ABSTRACT
Toxoplasma gondii is a pathogen relevant to psychiatric disorders. We recently showed that reactivation of chronic T. gondii infection induced depression-like behaviors in mice. Furthermore, it has been hypothesized that depression-like behaviors are mediated via a host defense mechanism against invading pathogens; proximate mechanisms of this behavioral hypothesis remain unclear. In the present study, we investigate the contribution of indoleamine 2,3-dioxygenase (IDO), inflammation, and interferon gamma (IFN-γ) to anhedonic and despair-related behaviors in T. gondii-infected mice by using sucrose preference and forced-swim tests, respectively. First, we confirmed that BALB/c mice exhibited both sickness and depression-like behaviors during acute infection. Treatment of infected wild-type mice with minocycline (anti-inflammatory drug) abated sickness and anhedonic and despair-like behaviors, whereas in T. gondii-infected mice, treatment normalized kynurenine/tryptophan (Kyn/Trp) ratios in both plasma and brain tissue. Additionally, T. gondii infection failed to induce anhedonic and despair-like behaviors or increase the Kyn/Trp ratio in immunocompromised (IFN-γ−/−) mice, whereas sickness behavior was observed in both immunocompetent and IFN-γ−/− mice following infection. Furthermore, treatment with 1-methyl tryptophan (an IDO inhibitor) did not affect locomotor activity, attenuated clinical scores and anhedonic and despair-like behaviors, and resulted in normal Kyn/Trp ratios in T. gondii-infected wild-type mice. Although low levels of serotonin and dopamine were observed in the brain during acute and chronic infections, anhedonic and despair-like behaviors were not detected in the chronic stage of infection. Collectively, our results demonstrated that immune enhancement in response to infection with T. gondii resulted in IFN-γ production, IDO activation, and inflammation associated with anhedonic and despair-like behaviors.
KEYWORDS: Toxoplasma gondii, 1-methyl tryptophan, minocycline, interferon gamma, sickness behavior, anhedonic behavior, despair-like behavior
INTRODUCTION
Toxoplasma gondii infection is linked to some mood and psychiatric disorders (1, 2). Furthermore, recent evidence indicated that reactivation of chronic T. gondii infection induces depression-related behaviors in mice (3). As the pathogenesis of infection relies largely upon host immunity, after T. gondii infection, peripheral macrophages and lymphocytes quickly become activated to kill intracellular tachyzoites (4). Sickness and depression-related behaviors are mediated by proinflammatory cytokines such as interleukin-1β (IL-1β), IL-6, tumor necrosis factor alpha (TNF-α), and interferon gamma (IFN-γ) (5–7). However, the distinction between sickness and depressive symptoms in T. gondii infection remains unclear. Mice given an experimental immune challenge with bacterial lipopolysaccharides (LPSs) or Mycobacterium bovis BCG vaccines exhibited behaviors specific for sickness and depression-like behaviors. For example, mice exhibited sickness symptoms in the form of reduced body weight and locomotor activity and depressive symptoms such as a reduced preference for sucrose and lower motility in forced-swim and tail suspension tests (8, 9).
Genes shown to promote major depressive disorder in humans were hypothesized to be associated with successful immune responses, protection from microbes, and enhanced survival in the ancestral environment. Therefore, specific depressive symptoms have been suggested to play roles in pathogen host defense (7). IFN-γ has been linked to depressive symptoms by inducing indoleamine 2,3-dioxygenase (IDO) activation and depleting tryptophan (Trp), the only known precursor of serotonin. Increased serum IFN-γ levels in response to peripheral immune stimulation enhanced cerebral IDO activity, which may reduce Trp levels in the brains of mice (10, 11). In addition, Trp depletion is caused by the stress-induced activation of tryptophan 2,3-dioxygenase (TDO), a hepatic enzyme, and/or the ubiquitous enzyme IDO (1, 2, 4, 5). IDO catabolizes Trp into the neurotoxic metabolites kynurenine (Kyn) and kynurenic acid (12, 13). Although the catabolism of Trp is stimulated by the induction of TDO and IDO, it is still argued that a reduction in Trp blood levels under conditions of stress and inflammation decreases the formation of cerebral serotonin (13). Furthermore, circulating Kyn crosses the blood-brain barrier (BBB), whereby it elevates cerebral Kyn levels (14). Hence, circulating Trp can cross the BBB by competing with other amino acids (13). Minocycline (Mino) (expanded-spectrum tetracycline) is widely used to block the expression of proinflammatory cytokines in peripheral and central organs (15–17) and prevent ischemic neuronal death (18, 19). 1-Methyl-dl-tryptophan (1-dl-MT) has become a reference drug for blocking IDO by competing with Trp. However, only 1-l-MT inhibits the enzyme activity of IDO, while 1-d-MT does not (20). Unlike Mino, 1-dl-MT blocks IDO-mediated immune events in rheumatoid arthritis (21–23) and inhibits T. gondii multiplication in vivo (20, 24). IDO activation in the brain has been proposed to induce Kyn, which may contribute to the depressive symptoms of epilepsy, Alzheimer's disease, and cerebral malaria (25–27).
We selected BALB/c mice to examine immune enhancement, as C57BL/6 mice have an insufficient intracerebral immune response (28, 29). BALB/c mice are considered genetically resistant to T. gondii infection and, instead of developing acute fatal toxoplasmic encephalitis, establish a chronic latent infection (30–32). Therefore, we predict that BALB/c mice will exhibit more depression-like behaviors than sickness symptoms. We hypothesized that T. gondii-induced depressive behaviors were based on immune enhancement in terms of IFN-γ production, the activation of IDO, and the disruption of serotonergic neurotransmission. To test this hypothesis, we used different approaches to investigate sickness (clinical score and locomotor activity) and depressive (anhedonic and despair-like) behaviors during the acute stage of T. gondii infection in wild-type and IFN-γ-deficient mice (BALB/c background) in the context of treatment with Mino or 1-dl-MT to inhibit inflammation or block IDO functions, respectively. Our findings provide insight into immune enhancement associated with the development of anhedonic and despair-like behaviors during the acute stage of T. gondii infection.
RESULTS
Attenuation of anhedonic behavior in T. gondii-infected mice with Mino or 1-dl-MT treatment or IFN-γ deficiency.
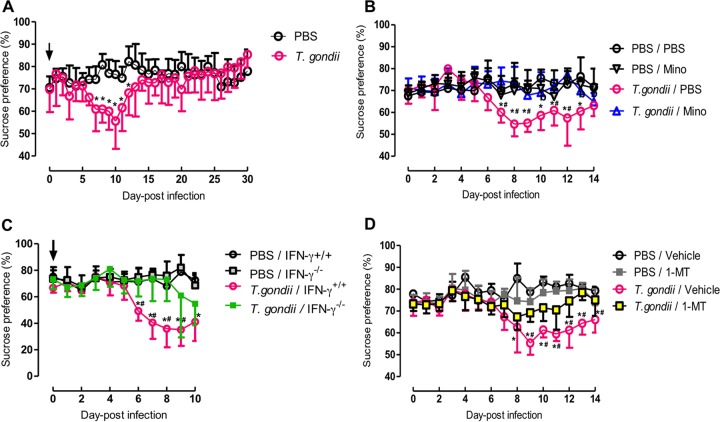
Reduced sucrose preference as a putative indicator of anhedonic behavior was measured by using a two-bottle preference test (Fig. 1). Initially, reduced sucrose consumption appeared 7 days after infection with T. gondii before reaching a peak level at 10 days postinfection (dpi) and returning to control levels after 2 weeks (Fig. 1A). This result indicates that anhedonic behavior was induced by acute infection with T. gondii. To test the role of the inflammatory response in anhedonic behavior, treatment of wild-type mice with an anti-inflammatory agent, Mino, was performed (Fig. 1B). Compared with the T. gondii–phosphate-buffered saline (PBS)-treated group, reduced sucrose consumption during acute infection was prevented by treatment with Mino. This indicates that the inflammatory response resulting from infection with T. gondii played a role in the anhedonic behavior observed in mice. Next, the effects of IFN-γ on anhedonic behavior were examined by using IFN-γ−/− mice (Fig. 1C), as IFN-γ is an important cytokine for inflammatory responses. Although reduced sucrose consumption was observed in T. gondii-infected wild-type mice from days 6 to 10 postinfection, infected IFN-γ−/− mice showed reduced consumption at levels similar to those of infected wild-type animals only at 10 dpi, suggesting a contribution of IFN-γ to anhedonic behavior. Since IFN-γ can activate IDO activity, we used an IDO inhibitor, 1-dl-MT, to treat anhedonic behavior in infected wild-type mice (Fig. 1D). Treatment with 1-dl-MT attenuated the reduction in the preference for sucrose from days 9 to 14 postinfection in T. gondii-infected mice. Collectively, these data suggest that anti-inflammatory treatment, deficiency of IFN-γ, or inactivation of IDO may prevent the onset of anhedonic behavior.
FIG 1.
Analysis of anhedonic behavior by sucrose consumption following T. gondii infection. (A) Sucrose consumption by wild-type BALB/c mice after injection with PBS or infection with T. gondii. Data from two independent experiments are summarized and presented as means ± standard deviations [PBS, n = 4 plus 6; T. gondii, n = 2 plus 6 (two mice died due to infection during trial 1); F(30, 496) = 4.15; P < 0.0001]. * indicates a significant difference between the PBS-injected and T. gondii-infected groups by two-way ANOVA plus Bonferroni post hoc analysis. (B) Sucrose consumption of wild-type BALB/c mice after injection with PBS or infection with T. gondii under treatment with Mino (10 mg/kg i.p.) or injection with PBS from days 4 to 7 postinfection. Data are representative of data from two independent experiments with similar results and are presented as means ± standard deviations [n = 6; F(42, 300) = 3.27; P < 0.0001]. * indicates a significant difference between the PBS-PBS and T. gondii-PBS groups, and # indicates a significant difference between the T. gondii-PBS and T. gondii-Mino groups by two-way ANOVA plus Bonferroni post hoc analysis. (C) Sucrose consumption of wild-type BALB/c mice (IFN-γ+/+) and IFN-γ−/− mice after injection with PBS or infection with T. gondii. Data are representative of data from four independent experiments with similar results and are presented as means ± standard deviations [n = 5; F(30, 176) = 3.77; P < 0.0001]. * indicates a significant difference between the PBS–IFN-γ+/+ and T. gondii–IFN-γ+/+ groups, and # indicates a significant difference between the T. gondii–IFN-γ+/+ and T. gondii–IFN-γ−/− groups by two-way ANOVA plus Bonferroni post hoc analysis. (D) Sucrose consumption of wild-type BALB/c mice after injection with PBS or infection with T. gondii under treatment with 1-MT (50 mg/kg subcutaneously) or injection with the vehicle from days 4 to 7 postinfection. Data are representative of data from two independent experiments with similar results and are presented as means ± standard deviations [n = 6; F(42, 300) = 4.24; P < 0.0001]. * indicates a significant difference between the PBS-vehicle and T. gondii-vehicle groups, and # indicates a significant difference between the T. gondii-vehicle and T. gondii–1-MT groups by two-way ANOVA plus Bonferroni post hoc analysis.
Attenuation of despair-like behavior in T. gondii-infected mice with Mino or 1-dl-MT treatment or IFN-γ deficiency.
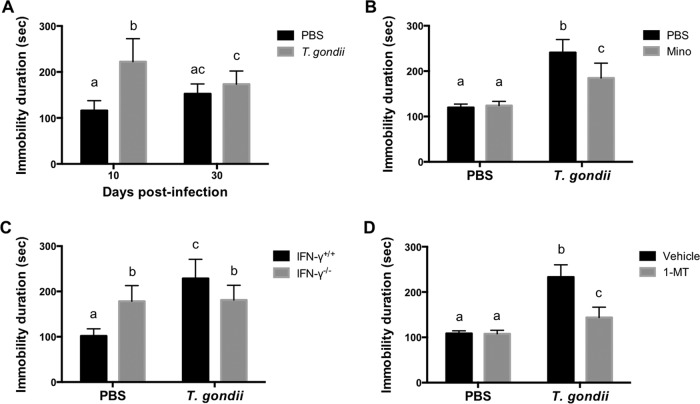
To test the effect of T. gondii infection on despair-like behavior, we measured the total time spent floating (immobility duration) in a forced-swim test (FST) at 10 and 30 dpi (Fig. 2A). At 10 dpi, but not at 30 dpi, an increased duration of immobility was observed, indicating despair-like behavior during acute infection. To determine whether T. gondii-induced despair-like behavior is dependent on inflammatory responses, wild-type mice were treated with Mino (Fig. 2B). Treatment of infected mice with Mino reduced the duration of immobility compared with that of untreated mice, indicating that despair-like behavior is mediated by inflammatory responses. In the case of IFN-γ−/− mice, there was no significant difference in durations of immobility between infected and noninfected mice, whereas the duration of immobility of T. gondii-infected IFN-γ−/− mice was shorter than that of infected wild-type mice (Fig. 2C), indicating IFN-γ-dependent despair-like behavior. However, the duration of immobility of IFN-γ−/− mice was longer than that of uninfected wild-type mice (Fig. 2C), suggesting a higher sensitivity of IFN-γ−/− mice to despair-like behavior. To evaluate the effects of IDO activity on despair-like behavior, wild-type mice were treated with 1-dl-MT (Fig. 2D). Similar to the results from Mino treatment, a reduced duration of immobility was observed for infected mice treated with 1-dl-MT compared with that for untreated infected animals. Taken together, despair-like behavior observed during acute infection may be induced by IDO activity via inflammatory responses.
FIG 2.
Analysis of despair behavior by duration of immobility following T. gondii infection. The total time spent floating (immobility duration) in the FST was measured as a putative indicator of despair behavior. (A) Duration of immobility of wild-type BALB/c mice after injection with PBS or infection with T. gondii at 10 and 30 dpi. Data from two independent experiments are summarized and presented as means ± standard deviations [PBS, n = 4 plus 6; T. gondii, n = 4 plus 6; F(1, 36) = 4.24; P = 0.0002]. (B) Duration of immobility of wild-type BALB/c mice after injection with PBS or infection with T. gondii at 10 dpi under treatment with Mino (10 mg/kg i.p.) or injection with PBS from days 4 to 7 postinfection. Data are representative of data from two independent experiments with similar results and are presented as means ± standard deviations [n = 5 or 6 (one mouse died due to infection in the T. gondii-infected and PBS-injected group); F(1, 19) = 10.54; P = 0.0042]. (C) Duration of immobility of wild-type BALB/c mice (IFN-γ+/+) and IFN-γ−/− mice after injection with PBS or infection with T. gondii at 10 dpi. Data from three independent experiments are summarized and presented as means ± standard deviations [n = 3 plus 5 plus 5; F(1, 48) = 46.50; P < 0.0001]. (D) Duration of immobility of wild-type BALB/c mice after injection with PBS or infection with T. gondii at 10 dpi under treatment with 1-MT (50 mg/kg subcutaneously) or injection with the vehicle from days 4 to 7 postinfection. Data are representative of data from two independent experiments with similar results and are presented as means ± standard deviations [n = 5 or 6 (one mouse died due to infection in the T. gondii-infected and vehicle-injected group); F(1, 19) = 35.81; P < 0.0001]. Different letters above bars in the graphs indicate statistically significant differences among the groups by two-way ANOVA plus Tukey-Kramer post hoc analysis.
Sickness behavior in IFN-γ−/− mice and wild-type mice treated with Mino and 1-MT.
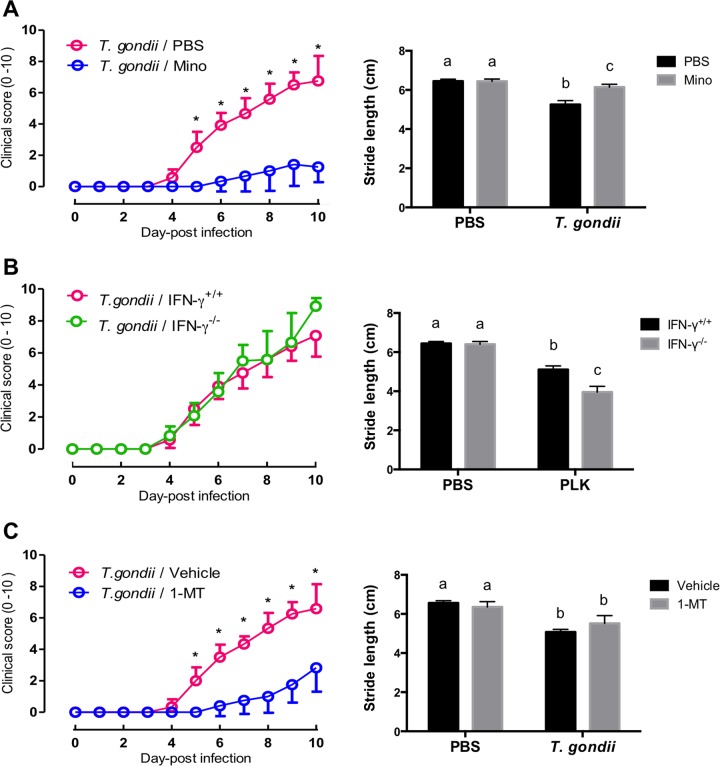
Acute T. gondii infection induces sickness behavior in terms of a considerably elevated clinical score (prepared ethogram) and reduced locomotor activity (stride length in a footprint test). Treatment of infected mice with Mino reduced clinical signs and improved the stride length of T. gondii-infected mice in the footprint test (Fig. 3A), suggesting that clinical symptoms and locomotor activity may be mediated by inflammatory responses. Conversely, IFN-γ−/− mice exhibited clinical symptoms similar to those of wild-type animals, including a marked reduction in locomotor activity, as indicated by a shortened stride in the footprint test in infected compared with uninfected wild-type mice (Fig. 3B), suggesting that IFN-γ may not be directly linked to sickness behavior. Treatment with 1-dl-MT attenuated T. gondii-induced sickness symptoms, as indicated by a reduction in the clinical score from 5 to 10 dpi, whereas 1-dl-MT did not alleviate the reduced stride length caused by infection (Fig. 3C), suggesting that IDO inhibition may reduce clinical symptoms but may not affect locomotor activity. Considered with observations of anhedonic and despair-like behaviors, inflammatory responses during acute infection (including IDO activation) may induce both sickness and depressive-like behaviors. However, sickness and depressive-like behaviors may result from independent pathways, as in the case of IFN-γ−/− mice.
FIG 3.
Clinical scores and locomotor activities of mice following T. gondii infection. (A) Clinical scores (left) and locomotor activities in terms of stride length in a footprint test (right) of wild-type BALB/c mice after infection with T. gondii and treatment with Mino (10 mg/kg i.p.) or injection with PBS from days 4 to 7 postinfection. Clinical scores from two independent experiments are summarized and presented as means ± standard deviations [n = 6 plus 6; F(10, 242) = 52.97; P < 0.0001]. Locomotor activity data are presented as means ± standard deviations [n = 5; F(1, 16) = 47.15; P < 0.0001]. (B) Clinical scores (left) and locomotor activities (right) of wild-type BALB/c mice (IFN-γ+/+) and IFN-γ−/− mice after infection with T. gondii. Data from two independent experiments are summarized and presented as means ± standard deviations [clinical score, n = 6 plus 6, F(10, 242) = 3.05, and P = 0.0012; locomotor activity, n = 5 plus 5, F(1, 36) = 81.69, and P < 0.0001]. (C) Clinical scores (left) and locomotor activities (right) of wild-type BALB/c mice after injection with PBS or infection with T. gondii under treatment with 1-MT (50 mg/kg subcutaneously) or injection with the vehicle from days 4 to 7 postinfection. Clinical score data from two independent experiments are summarized and presented as means ± standard deviations [n = 6 plus 6; F(10, 242) = 41.79; P < 0.0001]. Data for locomotor activity are presented as means ± standard deviations [n = 5; F(1, 16) = 7.877; P = 0.0127]. * indicates significant differences in clinical scores (left) between two groups by two-way ANOVA plus Bonferroni post hoc analysis. Different letters above bars in the graphs indicate statistically significant differences among the groups by two-way ANOVA plus Tukey-Kramer post hoc analysis (right).
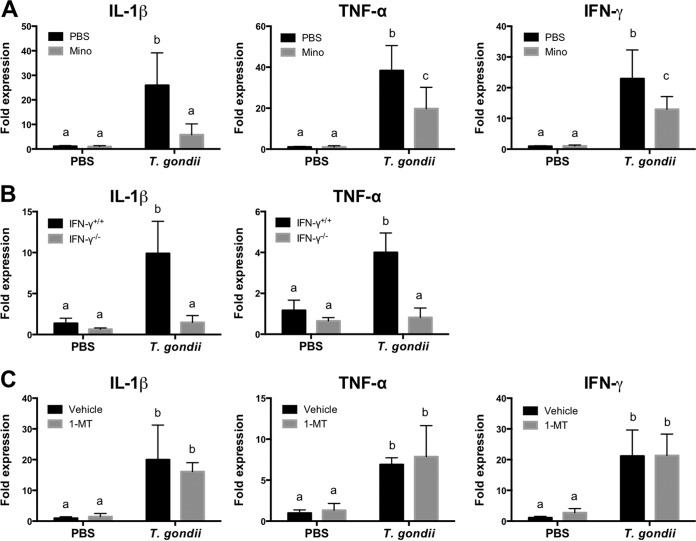
Expression of proinflammatory cytokines in IFN-γ−/− mice and wild-type mice treated with Mino and 1-dl-MT.
T. gondii infection increased the expression levels of IL-1β, TNF-α, and IFN-γ in the brain (Fig. 4). Correlation analysis of levels of proinflammatory cytokine expression and depressive-like behavior indicated anhedonic and despair-like behaviors, and levels of IFN-γ and IL-1β were strongly and moderately correlated, respectively (see Table S2 in the supplemental material). Furthermore, inhibitory effects of Mino on the expression of IL-1β, TNF-α, and IFN-γ mRNAs were observed in brains at 10 dpi (Fig. 4A). In addition, plasma levels of IL-1β and IFN-γ were reduced by Mino treatment in infected mice (Table 1). These results indicate that the changes in proinflammatory cytokine expression were associated with altered behaviors after T. gondii infection. IFN-γ−/− mice exhibited an attenuation of IL-1β and TNF-α mRNAs (Fig. 4B); however, 1-dl-MT treatment did not reduce mRNA expression levels of IL-1β, TNF-α, and IFN-γ in infected mice (Fig. 4C). In addition, compared with untreated and T. gondii-infected mice, 1-dl-MT treatment did not alter the production of IL-1β and IFN-γ after T. gondii infection (Table 1). Therefore, these results suggest an involvement of IDO activity in the induction of anhedonic and despair-like behaviors as well as clinical symptoms during acute T. gondii infection.
FIG 4.
Expression of cytokines in brains of mice following T. gondii infection. (A) Expression of IL-1β, TNF-α, and IFN-γ in brains of wild-type BALB/c mice after injection with PBS or infection with T. gondii at 10 dpi under treatment with Mino (10 mg/kg i.p.) or injection with PBS from days 4 to 7 postinfection. Data are representative of data from two independent experiments with similar results and are presented as means ± standard deviations [n = 5 or 6 (one mouse died due to infection in the T. gondii-infected and PBS-injected group); for IL-1β, F(1, 19) = 13.66 and P = 0.0015; for TNF-α, F(1, 19) = 8.235 and P = 0.0098; for IFN-γ, F(1, 19) = 6.325 and P = 0.0211]. (B) Expression of IL-1β and TNF-α in brains of wild-type BALB/c mice (IFN-γ+/+) and IFN-γ−/− mice after injection with PBS or infection with T. gondii at 10 dpi. Data from two independent experiments are summarized and presented as means ± standard deviations [n = 5 plus 5; for IL-1β, F(1, 36) = 35.98 and P < 0.0001; for TNF-α, F(1, 36) = 50.97 and P < 0.0001]. (C) Expression of IL-1β, TNF-α, and IFN-γ in brains of wild-type BALB/c mice after injection with PBS or infection with T. gondii at 10 dpi under treatment with 1-MT (50 mg/kg subcutaneously) or injection with the vehicle from days 4 to 7 postinfection. Data are representative of data from two independent experiments with similar results and are presented as means ± standard deviations [n = 5 or 6 (one mouse died in the T. gondii-infected and vehicle-injected group); for IL-1β, F(1, 19) = 0.937 and P = 0.3452; for TNF-α, F(1, 19) = 0.1289 and P = 0.7235; for IFN-γ, F(1, 19) = 0.1108 and P = 0.7429]. Different letters above bars in the graphs indicate statistically significant differences among the groups by two-way ANOVA plus Tukey-Kramer post hoc analysis.
TABLE 1.
Effects of minocycline and 1-methyl-dl-tryptophan treatments on plasma levels of the inflammatory cytokines IFN-γ and IL-1β in mice infected with T. gondii at 10 days postinfectiona
| Treatment | Mean level of protein (pg/ml) ± SD |
|
|---|---|---|
| IL-1β | IFN-γ | |
| PBS-PBS | ND | ND |
| PBS-Mino | 47.2 ± 4.6 a | 82.5 ± 28.4 a |
| T. gondii-PBS | 645.6 ± 34.6 b | 410.89 ± 87.7 b |
| T. gondii-Mino | 24.1 ± 4.4 a | 136.6 ± 7.6 c |
| PBS-vehicle | ND | ND |
| PBS–1-MT | ND | 26.5 ± 5.3 a |
| T. gondii-vehicle | 676.0 ± 32.5 | 459.6 ± 43.2 b |
| T. gondii–1-MT | 619.5 ± 26.7 | 433.3 ± 40.0 b |
Data represent average mean values ± standard deviations {n = 5 or 6; one mouse each died due to infection in the T. gondii-PBS group [for IL-1β, F(3, 22) = 231.7 and P = 0.0001; for IFN-γ, F(3, 22) = 329.4 and P = 0.0001] and the T. gondii-vehicle group [for IL-1β, F(3, 22) = 156.7 and P = 0.0001; for IFN-γ, F(3, 22) = 326.0 and P = 0.0001]}. Different letters indicate statistically significant differences among the groups by one-way ANOVA plus Tukey-Kramer post hoc analysis. ND, not detected.
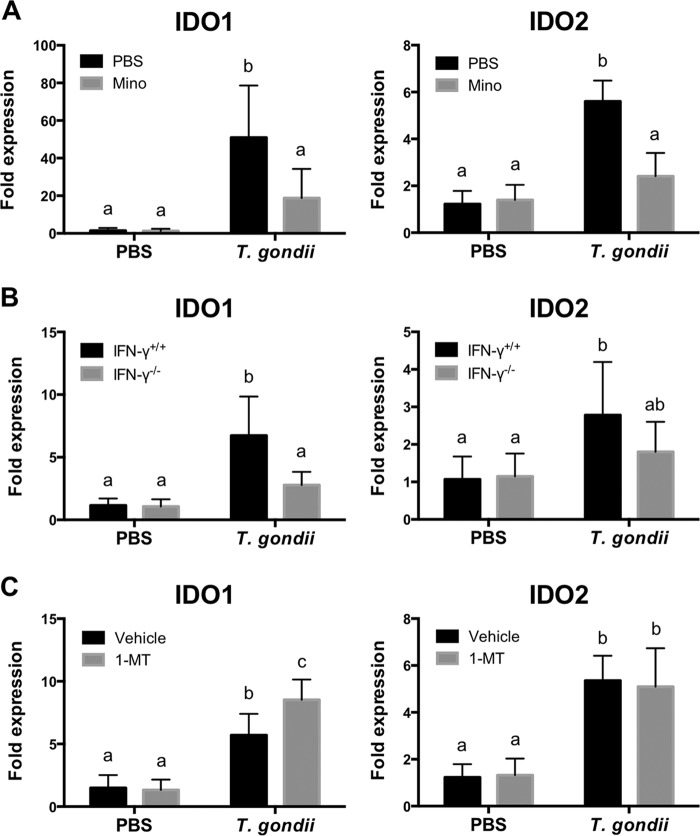
Expression of IDO in IFN-γ−/− mice and wild-type mice treated with Mino and 1-dl-MT.
The expression of IDO1 and its other form IDO2 has been described in both mice and humans (33). To determine whether the expression of IDO1 and IDO2 was correlated with altered behavior exhibited during T. gondii infection, quantitative reverse transcriptase PCR (qRT-PCR) of brains was performed (Fig. 5). As shown in Table S2 in the supplemental material, the expression levels of IDO1 and IDO2 in the brain correlated with the duration of immobility but not with sucrose preference, suggesting that the expression of IDO1 and IDO2 plays a role in the induction of despair-like behaviors. At 10 dpi, decreased expression levels of IDO1 and IDO2 were observed in Mino-treated wild-type mice compared with T. gondii-infected mice (Fig. 5A). A decreased expression level of IDO1 was also observed in IFN-γ−/− mice compared with infected wild-type mice (Fig. 5B). However, 1-dl-MT treatment did not affect IDO2 mRNA expression levels but significantly increased IDO1 expression levels in T. gondii-infected mice at 10 dpi (Fig. 5C). Thus, the genetic deletion of IFN-γ and the blockage of proinflammatory cytokines by Mino were associated with reduced IDO expression. However, 1-dl-MT treatment was not associated with reduced IDO expression in T. gondii-infected mice.
FIG 5.
Expression of IDO in brains of mice following T. gondii infection. (A) Expression of IDO1 and IDO2 in brains of wild-type BALB/c mice after injection with PBS or infection with T. gondii at 10 dpi under treatment with Mino (10 mg/kg i.p.) or injection with PBS from days for 4 to 7 postinfection. Data are representative of data from two independent experiments with similar results and are presented as means ± standard deviations [n = 5 or 6 (one mouse died due to infection in the T. gondii-infected and PBS-injected group); for IDO1, F(1, 19) = 6.480 and P = 0.0197; for IDO2, F(1, 19) = 26.09 and P < 0.0001]. (B) Expression of IDO1 and IDO2 in brains of wild-type BALB/c mice (IFN-γ+/+) and IFN-γ−/− mice after injection with PBS or infection with T. gondii at 10 dpi. Data from two independent experiments are summarized and presented as means ± standard deviations [n = 5 plus 5; for IDO1, F(1, 36) = 13.06 and P = 0.0009; for IDO2, F(1, 36) = 3.313 and P = 0.077]. (C) Expression of IDO1 and IDO2 in brains of wild-type BALB/c mice after injection with PBS or infection with T. gondii at 10 dpi under treatment with 1-MT (50 mg/kg subcutaneously) or injection with the vehicle from days 4 to 7 postinfection. Data are representative of data from two independent experiments with similar results and are presented as means ± standard deviations [n = 5 or 6 (one mouse died due to infection in T. gondii-infected and vehicle-injected group); for IDO1, F(1, 19) = 7.313 and P = 0.0141; for IDO2, F(1, 19) = 0.158 and P = 0.6954]. Different letters above bars in the graphs indicate statistically significant differences among the groups by two-way ANOVA plus Tukey-Kramer post hoc analysis.
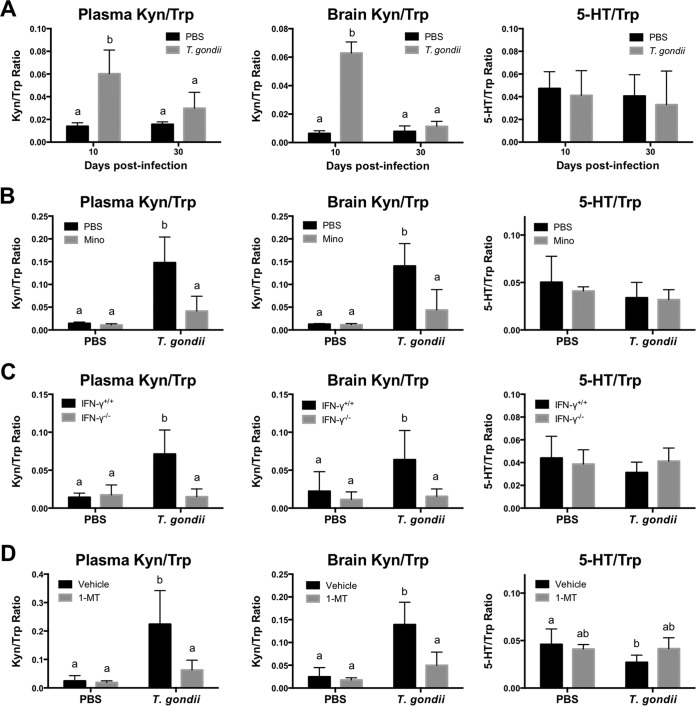
Tryptophan metabolism in IFN-γ−/− mice and wild-type mice treated with Mino or 1-dl-MT.
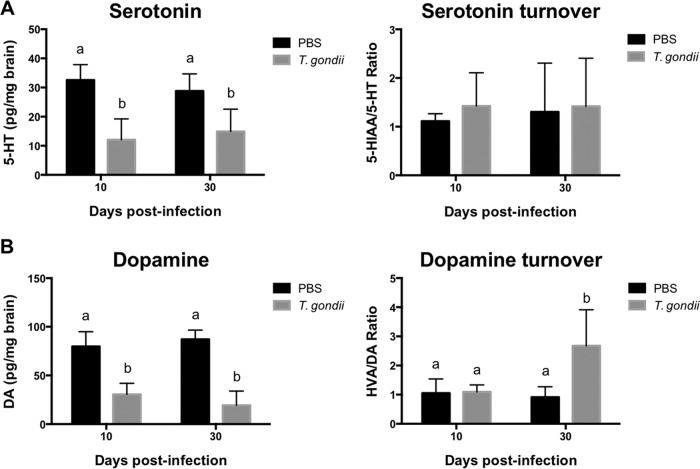
To determine the mechanism by which alterations in Trp degradation contribute to depressive-like behavior following T. gondii infection, we measured Trp, Kyn, and 5-hydroxytryptamine (5-HT) levels in brains as well as plasma levels of Trp and Kyn (Fig. 6). The calculated Kyn/Trp ratio in brains at 10 dpi was ∼3.6-fold higher than that at 30 dpi, whereas in plasma, it was ∼1.8-fold higher (Fig. 6A). It was noted that T. gondii infection did not affect Trp turnover to 5-HT at 10 and 30 dpi in wild-type mice, as measured by comparing the ratios of 5-HT/Trp (Fig. 6A). At 10 dpi, Kyn/Trp ratios in plasma and brain exhibited a weak to moderate correlation with anhedonic behaviors and a moderately strong correlation with despair-like behaviors (see Table S2 in the supplemental material). To further investigate the effects of the inflammatory response and IFN-γ action on Trp metabolism, we measured Kyn/Typ and 5-HT/Trp ratios in Mino-treated wild-type mice (Fig. 6B) and IFN-γ−/− mice (Fig. 6C). Reproducibly, T. gondii infection markedly increased the Kyn/Trp ratio in both plasma and brain. These altered ratios of Kyn/Trp were significantly reduced in the plasma and brains of Mino-treated wild-type mice and in IFN-γ−/− mice, whereas the 5-HT/Trp ratio was not affected (Fig. 6B and C). In parallel, treatment of T. gondii-infected wild-type mice with 1-dl-MT normalized the Kyn/Trp ratio in both the plasma and brain (Fig. 6D). Next, we examined the levels of 5-HT and dopamine (DA) in the brain (Fig. 7). Lower levels of 5-HT and dopamine were observed in infected wild-type mice at both 10 and 30 dpi. Dopamine metabolism was upregulated in infected mice at 30 dpi as the result of lower DA levels and higher DA turnover rates than those in uninfected mice (Fig. 7B). Thus, 5-HT and dopamine may not be directly involved in depression-like behavior because the behavioral changes were not seen at 30 dpi. Together, these findings indicate that T. gondii enhanced Trp catabolism toward Kyn and not 5-HT.
FIG 6.
Trp turnover to Kyn or 5-HT in mice following T. gondii infection. Trp turnover to Kyn (Kyn/Trp ratio) in plasma and brain and Trp turnover to brain serotonin (5-HT/Trp ratio) were determined. (A) Trp turnover in wild-type BALB/c mice after injection with PBS or infection with T. gondii at 10 and 30 dpi. Plasma Kyn/Trp ratios are presented as means ± standard deviations [n = 5; F(1, 16) = 8.039; P = 0.0119]. Data for brain Kyn/Trp and brain 5-HT/Trp ratios from two independent experiments are summarized and presented as means ± standard deviations [n = 4 or 5 plus 5; for the brain Kyn/Trp ratio, F(1, 32) = 274.6 and P < 0.0001; for Trp turnover, F(1, 36) = 0.01294 and P = 0.9101]. (B) Trp turnover in wild-type BALB/c mice after injection with PBS or infection with T. gondii at 10 dpi under treatment with Mino (10 mg/kg i.p.) or injection with PBS from days 4 to 7 postinfection. Data are presented as means ± standard deviations [n = 5 or 6 (one mouse died in the T. gondii-infected and PBS-injected group); for the plasma Kyn/Trp ratio, F(1, 19) = 15.91 and P = 0.0008; for the brain Kyn/Trp ratio, F(1, 19) = 12.44 and P = 0.0023; for Trp turnover, F(1, 19) = 0.2663 and P = 0.6118]. (C) Trp turnover of wild-type BALB/c mice (IFN-γ+/+) and IFN-γ−/− mice after injection with PBS or infection with T. gondii at 10 dpi. Data from two independent experiments are summarized and presented as means ± standard deviations [for the plasma Kyn/Trp ratio, n = 4 plus 4, F(1, 28) = 21.44, and P < 0.001; for the brain Kyn/Trp ratio, n = 5 plus 5, F(1, 36) = 6.016, and P = 0.0192; for the brain 5-HT/Trp ratio, n = 5 plus 5, F(1, 36) = 3.19, and P = 0.0825]. (D) Trp turnover of wild-type BALB/c mice after injection with PBS or infection with T. gondii at 10 dpi under treatment with 1-MT (50 mg/kg subcutaneously) or injection with the vehicle from days 4 to 7 postinfection. Data are presented as means ± standard deviations [n = 5 or 6 (one mouse died due to infection in the T. gondii-infected and vehicle-injected group); for the plasma Kyn/Trp ratio, F(1, 16) = 7.838 and P = 0.0129; for the brain Kyn/Trp ratio, F(1, 19) = 11.51 and P = 0.0031; for Trp turnover, F(1, 19) = 4.43 and P = 0.0489]. Different letters above bars in graphs indicate statistically significant differences among the groups by two-way ANOVA plus Tukey-Kramer post hoc analysis.
FIG 7.
Levels of serotonin and dopamine in brains of mice following T. gondii infection. 5-HT and its turnover to 5-hydroxyindoleacetic acid (5-HIAA) (5-HIAA/5-HT ratio) as well as DA and its turnover to homovanillic acid (HVA) (HVA/DA ratio) were measured in brains of mice. (A) 5-HT and its turnover in wild-type BALB/c mice after injection with PBS or infection with T. gondii at 10 and 30 dpi. Data from two independent experiments are summarized and presented as means ± standard deviations [n = 5 plus 5; for 5-HT, F(1, 36) = 2.538 and P = 0.1199; for the 5-HIAA/5-HT ratio, F(1, 36) = 0.1521 and P = 0.6989]. (B) DA and its turnover in wild-type BALB/c mice after injection with PBS or infection with T. gondii at 10 and 30 dpi. Data from two independent experiments are summarized and presented as means ± standard deviations [n = 5 plus 5; for DA, F(1, 36) = 5.27 and P = 0.0276; for the 5-HIAA/5-HT ratio, F(1, 36) = 0.1521 and P = 0.6989]. Different letters above bars in graphs indicate statistically significant differences among the groups by two-way ANOVA plus Tukey-Kramer post hoc analysis.
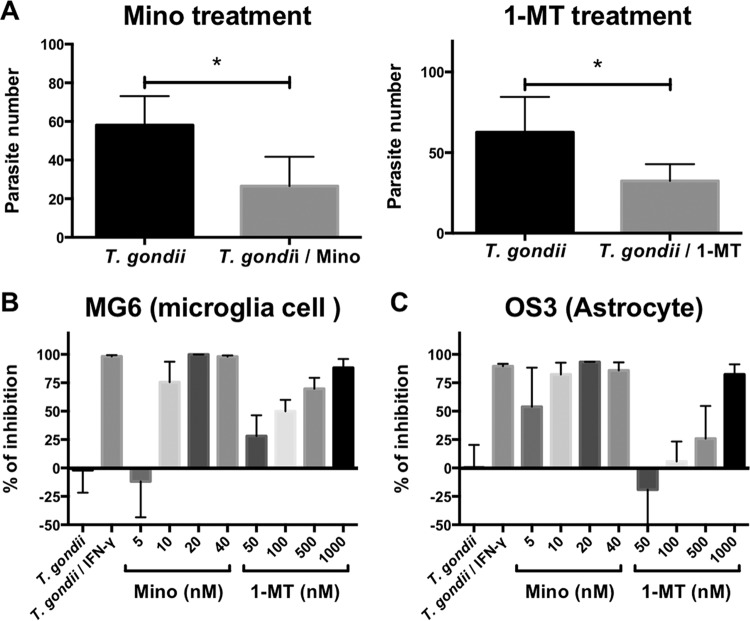
Effect of Mino and 1-dl-MT on parasite growth in vivo and in vitro.
At 14 dpi, the number of parasites in brain DNA was measured by quantitative PCR (qPCR) (Fig. 8). We found that treatment of infected mice with Mino and 1-MT reduced the brain parasite load (Fig. 8A). This effect was validated in vitro in T. gondii-infected microglia and astrocyte cell lines, MG6 and OS3, respectively. Pretreatment with Mino (5 to 40 nM) and 1-dl-MT (0.05 to 1 μM) had dose-dependent effects on restricting T. gondii replication in MG6 and OS3 cells (Fig. 8B and C). No effect on cell viability at the selected dose ranges was observed (data not shown). These findings indicate that both 1-dl-MT and Mino exhibit significant anti-Toxoplasma activities in vivo and in vitro.
FIG 8.
Effects of 1-MT and Mino on T. gondii growth in vivo and in vitro. (A) Numbers of parasites in the brains of wild-type BALB/c mice after infection with T. gondii at 14 dpi under treatment with Mino (10 mg/kg i.p.) or injection with PBS from days 4 to 7 postinfection and under treatment with 1-MT (50 mg/kg subcutaneously) or injection with the vehicle from days 4 to 7 postinfection. Data are presented as means ± standard deviations (n = 5 or 6; one mouse each died due to infection in the T. gondii-infected and PBS-injected groups and in the T. gondii-infected and vehicle-injected groups). * indicates significant differences between groups by Student's t test. (B and C) Effects of IFN-γ (250 U/ml), Mino (5 to 40 nM), and 1-MT (50 to 1,000 nM) on T. gondii growth in murine microglia MG6 (B) and murine astrocyte OS3 (C) cells. Data are presented as means ± standard deviations (n = 3).
DISCUSSION
Causes of depression do not stem from a single source; rather, depression is likely to be caused by a combination of factors. Perpetuation of the neurotropic parasite T. gondii may further complicate this syndrome. In the present study, we observed anhedonic and despair-like behaviors during the acute stage of T. gondii infection. Therefore, immune enhancement may be a prerequisite for the development of such a depressive phenotype. Here, we hypothesized that the activation of host immunity mediates depression-like behaviors in T. gondii-infected mice. Our results support the view that interactions of the immune system and IFN-γ, IDO, and Trp metabolism play crucial roles in developing T. gondii-induced depression-like behaviors. In animal models, studies of the Kyn pathway and the Trp catabolic shunt indicate that these mechanisms are conserved not only between humans and rodents but also in lower organisms such as yeast (34). Several lines of evidence illustrate that depression-like behavior is associated with an enhancement of Trp catabolism caused by either inflammation or the release of proinflammatory cytokines (34, 35). Investigation of this pathway following infection with a chronic pathogen, such as T. gondii, will likely continue to advance our understanding of mechanisms by which neurotropic parasites induce some psychiatric disorders (36). The induction of depressive-like behaviors in T. gondii-infected mice and enhancement of Trp catabolism toward Kyn may be relevant to interpreting studies of psychobehavioral disorders in T. gondii-infected humans.
In the present study, T. gondii infection induced depression-like behavior in the forms of anhedonic and despair-like behaviors in mice during the acute stage. Additionally, sickness behavior was observed in mice following acute infection. As behavioral symptoms of sickness and depression are triggered by proinflammatory cytokines (17), it may be difficult to determine whether T. gondii directly induced depression-like behavior or whether generalized inflammatory responses induced by infection triggered behavioral changes. Although both sickness and depression-like behaviors are induced by the same proinflammatory cytokines, IDO induction has been proposed to lie at the interface between chronic inflammatory disease and depression (17). Although T. gondii-infected IFN-γ−/− mice showed clinical scores similar to those of infected wild-type mice, infected IFN-γ−/− mice did not exhibit anhedonic behavior (reduced sucrose preference) at 6 to 9 dpi, except for 10 dpi, compared with uninfected IFN-γ−/− mice. At 10 dpi, both wild-type BALB/c and IFN-γ−/− mice showed comparably severe clinical symptoms following infection. These symptoms at 10 dpi were categorized as sickness-related symptoms. Additionally, the locomotor activity of IFN-γ−/− mice infected with T. gondii was reduced compared with that of infected wild-type BALB/c mice. In fact, IFN-γ−/− mice showed a reduced intake of both water and sucrose (total fluid intake), which ranges from 0 to 2 ml of each bottle during 24 h. Actually, these IFN-γ−/− mice mostly succumbed after 10 days of infection. Thus, the severe reduction of locomotor activity may result in a reduced sucrose preference (or total fluid intake) in IFN-γ−/− mice infected with T. gondii at 10 dpi. It should be noted that uninfected IFN-γ−/− mice showed a longer duration of immobility in the FST than did uninfected wild-type mice. Furthermore, T. gondii infection did not increase the duration of immobility in IFN-γ−/− mice at 10 dpi compared with uninfected IFN-γ−/− mice (Fig. 2C). This result suggests that sickness and anhedonic behaviors may occur in T. gondii-infected mice by independent mechanisms. However, at present, it is unclear why the genetic deletion of IFN-γ induced despair behavior (increased duration of immobility in the FST) in uninfected IFN-γ−/− mice compared with uninfected wild-type mice. Perhaps, the relationship between IFN-γ and Trp metabolism may be linked to the immunogenetics of major depression, as reported previously in some correlation studies (11). Similarly, a deficiency of Toll-like receptor 2 was found to be associated with the induction of schizophrenia-like symptoms in mice (37). Collectively, these results suggest that the deficiency of an immunity-related gene (IRG), such as IFN-γ, was associated with despair-like and anhedonic behaviors in uninfected mice.
IFN-γ is essential for host defense mechanisms and the survival of mice during T. gondii infection. IFN-γ-deficient mice succumb to acute infection (4). Furthermore, mice lacking IL-12 or T cells that regulate or produce IFN-γ, respectively, do not survive the chronic stage of T. gondii infection (38–40). IFN-γ can suppress symptoms in T. gondii infection and, in fact, can inhibit T. gondii proliferation through various mechanisms, including (i) the depletion of arginine and the production of free radical nitric oxide via enzymatic activation (41); (ii) the disruption of parasitophorous vacuoles via IFN-γ-inducible genes, such as IRGs and guanylate-binding proteins (GBPs), in murine macrophages (42–44); or (iii) the starvation of an essential amino acid, Trp, mediated through IDO activity, as shown in human fibroblasts (45), but a direct effect of IDO on parasites in mice remains unclear. Thus, the activation of IFN-γ and IDO was associated with the depletion of Trp, suggesting a correlation between host defense mechanisms and the display of anhedonic and despair-like behaviors. Therefore, it is plausible that anhedonic and despair-like behaviors were dependent on IFN-γ or IDO activation rather than simply being linked to sickness behaviors.
Aside from the association of sickness symptoms with depression-like behavior during the acute stage of T. gondii infection, our findings support a role for the Trp-to-Kyn shunt in depression pathophysiology induced by T. gondii infection. Anhedonic and despair-like behaviors during the acute stage were present with low 5-HT levels (associated with a high Kyn/Trp ratio in plasma and brain) and normal 5-HT turnover. However, such behaviors were not displayed at 30 dpi in the context of low brain 5-HT and DA levels. In support of these results, we found that Kyn/Trp ratios in the brain of infected mice at 60 dpi did not change from those in control uninfected mice, indicating lower IDO activity in the chronic stage (3). Thus, the development of anhedonic and despair-like behaviors may not be simply dependent on lower levels of serotonergic and dopaminergic neurotransmission during the acute stage of T. gondii infection in mice. It has been reported that IDO induction, Trp degradation, and Kyn formation are completely absent in T. gondii-infected IFN-γ-deficient mice (46, 47). Our data also confirmed the importance of IFN-γ for IDO activity induction and the resultant depression-like behaviors. Since IFN-γ-mediated IDO activation may induce such behaviors in mice following infection with T. gondii during the acute stage, T. gondii infection can induce changes in Trp metabolism via proinflammatory cytokines, especially IFN-γ.
However, at present, it is unclear why the sustained decreases in DA and 5-HT levels were not reflected in the precipitation of anhedonic and despair-like symptoms in T. gondii-infected mice during chronic infection. On the other hand, low levels of 5-HT and DA coexisted with anhedonic and despair-like behaviors during acute infection. Thus, the contribution of DA and 5-HT to depression-like behaviors could not be concluded. Compared with uninfected mice, the unchanged 5-HT/Trp ratio and serotonin turnover and the higher Kyn/Trp ratio indicated enhanced Trp catabolism toward Kyn in infected mice during acute infection. Together, these findings suggest that enhanced Trp catabolism toward Kyn resulting from the inflammatory response, IFN-γ action, and IDO activity-dependent mechanisms of T. gondii infection may be related to the appearance of anhedonic and despair-like behaviors during acute infection.
In the present study, Mino abrogated T. gondii-induced anhedonic and despair-like behaviors; it also normalized Kyn/Trp ratios in the brain and plasma in T. gondii-infected mice. It is well known that proinflammatory cytokines are involved in depression-like behaviors through the generation of a neuroreactive Trp metabolite, Kyn. A Kyn metabolite, quinolinic acid, acts as an N-methyl-d-aspartate (NMDA) receptor agonist (48) and is implicated in the development of core symptoms of depression-like anhedonia and behavioral despair following the peripheral administration of cytokine-inducing bacterial LPS (8). Our data showed that treatment with Mino inhibited IDO expression in the brains of T. gondii-infected mice, resulting in the normalization of Kyn/Trp ratios in both the plasma and brains of infected mice. Mino, a prototype anti-inflammatory drug, was used to prevent ischemic neuronal death. In addition, Mino protects neurons from glutamate toxicity and blocks the expression of proinflammatory cytokines in both peripheral and central organs (17–19). Mino is also a selective inhibitor of microglia activation, and it inhibits apoptosis by decreasing the levels of IL-1β, TNF-α, and their converting enzymes caspase 1 and caspase 3 (49, 50). Additionally, Mino reduced the permeability of the BBB by inhibiting IL-1β, TNF-α, matrix metalloproteinase 2 (MMP-2), MMP-9, and vascular cell adhesion molecule (VCAM) expression as well as reducing the transmigration of T cells across the fibronectin matrix barrier (51, 52). Therefore, Mino may be a potential therapeutic for psychiatric diseases, including early schizophrenia, multiple sclerosis, Huntington disease, and Parkinson's disease (49, 50, 52–54, 72). Activated microglia contribute to neuronal apoptosis; thus, the inhibition of microglia activation by Mino may represent a novel therapeutic strategy for treating neuronal apoptosis in cases of toxoplasmic encephalitis (55). Mino also prevented anhedonia and sickness behavior in mice challenged with LPS (56). Although LPS induces sickness behaviors followed by depression-like behaviors, this appeared within a relatively short time course compared with immune challenge with the BCG vaccine (9, 57) or T. gondii infection. In the present study, we treated T. gondii-infected mice with Mino for 4 days at doses of 10 mg/kg of body weight intraperitoneally (i.p.). Our treatment regimen decreased clinical symptoms induced by infection without inducing side effects. Furthermore, Mino decreased the brain parasite burden (Fig. 5A), thus conferring the strongest protective effect against T. gondii in murine microglia and astrocyte cells (Fig. 4B and C). T. gondii tachyzoites invade murine microglia, astrocytes, and neurons; thereafter, the parasite forms cysts within these cells (58). Our data for parasite growth indicated that microglia have 17 times the ability of astrocytes to control T. gondii. Within the brain, microglia are reported to be the major effector cells for the prevention of T. gondii tachyzoite proliferation (58). At the neuronal level, Mino salvaged glutamate toxicity in cultured primary neurons (16). Thus, Mino may have the potential to control acute toxoplasmosis, while the anti-Toxoplasma mechanism of Mino is still unknown.
1-dl-MT was previously used as a reference drug for blocking IDO activity in human and murine cells (21, 23, 59). In clinical trials, 1-dl-MT has been used as a vaccine adjuvant and an add-on immunotherapeutic agent for cancer patients (60). As IDO triggers Trp depletion in the cellular microenvironment, it can also inhibit the proliferation of T cells and permit tumor cells to escape the immune system (61). Therefore, IDO inhibition by 1-dl-MT reverses the immunosuppressive effects of IDO (62). Interestingly, chronic treatment with 1-l-MT or 1-dl-MT results in controversial antiparasitic activity, but subchronic treatment exerts some anti-Toxoplasma activity (20, 24). Furthermore, IDO−/− mice and mice with IDO inhibited by 1-dl-MT exhibit reduced T. gondii mRNA expression in the lungs (24). In our study, 1-dl-MT treatment attenuated anhedonic and despair-like behaviors in T. gondii-infected mice without an obvious effect on proinflammatory cytokine expression, suggesting that the effect of 1-dl-MT is independent of proinflammatory cytokine expression. Furthermore, IDO activity may be involved in anhedonic and despair-like behaviors, and such behaviors are unlikely to be linked to cytokine production in T. gondii-infected mice. Our results indicate that 1-dl-MT, like Mino, significantly reduced the parasite load in vivo and in vitro (Fig. 8). It would be of interest in future studies to examine mechanisms underlying the ability of Mino and 1-dl-MT to control the growth of T. gondii.
In summary, the present study suggests that depression-like behaviors are likely mediated by host defense mechanisms against T. gondii infection. As a host immune response against acute T. gondii infection, IFN-γ produced by T cells or natural killer cells stimulates IDO activity. Metabolites of IDO activity, Trp to Kyn, resulted in depression-like behaviors. Treatment with Mino and 1-dl-MT ameliorated T. gondii-induced anhedonic and despair-like behaviors, suggesting the potential of this drug to treat core depression symptoms. As these drugs are highly lipid soluble and capable of penetrating the BBB (17), they may be of potential interest for future clinical studies. Since these drugs inhibit Toxoplasma growth, their use for T. gondii infection may be beneficial. Moreover, their effect was associated with symptomatic relief of anhedonia and despair as well as an improvement of clinical symptoms or locomotor activity. Our results demonstrate that immune enhancement in response to infection of immunocompetent mice with T. gondii resulted in IFN-γ production, IDO activation, and inflammation associated with sickness and anhedonic and despair-like behaviors. In contrast, after inhibition of IFN-γ production, sickness symptoms were displayed without the other behaviors. The inactivation of IDO improved clinical symptoms and depression-like behaviors. In conclusion, host defense mechanisms against T. gondii infection may be involved in anhedonic and despair-like behaviors in mice.
MATERIALS AND METHODS
Ethics statement.
This study was performed in strict accordance with recommendations of the Guidelines for Proper Conduct of Animal Experiments (73). The protocol was approved by the Committee on the Ethics of Animal Experiments of the Obihiro University of Agriculture and Veterinary Medicine (permit numbers 24-13, 24-15, 25-59, and 25-61). All injections were performed under isoflurane anesthesia, and every effort was made to minimize animal suffering.
Animals.
According to our established experimental model (3, 63), experiments were performed by using wild-type female BALB/c mice (7 weeks old). Female IFN-γ−/− mice (BALB/c background) were obtained from Clea Japan (Tokyo, Japan) and maintained at the National Research Center for Protozoan Diseases (Obihiro University of Agriculture and Veterinary Medicine, Obihiro, Japan). Animals were examined after 1 week of accommodation under specific-pathogen-free conditions and under stable conditions (12-h light/dark cycles; light on from 07:00 h to 19:00 h). Food and water were administered ad libitum, and all behavioral experiments commenced at 09:00 h.
Toxoplasma gondii culture and infection.
The type II PLK strain of T. gondii was utilized; the parasites were maintained as tachyzoites in Vero cell culture. After syringe lysis purification, wild-type and IFN-γ−/− mice were either i.p. infected with 103 tachyzoites or injected with sterile PBS, as previously described (42, 63). At designated time points, mice were decapitated without anesthesia, blood was collected into heparinized tubes to obtain plasma, and organs were instantly frozen in liquid nitrogen for storage at −80°C until further analyses.
Treatments and experimental groups.
At 4 dpi, groups of T. gondii-infected and PBS-injected mice were treated with either Mino (Sigma-Aldrich, St. Louis, MO) (10 mg/kg i.p.) or PBS once a day for 4 days (16). Similarly, groups of T. gondii-infected and PBS-injected mice were subcutaneously treated with 50 mg/kg 1-dl-MT) (Sigma) or its vehicle once a day for 4 days starting at 4 dpi. Injections of 1-dl-MT were prepared in 0.1 N HCl, neutralized with an equal volume of 0.1 M NaOH, buffered with 2× PBS, and filtered through a 0.2-μm syringe filter.
Cell culture.
A murine microglia cell line (MG6) was cultured in Dulbecco's modified Eagle's medium (DMEM; Sigma) containing 10% heat-inactivated fetal bovine serum (FBS; Nichirei Biosciences, Tokyo, Japan), 10 μg/ml bovine insulin (Sigma), and 100 μM 2-mercaptoethanol (Sigma) (64, 65). An astrocyte cell line (OS3) was cultured in modified Eagle's medium (MEM; Sigma) containing 10% heat-inactivated FBS and 5 μg/ml bovine insulin (66). Both the MG6 and OS3 cell lines were provided by Riken BRC through the National Bio-Resource Project of MEXT, Japan.
Sucrose preference test.
Anhedonic behavior is putatively considered a reduction in sucrose preference (67). First, mice were habituated with two bottles of water for 1 week, followed by one bottle of 1% sucrose and one bottle of water 2 days before treatment. The bottle position was switched every day according to a reward test protocol (3, 67). The total consumption of each fluid was measured daily, and sucrose preference was calculated by using the following formula: [sucrose intake/(water intake + sucrose intake)] × 100.
FST.
FST evaluations were performed as described previously (68) and in our previous study (3). The FST was conducted under normal light for 6 min, and the time of immobility was then analyzed. Mice were individually placed into the water-filled FST cylinder (12-cm diameter, filled to a 25-cm water depth; Coulbourn Instruments, White Hall, PA). The water temperature was adjusted to within a thermoneutral range (31°C ± 1°C) for rodents. Immobility was defined as mice remaining motionless, except for necessary movements to maintain floating. The first 2 min of the test allowed accommodation. The duration of immobility within a 6-min session was recorded as an immobility score. Analysis was performed offline by an experienced observer blind to experimental groups. After the testing period, mice were towel dried and returned to their housing conditions.
Clinical score.
Ethograms were customized according to the appearance of clinical symptoms during T. gondii infection within home and naive cages, as previously reported (3). In brief, scores varied from 0 (no signs) to 10 (all signs). Recorded signs included hunching, piloerection, warmth-seeking behavior (hiding in the corner of the cage and beneath bedding), ptosis (drooping or falling of the upper eyelids), sunken eyes, ataxia, reluctant movement, deficient evacuation and touch reflexes, and lying on the belly. Each symptom was equivalent and worth 1 point, and there were exactly 10 measures.
Footprint test.
Each mouse was exposed to the footprint test (69) once a day, with some modifications. In brief, the feet of mice were brushed with black and red (right and left forefeet, respectively) and green and yellow (right and left hind feet, respectively) nontoxic paints. Animals were then allowed to walk along a 50-cm-long and 10-cm-wide enclosed runway with 10-cm-high walls. All mice performed three training runs before being subsequently tested with one run per day. Stride length was measured as the average distance of forward movement between each stride. For each step, three values from one run were measured, excluding footprints made at the beginning and the end of the run, where the animal was initiating and finishing movement, respectively. The mean value for each set of three values was used in subsequent analyses.
Quantitative reverse transcriptase PCR.
Total RNA was extracted from the left halves of brains by using Tri reagent (Sigma). Reverse transcription was performed by using Superscript III reverse transcriptase (Thermo Fisher Scientific, Waltham, MA), according to the manufacturer's instructions. Amplification was performed by using a standard protocol recommended by the manufacturer (2 min at 50°C, 10 min at 95°C, 40 cycles at 95°C for 15 s, and 60°C for 1 min). Samples were run in duplicate. Amplification, data acquisition, and data analysis were carried out in an ABI Prism 7900HT sequence detection system (Thermo Fisher), and calculated cycle threshold (CT) values were exported to Microsoft Excel for analysis. Expression levels of each gene relative to the value for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were calculated by using the 2ΔCT method (user bulletin no. 2; Perkin-Elmer Applied Biosystems, Waltham, MA). The optimal reference gene was selected based on the Cotton EST database. Specific primers for each gene were designed by using PRIMER EXPRESS software (Perkin-Elmer Applied Biosystems). A list of primer sequences is shown in Table S1 in the supplemental material.
DNA isolation and qPCR detection of T. gondii.
qPCR was performed on purified DNA from brain homogenates, cultured cells, and egressed parasites in the culture medium by using Tri reagent (Sigma), as previously described (42). Briefly, after DNA purification, 50 ng of DNA extracted from brain homogenates or total cellular DNA was used for the amplification of parasite DNA with primers specific for the T. gondii B1 gene (70). Parasite numbers were calculated by interpolation on a standard curve, with CT values plotted against a known parasite concentration. MG6 and OS3 cells were pretreated with 1-dl-MT (0.05 to 1.0 μM), Mino (5.0 to 40.0 nM), or IFN-γ (250 U/ml) for 24 h. After the medium was changed, cells were treated again with 1-dl-MT or Mino and infected with T. gondii (PLK strain; multiplicity of infection [MOI] of 0.25) for an additional 48 h. Data are presented as percentages of control inhibition in T. gondii-infected cells according to a previously described formula (42). The percentage of inhibition was calculated as {[(mean value of the control) − (value of the test sample)]/(mean value of the control)} × 100.
High-performance liquid chromatography (HPLC).
Major monoamines, l-Trp, and their metabolites were examined in supernatants obtained from the right brain hemispheres by using an SC-5ODS column (Eicompak) and an electrochemical detector according to the monoamine analysis application manual (Eicom, Kyoto, Japan) and as previously described (3). Wet samples (100 mg) were homogenized in 0.5 ml of 0.2 M perchloric acid (including 100 μM EDTA-2Na and 1 μg/ml isoproterenol as an internal standard). External calibration was done by using freshly prepared standards. Final standard concentrations (100 pg), prepared daily from a stock in 20 mM acetic acid and stored at 4°C until use, were injected into the system. The mobile phase (pH 3.5) consisted of 0.075 M NaH2PO4 and 25 mM EDTA in 0.1 M citrate acetate buffer (83%) and methanol (17%). The flow rate was maintained at 0.5 ml/min throughout chromatographic runs. Chromatographs were quantified by using PowerChrom software version 2.5 (eDAQ Pty. Ltd., Densitone East, Australia).
ELISA.
Levels of plasma cytokines (IFN-γ, IL-1β, IL-6, and TNF-α) were measured by using an enzyme-linked immunosorbent assay (ELISA) kit (BD Biosciences Pharmingen, Piscataway, NJ) according to the manufacturer's recommendations. The amount of cytokine produced was calculated by using a standard cytokine curve performed on each immunoplate. Plasma l-Trp was assayed with a Bridge-It l-Trp fluorescence assay (Mediomics, St. Louis, MO) by using a fluorescence microplate reader (the Trp detection limit was 4.114 μmol at an optical density [OD] value of 0.036, using 485-nm excitation and 665-nm emission). Plasma l-Kyn (Sigma) was assayed by using a competitive ELISA with mouse l-Kyn monoclonal conjugated antibody (Abcam, Tokyo, Japan) (the Kyn detection limit was 35.5 nmol at an OD value of 0.042); readings were plotted against an l-Kyn standard curve.
Kynurenine production assay.
IDO activity is directly correlated with the concentration of Kyn, its stable conversion product from Trp, as described previously (3, 42, 71).
Statistical analysis.
Statistical analyses were performed by using GraphPad Prism 5 software (GraphPad Software, La Jolla, CA). Results are presented as means ± standard deviations. The significance of differences was evaluated by two-way analysis of variance (ANOVA) followed by Tukey's multiple-comparisons procedure or Student's t test. Data for sucrose preference were analyzed by two-way ANOVA followed by Bonferroni's post hoc test. A P value of <0.05 was considered statistically significant.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by the Japan Society for the Promotion of Science's Funding Program for Next-Generation World-Leading Researchers (NEXT Program), initiated by the Council for Science and Technology Policy (2011/LS003). This work was also supported by a grant-in-aid for challenging exploratory research from MEXT KAKENHI (15K15118). Motamed Elsayed Mahmoud was supported by a postdoctoral fellowship from the Egyptian Ministry of Higher Education and Scientific Research.
We declare that there are no conflicts of interest to disclose.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00007-17.
REFERENCES
- 1.Pedersen MG, Mortensen PB, Norgaard-Pedersen B, Postolache TT. 2012. Toxoplasma gondii infection and self-directed violence in mothers. Arch Gen Psychiatry 69:1123–1130. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, Träskman-Bendz L, Janelidze S, Langenberg P, Saleh A, Constantine N, Okusaga O, Bay-Richter C, Brundin L, Postolache TT. 2012. Toxoplasma gondii immunoglobulin G antibodies and nonfatal suicidal self-directed violence. J Clin Psychiatry 73:1069–1076. doi: 10.4088/JCP.11m07532. [DOI] [PubMed] [Google Scholar]
- 3.Mahmoud ME, Ihara F, Fereig RM, Nishimura M, Nishikawa Y. 2016. Induction of depression-related behaviors by reactivation of chronic Toxoplasma gondii infection in mice. Behav Brain Res 298:125–133. doi: 10.1016/j.bbr.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki Y, Orellana MA, Schreiber R, Remington JS. 1988. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science 240:516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- 5.Dantzer R, Kelley KW. 2007. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun 21:153–160. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hauser P, Khosla J, Aurora H, Laurin J, Kling MA, Hill J, Gulati M, Thornton AJ, Schultz RL, Valentine AD, Meyers CA, Howell CD. 2002. A prospective study of the incidence and open-label treatment of interferon-induced major depressive disorder in patients with hepatitis C. Mol Psychiatry 7:942–947. doi: 10.1038/sj.mp.4001119. [DOI] [PubMed] [Google Scholar]
- 7.Raison CL, Miller AH. 2013. The evolutionary significance of depression in pathogen host defense (PATHOS-D). Mol Psychiatry 18:15–37. doi: 10.1038/mp.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Connor JC, André C, Wang Y, Lawson MA, Szegedi SS, Lestage J, Castanon N, Kelley KW, Dantzer R. 2009. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry 14:511–522. doi: 10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moreau M, André C, O'Connor JC, Dumich SA, Woods JA, Kelley KW, Dantzer R, Lestage J, Castanon N. 2008. Inoculation of Bacillus Calmette-Guerin to mice induces an acute episode of sickness behavior followed by chronic depressive-like behavior. Brain Behav Immun 22:1087–1095. doi: 10.1016/j.bbi.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lestage J, Verrier D, Palin K, Dantzer R. 2002. The enzyme indoleamine 2,3-dioxygenase is induced in the mouse brain in response to peripheral administration of lipopolysaccharide and superantigen. Brain Behav Immun 16:596–601. doi: 10.1016/S0889-1591(02)00014-4. [DOI] [PubMed] [Google Scholar]
- 11.Myint AM, Bondy B, Baghai TC, Eser D, Nothdurfter C, Schüle C, Zill P, Müller N, Rupprecht R, Schwarz MJ. 2013. Tryptophan metabolism and immunogenetics in major depression: a role for interferon-γ gene. Brain Behav Immun 31:128–133. doi: 10.1016/j.bbi.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Wichers MC, Koek GH, Robaeys G, Verkerk R, Scharpé S, Maes M. 2005. IDO and interferon-alpha-induced depressive symptoms: a shift in hypothesis from tryptophan depletion to neurotoxicity. Mol Psychiatry 10:538–544. doi: 10.1038/sj.mp.4001600. [DOI] [PubMed] [Google Scholar]
- 13.Russo S, Kema IP, Fokkema MR, Boon JC, Willemse PH, de Vries EG, den Boer JA, Korf J. 2003. Tryptophan as a link between psychopathology and somatic states. Psychosom Med 65:665–671. doi: 10.1097/01.PSY.0000078188.74020.CC. [DOI] [PubMed] [Google Scholar]
- 14.Fukui S, Schwarcz R, Rapoport SI, Takada Y, Smith QR. 1991. Blood-brain barrier transport of kynurenines: implications for brain synthesis and metabolism. J Neurochem 56:2007–2017. doi: 10.1111/j.1471-4159.1991.tb03460.x. [DOI] [PubMed] [Google Scholar]
- 15.Yrjänheikki J, Keinänen R, Pellikka M, Hökfelt T, Koistinaho J. 1998. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc Natl Acad Sci U S A 95:15769–15774. doi: 10.1073/pnas.95.26.15769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yrjänheikki J, Tikka T, Keinänen R, Goldsteins G, Chan PH, Koistinaho JA. 1999. Tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc Natl Acad Sci U S A 96:13496–13500. doi: 10.1073/pnas.96.23.13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. 2008. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rifkin BR, Vernillo A, Golub LM, Ramamurthy N. 1994. Modulation of bone resorption by tetracyclines. Ann N Y Acad Sci 732:165–180. doi: 10.1111/j.1749-6632.1994.tb24733.x. [DOI] [PubMed] [Google Scholar]
- 19.Amin AR, Attur MG, Thakker GD, Patel PD, Vyas PR, Patel RN, Patel IR, Abramson SB. 1996. A novel mechanism of action of tetracyclines: effects on nitric oxide synthases. Proc Natl Acad Sci U S A 93:14014–14019. doi: 10.1073/pnas.93.24.14014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Divanovic S, Sawtell NM, Trompette A, Warning JI, Dias A, Cooper AM, Yap GS, Arditi M, Shimada K, Duhadaway JB, Prendergast GC, Basaraba RJ, Mellor AL, Munn DH, Aliberti J, Karp CL. 2012. Opposing biological function of tryptophan degrading enzyme during intracellular infection. J Infect Dis 205:152–161. doi: 10.1093/infdis/jir621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munn DH, Sharma MD, Lee JR, Jhaver KG, Johnson TS, Keskin DB, Marshall B, Chandler P, Antonia SJ, Burgess R, Slingluff CL Jr, Mellor AL. 2002. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science 297:1867–1870. doi: 10.1126/science.1073514. [DOI] [PubMed] [Google Scholar]
- 22.Sakurai K, Zou JP, Tschetter JR, Ward JM, Shearer GM. 2002. Effect of indoleamine 2,3-dioxygenase on induction of experimental autoimmune encephalomyelitis. J Neuroimmunol 129:186–196. doi: 10.1016/S0165-5728(02)00176-5. [DOI] [PubMed] [Google Scholar]
- 23.Seo SK, Choi JH, Kim YH, Kang WJ, Park HY, Suh JH, Choi BK, Vinay DS, Kwon BS. 2004. 4-1BB-mediated immunotherapy of rheumatoid arthritis. Nat Med 10:1088–1094. doi: 10.1038/nm1107. [DOI] [PubMed] [Google Scholar]
- 24.Murakami Y, Hoshi M, Hara A, Takemura M, Arioka Y, Yamamoto Y, Matsunami H, Funato T, Seithima M, Saito K. 2012. Inhibition of increased indoleamine 2,3 dioxygenase activity attenuates Toxoplasma gondii replication in the lung during acute infection. Cytokine 59:245–251. doi: 10.1016/j.cyto.2012.04.022. [DOI] [PubMed] [Google Scholar]
- 25.Xie W, Cai L, Yu Y, Gao L, Xiao L, He Q, Ren Z, Liu Z. 2014. Activation of brain indoleamine 2,3-dioxygenase contributes to epilepsy-associated depressive-like behavior in rats with chronic temporal lobe epilepsy. J Neuroinflammation 11:41. doi: 10.1186/1742-2094-11-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guillemin GJ, Williams KR, Smith DG, Smythe GA, Croitoru-Lamoury J, Brew BJ. 2003. Quinolinic acid in the pathogenesis of Alzheimer's disease. Adv Exp Med Biol 527:167–176. doi: 10.1007/978-1-4615-0135-0_19. [DOI] [PubMed] [Google Scholar]
- 27.Sanni LA, Thomas SR, Tattam BN, Moore DE, Chaudhri G, Stocker R, Hunt NH. 1998. Dramatic changes in oxidative tryptophan metabolism along the kynurenine pathway in experimental cerebral and noncerebral malaria. Am J Pathol 152:611–619. [PMC free article] [PubMed] [Google Scholar]
- 28.McLeod R, Skamene E, Brown CR, Eisenhauer PB, Mack DG. 1989. Genetic regulation of early survival and cyst number after peroral Toxoplasma gondii infection of A × B/B × A recombinant inbred and B10 congenic mice. J Immunol 143:3031–3034. [PubMed] [Google Scholar]
- 29.Suzuki Y, Joh K, Orellana MA, Conley FK, Remington JS. 1991. A gene(s) within the H-2D region determines the development of toxoplasmic encephalitis in mice. Immunology 74:732–739. [PMC free article] [PubMed] [Google Scholar]
- 30.Brown CR, Hunter CA, Estes RG, Beckmann E, Forman J, David C, Remington JS, McLeod R. 1995. Definitive identification of a gene that confers resistance against Toxoplasma cyst burden and encephalitis. Immunology 85:419–428. [PMC free article] [PubMed] [Google Scholar]
- 31.Suzuki Y, Yang Q, Remington JS. 1995. Genetic resistance against acute toxoplasmosis depends on the strain of T. gondii. J Parasitol 81:1032–1034. doi: 10.2307/3284069. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki Y, Kang H, Parmley S, Lim S, Park D. 2000. Induction of tumor necrosis factor-alpha and inducible nitric oxide synthase fails to prevent toxoplasmic encephalitis in the absence of interferon-gamma in genetically resistant BALB/c mice. Microbes Infect 2:455–462. doi: 10.1016/S1286-4579(00)00318-X. [DOI] [PubMed] [Google Scholar]
- 33.Ball HJ, Yuasa HJ, Austin CJ, Weiser S, Hunt NH. 2009. Indoleamine 2,3-dioxygenase-2; a new enzyme in the kynurenine pathway. Int J Biochem Cell Biol 41:467–471. doi: 10.1016/j.biocel.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 34.Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ. 2012. Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci 13:465–477. doi: 10.1038/nrn3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parrott JM, O'Connor JC. 2015. Kynurenine 3-monooxygenase: an influential mediator of neuropathology. Front Psychiatry 6:116. doi: 10.3389/fpsyt.2015.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noakes R. 2015. The aryl hydrocarbon receptor: a review of its role in the physiology and pathology of the integument and its relationship to the tryptophan metabolism. Int J Tryptophan Res 8:7–18. doi: 10.4137/IJTR.S19985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park SJ, Lee JY, Kim SJ, Choi SY, Yune TY, Ryu JH. 2015. Toll-like receptor-2 deficiency induces schizophrenia-like behaviors in mice. Sci Rep 5:8502. doi: 10.1038/srep08502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khan IA, Matsuura T, Kasper LH. 1994. Interleukin-12 enhances murine survival against acute toxoplasmosis. Infect Immun 62:1639–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hunter CA, Subauste CS, Van Cleave VH, Remington JS. 1994. Production of gamma interferon by natural killer cells from Toxoplasma gondii-infected SCID mice: regulation by interleukin-10, interleukin-12, and tumor necrosis factor alpha. Infect Immun 62:2818–2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adams LB, Hibbs JB Jr, Taintor RR, Krahenbuhl JL. 1990. Microbiostatic effect of murine activated macrophages for Toxoplasma gondii: role for synthesis of inorganic nitrogen oxides from l-arginine. J Immunol 144:2725–2729. [PubMed] [Google Scholar]
- 41.Collazo CM, Yap GS, Sempowski GD, Lusby KC, Tessarollo L, Vande Woude GF, Sher A, Taylor GA. 2001. Inactivation of LRG-47 and IRG-47 reveals a family of interferon gamma-inducible genes with essential, pathogen-specific roles in resistance to infection. J Exp Med 194:181–188. doi: 10.1084/jem.194.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mahmoud ME, Ui F, Salman D, Nishimura M, Nishikawa Y. 2015. Mechanisms of interferon-beta-induced inhibition of Toxoplasma gondii growth in murine macrophages and embryonic fibroblasts: role of immunity-related GTPaseM1. Cell Microbiol 17:1069–1083. doi: 10.1111/cmi.12423. [DOI] [PubMed] [Google Scholar]
- 43.Butcher BA, Greene RI, Henry SC, Annecharico KL, Weinberg JB, Denkers EY, Sher A, Taylor GA. 2005. p47 GTPases regulate Toxoplasma gondii survival in activated macrophages. Infect Immun 73:3278–3286. doi: 10.1128/IAI.73.6.3278-3286.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pfefferkorn ER. 1984. Interferon-γ blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophan. Proc Natl Acad Sci U S A 81:908–912. doi: 10.1073/pnas.81.3.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chao CC, Anderson WR, Hu S, Martella A, Gekker G, Peterson PK. 1993. Activated microglia inhibit Toxoplasma gondii via a nitric oxide mechanism. Clin Immunol Immunopathol 67:178–183. doi: 10.1006/clin.1993.1062. [DOI] [PubMed] [Google Scholar]
- 46.Fujigaki S, Saito K, Takemura M, Maekawa N, Yamada Y, Wada H, Seishima M. 2002. l-Tryptophan-l-kynurenine pathway metabolism accelerated by Toxoplasma gondii infection is abolished in gamma interferon-gene-deficient mice: cross-regulation between inducible nitric oxide synthase and indoleamine-2,3-dioxygenase. Infect Immun 70:779–786. doi: 10.1128/IAI.70.2.779-786.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Silva NM, Rodrigues CV, Santoro MM, Reis LF, Alvarez-Leite JI, Gazzinelli RT. 2002. Expression of indoleamine 2,3-dioxygenase, tryptophan degradation, and kynurenine formation during in vivo infection with Toxoplasma gondii: induction by endogenous gamma interferon and requirement of interferon regulatory factor 1. Infect Immun 70:859–868. doi: 10.1128/IAI.70.2.859-868.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walker AK, Budac DP, Bisulco S, Lee AW, Smith RA, Beenders B, Kelley KW, Dantzer R. 2013. NMDA receptor blockade by ketamine abrogates lipopolysaccharide-induced depressive-like behavior in C57BL/6J mice. Neuropsychopharmacology 38:1609–1616. doi: 10.1038/npp.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu S, Stavrovskaya IG, Drozda M, Kim BY, Ona V, Li M, Sarang S, Liu AS, Hartley DM, Wu DC, Gullans S, Ferrante RJ, Przedborski S, Kristal BS, Friedlander RM. 2002. Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in mice. Nature 417:74–78. doi: 10.1038/417074a. [DOI] [PubMed] [Google Scholar]
- 50.Chen M, Ona VO, Li M, Ferrante RJ, Fink KB, Zhu S, Bian J, Guo L, Farrell LA, Hersch SM, Hobbs W, Vonsattel JP, Cha JH, Friedlander RM. 2000. Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nat Med 6:797–801. doi: 10.1038/77528. [DOI] [PubMed] [Google Scholar]
- 51.Giuliani F, Hader W, Yong VW. 2005. Minocycline attenuates T cell and microglia activity to impair cytokine production in T cell-microglia interaction. J Leukoc Biol 78:135–143. doi: 10.1189/jlb.0804477. [DOI] [PubMed] [Google Scholar]
- 52.Brundula V, Rewcastle NB, Metz LM, Bernard CC, Yong VW. 2002. Targeting leukocyte MMPs and transmigration: minocycline as a potential therapy for multiple sclerosis. Brain 125:1297–1308. doi: 10.1093/brain/awf133. [DOI] [PubMed] [Google Scholar]
- 53.Du Y, Ma Z, Lin S, Dodel RC, Gao F, Bales KR, Triarhou LC, Chernet E, Perry KW, Nelson DL, Luecke S, Phebus LA, Bymaster FP, Paul SM. 2001. Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson's disease. Proc Natl Acad Sci U S A 98:14669–14674. doi: 10.1073/pnas.251341998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zabad RK, Metz LM, Todoruk TR, Zhang Y, Mitchell JR, Yeung M, Patry DG, Bell RB, Yong VW. 2007. The clinical response to minocycline in multiple sclerosis is accompanied by beneficial immune changes: a pilot study. Mult Scler 13:517–526. doi: 10.1177/1352458506070319. [DOI] [PubMed] [Google Scholar]
- 55.Zhang YH, Chen H, Chen Y, Wang L, Cai YH, Li M, Wen HQ, Du J, An R, Luo QL, Wang XL, Lun ZR, Xu YH, Shen JL. 2014. Activated microglia contribute to neuronal apoptosis in toxoplasmic encephalitis. Parasit Vectors 7:372. doi: 10.1186/1756-3305-7-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carruthers VB, Suzuki Y. 2007. Effects of Toxoplasma gondii infection on the brain. Schizophr Bull 33:745–751. doi: 10.1093/schbul/sbm008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Henry CJ, Huang Y, Wynne A, Hanke M, Himler J, Bailey MT, Sheridan JF, Godbout JP. 2008. Minocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia. J Neuroinflammation 5:15. doi: 10.1186/1742-2094-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Frenois F, Moreau M, O'Connor J, Lawson M, Micon C, Lestage J, Kelley KW, Dantzer R, Castanon N. 2007. Lipopolysaccharide induces delayed FosB/DeltaFosB immunostaining within the mouse extended amygdala, hippocampus and hypothalamus, that parallel the expression of depressive-like behavior. Psychoneuroendocrinology 32:516–531. doi: 10.1016/j.psyneuen.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ueno A, Cho S, Cheng L, Wang J, Hou S, Nakano H, Nakano H, Santamaria P, Yang Y. 2007. Transient upregulation of indoleamine 2,3-dioxygenase in dendritic cells by human chorionic gonadotropin downregulates autoimmune diabetes. Diabetes 56:1686–1693. doi: 10.2337/db06-1727. [DOI] [PubMed] [Google Scholar]
- 60.Uyttenhove C, Pilotte L, Théate I, Stroobant V, Colau D, Parmentier N, Boon T, Van den Eynde BJ. 2003. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med 9:1269–1274. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 61.Muller AJ, DuHadaway JB, Donover PS, Sutanto-Ward E, Prendergast GC. 2005. Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat Med 11:312–319. doi: 10.1038/nm1196. [DOI] [PubMed] [Google Scholar]
- 62.Kwidzinski E, Bunse J, Aktas O, Richter D, Mutlu L, Zipp F, Nitsch R, Bechmann I. 2005. Indoleamine 2,3 dioxygenase is expressed in the CNS and down-regulates autoimmune inflammation. FASEB J 19:1347–1349. [DOI] [PubMed] [Google Scholar]
- 63.Tanaka S, Nishimura M, Ihara F, Yamagishi J, Suzuki Y, Nishikawa Y. 2013. Transcriptome analysis of mouse brain infected with Toxoplasma gondii. Infect Immun 81:3609–3619. doi: 10.1128/IAI.00439-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Takenouchi T, Ogihara K, Sato M, Kitani H. 2005. Inhibitory effects of U73122 and U73343 on Ca2+ influx and pore formation induced by the activation of P2X7 nucleotide receptors in mouse microglial cell line. Biochim Biophys Acta 1726:177–186. doi: 10.1016/j.bbagen.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 65.Nakamichi K, Saiki M, Kitani H, Kuboyama Y, Morimoto K, Takayama-Ito M, Kurane I. 2006. Suppressive effect of simvastatin on interferon-beta-induced expression of CC chemokine ligand 5 in microglia. Neurosci Lett 407:205–210. doi: 10.1016/j.neulet.2006.08.044. [DOI] [PubMed] [Google Scholar]
- 66.Ohtani K, Suzumura A, Sawada M, Marunouchi T, Nakashima I, Takahashi A. 1992. Establishment of mouse oligodendrocyte/type-2 astrocyte lineage cell line by transfection with origin-defective simian virus 40 DNA. Cell Struct Funct 17:325–333. doi: 10.1247/csf.17.325. [DOI] [PubMed] [Google Scholar]
- 67.Strekalova T, Spanagel R, Bartsch D, Henn FA, Gass P. 2004. Stress-induced anhedonia in mice is associated with deficits in forced swimming and exploration. Neuropsychopharmacology 29:2007–2017. doi: 10.1038/sj.npp.1300532. [DOI] [PubMed] [Google Scholar]
- 68.Lucki I. 1997. The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behav Pharmacol 8:523–532. doi: 10.1097/00008877-199711000-00010. [DOI] [PubMed] [Google Scholar]
- 69.Carter RJ, Lione LA, Humby T, Mangiarini L, Mahal A, Bates GP, Dunnett SB, Morton AJ. 1999. Characterization of progressive motor deficits in mice transgenic for the human Huntington's disease mutation. J Neurosci 19:3248–3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Contini C, Seraceni S, Cultrera R, Incorvaia C, Sebastiani A, Picot S. 2005. Evaluation of a real-time PCR-based assay using the light-cycler system for detection of Toxoplasma gondii bradyzoite genes in blood specimens from patients with toxoplasmic retinochoroiditis. Int J Parasitol 35:275–283. doi: 10.1016/j.ijpara.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 71.Däubener W, Wanagat N, Pilz K, Seghrouchni S, Fischer HG, Hadding U. 1994. A new, simple, bioassay for human IFN-gamma. J Immunol Methods 168:39–47. doi: 10.1016/0022-1759(94)90207-0. [DOI] [PubMed] [Google Scholar]
- 72.Levkovitz Y, Mendlovich S, Riwkes S, Braw Y, Levkovitch-Verbin H, Gal G, Fennig S, Treves I, Kron S. 2010. A double-blind, randomized study of minocycline for the treatment of negative and cognitive symptoms in early-phase schizophrenia. J Clin Psychiatry 71:138–149. doi: 10.4088/JCP.08m04666yel. [DOI] [PubMed] [Google Scholar]
- 73.Science Council of Japan. 2006. Guidelines for proper conduct of animal experiments. Science Council of Japan, Tokyo, Japan: http://www.scj.go.jp/ja/info/kohyo/pdf/kohyo-20-k16-2e.pdf. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.