Abstract
Pulmonary alveolar proteinosis (PAP) is classified as autoimmune, secondary, or genetic. We herein describe a 69-year-old man with autoimmune PAP, simultaneously diagnosed with myeloproliferative neoplasm (MPN). Two years after the diagnosis, the MPN progressed to acute myeloid leukemia, and the patient died from an alveolar hemorrhage during remission induction chemotherapy. Throughout the clinical course, no progression of PAP was observed, despite the progression to leukemia. There are few reports of autoimmune PAP with hematological malignancy, and this case demonstrated that an evaluation for GM-CSF autoantibodies is important for distinguishing the autoimmune and secondary forms of PAP, even if the patient has hematological malignancy.
Keywords: pulmonary alveolar proteinosis, GM-SCF autoantibody, myeloproliferative neoplasm
Introduction
Pulmonary alveolar proteinosis (PAP) is a rare disease characterized by the excessive accumulation of lipoproteins containing periodic acid/Schiff reagent (PAS)-positive surfactant in the lower respiratory tracts. It can be classified as autoimmune, secondary, or genetic. Regarding the secondary type, complications from blood disorders are well known causes. Secondary PAP is believed to be caused by a relative deficiency of granulocyte macrophage colony-stimulating factor (GM-CSF) and related macrophage dysfunction. In contrast, autoimmune PAP is thought to be primarily caused by anti-GM-CSF autoantibodies (1-3). In the present report, we describe a patient with PAP who was positive for GM-CSF autoantibodies and simultaneously exhibited a myeloproliferative neoplasm.
Case Report
A 69-year-old man experienced respiratory difficulty on exertion for 6 months. He underwent an examination at a local clinic after becoming aware of swelling of the dorsum of the right foot for 2 weeks. Leukocytosis was observed, and he was referred to our hospital. The patient had no pertinent underlying diseases, medical history, or family history. Regarding his lifestyle, he had smoked 30 cigarettes per day for 50 years and was never exposed to a dust explosion. On a physical examination, a fine crackle was audible on both sides of the back, and edema of the right dorsum of the foot was evident.
The blood test results were as follows: white blood cells (WBCs): 30,402 /μL (differential count = myeloblasts: 2%, promyelocytes: 1%, myelocytes: 3%, metamyelocytes: 2%, band cells: 9%, granulocytes: 68%, lymphocytes: 7%, basophils: 1%, and monocytes: 7%), Hb: 15.6 g/dL, reticulocytes (Ret): 2.2%, platelets (Plt): 195,000 /μL, total protein: 7.4 g/dL, albumin (Alb): 4.1 g/dL, blood urea nitrogen: 18.2 mg/dL, creatinine (Cr): 1.18 mg/dL, aspartate amino transferase: 22 IU/L, alanine amino transferase: 19 U/L, lactate dehydrogenase (LDH): 430 IU/L, total bilirubin: 0.5 mg/dL, uric acid: 6.6 mg/dL, sodium: 138 mEq/L, potassium: 4.0 mEq/L, chloride: 105 mEq/L, calcium: 9.3 mg/dL, C-reactive protein: 0.71 mg/dL, antinuclear antibody: (-), cytoplasmic antineutrophil cytoplasmic antibodies: (-), perinuclear antineutrophil cytoplasmic antibodies: (-), cardioembryonic antigen (CEA): 15.8 ng/mL, Krebs von den Lungen-6 (KL-6): 38,090 U/mL, surfactant protein D: 185.9 ng/mL, and β-D-glucan: 9.5 pg/mL.
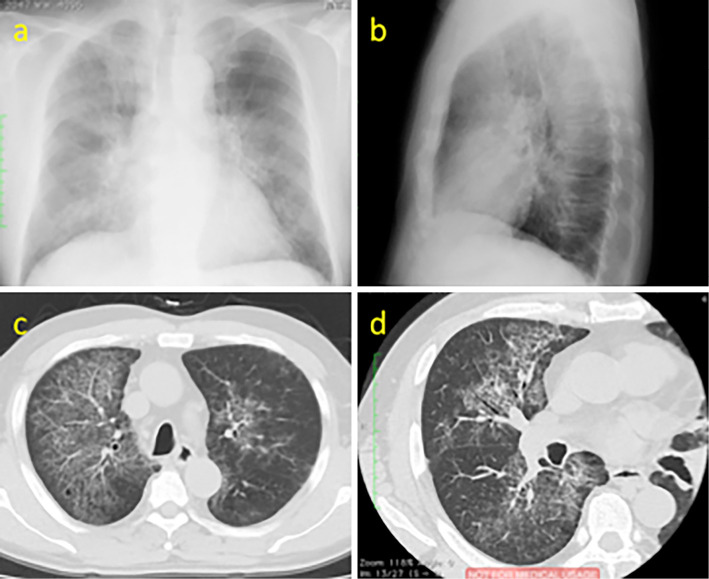
Plain chest X-ray and computed tomography (CT) showed ground glass opacity (GGO) accompanied by significant, diffuse hypertrophy of the interlobular septa of both lungs (Fig. 1). No lymph node enlargement was noted, but the spleen was mildly enlarged.
Figure 1.
(a, b) On plain chest radiography, bilateral alveolar opacities located centrally in mid and lower lung zones are found. (c, d) On high resolution CT reveals ground glass opacity accompanied by significant, diffuse hypertrophy of the interlobular septa of both lungs referred to as “crazy- paving”.
Respiratory function testing indicated that, on room air, the blood oxygen saturation level (SpO2) was 93%, blood gas was pH 7.437, partial pressure of carbon dioxide was 33.6 mmHg, partial pressure of oxygen was 72.2 mmHg, base excess was -0.7 mmol/L, bicarbonate was 22.3 mEq/L, vital capacity was 94.1%, forced expiratory volume in 1 second (FEV1%) was 80.2%, and diffusing capacity for carbon monoxide was 11.39 mL/min/mmHg (80.8%).
Regarding leukocytosis, bone marrow testing indicated that the numbers of all types of granulocytes were elevated in the hyperplasic bone marrow without any abnormalities in differentiation of any lineages or dysplastic features. The numbers of megakaryocytes were modestly increased, but no morphological abnormalities were apparent. A small increase in the numbers of reticular fibers was detected using the silver impregnation method. Chronic myelogenous leukemia (CML) was excluded because the G-banding chromosome analysis was 46,XY [20/20] and a fluorescence in situ hybridization analysis of the BCR-ABL fusion gene was 0%. It was difficult to make an accurate diagnosis according to the WHO Classification 2008 because mutations in genes such as JAK2, V617F, and CSF3R were not evaluated. On clinical presentation, there was no splenomegaly. On a peripheral blood analysis, there was no leukoerythroblastosis, anemia, polycythemia, thrombocythemia, or eosinophilia. These findings excluded primary myelofibrosis, polycythemia vera, essential thrombocythemia, and chronic eosinophilic leukemia. There was no bone marrow dysplasia, so we excluded myelodysplastic syndrome (MDS) and atypical CML. Chronic neutrophilic leukemia was also excluded because a peripheral blood analysis revealed that the band cell and granulocyte levels were less than 80% and myeloblast levels over 1%. Therefore, we diagnosed the patient with myeloproliferative neoplasm, unclassifiable (MPN U) by exclusion diagnosis.
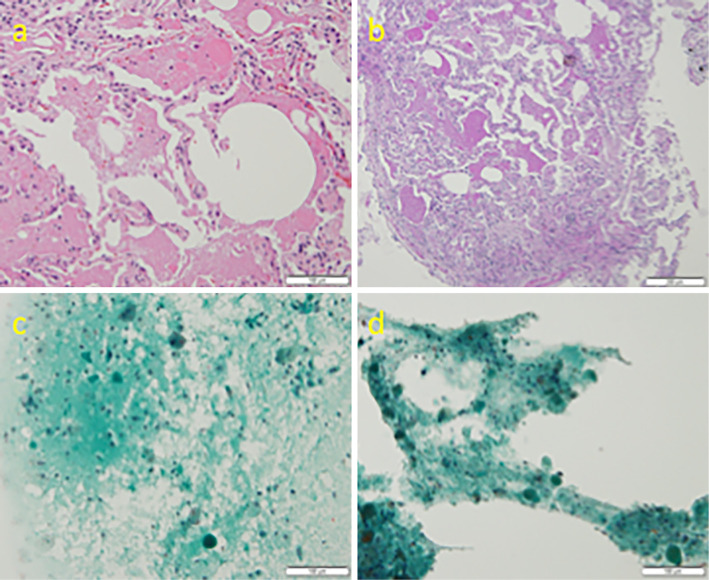
Lung shadows on X-ray imaging suggested PAP; therefore, bronchoscopy was performed. The bronchoalveolar lavage (BAL) had a milky appearance. BAL tests results were as follows: LDH level was 81 IU/L, leukocytes were 1.1×105/mL, quantitative method for Alb was 246.8 mg/Cr, quantitative method for urinary protein was 70 mg/dL, and CEA was 18.4 mg/mL. On transbronchial lung biopsy, the alveolar space was filled with PAS-positive eosinophilic granule-like substances, consistent with PAP (Fig. 2A and B). We noted substances that tended to stain light green in the BAL fluid and suctioned the sputum. Macrophages were also present in the BAL and sputum (Fig. 2C and D). The GM-CSF autoantibody levels were high, at 56.45 μg/mL. Taken together, these findings were consistent with PAP, and a diagnosis of PAP was made.
Figure 2.
(a, b) On trans bronchial lung biopsy, The terminal bronchioles and alveoli are filled with a PAS-positive eosinophilic material with a granular pattern. (c, d) On bronchoalveolar lavage fluid and suctioned sputum, we can find granule-like substances that tended to be stained light green and the presence of macrophages.
Our treatment strategy involved a conservative approach with regular follow-up observations for MPN. Regarding PAP, following discussion with respiratory specialists, given that the dyspnea on exertion was mild and did not interfere with the patient's daily activities, we decided not to perform alveolar lavage or GM-CSF inhalation therapy until more severe symptoms manifested.
Two years after the diagnosis, the WBC count reached over 70,000 /μL, and we began treatment with hydroxyurea (500 mg/day). At that time, the respiratory symptoms and chest X-ray showed no signs of progression of PAP. After that, the WBC count was gradually maintained at 15,000-45,000 /μL.
Three years after the diagnosis, the levels of blast cells in the peripheral blood increased suddenly to 18%. At that time, there were almost no respiratory symptoms like dyspnea on exertion. The SpO2 was 94% on room air, and plain chest X-ray and CT showed no marked changes from the initial diagnosis of PAP. The blood test results were as follows: WBCs: 13,710 /μL (differential count = myeloblasts: 18%, myelocytes: 1%, band cells: 2%, granulocytes: 42%, lymphocytes: 19%, basophils: 1%, eosinophils: 1%, and monocytes: 16%), Hb: 11.5 g/dL, Ret: 1.5%, and Plt: 43,000 /μL. Bone marrow testing showed that the blast cell levels were at 37%, and peroxidase staining results were positive. Blast cells were positive for CD13, CD33, and HLA-DR and negative for CD34 and CD117. The levels of monocytes also reached 10%, and a diagnosis of acute myeloid leukemia (AML) was made. The G-banding chromosome analysis was 46,XY[7/20], 46,XY,idic(17)[11/20], 47,XY,+21[2/20].
Soon after admission, remission induction therapy (30 mg/m2 daunorubicin for 3 days and 200 mg/m2 enocitabine for 8 days) was introduced in accordance with The Japan Adult Leukemia Study Group GML200 protocol (4). No severe adverse events occurred during the course, although on Day 27 of the recovery phase of remission induction therapy, bone marrow aspirate showed residual myeloid blast cells (about 5-10%), and the patient failed to achieve remission. We chose to start reinduction therapy. On Day 14 of the second induction, a large volume of hemoptysis began to continuously appear, and his oxygenation simultaneously worsened rapidly. Plain chest X-ray and CT showed progression of bilateral GGO and pleural effusion. A respiratory specialist determined the patient to have alveolar bleeding due to thrombocytopenia and infection. Despite intensive care, the patient died on Day 16 of the second induction. Throughout the course from diagnosis to death, we deemed the state of PAP to be unchanged, despite the progression to leukemia. This meant that we did not provide therapy for PAP, such as alveolar lavage. The GM-CSF autoantibody levels could not be evaluated periodically because this was not covered by his medical insurance.
Discussion
The abnormal GM-CSF function caused by autoimmune mechanisms is the main cause of adult PAP (5-7). Of the 248 reported cases of PAP in Japan, 223 (89.9%) had autoimmune PAP that was positive for GM-CSF autoantibodies. Twenty-four patients (9.7%) were diagnosed with secondary PAP that was negative for GM-CSF autoantibodies or an underlying illness. The remaining patient (0.4%) had disease of unknown cause (7). This report shows that autoimmune PAP is the most common form. In the present case, tests for GM-CSF autoantibodies were positive, and the disease was thus considered to be autoimmune PAP.
The causes of secondary PAP are likely related to relative decreases in the levels of GM-CSF and corresponding macrophage dysfunction. Only one case of secondary PAP related to indium-tin oxide exposure with GM-CSF autoantibodies was observed (8). Other reports on secondary PAP have shown that most cases were negative for GM-CSF autoantibodies and did not involve autoimmune mechanisms (1,3,9,10).
One report showed that 40 cases of PAP secondary to hematologic malignancy were negative for GM-CSF autoantibodies (3). In contrast, the present case of PAP was positive for GM-CSF autoantibodies and had MPN. Although the median antibody titer for autoimmune PAP was reported to be 15.29 μg/mL (7), the antibody titer in our case was 56.45 μg/mL, which was relatively high. We cannot exclude the possibility that the onset of PAP was a result of MPN, but we regarded this case as one of autoimmune PAP with independent MPN.
Secondary PAP with hematological disease and without autoimmune mechanisms is common, but autoimmune PAP with hematological disease, as in the present case, has rarely been reported. Previous case reports of PAP with MPN are listed in Table (11-14). As noted in other reports (9), CML and MDS are the most common cause of PAP, and there are no reports of PAP with MPN U such as ours. In one case of PAP, GM-CSF autoantibodies were detected, and it was speculated that ABL tyrosine kinase inhibitor-induced mechanisms were involved (14). Interestingly, three out of four cases listed in Table were diagnosed with PAP when MPN progressed or disseminated infection occurred. In the remaining case, MPN improved temporarily when whole-lung lavage was provided as treatment for PAP. However, in our case, the relationship between the clinical course of MPN and PAP was not apparent.
Table.
Case Reports of PAP with MPN.
| reference | type of MPN | MPN disease status at diagnosis of PAP | anti GM-SCF antibody | treatment | outcome |
|---|---|---|---|---|---|
| 11 | CML | Chronic phase | not evaluated | antibiotics of Mycobacterium kansasii | died due to septic shock |
| treated for 3years by interferon,imatinib and Hydroxyurea disseminated Mycobacterium kansasii infection | |||||
| 12 | MDS/myelopro liferative syndrome | diagnosed at the same time | not evaluated | WLL | improved dyspnea after WLL |
| 13 | CML | progress to accelerated phase occurred | died due to pneumonia | ||
| treated for 8years by interferon,imatinib and Hydroxyurea | negative | WLL | |||
| 14 | CML | progress to lymphoid blast crisis occurred | 34.9 μg/mL | Switched from imatinib to nilotinib | died due to leukemia |
| treated for 12 years by interferon and imatinib |
One report compared the CT findings between autoimmune PAP and secondary PAP and showed that the typical high-resolution CT (HRCT) findings for autoimmune PAP were GGO with a patchy geographic pattern, subpleural sparing, crazy-paving appearance, and predominance in the lower lung field. These findings are uncommon for secondary PAP (15). In the present case, the HRCT findings were a geographic GGO pattern, rather than diffuse GGO. Additionally, subpleural sparing and the crazy-paving appearance were seen, patterns which are more similar to autoimmune PAP. However, GGO was not predominant in the lower lung field, and we could not confirm the typical appearance of an autoimmune PAP pattern.
When reviewing the therapeutic strategies for PAP, it is important to note that the various forms of PAP may require different treatments, even though whole-lung lavage is the current therapeutic standard (16,17). GM-CSF inhalation therapy (18) and rituximab (19) may also be effective treatments for autoimmune PAP. Several reports have shown that alveolar lavage and serial measurements of serum GM-CSF autoantibodies may prove useful for monitoring disease activity and response to treatment (20).
Secondary PAP with hematological malignancy is considered to have poor prognosis (8). However, it has been reported that PAP may also improve if hematological malignancy can be controlled (2). PAP did not progress after MPN transformed to AML in our case, and this observation was consistent with autoimmune PAP.
This report shows that evaluating the levels of GM-CSF autoantibodies is critical for distinguishing secondary and autoimmune PAP, even if the patient has PAP with hematological disease.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Trapnell BC, Whitsett JA, Nakata K. Pulmonary alveolar proteinosis. N Engl J Med 349: 2527-2539, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Cordonnier C, Fleury-Feith J, Escudier E, Atassi K, Bernaudin J-F. Secondary alveolar proteinosis is a reversible cause of respiratory failure in leukemic patients. Am J Respir Crit Care Med 149: 788-794, 1994. [DOI] [PubMed] [Google Scholar]
- 3.Chakra PC, Monika P, Laurence B, Maher T, John KE. Secondary pulmonary alveolar proteinosis in hematologic malignancies. Hematol Oncol Stem Cell Ther 7: 127-135, 2014. [DOI] [PubMed] [Google Scholar]
- 4.Wakita A, Ohtake S, Takada S, et al. Randomized comparison of fixed-schedule versus response-oriented individualized induction therapy and use of ubenimex during and after consolidation therapy for elderly patients with acute myeloid leukemia: the JALSG GML200 Study. Int J Hematol 96: 84-93, 2012. [DOI] [PubMed] [Google Scholar]
- 5.Uchida K, Nakata K, Trapnell BC, et al. High-affinity autoantibodies specifically eliminate granulocyte-macrophage colony-stimulating factor activity in the lungs of patients with idiopathic pulmonary alveolar proteinosis. Blood 103: 1089-1098, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Kitamura T, Uchida K, Tanaka N, et al. Serological diagnosis of idiopathic pulmonary alveolar proteinosis. Am J Respir Crit Care Med 162: 658-662, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Inoue Y, Trapnell BC, Tazawa R, et al. Characteristics of a Large Cohort of Patients with Autoimmune Pulmonary Alveolar Proteinosis in Japan. Am J Respir Crit Care Med 177: 752-762, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cummings KJ, Donat WE, Ettensohn DB, Roggli VL, Ingram P, Kreiss K. Pulmonary alveolar proteinosis in workers at an indium processing facility. Am J Respir Crit Care Med 181: 458-464, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishii H, Tazawa R, Kaneko C, et al. Clinical features of secondary pulmonary alveolar proteinosis: pre-mortem cases in Japan. Eur Respir J 37: 465-468, 2011. [DOI] [PubMed] [Google Scholar]
- 10.Bonella F, Bauer PC, Griese M, Ohshimo S, Guzman J, Costabel U. Pulmonary alveolar proteinosis: new insights from a single-center cohort of 70 patients. Respir Med 105: 1908-1916, 2011. [DOI] [PubMed] [Google Scholar]
- 11.Goldschmidt N, Nusair S, Gural A, Amir G, Izhar U, Laxer U. Disseminated Mycobacterium kansasii infection with pulmonary alveolar proteinosis in a patient with chronic myelogenous leukemia. Am J Hematol 74: 221-223, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Pollack SM, Gutierrez G, Ascensao J. Pulmonary alveolar proteinosis with myeloproliferative syndrome with myelodysplasia: bronchoalveolar lavage reduces white blood cell count. Am J Hematol 81: 634-638, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Ohmachi K, Ogiya D, Morita F, et al. Secondary pulmonary alveolar proteinosis in a patient with chronic myeloid leukemia in the accelerated phase. Tokai J Exp Clin Med 33: 146-149, 2008. [PubMed] [Google Scholar]
- 14.Yoshimura M, Kojima K, Tomimasu R, et al. ABL tyrosine kinase inhibitor-induced pulmonary alveolar proteinosis in chronic myeloid leukemia. Int J Hematol 100: 611-614, 2014. [DOI] [PubMed] [Google Scholar]
- 15.Ishii H, Trapnell BC, Tazawa R, et al. Comparative study of high-resolution CT findings between autoimmune and secondary pulmonary alveolar proteinosis. Chest 136: 1348-1355, 2009. [DOI] [PubMed] [Google Scholar]
- 16.Campo I, Kadija Z, Mariani F, et al. Pulmonary alveolar proteinosis: diagnostic and therapeutic challenges. Multidiscip Respir Med 7: 4, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beccaria M, Luisetti M, Rodi G, et al. Long-term durable benefit after whole lung lavage in pulmonary alveolar proteinosis. Eur Respir J 23: 526-531, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Tazawa R, Trapnell BC, Inoue Y, et al. Inhaled granulocyte/macrophage-colony stimulating factor as therapy for pulmonary alveolar proteinosis. Am J Respir Crit Care Med 181: 1345-1354, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kavuru MS, Malur A, Marshall I, et al. An open-label trial of rituximab therapy in pulmonary alveolar proteinosis. Eur Respir J 38: 1361-1367, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonfield TL, Kavuru MS, Thomassen MJ. Anti-GM-CSF titer predicts response to GM-CSF therapy in pulmonary alveolar proteinosis. Clin Immunol 105: 342, 2002. [DOI] [PubMed] [Google Scholar]