Abstract
Targeting sperm ion channels and other sperm-specific proteins is an effective way to develop unisex contraceptives, as they should have decreased side effects. This Science & Society summarizes the current advances in human sperm physiology in attempts to evaluate what would be appropriate targets for unisex contraceptives.
Mammalian fertilization results in the fusion of the male gamete (the spermatozoon) with the female gamete (the ovum) that takes place in the specified region of the female reproductive tract (the ampulla). The ovulated egg arrives at the ampulla, where it awaits sperm cells. However, its survival time is limited and lasts from 12 to 24 h; after which, the ovulated egg disintegrates if it is not fertilized by spermatozoon. Mammalian sperm cells are infertile when initially deposited into female reproductive pathways. Human spermatozoa have to spend at least 3 h inside the female reproductive tract to undergo their final maturation before they become competent to fertilize an egg, in a process called capacitation. Therefore, to arrive at the ampulla in time, sperm cells must not only survive in the hostile environment of the female genital tract, but also possess robust motility and overcome numerous obstacles presented along their way. Not surprisingly, during natural conception only a few spermatozoa will reach the site of fertilization in time.
The principle of sperm motility has fascinated researchers since Antonie van Leeuwenhoek first discovered these cells in 1677. Spermatozoa are equipped with sophisticated molecular mechanisms that allow successful navigation in the female reproductive tract; however, these mechanisms vary among species. To succeed, a sperm cell must sense the environment and adapt its motility, which is controlled partially by ATP production, and partially by ion homeostasis, which is in turn under tight control of sperm ion channels and transporters. Sperm ATP production, intracellular alkalinity, membrane voltage, and intracellular calcium concentration are all important for sperm activity within the female reproductive tract. While all mammalian sperm are united in their goal to find and fertilize an egg, the molecular mechanisms they utilize for this purpose differ among species, especially at the level of ion channels.
The incredible diversity in the molecular mechanisms driving sperm motility has hindered our ability to understand and manipulate human sperm function. Our knowledge of the physiology of human spermatozoa, and in particular the sophisticated machinery that regulates human sperm motility and fertilization potential, is so limited that the etiology of ~80% of male infertility cases remain unknown, and the only available treatment is limited to assisted reproductive technologies (http://grants.nih.gov/grants/guide/rfa-files/RFA-HD-09-032.html). That mammalian spermatozoa are diverse in their choice of molecular regulators has also hindered multiple attempts to develop effective compounds to halt sperm fertilizing potential. Essentially, what works for rodents may not necessarily work for humans. Therefore, whether in search of an ideal contraceptive, or developing a universal male fertility diagnostic screen, a more detailed and thorough understanding of what is going on at the molecular level in human sperm cells is needed.
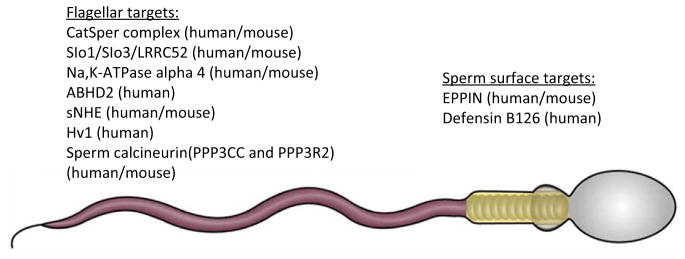
As mentioned above, sperm motility depends on sperm ion homeostasis, which is largely controlled by ion channels and transporters. Many of these proteins or their regulatory subunits are expressed exclusively in sperm cells, thus making them ideal targets for contraceptives (Figure 1). One such target is the flagellar calcium channel CatSper, which is indispensable for fertility in both mice and humans [1,2]. In spermatozoa, swimming behavior is tightly controlled by rises in flagellar calcium ([Ca2+]i) that change the basal flagellar beat pattern into an asymmetrical, whip-like bending of the flagellum, commonly referred to as hyperactivation. This high-amplitude asymmetric flagellar bending is essential for the sperm fertility, because it enables them to overcome the protective vestments of the egg. Male mice and men deficient in CatSper are infertile, while no other phenotypical abnormalities, specifically associated with CatSper deletion, are noted [1]. Human CatSper channel activation requires a combination of intracellular alkalinity and the presence of the female sex hormone progesterone [3,4], which is produced and released by the cumulus cells surrounding the ovulated egg. However, rodent CatSper is progesterone insensitive.
Figure 1.
Mammalian Sperm Targets for Contraceptive Development. Sperm-specific targets: Cat-Sper: cationic channel of sperm; Slo3: pore-forming subunit of potassium channel KSper; LRRC52: auxiliary subunit of potassium channel KSper; Na,K-ATPase isoform ∝4: Na,K pump; sNHE: sperm sodium–proton exchanger; PPP3CC and PPP3R2: catalytic and regulatory subunits of sperm calcineurin; defensin B126: sperm surface protein; EPPIN: epididymal protease inhibitor. Ubiquitously expressed targets: Slo1: calcium-activating potassium channel; ABHD2: ab domain like hydrolase 2; Hv1: voltage-gated proton channel 1.
The details of human CatSper activation by progesterone were recently revealed [5]. CatSper activation begins with the steroid binding to a serine hydrolase, ABHD2, thus triggering its activation and clearance of the endogenous CatSper inhibitor, endocannabinoid 2-arachodo-noylglycerol, from the sperm membrane. The regulation of [Ca2+]i is critical for proper sperm function and thus compounds that directly modulate CatSper or affect [Ca2+]i will also impact sperm fertilization potential. In contrast to CatS-per, ABHD2 is ubiquitously expressed suggesting it might be a less attractive target for contraception development than CatSper. However, the function of ABHD2 is progesterone dependent, which means this enzyme will only work if the female sex hormone progesterone is present. Given that progesterone is predominantly a female hormone, and its concentration in the male body is minimal, blocking ABHD2 action should not theoretically affect men, because the protein should be largely inactive in the male body. Thus, theoretically, an ABHD2 inhibitor could serve as effective male contraceptive pill. If ABHD2 is irreversibly blocked in sperm cells, they will not respond to progesterone once deposited in the female body, and therefore will not be able to fertilize an egg. Therefore, an evaluation of the progesterone-binding domain within ABHD2 could be a valuable first step in search of a male contraceptive that is based on ABHD2 targeting. Interestingly, mice deficient in ABHD2 are apparently normal and fertile [6]. The latter is explained by murine ABHD2 being expressed in a different sperm cellular compartment than murine CatSper, as well as murine CatSper insensitivity to progesterone, while in human spermatozoa, ABHD2 and CatSper are colocalized [5]. It is possible that the role of ABHD2 in other tissues is minor, and its inhibition may not result in potential undesirable health effects, at least in men.
Many other sperm-specific proteins are also being considered as valuable targets for contraceptive development. The mouse potassium channel Slo3 and its auxiliary subunit LRRC52 are sperm specific, and male mice deficient in each of these proteins are severely subfertile [7,8]. Potassium channels are vital for regulation of the sperm membrane electrical potential, because they influence the function of other channels, such as CatSper. Slo3 also forms the potassium channel of human sperm [9,10]; however, its properties are different from murine Slo3 [11], which has created a debate about the true molecular identity of the human sperm potassium channel. As with the CatSper channel, human genetics may provide a useful tool to answer this question.
Sperm cells are unique in that many ubiquitously expressed proteins have sperm-specific isoforms, which also could provide useful targets for contraceptives. For example, the calcium and calmodulin-dependent serine–threonine phosphatase calcineurin, which is essential for calcium signaling in many tissues, also has a sperm-specific isoform whose deficiency or deletion leads to profound male infertility [12]. It is remains to be shown whether selective targeting of sperm calcineurin would produce effective contraceptives because male patients treated with cyclosporine A–a well-known calcineurin inhibitor–do not suffer from infertility [12]. Other potential contraceptive targets could be sperm surface proteins, such as EPPIN (epididymal protease inhibitor) [13] or defensins. Antibodies derived against EPPIN have demonstrated effective and reversible contraception in primates [13]. In addition, mutation in the gene coding for the sperm surface protein defensin B126 is a cause of male subfertility in humans [14]. Among transporters, an interesting candidate for sperm-specific targets for contraceptive is Na,K-ATPase, which exists in several isoforms, among which isoform 4 is sperm specific [15]. Male mice deficient in Na,K-ATPase ∝4 gene are completely sterile, and their spermatozoa are unable to fertilize an egg in vitro [15].
In summary, idiopathic male infertility can be partially attributed to the malfunctioning of sperm regulatory units, their effector molecules, or sperm surface proteins. The molecular repertoire and mode of regulation is divergent between sperm cells of different species, therefore, to search for effective male contraceptives, we must pay significant attention to human sperm-specific regulatory proteins. Detailed characterization of human sperm regulatory units and, perhaps, a comprehensive database that combines the human sperm proteome, transcriptome, and even lipidome, is needed to define the ideal targets for human male contraceptives.
Acknowledgments
This work was supported by NIH R01GM111802, R21HD081403, Pew Biomedical Scholars Award from Pew Charitable Trust, Alfred P. Sloan Award, and Packer Wentz Endowment Will to P.V.L.
References
- 1.Ren D, et al. A sperm ion channel required for sperm motility and male fertility. Nature. 2001;413:603–609. doi: 10.1038/35098027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith JF, et al. Disruption of the principal, progesterone-activated sperm Ca2 + channel in a CatSper2-deficient infertile patient. Proc Natl Acad Sci USA. 2013;110:6823–6828. doi: 10.1073/pnas.1216588110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lishko PV, et al. Progesterone activates the principal Ca2 + channel of human sperm. Nature. 2011;471:387–391. doi: 10.1038/nature09767. [DOI] [PubMed] [Google Scholar]
- 4.Strünker T, et al. The CatSper channel mediates progesterone-induced Ca2 + influx in human sperm. Nature. 2011;471:382–386. doi: 10.1038/nature09769. [DOI] [PubMed] [Google Scholar]
- 5.Miller MR, et al. Unconventional endocannabinoid signaling governs sperm activation via sex hormone progesterone. Science. 2016;352:555–559. doi: 10.1126/science.aad6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miyata K, et al. Increase of smooth muscle cell migration and of intimal hyperplasia in mice lacking the alpha/beta hydrolase domain containing 2 gene. Biochem Biophys Res Commun. 2005;329:296–304. doi: 10.1016/j.bbrc.2005.01.127. [DOI] [PubMed] [Google Scholar]
- 7.Santi CM, et al. The SLO3 sperm-specific potassium channel plays a vital role in male fertility. FEBS Lett. 2010;584:1041–1046. doi: 10.1016/j.febslet.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeng XH, et al. SLO3 auxiliary subunit LRRC52 controls gating of sperm KSPER currents and is critical for normal fertility. Proc Natl Acad Sci USA. 2015;112:2599–2604. doi: 10.1073/pnas.1423869112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brenker C, et al. The Ca2 +-activated K + current of human sperm is mediated by Slo3. eLife. 2014;3:e01438. doi: 10.7554/eLife.01438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mansell SA, et al. Patch clamp studies of human sperm under physiological ionic conditions reveal three functionally and pharmacologically distinct cation channels. Mol Hum Reprod. 2014;20:392–408. doi: 10.1093/molehr/gau003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mannowetz N, et al. Slo1 is the principal potassium channel of human spermatozoa. eLife. 2013;2:e01009. doi: 10.7554/eLife.01009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyata H, et al. Sperm calcineurin inhibition prevents mouse fertility with implications for male contraceptive. Science. 2015;350:442–445. doi: 10.1126/science.aad0836. [DOI] [PubMed] [Google Scholar]
- 13.O’Rand MG, et al. Non-hormonal male contraception: a review and development of an Eppin based contraceptive. Pharmacol Ther. 2016;157:105–111. doi: 10.1016/j.pharmthera.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tollner TL, et al. A common mutation in the defensin DEFB126 causes impaired sperm function and sub-fertility. Sci Transl Med. 2011;3:92ra65. doi: 10.1126/scitranslmed.3002289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jimenez T, et al. Na,K-ATPase alpha4 isoform is essential for sperm fertility. Proc Natl Acad Sci USA. 2011;108:644–649. doi: 10.1073/pnas.1016902108. [DOI] [PMC free article] [PubMed] [Google Scholar]