ABSTRACT
Helminth infections are known to modulate cytokine responses in latent tuberculosis (LTB). However, very few studies have examined whether this modulation is reversible upon anthelmintic therapy. We measured the systemic and mycobacterial (TB) antigen-stimulated levels of type 1, type 2, type 17, and regulatory cytokines in individuals with LTB and with or without coexistent Strongyloides stercoralis infection before and after anthelmintic therapy. Our data reveal that individuals with LTB and coexistent S. stercoralis infection have significantly lower levels of systemic and TB antigen-stimulated type 1 (gamma interferon [IFN-γ], tumor necrosis factor alpha [TNF-α], and interleukin-2 [IL-2]) and type 17 (IL-17A and/or IL-17F) cytokines and significantly higher levels of systemic but not TB antigen-stimulated type 2 (IL-4 and IL-5) and regulatory (transforming growth factor beta [TGF-β]) cytokines. Anthelmintic therapy resulted in significantly increased systemic levels of type 1 and/or type 17 cytokines and in significantly decreased systemic levels of type 2 and regulatory (IL-10 and TGF-β) cytokines. In addition, anthelmintic therapy resulted in significantly increased TB antigen-stimulated levels of type 1 cytokines only. Our data therefore confirm that the modulation of systemic and TB antigen-stimulated cytokine responses in S. stercoralis-LTB coinfection is reversible (for the most part) by anthelmintic treatment.
KEYWORDS: tuberculosis, helminths, cytokines, immune responses, Strongyloides
INTRODUCTION
Helminth infections and tuberculosis (TB) are both common in low- and middle-income countries and share a great degree of geographical overlap (1). In addition, both helminth infection and TB are major health care problems worldwide and the cause of rising morbidity and mortality. Helminth infections are inducers both of type 2 immune responses, typically characterized by the induction of interleukin-4 (IL-4), IL-5, and IL-13 responses, and of regulatory responses characterized by the production of IL-10 and TGF-β (2). In contrast, immunity to TB is typically characterized by the presence of type 1-associated immune responses, with the cytokines gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α), and IL-2 all playing important roles (3). Moreover, type 17-associated cytokines (IL-17A, IL-17F, and IL-22) are also thought to play an accessory role in TB infection (4).
Clinically, TB is associated with a spectrum of clinical presentation ranging from asymptomatic, latent infection to active pulmonary or extrapulmonary disease (3). After initial infection, most individuals control bacterial replication and enter a period of infectious latency known as latent tuberculosis (LTB). Approximately 5 to 10% of those with LTB progress to active tuberculosis in their lifetime, a progression reflecting the failure of host immune responses in containing bacterial replication (3). Among the helminth parasites, Strongyloides stercoralis, the causative agent of strongyloidiasis, infects approximately 50 to 100 million people worldwide (5, 6). S. stercoralis infection is often clinically asymptomatic and longstanding due, in large part, to the parasites' autoinfective life cycle and their ability to modulate or evade the host immune system (2, 7). Recent epidemiological and experimental data have provided evidence that helminths (both systemic and intestinal) have a negative regulatory role in the immune response to TB infection and disease, although no evidence exists for their having a significant impact on clinical outcomes of TB (1, 8, 9).
Helminth infections are known to induce modulation of T cell-mediated immune responses to TB antigens in both LTB and active TB, a modulation at least partially dependent on the regulatory cytokines, IL-10 and TGF-β, and the coinhibitory molecules, cytotoxic-T-lymphocyte-associated antigen 4 (CTLA-4) and programmed cell death 1 (PD-1) (9). This modulation is known to extend to influencing the systemic cytokine responses as well (10). This has been demonstrated for filarial infections, hookworm infections, schistosomes, and S. stercoralis infection (11–14). However, very few studies have examined the effect of anthelmintic treatment on the cytokine profiles of helminth-TB coinfection (14). We therefore examined the systemic and TB antigen-stimulated levels of type 1, type 2, type 17, and regulatory cytokines in LTB individuals with or without S. stercoralis coinfection and in the coinfected individual before and after treatment. We found that anthelmintic therapy has a major impact on systemic and TB antigen-driven cytokine responses in the context of coinfection and that the results highlight the reversibility of helminth-modulated antigen-specific cytokine responses in LTB.
RESULTS
Study population characteristics.
The baseline hematological characteristics of the study population are shown in Table 1. As can be seen, individuals coinfected with LTB and S. stercoralis (LTB-S. stercoralis individuals) exhibited significantly higher levels of hemoglobin and erythrocyte and eosinophil counts and significantly lower levels of lymphocyte counts than LTB individuals. No significant differences in the other hematological parameters were observed.
TABLE 1.
Hematological parameters of the study populationa
| Parameter | Value(s) |
||
|---|---|---|---|
| LTB-S. stercoralis (n = 60) | LTB (n = 58) | P | |
| Hb gm/dl GM (range) | 12.48 (4.9–18.6) | 11.14 (4.9–16.3) | 0.0378 |
| RBC 106/ml GM (range) | 4.5 (3.5–6.06) | 4.067 (2.11–5.84) | 0.0391 |
| WBC 103/ml GM (range) | 8.83 (5.8–16.9) | 8.81 (5.8–13.7) | NS |
| HCT % GM (range) | 36.85 (19.5–53) | 33.55 (15–47.4) | NS |
| PLT 103/ml GM (range) | 261.91 (140–417) | 258.18 (198–363) | NS |
| Neutrophil 103/ml GM (range) | 5.3 (3.3–7.2) | 5.5 (4.2–6.89) | NS |
| Lymphocyte 103/ml GM (range) | 2.63 (1.45–3.71) | 3.04 (2.02–4.27) | 0.0248 |
| Monocyte 103/ml GM (range) | 0.67 (0.38–1.2) | 0.71 (0.43–1.09) | NS |
| Eosinophil 103/ml GM (range) | 0.68 (0.11–3.45) | 0.43 (0.18–1.1) | 0.0354 |
| Basophil 103/ml GM (range) | 0.09 (0.02–0.33) | 0.08 (0.05–0.35) | NS |
Values represent geometric means with 95% confidence intervals, and P values were calculated using the Mann-Whitney U test. Boldface characters represent statistically significant differences. Hb, hemoglobin; RBC, red blood cell; WBC, white blood cell; HCT, hematocrit test; PLT, platelet count; NS, not significant.
Coexistent S. stercoralis infection is associated with diminished systemic levels of type 1 and type 17 cytokines and elevated systemic levels of type 2 and regulatory cytokines in LTB.
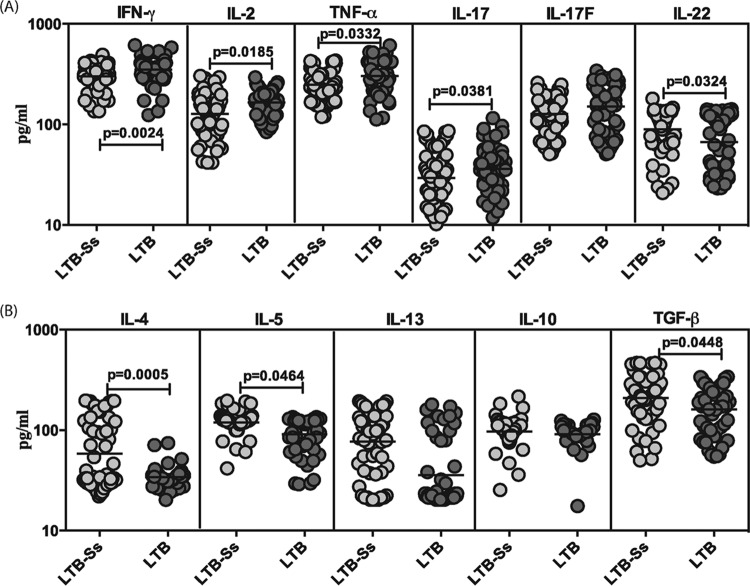
To determine the influence of coexistent S. stercoralis infection on type 1, type 17, and type 2 cytokines and regulatory cytokines in LTB, we measured the serum levels of IFN-γ, TNF-α, IL-2, IL-17A, IL-17F, IL-22, IL-4, IL-5, IL-13, IL-10, and transforming growth factor beta (TGF-β) in LTB-S. stercoralis and LTB individuals (Fig. 1). As shown in Fig. 1A, the systemic levels of IFN-γ (geometric mean [GM] of 300 pg/ml in LTB-S. stercoralis versus 351 pg/ml in LTB), TNF-α (GM of 249 pg/ml versus 302 pg/ml), and IL-2 (GM of 127 pg/ml versus 165 pg/ml) were significantly lower in LTB-S. stercoralis individuals than in LTB individuals. Similarly, the systemic levels of the prototypical type 17 cytokine—IL-17A (GM of 29 pg/ml versus 53 pg/ml)—were also significantly lower in LTB-S. stercoralis individuals than in LTB individuals. In contrast, the systemic levels of IL-22 were significantly higher in LTB individuals than in LTB-S. stercoralis individuals (GM of 90 pg/ml versus 67 pg/ml). Also, as shown in Fig. 1B, the systemic levels of type 2 cytokines—IL-4 (GM of 58 pg/ml in LTB-S. stercoralis individuals versus 34 pg/ml in LTB individuals) and IL-5 (GM of 120 pg/ml versus 91 pg/ml)—and the regulatory cytokine—TGF-β (GM of 209 pg/ml versus 160 pg/ml)—were significantly higher in LTB-S. stercoralis individuals than in LTB individuals. Thus, coexistent S. stercoralis infection is associated with diminished systemic levels of type 1 and type 17 cytokines and increased levels of type 2 and regulatory cytokines in LTB.
FIG 1.
S. stercoralis infection is associated with diminished systemic levels of type 1 and type 17 cytokines and elevated levels of type 2 cytokines and TGF-β in LTB. (A) The systemic (plasma) levels of type 1 (IFN-γ, TNF-α, IL-2) and type 17 (IL-17A, IL-17F, IL-22) cytokines were measured in LTB individuals with S. stercoralis coinfection (LTB-Ss, n = 60) or without S. stercoralis coinfection (LTB, n = 58). (B) The systemic (plasma) levels of type 2 (IL-4, IL-5, IL-13) and regulatory (IL-10, TGF-β) cytokines were measured in LTB individuals with S. stercoralis coinfection (LTB-Ss, n = 60) or without S. stercoralis coinfection (LTB, n = 58). The results are shown as scatterplots, with each circle representing a single individual and the bar representing the GM. P values were calculated using the Mann-Whitney test with Holm's correction for multiple comparisons.
Treatment modifies the systemic cytokine profile of LTB-S. stercoralis-coinfected individuals.
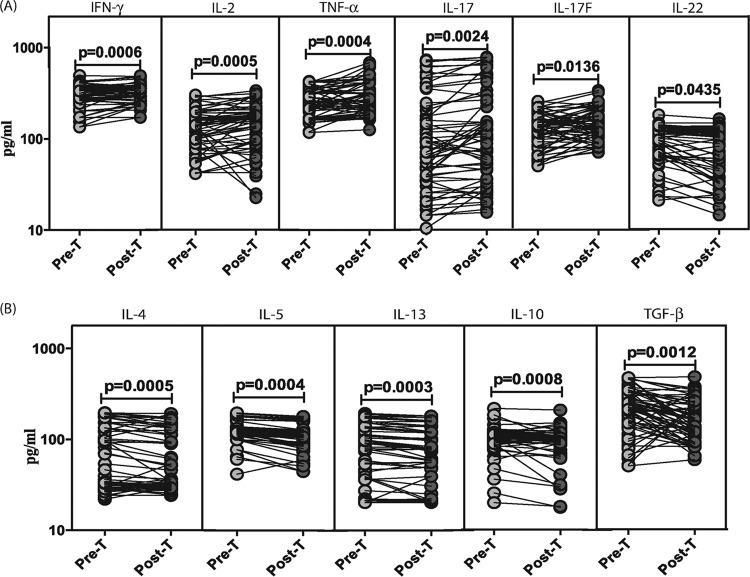
To determine the effect of anthelmintic therapy of LTB-S. stercoralis-coinfected individuals on type 1, type 17, and type 2 cytokines and regulatory cytokines, we measured the circulating levels of IFN-γ, TNF-α, IL-2, IL-17A, IL-17F, IL-22, IL-4, IL-5, IL-13, IL-10, and TGF-β in LTB-S. stercoralis individuals before (pre-T) and 6 months after (post-T) administration of treatment (Fig. 2). As shown in Fig. 2A, the systemic levels of IFN-γ (mean fold change of 1.2 at post-T compared to pre-T values), TNF-α (mean fold change of 1.2), and IL-2 (mean fold change of 1.2) were significantly increased in LTB-S. stercoralis individuals following treatment. Similarly, the systemic levels of type 17 cytokines—IL-17A (mean fold change of 1.2) and IL-17F (mean fold change of 1.2)—were also significantly increased following treatment. In contrast, the systemic levels of IL-22 (mean fold change of 0.8) were significantly decreased following treatment. In addition, as shown in Fig. 2B, the systemic levels of type 2 cytokines—IL-4 (mean fold change of 0.9), IL-5 (mean fold change of 0.9), and IL-13 (mean fold change of 0.9)—and the regulatory cytokines—IL-10 (mean fold change of 0.9) and TGF-β (mean fold change of 0.7)—were all significantly decreased in LTB-S. stercoralis individuals following treatment. Thus, treatment of coexistent S. stercoralis infection is associated with modulation of the systemic cytokine profile in LTB-S. stercoralis coinfection.
FIG 2.
Treatment modifies the systemic cytokine profile in LTB-S. stercoralis coinfection. (A) The systemic (plasma) levels of type 1 (IFN-γ, TNF-α, IL-2) and type 17 (IL-17A, IL-17F, IL-22) cytokines were measured in LTB-S. stercoralis individuals (n = 60) before (Pre-T) and 6 months after (Post-T) anthelmintic therapy. (B) The systemic (plasma) levels of type 2 (IL-4, IL-5, IL-13) and regulatory (IL-10, TGF-β) cytokines were measured in LTB-S. stercoralis individuals (n = 60) before (Pre-T) and 6 months after (Post-T) anthelmintic therapy. The results are shown as line diagrams, with each line representing a single individual. P values were calculated using the Wilcoxon signed-rank test with Holm's correction for multiple comparisons.
Coexistent S. stercoralis infection is associated with diminished baseline and/or TB antigen-stimulated levels of type 1 and type 17 cytokines in LTB.
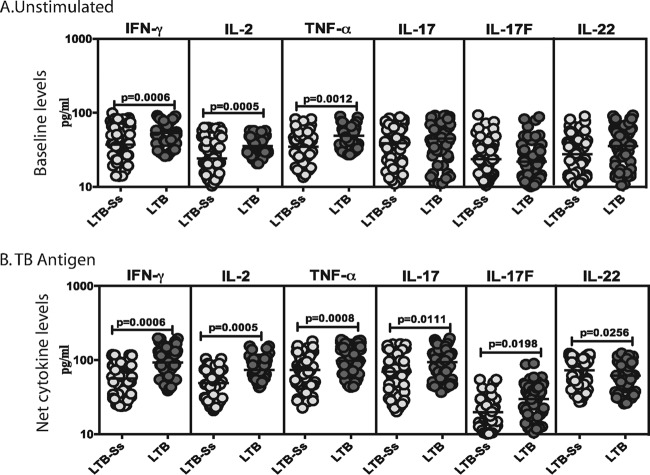
To determine the influence of coexistent S. stercoralis infection on baseline or TB antigen- or mitogen-stimulated type 1 and type 17 cytokine responses in LTB, we measured the levels of IFN-γ, TNF-α, IL-2, IL-17A, IL-17F, and IL-22 in whole-blood culture supernatants (Quantiferon supernatants) following incubation with no antigen, TB antigen, or mitogen in LTB-S. stercoralis and LTB individuals (Fig. 3). As shown in Fig. 3A, the baseline (antigen-unstimulated) levels of IFN-γ (GM of 36 pg/ml in LTB-S. stercoralis individuals versus 53 pg/ml in LTB individuals), TNF-α (GM of 24 pg/ml versus 55 pg/ml), and IL-2 (GM of 34 pg/ml versus 58 pg/ml) were significantly lower in LTB-S. stercoralis individuals than in LTB individuals. Similarly, as shown in Fig. 3B, the TB antigen-stimulated levels of IFN-γ (GM of 56 pg/ml in LTB-S. stercoralis individuals versus 92 pg/ml in LTB individuals), TNF-α (GM of 73 pg/ml versus 98 pg/ml), IL-2 (GM of 48 pg/ml versus 73 pg/ml), IL-17A (GM of 69 pg/ml versus 93 pg/ml), and IL-17F (GM of 19 pg/ml versus 39 pg/ml) were also significantly lower in LTB-S. stercoralis individuals than in LTB individuals. In contrast, TB antigen-stimulated levels of IL-22 were significantly higher in LTB individuals than in LTB-S. stercoralis individuals (GM of 72 pg/ml versus 51 pg/ml). In addition, as shown in Fig. S1, TB antigen stimulation resulted in significantly increased levels of type 1 and type 17 cytokines in both LTB-S. stercoralis individuals and LTB individuals. Thus, coexistent S. stercoralis infection is associated with downmodulation of both baseline and TB antigen-driven type 1 and/or type 17 cytokine responses in LTB.
FIG 3.
S. stercoralis infection is associated with diminished baseline and/or mycobacterial antigen-stimulated production of type 1 and type 17 cytokines in LTB. The baseline (A) or mycobacterial antigen-stimulated (B) levels (at 18 h) of type 1 (IFN-γ, TNF-α, IL-2) and type 17 (IL-17A, IL-17F, IL-22) cytokines were measured in LTB individuals with S. stercoralis coinfection (LTB-Ss, n = 60) or without S. stercoralis coinfection (LTB, n = 58). The results are shown as scatterplots, with each circle representing a single individual and the bar representing the GM. P values were calculated using the Mann-Whitney test with Holm's correction for multiple comparisons.
Treatment modifies the baseline and TB antigen-stimulated type 1 cytokine responses of LTB-S. stercoralis-coinfected individuals.
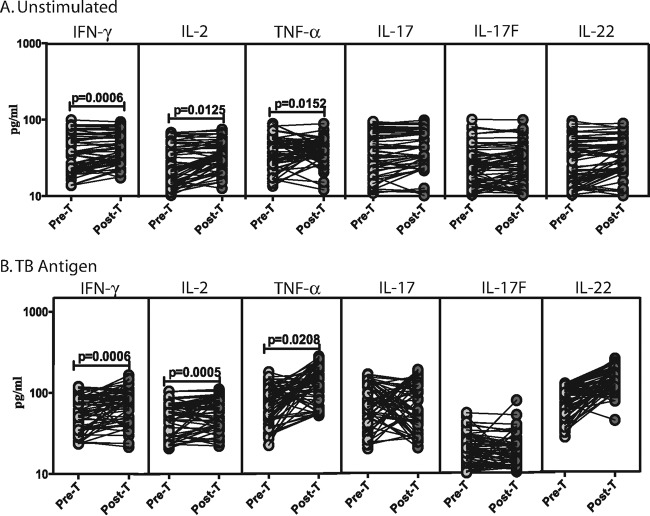
To determine the effect of anthelmintic therapy of LTB-S. stercoralis-coinfected individuals on type 1 and type 17 cytokine responses, we measured the circulating levels of IFN-γ, TNF-α, IL-2, IL-17A, IL-17F, and IL-22 in LTB-S. stercoralis individuals before (pre-T) and 6 months after (post-T) administration of treatment (Fig. 4). As shown in Fig. 4A, the baseline levels of IFN-γ (mean fold change of 1.2 at post-T compared to pre-T values), TNF-α (mean fold change of 1.2), and IL-2 (mean fold change of 1.2) were significantly increased in LTB-S. stercoralis individuals following treatment. However, there were no significant differences in the baseline levels of type 17 cytokines after treatment. Similarly, as shown in Fig. 4B, the TB antigen-stimulated levels of IFN-γ (mean fold change of 1.2), TNF-α (mean fold change of 1.7), and IL-2 (mean fold change of 1.2) were significantly increased in LTB-S. stercoralis individuals following treatment. In contrast, no significant differences were observed in TB antigen-stimulated levels of type 17 cytokines after treatment. However, even at posttreatment time points, per-individual analysis revealed that TB antigen-induced type 1 or type 17 cytokine production was significantly increased in LTB-S. stercoralis individuals (see Fig. S1C in the supplemental material). Thus, treatment modified the baseline and TB-driven type 1 cytokine responses in LTB-S. stercoralis coinfection.
FIG 4.
Treatment modifies the baseline and mycobacterial antigen-stimulated cytokine profile in LTB-S. stercoralis coinfection. The baseline (A) or mycobacterial antigen-stimulated (B) levels (at 18 h) of type 1 (IFN-γ, TNF-α, IL-2) and type 17 (IL-17A, IL-17F, IL-22) cytokines were measured in LTB-S. stercoralis individuals (n = 60) before (Pre-T) and 6 months after (Post-T) anthelmintic therapy. The results are shown as line diagrams, with each line representing a single individual. P values were calculated using the Wilcoxon signed-rank test with Holm's correction for multiple comparisons.
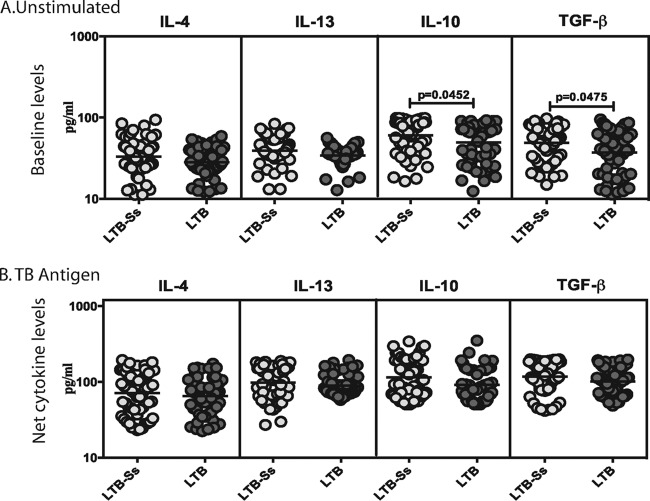
Coexistent S. stercoralis infection is associated with diminished baseline but not TB antigen-stimulated levels of regulatory cytokines in LTB.
To determine the influence of coexistent S. stercoralis infection on baseline or TB antigen- or mitogen-stimulated type 2 and regulatory cytokine responses in LTB, we measured the levels of IL-4, IL-5, IL-13, IL-10, and TGF-β in whole-blood culture supernatants following incubation with no antigen, TB antigen, or mitogen in LTB-S. stercoralis individuals and LTB individuals (Fig. 5). As shown in Fig. 5A, the baseline (or no antigen-stimulated) levels of IL-10 (GM of 60.6 pg/ml in LTB-S. stercoralis individuals versus 50.9 pg/ml in LTB individuals) and TGF-β (GM of 49 pg/ml versus 27 pg/ml) but not IL-4, IL-5, or IL-13 were significantly higher in LTB-S. stercoralis individuals than in LTB individuals. In contrast, as shown in Fig. 5B, no significant differences were observed in the TB antigen-stimulated levels of type 2 or regulatory cytokines between LTB-S. stercoralis individuals and LTB individuals. In addition, as shown in Fig. S1, TB antigen stimulation resulted in significantly increased levels of type 2 and regulatory cytokines in both LTB-S. stercoralis individuals and LTB individuals. Thus, coexistent S. stercoralis infection is associated with upregulation of baseline regulatory cytokine responses in LTB.
FIG 5.
S. stercoralis infection is associated with diminished baseline but not mycobacterial antigen-stimulated production of regulatory cytokines in LTB. The baseline (A) or mycobacterial antigen-stimulated (B) levels (18 h) of type 2 (IL-4, IL-5, IL-13) and regulatory (IL-10, TGF-β) cytokines were measured in LTB individuals with S. stercoralis coinfection (LTB-Ss, n = 60) or without S. stercoralis coinfection (LTB, n = 58). The results are shown as scatterplots, with each circle representing a single individual and the bar representing the GM. P values were calculated using the Mann-Whitney test with Holm's correction for multiple comparisons.
Treatment does not modify the baseline or TB antigen-stimulated type 2 or regulatory cytokine response of LTB-S. stercoralis-coinfected individuals.
To determine the effect of anthelmintic therapy of LTB-S. stercoralis-coinfected individuals on type 2 and regulatory cytokine responses, we measured the circulating levels of IL-4, IL-5, IL-13, IL-10, and TGF-β in LTB-S. stercoralis individuals before (pre-T) and 6 months after (post-T) administration of treatment (Fig. 6). As shown in Fig. 6, no significant increase or decrease in the baseline (Fig. 6A) or TB antigen-stimulated (Fig. 6B) levels of type 2 and regulatory cytokines were observed after treatment compared to pretreatment levels. Thus, treatment does not modify the baseline or TB antigen-stimulated type 2 or regulatory cytokine profile in LTB-S. stercoralis individuals. However, even at posttreatment time points, per-individual analysis revealed that TB antigen-induced type 2 or regulatory cytokine production was significantly increased in LTB-S. stercoralis individuals (Fig. S1C).
FIG 6.
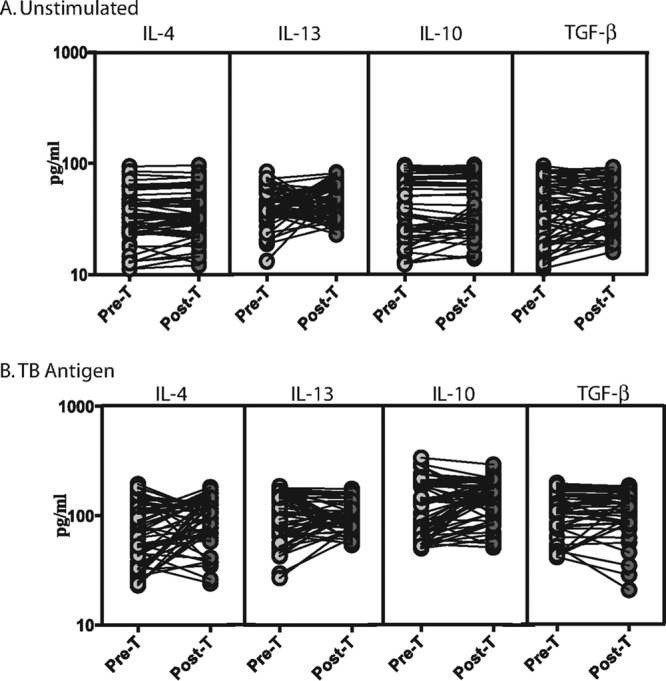
Treatment does not modify the baseline or mycobacterial antigen-stimulated cytokine profile in LTB-S. stercoralis coinfection. The baseline (A) or mycobacterial antigen-stimulated (B) levels (at 18 h) of type 2 (IL-4, IL-5, IL-13) and type 17 (IL-10, TGF-β) cytokines were measured in LTB-S. stercoralis individuals (n = 60) before (Pre-T) and 6 months after (Post-T) anthelmintic therapy. The results are shown as line diagrams, with each line representing a single individual. P values were calculated using the Wilcoxon signed-rank test with Holm's correction for multiple comparisons.
Coexistent S. stercoralis infection is not associated with any significant changes in mitogen-stimulated levels of type 1, type 17, type 2, or regulatory cytokines in LTB.
To determine the influence of coexistent S. stercoralis infection on mitogen-stimulated type 1, type 17, type 2, and regulatory cytokine responses in LTB, we measured the levels of IFN-γ, TNF-α, IL-2, IL-17A, IL-17F, IL-22, IL-4, IL-5, IL-13, IL-10, and TGF-β in whole-blood culture supernatants following incubation with mitogen in LTB-S. stercoralis individuals and LTB individuals (Fig. S2). As shown in Fig. S2A, no significant differences between LTB-S. stercoralis individuals and LTB individuals were observed in the mitogen-stimulated levels of type 1 or type 17 cytokines. Similarly, as shown in Fig. S2B, no significant differences between LTB-S. stercoralis individuals and LTB individuals were observed in the mitogen-stimulated levels of type 2 or regulatory cytokines. Thus, mitogen stimulation does not induce a differential cytokine response between LTB-S. stercoralis individuals and LTB individuals.
DISCUSSION
Helminth infections are powerful modulators of the immune response and typically elicit both type 2 and regulatory cytokine responses (2, 15). Due to their ability to manipulate the host immune system and their propensity to establish long-standing infections, helminths characteristically induce bystander or spillover suppression of immune responses to third-party antigens (1). In addition, helminths harbor and secrete a variety of immunomodulatory molecules that are known to dampen the inflammatory response in a variety of settings (16). Thus, numerous studies in humans as well as in animal models clearly suggest that helminth infections or their products can engender protection from a variety of inflammatory diseases such as allergic disease, autoimmune disease, and inflammatory bowel disease (17, 18). In addition, helminths are now known to exert major effects on host metabolic processes both by direct effects and indirectly through interactions with the bacterial microbiota (19).
We and others have previously shown that different helminth infections can influence the innate and adaptive immune response to mycobacterial antigens in latent TB (20). Thus, filarial infections are known to modulate antigen-specific type 1 and type 17 cytokine responses in latent TB and also to alter the expression and function of Toll-like receptors in this coinfection (11, 21). Similarly, hookworm infections are known to modulate Th1 and Th17 responses in an antigen-specific manner in latent TB (13). Both S. stercoralis and schistosomes are known to enhance regulatory T cell responses, which in turn downmodulate Th1 responses in latent TB (14). Finally, S. stercoralis infection is also known to influence the systemic responses of cytokines, including type 1, type 2, and type 17 cytokines, and other proinflammatory responses in coinfected individuals (10). The mechanism by which helminths drive this modulation is still not clearly understood, although the induction of alternatively activated macrophages and arginase-1 appears to play an important role (22, 23).
The results of our study expand on the findings described above and confirm the effect of S. stercoralis infection on systemic cytokine responses in latent TB. Thus, in the presence of S. stercoralis infection, type 1 and type 17 responses are downmodulated whereas type 2 and regulatory cytokine responses are upregulated. This is further confirmed by our data on whole-blood culture supernatants. Our study data add to the previous literature in also demonstrating an important effect of TB antigen stimulation on cytokine responses in latent TB. First, activities of TB antigen-specific type 1 cytokines normally associated with protective immunity in LTB (3), including IFN-γ, TNF-α, and IL-2, are clearly shown to be downmodulated with S. stercoralis coinfection. This is the most direct associative evidence that coexistent helminth infection can indeed alter the protective immune response in TB infection. Second, activities of TB antigen-specific type 17 cytokines, especially IL-17A, were also downmodulated in S. stercoralis infection. Among the type 17 cytokines, only IL-17A has been shown to clearly exert protective immunity to TB infection in mice (24). The role of IL-17F and IL-22 in TB infection remains poorly explored. Our data would therefore suggest that, while the prototypical Th17 cytokine (IL-17A) follows the same pattern as type 1 cytokines, IL-22 and, to a lesser extent, IL-17F do not. Third, our data reveal that, while baseline or systemic levels of type 2 or regulatory cytokines are also altered in latent TB, TB antigen-specific cytokine responses are not. This implies that alterations in TB antigen-driven type 2 or regulatory cytokines are not major hallmarks of LTB-S. stercoralis coinfections. They also highlight a potential mechanism by which helminth coinfections could deleteriously alter the course of latent infection, i.e., by compromising the ability to contain infection by direct inhibition of protective type 1/type 17 responses. The baseline elevation of type 2 and/or regulatory cytokines could also potentially influence the pathogenesis of TB by other mechanisms, including induction of alternative activation of macrophages, abrogation of host protective autophagy, and other intracellular protective responses (25).
Very few clinical studies have examined the effect of treatment on the immune response in helminth-TB coinfection. Previous studies examining the effect of anthelmintic treatment on clinical outcomes (8, 26) as well as on Mycobacterium bovis BCG vaccination (27, 28) have yielded conflicting results. However, examination of T cell responses in helminth-infected individuals clearly showed diminished T cell proliferation and IFN-γ production in response to purified protein derivative (PPD) and partial reversal of the diminished levels following albendazole therapy, although the LTB status of the population was not ascertained (29). We have previously shown that downmodulation of TLR expression and function was partially reversible by anti-filarial treatment in filarial-LTB coinfection (21). More recently, anthelmintic treatment was shown to significantly improve the Th1 response and downregulate the regulatory T cell response in helminth-LTB coinfection (14). Our study data thus add to the literature on the modulation of immune response following treatment of helminth infection in the setting of coinfections. Our data extend to S. stercoralis a major effect of anthelmintic treatment on the systemic cytokine response prevalent in the setting of LTB-S. stercoralis coinfection. Thus, while type 1 and type 17 responses are significantly upregulated, type 2 and regulatory cytokines are significantly downregulated, and data from this study provide direct evidence for a role of helminth infection in actively modulating the cytokine milieu in the context of latent TB. The data also suggest that intestinal helminth infections such as those by S. stercoralis parasites could exert profound systemic effects, possibly due to their migratory capacity or due to translocation of parasite products. In addition, our data on treatment-induced TB antigen-specific responses suggest that the downmodulation of antigen-specific immune responses (especially those of type 1 and type 17 cytokines) engendered by helminth parasites is also reversible for the most part. While the importance of this reversal in the continued maintenance of protective immunity for the development of active TB is still unclear, the data might imply that anthelmintic treatment preceding vaccine efficacy studies in areas of helminth-TB coendemicity would offer great benefits. This is because almost all vaccine studies of novel TB vaccine candidates have relied on the examination of type 1 and type 17 cytokine responses as correlates of protective immunity, and the confounding presence of helminths could spuriously skew the cytokine profile in vaccinees. Interestingly, while antigen-specific type 1 and type 17 responses exhibited major changes, anthelmintic treatment had very little to no effect on antigen-specific type 2 and regulatory cytokine responses. This could possibly reflect different kinetics in the restoration of cytokine responses (type 1 versus type 2) following therapy or failure to optimally downregulate type 2 responses following treatment in these coinfected individuals.
Understanding the balance between type 1/type 17 cytokines and type 2/regulatory cytokines is crucial for developing more-effective measures to combat TB infection and disease, and the lack of knowledge of the mechanisms that mediate protection and pathogenesis is a major hurdle in improving vaccination and therapeutic strategies (30). Clearly, coexistent helminth infection can significantly alter the systemic immune response in TB infections or disease and the immune response to TB antigens. Limitations of our study were that cytokine levels exhibit a great degree of overlap between groups and that there is variability in the responses of different individuals in the same group; thus, cytokine levels cannot be utilized as biomarkers for diagnosis or therapeutic monitoring. In addition, administration of ivermectin per se has been shown to exert anti-mycobacterial effects and could thus serve as a confounding factor (31). While our report is clearly preliminary and the data need to be confirmed in a much larger setting with additional examination of local cytokine responses—and while longitudinal studies examining the effect of anthelmintic treatment on systemic and antigen-specific cytokine responses in TB need to be performed—our data clearly highlight the major regulatory effects that helminth infections can exert on the immune response to coexisting infections.
MATERIALS AND METHODS
Ethics statement.
All individuals were examined as part of a natural history study protocol (NCT 01547884) approved by Institutional Review Boards of the National Institute of Allergy and Infectious Diseases (USA) and the National Institute for Research in Tuberculosis (India), and informed written consent was obtained from all participants.
Study population.
We studied a group of 118 individuals with LTB, 60 of whom had S. stercoralis infection (here LTB-S. stercoralis) and 58 of whom had latent TB alone (LTB) (Table 2). All the study individuals were recruited from villages in the Kanchipuram District of Tamil Nadu state, on the outskirts of the city of Chennai, India. This was a cross-sectional study, and a convenience sampling methodology was used. LTB individuals were asymptomatic, with positive Quantiferon gold in-tube tests and normal chest radiographs. S. stercoralis infection was diagnosed by the presence of IgG antibodies to the 31-kDa recombinant NIE antigen by the luciferase immunoprecipitation system assay (LIPS assay) as described previously (32). Individuals with other helminth infections were excluded. All individuals were negative for filarial infection by filarial antigen tests (immunochromatographic card test [ICT] and TropBio enzyme-linked immunosorbent assay [ELISA]), and none had other intestinal helminths by stool microscopy. All individuals were nondiabetic (determined by fasting blood glucose) and antituberculous and anthelmintic treatment naive. All S. stercoralis-infected individuals were treated with single doses of ivermectin (12 mg) and albendazole (400 mg), and followup blood draws were obtained 6 months later. End-of-treatment serology revealed significantly decreased anti-NIE IgG levels, and stool examination did not reveal any intestinal helminths.
TABLE 2.
Demographics of the study population
| Parameter | Result |
|
|---|---|---|
| LTB-S. stercoralis (n = 60) | LTB (n = 58) | |
| No. of males/no. of females | 37/23 | 32/26 |
| Median age (yrs) (range) | 36 (20–61) | 40 (20–60) |
| Tuberculin skin test (diam) | ≥12 mm | ≥12 mm |
| Quantiferon ELISA | Positive (≥0.35 IU/ml) | Positive (≥0.35 IU/ml) |
| NIE ELISA | Positive | Negative |
ELISA.
Plasma cytokines were measured using a Bioplex multiplex cytokine assay system (Bio-Rad, Hercules, CA). The parameters analyzed were IFN-γ, TNF-α, IL-2, IL-17A, IL-4, IL-5, IL-13, and IL-10 levels. Plasma levels of TGF-β (R& D Systems), IL-17F (Biolegend), and IL-22 (eBioscience) were measured by ELISA. All samples were run in duplicate.
Quantiferon supernatant ELISA.
Whole blood from LTB-S. stercoralis individuals and LTB individuals was incubated with either no antigen or TB antigen (ESAT-6, CFP-10, TB 7.7) or mitogen according to the instructions of the manufacturers using a Quantiferon gold in-tube kit. The unstimulated or TB antigen- or mitogen-stimulated whole-blood supernatants were then used to analyze the levels of IFN-γ, TNF-α, IL-2, IL-17A, IL-17F, IL-22, IL-4, IL-5, IL-13, IL-10, and TGF-β. The net cytokine values for TB antigen or mitogen stimulation were calculated by subtracting the baseline (no-antigen) values for each individual.
Statistical analysis.
Data analyses were performed using GraphPad PRISM (GraphPad Software, Inc., San Diego, CA, USA). Geometric means (GM) were used for measurements of central tendencies. Comparisons between two groups were made by using the Mann-Whitney U test followed by Holm's correction for multiple comparisons. Similarly, comparisons before and after treatment were made using the Wilcoxon signed-rank test followed by Holm's correction for multiple comparisons.
Supplementary Material
ACKNOWLEDGMENTS
We thank R. Satiswaran and Prabbu Balakrishnan for valuable assistance in collecting the clinical data for this study. We thank the staff of the Department of Epidemiology, NIRT, for valuable assistance in recruiting the individuals for this study.
This work was funded by the Division of Intramural Research, NIAID, NIH. The funders had no role in the study design, collection of data, analysis and interpretation of data, writing of the manuscript, or decision to submit for publication.
We declare that we have no conflicts of interest.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00973-16.
REFERENCES
- 1.Salgame P, Yap GS, Gause WC. 2013. Effect of helminth-induced immunity on infections with microbial pathogens. Nat Immunol 14:1118–1126. doi: 10.1038/ni.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen JE, Maizels RM. 2011. Diversity and dialogue in immunity to helminths. Nat Rev Immunol 11:375–388. doi: 10.1038/nri2992. [DOI] [PubMed] [Google Scholar]
- 3.O'Garra A, Redford PS, McNab FW, Bloom CI, Wilkinson RJ, Berry MP. 2013. The immune response in tuberculosis. Annu Rev Immunol 31:475–527. doi: 10.1146/annurev-immunol-032712-095939. [DOI] [PubMed] [Google Scholar]
- 4.Cooper AM, Khader SA. 2008. The role of cytokines in the initiation, expansion, and control of cellular immunity to tuberculosis. Immunol Rev 226:191–204. doi: 10.1111/j.1600-065X.2008.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Babu S, Nutman TB. 2014. Immunology of lymphatic filariasis. Parasite Immunol 36:338–346. doi: 10.1111/pim. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonne-Année S, Hess JA, Abraham D. 2011. Innate and adaptive immunity to the nematode Strongyloides stercoralis in a mouse model. Immunol Res 51:205–214. doi: 10.1007/s12026-011-8258-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maizels RM, Yazdanbakhsh M. 2003. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nat Rev Immunol 3:733–744. doi: 10.1038/nri1183. [DOI] [PubMed] [Google Scholar]
- 8.Chatterjee S, Kolappan C, Subramani R, Gopi PG, Chandrasekaran V, Fay MP, Babu S, Kumaraswami V, Nutman TB. 2014. Incidence of active pulmonary tuberculosis in patients with coincident filarial and/or intestinal helminth infections followed longitudinally in South India. PLoS One 9:e94603. doi: 10.1371/journal.pone.0094603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chatterjee S, Nutman TB. 2015. Helminth-induced immune regulation: implications for immune responses to tuberculosis. PLoS Pathog 11:e1004582. doi: 10.1371/journal.ppat.1004582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.George PJ, Pavan Kumar N, Jaganathan J, Dolla C, Kumaran P, Nair D, Banurekha VV, Shen K, Nutman TB, Babu S. 2015. Modulation of pro- and anti-inflammatory cytokines in active and latent tuberculosis by coexistent Strongyloides stercoralis infection. Tuberculosis (Edinb) 95:822–828. doi: 10.1016/j.tube.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Babu S, Bhat SQ, Kumar NP, Jayantasri S, Rukmani S, Kumaran P, Gopi PG, Kolappan C, Kumaraswami V, Nutman TB. 2009. Human type 1 and 17 responses in latent tuberculosis are modulated by coincident filarial infection through cytotoxic T lymphocyte antigen-4 and programmed death-1. J Infect Dis 200:288–298. doi: 10.1086/599797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chatterjee S, Clark CE, Lugli E, Roederer M, Nutman TB. 2015. Filarial infection modulates the immune response to Mycobacterium tuberculosis through expansion of CD4+ IL-4 memory T cells. J Immunol 194:2706–2714. doi: 10.4049/jimmunol.1402718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.George PJ, Anuradha R, Kumaran PP, Chandrasekaran V, Nutman TB, Babu S. 2013. Modulation of mycobacterial-specific Th1 and Th17 cells in latent tuberculosis by coincident hookworm infection. J Immunol 190:5161–5168. doi: 10.4049/jimmunol.1203311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toulza F, Tsang L, Ottenhoff TH, Brown M, Dockrell HM. 2016. Mycobacterium tuberculosis-specific CD4+ T-cell response is increased, and Treg cells decreased, in anthelmintic-treated patients with latent TB. Eur J Immunol 46:752–761. doi: 10.1002/eji.201545843. [DOI] [PubMed] [Google Scholar]
- 15.Mishra PK, Palma M, Bleich D, Loke P, Gause WC. 2014. Systemic impact of intestinal helminth infections. Mucosal Immunol 7:753–762. [DOI] [PubMed] [Google Scholar]
- 16.Weinstock JV, Elliott DE. 2014. Helminth infections decrease host susceptibility to immune-mediated diseases. J Immunol 193:3239–3247. doi: 10.4049/jimmunol.1400927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harnett W, Harnett MM. 2010. Helminth-derived immunomodulators: can understanding the worm produce the pill? Nat Rev Immunol 10:278–284. doi: 10.1038/nri2730. [DOI] [PubMed] [Google Scholar]
- 18.van Riet E, Hartgers FC, Yazdanbakhsh M. 2007. Chronic helminth infections induce immunomodulation: consequences and mechanisms. Immunobiology 212:475–490. doi: 10.1016/j.imbio.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 19.Gause WC, Maizels RM. 2016. Macrobiota - helminths as active participants and partners of the microbiota in host intestinal homeostasis. Curr Opin Microbiol 32:14–18. doi: 10.1016/j.mib.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Metenou S, Babu S, Nutman TB. 2012. Impact of filarial infections on coincident intracellular pathogens: Mycobacterium tuberculosis and Plasmodium falciparum. Curr Opin HIV AIDS 7:231–238. doi: 10.1097/COH.0b013e3283522c3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Babu S, Bhat SQ, Kumar NP, Anuradha R, Kumaran P, Gopi PG, Kolappan C, Kumaraswami V, Nutman TB. 2009. Attenuation of Toll-like receptor expression and function in latent tuberculosis by coexistent filarial infection with restoration following antifilarial chemotherapy. PLoS Negl Trop Dis 3:e489. doi: 10.1371/journal.pntd.0000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monin L, Griffiths KL, Lam WY, Gopal R, Kang DD, Ahmed M, Rajamanickam A, Cruz-Lagunas A, Zuniga J, Babu S, Kolls JK, Mitreva M, Rosa BA, Ramos-Payan R, Morrison TE, Murray PJ, Rangel-Moreno J, Pearce EJ, Khader SA. 2015. Helminth-induced arginase-1 exacerbates lung inflammation and disease severity in tuberculosis. J Clin Invest 125:4699–4713. doi: 10.1172/JCI77378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Potian JA, Rafi W, Bhatt K, McBride A, Gause WC, Salgame P. 2011. Preexisting helminth infection induces inhibition of innate pulmonary anti-tuberculosis defense by engaging the IL-4 receptor pathway. J Exp Med 208:1863–1874. doi: 10.1084/jem.20091473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gopal R, Monin L, Slight S, Uche U, Blanchard E, Fallert Junecko BA, Ramos-Payan R, Stallings CL, Reinhart TA, Kolls JK, Kaushal D, Nagarajan U, Rangel-Moreno J, Khader SA. 2014. Unexpected role for IL-17 in protective immunity against hypervirulent Mycobacterium tuberculosis HN878 infection. PLoS Pathog 10:e1004099. doi: 10.1371/journal.ppat.1004099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rook GA. 2007. Th2 cytokines in susceptibility to tuberculosis. Curr Mol Med 7:327–337. doi: 10.2174/156652407780598557. [DOI] [PubMed] [Google Scholar]
- 26.Abate E, Elias D, Getachew A, Alemu S, Diro E, Britton S, Aseffa A, Stendahl O, Schon T. 2015. Effects of albendazole on the clinical outcome and immunological responses in helminth co-infected tuberculosis patients: a double blind randomised clinical trial. Int J Parasitol 45:133–140. doi: 10.1016/j.ijpara.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 27.Elias D, Britton S, Aseffa A, Engers H, Akuffo H. 2008. Poor immunogenicity of BCG in helminth infected population is associated with increased in vitro TGF-beta production. Vaccine 26:3897–3902. doi: 10.1016/j.vaccine.2008.04.083. [DOI] [PubMed] [Google Scholar]
- 28.Ndibazza J, Mpairwe H, Webb EL, Mawa PA, Nampijja M, Muhangi L, Kihembo M, Lule SA, Rutebarika D, Apule B, Akello F, Akurut H, Oduru G, Naniima P, Kizito D, Kizza M, Kizindo R, Tweyongere R, Alcock KJ, Muwanga M, Elliott AM. 2012. Impact of anthelminthic treatment in pregnancy and childhood on immunisations, infections and eczema in childhood: a randomised controlled trial. PLoS One 7:e50325. doi: 10.1371/journal.pone.0050325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elias D, Wolday D, Akuffo H, Petros B, Bronner U, Britton S. 2001. Effect of deworming on human T cell responses to mycobacterial antigens in helminth-exposed individuals before and after bacille Calmette-Guerin (BCG) vaccination. Clin Exp Immunol 123:219–225. doi: 10.1046/j.1365-2249.2001.01446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Etna MP, Giacomini E, Severa M, Coccia EM. 2014. Pro- and anti-inflammatory cytokines in tuberculosis: a two-edged sword in TB pathogenesis. Semin Immunol 26:543–551. doi: 10.1016/j.smim.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 31.Lim LE, Vilcheze C, Ng C, Jacobs WR Jr, Ramon-Garcia S, Thompson CJ. 2013. Anthelmintic avermectins kill Mycobacterium tuberculosis, including multidrug-resistant clinical strains. Antimicrob Agents Chemother 57:1040–1046. doi: 10.1128/AAC.01696-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramanathan R, Burbelo PD, Groot S, Iadarola MJ, Neva FA, Nutman TB. 2008. A luciferase immunoprecipitation systems assay enhances the sensitivity and specificity of diagnosis of Strongyloides stercoralis infection. J Infect Dis 198:444–451. doi: 10.1086/589718. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.