ABSTRACT
Leptospirosis is potentially a fatal zoonosis acquired by contact of skin and mucosal surfaces with soil and water contaminated with infected urine. We analyzed the outcome of infection of C3H/HeJ mice with Leptospira interrogans serovar Copenhageni using an enzootic mode of transmission, the conjunctival route. Infection led to weight loss and L. interrogans dissemination from blood to urine, and spirochetes were detected in blood and urine simultaneously. The infectious dose that led to consistent dissemination to kidney after conjunctival infection was ∼108 leptospires. Interestingly, a lower number of spirochetes appeared to colonize the kidney, given that we quantified ∼105 and ∼10 leptospires per μl of urine and per μg of kidney, respectively. Leptospira-specific IgM and IgG were detected at 15 days postinfection, and isotyping of the Ig subclass showed that the total IgG response switched from an IgG1 response to an IgG3 response after infection with L. interrogans. Histological periodic acid-Schiff D staining of infected kidney showed interstitial nephritis, mononuclear cell infiltrates, and reduced size of glomeruli. Quantification of proinflammatory immunomediators in kidney showed that keratinocyte-derived chemokine, macrophage inflammatory protein 2, RANTES, tumor necrosis factor alpha, gamma interferon, and interleukin-10 were upregulated in infected mice. We show that the kinetics of disease progression after infection via the ocular conjunctiva is delayed compared with infection via the standard intraperitoneal route. Differences may be related to the number of L. interrogans spirochetes that succeed in overcoming the natural defenses of the ocular conjunctiva and transit through tissue.
KEYWORDS: C3H/HeJ, Leptospira, conjunctiva, enzootic transmission, inflammation, mouse model, sublethal infection
INTRODUCTION
Leptospirosis is a reemerging underdiagnosed worldwide zoonotic disease, with an estimated 1.03 million cases and 60,000 deaths occurring per year (1, 2). It places a substantial burden on human and animal health (3). Tropical regions are favorable to leptospirosis transmission due to the abundance of water (4). Humans are accidental hosts that acquire leptospirosis via contact with infected urine shed by dogs, pigs, cattle, and rodents or through contact with contaminated soil and water during daily activities, like washing, fishing, and swimming, or during recreational activities (5–7). The outcome of the disease is greatly influenced by the pathogen serovar and the host's age and species (8–11). The ensuing immune response and inflammation may lead to bacterial clearance, limited bacterial colonization of a few target organs, or the induction of sepsis as the host succumbs to infection and dies (12). Thus, accidental hosts infected with pathogenic strains of Leptospira may develop conditions ranging from a mild febrile illness to a potentially fatal acute infection. Rodents, such as rats and mice, are common reservoir hosts (13, 14). The infection of wild outbred reservoir host species is largely asymptomatic (15, 16). However, we (14) and others (10, 11, 17, 18) have shown that a number of inbred mouse strains are susceptible to infection with Leptospira interrogans and that the disease course, bacterial dissemination, kidney histopathology, and immune responses to the pathogen recapitulate the natural disease in nonreservoir hosts.
Given a mortality rate of about 5 to 10% in humans (1), it is reasonable to expect that about 90% of people will go on to develop sublethal cases of leptospirosis. The mouse is a versatile animal model with which to study the pathogenesis caused by L. interrogans. More importantly, the immune response to the pathogen and to putative vaccines can only be studied in this biological system due to the availability of reagents and mouse genetic backgrounds for immunology investigation.
One area that remains to be explored is the characterization of L. interrogans dissemination and disease progression in mice after infection via a natural route of transmission, such as through the skin and mucosal surfaces. Our goal was to analyze differences between the transmission of L. interrogans via an enzootic relevant route and transmission of the same serovar via the standard intraperitoneal (i.p.) route in our previously established mouse model of sublethal infection (14). We first titrated the conjunctival infectious dose and followed the disease course by assessment of clinical scores and histopathology. We tracked pathogen dissemination by quantification of the L. interrogans burden in body fluids and tissues, and we finished the study with an analysis of the immune responses and inflammation by profiling the immunomediators of infection in blood, urine, and kidney.
RESULTS
Titration of infectious dose of Leptospira via the conjunctival route.
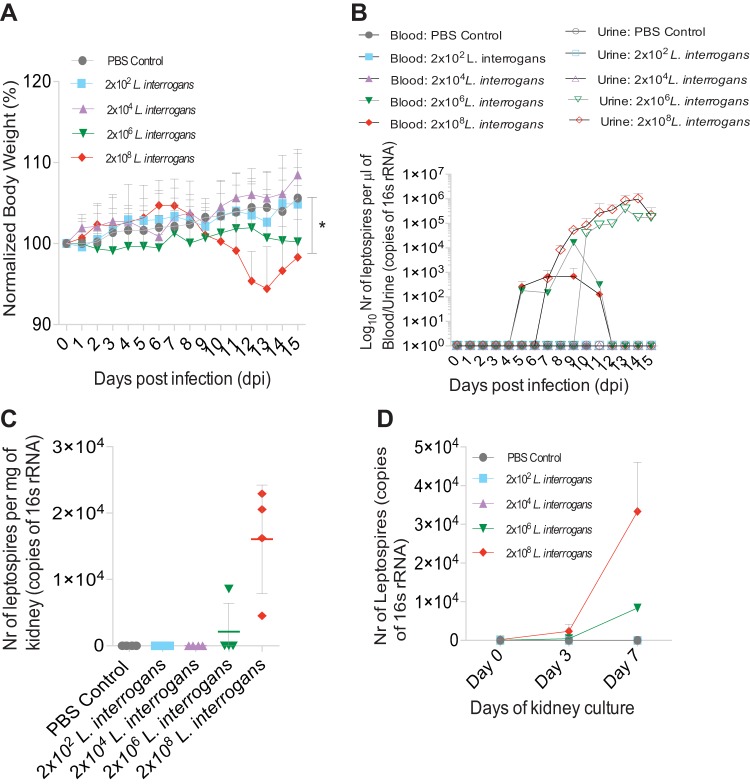
The infectious dose of Leptospira was titrated using a range of concentrations of spirochetes of L. interrogans serovar Copenhageni Fiocruz L1-130 (passage 4) (2 × 108, 2 × 106, 2 × 104, and 2 × 102) to inoculate 10-week-old C3H/HeJ mice via the ocular conjunctiva. Body weight records showed that all 4 mice infected with 2 × 108 leptospires lost a significant amount of weight from days 10 to 15 (up to an average of 6%) compared to the control mice (P = 0.0395). In the group infected with 2 × 106 leptospires, only 1 mouse lost a significant amount of body weight (15%), and the other 3 mice in that group lost ∼2% of their body weight overall. The mice that lost weight were less active than the uninfected mice. The mice in the control group receiving phosphate-buffered saline (PBS) and in the groups infected with 2 × 102 and 2 × 104 leptospires did not lose weight and did not show symptoms of infection (Fig. 1A). Dissemination and shedding were analyzed by the presence of leptospiral DNA in blood and urine by quantitative PCR (qPCR). L. interrogans was first detected in blood from days 5 to 11 postinfection and then in urine from day 7 to day 15. qPCR of genomic DNA showed an average of 444 ± 291 leptospires in blood and an average of 3 × 105 ± 3.5 × 105 leptospires in urine (Fig. 1B) in all 4 infected mice. From day 7 through day 11, blood and urine tested positive for L. interrogans. Quantitative PCR of genomic DNA extracted from kidney tissue revealed an average of ∼104 spirochetes per mg of tissue (10 spirochetes/μg of tissue) in all the mice that showed previous evidence of infection (Fig. 1C). The viability of L. interrogans in the kidney was tested by qPCR analysis of Ellinghausen-McCullough-Johnson-Harris (EMJH) medium cultures of kidney tissue collected at termination on day 15. Cultures were grown for 7 days, and 2-μl samples were saved on days 0, 3, and 7 for analysis by qPCR. A growth curve of L. interrogans was obtained for all the mice that showed previous evidence of infection (Fig. 1D).
FIG 1.
Titration of infectious dose of Leptospira interrogans administered through the conjunctival route. Groups of mice (n = 4) were inoculated with sterile 1× PBS and with 2 × 102, 2 × 104, 2 × 106, and 2 × 108 L. interrogans bacteria (A to C). At 15 days postinfection, we analyzed body weight (A) and the bacterial burden in blood and urine (B), as well as kidney (C). Kidney tissue collected at termination on day 15 was cultured for 7 days to assess L. interrogans viability (D). P values were determined by a two-tailed unpaired t test with Welch's correction. *, P < 0.05. Nr, number.
Reproducibility of infection.
The results from the infectious dose titration curve using 2 × 108 leptospires were reproduced in two additional independent experiments by a different laboratory member. In contrast to the controls, infected mice lost a significant amount of weight by day 15 postinfection (group average, 11%; P = 0.0049) (see Fig. S1A in the supplemental material), and the body temperature associated with hypothermia reached 33.6°C in some mice of this group (P = 0.0153) (Fig. S1B). L. interrogans was detected in blood from day 5 to day 11, and shedding in urine started at days 8 and 9, although one of the mice started shedding on day 7 (Fig. S1C). The simultaneous presence of L. interrogans DNA in the blood and urine between days 7 and 11 was reproduced twice using 2 × 108 L. interrogans spirochetes via conjunctival inoculation.
Infection induced the production of L. interrogans-specific IgM and IgG antibodies in blood and IgG isotype switching to IgG3.
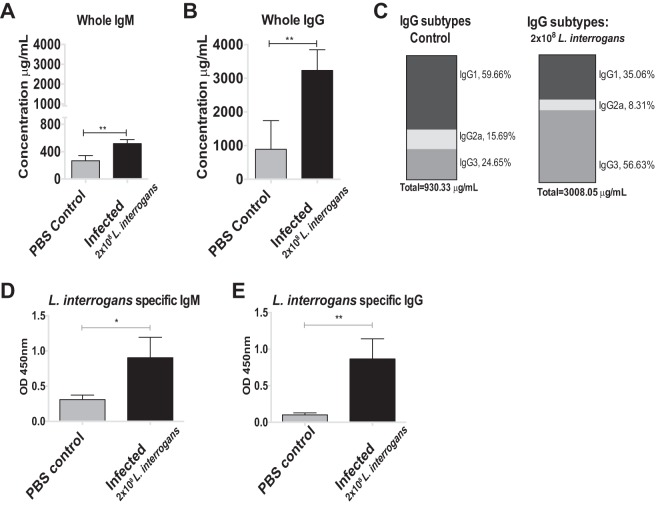
Serum collected at termination at 2 weeks postinfection was assessed for the presence of total immunoglobulin as well as L. interrogans-specific IgM and IgG. The concentrations of total IgM and IgG were ∼1.5 times and 3.5 times greater in the infected group (2 × 108 leptospires) than the control group (Fig. 2A and B). Subtyping of total IgG revealed isotype switching in response to infection. Serum from control mice was enriched for IgG1 (59.66%), followed by IgG3 (24.65%) and IgG2a (15.69%), whereas infection led to an enrichment of total IgG3 (56.63%), and the proportions of IgG1 and IgG2a declined to 35.06% and 8.31%, respectively (Fig. 2C). Determination of the leptospira-specific immunoglobulin showed that infected mice produced larger quantities of both IgM and IgG antibodies at 15 days postinfection (for IgM, P = 0.0148 [Fig. 2D]; for IgG, P = 0.0092 [Fig. 2E]).
FIG 2.
Antibody response in serum following L. interrogans infection through the conjunctival route (A to C). The total concentrations of IgM and IgG in serum from C3H/HeJ mice were quantified (A, B), and the proportions of subtypes IgG1, IgG2a, and IgG3 were measured (C). L. interrogans-specific IgM and IgG levels in serum were also quantified by ELISA (D and E). OD, optical density. P values were determined by a two-tailed unpaired t test with Welch's correction. *, P < 0.05; **, P < 0.005. Data represent one of two independent studies.
Infection of mice through the ocular conjunctiva triggers inflammation in the kidney.
Histopathological analysis of the kidneys from infected mice by periodic acid-Schiff D (PAS-D) staining showed scattered interstitial nephritis with infiltrates of mononuclear cells, tubular damage, and glomerular atrophy in contrast to the uninfected controls (Fig. 3A). Measurement of kidney glomeruli showed a significant decrease in the sizes of these structures in infected mice compared to the controls (P = 0.0131) (Fig. 3B). The extent of damage correlated with a marked increase in the level of inducible nitric oxide synthase (iNOS) mRNA expression in the infected mice compared to the controls (P = 0.0046) (Fig. 3C).
FIG 3.
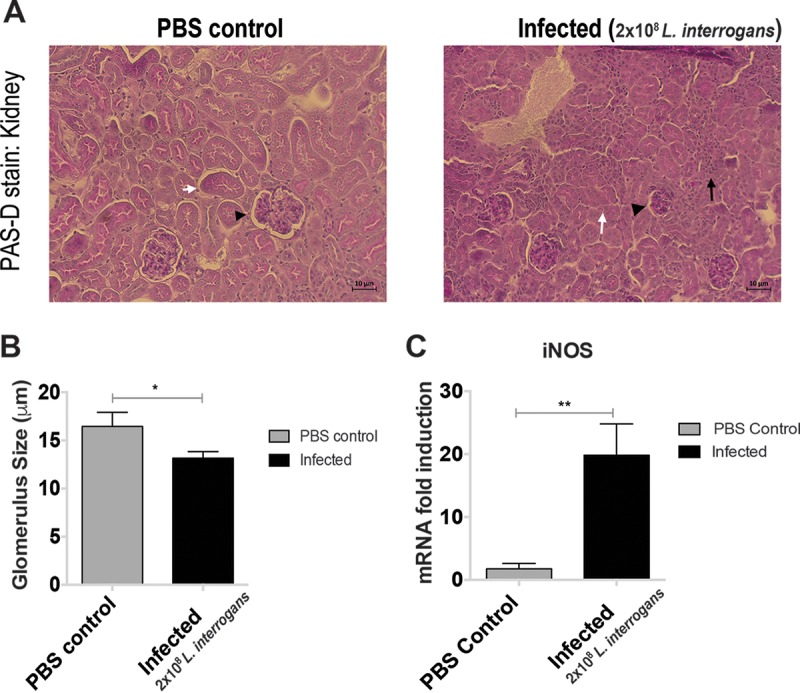
Interstitial nephritis and immune cell infiltration in mice infected with L. interrogans through the conjunctival route. PAS-D staining shows increased cellular infiltration, reduced size of glomeruli, and loss of tubular structure in infected mouse kidneys observed under a light microscope (Zeiss Axio microscope [×20]; white arrows, tubules; arrowheads, glomeruli; black arrows, immune cells) (A). The glomerulus size in the infected kidney tissue was reduced. The histogram represents the average size of 10 randomly selected glomeruli per 5 fields per animal ± SD (B). High levels of iNOS were observed in the kidney tissue after infection (C). P values were determined using a two-tailed unpaired t test with Welch's correction. *, P < 0.05; **, P < 0.005. Data represent one of two independent studies.
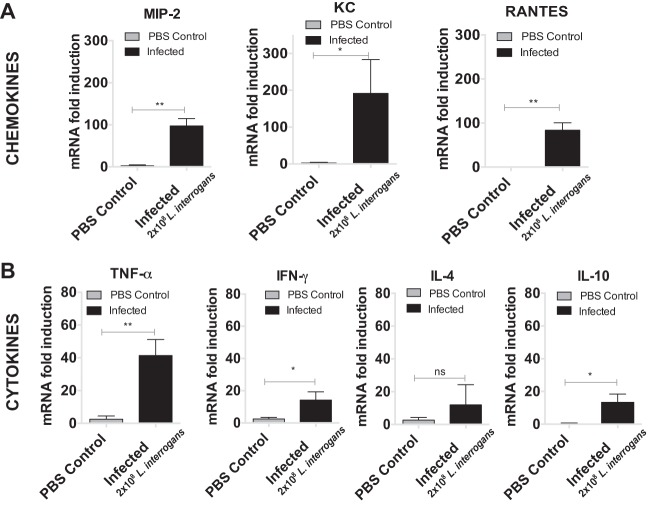
Conjunctival infection leads to induction of pro- and anti-inflammatory cytokines and chemokines by immune cell infiltrates in tissue.
Kidney was processed for quantification of the mRNA of pro- and anti-inflammatory mediators. The levels of expression of mRNA for the chemoattractant chemokines macrophage inflammatory protein 2 (MIP-2), keratinocyte-derived chemokine (KC), and RANTES in kidneys from infected mice were over 100-fold greater (MIP-2 and KC) and 80-fold greater (RANTES) than those in kidneys from mice in the control group (Fig. 4A). Among the cytokines, the levels of tumor necrosis factor alpha (TNF-α) and gamma interferon (IFN-γ) (which are involved in a proinflammatory Th1 response) and interleukin-10 (IL-10; which is involved in an anti-inflammatory response) were significantly increased in the infected mice compared with the control mice; in contrast, the level of IL-4 (which is involved in a proinflammatory Th2 response) was not different from the control (Fig. 4B). The cytokine and chemokine results for the mouse infected with 2 × 106 leptospires were similar to those for mice infected with 2 × 108 leptospires. The cytokine and chemokine results for the groups infected with 2 × 102 and 2 × 104 spirochetes were comparable to those for the PBS-treated control group (data not shown).
FIG 4.
Infection of L. interrogans through the conjunctival route triggers inflammation in the kidney. The plots represent analyses of the proinflammatory mediators, chemokines (A) and cytokines (B), of the innate and adaptive immune responses isolated from the kidneys of control and infected mice. Histograms show the mean ± SD mRNA transcript levels for mice (n = 4) sacrificed at day 15 after conjunctival infection. P values were determined using a two-tailed unpaired t test with Welch's correction. *, P < 0.05; **, P < 0.005; ns, not significant. Data represent one of two independent studies.
DISCUSSION
One of our goals is to develop mouse models of experimental infection that recapitulate the natural disease in reservoir and nonreservoir hosts. In this study, we found that infection of C3H/HeJ mice with pathogenic Leptospira via a natural transmission route, the ocular conjunctiva, led to differences in the kinetics of pathogen dissemination as well as in the bacterial burden in the kidney from those previously measured when the intraperitoneal (i.p.) route of infection was used.
Outbred wild mice are reservoir hosts of L. interrogans. As such, they develop tolerance or resistance to the spirochete and are usually asymptomatic when infected (19). Leptospiral lipopolysaccharide (LPS) activates the innate immune system mostly via Toll-like receptor 2 (TLR2) but also via TLR4 (20, 21). However, human TLR4 does not recognize leptospiral LPS (22) and as such might be less likely to engage a protective immune response against this spirochete. Inbred C3H/HeJ mice have a point mutation in a TLR4 gene (23, 24) which results in a dysfunctional receptor for LPS. Furthermore, signaling through TLR4 has been shown to decrease the expression of TLR2 in spleen and liver in C3H/HeJ mice infected with Salmonella enterica serovar Typhimurium (25). In fact, Chassin et al. showed that TLR2 and TLR4 double-knockout (KO) mutants of another strain of mice (C57BL/6J) rapidly died from severe organ failure following L. interrogans inoculation, showing that TLR2/TLR4-mediated B cell responses lead to the clearance of this pathogen (21). C3H/HeJ mice are susceptible to leptospirosis and have been used to develop models of infection that recapitulate the disease in nonreservoir hosts, including histopathology features observed in accidental hosts, such as humans (10, 14, 17). The intraperitoneal route of infection has been used to establish the initial proof-of-principle mouse models of most human diseases because it is a reliable method of delivering a known infectious dose of the pathogen to the host. This method, however, falls short of achieving what might happen in nature. Pathogenic strains of Leptospira infect mice or any other mammal, including humans, via mucosal or transdermal routes (16, 26). In this study, we evaluated the pathogenesis of L. interrogans in mice infected via a natural route of transmission. Our ultimate goal was to evaluate if infection of mice via the ocular conjunctiva reproduced the disease metrics, histopathology, and immunomediator markers that we, and others, previously established by infection of mice by the intraperitoneal route.
Several studies have evaluated a number of doses of different Leptospira interrogans serovars injected intraperitoneally into mice of different strains and ages (10–12, 14). Intraperitoneal infectious doses ranging from 103 to 109 have been used in mice aged 3 to 14 weeks (27–30). In our study, we determined that ∼108 cells of L. interrogans serovar Copenhageni were needed to establish infection consistently in 100% of the C3H/HeJ mice at 10 weeks of age. A conjunctival infectious dose such as ∼106 established infection in 25% of the mice in the cohort tested. Doses of infectious Leptospira ranging from 102 to 5 × 103 leptospires were previously quantified in urban slum water gutters in the Amazon region of Iquitos, Peru (31), known for its high incidence of human leptospirosis. The lower infectious dose range (102 to 104) tested in our study failed to establish infection in C3H/HeJ mice. However, there are examples of other diseases, such as melioidosis, in which humans can acquire infection after being exposed to extra low doses of bacteria via one route (32) but much higher doses are needed to replicate the disease in mouse models of infection (33–35).
Sublethal signs of leptospirosis have been described in different strains of mice (strains A, CBA, C57BL/6, and C3H/HeJ) and were determined by quantification of the leptospires in blood, urine, and kidney and weight loss (14, 29), nephritis (14, 36), and fibrosis in the kidney (14, 18). One interesting difference between mice infected via the conjunctiva and mice infected via the intraperitoneal route was that there was a large overlap between the period in which the spirochete was detected in blood and the period in which it was detected in urine from days 7 to 11 postinfection. This overlap forms a plot of the time of disease progression that looks more unimodal, unlike the bimodal graph produced by i.p. infections with very high doses of spirochetes (>107). This suggests that the natural history of infection in mammals may account for a period in which the spirochete may be detected in both body fluids simultaneously. Colonization of the kidney by live L. interrogans spirochetes was demonstrated by direct detection of the 16S rRNA gene in kidney and by the subsequent recovery and quantification of live bacteria from kidney tissue cultures. Histopathology of the kidney showed nephritis in the infected mice similar to that which was previously seen in C57BL/6 mice (27), and the tubular damage and atrophied glomeruli of various degrees were equivalent to those seen in C3H/HeJ mice infected via the intraperitoneal route (10, 14, 36). Furthermore, the clinical scores of disease, such as loss of weight and hypothermia, correlated with colonization of the kidney at about day 10 or 11 postinfection.
A stark difference observed between mice infected by the two routes was that in the intraperitoneal infection model, the initial infectious dose of 106 led to the shedding of 106 leptospires per μl of urine and to colonization of the kidney with 106 leptospires per μg of tissue at 15 days postinfection (14). In the conjunctival infection study reported here, an initial infectious dose of 108 leptospires led to the shedding of ∼105 leptospires per μl of urine and to colonization of the kidney with 10 leptospires per μg of tissue in the same time frame. Another interesting observation is that dissemination in blood and urine happened 24 h to 48 h later when mice were infected by the conjunctival route. This may be related to the fact that after intraperitoneal inoculation all bacteria are available for dissemination, whereas after conjunctival inoculation, spirochetes must overcome the natural defenses of the ocular conjunctiva and the lower numbers that succeed in crossing that barrier still have to transit through tissue before dissemination takes place.
The humoral immune response to L. interrogans infection led to increases in total and L. interrogans-specific IgM and IgG concentrations at 2 weeks postinfection. Uninfected mice had higher concentrations of IgG1 in serum, whereas serum from infected mice was enriched for the IgG3 isotype. This was a puzzling finding, given that it has been reported that mice lacking functional TLR4 (such as C3H/HeJ mice) or its adaptor molecule, MyD88, had significantly smaller amounts of IgG3 natural antibodies to LPS (37). However, Leptospira LPS is also known to activate the host immune system via TLR2 (21). It is tempting to speculate that associations between Leptospira LPS and TLR2 might lead to increases in the amount of total IgG3, which synergizes with IgM early in infection to help clear L. interrogans independently of comprehensive downstream T cell responses. We did not test IgG3 in our proof-of-principle model of sublethal infection by the intraperitoneal route and therefore cannot draw comparative conclusions. However, we do not expect to see differences in IgG3 enrichment in serum between mice infected by the two routes.
There was evidence of involvement of early innate immune responses (indicated by the presence of MIP-2, KC, RANTES, and TNF-α) and cytokines associated with adaptive responses (IFN-γ, Th1) in the kidney, despite the presence of viable L. interrogans in this organ. These data provide molecular support for our histopathology findings (14) and those of other investigators (18). Tumor necrosis factor alpha receptor (TNFR) and IFN-γ knockout (KO) did not eliminate L. interrogans infection in the kidneys of C57BL/6 mice, and mice with TNFR KO had higher scores for interstitial nephritis than wild-type mice, whereas nephritis was reduced in the mice with INF-γ KO (38). These data suggest that while the L. interrogans burden in the kidney may not be affected, the proinflammatory cytokines produced might contribute to inflammation and nephritis. We did not observe major differences in the levels of production of immunomediator markers between mice infected via the intraperitoneal route and those infected via the conjunctival route.
Our findings suggest that infection of C3H/HeJ mice with pathogenic Leptospira via a natural transmission route, such as the ocular conjunctiva, leads to some differences from the findings observed when the intraperitoneal route of infection was used. L. interrogans disseminated from blood to urine in a sequential manner, but we observed a nonbimodal, nearly simultaneous presence of the spirochete in blood and in urine. Another significant difference was measured between the initial infectious dose and the burden of bacterial shedding in urine, and the number of bacteria quantified in colonized kidneys 2 weeks postinfection. In the model of infection by the i.p. route, the same number of 106 L. interrogans bacteria used to infect mice was shed per μl of urine and quantified per μg of kidney, whereas in the conjunctival model of infection, of the 108 leptospires used to infect mice, ∼105 leptospires were shed per μl of urine and 10 leptospires were detected in 1 μg of kidney tissue. Furthermore, in the conjunctival model of infection, dissemination in blood and urine happened 24 to 48 h later than in the intraperitoneal model of infection. Our data suggest that higher numbers of spirochetes could be necessary to overcome the natural defenses of the ocular conjunctiva and transit through tissue before systemic dissemination takes place. We found that the kinetics of disease progression after infection of mice using an enzootic transmission route was different from that obtained using the traditional intraperitoneal route. The conjunctival route of infection may better recapitulate the natural history of the disease. Testing of vaccines and therapeutics in these two mouse models of infection could yield different efficacy outcomes.
MATERIALS AND METHODS
Animals and facility.
Female C3H/HeJ mice (age, 10 weeks; n = 36) from The Jackson Laboratory (Bar Harbor, ME) were housed in an animal biosafety level 2 environment within the Laboratory Animal Care Unit of the University of Tennessee Health Science Center. Animal experimentation was conducted in compliance with University of Tennessee Health Science Center IACUC protocol number 14-018.
Bacterial strain and culture conditions.
Leptospira interrogans serovar Copenhageni strain Fiocruz L1-130 was cultivated in Ellinghausen-McCullough-Johnson-Harris (EMJH) medium supplemented with Difco Leptospira enrichment EMJH (Becton, MD) and 100 μg/ml 5-fluorouracil (MP Chemicals, CA) at 30°C. 5-Fluorouracil prevents contamination of the culture by inhibiting the growth of other organisms but does not affect the growth of L. interrogans. The culture (passage 4) was allowed to reach log phase of growth, pelleted by centrifugation at 3,000 × g for 5 min, washed, and resuspended in sterile 1× PBS. The cells were diluted 1:100 and 1:1,000, counted under a dark-field microscope (Zeiss USA, NY) using a Petroff-Hausser counting chamber, and confirmed by qPCR (Life Technologies, NY).
Experimental infection.
Groups of mice (n = 4) were anesthetized and infected by application of a drop of medium (10 μl) containing L. interrogans (3.33 × 109 leptospires/ml) on the medial canthus of each eye 3 different times separated by 20 min (total, 60 μl). The control group received the same amount of sterile 1× PBS. Following inoculation, the eyelid was gently massaged to spread the liquid evenly on the conjunctival tissue. For the infectivity titration study, 4 groups of mice (age, 10 weeks; n = 4) were infected with 2 × 108, 2 × 106, 2 × 104, and 2 × 102 L. interrogans spirochetes; the control group received 1× PBS (n = 20 mice). Conjunctival infection with 2 × 108 leptospires per mouse was done in two additional independent experiments to address the reproducibility of the results (n = 16 mice). Mice were weighed, the body temperature was recorded rectally, and urine was collected daily for 2 weeks. Blood was collected daily until day 5 and on alternate days until day 15. Animals were sacrificed on day 15 postinfection, kidneys were collected, and one half of the kidney was stored in RNAlater (Ambion, TX) for the quantification of leptospires from genomic DNA extracts, PAS-D staining, and quantification of immune marker RNA. One half of the kidney was placed in supplemented EMJH medium to check the viability of L. interrogans. The minimum infectious dose was defined as the number of L. interrogans spirochetes that caused disease, as measured by scoring clinical symptoms as well as bacterial dissemination through blood and establishment of kidney colonization.
Serological assays.
Analysis of the total concentrations of IgM and IgG in mouse serum was performed using a Ready-Set-Go enzyme-linked immunosorbent assay (ELISA) (eBioscience) according to the manufacturer's instructions. To determine leptospira-specific IgM and IgG, 96-well microtiter plates (MaxiSorp; Thermo Fisher, Waltham, MA) were coated with heat-killed L. interrogans bacteria (0.5 mg/ml), 4 μl of which was diluted in 100 mM sodium carbonate (pH 9.7) to obtain 2 μg/100 μl/well of total protein. Heat-killed leptospires were prepared as follows: pellets from the culture, which were washed with 1× PBS, were harvested by centrifugation at 12,000 × g and heat killed by exposure to heat for 10 min at 95°C. The supernatant was discarded, and the pellet was resuspended in PBS. The protein concentration was determined by a Lowry assay, and 2 μg of protein was used to coat each well of the plate. Anti-Leptospira IgM and IgG antibody levels were measured using serum from infected and uninfected mice (dilution, 1:100) and horseradish peroxidase-conjugated anti-mouse immunoglobulin secondary antibodies (1:50,000; Jackson ImmunoResearch, PA). 3,3′,5,5′-Tetramethylbenzidine was used as a peroxidase substrate. The optical density at 450 nm was measured using a SpectraMax microplate reader (Molecular Devices, CA).
qPCR.
Genomic DNA was extracted from blood, urine, and kidney with a NucleoSpin tissue kit (Clontech, CA) according to the manufacturer's instructions, and purified DNA was stored at −20°C. The leptospiral burden was quantified by the amplification of the 16S rRNA gene (rrm locus), using the specific 6-carboxytetramethylrhodamine (TAMRA) probe and primers (Eurofins, AL), on a StepOne Plus real-time PCR system as described previously (23). Quantification of the leptospires was done by running a standard curve prepared from serial dilutions of DNA from known numbers of L. interrogans bacteria. The PCR mixture contained 25 μM each primer, 100 nM the specific probe, and 2 μl of DNA sample in a total volume of 20 μl. The reactions were performed in duplicate. The amplification protocol consisted of 10 min at 95°C, followed by 40 cycles of amplification (95°C for 15 s and 60°C for 1 min). The analysis was done using the comparative threshold cycle (CT) method. A negative result was assigned when no amplification occurred or if the CT value was >35.
Quantitative reverse transcription-PCR.
An RNeasy minikit (Qiagen) was used to extract total RNA from approximately 20 mg of kidney tissue; DNase was used to eliminate DNA contamination, and the RNA was reverse transcribed using a high-capacity cDNA reverse transcription kit (Applied Biosystems). 6-Carboxyfluorescein–TAMRA probes and primers (Eurofins, AL) specific to the following chemokines and cytokines were generated: KC, MIP-2, RANTES, iNOS, TNF-α, IFN-γ, IL-4, IL-10, and β-actin. Cytokine data are reported as the relative increase in mRNA transcript levels in infected mice compared with that in control mice corrected by the respective levels of β-actin, used as an internal control.
Histopathology.
Formalin-fixed tissues of kidney were embedded in paraffin, sectioned, and stained with periodic acid-Schiff D (PAS-D). Samples were evaluated for interstitial inflammation, glomerular size, and tubular damage.
Statistical analysis.
Statistical analysis was performed using GraphPad Prism software. Data for each group are means for 4 mice ± standard deviations (SDs). Statistical analyses were performed using a two-tailed unpaired t test with Welch's correction with a cutoff P value of <0.05 for all values for the control versus the infected groups (Fig. 1A, 2A, B, D, and E, 3B and C, 4, and S1A and B).
Supplementary Material
ACKNOWLEDGMENTS
We thank David Haake at the VA Greater Los Angeles Healthcare System for kindly providing hamster-passaged Leptospira interrogans serovar Copenhageni strain Fiocruz L1-130. We thank Luciana Richer at US Biologic, Inc., for helping to set up murine conjunctival infections. We thank the Integrated Microscopy Center at the University of Memphis for its histopathology services.
This work was supported by Public Health Service grants R01 AI034431 (to David Haake and Maria Gomes-Solecki) and R44 AI096551 (to Maria Gomes-Solecki) from the National Institutes of Health.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.01050-16.
REFERENCES
- 1.Costa F, Hagan JE, Calcagno J, Kane M, Torgerson P, Martinez-Silveira MS, Stein C, Abela-Ridder B, Ko AI. 2015. Global morbidity and mortality of leptospirosis: a systematic review. PLoS Negl Trop Dis 9:e0003898. doi: 10.1371/journal.pntd.0003898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bharti AR, Nally JE, Ricaldi JN, Matthias MA, Diaz MM, Lovett MA, Levett PN, Gilman RH, Willig MR, Gotuzzo E, Vinetz JM, Peru-United States Leptospirosis Consortium. 2003. Leptospirosis: a zoonotic disease of global importance. Lancet Infect Dis 3:757–771. doi: 10.1016/S1473-3099(03)00830-2. [DOI] [PubMed] [Google Scholar]
- 3.Abela-Ridder B, Sikkema R, Hartskeerl RA. 2010. Estimating the burden of human leptospirosis. Int J Antimicrob Agents 36(Suppl 1):S5–S7. doi: 10.1016/j.ijantimicag.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 4.Haake DA, Levett PN. 2015. Leptospirosis in humans. Curr Top Microbiol Immunol 387:65–97. doi: 10.1007/978-3-662-45059-8_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caldas EM, Sampaio MB. 1979. Leptospirosis in the city of Salvador, Bahia, Brazil: a case-control seroepidemiologic study. Int J Zoonoses 6:85–96. [PubMed] [Google Scholar]
- 6.Monahan AM, Miller IS, Nally JE. 2009. Leptospirosis: risks during recreational activities. J Appl Microbiol 107:707–716. doi: 10.1111/j.1365-2672.2009.04220.x. [DOI] [PubMed] [Google Scholar]
- 7.Lehmann JS, Matthias MA, Vinetz JM, Fouts DE. 2014. Leptospiral pathogenomics. Pathogens 3:280–308. doi: 10.3390/pathogens3020280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vernel-Pauillac F, Goarant C. 2010. Differential cytokine gene expression according to outcome in a hamster model of leptospirosis. PLoS Negl Trop Dis 4:e582. doi: 10.1371/journal.pntd.0000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silva EF, Santos CS, Athanazio DA, Seyffert N, Seixas FK, Cerqueira GM, Fagundes MQ, Brod CS, Reis MG, Dellagostin OA, Ko AI. 2008. Characterization of virulence of Leptospira isolates in a hamster model. Vaccine 26:3892–3896. doi: 10.1016/j.vaccine.2008.04.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nally JE, Fishbein MC, Blanco DR, Lovett MA. 2005. Lethal infection of C3H/HeJ and C3H/SCID mice with an isolate of Leptospira interrogans serovar Copenhageni. Infect Immun 73:7014–7017. doi: 10.1128/IAI.73.10.7014-7017.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Viriyakosol S, Matthias MA, Swancutt MA, Kirkland TN, Vinetz JM. 2006. Toll-like receptor 4 protects against lethal Leptospira interrogans serovar Icterohaemorrhagiae infection and contributes to in vivo control of leptospiral burden. Infect Immun 74:887–895. doi: 10.1128/IAI.74.2.887-895.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zuerner RL. 2015. Host response to leptospira infection. Curr Top Microbiol Immunol 387:223–250. doi: 10.1007/978-3-662-45059-8_9. [DOI] [PubMed] [Google Scholar]
- 13.Bonilla-Santiago R, Nally JE. 2011. Rat model of chronic leptospirosis. Curr Protoc Microbiol Chapter 12:Unit 12E.3. doi: 10.1002/9780471729259.mc12e03s20. [DOI] [PubMed] [Google Scholar]
- 14.Richer L, Potula HH, Melo R, Vieira A, Gomes-Solecki M. 2015. Mouse model for sublethal Leptospira interrogans infection. Infect Immun 83:4693–4700. doi: 10.1128/IAI.01115-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adler B, Faine S. 1977. Host immunological mechanisms in the resistance of mice to leptospiral infections. Infect Immun 17:67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zilber AL, Belli P, Grezel D, Artois M, Kodjo A, Djelouadji Z. 2016. Comparison of mucosal, subcutaneous and intraperitoneal routes of rat Leptospira infection. PLoS Negl Trop Dis 10:e0004569. doi: 10.1371/journal.pntd.0004569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pereira MM, Andrade J, Marchevsky RS, Ribeiro dos Santos R. 1998. Morphological characterization of lung and kidney lesions in C3H/HeJ mice infected with Leptospira interrogans serovar Icterohaemorrhagiae: defect of CD4+ and CD8+ T-cells are prognosticators of the disease progression. Exp Toxicol Pathol 50:191–198. doi: 10.1016/S0940-2993(98)80083-3. [DOI] [PubMed] [Google Scholar]
- 18.Fanton d'Andon M, Quellard N, Fernandez B, Ratet G, Lacroix-Lamande S, Vandewalle A, Boneca IG, Goujon JM, Werts C. 2014. Leptospira interrogans induces fibrosis in the mouse kidney through Inos-dependent, TLR- and NLR-independent signaling pathways. PLoS Negl Trop Dis 8:e2664. doi: 10.1371/journal.pntd.0002664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsui M, Roche L, Geroult S, Soupe-Gilbert ME, Monchy D, Huerre M, Goarant C. 2016. Cytokine and chemokine expression in kidneys during chronic leptospirosis in reservoir and susceptible animal models. PLoS One 11:e0156084. doi: 10.1371/journal.pone.0156084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Werts C, Tapping RI, Mathison JC, Chuang TH, Kravchenko V, Saint Girons I, Haake DA, Godowski PJ, Hayashi F, Ozinsky A, Underhill DM, Kirschning CJ, Wagner H, Aderem A, Tobias PS, Ulevitch RJ. 2001. Leptospiral lipopolysaccharide activates cells through a TLR2-dependent mechanism. Nat Immunol 2:346–352. doi: 10.1038/86354. [DOI] [PubMed] [Google Scholar]
- 21.Chassin C, Picardeau M, Goujon JM, Bourhy P, Quellard N, Darche S, Badell E, d'Andon MF, Winter N, Lacroix-Lamande S, Buzoni-Gatel D, Vandewalle A, Werts C. 2009. TLR4- and TLR2-mediated B cell responses control the clearance of the bacterial pathogen, Leptospira interrogans. J Immunol 183:2669–2677. doi: 10.4049/jimmunol.0900506. [DOI] [PubMed] [Google Scholar]
- 22.Nahori MA, Fournie-Amazouz E, Que-Gewirth NS, Balloy V, Chignard M, Raetz CR, Saint Girons I, Werts C. 2005. Differential TLR recognition of leptospiral lipid A and lipopolysaccharide in murine and human cells. J Immunol 175:6022–6031. doi: 10.4049/jimmunol.175.9.6022. [DOI] [PubMed] [Google Scholar]
- 23.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. 1999. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol 162:3749–3752. [PubMed] [Google Scholar]
- 24.Qureshi ST, Lariviere L, Leveque G, Clermont S, Moore KJ, Gros P, Malo D. 1999. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4). J Exp Med 189:615–625. doi: 10.1084/jem.189.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Totemeyer S, Foster N, Kaiser P, Maskell DJ, Bryant CE. 2003. Toll-like receptor expression in C3H/HeN and C3H/HeJ mice during Salmonella enterica serovar Typhimurium infection. Infect Immun 71:6653–6657. doi: 10.1128/IAI.71.11.6653-6657.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wunder EA Jr, Figueira CP, Santos GR, Lourdault K, Matthias MA, Vinetz JM, Ramos E, Haake DA, Picardeau M, Dos Reis MG, Ko AI. 2016. Real-time PCR reveals rapid dissemination of Leptospira interrogans after intraperitoneal and conjunctival inoculation of hamsters. Infect Immun 84:2105–2115. doi: 10.1128/IAI.00094-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santos CS, Macedo JO, Bandeira M, Chagas-Junior AD, McBride AJ, McBride FW, Reis MG, Athanazio DA. 2010. Different outcomes of experimental leptospiral infection in mouse strains with distinct genotypes. J Med Microbiol 59:1101–1106. doi: 10.1099/jmm.0.021089-0. [DOI] [PubMed] [Google Scholar]
- 28.Bandeira M, Santos CS, de Azevedo EC, Soares LM, Macedo JO, Marchi S, da Silva CL, Chagas-Junior AD, McBride AJ, McBride FW, Reis MG, Athanazio DA. 2011. Attenuated nephritis in inducible nitric oxide synthase knockout C57BL/6 mice and pulmonary hemorrhage in CB17 SCID and recombination activating gene 1 knockout C57BL/6 mice infected with Leptospira interrogans. Infect Immun 79:2936–2940. doi: 10.1128/IAI.05099-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ratet G, Veyrier FJ, Fanton d'Andon M, Kammerscheit X, Nicola MA, Picardeau M, Boneca IG, Werts C. 2014. Live imaging of bioluminescent Leptospira interrogans in mice reveals renal colonization as a stealth escape from the blood defenses and antibiotics. PLoS Negl Trop Dis 8:e3359. doi: 10.1371/journal.pntd.0003359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferrer MF, Scharrig E, Alberdi L, Cedola M, Pretre G, Drut R, Song WC, Gomez RM. 2014. Decay-accelerating factor 1 deficiency exacerbates leptospiral-induced murine chronic nephritis and renal fibrosis. PLoS One 9:e102860. doi: 10.1371/journal.pone.0102860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ganoza CA, Matthias MA, Collins-Richards D, Brouwer KC, Cunningham CB, Segura ER, Gilman RH, Gotuzzo E, Vinetz JM. 2006. Determining risk for severe leptospirosis by molecular analysis of environmental surface waters for pathogenic Leptospira. PLoS Med 3:e308. doi: 10.1371/journal.pmed.0030308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tuanyok A, Tom M, Dunbar J, Woods DE. 2006. Genome-wide expression analysis of Burkholderia pseudomallei infection in a hamster model of acute melioidosis. Infect Immun 74:5465–5476. doi: 10.1128/IAI.00737-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sarkar-Tyson M, Titball RW. 2010. Progress toward development of vaccines against melioidosis: a review. Clin Ther 32:1437–1445. doi: 10.1016/j.clinthera.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 34.Titball RW, Russell P, Cuccui J, Easton A, Haque A, Atkins T, Sarkar-Tyson M, Harley V, Wren B, Bancroft GJ. 2008. Burkholderia pseudomallei: animal models of infection. Trans R Soc Trop Med Hyg 102(Suppl 1):S111–S116. doi: 10.1016/S0035-9203(08)70026-9. [DOI] [PubMed] [Google Scholar]
- 35.Razak CEIG, Embim N, Omar O. 1986. Protection studies using whole cells and partially purified toxic material (PPTM) of Pseudomonas pseudomallei. Malays Appl Biol 15:105–111. [Google Scholar]
- 36.da Silva JB, Carvalho E, Covarrubias AE, Ching AT, Mattaraia VG, Paiva D, de Franco M, Favaro RD, Pereira MM, Vasconcellos S, Zorn TT, Ho PL, Martins EA. 2012. Induction of TNF-alfa and CXCL-2 mRNAs in different organs of mice infected with pathogenic Leptospira. Microb Pathog 52:206–216. doi: 10.1016/j.micpath.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 37.Quintana FJ, Solomon A, Cohen IR, Nussbaum G. 2008. Induction of IgG3 to LPS via Toll-like receptor 4 co-stimulation. PLoS One 3:e3509. doi: 10.1371/journal.pone.0003509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Athanazio DA, Santos CS, Santos AC, McBride FW, Reis MG. 2008. Experimental infection in tumor necrosis factor alpha receptor, interferon gamma and interleukin 4 deficient mice by pathogenic Leptospira interrogans. Acta Trop 105:95–98. doi: 10.1016/j.actatropica.2007.09.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.