Abstract
Maternal transmission is the main transmission pathway of facultative bacterial endosymbionts, but phylogenetically distant insect hosts harbor closely related endosymbionts, suggesting that horizontal transmission occurs in nature. Here we report the first case of plant-mediated horizontal transmission of Wolbachia between infected and uninfected Bemisia tabaci AsiaII7 whiteflies. After infected whiteflies fed on cotton leaves, Wolbachia was visualized, both in the phloem vessels and in some novel ‘reservoir' spherules along the phloem by fluorescence in situ hybridization using Wolbachia-specific 16S rRNA probes and transmission electron microscopy. Wolbachia persisted in the plant leaves for at least 50 days. When the Wolbachia-free whiteflies fed on the infected plant leaves, the majority of them became infected with the symbiont and vertically transmitted it to their progeny. Multilocus sequence typing and sequencing of the wsp (Wolbachia surface protein) gene confirmed that the sequence type of Wolbachia in the donor whiteflies, cotton phloem and the recipient whiteflies are all identical (sequence type 388). These results were replicated using cowpea and cucumber plants, suggesting that horizontal transmission is also possible through other plant species. Our findings may help explain why Wolbachia bacteria are so abundant in arthropods, and suggest that in some species, Wolbachia may be maintained in populations by horizontal transmission.
Introduction
Offspring vertically inherit both nuclear and non-nuclear genetic material from their mothers. Intracellular bacteria are the non-nuclear materials inherited vertically from mother to offspring (Oliver et al., 2010). There has been an increasing interest in intracellular bacteria over the past two decades, because of their widespread distribution in nature and their significance to the ecology, evolution and reproductive biology of their hosts (Gotoh et al., 2007; Himler et al., 2011; Segoli et al., 2013; Baldini et al., 2014). Innumerable species of insects and other arthropods are associated with various intracellular bacteria (Hilgenboecker et al., 2008; Watts et al., 2009; Jaenike and Brekke, 2011). These bacteria often live in symbioses with their hosts (Oliver et al., 2010), and may be obligate (that is, primary endosymbionts essential for host survival) or facultative (that is, secondary endosymbionts that can increase or decrease host fitness; Himler et al., 2011; Jiggins and Hurst, 2011). The obligate symbionts are found within specialized cells and typically share a long evolutionary history with their hosts (Buchner, 1965), whereas the facultative symbionts tend to have more recently formed associations with their hosts. Wolbachia (Alphaproteobacteria: Rickettsiales) is a genus of facultative endosymbionts common among arthropods and is estimated to have infected the majority of arthropods and filarial nematodes. In arthropods, Wolbachia most commonly interact with their hosts via a parasitic manipulation of the reproductive system (Werren et al., 2008). As with other facultative endosymbionts, Wolbachia have been thought to undergo primarily vertical transmission from mother to offspring with high fidelity. The horizontal transmission pathway is thought to be the most likely explanation for closely related symbionts occurring in phylogenetically distant insects (Werren et al., 1995; Vavre et al., 1999; Noda et al., 2001; Baldo et al., 2008; Ahmed et al., 2013). Over the past two decades, there have been multiple phylogenetic and transinfection studies reporting evidence of Wolbachia transmission between both phylogenetically close and phylogenetically distant species (Boyle et al., 1993; Heath et al., 1999; Vavre et al., 1999). Thus, it is probable that Wolbachia horizontal transmission is occurring between some arthropod taxa (Ahmed et al., 2015). Although Wolbachia has been shown to undergo extensive horizontal transmission between several host taxa (Werren et al., 1995; Baldo et al., 2006; Chiel et al., 2009; Raychoudhury et al., 2009; Oliver et al., 2010; Ahmed et al., 2015), the mechanisms for this are poorly understood.
There is growing evidence for common horizontal transmission of Wolbachia from one species to another (Ahmed et al., 2013; Gerth et al., 2013; Brown and Lloyd, 2015), and the transmission route is becoming a hotspot of research, given its importance in ecological and evolutionary biology (Vavre et al., 1999; Sintupachee et al., 2006; Caspi-Fluger et al., 2012; Gehrer and Vorburger, 2012). Recently, Wolbachia horizontal transmission through invertebrate predators and parasitoids has been revealed (Huigens et al., 2000, 2004; Le Clec'h et al., 2013; Ahmed et al., 2015). However, the presence of identical strains of Wolbachia among species that do not share predators and parasitoids but have similar habitats, such as shared host plants or food sources, suggests that plants or food may be involved in Wolbachia horizontal transmission (Sintupachee et al., 2006; Caspi-Fluger et al., 2012; Weinert et al., 2015).
The whitefly Bemisia tabaci (Hemiptera: Aleyrodidae) is a small hemipterous insect that feeds on phloem sap of numerous host plants; it has a very wide host plant range of over 500 species worldwide (Stansly and Naranjo, 2010). Bemisia tabaci is a complex of distinct cryptic species, harboring various bacterial symbionts such as Wolbachia, Arsenophonus, Cardinium, Hamiltonella and Rickettsia, but endosymbionts vary largely among the different whitefly species (Chiel et al., 2007; Ahmed et al., 2010; Skaljac et al., 2013). Here, we investigated if host plant had a role in the horizontal transmission of Wolbachia between whiteflies. We also studied the transfer dynamics of Wolbachia during this plant-mediated transmission.
Materials and methods
Plants and insects
Cotton plants (Gossypium hirsutum L. var. Lumianyan no. 32) were used in this study. Cotton seeds were sown in 15-cm-diameter plastic pots containing a soil–sand mixture (10% sand, 5% clay and 85% peat) in a greenhouse at ambient temperature and photoperiod. Plants were watered as necessary before being used in experiments at the 6–8 expanded leaf stage.
The whiteflies used in the study were Bemisia tabaci AsiaII7 (formerly known as Cv biotype), which is an indigenous cryptic species in South China (De Barro and Ahmed, 2011). Details regarding the collection, rearing and Wolbachia infection monitoring of AsiaII7 whiteflies are shown in the Supplementary Method S1.
Wolbachia transmission from whiteflies to cotton plants
We investigated the effects of the number and feeding time of Wolbachia-positive AsiaII7 on the efficiency of Wolbachia transmission from whiteflies to cotton plants. We collected AsiaII7 adults 24–48 h after they emerged from the pupal stage, using a hand aspirator from the Wolbachia-positive subcolony. In AsiaII7, Wolbachia created the scattered infection pattern described by Ahmed et al. (2015). Whiteflies were released into leaf cages (2 cm high, 3 cm diameter) covered on the undersurface of clean, healthy cotton leaves (as shown in Supplementary Figure S5). We studied three treatments, each with a different amount of whiteflies per cage (1 pair, 5 pairs and 10 pairs), and performed 10 replicates per treatment.
Two days after the AsiaII7 B. tabaci were released into the leaf cages, we recorded which leaves were being fed upon by the whiteflies (herein referred to as ‘fed leaves'). We then began cutting ~0.01 g of leaf material (equivalent to leaf surface area of ~50 mm2) every 24 h from both the fed leaf and from the leaf immediately below it on the same stem (~2 cm distance between the bases of the two leaves). The presence of Wolbachia in the cotton leaves was detected by PCR using the Wolbachia-specific primers of Wolbachia surface protein (wsp) gene and 16S rRNA genes (O'Neill et al., 1992; Braig et al., 1998); the primers and protocols for PCR detection are shown in Supplementary Table S1 and Supplementary Method S2. The initial time when Wolbachia was positively detected in cotton leaves was recorded for each replicate. For negative controls, the same procedure was followed using whiteflies that were collected from the Wolbachia-free subcolony.
Visualization of Wolbachia in plants
Fluorescence in situ hybridization (FISH) was used to identify the location of Wolbachia in cotton plants. Ten pairs of AsiaII7 were released onto a single leaf of each plant and were allowed to feed for 25–28 days. Then, a 50 mm2 leaf section (10 mm × 5 mm) was removed by cutting longitudinally along the leaf vein; an equally sized section was also removed from the leaf immediately below the fed leaf. These leaf samples were placed in Carnoy's fixative. FISH detection was then performed following the method of Sakurai et al. (2005; see Supplementary Method S3), using a Wolbachia-specific 16S rRNA probe (W2-Cy3: 5′-CTTCTGTGAGTACCGTCATTATC-3′) that had its specificity tested with the Ribosomal Database Project II ‘probe match' analysis tool (http://rdp.cme.msu.edu) by Gottlieb et al. (2008). The stained leaf samples were mounted and viewed under a Nikon eclipse Ti-U FluoView inverted microscope (Nikon Instruments Inc., Tokyo, Japan). Specificity of Wolbachia detection was confirmed using two controls: (1) Wolbachia-infected cotton leaves without the Wolbachia 16S rRNA probe and (2) Wolbachia-free cotton leaves with the Wolbachia 16S rRNA probe. FISH visualization experiments were also performed on cowpea and cucumber plants, with the same protocol and primers used in the cotton plant experiment.
Transmission electron microscopy was used to confirm the location of Wolbachia in the cotton leaves. Samples of Wolbachia-deposited cotton leaves (1.0 mm × 0.5 mm) were fixed in 4% glutaraldehyde in cacodylate buffer (pH 7.4) at 4 °C for 24 h, and then overnight in 1% osmium tetroxide. The fixed leaf samples were dehydrated through an alcohol series and embedded in Spurr's resin. Ultrathin sections were collected on copper grids with a single slot, stained with 1% uranyl acetate and lead citrate, and finally examined under a Transmission electron microscopy (JEOL, Tokyo, Japan).
Persistence of Wolbachia in cotton plants
Two experiments, each with three replicates, were performed to study the persistence of Wolbachia in the cotton leaves. In the first experiment, 10 two-day-old pairs of Wolbachia-positive AsiaII7 adults were released into a leaf cage to feed on the cotton leaves for 24 days (we have already shown that the 24th day is approximately the earliest day in which Wolbachia can be detected in plant leaves). On day 25th, the adult whiteflies and their immature progeny were collected and 0.01 g of the fed leaves were cut for DNA extraction and the detection of Wolbachia presence using PCR and quantitative real-time PCR (q-PCR) with the wsp primers. For the next 50 days, additional 0.01 g leaf segments were cut every 5 days and tested for the presence of Wolbachia; a total of 11 sets of leaf samples were tested in this experiment. The second experiment was run with a nearly identical protocol, except the 10 pairs of AsiaII7 whiteflies and their offspring were not collected on day 25th. Instead, they were allowed to feed continuously on the cotton leaves during the experiment. The wsp primers used in PCR detection were wsp 81F and wsp 691R (Supplementary Table S1) and wsp primers used in q-PCR were wsp-QF and wsp-QR (Supplementary File Supplementary Method S2). A UBQ7 gene of cotton fiber (DQ116441) was used as an internal control for data normalization and quantification (Tu et al., 2007). Detailed procedures for PCR and q-PCR Wolbachia detection are shown in Supplementary Method S2.
To test whether the Wolbachia detected in the plants was still alive, RNA was extracted from leaf discs (2 cm diameter) of five cotton plants that had each been exposed to Wolbachia-positive AsiaII7 whiteflies for a different amount of time (24, 34, 44, 54 or 64 days). RNA was also extracted from a cotton plant that had not been exposed to whiteflies, as a negative control. Extractions were performed using the Trizol RNA Extraction Kit (Omega, Stamford, CT, USA) following the protocol in the instruction manual. The RNA was then reverse transcribed to cDNA using Moloney murine leukemia virus (Invitrogen, Carlsbad, CA, USA) and the specific 16S rRNA primers (Supplementary Table S1).
Wolbachia transmission from cotton to whitefly and its subsequent vertical transmission
To detect the horizontal transmission of Wolbachia from cotton plants to whiteflies, 10 newly emerged adults collected from the Wolbachia-negative colony were released into 10 different leaf cages. They were allowed to feed on the Wolbachia-positive cotton leaves for 20 days, and then collected for Wolbachia PCR detection. This experiment was repeated 10 times.
To evaluate the Wolbachia acquisition efficiency of recipient whiteflies, 100 pairs of AsiaII7 adults from the Wolbachia-negative colony were introduced into 20 leaf cages (five pairs per cage) covered on the Wolbachia-positive cotton leaves. Additional Wolbachia-negative whitefly adults were released into a leaf cage with Wolbachia-free leaves, as a negative control. Every 2 days, five randomly selected pairs of adults were collected for Wolbachia PCR and q-PCR using the wsp primers (see Supplementary Table S1 and Supplementary Method S4, respectively). A β-actin gene of B. tabaci was used as an internal control for data normalization and quantification in q-PCR (Ghanim and Kontsedalov, 2009); the detailed procedure for q-PCR is described in Supplementary Method S4. This q-PCR experiment was repeated three times.
In the instances where Wolbachia was detected in a recipient whitefly, RNA was extracted from the whitefly to test whether the Wolbachia was still alive. Extractions were performed using the Total RNA Mini Kit (Bio-Rad, Hercules, CA, USA) following the procedure in the instruction manual. DNase was added to remove DNA contamination, and cDNA was synthesized using a Verso cDNA Kit (Thermo Scientific, Waltham, MA, USA). Reactions without the reverse transcriptase enzyme (to exclude DNA contamination) were used as negative controls for the RT-PCR.
To test if the Wolbachia acquired by AsiaII7 during feeding can be vertically transferred in subsequent generations of AsiaII7, we randomly selected 10 pairs of adults that had been feeding on Wolbachia-positive leaves for 15 days (Wolbachia can be initially detected in a recipient whitefly after 10 days). Each pair was then introduced into a separate leaf cage containing new healthy cotton leaves to encourage oviposition. After 24 h, the adult whiteflies were removed from the cages and used for Wolbachia PCR detection; all eggs (the F1 generation) were left in the cages and allowed to fully mature. Twenty randomly selected specimens of the offspring adults were then used to examine the Wolbachia distribution pattern, and the others were used to determine the percentage of F1 whiteflies that retained Wolbachia. This experiment was repeated 10 times.
Multilocus sequence typing and phylogenetic analysis of Wolbachia
Multilocus sequence typing (MLST) was used to identify Wolbachia strains in (1) donor Wolbachia-positive whiteflies, (2) recipient Wolbachia-negative whiteflies, (3) the F1 generation progeny of the newly infected whiteflies and (4) cotton leaves. Five MLST genes (gatB, coxA, hcpA, ftsZ and fbpA) as well as the wsp gene were sequenced following the methods of Baldo et al. (2006). The five MLST genes were concatenated using Geneious (version r8; Kearse et al., 2012). MLST loci were blasted against the Wolbachia MLST database (http://pubmlst.org/Wolbachia). Two MLST loci from supergroup A, four from supergroup B, one from supergroup D and our MLST loci were analyzed, using maximum likelihood (ML) in RAxML (Stamatakis, 2006), to construct a phylogenetic tree (Supplementary Table S2 and Supplementary Method S5).
Transmission of Wolbachia through other plant species
To examine Wolbachia transmission through other plant species, we repeated the Wolbachia horizontal transmission and FISH visualization experiments using cucumber, Cucumis sativus L. (var. Xiayou168), and cowpea, Vigna unguiculata (L.) Walp (var. Kefeng), instead of cotton. Wolbachia was transmitted from Wolbachia-positive AsiaII7 to cucumber and cowpea plants, and then to the Wolbachia-negative AsiaII7 individuals and their F1 generation offspring. PCR detection of Wolbachia in the new host plant species, determinations of the strain genotypes and phylogenetic analyses were all conducted using the same methodology as in the cotton plant experiments.
Results
Wolbachia can be transmitted to cotton plants by whiteflies
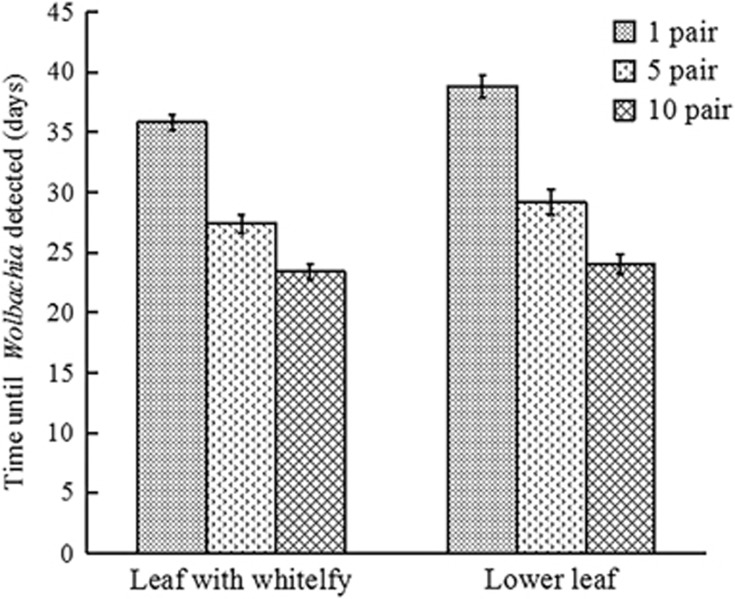
Results of the Wolbachia PCR detection using wsp and 16S rRNA gene primers (Supplementary Table S1) revealed that this endosymbiont could be detected in cotton plants after several weeks of Wolbachia-positive whitefly feeding. In samples taken from leaves that the whiteflies were directly feeding on (that is, the fed leaves), the time interval from when the whiteflies began feeding to when Wolbachia was initially detected in the leaf was inversely correlated with the number of infected whiteflies in the leaf cage (Figure 1, 35.8±0.6, 27.4±0.8 and 23.4±0.7 days for the groups of 1, 5 and 10 pairs of insects, respectively, mean±s.e.). Wolbachia was also detected in samples taken from leaves immediately below the fed leaves; that is, in each treatment, Wolbachia was positively detected 1–3 days after Wolbachia was detectable on the corresponding fed leaf (Figure 1). In the negative control treatments, which only involved Wolbachia-free whiteflies, Wolbachia was not detected on any of the cotton leaves.
Figure 1.
Correlation between the number of Wolbachia-infected whiteflies (AsiaII7 Bemisia tabaci) feeding on cotton plants and the amount of time (as of the initial whitefly introduction) before Wolbachia was initially detected in the cotton leaves. The column and error bars are the mean ±s.e. of time (in days) and 10 replicated were repeated in each column.
The localization of Wolbachia in cotton leaves
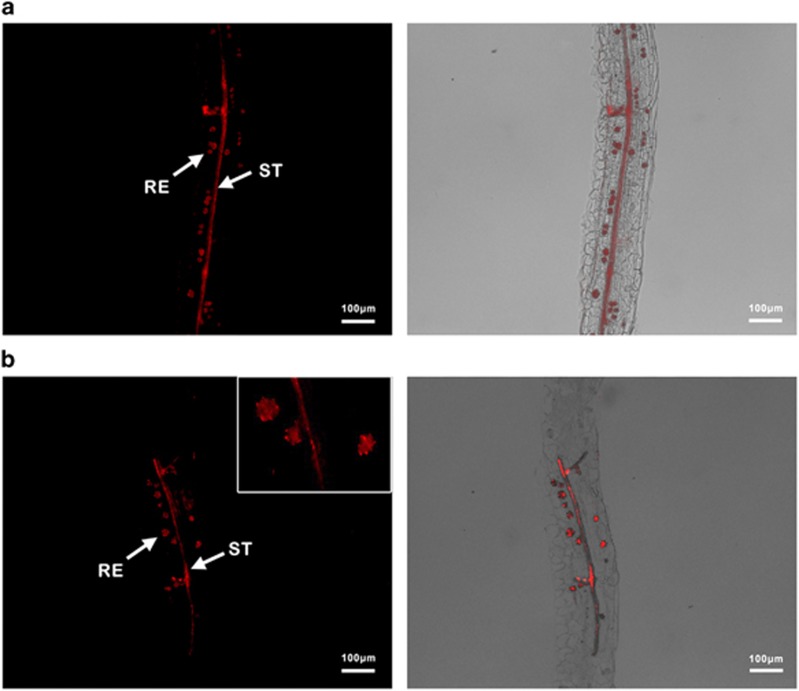
Using a cyanine3-labeled Wolbachia-specific 16S rRNA probe (Supplementary Method S3), the FISH visualization of the fed leaves revealed that Wolbachia could be found in most parts of the phloem sieve tube, which is usually 5–8 cm from the whitefly feeding site. Wolbachia was unexpectedly also found in some novel globular regions along the phloem sieve tube (Figure 2a and Supplementary Figure S1). Wolbachia was also found in the phloem of the leaves immediately below the fed leaves (Figure 2b). This presence of Wolbachia in cotton leaves that had not been directly fed on by whiteflies suggests that Wolbachia can move between leaves after the initial transmission. As with the cotton leaves, spherules of Wolbachia were also in the phloem of the cowpea and cucumber leaves that had been exposed to Wolbachia-positive whiteflies (Supplementary Figure S2). However, the quantities and sizes of these spherules were different from those found in the cotton leaves (Supplementary Figure S2). Wolbachia was not observed in any leaves from the negative controls (Supplementary Figure S3).
Figure 2.
FISH visualization of Wolbachia in cotton leaves. Figures show the leaf tissue longitudinally along the leaf phloem. (a) Wolbachia in a leaf directly fed on by AsiaII7 whiteflies; (b) Wolbachia in the leaf immediately below the fed leaf; ST, phloem sieve tube; RE, reservoir of Wolbachia along the phloem; left panels: fluorescence in the dark field; right panels: fluorescence in the bright field.
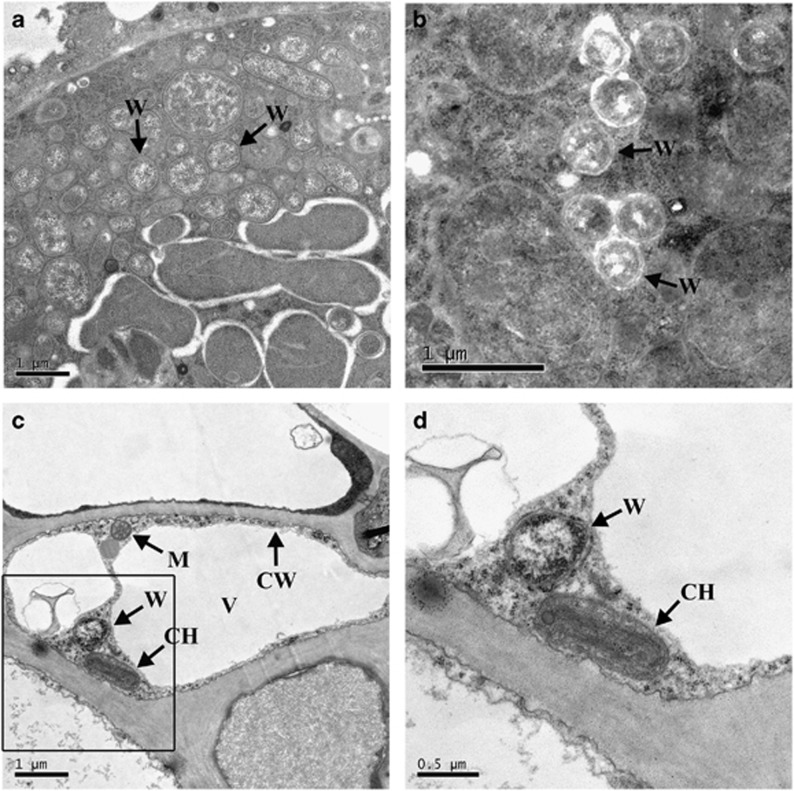
Transmission electron microscopy images were used to visualize Wolbachia morphology in the bacteriocytes located in the adult whitefly abdomen and in the cotton leaf phloem. In the whitefly bacteriocytes, most individual Wolbachia have a small (0.5–1 μm), irregular coccoid form with a double membrane (Figures 3a and b). In the phloem, Wolbachia was found in the vacuole of a plant cell and was morphologically similar to those in whitefly adult abdomens (Figures 3c and d).
Figure 3.
TEM images of Wolbachia in the bacteriocyte located in the abdomen of an adult AsiaII7 whitefly (a, b) and in the phloem sieve tube of a cotton leaf (c, d). CW, cell wall of the plant phloem; CH, chloroplast; M, mitochondrion; V, vacuole of the plant cell; W, presence of Wolbachia in a double membrane cell.
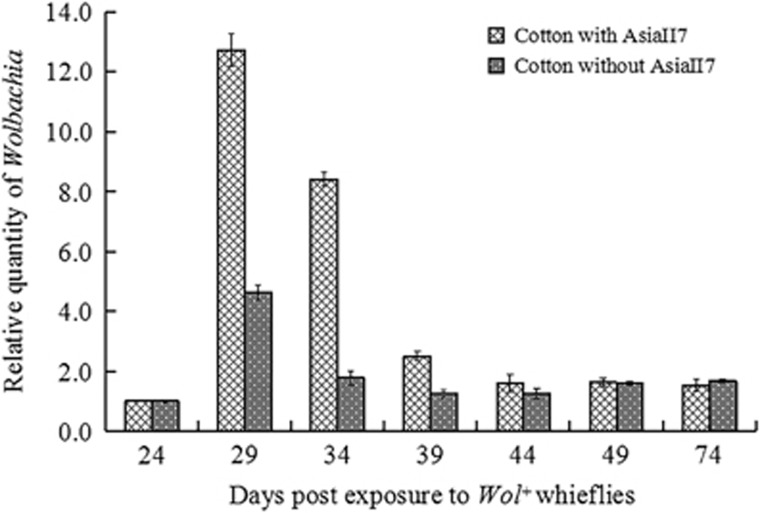
Changes in the amount of Wolbachia in cotton leaves over time
In this study, two plant groups were exposed to Wolbachia-infected AsiaII7 whiteflies. In one group, the whiteflies were removed after the first 24 days, whereas in the other group, the whiteflies were left on the plants for the entire duration of the experiment (74 days). Wolbachia persisted in cotton leaves for at least 50 days after its first detection (that is, 74 days after the Wolbachia-positive whiteflies first fed on the leaf) in both of the plant groups, regardless of whether the whiteflies had been removed (Figure 4). The relative quantity of Wolbachia in all treatments increased to its highest amount during the first 5–10 days after it was positively detected (that is, days 25th–34th), and during this period the leaves that were continuously fed on by whiteflies had significantly higher quantities of Wolbachia than the leaves that had the whiteflies removed. After reaching this peak quantity, the amount of Wolbachia reduced gradually in leaves from both groups.
Figure 4.
Retention time (days) and relative quantity of Wolbachia endosymbiont in cotton leaves since the initial introduction of Wolbachia-positive whiteflies to the 50th day after introduction. The relative quantity (rq) of Wolbachia was calculated based on its wsp gene using the formula rq=2−ΔΔct. The column and error bars were the mean±s.e. of time (days) with three replicates for each column.
Wolbachia transmission from cotton to whiteflies and its subsequent vertical transmission
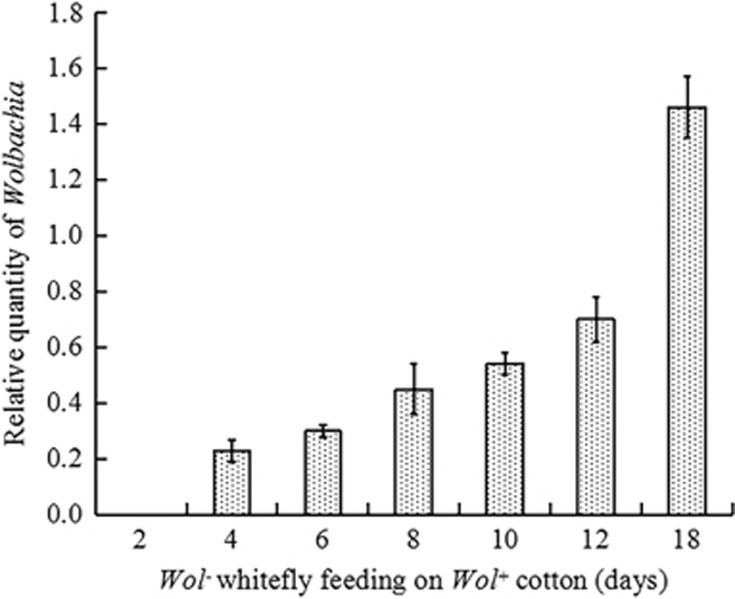
When uninfected adult whiteflies continuously fed on the Wolbachia-infected cotton leaves, Wolbachia was initially detected in the recipient whiteflies after 10.0±0.3 days. After 20 days of feeding, Wolbachia was detected in 62.0±5.5% of the recipient female whiteflies. In the more sensitive q-PCR evaluation, Wolbachia was initially detected in the recipient whiteflies after 4 days of feeding on the Wolbachia-infected cotton leaves, and the relative quantity of Wolbachia in female adults greatly increased as the feeding time increased (Figure 5). We also found that 95.0±1.67% of the F1 whitefly adults tested positive for Wolbachia, indicating that Wolbachia can be vertically transmitted from newly infected female whiteflies to their offspring (Supplementary Figure S4).
Figure 5.
Dynamics of Wolbachia in recipient AsiaII7 whiteflies that have fed on Wolbachia-positive cotton leaves. The column and error bars are the mean±s.e. of time (in days) with three replicates repeated in each column.
Multilocus sequence typing and phylogenetic analysis of Wolbachia from plants and whiteflies
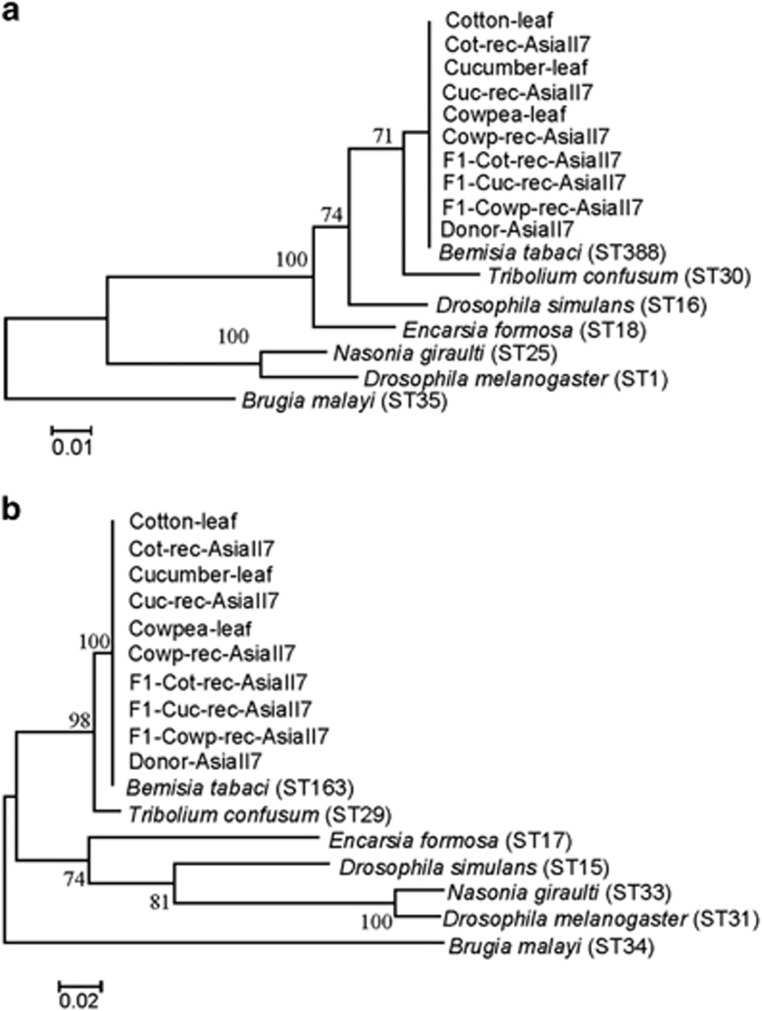
Our genetic analysis of the Wolbachia strain being transmitted between cotton plants and AsiaII7 whiteflies revealed that all of the tested Wolbachia samples were identical. The Wolbachia from the donor whiteflies, the infected cotton leaves, the recipient AsiaII7 whiteflies and the recipient whitefly offspring are all Wolbachia ST388 (Figure 6a and Supplementary Table S3). These results were confirmed by DNA sequencing and by our phylogenetic analysis (Figure 6b). Furthermore, all of the tested Wolbachia endosymbionts belong to the Con group within Supergroup B (Figure 6). No sequence variation was found in the wsp gene of these Wolbachia endosymbionts.
Figure 6.
Phylogenetic analysis of Wolbachia strains detected from different hosts. (a) The ML phylogenetic tree based on five MLST genes, using a GTR model. Support values are based on 1000 bootstrap iterations. The numbers within parentheses represent the sequence types of Wolbachia in the Wolbachia MLST database. The capital letters represent the supergroups of different Wolbachia resources. (b) The ML tree based on wsp gene sequences using a Tamura 3-parameter model. Support values are based on 1000 bootstrap iterations. Wolbachia hosts: ‘Donor AsiaII7': Wolbachia-positive AsiaII7 B. tabaci; ‘Cotton/Cucumber/Cowpea leaf': Wolbachia in the infected cotton/cucumber/cowpea plants; ‘Cot/Cuc/Cowp-rec AsiaII7': recipient AsiaII7 B. tabaci that became infected with Wolbachia after feeding on Wolbachia-positive cotton/cucumber/cowpea leaves; ‘F1 Cot/Cuc/Cowp-rec AsiaII7': progeny of the Cot/Cuc/Cowp-rec AsiaII7 whiteflies.
Transmission of Wolbachia through different plant species
After repeating our Wolbachia transmission and detection experiments with two additional types of plants, cucumber and cowpea, we found evidence that the whiteflies' choice of host plant does not have any effect on the sequence type or the fidelity of Wolbachia during horizontal transmission (Figure 6). This demonstrates that this particular Wolbachia strain (ST388) remains highly conservative during its plant-mediated horizontal transmission.
Discussion
There has been an increased interest in studying the horizontal transmission of intracellular bacterial endosymbionts over the past two decades, particularly Wolbachia and Rickettsia because of their widespread distribution and significant role in the ecology and evolution of their hosts (Vavre et al., 1999; Sintupachee et al., 2006; Gotoh et al., 2007; Himler et al., 2011; Caspi-Fluger et al., 2012). The vector-mediated interspecific transmission of intracellular bacterial endosymbionts was observed through shared food sources (in Wolbachia, Spiroplasma, Hamiltonella defensa; Rigaud and Juchault, 1995; Oliver et al., 2010; Caspi-Fluger et al., 2012), ectoparasitic mites (Jaenike et al., 2007; Gehrer and Vorburger, 2012), host plants (in Rickettsia, Arsenophonus; Caspi-Fluger et al., 2012; Bressan, 2014) and parasitoids (in Arsenophonus, Wolbachia; Duron et al., 2010; Ahmed et al., 2015). The hypothesis that Wolbachia can be horizontally transmitted between two insect species has been supported by both phylogenetic and experimental analyses (Vavre et al., 1999; Huigens et al., 2000, 2004; Sintupachee et al., 2006; Ahmed et al., 2013; Yang et al., 2013). Ahmed et al. (2013) compared the phylogeny of different Bemisia species and their endosymbionts, revealing the incongruence of Wolbachia with their whitefly hosts and suggesting a host shift of Wolbachia through horizontal transmission. Recently, Ahmed et al. (2015) revealed that parasitoids can transmit Wolbachia by feeding and probing their uninfected hosts. Both the phylogenetic analysis and transmission experiments demonstrated that parasitoids could serve as potential routes for horizontal transmission of Wolbachia between different hosts. Sintupachee et al. (2006) discovered a potential route for lateral transmission of Wolbachia between different insects that share the same leaf substrate in pumpkin plants. Huigens et al. (2000, 2004) discovered a frequent horizontal transmission of Wolbachia from infected to uninfected wasp larvae (Trichogramma kaykai) when they feed on a common food source: eggs of the butterfly Apodemia mormo deserti. After the wasps matured, the females then vertically transmitted Wolbachia to their offspring. Sintupachee et al. (2006) showed that four taxonomically diverse insects feeding on the same host plant contained very closely related Wolbachia, suggesting the potential role of host plants in Wolbachia horizontal transmission (Sintupachee et al., 2006). Work by Yang et al. (2013) also showed that identical strains of Wolbachia can be shared by two species that live in the same plant tissue: the gall wasp Andricus mukaigawae and its inquiline wasp Synergus japonicas. Stahlhut et al. (2010) used a multigene approach to provide evidence that ecological associations can facilitate horizontal transmission of Wolbachia within mycophagous fly communities. Our current study supplements the findings on Wolbachia transmission by providing direct evidence of plant-mediated transmission of Wolbachia, including the transmission efficiency, distribution pattern and persistence of Wolbachia in both plant tissues and in the progeny of its recipient host.
The distribution patterns of endosymbionts in their hosts may have important influences on their transmission ability. Caspi-Fluger et al. (2011) found that another endosymbiont, Rickettsia, can have two distribution patterns in its whitefly host: a scattered pattern localized in the whitefly hemocoel and a confined pattern restricted to the bacteriocytes. Moreover, Chiel et al. (2009) found that the scattered pattern of Rickettsia facilitates its transmission to whitefly parasitoids. Previous work by Ahmed et al. (2015) showed that, like Rickettsia, Wolbachia can also be found in scattered and confined distribution patterns. The scattered Wolbachia have the potential to be transmitted horizontally between whiteflies through the feeding or oviposition probing of an Eretmocerus parasitoid. In the current study, we found that Wolbachia-positive AsiaII7 whiteflies with a scattered Wolbachia pattern can transmit Wolbachia to cotton leaves, and Wolbachia-negative AsiaII7 whiteflies can become infected with scattered-pattern Wolbachia after feeding on Wolbachia-positive cotton leaves for at least 10 days. This suggests similarities between the plant-mediated transmission routes of Wolbachia and Rickettsia.
The distribution pattern of Wolbachia in plant tissues has not been previously demonstrated. Wolbachia could not be detected in plant tissue until the Wolbachia-positive whiteflies had been feeding for 24 days, at which point the amount of Wolbachia noticeably increased for the ensuing 5 days. We surmise that Wolbachia requires 24 days to accumulate of a titer, with the subsequent amplification being a barrier break in the interaction between invasive Wolbachia and the physiological contents of the plant leaf. Here, for the first time, we have shown that Wolbachia can persist in cotton leaves for more than 50 days; our RNA RT experiments revealed that all of the Wolbachia endosymbionts present in the cotton leaves for 24–64 days were alive. In order for Wolbachia to live that long in the plant, there should be some sort of interaction effect or nutritional support. This requires further investigation. Interestingly, we found that some Wolbachia cluster in an irregular globular shape along the leaf phloem. The biological role of these globular clusters is unclear, but we suspect that these may be the reservoirs of Wolbachia in plant leaves. Purcell et al. (1994) found that a bacterial parasite of the leafhopper Euscelidius variegatus can be horizontally transmitted between different individuals of E. variegatus that feed on the same plant leaves. This particular bacterium did not multiply or move within the plant. In contrast, we found that Wolbachia can move within its plant after transmission; FISH visualization showed the presence of Wolbachia in leaves that had not been fed on by whiteflies.
Facultative endosymbionts have already been shown to change host fitness or biology for multiple reasons, including host protection against entomopathogenic fungi and parasitic wasps, amelioration of the detrimental effects of heat and influence on host plant suitability (Oliver et al., 2003, 2005, 2010; Scarborough et al., 2005). One main consequence of Wolbachia horizontal transmission is the induction of unknown phenotypes in the novel host (Werren et al., 2008; Ahmed et al., 2015). Wolbachia can confer positive fitness benefits by increasing the resistance against natural pathogens in fruit flies (Teixeira et al., 2008). Hornett et al. (2006) revealed that Wolbachia, in some host species, do not currently induce any phenotype but may have done so in the past, implying that more species have had their biology affected by Wolbachia than previously estimated. In other situations, after transinfection of Wolbachia, the newly induced phenotype can be suppressed by its novel host (Hornett et al., 2008) and can also be changed into a completely different phenotype (Sasaki et al., 2002). It is therefore necessary to investigate each strain's genotype and phenotype in its natural host, as well as other possible hosts in which it may have been transferred through shared host plants.
Plant-mediated transmission might explain the widespread abundance of Wolbachia infection in phytophagous arthropods and the presence of its identical strains in evolutionarily distant species. Overall, plant-mediated transmission might be having a crucial unknown role in ecological and evolutionary biology. In this study, some novel ‘reservoir' spherules were found along the cotton leaf phloem using FISH, but this kind of spherule was not found when the Rickettsia-infected B. tabaci MEAM1 species fed upon cotton leaves while contaminating the phloem with Rickettsia (An et al., 2015). The biological roles of the Wolbachia ‘reservoir' in plant leaves, and the consequent plant-mediated transmission, need to be further investigated to fully understand the dynamics of Wolbachia infection.
Acknowledgments
We are very grateful to Francis M Jiggins, John J Welch (University of Cambridge) and David Plotkin (University of Florida) for their constructive responses on an earlier version of this manuscript and review the language. This research was funded by the National Basic Research Program of China (2013CB127600), the National Department Public Benefit Research Foundation (201303019) and the Guangdong Province Universities and Colleges Pearl River Scholar Funded Scheme (201419) to BLQ.
Author contributions
BLQ and SJL designed the study; SJL, MZA, NL, PQS and JLH performed the experiments; BLQ, SJL, XMW and MZA analyzed the data; BLQ and MZA wrote the paper; BLQ supported the grants.
Footnotes
Supplementary Information accompanies this paper on The ISME Journal website (http://www.nature.com/ismej)
The authors declare no conflict of interest.
Supplementary Material
References
- Ahmed MZ, De Barro PJ, Ren SX, Greeff JM, Qiu BL. (2013). Evidence for horizontal transmission of secondary endosymbionts in the Bemisia tabaci Cryptic Species Complex. PLoS One 8: e53084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed MZ, Li SJ, Xue X, Yin XJ, Ren SX, Jiggins FM et al. (2015). The intracellular bacterium Wolbachia uses parasitoid wasps as phoretic vectors for efficient horizontal transmission. PLoS Pathog 10: e1004672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed MZ, Ren SX, Xue X, Li XX, Jin GH, Qiu BL. (2010). Prevalence of endosymbionts in Bemisia tabaci populations and their in vivo sensitivity to antibiotics. Curr Microbiol 61: 322–328. [DOI] [PubMed] [Google Scholar]
- An X, Li YH, Li SJ, Guo CF, Ren SX, Qiu BL. (2015). Preliminary research on the distribution and transmission efficiency of Rickettsia, an endosymbiont of whitefly Bemisia tabaci. Chinese J Appl Entomol 52: 135–142. [Google Scholar]
- Baldini F, Segata N, Pompon J, Marcenac P, Shaw WR, Dabire RK et al. (2014). Evidence of natural Wolbachia infections in field populations of Anopheles gambiae. Nat Commun 5: 3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldo L, Ayoub NA, Hayashi CY, Russell JA, Stahlhut JK, Werren JH. (2008). Insight into the routes of Wolbachia invasion: high levels of horizontal transfer in the spider genus Agelenopsis revealed by Wolbachia strain and mitochondrial DNA diversity. Mol Ecol 17: 557–569. [DOI] [PubMed] [Google Scholar]
- Baldo L, Hotopp JCD, Jolley KA, Bordenstein SR, Biber SA, Choudhury RR et al. (2006). Multilocus sequence typing system for the endosymbiont Wolbachia pipientis. Appl Environ Microb 72: 7098–7110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle L, Oneill SL, Robertson HM, Karr TL. (1993). Interspecific and intraspecific horizontal transfer of Wolbachia in Drosophila. Science 260: 1796–1799. [DOI] [PubMed] [Google Scholar]
- Braig HR, Zhou W, Dobson SL, O'Neill SL. (1998). Cloning and characterization of a gene encoding the major surface protein of the bacterial endosymbiont Wolbachia pipientis. J Bacteriol 180: 2373–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressan A. (2014). Emergence and evolution of Arsenophonus bacteria as insect-vectored plant pathogens. Infect Genet Evol 22: 81–90. [DOI] [PubMed] [Google Scholar]
- Brown AN, Lloyd VK. (2015). Evidence for horizontal transfer of Wolbachia by a Drosophila mite. Exp Appl Acarol 66: 301–311. [DOI] [PubMed] [Google Scholar]
- Buchner P. (1965) Endosymbiosis of Animals with Plant Microorganisms. John Wiley and Sons: New York, NY, USA. [Google Scholar]
- Caspi-Fluger A, Inbar M, Mozes-Daube N, Katzir N, Portnoy V, Belausov E et al. (2012). Horizontal transmission of the insect symbiont Rickettsia is plant-mediated. Proc R Soc Ser B 279: 1791–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi-Fluger A, Inbar M, Mozes-Daube N, Mouton L, Hunter MS, Zchori-Fein E. (2011). Rickettsia 'in' and 'out': two different localization patterns of a bacterial symbiont in the same insect species. PLoS One 6: e21096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiel E, Gottlieb Y, Zchori-Fein E, Mozes-Daube N, Katzir N, Inbar M et al. (2007). Biotype-dependent secondary symbiont communities in sympatric populations of Bemisia tabaci. Bull Entomol Res 97: 407–413. [DOI] [PubMed] [Google Scholar]
- Chiel E, Zchori-Fein E, Inbar M, Gottlieb Y, Adachi-Hagimori T, Kelly SE et al. (2009). Almost there: transmission routes of bacterial symbionts between trophic levels. PLoS One 4: e4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Barro PJ, Ahmed MZ. (2011). Genetic networking of the Bemisia tabaci cryptic species complex reveals patterns of biological invasions. PLoS One 6: e25579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duron O, Wilkes TE, Hurst GDD. (2010). Interspecific transmission of a male-killing bacterium on an ecological timescale. Ecol Lett 13: 1139–1148. [DOI] [PubMed] [Google Scholar]
- Gehrer L, Vorburger C. (2012). Parasitoids as vectors of facultative bacterial endosymbionts in aphids. Biol Lett 8: 613–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerth M, Roethe J, Bleidorn C. (2013). Tracing horizontal Wolbachia movements among bees (Anthophila): a combined approach using multilocus sequence typing data and host phylogeny. Mol Ecol 22: 6149–6162. [DOI] [PubMed] [Google Scholar]
- Ghanim M, Kontsedalov S. (2009). Susceptibility to insecticides in the Q biotype of Bemisia tabaci is correlated with bacterial symbiont densities. Pest Manag Sci 65: 939–942. [DOI] [PubMed] [Google Scholar]
- Gotoh T, Sugasawa J, Noda H, Kitashima Y. (2007). Wolbachia-induced cytoplasmic incompatibility in Japanese populations of Tetranychus urticae (Acari: Tetranychidae). Exp Appl Acarol 42: 1–16. [DOI] [PubMed] [Google Scholar]
- Gottlieb Y, Ghanim M, Gueguen G, Kontsedalov S, Vavre F, Fleury F et al. (2008). Inherited intracellular ecosystem: symbiotic bacteria share bacteriocytes in whiteflies. FASEB J 22: 2591–2599. [DOI] [PubMed] [Google Scholar]
- Heath BD, Butcher RDJ, Whitfield WGF, Hubbard SF. (1999). Horizontal transfer of Wolbachia between phylogenetically distant insect species by a naturally occurring mechanism. Curr Biol 9: 313–316. [DOI] [PubMed] [Google Scholar]
- Hilgenboecker K, Hammerstein P, Schlattmann P, Telschow A, Werren JH. (2008). How many species are infected with Wolbachia?—a statistical analysis of current data. FEMS Microbiol Lett 281: 215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himler AG, Adachi-Hagimori T, Bergen JE, Kozuch A, Kelly SE, Tabashnik BE et al. (2011). Rapid spread of a bacterial symbiont in an invasive whitefly is driven by fitness benefits and female bias. Science 332: 254–256. [DOI] [PubMed] [Google Scholar]
- Hornett EA, Charlat S, Duplouy AMR, Davies N, Roderick GK, Wedell N et al. (2006). Evolution of male-killer suppression in a natural population. PLoS Biol 4: 1643–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornett EA, Duplouy AMR, Davies N, Roderick GK, Wedell N, Hurst GDD et al. (2008). You can't keep a good parasite down: evolution of a male-killer suppressor uncovers cytoplasmic incompatibility. Evolution 62: 1258–1263. [DOI] [PubMed] [Google Scholar]
- Huigens ME, De Almeida RP, Boons PAH, Luck RF, Stouthamer R. (2004). Natural interspecific and intraspecific horizontal transfer of parthenogenesis-inducing Wolbachia in Trichogramma wasps. Proc R Soc Ser B 271: 509–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huigens ME, Luck RF, Klaassen RHG, Maas MFPM, Timmermans MJTN, Stouthamer R. (2000). Infectious parthenogenesis. Nature 405: 178–179. [DOI] [PubMed] [Google Scholar]
- Jaenike J, Polak M, Fiskin A, Helou M, Minhas M. (2007). Interspecific transmission of endosymbiotic Spiroplasma by mites. Biol Lett 3: 23–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenike J, Brekke TD. (2011). Defensive endosymbionts: a cryptic trophic level in community ecology. Ecol Lett 14: 150–155. [DOI] [PubMed] [Google Scholar]
- Jiggins FM, Hurst GDD. (2011). Rapid insect evolution by symbiont transfer. Science 332: 185–186. [DOI] [PubMed] [Google Scholar]
- Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S et al. (2012). Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28: 1647–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Clec'h W, Chevalier FD, Genty L, Bertaux J, Bouchon D, Sicard M. (2013). Cannibalism and predation as paths for horizontal passage of Wolbachia between terrestrial isopods. PLoS One 8: e60232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda H, Miyoshi T, Zhang Q, Watanabe K, Deng K, Hoshizaki S. (2001). Wolbachia infection shared among planthoppers (Homoptera: Delphacidae) and their endoparasite (Strepsiptera: Elenchidae): a probable case of interspecies transmission. Mol Ecol 10: 2101–2106. [DOI] [PubMed] [Google Scholar]
- O'Neill SL, Giordano R, Colbert AM, Karr TL, Robertson HM. (1992). 16S rRNA phylogenetic analysis of the bacterial endosymbionts associated with cytoplasmic incompatibility in insects. Proc Natl Acad Sci USA 89: 2699–2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver KM, Degnan PH, Burke GR, Moran NA. (2010). Facultative symbionts in aphids and the horizontal transfer of ecologically important traits. Annu Rev Entomol 55: 247–266. [DOI] [PubMed] [Google Scholar]
- Oliver KM, Moran NA, Hunter MS. (2005). Variation in resistance to parasitism in aphids is due to symbionts not host genotype. Proc Natl Acad Sci USA 102: 12795–12800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver KM, Russell JA, Moran NA, Hunter MS. (2003). Facultative bacterial symbionts in aphids confer resistance to parasitic wasps. Proc Natl Acad Sci USA 100: 1803–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell AH, Suslow KG, Klein M. (1994). Transmission via plants of an insect pathogenic bacterium that does not multiply or move in plants. Microbiol Ecol 27: 19–26. [DOI] [PubMed] [Google Scholar]
- Raychoudhury R, Baldo L, Oliveira DCSG, Werren JH. (2009). Modes of acquisition of Wolbachia: horizontal transfer, hybrid introgression, and codivergence in the Nasonia species complex. Evolution 63: 165–183. [DOI] [PubMed] [Google Scholar]
- Rigaud T, Juchault P. (1995). Success and failure of horizontal transfers of feminizing Wolbachia endosymbionts in woodlice. J Evol Biol 8: 249–255. [Google Scholar]
- Sakurai M, Koga R, Tsuchida T, Meng XY, Fukatsu T. (2005). Rickettsia symbiont in the pea aphid Acyrthosiphon pisum: novel cellular tropism, effect on host fitness, and interaction with the essential symbiont Buchnera. Appl Environ Microb 71: 4069–4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Kubo T, Ishikawa H. (2002). Interspecific transfer of Wolbachia between two lepidopteran insects expressing cytoplasmic incompatibility: a Wolbachia variant naturally infecting Cadra cautella causes male killing in Ephestia kuehniella. Genetics 162: 1313–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarborough CL, Ferrari J, Godfray HCJ. (2005). Aphid protected from pathogen by endosymbiont. Science 310: 1781–1781. [DOI] [PubMed] [Google Scholar]
- Segoli M, Stouthamer R, Stouthamer CM, Rugman-Jones P, Rosenheim JA. (2013). The effect of Wolbachia on the lifetime reproductive success of its insect host in the field. J Evolution Biol 26: 2716–2720. [DOI] [PubMed] [Google Scholar]
- Sintupachee S, Milne JR, Poonchaisri S, Baimai V, Kittayapong P. (2006). Closely related Wolbachia strains within the pumpkin arthropod community and the potential for horizontal transmission via the plant. Microbiol Ecol 51: 294–301. [DOI] [PubMed] [Google Scholar]
- Skaljac M, Zanic K, Hrncic S, Radonjic S, Perovic T, Ghanim M. (2013). Diversity and localization of bacterial symbionts in three whitefly species (Hemiptera: Aleyrodidae) from the east coast of the Adriatic Sea. Bull Entomol Res 103: 48–59. [DOI] [PubMed] [Google Scholar]
- Stahlhut JK, Desjardins CA, Clark ME, Baldo L, Russell JA, Werren JH et al. (2010). The mushroom habitat as an ecological arena for global exchange of Wolbachia. Mol Ecol 19: 1940–1952. [DOI] [PubMed] [Google Scholar]
- Stamatakis A. (2006). RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22: 2688–2690. [DOI] [PubMed] [Google Scholar]
- Stansly PA, Naranjo SE. (2010) Bemisia: Bionomic and Management of a Global Pest. Springer: New York, NY, USA. [Google Scholar]
- Teixeira L, Ferreira A, Ashburner M. (2008). The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol 6: 2753–2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu LL, Zhang XL, Liu DQ, Jin SX, Cao JL, Zhu LF et al. (2007). Suitable internal control genes for qRT-PCR normalization in cotton fiber development and somatic embryogenesis. Chinese Sci Bull 52: 3110–3117. [Google Scholar]
- Vavre F, Fleury F, Lepetit D, Fouillet P, Bouletreau M. (1999). Phylogenetic evidence for horizontal transmission of Wolbachia in host-parasitoid associations. Mol Biol Evol 16: 1711–1723. [DOI] [PubMed] [Google Scholar]
- Watts T, Haselkorn TS, Moran NA, Markow TA. (2009). Variable incidence of Spiroplasma infections in natural populations of Drosophila species. PLoS One 4: e5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinert LA, Araujo-Jnr EV, Ahmed MZ, Welch JJ. (2015). The incidence of bacterial endosymbionts in terrestrial arthropods. Proc R Soc Ser B 282: 20150249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werren JH, Baldo L, Clark ME. (2008). Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol 6: 741–751. [DOI] [PubMed] [Google Scholar]
- Werren JH, Zhang W, Guo LR. (1995). Evolution and phylogeny of Wolbachia: reproductive parasites of arthropods. Proc R Soc Ser B 261: 55–63. [DOI] [PubMed] [Google Scholar]
- Yang XH, Zhu DH, Liu ZW, Zhao L, Su CY. (2013). High levels of multiple infections, recombination and horizontal transmission of Wolbachia in the Andricus mukaigawae (Hymenoptera; Cynipidae) communities. PLoS One 8: e78970. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.