
| Yes | 1939 | 3.2 | 2995 | 2.5 |
Afib, atrial fibrillation; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus.
The Kaplan-Meier survival analysis is shown in Figure 2, and indicates that the atrial fibrillation rate was significantly higher in insomnia patient than in the comparison cohort (log-rank test; p < 0.001). The results of the Cox regression model for predictors of atrial fibrillation development are shown in Table 3. Male gender, advanced age, hypertension, COPD, congestive heart failure, and coronary artery disease were associated with a higher rate of atrial fibrillation development. Insomnia was also associated with an increased risk of atrial fibrillation (HR = 1.08, 95% CI: 1.01-1.14 and by using propensity score HR = 1.33, 95% CI: 1.25-1.41). After insomnia frequency analysis, patients diagnosed with insomnia who had fewer than 10 episodes of the condition did have a higher risk of atrial fibrillation development than those patients with > 10 episodes of insomnia. Patient with dyslipidemia had a lower rate of atrial fibrillation development.
Figure 2.
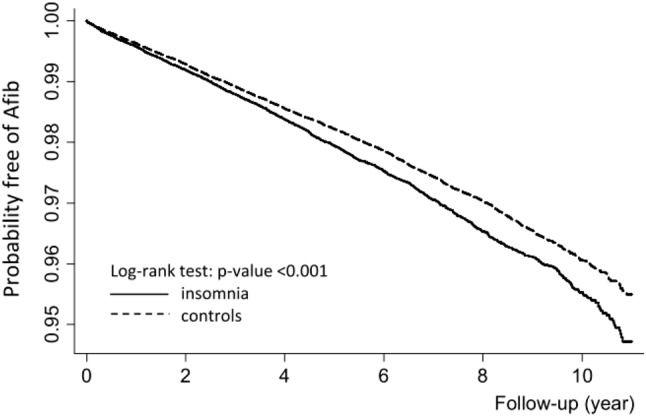
Kaplan-Meier analysis comparing probabilities of atrial fibrillation between patients with insomnia and controls.
Table 3. Cox regression model for predictor of atrial fibrillation development.
| Variables | Model 1 | Model 2 | Model 3 | ||||||
| HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Gender | |||||||||
| Female | 1.00 | 1.00 | 1.00 | ||||||
| Male | 1.25 | 1.18-1.33 | < 0.001 | 1.2 | 1.14-1.27 | < 0.001 | 1.25 | 1.18-1.33 | < 0.001 |
| Age, y | |||||||||
| 18-40 | 1.00 | 1.00 | 1.00 | ||||||
| 41-65 | 6.45 | 5.05-8.24 | < 0.001 | 7.62 | 5.99-9.69 | < 0.001 | 6.47 | 5.07-8.27 | < 0.001 |
| ≥ 65 | 26.95 | 21.11-34.42 | < 0.001 | 33.1 | 26.01-42.11 | < 0.001 | 27.09 | 21.21-34.59 | < 0.001 |
| Comorbidity | |||||||||
| DM | 0.95 | 0.89-1.02 | 0.15 | 0.96 | 0.90-1.02 | 0.22 | 0.95 | 0.89-1.02 | 0.15 |
| Hypertension | 1.25 | 1.16-1.34 | < 0.001 | 1.23 | 1.15-1.32 | < 0.001 | 1.25 | 1.17-1.35 | < 0.001 |
| Dyslipidemia | 0.66 | 0.62-0.70 | < 0.001 | 0.62 | 0.59-0.66 | < 0.001 | 0.66 | 0.62-0.70 | < 0.001 |
| COPD | 1.15 | 1.07-1.25 | < 0.001 | 1.16 | 1.08-1.24 | < 0.001 | 1.15 | 1.07-1.25 | < 0.001 |
| Congestive heart failure | 2.20 | 2.05-2.36 | < 0.001 | 2.14 | 2.00-2.28 | < 0.001 | 2.20 | 2.05-2.36 | < 0.001 |
| Coronary artery disease | 1.72 | 1.61-1.84 | < 0.001 | 1.66 | 1.57-1.77 | < 0.001 | 1.73 | 1.62-1.85 | < 0.001 |
| Chronic kidney disease | 1.02 | 0.92-1.13 | 0.77 | 0.93 | 0.84-1.03 | 0.16 | 1.02 | 0.92-1.13 | 0.77 |
| Stroke | 0.94 | 0.86-1.02 | 0.15 | 0.82 | 0.75-0.89 | < 0.001 | 0.94 | 0.86-1.02 | 0.14 |
| Peripheral artery disease | 0.93 | 0.76-1.13 | 0.45 | 0.92 | 0.76-1.11 | 0.38 | 0.93 | 0.76-1.13 | 0.46 |
| Sleep apnea | 0.81 | 0.42-1.56 | 0.53 | 0.47 | 0.21-1.04 | 0.06 | 0.81 | 0.42-1.56 | 0.53 |
| Valvular heart diseases | 0.99 | 0.83-1.19 | 0.93 | 1.08 | 0.92-1.27 | 0.35 | 0.99 | 0.83-1.19 | 0.95 |
| Depression | 0.89 | 0.69-1.08 | 0.12 | 0.87 | 0.65-1.12 | 0.14 | 0.89 | 0.69-1.08 | 0.12 |
| Bipolar disorder | 0.95 | 0.78-1.11 | 0.22 | 0.93 | 0.74-1.15 | 0.25 | 0.95 | 0.78-1.11 | 0.23 |
| Insomnia | |||||||||
| No | 1.00 | 1.00 | |||||||
| Yes | 1.08 | 1.01-1.14 | 0.02 | 1.33 | 1.25-1.41 | < 0.001 | |||
| Insomnia | |||||||||
| No | 1.00 | ||||||||
| < 5 visits | 1.16 | 1.05-1.28 | 0.004 | ||||||
| 5-9 visits | 1.14 | 1.03-1.26 | 0.009 | ||||||
| ≥ 10 visits | 1.00 | 0.92-1.08 | 0.93 |
Model 1, Non-insomnia cohort matched on sex, age, index date; Model 2, Non-insomnia cohort matched on sex, age, co-morbidity by using propensity score; Model 3, Insomnia frequency analysis between insomnia and Afib. Abbreviations are in Table 1.
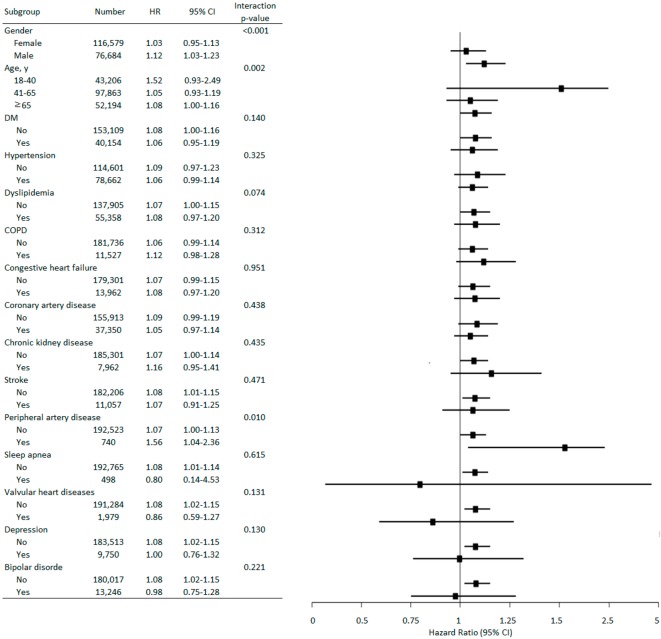
The multivariate stratified analysis for atrial fibrillation development is shown in Figure 3. Male patients with insomnia had a higher risk of atrial fibrillation development than female patients with insomnia (HR = 1.12, 95% CI 1.03-1.23). Among those patients older than 65 years of age, patients with insomnia seemed to have a higher risk of atrial fibrillation development (HR = 1.08, 95% CI 1.00-1.16). Among patients with peripheral artery disease, patients with insomnia had a higher risk of atrial fibrillation development (HR = 1.56, 95% CI 1.04-2.36).
Figure 3.
Adjusted hazard ratios (HRs) for atrial fibrillation development. In each stratum, the HRs were compared between patients with insomnia and controls. COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus.
DISCUSSION
This study is the first study to examine the effects of insomnia on the risk of developing atrial fibrillation. It is the first large-scale nationwide analysis to demonstrate that insomnia is associated with a higher rate of atrial fibrillation in a long follow-up period (adjusted HR = 1.08). As in many other industrialized countries, atrial fibrillation is the most commonly diagnosed sustained cardiac arrhythmia in the Taiwan population.6 In this study, atrial fibrillation cases were identified through a search of the Database of the NHI Bureau, which covers most of the Taiwanese population. The quality of its information on prescription use, diagnoses, and hospitalizations has been shown to be excellent.15 To ensure the accuracy of the claim files, the Bureau of NHI (BNHI) performs quarterly expert reviews on a random sample of every 50-100 ambulatory and inpatient claims. False reports of diagnostic information result in a severe penalty from the BNHI.16 The homogeneous patient population (98% of Taiwanese residents are of Han Chinese ethnicity) also may help to avoid the possible confounding effects of race.17
There is increasing evidence of an important association between sleep apnea and atrial fibrillation.18 The possible speculative mechanisms of sleep apnea on atrial fibrillation development include hypoxia, impaired autonomic nervous control, and inflammation.13 Insomnia is also associated with transient hypoxia, impaired autonomic nervous function, and inflammation.19-21 Moreover, is has been shown that insomnia can cause hypertension and activation of the renin-aldosterone-angiotensin system, which could lead to atrial remodeling and fibrosis with the loss of atrial muscle mass.22 Our study showed an association between atrial fibrillation and insomnia. Although the mechanism by which insomnia leads to atrial fibrillation is rarely discussed, we believe that the mechanism described above may be responsible for insomnia leading to atrial fibrillation development.
In the insomnia group, women (60.3%) and patients 41-65 years of aged were predominant (50.6%). The prevalence of comorbidities such as hypertension, COPD, congestive heart failure, and coronary artery disease was significantly higher in patients with insomnia compared to control cases without insomnia (p < 0.001). On the contrary, insomnia cases with dyslipidemia had a lower rate of atrial fibrillation development (p < 0.001). This may be due to the use of statin, which has been shown to have a protective effect on atrial fibrillation development.23 This finding was consistent with results from previous epidemiology reports.24,25 We assessed the risk of atrial fibrillation development based on gender, age, and comorbidities (Table 2, Figure 3). Among each subgroup, an association between insomnia and increased risk for atrial fibrillation development was observed. Patients with insomnia > 65 years of age had a greater risk of atrial fibrillation development than the comparison cohort. Men had a greater risk of atrial fibrillation development than women (adjusted HR = 1.12 vs. 1.03, respectively). This inconsistency might be due to other important factors such as lifestyle, alcohol use, smoking, and body weight, which were not available in our dataset. In Taiwan the prevalence of smoking and alcohol use are higher in men than woman, which might have influenced the study results.26,27 That male patients and those > 65 years of age with insomnia diagnosis seemed to have higher risk of atrial fibrillation particularly was found in our analysis. However, the patient numbers were relative few in our subgroup analysis. Consequently, further studies are necessary to support the finding.
In terms of dose-dependent response, an incremental effect was not found as the times of insomnia diagnosis increased. In this study, patients with times of insomnia diagnosis less than 10 did have a higher risk of atrial fibrillation development, but those with times of insomnia diagnosis more than 10 did not. Otherwise, there was no dose-response phenomenon. As the times of insomnia diagnosis increased, more and more medical examination were done as well as treatment. That more education for life modification and risk factors modification as the times of insomnia diagnosis increased might cause the result of insomnia frequency analysis. Additional study is appropriate to interpret the finding.
This study had some potential limitations. First, although we did adequate adjustment for confounding factors, a number of possible confounding variables associated with atrial fibrillation development including blood pressure, smoking, family history, and alcohol consumption were not included in our database. This major limitation is inherent in many similar studies, and could have compromised our findings. The diagnosis of COPD was adjusted because the link between COPD and smoking is well-established,28 as well as the link between dyslipidemia and obesity.29 Second, it was impossible for us to contact the patients directly about their diagnosis of insomnia because identifying information was removed from the database. Therefore, the lack of objective measures of sleep duration and subjectively reported sleep quality is another limitation. Nevertheless, the study data regarding insomnia and atrial fibrillation diagnoses remained highly reliable due to the validity of the database, large sample size, and long follow-up period. Third, although the aim of this study was to exam whether insomnia was associated with atrial fibrillation, the fact that we did not test the effect between drugs of comorbidities and atrial fibrillation is one of the limitations in our study. Adding drug use to comorbidities in the Cox regression models might help to distinguish the effects between drugs and diseases. However, many similar studies such as the association between sleep apnea on atrial fibrillation30,31 did not do such test and the possible bias might not be statistically obvious.
CONCLUSIONS
In conclusion, we determined that patients with insomnia have a higher risk for atrial fibrillation development. This is the first population-based retrospective cohort study about the effects of insomnia on atrial fibrillation. Although the main finding of the study is hypothesis-generating, it is potentially important in the prevention of atrial fibrillation. We hope that more emphasis will be placed on patients with insomnia, and this study will make a strong case for more studies to confirm the mechanism and the effects of insomnia on atrial fibrillation development.
Acknowledgments
This study was supported by the Department of Health, Taipei City Government (TPCH-104-005). This study is based in part on data obtained from the National Health Insurance Research Database provided by the Bureau of National Health Insurance, Department of Health and managed by the National Health Research Institutes. The interpretation and conclusions contained herein do not represent those of the Bureau of National Health Insurance, Department of Health, or National Health Research Institutes.
REFERENCES
- 1.Roth T, Coulouvrat C, Hajak G, et al. Prevalence and perceived health associated with insomnia based on DSM-IV-TR; International Statistical Classification of Diseases and Related Health Problems, Tenth Revision; and Research Diagnostic Criteria/International Classification of Sleep Disorders, Second Edition criteria: results from the America Insomnia Survey. Biol Psychiatry. 2011;69:592–600. doi: 10.1016/j.biopsych.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 2.Kao CC, Huang CJ, Wang MY, et al. Insomnia: prevalence and its impact on excessive daytime sleepiness and psychological well-being in the adult Taiwanese population. Qual Life Res. 2008;17:1073–1080. doi: 10.1007/s11136-008-9383-9. [DOI] [PubMed] [Google Scholar]
- 3.Roth T. Insomnia: definition, prevalence, etiology, and consequences. J Clin Sleep Med. 2007;3:S7–10. [PMC free article] [PubMed] [Google Scholar]
- 4.Becker PM. Insomnia: prevalence, impact, pathogenesis, differential diagnosis, and evaluation. Psychiatr Clin North Am. 2006;29:855–870, abstract vii. doi: 10.1016/j.psc.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 5.European Heart Rhythm A; European Association for Cardio-Thoracic S. Camm AJ, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J. 2010;31:2369–2429. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- 6.Lin LY, Lee CH, Yu CC, et al. Risk factors and incidence of ischemic stroke in Taiwanese with nonvalvular atrial fibrillation -- a nation wide database analysis. Atherosclerosis. 2011;217:292–295. doi: 10.1016/j.atherosclerosis.2011.03.033. [DOI] [PubMed] [Google Scholar]
- 7.Kirchhof P, Auricchio A, Bax J, et al. Outcome parameters for trials in atrial fibrillation: executive summary. Eur Heart J. 2007;28:2803–2817. doi: 10.1093/eurheartj/ehm358. [DOI] [PubMed] [Google Scholar]
- 8.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 9.Thrall G, Lane D, Carroll D, et al. Quality of life in patients with atrial fibrillation: a systematic review. Am J Med. 2006;119:448 e1–e19. doi: 10.1016/j.amjmed.2005.10.057. [DOI] [PubMed] [Google Scholar]
- 10.Wu VC, Hu YH, Wu CH, et al. Administrative data on diagnosis and mineralocorticoid receptor antagonist prescription identified patients with primary aldosteronism in Taiwan. J Clin Epidemiol. 2014;67:1139–1149. doi: 10.1016/j.jclinepi.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 11.Lin CC, Lai MS, Syu CY, et al. Accuracy of diabetes diagnosis in health insurance claims data in Taiwan. J Formos Med Assoc. 2005;104:157–163. [PubMed] [Google Scholar]
- 12.Little RJ, Rubin DB. Causal effects in clinical and epidemiological studies via potential outcomes: concepts and analytical approaches. Annu Rev Public Health. 2000;21:121–145. doi: 10.1146/annurev.publhealth.21.1.121. [DOI] [PubMed] [Google Scholar]
- 13.Digby GC, Baranchuk A. Sleep apnea and atrial fibrillation; 2012 update. Curr Cardiol Rev. 2012;8:265–272. doi: 10.2174/157340312803760811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whang W, Davidson KW, Conen D, et al. Global psychological distress and risk of atrial fibrillation among women: The Women’s Health Study. J Am Heart Assoc. 2012;1:e001107. doi: 10.1161/JAHA.112.001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsieh CY, Chen CH, Li CY, et al. Validating the diagnosis of acute ischemic stroke in a National Health Insurance claims database. J Formos Med Assoc. 2013 doi: 10.1016/j.jfma.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 16.Tseng CH. Mortality and causes of death in a national sample of diabetic patients in Taiwan. Diabetes Care. 2004;27:1605–1609. doi: 10.2337/diacare.27.7.1605. [DOI] [PubMed] [Google Scholar]
- 17.Chiu WC, Ho WC, Lin MH, et al. Angiotension receptor blockers reduce the risk of dementia. J Hypertens. 2014 doi: 10.1097/HJH.0000000000000086. [DOI] [PubMed] [Google Scholar]
- 18.Gami AS, Pressman G, Caples SM, et al. Association of atrial fibrillation and obstructive sleep apnea. Circulation. 2004;110:364–367. doi: 10.1161/01.CIR.0000136587.68725.8E. [DOI] [PubMed] [Google Scholar]
- 19.Pappenheimer JR. Hypoxic insomnia: effects of carbon monoxide and acclimatization. J Appl Physiol Respir Environ Exerc Physiol. 1984;57:1696–1703. doi: 10.1152/jappl.1984.57.6.1696. [DOI] [PubMed] [Google Scholar]
- 20.Stein PK, Pu Y. Heart rate variability, sleep and sleep disorders. Sleep Med Rev. 2012;16:47–66. doi: 10.1016/j.smrv.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Zhang J, Lamers F, Hickie IB, et al. Differentiating nonrestorative sleep from nocturnal insomnia symptoms: demographic, clinical, inflammatory, and functional correlates. Sleep. 2013;36:671–679. doi: 10.5665/sleep.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grandner MA, Perlis ML. Short sleep duration and insomnia associated with hypertension incidence. Hypertens Res. 2013;36:932–933. doi: 10.1038/hr.2013.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hung CY, Lin CH, Wang KY, et al. Dosage of statin, cardiovascular comorbidities, and risk of atrial fibrillation: a nationwide population-based cohort study. Int J Cardiol. 2013;168:1131–1136. doi: 10.1016/j.ijcard.2012.11.087. [DOI] [PubMed] [Google Scholar]
- 24.Zhang B, Wing YK. Sex differences in insomnia: a meta-analysis. Sleep. 2006;29:85–93. doi: 10.1093/sleep/29.1.85. [DOI] [PubMed] [Google Scholar]
- 25.Mai E, Buysse DJ. Insomnia: prevalence, impact, pathogenesis, differential diagnosis, and evaluation. Sleep Med Clin. 2008;3:167–174. doi: 10.1016/j.jsmc.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin CM, Wen TH. Temporal changes in geographical disparities in alcohol-attributed disease mortality before and after implementation of the alcohol tax policy in Taiwan. BMC Public Health. 2012;12:889. doi: 10.1186/1471-2458-12-889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levy DT, Wen CP, Chen TY, et al. Increasing taxes to reduce smoking prevalence and smoking attributable mortality in Taiwan: results from a tobacco policy simulation model. Tob Control. 2005;14 Suppl 1:i45–i50. doi: 10.1136/tc.2003.005660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Zyl Smit RN, Pai M, Yew WW, et al. Global lung health: the colliding epidemics of tuberculosis, tobacco smoking, HIV and COPD. Eur Respir J. 2010;35:27–33. doi: 10.1183/09031936.00072909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ishigami M, Matsuzawa Y. Hyperlipidemia and obesity. Nihon Rinsho. 2003;61 Suppl 1:241–246. [PubMed] [Google Scholar]
- 30.Padeletti L, Gensini GF, Pieragnoli P, et al. The risk profile for obstructive sleep apnea does not affect the recurrence of atrial fibrillation. Pacing Clin Electrophysiol. 2006;29:727–732. doi: 10.1111/j.1540-8159.2006.00426.x. [DOI] [PubMed] [Google Scholar]
- 31.Gami AS, Hodge DO, Herges RM, et al. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol. 2007;49:565–571. doi: 10.1016/j.jacc.2006.08.060. [DOI] [PubMed] [Google Scholar]