ABSTRACT
Pneumocystis pneumonia (PcP) is a life-threatening infection that affects immunocompromised individuals. Nearly half of all PcP cases occur in those prescribed effective chemoprophylaxis, suggesting that additional preventive methods are needed. To this end, we have identified a unique mouse Pneumocystis surface protein, designated Pneumocystis cross-reactive antigen 1 (Pca1), as a potential vaccine candidate. Mice were immunized with a recombinant fusion protein containing Pca1. Subsequently, CD4+ T cells were depleted, and the mice were exposed to Pneumocystis murina. Pca1 immunization completely protected nearly all mice, similar to immunization with whole Pneumocystis organisms. In contrast, all immunized negative-control mice developed PcP. Unexpectedly, Pca1 immunization generated cross-reactive antibody that recognized Pneumocystis jirovecii and Pneumocystis carinii. Potential orthologs of Pca1 have been identified in P. jirovecii. Such cross-reactivity is rare, and our findings suggest that Pca1 is a conserved antigen and potential vaccine target. The evaluation of Pca1-elicited antibodies in the prevention of PcP in humans deserves further investigation.
KEYWORDS: Pneumocystis, PcP, vaccine, antibody, pneumonia, immunization
INTRODUCTION
Pneumocystis pneumonia (PcP) is a common opportunistic infection, with 10,000 estimated cases each year in the United States and more than 400,000 cases worldwide (1, 2). The need for admission to the intensive care unit and ventilator support is common. Mortality rates of up to 40% are typical in high-risk patient populations despite first-line treatment. PcP affects immunosuppressed hosts, with cancer patients and organ transplant recipients accounting for the majority of cases in developed countries (3, 4). Patients receiving immunomodulatory agents and those with preexisting lung disease represent growing patient populations at risk of developing PcP. These factors contributed to an overall increase in the incidence of PcP in England from 2000 to 2010, despite a decline in the number of HIV-associated cases during that time period (3).
While effective chemoprophylaxis for PcP exists, half of all PcP cases occur in those prescribed adequate prophylaxis, most commonly a result of noncompliance (5). Another threat to the effectiveness of chemoprophylaxis is the potential for the development of resistance to trimethoprim-sulfamethoxazole, unquestionably our most effective prophylactic and therapeutic agent (6). Mortality rates have changed little over the past few decades, emphasizing the need for additional treatment options. Immunization, passive or active, is a viable approach. Active immunization of children and adults with cancer against bacterial and viral pathogens during the initial phases of chemotherapy has been shown to protect them through periods of immunosuppression (7–9). Pneumocystis is an attractive target for vaccine-based prevention, since patient populations at risk for PcP can often be identified prior to patients becoming immunosuppressed.
Active immunization with whole organisms is uniformly protective in animal models of PcP (10–12). However, the antigenic profile of Pneumocystis infecting each host is so distinct from that of Pneumocystis infecting any other host species that cross-protective immunity is not induced. For example, immunization of mice with mouse-derived Pneumocystis (P. murina) protects them from subsequent infection, while immunization with ferret-derived Pneumocystis fails to protect (13). The inability to cultivate the organism is a further impediment to vaccine development. An alternative approach is to use molecular techniques to develop a subunit vaccine, especially one that contains cross-reactive epitopes. Such antigens are rare but do exist (13, 14). Thus far, the efficacy of subunit vaccines for Pneumocystis has not matched that observed with whole-cell vaccination.
We previously identified a protective monoclonal antibody (MAb), 4F11, that is cross-reactive with other Pneumocystis species, including P. jirovecii (13). Active immunization with a 142-amino-acid polypeptide (A12) that contains a 4F11 epitope elicits a protective response, decreasing organism burden and lung inflammation (15). We have now isolated and partially characterized the full-length cDNA from which the A12 C-terminal polypeptide was derived. On the basis of the findings described herein, we suggest the name Pneumocystis cross-reactive antigen 1 (Pca1) for this molecule. Here, we show that active immunization with the N-terminal half of Pca1 protected against infection in a CD4+ T cell-depleted mouse model of PcP. Furthermore, antibody generated from the immunization of mice with this protein also recognizes epitopes on the surface of the human pathogen, P. jirovecii, highlighting the possibility of developing Pca1 as a human vaccine candidate.
(Part of this work was presented in poster format at the 54th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, DC, 2014.)
RESULTS
Pca1 immunization provides protection against infection.
After immunizing immunocompetent mice with Pca1, whole Pneumocystis, or control proteins, we mimicked the human susceptibility to PcP through depletion of CD4+ T cells with anti-CD4 antibody (clone GK1.5). Circulating CD4+ T cells were reduced from 13 to 18% to 0 to 1% of the total lymphocyte population sampled from the spleen. We then used an established mouse model of PcP that simulates natural infection by cohousing the susceptible, immunized mice with actively infected SCID mice (15). Pca1 immunization provided protection against subsequent infectious challenge (Table 1), with complete protection being defined as undetected organism burden by quantitative PCR (qPCR) (total lung organism burden below 104, the lower limit of detection). All unimmunized control mice developed PcP, confirming the functional efficacy of our CD4+ cell depletion regimen and infection strategy. Nearly all mice (32/37) immunized with Pca1 were completely protected against infection, with undetected organism burden at the time of sacrifice, whereas none of the 28 control protein-immunized mice were protected (Table 1) (P < 0.0001 by Fisher's exact test). The proportion of mice protected by Pca1 immunization was statistically indistinguishable from the proportion protected by whole-cell Pneumocystis immunization (Table 1). These results were confirmed by examining the lung homogenates after silver staining to identify Pneumocystis cysts. Furthermore, PCR for the multicopy glycoprotein A (gpA) gene failed to detect any target DNA in lung samples from protected mice (data not shown). The finding of reduced organism burden compared to that in experimental controls in the few mice immunized with Pca1 but not completely protected may be an in vivo demonstration of dose-dependent response to immunization (Fig. 1A). Although not statistically significant, likely due to sample size, this provides additional evidence for vaccine efficacy.
TABLE 1.
Summary of protection by Pca1 immunizationa
| Parameter | Value for parameter for mice immunized with the following immunogenb: |
||
|---|---|---|---|
| Negative-control protein | Pca1 fusion protein | Whole Pneumocystis (positive control) | |
| No. of mice | |||
| Not protected | 23 | 4 | 1 |
| Protected | 0 | 33 | 11 |
| Total | 23 | 37 | 12 |
| % mice protected | 0 | 89*** | 92*** |
Protection defined as an organism burden below the limit of detection by qPCR.
***, P < 0.0001 for mice immunized with Pca1 or whole Pneumocystis compared to the negative-control value by Fisher's exact test.
FIG 1.
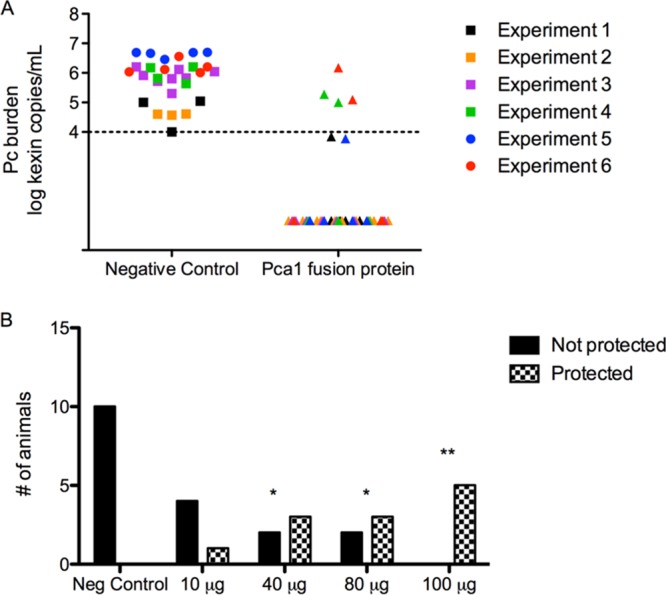
Pca1 immunization reduces organism burden in a dose-dependent trend. (A) Mice immunized with Pca1 fusion protein and with Pneumocystis detected by qPCR (n = 37) (triangles) had reduced Pneumocystis (Pc) organism burdens compared to mice immunized with the fusion partner (n = 10) (circles) or an irrelevant protein (n = 18) (squares). Data points represent log transformation of qPCR, with the limit of assay detection marked by the dashed line. Data from six experiments are shown, and the data are color coded. (B) Increasing doses of Pca1 immunization resulted in an increased number of mice protected from infection. Values that are significantly different from the value for the negative control are indicated by asterisks as follows: **, P < 0.01; *, P < 0.05. Data from two pooled experiments are shown.
To determine a threshold dose, mice were immunized with different doses of Pca1. As expected, the efficacy of Pca1 was dependent on the dose. All five mice immunized with 100 μg of Pca1 were completely protected against infection, whereas only three of five mice immunized with either 80 μg or 40 μg and one of five mice immunized with 10 μg of Pca1 had undetected burden (Fig. 1B).
Molecular characterization of P. murina Pca1.
Nucleotide sequence analysis (GenBank accession no. KX011348) and Southern blotting (data not shown) demonstrated that the Pca1 antigen gene is present in a single copy. It encodes a 1,099-amino-acid protein of unknown function. Specifically, the protein does not have characteristics typical of fungal adhesins (16). Nor was there significant homology with closely related fungal species such as Schizosaccharomyces pombe. Significant protein sequence homology was found in the genomes of rat Pneumocystis (P. carinii) and human Pneumocystis (P. jirovecii) sequences deposited in data banks. A single protein with identity and similarity to Pca1 of 39% and 61%, respectively, was identified in the P. carinii genome (accession no. KTW27087.1) Five putative Pca1 orthologs were identified in the genome of P. jirovecii (KTW31106.1, KTW25468.1, KTW32226.1, KTW25482.1, and KTW25472.1). These proteins displayed identity and similarity to Pca1 of up to 26% and 45%, respectively, and 48 of the 49 cysteine residues in mouse Pneumocystis Pca1 are conserved in these human Pneumocystis sequences. Importantly, these five P. jirovecii proteins were highly similar to each other, with identity and similarity scores ranging from 78 to 89% and 87 to 93%, respectively.
Pca1 immunization generates antigen-specific antibody that cross-reacts with the human pathogen, P. jirovecii.
Pca1 immunization resulted in production of antibody to the Pca1 portion of the fusion protein (Fig. 2A) in a dose-dependent fashion after a three-dose immunization series as measured by an enzyme-linked immunosorbent assay (ELISA) to a Pca1 polypeptide lacking the fusion partner. Little Pca1-specific antibody could be detected in sera from mice immunized with a low dose (10 μg) of Pca1 or the fusion partner alone. Pca1-specific antibody was detected in high-dose Pca1-immunized mice following Pneumocystis exposure and sacrifice approximately 9 weeks after the last immunization (Fig. 2B).
FIG 2.
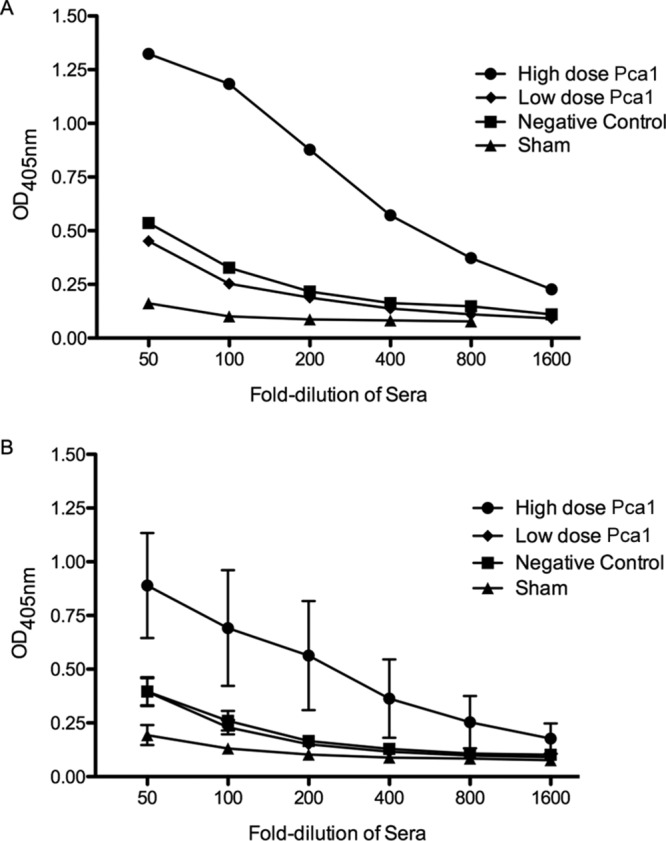
Pca1-specific antibody development in immunized mice. (A) Pooled sera from five mice bled 3 weeks after completing a three-dose immunization series of 80 μg of Pca1 fusion protein (high-dose Pca1) demonstrated antigen-specific antibody as measured by ELISA to a Pca1 polypeptide. Pooled sera from five mice immunized with only 10 μg of the Pca1 fusion protein (low-dose Pca1) or 80 μg of the fusion partner only (negative control) did not demonstrate significant antibody development above PBS-immunized mice (sham). OD405nm, optical density at 405 nm. (B) Pca1-specific antibody was detected in high-dose Pca1-immunized mice approximately 6 weeks following CD4+ T cell depletion and subsequent exposure to Pneumocystis, corresponding to approximately 9 weeks after the last immunization. Values are means ± standard errors of the means (SEM) (error bars) for the groups (five animals in each group).
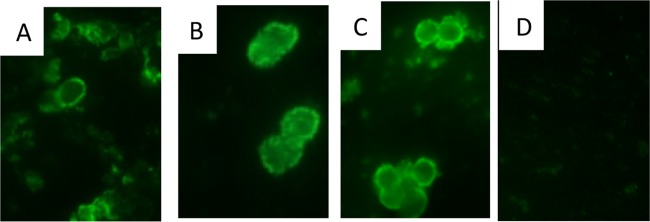
To evaluate cross-reactivity, indirect immunofluorescence assays (IFA) were performed with antisera from Pca1- and control protein-immunized mice and tested against Pneumocystis isolated from three different host species. Antisera from Pca1-immunized mice contained antibody that bound to not only the mouse-derived P. murina (Fig. 3A) cysts but also rat-derived P. carinii (Fig. 3B) and, importantly, to four distinct clinical isolates of P. jirovecii isolated from infected patients (Fig. 3C). This binding to P. jirovecii was similar to that seen with positive-control MAb 4F11, known to be cross-reactive (data not shown). No such binding to P. jirovecii was observed with the antisera from mice immunized with the fusion partner trigger factor (Fig. 3D).
FIG 3.
Pca1 antisera recognize both mouse and human Pneumocystis cysts by IFA. Antisera from mice immunized with the Pca1 fusion protein bound not only to the surface of mouse species-specific Pneumocystis (P. murina) cysts (A) but also to the surfaces of rat-specific P. carinii (B) and the human pathogen P. jirovecii (C). No such binding was observed with fusion partner-immunized antisera (D).
DISCUSSION
We describe here the characteristics of a novel Pneumocystis antigen, Pca1, isolated from P. murina with an ortholog identified in P. jirovecii. Immunization with the N-terminal half of Pca1 protects in a mouse model of PcP and has the unique characteristic of inducing antibody that cross-reacts with human Pneumocystis, P. jirovecii. The host species specificity of Pneumocystis impedes the human translation of many animal model observations, and previous studies have failed to demonstrate serologic cross-reactivity or protection (13). Only three cross-reactive MAbs have been identified to our knowledge (13, 14). One of these antibodies, 4F11, has a known antibody epitope in the C terminus of the full-length Pca1. Since immunization with this polypeptide was only partially protective in the mouse model (17), we set out to clone the entire gene. The cross-reactivity of N-terminal Pca1 antisera opens the possibility of using heterologous Pneumocystis antigens to protect humans against the development of PcP.
The extent of protection that resulted from immunization with Pca1 was unexpected, since partial protection after immunization appears to be the typical outcome of immunization against fungal pathogens such as Candida, Aspergillus, and Cryptococcus (18–22). A number of potential vaccine candidates have been evaluated in animal models of PcP as well, but at best, they achieved only partial protection (15, 23–27). A recent publication also describes boosting naturally acquired antibody in Pneumocystis-exposed animals with a fragment of kexin as a means to enhance immune response to Pneumocystis (28). The presence of shared antibody epitopes between kexin and Pca1 warrants further analysis of vaccine immunogenicity and efficacy (17). The complete clearance of Pneumocystis from Pca1-immunized and -exposed animals reinforces the role Pca1 may have in PcP protection.
While these experiments were not designed to determine the mechanism of vaccine-induced protection, several observations are consistent with antibody-mediated protection. We have previously shown that administration of a Pca1-binding MAb (4F11) reduces organism burden, and maximal reduction of Pneumocystis after MAb infusion requires an intact Fc region and a functional complement system (29). Persistence of protection well after immunization and in the absence of CD4+ T cells also is most consistent with antibody-mediated protection. CD8+ T cells do not appear to have a major role in defense against Pneumocystis and are not necessary for organism clearance. Therefore, they are unlikely to play a role in Pca1-mediated protection (30).
To circumvent the difficulties in synthesizing full-length Pneumocystis proteins, we have examined the immunogenicity of fragments of Pca1. We previously demonstrated that the C-terminal end was partially protective (15) and have now characterized the efficacy of immunization with the N-terminal half of the protein. In addition to completely protecting nearly all animals from subsequent infection, Pca1 immunization produced antibodies that reacted with P. jirovecii, a rare finding. The ability of Pca1 immunization to protect mice from infection and induce antibody that binds to P. jirovecii makes Pca1 a leading candidate for further development into a potential human subunit vaccine.
While Pca1 was isolated from P. murina, it may prove valuable in developing a vaccine for use in humans. From a technical standpoint, the potential advantage of Pca1 is that it could be tested in animal models and, if warranted, based on animal model results, it could be moved to clinical trial with supporting data for its activity in humans. It would be unlikely that a vaccine based on human pneumocystis could be utilized in both animals and humans unless it too was based on a cross-reactive antigen (13). Pca1 provides a potential immunological advantage as well. The antigenic diversity of P. jirovecii has not been well studied. However, if Pca1 induces antibody that is cross-reactive with pneumocystis from a range of mammalian hosts, it may be more likely that the vaccine would induce an immune response broadly reactive with most, if not all, human pneumocystis.
Finally, we appear to have identified the ortholog of mouse Pca1 in the human pathogen, P. jirovecii. The degrees of identity and of similarity observed between mouse Pneumocystis-derived Pca1 and candidate ortholog sequences identified in rat and human Pneumocystis are similar to those observed between Pneumocystis surface glycoprotein A sequences from different mammalian host species (31, 32). The additional observation of cysteine conservation between mouse Pca1 and the candidate human Pneumocystis ortholog suggests similar protein structure. The identification of a human Pneumocystis ortholog and demonstration of cross-reactive antibody production overcome the host species specificity impediment to translation of Pneumocystis animal models.
We have demonstrated that active immunization with mouse Pca1 protects mice against subsequent infectious challenge. The mice were immunocompetent at the time of immunization. However, most patients at risk for PcP can be identified during periods of relative immunocompetence, such as during early HIV infection or prior to the administration of immunosuppressive agents for malignancy or autoimmune conditions. These patients would be ideal candidates for active immunization. Even patients undergoing chemotherapy have been shown to demonstrate adequate antibody responses to polysaccharide, protein, and conjugated vaccines (7, 8, 33). The vaccination strategy could also be optimized to function in a T cell-independent fashion through adjuvants of conjugation. Additionally, it could be used in CD4+ T cell-independent platforms such as DNA vaccination (27).
Immune-mediated inflammation is a key component in the pathophysiology of PcP. Passive treatment with pools of anti-Pneumocystis monoclonal antibodies, including 4F11, has previously been shown to reduce both organism burden and inflammation (34–36). Therefore, in addition to the antimicrobial effects, the immunoregulatory effects of high-dose intravenous immunoglobulin (IVIG) may provide additional benefit in the treatment of a patient with PcP. Pca1 or the human ortholog could be used to generate high-titer antisera for passive administration, as is done for diseases such as tetanus and rabies. Patients likely to have a poor prognosis, usually as a result of inflammatory injury, can frequently be identified on admission to the hospital and could be targeted for treatment (37). These studies support further investigation into the development of Pca1-based prophylactic and therapeutic immunotherapies. With PcP-related mortality remaining high and relatively unchanged, Pca1-based immunotherapy could provide a novel therapeutic approach to our current management of PcP.
MATERIALS AND METHODS
Mice.
Pathogen-free CB.17 and SCID mice on a CB.17 background were obtained from a breeding colony at the University of Rochester animal care facilities (originally purchased from Taconic Biosciences, Germantown, NY) and housed in microisolator cages, given sterile food and water ad lib.
Cloning and characterization of the Pca1 antigen cDNA and gene.
Using the Pca1 partial cDNA sequence (GenBank accession no. AY371664.1) as a starting point, we used GeneRacer (Invitrogen, Carlsbad, CA) reactions to obtain the full-length sequence of the cDNA (GenBank accession no. KX011348). Based on the cDNA sequence, primers were designed to amplify the full-length Pca1 gene. All procedures for cDNA synthesis and genomic DNA isolation have been described previously (15, 17). Southern blotting was used to determine the copy number of the Pca1 gene in the Pneumocystis murina genome as described previously (38).
Cloning and expression in Escherichia coli.
Expressing the recombinant full-length Pca1 protein proved difficult for our laboratory and a commercial contractor. For the immunization model, a 544-amino-acid (aa) N-terminal portion of the Pca1 protein (19 to 1,650 bp) was produced by GenScript (Piscataway, NJ) as a fusion protein with trigger factor (TF) using proprietary technology to produce a codon-optimized synthetic gene based on the Pca1 amino acid sequence. TF was also expressed and purified using the same system to serve as a control. To express a portion of the Pca1 gene without the fusion partner, DNA was generated from the codon-optimized synthetic gene by PCR using a 44-bp primer pair which introduced unique XhoI restriction sites. The resulting PCR product was subcloned into the pET14b (Novagen, Gibbstown, NJ) expression vector, forming a sequence encoding a 388-aa portion (274 to 1,439 bp) of Pca1 with an N-terminal His6 tag. It was then transformed in the E. coli BL21(DE3) RIL CodonPlus (New England BioLabs, Ipswich, MA) host to allow for optimal expression of AT-rich genes.
Immunization and infectious challenge model.
To investigate the immunogenicity and efficacy of the recombinant Pca1 fusion protein, groups of 6- to 8-week-old female CB.17 mice (5 to 10 mice in a group) were immunized subcutaneously with three doses of 100 μg of Pca1 emulsified in TiterMax Gold adjuvant (Sigma, St. Louis, MO) given in 3-week intervals. To evaluate the dose response, additional mice were immunized with 10, 40, or 80 μg of Pca1. Control mice were immunized with the fusion partner alone, an unrelated protein, or whole Pneumocystis. Sera were obtained from the mice by submandibular venipuncture 3 weeks after the final dose and stored at −80°C until use. Two weeks after the final immunization, all mice were depleted of CD4+ T cells through twice-weekly injections of 250 μg anti-CD4 monoclonal antibody (clone GK1.5; ATCC, Manassas, VA). CD4+ T cell depletion was evaluated by flow cytometry analysis of lymphocytes present in the spleen and bronchoalveolar lavage fluid. The mice were subsequently exposed to Pneumocystis while being cohoused with Pneumocystis-infected SCID mice for 2 weeks. This simulates the natural route of Pneumocystis infection. The mice were monitored for signs of infection, including weight loss and increased respiratory rate, while CD4+ T cell depletion was maintained. The mice were sacrificed after they demonstrated signs of active infection, 4 to 6 weeks after Pneumocystis exposure. At the time of sacrifice, serum was collected via cardiac puncture, spleens were removed, bronchoalveolar lavage was performed, and lungs were removed and flash frozen for Pneumocystis burden enumeration.
Serology.
Sera from immunized mice were assayed by enzyme-linked immunosorbent assay (ELISA) as previously described (10) for Pca1-specific antibody production by coating 96-well plates with 1 μg/ml of the 388-aa portion of the Pca1 protein not containing the fusion partner. An immunofluorescence assay was used to determine whether immunization induced antibody to P. murina, P. carinii, and P. jirovecii as previously described (34).
Quantitation of P. murina.
Homogenized lung tissue was prepared, and P. murina burden was calculated using quantitative real-time PCR of the single-copy kex1 gene as previously described (39). The limit of detection for this assay has been determined to be 4 log10 copies of kex1 gene per total lung volume.
Statistical analysis.
Categorical data were analyzed by Fisher's exact test. Continuous data were analyzed by t test. P values of <0.05 were considered significant. Analysis was performed with GraphPad Prism v.6 software (La Jolla, CA).
Study approval.
All procedures performed were subject to University of Rochester Committee on Animal Resources approval.
Accession number(s).
The genomic DNA sequence of Pca1 has been deposited in GenBank under accession number KX011348.
ACKNOWLEDGMENTS
We thank Jing Wang, Nabilah Khan, Samir Bhagwat, and Bradley Buchheit for exceptional technical assistance.
This work was supported by the National Institute of Allergy and Infectious Diseases (grants T32AI007464 [B.L.T.] and R01AI023302 [F.G.]), National Heart, Lung, and Blood Institute (grant R01HL092797 [F.G. and T.W.W.]), and Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant K12HD068373 [B.L.T.]), National Institutes of Health, Department of Health and Human Services.
We have no conflicts of interest or financial relationships relevant to this article to disclose.
Footnotes
For a commentary on this article, see https://doi.org/10.1128/IAI.00035-17.
REFERENCES
- 1.Kovacs JA, Gill VJ, Meshnick S, Masur H. 2001. New insights into transmission, diagnosis, and drug treatment of Pneumocystis carinii pneumonia. JAMA 286:2450–2460. doi: 10.1001/jama.286.19.2450. [DOI] [PubMed] [Google Scholar]
- 2.Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. 2012. Hidden killers: human fungal infections. Sci Transl Med 4:165rv113. doi: 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- 3.Maini R, Henderson KL, Sheridan EA, Lamagni T, Nichols G, Delpech V, Phin N. 2013. Increasing Pneumocystis pneumonia, England, UK, 2000-2010. Emerg Infect Dis 19:386–392. doi: 10.3201/eid1903.121151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saltzman RW, Albin S, Russo P, Sullivan KE. 2012. Clinical conditions associated with PCP in children. Pediatr Pulmonol 47:510–516. doi: 10.1002/ppul.21577. [DOI] [PubMed] [Google Scholar]
- 5.Morris A, Lundgren JD, Masur H, Walzer PD, Hanson DL, Frederick T, Huang L, Beard CB, Kaplan JE. 2004. Current epidemiology of Pneumocystis pneumonia. Emerg Infect Dis 10:1713–1720. doi: 10.3201/eid1010.030985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang L, Crothers K, Atzori C, Benfield T, Miller R, Rabodonirina M, Helweg-Larsen J. 2004. Dihydropteroate synthase gene mutations in Pneumocystis and sulfa resistance. Emerg Infect Dis 10:1721–1728. doi: 10.3201/eid1010.030994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feldman S, Gigliotti F, Shenep JL, Roberson PK, Lott L. 1990. Risk of Haemophilus influenzae type b disease in children with cancer and response of immunocompromised leukemic children to a conjugate vaccine. J Infect Dis 161:926–931. doi: 10.1093/infdis/161.5.926. [DOI] [PubMed] [Google Scholar]
- 8.Nordoy T, Aaberge IS, Husebekk A, Samdal HH, Steinert S, Melby H, Kolstad A. 2002. Cancer patients undergoing chemotherapy show adequate serological response to vaccinations against influenza virus and Streptococcus pneumoniae. Med Oncol 19:71–78. doi: 10.1385/MO:19:2:71. [DOI] [PubMed] [Google Scholar]
- 9.LaRussa P, Steinberg S, Gershon AA. 1996. Varicella vaccine for immunocompromised children: results of collaborative studies in the United States and Canada. J Infect Dis 174(Suppl 3):S320–S323. doi: 10.1093/infdis/174.Supplement_3.S320. [DOI] [PubMed] [Google Scholar]
- 10.Harmsen AG, Chen W, Gigliotti F. 1995. Active immunity to Pneumocystis carinii reinfection in T-cell-depleted mice. Infect Immun 63:2391–2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garvy BA, Wiley JA, Gigliotti F, Harmsen AG. 1997. Protection against Pneumocystis carinii pneumonia by antibodies generated from either T helper 1 or T helper 2 responses. Infect Immun 65:5052–5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pascale JM, Shaw MM, Durant PJ, Amador AA, Bartlett MS, Smith JW, Gregory RL, McLaughlin GL. 1999. Intranasal immunization confers protection against murine Pneumocystis carinii lung infection. Infect Immun 67:805–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gigliotti F, Harmsen AG. 1997. Pneumocystis carinii host origin defines the antibody specificity and protective response induced by immunization. J Infect Dis 176:1322–1326. doi: 10.1086/514128. [DOI] [PubMed] [Google Scholar]
- 14.Gigliotti F, Stokes DC, Cheatham AB, Davis DS, Hughes WT. 1986. Development of murine monoclonal antibodies to Pneumocystis carinii. J Infect Dis 154:315–322. doi: 10.1093/infdis/154.2.315. [DOI] [PubMed] [Google Scholar]
- 15.Wells J, Haidaris CG, Wright TW, Gigliotti F. 2006. Active immunization against Pneumocystis carinii with a recombinant P. carinii antigen. Infect Immun 74:2446–2448. doi: 10.1128/IAI.74.4.2446-2448.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramana J, Gupta D. 2010. FaaPred: a SVM-based prediction method for fungal adhesins and adhesin-like proteins. PLoS One 5:e9695. doi: 10.1371/journal.pone.0009695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wells J, Gigliotti F, Simpson-Haidaris PJ, Haidaris CG. 2004. Epitope mapping of a protective monoclonal antibody against Pneumocystis carinii with shared reactivity to Streptococcus pneumoniae surface antigen PspA. Infect Immun 72:1548–1556. doi: 10.1128/IAI.72.3.1548-1556.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rachini A, Pietrella D, Lupo P, Torosantucci A, Chiani P, Bromuro C, Proietti C, Bistoni F, Cassone A, Vecchiarelli A. 2007. An anti-beta-glucan monoclonal antibody inhibits growth and capsule formation of Cryptococcus neoformans in vitro and exerts therapeutic, anticryptococcal activity in vivo. Infect Immun 75:5085–5094. doi: 10.1128/IAI.00278-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Torosantucci A, Bromuro C, Chiani P, De Bernardis F, Berti F, Galli C, Norelli F, Bellucci C, Polonelli L, Costantino P, Rappuoli R, Cassone A. 2005. A novel glyco-conjugate vaccine against fungal pathogens. J Exp Med 202:597–606. doi: 10.1084/jem.20050749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xin H, Cutler JE. 2011. Vaccine and monoclonal antibody that enhance mouse resistance to candidiasis. Clin Vaccine Immunol 18:1656–1667. doi: 10.1128/CVI.05215-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spellberg BJ, Ibrahim AS, Avanesian V, Fu Y, Myers C, Phan QT, Filler SG, Yeaman MR, Edwards JE Jr. 2006. Efficacy of the anti-Candida rAls3p-N or rAls1p-N vaccines against disseminated and mucosal candidiasis. J Infect Dis 194:256–260. doi: 10.1086/504691. [DOI] [PubMed] [Google Scholar]
- 22.Cassone A, Casadevall A. 2012. Recent progress in vaccines against fungal diseases. Curr Opin Microbiol 15:427–433. doi: 10.1016/j.mib.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duan YN, Yi LH, Chen JL, Zhu DD, Wang JX, Feng JR, Qin YW, Zhu Y. 2011. Protective effect of DNA vaccine with the gene encoding 55kDa antigen fragment against Pneumocystis carinii in mice. Asian Pac J Trop Med 4:353–356. doi: 10.1016/S1995-7645(11)60102-8. [DOI] [PubMed] [Google Scholar]
- 24.Feng Y, Guo S, Jiang T, Han X, Liu P, Wu T, Luo Y. 2011. Active immunization against Pneumocystis carinii with p55-v3 DNA vaccine in rats. Can J Microbiol 57:375–381. doi: 10.1139/w11-023. [DOI] [PubMed] [Google Scholar]
- 25.Gigliotti F, Wiley JA, Harmsen AG. 1998. Immunization with Pneumocystis carinii gpA is immunogenic but not protective in a mouse model of P. carinii pneumonia. Infect Immun 66:3179–3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smulian AG, Sullivan DW, Theus SA. 2000. Immunization with recombinant Pneumocystis carinii p55 antigen provides partial protection against infection: characterization of epitope recognition associated with immunization. Microbes Infect 2:127–136. doi: 10.1016/S1286-4579(00)00275-6. [DOI] [PubMed] [Google Scholar]
- 27.Zheng M, Ramsay AJ, Robichaux MB, Kliment C, Crowe C, Rapaka RR, Steele C, McAllister F, Shellito JE, Marrero L, Schwarzenberger P, Zhong Q, Kolls JK. 2005. CD4+ T cell-independent DNA vaccination against opportunistic infections. J Clin Invest 115:3536–3544. doi: 10.1172/JCI26306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kling HM, Norris KA. 2016. Vaccine-induced immunogenicity and protection against Pneumocystis pneumonia in a nonhuman primate model of HIV and Pneumocystis coinfection. J Infect Dis 213:1586–1595. doi: 10.1093/infdis/jiw032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wells J, Haidaris CG, Wright TW, Gigliotti F. 2006. Complement and Fc function are required for optimal antibody prophylaxis against Pneumocystis carinii pneumonia. Infect Immun 74:390–393. doi: 10.1128/IAI.74.1.390-393.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gigliotti F, Crow EL, Bhagwat SP, Wright TW. 2006. Sensitized CD8+ T cells fail to control organism burden but accelerate the onset of lung injury during Pneumocystis carinii pneumonia. Infect Immun 74:6310–6316. doi: 10.1128/IAI.00668-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wright TW, Simpson-Haidaris PJ, Gigliotti F, Harmsen AG, Haidaris CG. 1994. Conserved sequence homology of cysteine-rich regions in genes encoding glycoprotein A in Pneumocystis carinii derived from different host species. Infect Immun 62:1513–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wright TW, Gigliotti F, Haidaris CG, Simpson-Haidaris PJ. 1995. Cloning and characterization of a conserved region of human and rhesus macaque Pneumocystis carinii gpA. Gene 167:185–189. doi: 10.1016/0378-1119(95)00704-0. [DOI] [PubMed] [Google Scholar]
- 33.Berglund A, Willen L, Grodeberg L, Skattum L, Hagberg H, Pauksens K. 2014. The response to vaccination against influenza A(H1N1) 2009, seasonal influenza and Streptococcus pneumoniae in adult outpatients with ongoing treatment for cancer with and without rituximab. Acta Oncol 53:1212–1220. doi: 10.3109/0284186X.2014.914243. [DOI] [PubMed] [Google Scholar]
- 34.Gigliotti F, Haidaris CG, Wright TW, Harmsen AG. 2002. Passive intranasal monoclonal antibody prophylaxis against murine Pneumocystis carinii pneumonia. Infect Immun 70:1069–1074. doi: 10.1128/IAI.70.3.1069-1074.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gigliotti F, Hughes WT. 1988. Passive immunoprophylaxis with specific monoclonal antibody confers partial protection against Pneumocystis carinii pneumonitis in animal models. J Clin Invest 81:1666–1668. doi: 10.1172/JCI113503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Empey KM, Hollifield M, Schuer K, Gigliotti F, Garvy BA. 2004. Passive immunization of neonatal mice against Pneumocystis carinii f. sp. muris enhances control of infection without stimulating inflammation. Infect Immun 72:6211–6220. doi: 10.1128/IAI.72.11.6211-6220.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Armstrong-James D, Copas AJ, Walzer PD, Edwards SG, Miller RF. 2011. A prognostic scoring tool for identification of patients at high and low risk of death from HIV-associated Pneumocystis jirovecii pneumonia. Int J STD AIDS 22:628–634. doi: 10.1258/ijsa.2011.011040. [DOI] [PubMed] [Google Scholar]
- 38.Lee LH, Gigliotti F, Wright TW, Simpson-Haidaris PJ, Weinberg GA, Haidaris CG. 2000. Molecular characterization of KEX1, a kexin-like protease in mouse Pneumocystis carinii. Gene 242:141–150. doi: 10.1016/S0378-1119(99)00533-8. [DOI] [PubMed] [Google Scholar]
- 39.Gigliotti F, Harmsen AG, Wright TW. 2003. Characterization of transmission of Pneumocystis carinii f. sp. muris through immunocompetent BALB/c mice. Infect Immun 71:3852–3856. doi: 10.1128/IAI.71.7.3852-3856.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]