Abstract
Hepatoid adenocarcinoma (HAC) is a rare subtype of extrahepatic adenocarcinoma that is characterized by its morphological and functional similarities to hepatocellular carcinoma. We herein present a novel case of HAC arising from the extrahepatic bile duct in a 75-year-old Japanese woman with polysplenia syndrome. This is the second reported case of HAC arising from this site. The tumor induced jaundice and hemobilia. A total of four isolated intraductal polypoid masses of HAC were found. No recurrence was seen five months after surgery. Further reports of similar cases will be needed to clarify the clinical characteristics and the prognosis of this malignancy.
Keywords: hepatoid adenocarcinoma, extrahepatic bile duct, polysplenia syndrome
Introduction
Hepatoid adenocarcinoma (HAC) is a unique subtype of hepatocellular carcinoma (HCC)-like adenocarcinoma. It usually occurs in the stomach; however, the extrahepatic bile duct is a very unusual site of HAC, and to our knowledge, the case we report here is only the second to involve HAC originating in this tissue. This case is also unique, as it involved multiple polypoid intraductal tumors and polysplenia syndrome. We herein present the detailed clinicopathological findings of this rare tumor and discuss the differential diagnosis.
Case Report
Clinicoradiological findings
A 75-year-old Japanese woman was admitted to our hospital with jaundice. General malaise and anorexia had persisted for one month. She had jaundice, pericardiac pain, and tenderness of the right upper abdomen. She had never consumed alcohol and had no history of treatment for hepatitis or malignancy. Her body mass index was 24 kg/m2, and she had high serum levels of total bilirubin (7.0 mg/dL; normal value, 0.3-1.3 mg/dL), direct bilirubin (4.1 mg/dL; normal value, 0.0-0.2 mg/dL), gamma-glutamyl transpeptidase (GTP) (1,530 IU/L; normal value, 11-64 IU/L), aspartate aminotransferase (214 IU/L; normal value, 10-32 IU/L), and alanine aminotransferase (149 IU/L; normal value, 5-27 IU/L), and tested negative for hepatitis B surface antigen and antibody. Although she tested positive for hepatitis C virus (HCV) antibody, no HCV RNA was detected. Her carcinoembryonic antigen (CEA) level was normal, but her carbonic anhydrase 19-9 (CA 19-9) level was markedly elevated at 78,366 U/mL (normal value, 37 U/mL). Preoperative serum alpha-fetoprotein (AFP) was not tested. Her complete blood count results were normal.
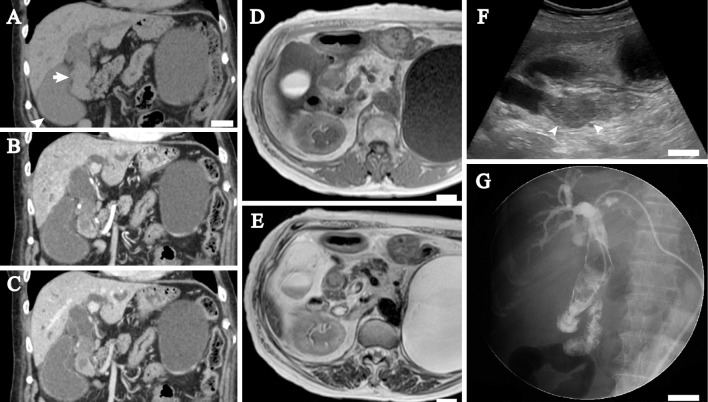
Computed tomography (CT, Fig. 1A-C) and magnetic resonance imaging (MRI) showed a mass that was mainly located in the common bile duct, with marked dilatation of the intrahepatic bile ducts and the extrahepatic bile tract. A precontrast CT image (Fig. 1A) revealed that the intraductal mass was slightly hypodense relative to the hepatic parenchyma, with a mean Hounsfield units value of 38. An arterial-phase contrast-enhanced CT image (Fig. 1B) obtained 35 seconds after contrast administration demonstrated that the mass had a slight and heterogeneous enhancement pattern; the mean Hounsfield units value of the mass was 53. A delayed-phase contrast-enhanced CT image (Fig. 1C) obtained 90 seconds after contrast administration showed the mass to be more enhanced, with a mean Hounsfield units value of 65. MRI revealed that the mass was heterogeneous and somewhat hypointense relative to the bile duct wall on a T1-weighted image (Fig. 1D) and partially hyperintense on a T2-weighted image (Fig. 1E). Ultrasonography revealed a smooth mass with a maximum size of approximately 7 cm, in which a low echoic lesion 3 cm in diameter (Fig. 1F) was seen. The liver had a smooth surface and a sharp edge and did not contain a tumor by ultrasonography or CT preoperatively or postoperatively. Endoscopically, hemorrhaging was seen on the non-ulcerative mucosa of the descending portion of the duodenum, and there appeared to be a discharge of hemorrhagic, non-mucinous materials from the ampulla of Vater. Endoscopic retrograde cholangiopancreatography and intraductal ultrasonography failed due to marked hemorrhaging from the ampulla of Vater during the procedure. Percutaneous transhepatic cholangiography as well as magnetic resonance cholangiopancreatography (MRCP) demonstrated that the extrahepatic bile duct lesion was a filling defect (Fig. 1G), and no pancreaticobiliary maljunction was observed. A cytological analysis of the bile fluid revealed clusters of polygonal cells with centrally or eccentrically located hyperchromatic nuclei and single or multiple distinct nucleoli. There were no salt-and-pepper-like nuclei that would have suggested a neuroendocrine tumor. A biopsy specimen of the bile duct lesion also contained clusters of polygonal carcinoma cells. As a result, extrahepatic bile duct cancer was diagnosed. A contrast-enhanced CT image suggested that the bile duct tumor was confined to the common bile duct, and no metastatic lesions were found on CT or MRI from the thorax to the abdomen.
Figure 1.
Radiographic images of the extrahepatic bile duct tumor. A-C: Coronal plane of computed tomography (CT) images. A precontrast CT image (A) shows the extrahepatic bile duct mass (arrow) and the dilated gallbladder (arrowhead). The intrahepatic and extrahepatic bile ducts are dilated. A large renal cyst is present in the left kidney. An arterial phase CT image (B) shows that the mass has slight and heterogeneous enhancement. A delayed phase CT image (C) shows that the mass is further enhanced. D-E: Magnetic resonance imaging shows that the mass is heterogeneous and somewhat hypointense on a T1-weighted image (D) and partially hyperintense (arrow) on a T2-weighted image (E). The hyperintense area probably corresponds to the largest tumor. F: Sagittal plane of the ultrasonography image. An intraductal nodule 7 cm in length with dilatation of the extrahepatic bile duct is seen. The nodule includes a hypoechoic lesion (arrowheads) that probably corresponds to the largest tumor of four polypoid lesions. G: Percutaneous transhepatic cholangiography. A filling defect is seen in the extrahepatic bile duct. The paucity of the transverse portion of the duodenum suggests intestinal malrotation. White bar, 2 cm in A, D-G.
Subtotal stomach-preserving pancreaticoduodenectomy and lymphadenectomy were then performed. In addition, a radiological study confirmed various developmental abnormalities, including polysplenia, non-rotation of the midgut, loss of the tail of the pancreas, and an absence of the suprarenal segment of the inferior vena cava with azygous continuation.
Pathological findings
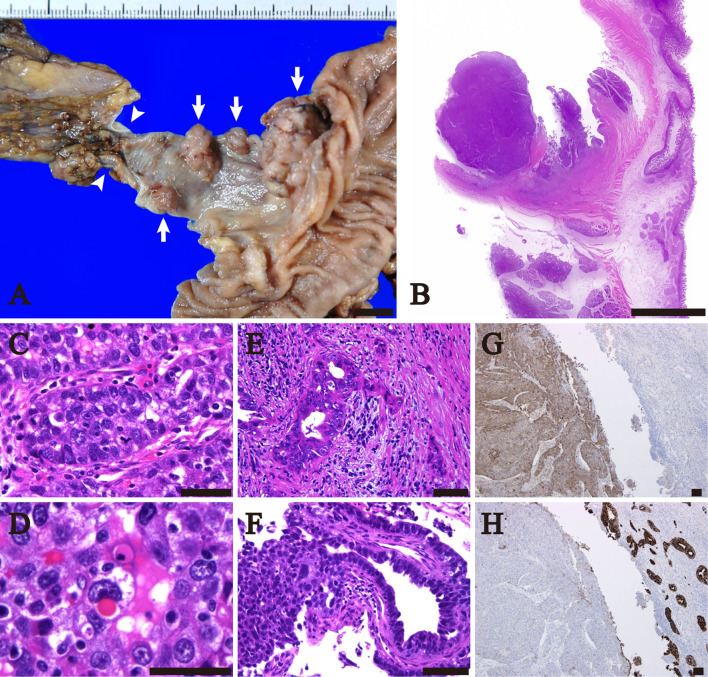
Grossly, hemorrhagic substances filled the extrahepatic bile duct. After the intraductal materials were removed, a total 4 polypoid lesions with maximum diameters of 27 mm (Fig. 2A), 16 mm, 9 mm, and 8 mm were seen in the upper portion of the common bile duct. The background mucosa was smooth.
Figure 2.
The macroscopic and microscopic findings of the extrahepatic bile duct tumor. A: The macroscopic appearance of the formalin-fixed tissue. Four mural nodules (arrows) were present in the upper portion of the common bile duct. The cystic duct is indicated by arrowheads. B-F: Low to high magnification of the Hematoxylin and Eosin staining sections of the largest tumor. B: The largest mass showed a polypoid and solid lesion. C: The largest tumor mostly consisted of carcinoma cells in a trabecular growth pattern. D: Hyaline globules were present in some tumor cells. E: A tubular adenocarcinoma component was present at the base of the largest tumor. F: There was a transition between the trabecular and adenocarcinoma components. G-H: Immunohistochemistry findings. G: alpha-fetoprotein staining revealed diffuse positivity in the solid component (left) and no staining in the adenocarcinoma portion (right). H: Cytokeratin 7 staining was almost negative in the solid component (left) and diffusely positive in the tubular adenocarcinoma portion (right). Bar, 1 cm in A-B; 100 μm in C-H.
A pathological analysis revealed that the four polypoid lesions consisted of trabecular arrangements of polygonal cells with hyperchromatic nuclei and some central nucleoli(Fig. 2B and C). The intraductal materials consisted of fibrinous exudate and necrotic substances, suggesting coagulation necrosis of the tumor cells. There were no glandular or papillary structures and staining with Alcian Blue and Periodic acid Schiff failed to reveal any cytoplasmic mucin. Hyaline globules (Fig. 2D) positive for Periodic acid Schiff staining with diastase treatment were present in the cytoplasm of some tumor cells. Thin fibrovascular septa were intimately associated with the tumor trabeculae, although there was no conspicuous sinusoidal growth pattern.
Well to moderately differentiated tubular adenocarcinoma (Fig. 2E) was confined to the base of the largest polypoid lesion and merged with the trabecular nests (Fig. 2F), while neither dysplastic epithelium nor tubular adenocarcinoma was present in the other smaller tumors or the surrounding biliary mucosa. The four tumors were isolated from each other and were mostly confined to the bile duct mucosa, although the adenocarcinoma component of the largest tumor had partly invaded the muscularis propria. There was relatively little lymphovascular invasion, but lymphatic permeation with a poorly differentiated component was found in one of the smaller nodules. Intratumoral inflammatory infiltrates consisting mainly of lymphocytes and plasma cells were noted in parts of the largest tumor.
Overall, the largest tumor was classified as being poorly differentiated adenocarcinoma, and the differential diagnosis included HAC and neuroendocrine carcinoma (mixed adenoneuroendocrine carcinoma). The other smaller masses were each considered to be a primary or intraductal metastasis of the poorly differentiated component of the largest tumor. Hall's staining did not reveal bile production by the tumor cells, but argyrophilic fibers were focally associated within some trabecular nests.
An immunohistochemical analysis showed the poorly differentiated component to be positive for CAM5.2 (70%), cytokeratin 19 (CK19, 60%), AFP (95%, Fig. 2G), glypican-3 (70%), SALL4 (100%), hepatocyte paraffin 1 (HepPar1, 5%), CD10 (<5%, canalicular staining), and thyroid transcription factor-1 (dot-like cytoplasmic staining, <5%) and negative for CK7 (Fig. 2H), CK20, CA19-9, CEA, chromogranin A, synaptophysin, and CD56. The adenocarcinoma component showed diffuse immunoreactivity for CK7 (Fig. 2H), CK19, CEA, CA19-9, and CAM5.2 and was partially positive for CD10, but showed no immunoreactivity for CK20, SALL4, HCC markers, or neuroendocrine markers. The proportions of Ki-67 positive cells in the poorly differentiated portion and tubular component was 80% and 25%, respectively. Epstein-Barr virus-encoded small RNAs were not detected in tumor cells by in situ hybridization. No ectopic liver tissue was present around the tumor or in the resected tissue. Thus, the poorly differentiated tumors were considered to be HAC. No lymph node metastasis was found.
Clinical follow-up
On postoperative day 30, the serum AFP level was 38.2 ng/mL (normal value, <20 ng/mL). Five months after surgery without additional therapy, no recurrence was seen by contrast-enhanced CT, and the serum AFP level had normalized (2.5 ng/mL). The serum CA19-9 level had normalized by about three months after surgery, and the postoperative serum CEA level was within normal limits.
Discussion
We herein report a case of multiple polypoid tumors of HAC originating in the extrahepatic bile duct, the first report of such a rare tumor in a patient with polysplenia syndrome. The preoperative radiological study including CT, MRI, MRCP, and percutaneous transhepatic cholangiography failed to reveal multiple tumors, probably because all but the largest tumor were too small to be detected and were also obscured by a covering of hemorrhagic and necrotic materials. The contrast-enhanced area of CT images might have corresponded to the largest tumor, but the heterogeneous enhancement obscured its detection. Given the radio-pathological correlation, the 3-cm-hypoechoic lesion and the hyperintense lesion on T2-weighted images were likely identical and corresponded to the largest tumor in the bile duct mass. Thus, ultrasonography and T2-weighted MRI were superior imaging modalities for the detection of the largest tumor.
The clinical diagnosis of HAC of the extrahepatic bile duct is challenging (1). Gross tumor polypoid morphology is a typical feature of cholangiocarcinoma (2) and intraductal papillary neoplasms of the extrahepatic bile duct (3). Cholangiocarcinoma is frequently associated with a high CEA level and can be associated with thickened and/or stricture-like bile duct wall (2), and those findings were not obtained in the present case. Although the adenocarcinoma component of the tumor immunohistochemically expressed CEA, the serum CEA level was normal, possibly because only a very small adenocarcinoma component was present. It is unusual to find hemobilia and tumor multiplicity in intraductal papillary neoplasms of the bile duct that typically show mucinous hypersecretion and a low rate (5%) of tumor multiplicity (3). Thus, the present case may be uncommon among the known cases of cholangiocarcinoma and precursor lesions of the extrahepatic bile duct.
HAC is considered to be an aggressive tumor with extensive hematogenous metastasis and early lymph node involvement (4,5). HAC was initially described as an AFP-producing adenocarcinoma with morphologically and immunohistochemically distinct foci of hepatic differentiation (6). Further studies suggested that the immunohistochemical expression of AFP by HAC had little prognostic significance (7), and HAC with no evidence of AFP production can still express albumin mRNA (8). Thus, AFP production is not necessary for the diagnosis of HAC, and its definitive diagnosis is instead made on the basis of its histopathologic and immunohistochemical similarities to HCC. The present case showed morphological, immunohistochemical, and functional similarities to HCC with respect to features such as a trabecular growth pattern, paucity of mucin, widespread positive staining for AFP and glypican-3, negative staining for CK7 and CK20, and elevated serum AFP. HAC usually occurs in the stomach, but is also reported to arise in other organs, such as the ovary, lung, gallbladder, pancreas, and uterus (9).
Although the gallbladder is the fourth-most common primary site of HAC, these tumors rarely arise in other parts of the extrahepatic bile tract, with only three reported cases found in a search of the PubMed database (10-12). No developmental abnormalities were described in these cases. Furthermore, in two of the three cases, the tumor originated in the ampulla of Vater (11,12), so the case described here is only the second to include HAC of the extrahepatic bile duct. Abdullah et al. (13) presented a case report of hepatoid carcinoma of the proximal portion of the extrahepatic bile duct, but the tumor they described could not be differentiated from HCC because the morphological and immunohistochemical findings of the tumor were consistent with HCC and no adenocarcinoma component was reported.
Wang et al. (10) described the first case of HAC of the extrahepatic bile duct that involved six intraluminal masses in the bile tract. This is very similar to the case we presented here, although they did not describe the relationship between the tumors and the wall of the bile tract, nor did they report whether there was tumor hemorrhaging (hemobilia) or whether all masses were primary or not. The present case showed four bile duct tumors that were mostly confined to the bile duct mucosa and were isolated from each other. Only the largest tumor was accompanied by intramucosal tubular adenocarcinoma, and the three smaller tumors consisted entirely of carcinoma showing the same histology of the solid component of the largest mass. As expected, the adenocarcinoma cells of the largest tumor underwent hepatic differentiation as it progressed.
The largest tumor was considered to be the primary lesion, although it was not possible to determine whether the other three tumors were also primary tumors or were instead metastatic lesions originating from the largest tumor. If the smaller three tumors were indeed metastatic lesions from the largest tumor, then three pathogeneses are possible: a hematogenous pathway, a lymphatic pathway, or intraductal dissemination (14). The hematogenous and lymphatic pathways may be unlikely because venous permeation was absent in all tumors and no lymphatic permeation was seen in the largest tumor or the other two smaller nodules. The empirically suggested mechanism of intraductal dissemination involves the spread of cancer cells through ducts such as the pancreatic ducts, bile ducts, or mammary ducts (14) and would seem to be the most probable explanation for the origin of the three smaller tumors. However, a genomic study will be needed to confirm whether the three polypoid lesions are disseminated foci or primary tumors (14).
The mechanism by which carcinoma cells undergo hepatoid differentiation has been addressed in a number of studies. Both cancer and normal cells can undergo tissue lineage changes and acquire new differentiated characteristics in response to their microenvironment, in a process that is termed transdifferentiation. In fact, a recent study (15) demonstrated that peribiliary glands located within bile duct walls contain multipotent stem cells that can differentiate into hepatocytes, cholangiocytes, or pancreatic islets depending on their microenvironment. The present case appeared to involve the transdifferentiation of cholangiocarcinoma into hepatoid carcinoma. Common sites of HAC such as the stomach, lung, gallbladder, and pancreas (9) are derived from the primitive embryonic foregut that can give rise to the liver as well as the extrahepatic bile duct. Thus, carcinoma cells arising in an organ that originates from the embryonal foregut may be able to transdifferentiate to a hepatoid phenotype more easily than those from other organs.
The differential diagnosis of HAC should include the intraductal recurrence of HCC (16) or primary HCC of the extrahepatic bile duct (17-19), because these can occur without a detectable liver tumor and demonstrate intraductal nodules. It is of interest that intraductal HCC can be associated with hemorrhagic substances, as in the present case. Thus, an examination of the history for any previous treatment for liver tumors, alcohol abuse, or hepatitis virus infection is necessary. Although the patient in the present case showed positivity for an HCV antibody, no liver tumor was detected, and chronic hepatitis due to HCV was unlikely, as radiological studies revealed a normal liver morphology, no HCV RNA was detected, and serological liver function normalized after surgery. Intraductal HCC without an obvious intrahepatic mass appears isodense or mildly hyperdense relative to the hepatic parenchyma during the arterial phase of contrast-enhanced CT (19), while the case described here involved a tumor that was relatively hypodense to the hepatic parenchyma during the arterial phase. The present case did not have typical pathological features of HCC, which are a sinusoidal growth pattern, bile production, loss of CK19, and diffuse immunoreactivity for HepPar1. Terracciano et al. (5) reported that tumor cells positive for CK19 and CK20 and negative for HepPar1 were more likely in HAC than in HCC. SALL4, which is a novel stem cell marker and is usually expressed in germ cell tumors, may be diagnostically useful in distinguishing gastric HAC from HCC because the latter shows low sensitivity for SALL4 while gastric HAC shows high sensitivity for SALL4 (20,21). The present case showed diffuse immunoreactivity for CK19 and SALL4 and was almost completely negative for HepPar1, suggestive of HAC rather than HCC. The pathological differential diagnosis should also include neuroendocrine carcinoma, as HAC can show neuroendocrine differentiation and neuroendocrine carcinoma can produce AFP (22,23). In the present case, there was no immunohistochemical expression of neuroendocrine markers, ruling out neuroendocrine carcinoma.
Did the patient's developmental abnormalities influence the progression of the bile duct cancer? The patient in the case described here had several developmental anomalies suggestive of polysplenia syndrome, which is a lateral development disorder characterized by interruption of the suprarenal segment of the inferior vena cava with azygous continuation, a preduodenal portal vein, a symmetric liver, intestinal malrotation, bronchial anomalies, and polysplenia. Asymptomatic cases of polysplenia syndrome are rare because it usually involves severe congenital heart disease, although the patient in the present case showed no apparent structural anomaly of the heart. To our knowledge, there have been three reported cases of bile duct cancer in patients with polysplenia syndrome (24-26). Each of these cases involved a single tumor, which was papillary adenocarcinoma in two cases and tubular adenocarcinoma in the other case. Kohara et al. (25) suggested that the hilar bile duct cancer in a patient with polysplenia syndrome was probably induced by the pancreaticobiliary maljunction, which promotes higher rates of bile cancer (27) and may induce multiple cancers of the extrahepatic bile tract (28). Polysplenia syndrome may also be associated with biliary atresia (29). However, the patient in the present case was not found to have biliary atresia or malfusion of the pancreaticobiliary ducts, and it is therefore unclear whether or not the pathogenesis of the HAC was related to the congenital anomaly.
The prognosis of HAC of the extrahepatic bile duct is unknown, and previous reports of HAC originating at this site or in the ampulla of Vater (10-12) did not include any prognostic information. The tumor in the case described here might have been non-aggressive, due to the notable exophytic growth, inconspicuous vascular permeation, and the absence of metastasis. HAC of the gallbladder is considered to be an aggressive tumor, similar to conventional gallbladder adenocarcinoma, which might be diagnosed late compared to HAC of the extrahepatic bile duct initially presenting as jaundice.
In summary, we have described a case of HAC of the extrahepatic bile duct in a patient with polysplenia syndrome. To our knowledge, this is only the second case of HAC arising at this site, and the fourth case of bile duct cancer with polysplenia syndrome. Tumor multiplicity, intraductal polypoid growth, and hemobilia might be characteristic of HAC of the extrahepatic bile duct; however, further reports of similar cases will be needed in order to clarify the clinical characteristics and the prognosis of this rare malignant tumor.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
The authors would like to thank Ms. Keiko Mizuno, Mr. Masahiko Ohara, Ms. Yukari Wada, Ms. Yoshiko Agatsuma, and Ms. Kaoru Yasuoka for the preparation of the histological and immunohistochemical specimens.
References
- 1.Palas J, Ramalho M, Matos AP, Heredia V. Case 194: periampullary hepatoid adenocarcinoma with duodenal invasion. Radiology 267: 959-963, 2013. [DOI] [PubMed] [Google Scholar]
- 2.Chung YE, Kim M-J, Park YN, et al. Varying appearances of cholangiocarcinoma: radiologic-pathologic correlation. Radiographics 29: 683-700, 2009. [DOI] [PubMed] [Google Scholar]
- 3.Wang X, Cai YQ, Chen YH, Liu XB. Biliary tract intraductal papillary mucinous neoplasm: report of 19 cases. World J Gastroenterol 21: 4261-4267, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ishikura H, Kishimoto T, Andachi H, Kakuta Y, Yoshiki T. Gastrointestinal hepatoid adenocarcinoma: venous permeation and mimicry of hepatocellular carcinoma, a report of four cases. Histopathology 31: 47-54, 1997. [DOI] [PubMed] [Google Scholar]
- 5.Terracciano LM, Glatz K, Mhawech P, et al. Hepatoid adenocarcinoma with liver metastasis mimicking hepatocellular carcinoma: an immunohistochemical and molecular study of eight cases. Am J Surg Pathol 27: 1302-1312, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Ishikura H, Fukasawa Y, Ogasawara K, Natori T, Tsukada Y, Aizawa M. An AFP-producing gastric carcinoma with features of hepatic differentiation. A case report. Cancer 56: 840-848, 1985. [DOI] [PubMed] [Google Scholar]
- 7.Nagai E, Ueyama T, Yao T, Tsuneyoshi M. Hepatoid adenocarcinoma of the stomach. A clinicopathologic and immunohistochemical analysis. Cancer 72: 1827-1835, 1993. [DOI] [PubMed] [Google Scholar]
- 8.Supriatna Y, Kishimoto T, Uno T, Nagai Y, Ishikura H. Evidence for hepatocellular differentiation in alpha-fetoprotein-negative gastric adenocarcinoma with hepatoid morphology: a study with in situ hybridisation for albumin mRNA. Pathology 37: 211-215, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Metzgeroth G, Strobel P, Baumbusch T, Reiter A, Hastka J. Hepatoid adenocarcinoma - review of the literature illustrated by a rare case originating in the peritoneal cavity. Onkologie 33: 263-269, 2010. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Liu YY, Han GP. Hepatoid adenocarcinoma of the extrahepatic duct. World J Gastroenterol 19: 3524-3527, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takahashi N, Aoyama F, Hiyoshi M, Kataoka H, Sawaguchi A. Establishment and biological characterization of a novel cell line derived from hepatoid adenocarcinoma originated at the ampulla of Vater. Int J Oncol 44: 1139-1145, 2014. [DOI] [PubMed] [Google Scholar]
- 12.Gardiner GW, Lajoie G, Keith R. Hepatoid adenocarcinoma of the papilla of Vater. Histopathology 20: 541-544, 1992. [DOI] [PubMed] [Google Scholar]
- 13.Abdullah A, Jenkins-Mosure K, Lewis T, Patel Y, Strobel S, Pepe L. Primary hepatoid carcinoma of the biliary tree: a radiologic mimicker of Klatskin-type tumor. Cancer Imaging 10: 198-201, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsubara A, Nara S, Sekine S, et al. Intraductal dissemination of papillary adenocarcinoma of the ampulla of Vater in the pancreatic duct. Pathol Int 64: 39-44, 2014. [DOI] [PubMed] [Google Scholar]
- 15.Cardinale V, Wang Y, Carpino G, et al. The biliary tree-a reservoir of multipotent stem cells. Nat Rev Gastroenterol Hepatol 9: 231-240, 2012. [DOI] [PubMed] [Google Scholar]
- 16.Abe T, Kajiyama K, Harimoto N, Gion T, Shirabe K, Nagaie T. Intrahepatic bile duct recurrence of hepatocellular carcinoma without a detectable liver tumor. Int J Surg Case Rep 3: 275-278, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park CM, Cha IH, Chung KB, et al. Hepatocellular carcinoma in extrahepatic bile ducts. Acta Radiol 32: 34-36, 1991. [PubMed] [Google Scholar]
- 18.Tsushimi T, Enoki T, Harada E, et al. Ectopic hepatocellular carcinoma arising in the bile duct. J Hepatobiliary Pancreat Surg 12: 266-268, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Long XY, Li YX, Wu W, Li L, Cao J. Diagnosis of bile duct hepatocellular carcinoma thrombus without obvious intrahepatic mass. World J Gastroenterol 16: 4998-5004, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ushiku T, Shinozaki A, Shibahara J, et al. SALL4 represents fetal gut differentiation of gastric cancer, and is diagnostically useful in distinguishing hepatoid gastric carcinoma from hepatocellular carcinoma. Am J Surg Pathol 34: 533-540, 2010. [DOI] [PubMed] [Google Scholar]
- 21.Shibahara J, Ando S, Hayashi A, et al. Clinicopathologic characteristics of SALL4-immunopositive hepatocellular carcinoma. Springerplus 3: 721, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu X, Yong H, Zhang L, et al. Pure alpha-fetoprotein-producing neuroendocrine carcinoma of the pancreas: a case report. BMC Gastroenterol 15: 16, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xin BB, Li JA, Han X, et al. Successful treatment of a case with pancreatic neuroendocrine carcinoma with focal hepatoid differentiation: a case report and literature review. Int J Clin Exp Med 7: 3588-3594, 2014. [PMC free article] [PubMed] [Google Scholar]
- 24.Fukushima M, Morita T, Fujita M, Okamura K, Yamaguchi K, Ichimura T. A case of polysplenia syndrome with common bile duct cancer. Nihon Rinsho Geka Gakkai Zasshi 72: 466-471, 2011(in Japanese, Abstract in English). [Google Scholar]
- 25.Kohara N, Motoshima K, Mori N, et al. A case of the extrahepatic bile duct carcinoma combined with situs inversus and preduodenal portal vein. Tando 4: 186-192, 1990. [Google Scholar]
- 26.Chirica M, Vullierme MP, Sibert A, et al. Major hepatectomy for peripheral papillary cholangiocarcinoma with hilar extension in a patient with situs ambiguous. Gastroenterol Clin Biol 29: 456-460, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Kamisawa T, Kuruma S, Tabata T, et al. Pancreaticobiliary maljunction and biliary cancer. J Gastroenterol 50: 273-279, 2015. [DOI] [PubMed] [Google Scholar]
- 28.Koizumi K, Sasajima J, Kawamoto T, et al. Multiple cancers of the biliary tract and pancreatic duct after cholecystectomy for gallbladder cancer in a patient with pancreaticobiliary maljunction. Intern Med 55: 141-146, 2016. [DOI] [PubMed] [Google Scholar]
- 29.Karrer FM, Hall RJ, Lilly JR. Biliary atresia and the polysplenia syndrome. J Pediatr Surg 26: 524-527, 1991. [DOI] [PubMed] [Google Scholar]