Abstract
Brain lateralization is a ubiquitous feature of neural organization across the vertebrate spectrum. We have developed a model of motor lateralization that attributes different motor control processes to each cerebral hemisphere. This bilateral hemispheric model of motor control has successfully predicted hemisphere-specific motor control and motor learning deficits in the ipsilesional, or non-paretic, arm of patients with unilateral stroke. We now show across large number and range of stroke patients that these motor performance deficits in the non-paretic arm of stroke patients vary with both the side of the lesion, as well as with the severity of contralesional impairment. This last point can be functionally devastating for patients with severe contralesional paresis because for these individuals, performance of upper extremity activities of daily living depends primarily and often exclusively on ipsilesional arm function. We present a pilot study focused on improving the speed and coordination of ipsilesional arm function in a convenience sample of three stroke patients with severe contralesional impairment. Over a three-week period, patients received a total of nine 1.5 h sessions of training that included intense practice of virtual reality and real-life tasks. Our results indicated substantial improvements in ipsilesional arm movement kinematics, functional performance, and that these improvements carried over to improve functional independence. In addition, the contralesional arm improved in our measure of contralesional impairment, which was likely due to improved participation in activities of daily living. We discuss of our findings for physical rehabilitation.
Keywords: Bilateral hemispheric model, Contralesional, Paresis, Ipsilesional, Rehabilitation, Dynamic dominance, Jebsen-Taylor hand function test, Interlimb transfer
Neural Lateralization for Motor Control Processes
The seminal research of Gazzaniga (1998) on disconnection syndrome in split brain patients established neural lateralization as a fundamental feature of brain organization. They proposed that distributing different neural processes across the hemispheres was a natural consequence of developing complex functions during the course of evolution. Gazzaniga’s research provided elegant support for this view of cerebral lateralization as a neural optimization process. The research of MacNeilage, Rogers, and Vallortigara (Bisazza et al. 1998; MacNeilage et al. 2009) demonstrated that brain lateralization served as a basic organizational principle over the course of vertebrate evolution and showed that neural lateralization is found throughout the vertebrate spectrum. While a great deal of research on brain lateralization has been conducted in humans, early research was largely limited to cognitive and perceptual processes, with little attention to the motor systems.
Based on asymmetries in reaching performance and adaptation to novel dynamic and visual motor environments in healthy adults, we introduced the dynamic dominance hypothesis of motor lateralization (Sainburg 2002). The dynamic dominance model proposes that the left hemisphere, in right-handers, is specialized for predictive processes that specify smooth and efficient movement trajectories under mechanically stable environmental circumstances, while the right hemisphere is specialized for impedance control mechanisms that confer robustness to movements performed under unpredictable and mechanically unstable environmental conditions. This hypothesis has been supported by empirical studies (Sainburg 2002), computational modeling studies (Yadav and Sainburg 2011, 2014) and studies in patients with unilateral brain lesions (Schaefer et al. 2007, 2012; Haaland et al. 2009; Mutha et al. 2012; Mani et al. 2013, 2014). This hypothesis of motor lateralization is consistent with the broader model of brain lateralization proposed by Rogers and colleagues (Bisazza et al. 1998). According to their model, the left hemisphere is “specialized for control of well-established patterns of behavior, under ordinary and familiar circumstances,” while the right hemisphere is specialized for “detecting and responding to unexpected stimuli in the environment” (MacNeilage et al. 2009). The dynamic dominance model provides the movement analog to Roger’s model, and thus places handedness in the context of a larger array of neurobehavioral asymmetries across the animal kingdom (Sainburg 2014).
Non-paretic Arm Motor Deficits in Stroke
The dynamic dominance hypothesis is a bihemispheric model of motor control, in which each hemisphere contributes different control mechanisms to voluntary movement. One of the strongest predictions of this model is that unilateral brain damage affecting sensorimotor centers should produce hemisphere-specific deficits in the non-paretic ipsilesional arm of stroke patients. The role of contralateral motor areas in controlling limb movements has been well-established (Lawrence and Kuypers 1968; Kuypers 1978, 1982). The role the ipsilateral hemisphere has been implicated by the robust occurrence of ipsilesional motor deficits in both animal models of unilateral brain damage (Grabowski et al. 1993) as well as human stroke survivors (Wyke 1967; Fisk and Goodale 1988; Winstein and Pohl 1995; Desrosiers et al. 1996; Pohl and Winstein 1999; Yarosh et al. 2004; Schaefer et al. 2009a). In addition, both electrophysiological and neural imaging studies have shown that unilateral arm and hand movements recruit motor-regions in both cerebral hemispheres (Tanji et al. 1988; Kawashima et al. 1993, 1998; Dassonville et al. 1997). Motor deficits in the non-paretic arm of patients with unilateral stroke have been documented as early as 1967 (Wyke 1967). More recent research has shown that these deficits are functionally limiting and that they persist throughout the chronic phase of stroke (Pohl and Winstein 1999; Winstein et al. 1999; Haaland et al. 2000, 2004, 2009; Prestopnik et al. 2003a, b; Wetter et al. 2005; Haaland 2006; Schaefer et al. 2007, 2009a, b, 2012; Chestnut and Haaland 2008; Poole et al. 2009; Mutha et al. 2010, 2011a, b, 2013). In fact, studies of non-paretic arm function in chronic stroke patients have reported performance deficiencies on a number of clinical tests, including the Purdue Pegboard Test (Rapin et al. 1966), the Jebsen-Taylor Hand Function Test (Schaefer et al. 2009b), and a variety of tests that directly assess or simulate activities of daily living (Desrosiers et al. 1996; Wetter et al. 2005; Haaland et al. 2012). In addition, significant deficits in movement coordination and accuracy have been revealed by studies that have employed motion analysis (Haaland and Harrington 1989a, b, 1996; Winstein and Pohl 1995; Winstein et al. 1996; Prestopnik et al. 2003a; Haaland et al. 2004, 2009; Schaefer et al. 2009a, b, 2012; Mutha et al. 2010, 2011a, b; 2012a, b; 2013; Mani et al. 2014).
Over the past decade, our laboratory has systematically assessed the hemisphere specificity, neural foundations, and functional implications of non-paretic arm motor deficits. In brief, our findings have indicated that non-paretic arm motor deficits in stroke result from a loss of the specific contributions of the ipsilateral hemisphere to motor control. Our research has established that right and left hemisphere damage lead to deficits in different motor control processes (Winstein and Pohl 1995; Schaefer et al. 2012; Mutha et al. 2013; Sainburg 2014).
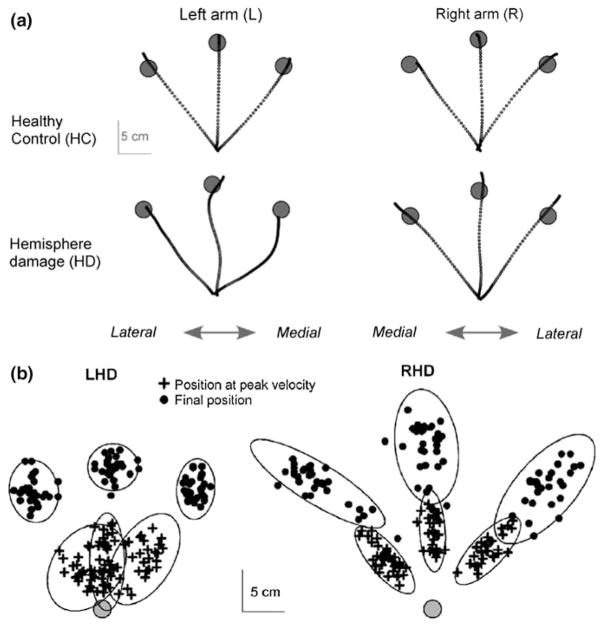
Two important aspects of non-paretic arm deficits in stroke patients are that the quality of the motor deficits depends on the hemisphere that has been damaged by stroke, and the extent of the deficits depends on the severity of paretic arm motor impairment. Figure 1 shows data from a study of multidirection reaching movements in patients with unilateral stroke (Schaefer et al. 2009b). Figure 1a shows reaching movements in the non-paretic arm of patients with left and right hemisphere damage. While patients with left hemisphere damage (LHD) made highly curved movements with accurate final positions, patients with right hemisphere damage (RHD) made straight movements with poor final position accuracies. Figure 1b shows the variance in hand positions during the initial trajectory phase (bottom ellipses), and in the final position (top ellipses) of the movement. These findings demonstrate a robust double dissociation between hemisphere of damage and motor control process effected by a unilateral hemisphere lesion: While RHD patients have deficits in stabilizing accurate final positions across trials, LHD patients have deficits in stabilizing accurate initial trajectories across trials. These results provide evidence that each hemisphere contributes different control mechanisms to both arms, and have been corroborated by studies that examined the effects of unilateral brain damage on adaptation to novel visuomotor rotations (Mutha et al. 2011a, b), and studies that have examined responses to sudden visual perturbations (Schaefer et al. 2012). This bihemispheric model of arm control also predicts hemisphere specific deficits in the contralesional arm of stroke patients, a prediction that has been supported in patients with mild paresis (Mani et al. 2013).
Fig. 1.
Multidirection reaching movements in patients with unilateral stroke (Schaefer et al. 2009b). a Reaching movements in the non-paretic arm of patients with left (LHD) and right (RHD) hemisphere damage. b Variance in hand positions during the initial trajectory phase (bottom ellipses), and in the final position (top ellipses) of the movement
Relevance of Motor Lateralization to Stroke Rehabilitation
Regardless of the robust occurrence of ipsilesional, non-paretic arm, motor deficits in patients with unilateral stroke, Occupational and Physical Therapy motor remediation protocols continue to either completely focus on the paretic arm, or employ bilateral movements that do not challenge non-paretic arm coordination. As stated above, the non-paretic arm tends to have substantial deficits in movement coordination, speed, and accuracy that limit both performance of activities of daily living (ADL) and functional independence. Specifically, non-paretic arm motor deficits are most severe and debilitating in patients with severe paresis. In fact, the usual standard of care in rehabilitation for these patients with severe contralesional paresis is task training in essential ADL rather than intensive remediation of either arm. It is important to stress that in these patients, performance of ADL depends on non-paretic arm function. We propose that intense remediation, focused on improving the speed, coordination, and accuracy of the non-paretic arm can improve motor performance and functional independence in stroke patients with severe hemiparesis. Currently, it is not known whether remedial treatment can improve non-paretic arm function. We are beginning to address this issue by examining the effects of non-paretic arm training on functional independence.
Non-paretic Arm Functional Deficits
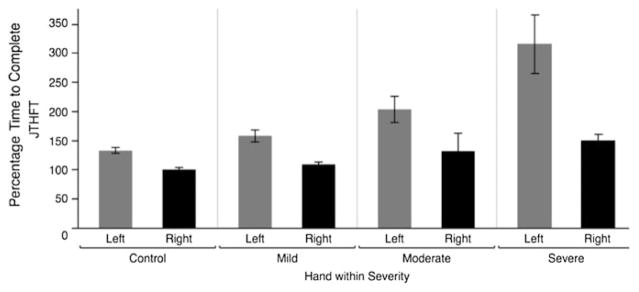
We recently tested how non-paretic arm motor function might depend on the severity of paresis in the contralesional arm, and on the side of the brain that is damaged. Figure 2 shows data from 72 age and gender-matched control participants (36 using the right arm, and 36 using the left arm), 22 LHD survivors, and 29 RHD survivors. The y-axis represents the Jebsen-Taylor Hand Function Test score, taken as a percentage of right dominant arm function in our control group. The JTHFT is a clinical assessment of unilateral arm function, validated in stroke survivors (Beebe and Lang 2009), that includes a range of tasks that elicit the coordination requirements of functional daily activities, including writing, turning pages, placing large and small objects on a table, stacking small objects, and feeding (Jebsen et al. 1969, 1971). The left column (control) shows the difference between healthy participants performing with the left arm (Gray) and the right arm (Black). The data are normalized to control subjects’ dominant arm performance (100 %). The data are stratified on the x-axis by hand (in the case of stroke survivors, this is always the non-paretic, ipsilesional arm), and severity of contralesional paresis, as measured by the upper extremity component of the Fugl-Meyer et al. (1975) assessment of motor impairment (mild > 55, moderate > 35, Severe ≤ 35).
Fig. 2.
Data from 72 age and gender matched control participants (36 using the right arm, and 36 using the left arm), 22 LHD survivors, and 29 RHD survivors. Y-axis represents the Jebsen-Taylor Hand Function Test score. Data are normalized to control subjects’ dominant arm performance (100 %). X-axis stratified by hand (in the case of stroke survivors, this is always the non-paretic, ipsilesional arm), and severity of contralesional deficit
As illustrated in Fig. 2, in stroke survivors, JTHFT performance with the non-paretic arm was impacted substantially by both the severity of paretic arm impairment and the side of the brain that was damaged. Participants with the most severe paresis in the contralesional arm had the greatest motor deficit in the non-paretic arm. This effect depended on the side of the lesion, such that LHD was associated with greater non-paretic arm deficits than RHD. More specifically, LHD survivors took, on average, 300 % longer to complete the tasks than age matched control participants. Thus, stroke survivors who are forced to rely most extensively on their non-paretic arms for performance of ADL have the greatest deficits in coordination in the non-paretic arm. The fact that we tested these stroke survivors 1.8 years (±0.3 SE) on average after their stroke suggests that these deficits do not spontaneously improve over time.
Remediation of the Non-paretic Arm
Remediation of the non-paretic arm is so novel that little empirical evidence exists as to whether such intervention could affect non-paretic arm control and coordination, and functional independence. We recently conducted a pilot study to test this hypothesis, which suggests that intense non-paretic arm training not only improves motor coordination and functional performance in the trained arm, but also improves functional independence and modestly improves paretic arm function. This is consistent with another recent pilot randomized clinical study that indicated that patients who received therapy that included training of the non-paretic arm improved functional independence greater than those receiving traditional therapy alone (Pandian et al. 2015). These studies provide preliminary evidence that non-paretic arm remediation might improve functional independence and even functioning in the paretic arm, possibly by increasing participation in ADL.
Our preliminary study was done with a three stroke survivor convenience sample. All participants showed significant contralesional paresis and deficits in performance of the non-paretic arm. All three participants were male. Details about age, initial Fugl-Meyer and initial JTHFT scores, and time since stroke are listed below:
SS 1: Age 58 Right MCA. Fugl-Meyer: 14, JTHFT: 69 s., TSS: 2 yr, 1 month.
SS 2: Age 66, Left MCA. Fugl-Meyer: 27, JTHFT: 125 s., TSS: 7 yrs, 0 month.
SS 3: Age 75, Left MCA. Fugl-Meyer: 45, JTHFT: 96 s., TSS: 3 yrs, 1 month.
We chose our outcome measures using the criteria that the tests can easily and quickly be administered in our environment, reflect the performance variables of interest, and have reported psychometrics in stroke survivors that include validity, reliability, and “responsiveness” or sensitivity for tracking changes in performance over time. These included the Functional Independence Measure (FIM), focusing on the components relevant to performance of ADL. In order to quantify paretic arm impairment, we used the upper extremity section of the Fugl-Meyer assessment. Lin et al. (2009) reported psychometric characteristics of the Fugl-Meyer, demonstrating substantial responsiveness (Effect Size 0.37–0.52), as well as high inter-rater reliability (ICC = 0.92–0.98). Our primary measures of non-paretic arm function include the Jebsen-Taylor Hand Function Test (JTHFT) and kinematic measurements, based on motion analysis of reaching movements. The JTHFT was discussed above, and we will describe our kinematic measures below.
Our kinematic recording and experimental setup is shown in Fig. 3. This set-up was used for both kinematic testing and virtual reality based movement training. Kinematic testing was be done for 2D, arm-supported reaching tasks, while training was done in 3-dimensions.
Fig. 3.
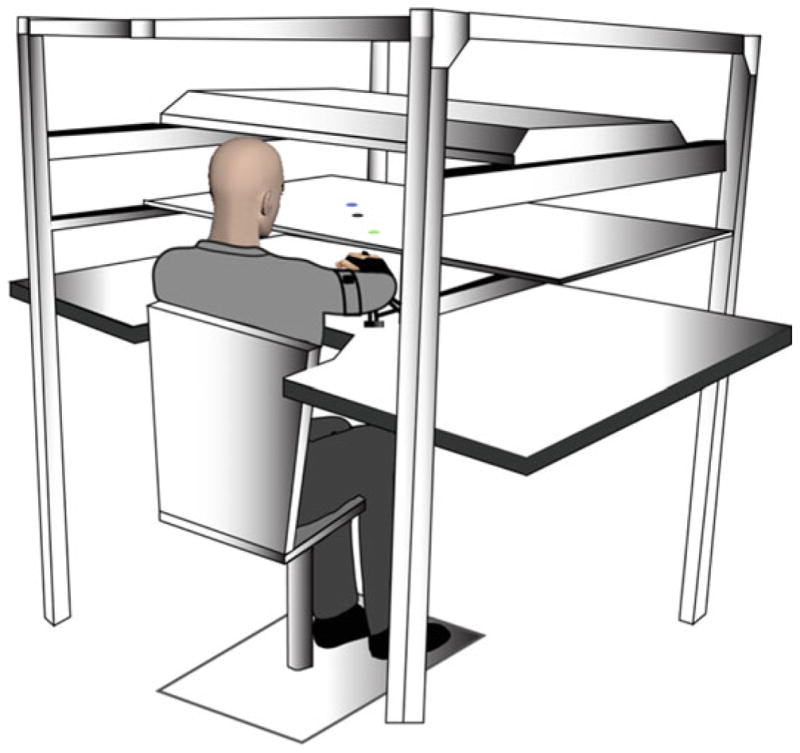
Participants were positioned facing a horizontally positioned mirror that reflected the 55″ monitor. The arm was supported over a horizontal table top, positioned just below shoulder height (adjusted to each individual’s comfort), by an air-jet system, which reduced the effects of gravity and friction
This custom designed apparatus allows participants to perform tasks in 2-dimensions as well as tasks that require participants to move in 3-dimensions and support their arms against gravity. For arm-supported tasks (2D), the forearms rest on air-cushion sleds that support the arms against gravity and nearly eliminate friction. For 3D tasks, the arm is held above the table top, and a cursor, representing hand position, can only be seen when the arm maintained off the table top. Task and movement feedback is displayed on a horizontal mirror positioned 35 cm above the table surface. This mirror reflects the stimuli presented on a horizontal, inverted, 60″ HDTV display. The proximal interphangeal joint of the index finger is reflected by the position of the cursor. Six Degree of freedom Trackstar® magnetic sensors are attached to the limbs, while positions of boney landmarks are used to digitize the hand, forearm, and upper arm segments, allowing calculation of 10 degrees of freedom per arm, recorded at 116 Hz. Data are low-pass filtered using a 12 Hz zero-lag Butterworth filter, prior to differentiating to yield velocity and acceleration profiles.
Testing of non-paretic arm kinematics were done during arm-supported reaching tasks made in multiple directions, in order to be consistent with our previous characterization of non-paretic arm motor control deficits (see Fig. 1) (Schaefer et al. 2007, 2009b, 2012; Mutha et al. 2010, 2012, 2014). This study will use the same three direction reaching paradigm that was described in Fig. 1. Our primary kinematic measures included Distance Error, the vector magnitude difference between the two vectors, defined by (1) The beginning and end of the hand path, and (2) The specified start position and the target, Direction Error, the angular difference between the two vectors defined above, Hand path aspect ration, a measure of the hand path’s deviation from linearity, measured as the ratio between the minor axis and the major axis of the hand path, maximum tangential velocity, and movement duration.
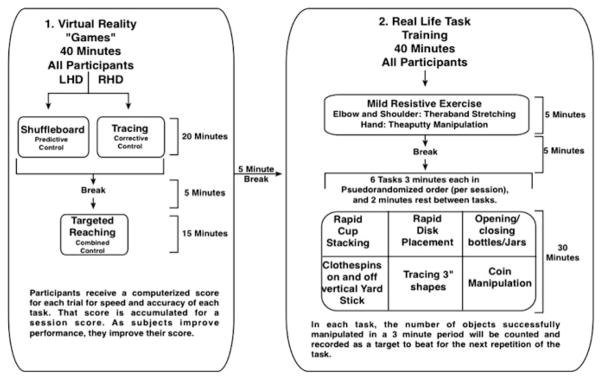
Figure 4 shows the timeline for the intervention. This preliminary study included a baseline period of 2 weeks, during which no intervention was done. This established stability of our dependent measures. Two baseline tests (Test 1 and Test 2) were given (2 weeks apart) prior to non-paretic arm training, and Test 3 was given immediately after the three-week training period. Subjects received 3 1.5 h sessions per week for 3 weeks. Figure 5 shows the 90 min intervention session. Participants were first exposed to virtual reality ‘games’ designed to focus on specific components of control that we have previously shown to be deficient in the non-paretic arm of right or left hemisphere damaged stroke patients. For the first 20 min of training, patients practiced tasks adapted to the motor control deficits associated with each hemisphere. These included virtual shuffleboard, which focuses on predictive aspects of trajectory control, tracing games that focused on feedback-mediated position control, and targeted reaching movements that incorporate both aspects of motor control. These tasks were performed in our VR environment, but with the arm unsupported and held above the table top. For the next 40-minute phase of the session, participants practiced real-life tasks: First, they engaged in mild resistive exercises, using elastic putty and elastic bands, designed for resistive exercises of the hand and arm, respectively. After 5 min of preparation and a 5-minute break, all participants engaged in a series of six tasks, randomized in order between sessions. These tasks are shown in the flow chart in Fig. 5.
Fig. 4.
Timeline for the intervention—baseline period of 2 weeks, during which no intervention was done. Two baseline tests (Test 1 and Test 2) were given (2 weeks apart) prior to Non-Paretic arm training, and Test 3 was given immediately after the 3-week training period
Fig. 5.
Participants were initially exposed to virtual reality games for 40 min, followed by real-life tasks for another 40 min
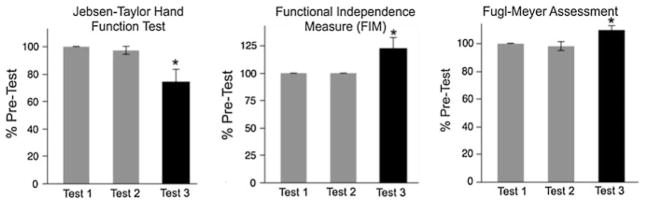
The results of this pilot study are shown in Figs. 6 and 7. As predicted, there were no significant differences between our baseline measures, for Test 1 and Test 2. This demonstrates stability in baseline performance over time. The effect of training, however, was reflected by a 25.8 % reduction in performance time for the Jebsen-Taylor Hand Function Test, and a 9.8 % reduction in impairment, as measured by the Fugl-Meyer Assessement. Most importantly, the FIM score improved by 22.6 %, representing an increase of 3.3 ± (SE = 0.88) points out of 28 points on the upper extremity self-care components of the FIM.
Fig. 6.
Mean ± SE for clinical assessments
Fig. 7.
Sample Handpaths prior to and following training, and associated measures (Mean ± SE) for the two pretests (test1, test2), and the post test (test3)
Our kinematic measures in the non-paretic arm also showed improvements. Figure 7 shows example hand paths from a left hemisphere damaged patient using the left non-paretic arm, prior to training (Test 1) and following training (Test 2). Bar graphs show average kinematic measures (±SE), across all participants and test-sessions. Movements became straighter and more accurately directed toward the target (upper circle) following training, but the distance of the movements was accurate even in baseline movements. These effects were consistent across all subjects, as shown by the significant reduction in direction error and aspect ratio (a measure of hand path straightness), only for Test 3, post-training.
In summary, our pilot data showed promising improvements in non-paretic arm motor coordination and function. In addition, modest improvements in paretic arm impairment, measured by Fugl-Meyer score were also shown. Most importantly, these intervention related changes in performance translated to improvements in functional independence. Whether these effects are specific to the training paradigm, robust across a range of patients, and durable will need to be examined in future studies with more extensive recruitment.
Discussion and Implications for Rehabilitation
For the most part, physical rehabilitation of upper extremity function following stroke has understandably been focused on training movements in the contralesional arm. However, the research discussed above provides compelling evidence that ipsilesional assessment and intervention also needs to be addressed. When patients have severe contralesional paresis, the ipsilesional arm is often the primary manipulator, or even the sole manipulator. Therefore, effective performance of ADL relies upon efficient coordination of this arm and hand (Haaland et al. 2012). We provided evidence that this arm shows substantial coordination deficits that limit performance of ADL and functional independence. Patients who must live with a severely paretic dominant arm, unfortunately have the most severe non-paretic arm deficits in coordination and functional performance.
We have provided preliminary evidence that intensive training of this ipsilesional, non-paretic arm could substantially improve functional independence in patients with hemiparesis. Although preliminary, this is among the first empirical evidence indicating that such intervention might lead to positive effects on motor performance and functional independence. However, our findings are consistent with a recent pilot intervention study that compared a group of patients who received therapy that included training of the non-paretic arm and the paretic arm to another group who only received “traditional” therapy for only the paretic arm (Pandian et al. 2015). The results indicated that when non-paretic intervention was combined with paretic arm training, the speed and accuracy of non-paretic arm movements improved, and the impairment level of the paretic arm modestly improved, when compared to patients who received traditional therapy alone. Combined with our current results, these findings indicate that focused non-paretic arm training might produce both improvements in non-paretic arm motor performance and in paretic arm function, both of which should facilitate improvements in functional independence. This finding appears at odds with the well-studied phenomenon of learned non-use of the paretic arm, an effect that has been successfully addressed by constraining the non-paretic arm in patients with moderate to mild paresis (Wolf et al. 1989, 2002, 2005, 2006). Constraint induced movement therapy uses constraint of the non-paretic arm in combination with intense practice of the paretic arm to facilitate recovery of paretic arm function. However, it should be stressed that this approach is most effective in patients with high-moderate to mild paresis, who are able to manipulate objects with the paretic arm, and engage in unilateral functional activities with that arm. The current study addresses recovery of function in patients with severe contralesional paresis, who generally have no voluntary movement of the wrist and fingers. Nevertheless, there is no conclusive evidence to predict whether non-paretic arm training will influence paretic arm function, either positively or negatively. Further and more extensive intervention research is necessary to address this question.
It would be inaccurate to suggest that the non-paretic arm is not addressed at all in rehabilitation. In fact, bilateral arm training has a long history in rehabilitation practice. Because most ADL are performed with both hands contributing to different aspects of the activity (Wetter et al. 2005; Haaland et al. 2012), bilateral training should be an important component to therapeutic intervention in stroke patients. It should also be stressed that bilateral training may be necessary because unilateral training may not spontaneously carry-over to bilateral activities. Recent research has indicated that in healthy young participants, adaptation to novel force fields imposed with robotic devices, and to distorted visual feedback, transfers only partially to bilateral movements, when the same arm experiences the imposed environments (Nozaki et al. 2006; Wang and Sainburg 2009). This suggests that learning a new task unilaterally (with each hand separately) may not spontaneously transfer to bilateral performance. These findings emphasize the importance of bilateral coordination in rehabilitation. Indeed, bilateral training has a long history in Occupational Therapy treatment, where manipulation of dowels and rolling pins has often been used to encourage bilateral arm use.
However, notwithstanding the importance of bilateral movement intervention, our current results indicate the importance of intense non-paretic arm remediation in patients with severe contralesional impairment. Bilateral training cannot challenge the non-paretic arm to the extent required for remediation. As Winstein and colleagues (Rose and Winstein 2005) demonstrated, when using the two arms together, the non-paretic arm slows down and adapts its movement characteristics to match that of the paretic arm. Obviously, this cannot challenge the speed and accuracy requirements of non-paretic arm coordination to the extent necessary for improvement of non-paretic function. It should also be stressed that in patients with severe contralesional paresis, many ADL must be performed unilaterally with the non-paretic arm. The fact that non-paretic arm deficits can be extensive and can persist throughout the chronic phase of stroke (see Fig. 2) indicates that forced use of the non-paretic arm does not spontaneously lead to amelioration of non-paretic arm motor deficits. We suggest that this is because the patients are not exposed to graded difficulty in challenging tasks, such as was done in the current training paradigm.
Take Home Message
The research presented in this chapter has demonstrated that many stroke patients with severe moderate to paresis in the contralesional arm have substantial and functionally limiting motor coordination deficits in the non-paretic ipsilesional arm. Furthermore, the combination of moderate to severe paresis with persistent motor deficits in the non-paretic arm limits performance of and participation in ADL. Based on our current results we recommend intense rehabilitation, sequentially focused on each arm, followed by practice in bilateral ADL when possible. It is critical to test this approach in a broader sample of stroke patients. A potential caveat to such a training scheme is reflected by the work of Jones et al. (1989). They showed, in an acute model of stroke in rats, that initial training of ipsilesional forelimb reaches limits the subsequent response to training in the contralesional forelimb. While this study was done in an acute lesion model in rats that bears only partial resemblance to the impairments seen in humans, it is possible that training the ipsilesional arm in the acute phase of stroke could be counter productive. To date, no studies of ipsilesional training in acute stroke are available in the literature. In direct contrast to the interpretation of the study of Jones et al., studies of motor learning in humans often demonstrate transfer of learning between the arms, (Wang and Sainburg a, b), and mirror training intervention has shown positive transfer between the arms in stroke patients (Stevens and Stoykov 2004; Oujamaa et al. 2009; Selles et al. 2014). We recommend further studies of ipsilesional arm intervention in stroke survivors with moderate to severe contralesional paresis to determine whether such training can positively affect functional outcomes and participation in human stroke survivors.
Contributor Information
Robert L. Sainburg, Department of Kinesiology, The Pennsylvania State University, 29 Rec Building, Biomechanics Laboratory, University Park, PA 16802, USA. Department of Neurology, Penn State Milton S. Hershey College of Medicine, Hershey, USA
Candice Maenza, Department of Kinesiology, The Pennsylvania State University, 29 Rec Building, Biomechanics Laboratory, University Park, PA 16802, USA. Department of Neurology, Penn State Milton S. Hershey College of Medicine, Hershey, USA.
Carolee Winstein, Department of Biokinesiology and Physical Therapy, University of Southern California, Los Angeles, USA.
David Good, Department of Neurology, Penn State Milton S. Hershey College of Medicine, Hershey, USA.
References
- Beebe JA, Lang CE. Relationships and responsiveness of six upper extremity function tests during the first six months of recovery after stroke. J Neurol Phys Ther. 2009;33:96–103. doi: 10.1097/NPT.0b013e3181a33638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisazza A, Rogers LJ, Vallortigara G. The origins of cerebral asymmetry: a review of evidence of behavioural and brain lateralization in fishes, reptiles and amphibians. Neurosci Biobehav Rev. 1998;22:411–426. doi: 10.1016/s0149-7634(97)00050-x. [DOI] [PubMed] [Google Scholar]
- Chestnut C, Haaland KY. Functional significance of ipsilesional motor deficits after unilateral stroke. Arch Phys Med Rehabil. 2008;89:62–68. doi: 10.1016/j.apmr.2007.08.125. [DOI] [PubMed] [Google Scholar]
- Dassonville P, Zhu XH, Uurbil K, Kim SG, Ashe J. Functional activation in motor cortex reflects the direction and the degree of handedness. Proc Natl Acad Sci USA. 1997;94:14015–14018. doi: 10.1073/pnas.94.25.14015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrosiers J, Bourbonnais D, Bravo G, Roy PM, Guay M. Performance of the ‘unaffected’ upper extremity of elderly stroke patients. Stroke. 1996;27:1564–1570. doi: 10.1161/01.str.27.9.1564. [DOI] [PubMed] [Google Scholar]
- Fisk JD, Goodale MA. The effects of unilateral brain damage on visually guided reaching: hemispheric differences in the nature of the deficit. Exp Brain Res. 1988;72:425–435. doi: 10.1007/BF00250264. [DOI] [PubMed] [Google Scholar]
- Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand J Rehabil Med. 1975;7:13–31. [PubMed] [Google Scholar]
- Gazzaniga MS. The split brain revisited. Sci Am. 1998;279:50–55. doi: 10.1038/scientificamerican0798-50. [DOI] [PubMed] [Google Scholar]
- Grabowski M, Brundin P, Johansson BB. Paw-reaching, sensorimotor, and rotational behavior after brain infarction in rats. Stroke. 1993;24:889–895. doi: 10.1161/01.str.24.6.889. [DOI] [PubMed] [Google Scholar]
- Haaland KY. Left hemisphere dominance for movement. Clin Neuropsychol. 2006;20:609–622. doi: 10.1080/13854040590967577. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Harrington D. The role of the hemispheres in closed loop movements. Brain & Cogn. 1989a;9:158–180. doi: 10.1016/0278-2626(89)90027-4. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Harrington DL. Hemispheric control of the initial and corrective components of aiming movements. Neuropsychologia. 1989b;27:961–969. doi: 10.1016/0028-3932(89)90071-7. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Harrington DL. Hemispheric asymmetry of movement. Curr Opin Neurobiol. 1996;6:796–800. doi: 10.1016/s0959-4388(96)80030-4. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Harrington DL, Knight RT. Neural representations of skilled movement. Brain. 2000;123(Pt 11):2306–2313. doi: 10.1093/brain/123.11.2306. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Mutha PK, Rinehart JK, Daniels M, Cushnyr B, Adair JC. Relationship between arm usage and instrumental activities of daily living after unilateral stroke. Arch Phys Med Rehabil. 2012;93:1957–1962. doi: 10.1016/j.apmr.2012.05.011. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Prestopnik JL, Knight RT, Lee RR. Hemispheric asymmetries for kinematic and positional aspects of reaching. Brain. 2004;127:1145–1158. doi: 10.1093/brain/awh133. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Schaefer SY, Knight RT, Adair J, Magalhaes A, Sadek J, Sainburg RL. Ipsilesional trajectory control is related to contralesional arm paralysis after left hemisphere damage. Exp Brain Res. 2009;196:195–204. doi: 10.1007/s00221-009-1836-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jebsen RH, Griffith ER, Long EW, Fowler R. Function of “normal” hand in stroke patients. Arch Phys Med Rehabil. 1971;52:170–174. (passim) [PubMed] [Google Scholar]
- Jebsen RH, Taylor N, Trieschmann RB, Trotter MJ, Howard LA. An objective and standardized test of hand function. Arch Phys Med Rehabil. 1969;50:311–319. [PubMed] [Google Scholar]
- Jones RD, Donaldson IM, Parkin PJ. Impairment and recovery of ipsilateral sensory-motor function following unilateral cerebral infarction. Brain. 1989;112(Pt 1):113–132. doi: 10.1093/brain/112.1.113. [DOI] [PubMed] [Google Scholar]
- Kawashima R, Matsumura M, Sadato N, Naito E, Waki A, Nakamura S, Matsunami K, Fukuda H, Yonekura Y. Regional cerebral blood flow changes in human brain related to ipsilateral and contralateral complex hand movements—a PET study. Eur J Neurosci. 1998;10:2254–2260. doi: 10.1046/j.1460-9568.1998.00237.x. [DOI] [PubMed] [Google Scholar]
- Kawashima R, Yamada K, Kinomura S, Yamaguchi T, Matsui H, Yoshioka S, Fukuda H. Regional cerebral blood flow changes of cortical motor areas and prefrontal areas in humans related to ipsilateral and contralateral hand movement. Brain Res. 1993;623:33–40. doi: 10.1016/0006-8993(93)90006-9. [DOI] [PubMed] [Google Scholar]
- Kuypers HG. The motor system and the capacity to execute highly fractionated distal extremity movements. Electroencephalogr Clin Neurophysiol (Suppl) 1978:429–431. [PubMed] [Google Scholar]
- Kuypers HG. A new look at the organization of the motor system. Prog Brain Res. 1982;57:381–403. doi: 10.1016/S0079-6123(08)64138-2. [DOI] [PubMed] [Google Scholar]
- Lawrence DG, Kuypers HG. The functional organization of the motor system in the monkey. I. The effects of bilateral pyramidal lesions. Brain. 1968;91:1–14. doi: 10.1093/brain/91.1.1. [DOI] [PubMed] [Google Scholar]
- Lin JH, Hsu MJ, Sheu CF, Wu TS, Lin RT, Chen CH, Hsieh CL. Psychometric comparisons of 4 measures for assessing upper-extremity function in people with stroke. Phys Ther. 2009;89:840–850. doi: 10.2522/ptj.20080285. [DOI] [PubMed] [Google Scholar]
- MacNeilage PF, Rogers LJ, Vallortigara G. Origins of the left & right brain. Sci Am. 2009;301:60–67. doi: 10.1038/scientificamerican0709-60. [DOI] [PubMed] [Google Scholar]
- Mani S, Mutha PK, Przybyla A, Haaland KY, Good DC, Sainburg RL. Contralesional motor deficits after unilateral stroke reflect hemisphere-specific control mechanisms. Brain. 2013;136:1288–1303. doi: 10.1093/brain/aws283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani S, Przybyla A, Good DC, Haaland KY, Sainburg RL. Contralesional arm preference depends on hemisphere of damage and target location in unilateral stroke patients. Neurorehabil Neural Repair. 2014;28:584–593. doi: 10.1177/1545968314520720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutha PK, Haaland KY, Sainburg RL. The effects of brain lateralization on motor control and adaptation. J Mot Behav. 2012;44:455–469. doi: 10.1080/00222895.2012.747482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutha PK, Haaland KY, Sainburg RL. Rethinking motor lateralization: specialized but complementary mechanisms for motor control of each arm. PLoS ONE. 2013;8:e58582. doi: 10.1371/journal.pone.0058582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutha PK, Sainburg RL, Haaland KY. Coordination deficits in ideomotor apraxia during visually targeted reaching reflect impaired visuomotor transformations. Neuropsychologia. 2010;48:3855–3867. doi: 10.1016/j.neuropsychologia.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutha PK, Sainburg RL, Haaland KY. Critical neural substrates for correcting unexpected trajectory errors and learning from them. Brain. 2011a;134:3647–3661. doi: 10.1093/brain/awr275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutha PK, Sainburg RL, Haaland KY. Left parietal regions are critical for adaptive visuomotor control. J Neurosci. 2011b;31:6972–6981. doi: 10.1523/JNEUROSCI.6432-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutha PK, Stapp LH, Sainburg RL, Haaland KY. Frontal and parietal cortex contributions to action modification. Cortex. 2014;57:38–50. doi: 10.1016/j.cortex.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki D, Kurtzer I, Scott S. Limited transfer of learning between unimanual and bimanual skills within the same limb. Nat Neurosci. 2006;9:3. doi: 10.1038/nn1785. [DOI] [PubMed] [Google Scholar]
- Oujamaa L, Relave I, Froger J, Mottet D, Pelissier JY. Rehabilitation of arm function after stroke. Literature review. Ann Phys Rehabil Med. 2009;52:269–293. doi: 10.1016/j.rehab.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Pandian S, Arya KN, Kumar D. Effect of motor training involving the less-affected side (MTLA) in post-stroke subjects: a pilot randomized controlled trial. Topics in stroke rehabilitation. 2015 doi: 10.1179/1074935714Z.0000000022. [DOI] [PubMed] [Google Scholar]
- Pohl PS, Winstein CJ. Practice effects on the less-affected upper extremity after stroke. Arch Phys Med Rehabil. 1999;80:668–675. doi: 10.1016/s0003-9993(99)90170-3. [DOI] [PubMed] [Google Scholar]
- Poole JL, Sadek J, Haaland KY. Ipsilateral deficits in 1-handed shoe tying after left or right hemisphere stroke. Arch Phys Med Rehabil. 2009;90:1800–1805. doi: 10.1016/j.apmr.2009.03.019. [DOI] [PubMed] [Google Scholar]
- Prestopnik J, Haaland K, Knight R, Lee R. Hemispheric dominance for open and closed loop movements. Soc Neurosci Abstr. 2003a:30. [Google Scholar]
- Prestopnik J, Haaland K, Knight R, Lee R. Hemispheric dominance in the parietal lobe for open and closed loop movements. J Int Neuropsychol Soc. 2003b;9:1–2. [Google Scholar]
- Rapin I, Tourk LM, Costa LD. Evaluation of the Purdue Pegboard as a screening test for brain damage. Dev Med Child Neurol. 1966;8:45–54. doi: 10.1111/j.1469-8749.1966.tb08272.x. [DOI] [PubMed] [Google Scholar]
- Rose DK, Winstein CJ. The co-ordination of bimanual rapid aiming movements following stroke. Clin Rehabil. 2005;19:452–462. doi: 10.1191/0269215505cr806oa. [DOI] [PubMed] [Google Scholar]
- Sainburg RL. Evidence for a dynamic-dominance hypothesis of handedness. Exp Brain Res. 2002;142:241–258. doi: 10.1007/s00221-001-0913-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL. Convergent models of handedness and brain lateralization. Front Psychol. 2014;5:1092. doi: 10.3389/fpsyg.2014.01092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer SY, Haaland KY, Sainburg RL. Ipsilesional motor deficits following stroke reflect hemispheric specializations for movement control. Brain. 2007;130:2146–2158. doi: 10.1093/brain/awm145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer SY, Haaland KY, Sainburg RL. Dissociation of initial trajectory and final position errors during visuomotor adaptation following unilateral stroke. Brain Res. 2009a;1298:78–91. doi: 10.1016/j.brainres.2009.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer SY, Haaland KY, Sainburg RL. Hemispheric specialization and functional impact of ipsilesional deficits in movement coordination and accuracy. Neuropsychologia. 2009b;47:2953–2966. doi: 10.1016/j.neuropsychologia.2009.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer SY, Mutha PK, Haaland KY, Sainburg RL. Hemispheric specialization for movement control produces dissociable differences in online corrections after stroke. Cereb Cortex. 2012;22:1407–1419. doi: 10.1093/cercor/bhr237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selles RW, Michielsen ME, Bussmann JB, Stam HJ, Hurkmans HL, Heijnen I, de Groot D, Ribbers GM. Effects of a mirror-induced visual illusion on a reaching task in stroke patients: implications for mirror therapy training. Neurorehabil Neural Repair. 2014;28:652–659. doi: 10.1177/1545968314521005. [DOI] [PubMed] [Google Scholar]
- Stevens JA, Stoykov ME. Simulation of bilateral movement training through mirror reflection: a case report demonstrating an occupational therapy technique for hemiparesis. Topics Stroke Rehabil. 2004;11:59–66. doi: 10.1310/GCFE-QA7A-2D24-KHRU. [DOI] [PubMed] [Google Scholar]
- Tanji J, Okano K, Sato KC. Neuronal activity in cortical motor areas related to ipsilateral, contralateral, and bilateral digit movements of the monkey. J Neurophysiol. 1988;60:325–343. doi: 10.1152/jn.1988.60.1.325. [DOI] [PubMed] [Google Scholar]
- Wang J, Sainburg RL. Generalization of visuomotor learning between bilateral and unilateral conditions. J Neurophysiol. 2009;102:2790–2799. doi: 10.1152/jn.00444.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetter S, Poole JL, Haaland KY. Functional implications of ipsilesional motor deficits after unilateral stroke. Arch Phys Med Rehabil. 2005;86:776–781. doi: 10.1016/j.apmr.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Winstein CJ, Merians AS, Sullivan KJ. Motor learning after unilateral brain damage. Neuropsychologia. 1999;37:975–987. doi: 10.1016/s0028-3932(98)00145-6. [DOI] [PubMed] [Google Scholar]
- Winstein CJ, Pohl PS. Effects of unilateral brain damage on the control of goal-directed hand movements. Exp Brain Res. 1995;105:163–174. doi: 10.1007/BF00242191. [DOI] [PubMed] [Google Scholar]
- Winstein CJ, Pohl PS, Cardinale C, Green A, Scholtz L, Waters CS. Learning a partial-weight-bearing skill: effectiveness of two forms of feedback [published erratum appears in Phys Ther 1997 Mar; 77(3):328] Phys Ther. 1996;76:985–993. doi: 10.1093/ptj/76.9.985. [DOI] [PubMed] [Google Scholar]
- Wolf SL, Blanton S, Baer H, Breshears J, Butler AJ. Repetitive task practice: a critical review of constraint-induced movement therapy in stroke. Neurologist. 2002;8:325–338. doi: 10.1097/01.nrl.0000031014.85777.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf SL, Lecraw DE, Barton LA, Jann BB. Forced use of hemiplegic upper extremities to reverse the effect of learned nonuse among chronic stroke and head-injured patients. Exp Neurol. 1989;104:125–132. doi: 10.1016/s0014-4886(89)80005-6. [DOI] [PubMed] [Google Scholar]
- Wolf SL, Thompson PA, Morris DM, Rose DK, Winstein CJ, Taub E, Giuliani C, Pearson SL. The EXCITE trial: attributes of the Wolf motor function test in patients with subacute stroke. Neurorehabil Neural Repair. 2005;19:194–205. doi: 10.1177/1545968305276663. [DOI] [PubMed] [Google Scholar]
- Wolf SL, Winstein CJ, Miller JP, Taub E, Uswatte G, Morris D, Giuliani C, Light KE, Nichols-Larsen D. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA. 2006;296:2095–2104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]
- Wyke M. Effect of brain lesions on the rapidity of arm movement. Neurology. 1967;17:1113–1120. doi: 10.1212/wnl.17.11.1113. [DOI] [PubMed] [Google Scholar]
- Yadav V, Sainburg RL. Motor lateralization is characterized by a serial hybrid control scheme. Neuroscience. 2011;196:153–167. doi: 10.1016/j.neuroscience.2011.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav V, Sainburg RL. Handedness can be explained by a serial hybrid control scheme. Neuroscience. 2014;278:385–396. doi: 10.1016/j.neuroscience.2014.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarosh CA, Hoffman DS, Strick PL. Deficits in movements of the wrist ipsilateral to a stroke in hemiparetic subjects. J Neurophysiol. 2004;92:3276–3285. doi: 10.1152/jn.00549.2004. [DOI] [PubMed] [Google Scholar]